پیوند اتوگرافت ملتحمه در درمان تریژیوم (pterygium)
چکیده
پیشینه
تریژیوم (pterygium) یا ناخنک، ضایعهای گوشتی و مثلثیشکل (wing‐shaped) از ملتحمه است که از محل لیمبوس (limbus) تا قرنیه کشیده میشود. شیوع آن در سراسر جهان بسیار گسترده است. شواهد موجود حاکی از آن هستند که پرتوهای فرابنفش از مهمترین عوامل مساعد کننده در تشکیل تریژیومها هستند. تریژیوم باعث اختلال بینایی و محدودیت حرکات چشم میشود و میتواند در چشم، آزردگی و احساس وجود جسم خارجی و خشکی ایجاد کند. در گروهی از بیماران مستعد، تریژیوم ممکن است با رشد و پوشاندن تمام قرنیه، محور بینایی را به کلی مسدود کند.
هر چند جراحی تنها درمان اثربخش برای تریژیوم است، اما عود پس از درمان شایع است. در بیمارانی که تحت جراحی با تکنیک اکسیزیون ساده (simple excision) قرار گرفتهاند (در این نوع جراحی، پس از خارج کردن تریژیوم، صلبیه (sclera) بدون پوشش (bare) رها میشود)، خطر عود 80% گزارش شده است. اما خطر عود در اکسیزیون ضایعه همراه با استفاده از بافت پیوندی یا گرافت (graft)، کمتر است. در جراحی اتوگرافت (autograft) ملتحمه، برای پوشش ناحیهای که تریژیوم از روی آن برداشته شده، از بافت ملتحمه ناحیه دیگری از چشم خود فرد که همراه با بافت لیمبوس در قالب یک قطعه جدا (resect) شده، استفاده میشود. نمونه دیگر از جراحی تریژیوم، با استفاده از گرافتی انجام میشود، که از بافت پرده آمنیوتیک (amniotic membrane) تهیه شده است، در این روش گرافت نامبرده در ناحیهای که پس از برداشتن تریژیوم بدون پوشش مانده به باقیمانده بافت لیمبوس متصل میشود.
اهداف
هدف از این مرور، ارزیابی اثربخشی و بیخطری پیوند اتوگرافت ملتحمه (با یا بدون درمان کمکی (adjunctive)) در مقایسه با پیوند پرده آمنیوتیک (با یا بدون درمان کمکی) در جراحی تریژیوم است. از اهداف دیگر این مطالعه همچنین، تعیین تاثیر احتمالی استفاده از MMC در بهبود نتایج جراحی و ارزیابی هزینههای مستقیم و غیر‐مستقیم این پروسیجرهاست.
روشهای جستوجو
جستوجوی خود را در منابع زیر انجام دادیم: CENTRAL (شامل پايگاه ثبت تخصصی گروه چشم و بینایی در کاکرین (Cochrane Eyes and Vision Trials Register)) (شماره 10؛ 2015)؛ Ovid MEDLINE؛ Ovid MEDLINE برای استنادات در حال انجام و سایر استنادات نمایه نشده؛ Ovid MEDLINE Daily؛ Ovid OLDMEDLINE (از ژانویه 1946 تا نوامبر 2015)؛ EMBASE (از ژانویه 1980 تا نوامبر 2015)؛ PubMed (از 1948 تا نوامبر 2015)؛ بانک اطلاعاتی منابع علمی سلامت آمریکای لاتین و کارائیب (LILACS) (از 1982 تا نوامبر 2015)؛ متارجیستری از کارآزماییهای کنترل شده (mRCT)؛ (www.controlled-trials.com) (آخرین جستوجو در 21 نوامبر 2014)؛ ClinicalTrials.gov (درwww.clinicaltrials.gov) و پلتفرم بینالمللی پایگاه ثبت کارآزماییهای بالینی (ICTRP) سازمان جهانی بهداشت (WHO) (www.who.int/ictrp/search/en). در جستوجوی الکترونیکی برای یافتن کارآزماییها، هیچ محدودیتی را از نظر تاریخ یا زبان مطالعه اعمال نکردیم. آخرین جستوجوی ما در بانکهای اطلاعاتی الکترونیکی به تاریخ 23 نوامبر 2015 برمیگردد.
