Autoinjerto conjuntival para el pterigión
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011349.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 11 febrero 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud ocular y de la visión
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Conceiving the review: ICK, VM, XW, CEVG
Designing the review: ICK, VM, XW
Drafting the protocol: ICK, VM, XW
Co‐ordinating the review: EC
Undertaking electronic and manual searches: CEVG
Screening search results: ICK, EC
Organizing retrieved papers against inclusion criteria: EC
Appraising methodological quality of papers: ICK, VM, EC
Abstracting data from papers: ICK, VM, EC, CEVG
Data management of the review: EC
Entering data into RevMan: EC
Analyzing and presenting results: ICK, EC
Interpreting results: ICK, EC
Writing the review: ICK, EC
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Eye Institute, National Institutes of Health, USA.
Elizabeth Clearfield is supported by the Cochrane Eyes and Vision Group US project Grant 1 U01 EY020522
-
National Institute for Health Research, UK.
-
Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Grou (CEV), acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
-
Declarations of interest
EC: No conflict of interest or financial interest.
VM: No conflict of interest or financial interest.
XW: No conflict of interest or financial interest.
ICK: No conflict of interest or financial interest.
Acknowledgements
The review authors would like to thank Claire Twose and Lori Rosman, Trials Search Co‐ordinators, Cochrane Eyes and Vision Group (CEVG), for designing the search strategies. We thank Sonal Singh and Barbara Hawkins for their comments during the preparation of the review. We also acknowledge the CEVG editorial team's support and peer reviewers for their comments on the review.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Feb 11 | Conjunctival autograft for pterygium | Review | Elizabeth Clearfield, Valliammai Muthappan, Xue Wang, Irene C Kuo | |
| 2014 Oct 20 | Conjunctival autograft for pterygium | Protocol | Irene C Kuo, Valliammai Muthappan, Xue Wang | |
Differences between protocol and review
To meet Cochrane standards, we modified our methods to include an assessment of the certainty of the evidence using the GRADE classification. These assessments are presented in the Summary of Findings table.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
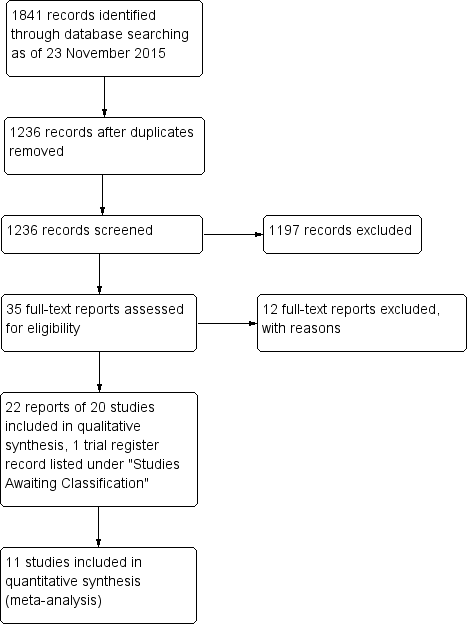
Study flow diagram.
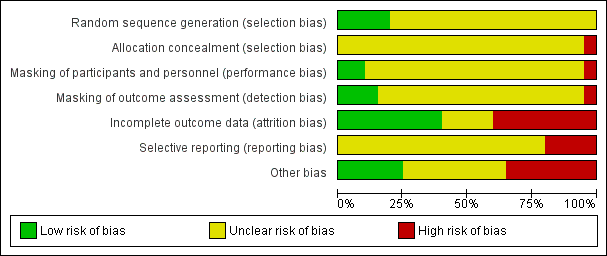
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Forest plot of comparison: 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), outcome: 1.1 Recurrence of pterygium at 3 months.
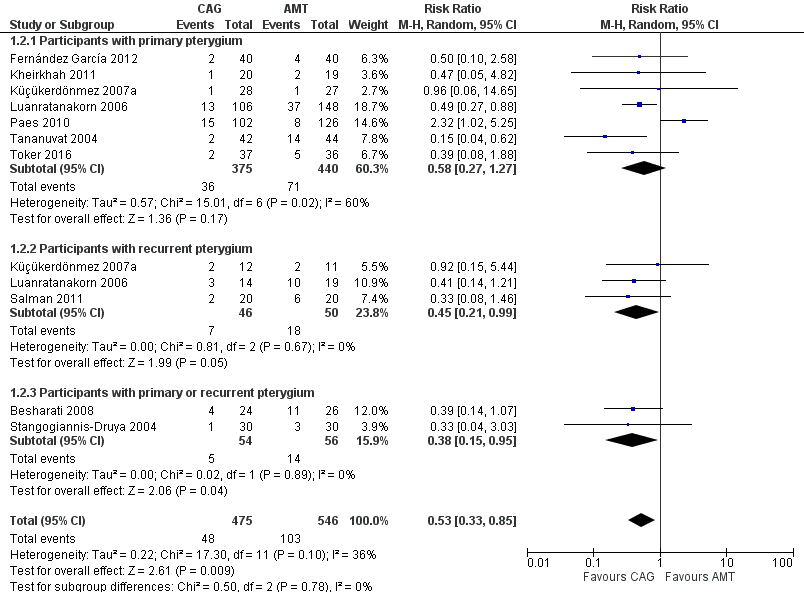
Forest plot of comparison: 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), outcome: 1.2 Recurrence of pterygium at 6 months.