معیارهای انتخاب
موارد کارآزماییهای تصادفیسازی و کنترل شده، که در آنها جراحی پیوند اتوگرافت ملتحمه (با یا بدون درمان کمکی) با جراحی پیوند پرده آمنیوتیک (با یا بدون درمان کمکی) در افراد دارای موارد اولیه یا عود کرده تریژیوم مقایسه شده بود، را وارد مرور کردیم.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم، نتایج جستوجو را غربالگری و متن کامل گزارشاتی را که از کارآزماییهای بالقوه واجد شرایط بودند، ارزیابی کردند. دو نویسنده مرور بهطور مستقل از هم ، دادهها را از کارآزماییهای وارد شده استخراج و ویژگیها و خطر سوگیری (bias) را در آنها ارزیابی کردند. پیامد اولیه، خطر عود تریژیوم در 3 ماه و 6 ماه پس از جراحی بود. نتایج حاصل از مطالعات مجزا را در متاآنالیزها با استفاده از مدلهای اثرات‐تصادفی ترکیب کردیم. در نهایت خطر عود تریژیوم با معیار خطر نسبی برای مقایسه اتوگرافت ملتحمه و پیوند پرده آمنیوتیک بیان شد.
نتایج اصلی
در 20 مطالعه شناسایی شده، در مجموع 1947 چشم از 1866 شرکتکننده بررسی شده بود (دامنه تعداد این شرکتکنندگان، که با روش تصادفیسازی (randomization) مطالعه شده بودند، از 8 تا 346 نفر در هر مطالعه بود). این مطالعات در هشت کشور مختلف طراحی و اجرا شده بودند: یک مورد در برزیل، سه مورد در چین، سه مورد در کوبا، یک مورد در مصر، دو مورد در تایلند، هفت مورد در ترکیه و سه مورد در ونزوئلا. خطر کلی سوگیری نامشخص بود، زیرا بسیاری از مطالعات اطلاعاتی در مورد روشهای تصادفیسازی یا ماسکه کردن (masking) برای پیشگیری از سوگیری عملکرد و تشخیص ارائه نکردند.
خطر نسبی (RR) برای عود تریژیوم در جراحی اتوگرافت ملتحمه، در برابر جراحی پیوند پرده آمنیوتیک، برای 3 ماه و 6 ماه پس از جراحی به ترتیب 0.87؛ (95% فاصله اطمینان (CI): 0.43 تا 1.77) و 0.53؛ (95% CI؛ 0.33 تا 0.85) محاسبه شد. این تخمینها شامل شرکتکنندگان با تریژیوم اولیه و عود کننده است. تجزیهوتحلیل زیر‐گروه را برای مقایسه شرکتکنندگان دارای تریژیوم اولیه و شرکتکنندگان دارای تریژیوم عود کننده انجام دادیم. در شرکتکنندگان مبتلا به تریژیوم اولیه، خطر نسبی در 3 و 6 ماه به ترتیب 0.92؛ (95% CI؛ 0.37 تا 2.30) و 0.58؛ (95% CI؛ 0.27 تا 1.27) محاسبه شد. ما فقط توانستیم عود تریژیوم را در 6 ماه برای شرکتکنندگان با تریژیوم عود کننده تخمین بزنیم، خطر نسبی در مقایسه اتوگرافت ملتحمه با پیوند پرده آمنیوتیک برابر 0.45 بود (95% CI؛ 0.21 تا 0.99). یک مطالعه پایان نامه دکتری بود و از پنهانسازی تخصیص استفاده نکرد. پس از کنارگذاری این مطالعه در فرآیند آنالیز حساسیت، خطر نسبی برای عود تریژیوم پس از 6 ماه پیگیری، برای شرکتکنندگان مبتلا به تریژیوم اولیه و عود کننده برابر 0.43 بود (95% CI؛ 0.30 تا 0.62). یکی از پیامدهای ثانویه مد نظر، نسبت شرکتکنندگان با بهبود بالینی بود که این پیامد فقط در یک مطالعه آنالیز شد. در این مطالعه، پیامد بالینی به صورت خطر عدم عود گزارش شده بود که تا 3 ماه پس از جراحی برای شرکتکنندگان، در جراحی اتوگرافت ملتحمه و لیمبوس برابر 93.8% و در جراحی پیوند پرده آمنیوتیک برابر 93.3% بود.
دادههای مربوط به نیاز به جراحی مجدد، کیفیت زندگی مرتبط با بینایی، و هزینههای مستقیم و غیر‐مستقیم جراحی را به دلیل تعداد ناکافی مطالعاتی که این پیامدها را گزارش میکنند، آنالیز نکردیم.
در سیزده مطالعه عوارض جانبی جراحیهای اتوگرافت ملتحمه و جراحی پیوند پرده آمنیوتیک گزارش شده بود. عوارض جانبی که در بیش از یک مطالعه گزارش شدند شامل گرانولوم (granuloma) گرانولوم چرکی و افزایش فشار داخل کره چشم بودند. هیچ کدام از مطالعات وارد شده، گزارشی از آستیگماتیسم القایی (induced astigmatism) نداشتند.
نتیجهگیریهای نویسندگان
در ارتباط با اکسیزیون تریژیوم، جراحی اتوگرافت ملتحمه خطر عود کمتری تا شش ماه پس از جراحی، در مقابل جراحی پیوند پرده آمنیوتیک دارد. به ویژه شرکتکنندگانی که تریژیوم عود کننده دارند، در مقایسه با پیوند غشای آمنیوتیک، خطر عود کمتری در هنگام دریافت جراحی اتوگرافت ملتحمه دارند. تعداد کمی از مطالعات هستند که این دو تکنیک جراحی را از منظر پیامدهای حدت بینایی مقایسه میکنند و در این مرور هیچ مطالعهای که گزارشی از کیفیت زندگی متاثر از بینایی مطلوب و همچنین هزینههای مستقیم یا غیر‐مستقیم داشته باشد، وجود نداشت. مقایسه این دو پروسیجر از منظر این معیارهای پیامد، تحقیقات بیشتری را میطلبد. تعداد مطالعاتی که تاثیر میتومایسین سی (mitomycin c) را به عنوان درمان کمکی به دنبال هر یک از این دو تکنیک تخمین زده بودند، برای بررسی کافی نبودند.
PICO
خلاصه به زبان ساده
جراحی پیوند بافت برای درمان رشد مثلثیشکل (تریژیوم) در چشم
سوال مطالعه مروری
در این مطالعه شواهد حاصل از مطالعات قبلی را برای یافتن اینکه کدام روش جراحی در درمان تریژیوم (pterygium) (که یک ضایعه در ملتحمه چشم است) کمعارضهتر و بهتر است، مرور کردیم. میخواستیم بدانیم کدام جراحی، بهتر از رشد دوباره تریژیوم پیشگیری میکند.
پیشینه
تریژیوم یک ضایعه مثلثیشکل در بیرونیترین لایه کره چشم است که از گوشه چشم، تا مرز بین سفیدی و عنبیه (ناحیه رنگی چشم) کشیده میشود. علت آن را مواجهه با پرتوهای فرابنفش نور خورشید میدانند. این ضایعه در مردان و افراد مسن شایعتر است. تریژیومهای بزرگ، دید را با مشکل مواجه میکند. چشم بیمار را آزار میدهند و با خشکی چشم باعث میشوند که فرد احساس کند یا احساس کند چیزی در چشمش رفته است. همچنین از نظر زیبایی هم میتوانند مشکلساز باشند. در بعضی افراد، تریژیوم آنقدر رشد میکند که با پوشاندن تمام سطح جلوی چشم، دید بیمار را بسیار کم میکند.