Forest plot of comparison: 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), outcome: 1.3 Adverse events.

Comparison 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), Outcome 1 Recurrence of pterygium at 3 months.

Comparison 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), Outcome 2 Recurrence of pterygium at 6 months.

Comparison 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), Outcome 3 Adverse events.
| Conjunctival autograft compared to amniotic membrane transplant for pterygium | ||||||
| Patient or population: people with primary or recurrent pterygium | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of eyes | Quality of the evidence | Comment | |
| Risk with amniotic membrane transplant | Risk with conjunctival autograft | |||||
| Recurrence of pterygium | Study population | RR 0.87 (0.43 to 1.77) | 538 | ⊕⊝⊝⊝ | ||
| 89 per 1000 | 77 per 1000 | |||||
| Recurrence of pterygium | Study population | RR 0.53 (0.33 to 0.85) | 1021 | ⊕⊕⊕⊝ | ||
| 189 per 1000 | 100 per 1000 | |||||
| Clinical improvement (non‐recurrence risk) follow‐up: 3 months | See comment | One study reported the risk of non‐recurrence as 93.8% for participants in the conjunctival limbal autograft group and 93.3% in the amniotic membrane transplant group at 3 months after surgery | ||||
| Need for repeat surgery | See comment | 2 studies reported the need for repeat surgery but did not provide time points. In 1 study, 1 participant in the amniotic membrane transplant group developed suture lysis, and amniotic membrane revision was performed. In the other study, 1 participant in each surgical group had surgery again | ||||
| Mean change in visual acuity | See comment | No study reported mean change. 1 study reported the logMAR at baseline and postoperatively, and there was no difference (mean difference 0.00, 95% CI ‐0.66 to 0.66) | ||||
| Quality of life | None of the included studies reported on quality of life measures after the 2 surgeries | |||||
| Direct and indirect costs | None of the included studies reported on direct or indirect costs after the 2 surgeries | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Allocation concealment unclear in every study and evidence of possible attrition bias. | ||||||
| Estimates Including Paes 2010 Study | Estimates Not Including Paes 2010 Study | |||
| Outcome | Number of Studies (Participants) | Risk Ratio (M‐H, Random, 95% CI) | Number of Studies (Participants) | Risk Ratio (M‐H, Random, 95% CI) |
| Recurrence of pterygium at 3 months | 6 (538) | 0.87 [0.43, 1.77] | 5 (310) | 0.62 [0.30, 1.27] |
| Participants with primary pterygium | 5 (488) | 0.92 [0.37, 2.30] | 4 (260) | 0.62 [0.24, 1.60] |
| Participants with primary or recurrent pterygium | 1 (50) | 0.62 [0.21, 1.85] | 1 (50) | 0.62 [0.21, 1.85] |
| Recurrence of pterygium at 6 months | 10 (1,021) | 0.53 [0.33, 0.85] | 9 (793) | 0.43 [0.30, 0.62] |
| Participants with primary pterygium | 7 (815) | 0.58 [0.27, 1.27] | 6 (587) | 0.43 [0.27, 0.69] |
| Participants with recurrent pterygium | 3 (96) | 0.45 [0.21, 0.99] | 3 (96) | 0.45 [0.21, 0.99] |
| Participants with primary or recurrent pterygium | 2 (110) | 0.38 [0.15, 0.95] | 2 (110) | 0.38 [0.15, 0.95] |
| Event | Time point | Number of studies (reference) | Conjunctival autograft, n (%) | Amniotic membrane transplant, n (%) | Risk ratio (95% CI) |
| Chemosis | 6 months | 1 (Salman 2011) | 4 (20.0) | 2 (10.0) | 2.00 (0.41 to 9.71) |
| Conjunctival contraction | 1 month | 1 (Besharati 2008) | 3 (12.5) | 4 (15.4) | 0.81 (0.20 to 3.26) |
| 3 months | 1 (Besharati 2008) | 1 (4.2) | 3 (11.5) | 0.36 (0.04 to 3.24) | |
| 6 months | 1 (Besharati 2008) | 2 (8.5) | 5 (19.2) | 0.43 (0.09 to 2.03) | |
| Conjunctival inflammation ‐ grade 1 to 3 | During study | 1 (Kheirkhah 2011) | 3 (15) | 16 (84.2) | 0.18 (0.06 to 0.51) |
| Corneal scar | 1 month | 1 (Besharati 2008) | 16 (66.7) | 16 (61.5) | 1.08 (0.72 to 1.64) |
| 3 months | 1 (Besharati 2008) | 16 (66.7) | 16 (61.5) | 1.08 (0.72 to 1.64) | |
| 6 months | 1 (Besharati 2008) | 16 (66.7) | 16 (61.5) | 1.08 (0.72 to 1.64) | |