درمان تریژیوم، جراحی است. اما حتی پس از جراحی هم امکان بازگشت آن وجود دارد. وقتی پزشک فقط خود ضایعه را برداشته و سطح زیر آن را بدون پوشش رها میکند، در 80% بیماران ضایعه برمیگردد. در تکنیک جدید جراحی، پس از برداشتن ضایعه سطح زیر آن را با بافتی میپوشانند. جراحی، بافت پیوندی یا گرافت خوانده میشود. وقتی این سطح با گرافت پوشانده میشود، بازگشت تریژیوم به اندازه زمانی نیست که سطح، بدون پوشش باقی بماند.
دو روش برای جراحی با بافت پیوندی وچود دارد: جراحی اتوگرافت (پیوند از خود) ملتحمه (conjunctival autograft surgery; CAG)، و جراحی پیوند از پرده آمنیوتیک (amniotic membrane transplant; AMT). هدف از این مرور مقایسه عود تریژیوم پس از انجام جراحی با هر یک از این دو روش بود.
در روش CAG، بافت از ناحیه دیگری از چشم خود بیمار جدا شده و در ناحیه بدون پوشش حاصل از برداشتن تریژیوم قرار داده میشود. اما در روش AMT، از بافت جفت نوزاد تازه متولد شده استفاده میشود. جراح آن را از بانک پیوند (tissue bank) تهیه میکند.
ویژگیهای مطالعه
روش جراحی را بهتر در نظر گرفتیم که در آن تریژیوم پس از سه و شش ماه پس از جراحی بازگشت کمتری در افراد داشته باشد. بانکهای اطلاعاتی آنلاین شامل مقالات پزشکی منتشر شده را جستوجو کردیم تا مطالعاتی را پیدا کنیم که شرکتکنندگان را به یکی از این دو جراحی اختصاص داده بود. در نهایت تنها مواردی را برای مرورمان انتخاب کردیم که قرارگیری شرکتکنندگان در هر یک از این دو گروه جراحی، به طور تصادفی (random) انجام شده بود، به این ترتیب شرکتکنندگان در هر یک از این مطالعات از شانس مساوی برای قرارگیری در هر یک از گروهها برخوردار بودند. شرکتکنندگان مطالعه، ممکن بود برای اولین بار تحت جراحی برای تریژیوم قرار گرفته باشند (موارد تریژیوم اولیه) یا به علت عود ضایعه به جراحی مجدد نیاز پیدا کرده باشند. شواهد تا نوامبر 2015 بهروز است.
نتایج کلیدی
20 مطالعه پیدا شد، که در مجموع این دو روش جراحی در 1947 چشم مقایسه شده بود. برای تعیین روش جراحی بهتر، اطلاعات این مطالعات را ادغام کردیم. بازگشت تریژیوم شش ماه پس از جراحی در افراد تحت جراحی CAG نسبت به افراد تحت جراحی AMT، به اندازه یک سوم تا نصف بود. این تفاوت را نمیشود فقط با شانس توجیه کرد.
مطالعات تحت بررسی، به همه سوالات ما پاسخ ندادند. ما هنوز هم میخواهیم تاثیر این دو روش را در مسائلی نظیر شفافیت و کیفیت بینایی و کیفیت زندگی پس از جراحی و همچنین در مورد هزینههای آنها بدانیم. پژوهش بیشتری لازم است تا به این سوالات پاسخ داده شود.
کیفیت شواهد
کیفیت کلی شواهد به دست آمده را که به نفع روش جراحی CAG بودند، میتوان پائین تا متوسط ارزیابی کرد، علت این موضوع، تفاوت و ناهمگونیهایی است که گاهی بین اجرا و نتایج مطالعات تحت بررسی وجود داشت. پژوهش بیشتری که در این زمینه در آینده منتشر خواهند شد، ممکن است بر نتیجهگیریهای انجام شده در این مرور تاثیر بگذارند.
Authors' conclusions
Summary of findings
| Conjunctival autograft compared to amniotic membrane transplant for pterygium | ||||||
| Patient or population: people with primary or recurrent pterygium | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of eyes | Quality of the evidence | Comment | |
| Risk with amniotic membrane transplant | Risk with conjunctival autograft | |||||
| Recurrence of pterygium | Study population | RR 0.87 (0.43 to 1.77) | 538 | ⊕⊝⊝⊝ | ||
| 89 per 1000 | 77 per 1000 | |||||
| Recurrence of pterygium | Study population | RR 0.53 (0.33 to 0.85) | 1021 | ⊕⊕⊕⊝ | ||
| 189 per 1000 | 100 per 1000 | |||||
| Clinical improvement (non‐recurrence risk) follow‐up: 3 months | See comment | One study reported the risk of non‐recurrence as 93.8% for participants in the conjunctival limbal autograft group and 93.3% in the amniotic membrane transplant group at 3 months after surgery | ||||
| Need for repeat surgery | See comment | 2 studies reported the need for repeat surgery but did not provide time points. In 1 study, 1 participant in the amniotic membrane transplant group developed suture lysis, and amniotic membrane revision was performed. In the other study, 1 participant in each surgical group had surgery again | ||||
| Mean change in visual acuity | See comment | No study reported mean change. 1 study reported the logMAR at baseline and postoperatively, and there was no difference (mean difference 0.00, 95% CI ‐0.66 to 0.66) | ||||
| Quality of life | None of the included studies reported on quality of life measures after the 2 surgeries | |||||
| Direct and indirect costs | None of the included studies reported on direct or indirect costs after the 2 surgeries | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Allocation concealment unclear in every study and evidence of possible attrition bias. | ||||||
Background
Description of the condition
A pterygium is a fleshy, wing‐shaped growth from the conjunctiva, crossing over the limbus onto the cornea. The tissue is fibrovascular and can occur over the nasal or temporal cornea. It can be a bilateral process and asymmetric with one eye affected by a larger pterygium than the other. In addition, two pterygia can affect a single eye, one nasally and the other temporally. The pterygium consists of collagen tissue that is hyperplastic and denatured, marked by elastotic degeneration (degeneration of collagen fibers). It often is preceded by a growth of the conjunctiva without extension onto the cornea, known as a pingueculum. It is believed that ultraviolet light is a major contributor in the formation of pterygia, though the finding that a high prevalence of pterygium exists outside inhabitants of equatorial regions strongly suggests that reflected, scattered light is also critical (Droutsas 2010). A study in Japan found a high prevalence of pterygium in welders, despite the wearing of eye protection with ultraviolet light filtration (Karai 1984). Both individual susceptibility and environmental exposure appear to be risk factors responsible for the occurrence of pterygium.
Pterygium is twice as common in men as in women, except in Aruba, where it is equally common between the sexes (Singh 2005). It is more prevalent in older age groups, but the incidence is higher in younger individuals. It is uncommon in people younger than 20 years of age and among people who wear glasses (Singh 2005). The incidence increases greatly in people between 20 and 40 years of age (Hill 1989). The prevalence is 23% in Aruba, 18% in Puerto Rico, 5% to 15% in Texas, Florida, California, Arizona, and New Mexico, and drops to 2% or less in areas more than 40 degrees from the equator (Raj 2010). A recent meta‐analysis of 20 studies, mostly from Asia, estimated the prevalence of pterygium to be 10.2% (95% confidence interval (CI) 6.3% to 16.1%) (Liu 2013). The pooled prevalence among men was higher than among women (14.5% versus 13.6%), and the proportion of participants with unilateral cases of pterygium was higher than those with bilateral cases of pterygium (8% versus 6.2%; no P value or CI reported). A greater prevalence of pterygium was associated with increasing geographical latitude and age. Risk factors for pterygium include male gender (odds ratio 2.32, 95% CI 1.66 to 3.23) and outdoor activity (odds ratio 1.76, 95% CI 1.55 to 2.00) (Liu 2013).