| Conjunctivitis | Overall | 0 (0) | 1 (3.6) | 0.33 (0.01 to 7.85) | |
| Diplopia | 3 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| 6 months | 1 (Besharati 2008) | 0 (0) | 2 (7.7) | 0.22 (0.01 to 4.28) | |
| Epithelial defect (lasting more than 5 days) | During study | 1 (Katircioglu 2014) | 1 (3.3) | 0 (0) | 0.67 (0.20 to 2.22) |
| Eye movement restriction | 3 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| 6 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) | |
| Eyelid edema and conjunctival hyperemia edema | 12 months | 1 (Liang 2012) | 8 (9.9) | 12 (23.1) | 0.43 (0.19 to 0.98) |
| Foreign body sensation or discomfort | 12 months | 1 (Liang 2012) | 11 (13.6) | 17 (32.7) | 0.42 (0.21 to 0.81) |
| Graft dehiscence/suture dehiscence | 1 month | 1 (Besharati 2008) | 3 (12.5) | 1 (3.8) | 3.25 (0.36 to 29.16) |
| 3 months | 1 (Besharati 2008) | 3 (12.5) | 1 (3.8) | 3.25 (0.36 to 29.16) | |
| 6 months | 1 (Besharati 2008) | 3 (12.5) | 1 (3.8) | 3.25 (0.36 to 29.16) | |
| During study | 1 (Toker 2016) | 2 (5.4) | 2 (5.6) | 0.97 (0.14 to 6.54) | |
| Overall | 0 (0) | 1 (3.6) | 0.33 (0.01 to 7.85) | ||
| Graft reaction | During study | 1 (7.1) | 0 (0) | 2.80 (0.12 to 63.20) | |
| Granuloma | During study | 4 (4.3) | 6 (6.5) | 0.71 (0.23 to 2.18) | |
| Increased intraocular pressure | 6 months | 8 (6.7) | 5 (3.0) | 2.52 (0.91 to 7.00) | |
| During study | 1 (Tananuvat 2004) | 5 (11.9) | 4 (9.1) | 1.31 (0.38 to 4.55) | |
| Infection | 1 month | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| Inflammation | During study | 1 (Pérez Parra 2008) | 2 (4.4) | 0 (0) | 5.00 (0.25 to 101.31) |
| Graft edema | During study | 0 (0) | 2 (15.4) | 0.19 (0.01 to 3.56) | |
| Other defect taking > 10 days to heal | During study | 1 (Katircioglu 2014) | 4 (13.4) | 5 (20) | 2.52 (0.11 to 59.18) |
| Pyogenic granuloma | 1 month | 1 (Besharati 2008) | 4 (16.7) | 2 (7.7) | 2.17 (0.44 to 10.78) |
| 3 months | 1 (Besharati 2008) | 4 (16.7) | 2 (7.7) | 2.17 (0.44 to 10.78) | |
| 6 months | 8 (5.6) | 5 (2.6) | 1.99 (0.67 to 5.90) | ||
| During study | 1 (1.3) | 5 (7.0) | 0.33 (0.07 to 1.55) | ||
| Severe pain | During study | 1 (Katircioglu 2014) | 4 (13.4) | 2 (8.0) | 1.67 (0.33 to 8.36) |
| Subconjunctival hemorrhage | Overall | 4 (14.2) | 0 (0) | 9.00 (0.51 to 159.70) | |
| Superficial punctate keratitis | Overall | 3 (10.7) | 3 (10.7) | 1.00 (0.22 to 4.54) | |
| 2 years | 1 (Chen 2012) | 1 (2.2) | 2 (4.4) | 0.50 (0.05 to 5.32) | |
| Symblepharon | 6 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| 2 years | 1 (Chen 2012) | 1 (2.2) | 1 (2.2) | 1.00 (0.06 to 15.50) | |
| During study | 1 (Perry 2000) | 1 (12.5) | 0 (0) | 3.00 (0.14 to 64.26) | |
| Wound healing | 2 years | 1 (Chen 2012) | 3 (6.7) | 3 (6.7) | 1.00 (0.21 to 4.69) |
| CI: confidence interval | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of pterygium at 3 months Show forest plot | 6 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.77] |
| 1.1 Participants with primary pterygium | 5 | 488 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.37, 2.30] |
| 1.2 Participants with primary or recurrent pterygium | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.21, 1.85] |
| 2 Recurrence of pterygium at 6 months Show forest plot | 10 | 1021 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.33, 0.85] |
| 2.1 Participants with primary pterygium | 7 | 815 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.27, 1.27] |
| 2.2 Participants with recurrent pterygium | 3 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.21, 0.99] |
| 2.3 Participants with primary or recurrent pterygium | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 0.95] |
| 3 Adverse events Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Pyogenic granuloma during the study | 3 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.55] |
| 3.2 Pyogenic granuloma at 6 months | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.67, 5.90] |
| 3.3 Granuloma during study | 3 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.18] |
| 3.4 Increased IOP at 6 months | 2 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.91, 7.00] |