Pterygia can impair vision through altered tear film, induced astigmatism, photophobia, epiphora, and binocular diplopia due to contraction of the Tenon's capsule, which limits eye movements. Pterygia can cause symptoms such as eye irritation, foreign body sensation, and dryness. In mild climates, it is unusual for a pterygium to grow over the visual axis, but patients are often concerned about the cosmetic appearance of their eye. In potentially susceptible individuals, the pterygium can grow across the entire corneal surface, impairing vision.
Description of the intervention
Surgery is the only effective treatment for pterygia, and many techniques have been described. No technique prevents recurrences, which are often associated with more ocular morbidity than the primary occurrence. The most common techniques in use include bare sclera excision, first described in 1948 (D'Ombrain 1948), and tissue grafting. These surgeries may be combined with adjunctive radiation or chemotherapy agents such as mitomycin C (MMC) (Kirwan 2003). The risk of recurrence after simple excision is reported to be as high as 88% in certain populations (Chen 1995). Graft tissue use is believed to have been first described in 1876, in which a mucous membrane was used to cover the defect left by pterygium surgery (Klein 1876).
In this review, we focused on two types of tissue grafting procedures: conjunctival autograft versus amniotic membrane graft. Conjunctival limbal autograft (hereafter referred to as "conjunctival autograft") involves removing limbal tissue and adjacent conjunctiva in one piece from another part of the person's eye and using the tissue to cover the area from which the pterygium was excised. Careful attention is paid to aligning the limbal tissue of the autograft to the limbal area over which the pterygium used to lie and which is denuded in the process of removing the pterygium. Conjunctival autograft has been the most popular method of pterygium surgery since it was re‐introduced in the 1980s (Kenyon 1985). A similar method was described at a meeting in 1965 (Barraquer 1965). The size of the autograft tissue is determined by the size of the pterygium tissue that is excised. The orientation of the autograft tissue is such that the limbal side of the transplant is fixed to the limbal area from where the pterygium was excised. The method has been modified over time to include adjunctive use of MMC (an alkylating agent that crosslinks DNA and thus inhibits mitosis) or use of tissue adhesive instead of sutures to attach the conjunctival autograft to the underlying tissue. Though the technique is significantly more lengthy and difficult compared to simple excision, it has the benefit of reducing the recurrence risk to 5% to 15% (Chen 1995).
Amniotic membrane graft, fixed either with sutures or tissue adhesive, is a relatively new technique used increasingly in recent years. A piece of amniotic membrane from a tissue bank or other organization is centered over the area from which the pterygium was removed then sutured or "glued" over the bare scleral area. The tissue can be cryopreserved, dehydrated, and sterilized; if dehydrated, it is re‐hydrated, then cut to the proper size and sutured in such a way to cover the bare sclera left after excision of the pterygium (Prabhasawat 1997; Shimazaki 1998). Possible reasons for the effectiveness of amniotic membrane graft in pterygium surgery include inhibition of pathological neovascularization (Hao 2000; Kim 1995; Kobayashi 1999), scar formation (Tseng 1999), and inflammation (Bultmann 1999; Solomon 1999).
How the intervention might work
Pterygium pathogenesis can be conceptualized as occurring in two stages:
-
initial and progressive disruption of the limbal corneal‐conjunctival epithelial barrier; and
-
progressive active "conjunctivalization" of the cornea by tissue characterized by extensive cellular proliferation, inflammation, connective tissue remodeling, and angiogenesis (Coroneo 1999).
The corneal limbal stem cells are usually abnormal in pterygia (Kwok 1994), such that the limbal corneal‐conjunctival epithelial barrier is disrupted, and the cornea assumes conjunctival characteristics in a process marked by extensive cellular proliferation, inflammation, connective tissue remodeling, and angiogenesis. A healthy limbus acts as a barrier to conjunctival overgrowth (Coroneo 1999). Inclusion of healthy tissue from grafting may reduce pterygium recurrence both physically and physiologically. It is unlikely that moving a limited area of limbus stem cells to another site would damage the ocular surface.
Why it is important to do this review
Conjunctival autograft may be the most common surgical technique for pterygium, but it is not universally accepted that it is the technique with the lowest risk of recurrence. Unlike newer methods, it does not require tissue allograft (tissue from another person). Amniotic membrane transplantation is an alternative, but it is more expensive and utilizes more resources. It is unclear how conjunctival autograft outcomes compare with those of amniotic membrane grafts. Moreover, it is unclear whether these procedures yield better results when combined with MMC. The parameters surrounding use of MMC, a potent antimetabolite that alkylates and crosslinks DNA, must be carefully identified and evaluated, as complications such as corneoscleral melt, necrosis, and perforation have been reported with MMC.
Objectives
The objective of this review was to assess the safety and effectiveness of conjunctival autograft (with or without adjunctive therapy) compared with amniotic membrane graft (with or without adjunctive therapy) for pterygium. We also planned to determine whether use of MMC yielded better surgical results and to assess the direct and indirect comparative costs of these procedures.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs) in this review.
Types of participants
We included studies of participants with primary or recurrent pterygium. We used no restrictions with respect to age, gender, co morbidities, or use of adjunctive therapy.
Types of interventions
We included studies in which conjunctival autograft was compared with amniotic membrane transplantation. We included studies in which intraoperative or postoperative MMC was used in both treatment arms.
Types of outcome measures
Primary outcomes
The primary outcome for comparison of the treatments was the recurrence of pterygium, as defined by the included studies, between three and six months' follow‐up. Recurrence is commonly defined as a recurrent pterygium greater than 1 mm in size anterior to the limbus; in different studies, the definition varied. We used and recorded the definition from each individual study.
Secondary outcomes
We considered the following secondary outcomes for comparison of the treatments for this review, assessed at six months and at one year.
-
The proportion of participants with clinical improvement as defined in the included studies. Clinical improvement typically is defined as 1) lack of recurrence and 2) resolution of pre‐operative symblepharon, binocular diplopia, hyperemia, or vascularization or improvement of ocular motility (involvement of ocular motility is rare and would be lumped with binocular diplopia).
-
The proportion of participants requiring repeat surgery for pterygium.
-
Mean change in best corrected and uncorrected visual acuity from baseline to a follow‐up time point, as reported by individual studies.
-
Vision‐related quality of life as assessed by a validated questionnaire, such as the National Eye Institute Visual Functioning Questionnaire (NEI VFQ).
-
The direct and indirect costs of the interventions.
Adverse outcomes
We compared the proportion of participants experiencing adverse outcomes such as new diplopia, induced astigmatism, and other complications as reported from the included studies.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (Issue 10, 2015), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to November 2015), EMBASE (January 1980 to November 2015), PubMed (1948 to November 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to November 2015), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com) (last searched 25 November 2013), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic search for trials. We last searched the electronic databases on 23 November 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3, PubMed (Appendix 4), LILACS (Appendix 5), mRCT (Appendix 6), ClinicalTrials.gov (Appendix 7), and the ICTRP (Appendix 8).
Searching other resources
We searched reference lists of included studies to identify any additional eligible studies. We also used the Science Citation Index Expanded database to identify additional studies that may have cited trials included in this review. We did not handsearch conference proceedings or journals specifically for this review; many conference abstracts have been handsearched by Cochrane and included in CENTRAL.
Data collection and analysis
Selection of studies
Two review authors independently reviewed titles and abstracts resulting from the literature searches against the eligibility criteria for this review. Each review author classified each record as "definitely relevant," "possibly relevant,"' or "definitely not relevant." We resolved any disagreements through discussion. After reaching a consensus, we retrieved full‐text reports for all records that both review authors classified as "definitely relevant" or "possibly relevant." The two review authors independently re‐assessed each full‐text report for inclusion of the study described therein. We contacted the investigators of studies in which eligibility was uncertain for further information, as required, after examining the full‐text reports. We resolved any disagreements through discussion. We documented the reasons for exclusion of studies after review of full‐text reports by both review authors. For reports written in languages not read by the review authors, we used Google Translate or consulted with a translator, or both to assess studies for eligibility.
Data extraction and management
Two review authors, using data extraction forms developed by the Cochrane Eyes and Vision Group, independently extracted characteristics of each eligible study including the setting, methods, participants, interventions, comparisons, and outcomes. One review author entered data into The Cochrane Collaboration's statistical software, Review Manager (RevMan 2014), and a second review author verified the entered data. We resolved any disagreements after data abstraction or data entry through discussion. We contacted study investigators to request unreported information. If after two weeks we did not receive a response, we proceeded with available information in the published reports. Data from included studies written in languages other than English were abstracted by at least one native reader and confirmed using Google Translate by a review author.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for potential sources of bias according to the guidelines in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We evaluated the following risk of bias domains: random sequence generation and allocation concealment (selection bias), masking of participants and personnel (performance bias), masking of outcome assessors (detection bias), missing data and intention‐to‐treat analysis (attrition bias), selective outcome reporting (reporting bias), and other potential sources of bias. We assessed each study for each bias parameter as either "low risk," "high risk," or "unclear risk" (insufficient information to permit judgment of low or high risk of bias).
We resolved disagreements through discussion. Whenever the methods were unclearly reported or additional information was required to assess the risk of bias, we contacted study investigators. If the investigators did not respond within two weeks, we assessed the risk of bias based on the descriptions as reported in the published reports.
Measures of treatment effect
We calculated summary risk ratios with 95% confidence intervals for dichotomous outcomes. These included proportion of participants who had post‐surgery recurrence of pterygium, had clinical improvement, who required repeat surgery, and had adverse outcomes. For risk of recurrence of pterygium, we planned to include summary reported data by estimating the hazard ratio if possible.
We planned to assess normality of continuous outcomes and calculate mean differences and 95% confidence intervals for vision‐related quality of life data and costs of interventions.
Unit of analysis issues
The unit of analysis was the eye for all outcomes, with the exception of vision‐related quality of life, where the unit of analysis was the person. We planned to attempt to extract or request from the investigators of within‐person trials the data needed to account for the design. If we were unable to retrieve these data, we planned to incorporate statistical techniques to approximate a paired analysis as outlined in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Whenever two eyes of a participant were enrolled in studies included in this review, we reported whether or not appropriate statistical techniques were employed to account for within‐person correlation.
Dealing with missing data
We contacted study investigators to provide more information on desired data that had not been reported such as standard deviations. If we received no response within two weeks, we used the data as reported in the published reports. We did not impute data for the purposes of this review.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by examining variations in participant characteristics, inclusion/exclusion criteria, and methods of assessments of primary and secondary outcomes. We calculated the I2 statistic (%) to determine the proportion of variation in outcomes due to heterogeneity, with a value above 50% suggesting substantial statistical heterogeneity. We also examined the result of the Chi2 test for heterogeneity and the degree of overlap in confidence intervals of included studies. Poor overlap also could suggest the presence of heterogeneity.
Assessment of reporting biases
To assess for selective outcome reporting, we compared the outcomes prespecified in protocols and the outcomes in the published report(s) from included studies. To assess potential publication bias, we planned to examine the symmetry of a funnel plot when 10 or more studies were included in meta‐analysis. We examined study characteristics or other factors that could contribute to asymmetry of the funnel plot.
Data synthesis
We performed meta‐analysis when clinical and methodological heterogeneity was minimal. We combined the results of included trials in a meta‐analysis using a random‐effects model, unless fewer than three studies were included, in which case we used a fixed‐effect model. If the I2 statistic suggested substantial heterogeneity (greater than 50%) and the direction of treatment effects were inconsistent across studies, we did not combine results in a meta‐analysis and instead presented a narrative summary.
Subgroup analysis and investigation of heterogeneity
Whenever sufficient data were available, we conducted subgroup analyses by stratifying participants based on whether they had primary pterygia or recurrent pterygia at study entry. As the characteristics of primary and recurrent pterygia are different, the surgeon may choose a different surgical approach for treating recurrent pterygia. Recurrent pterygia may be more difficult to remove because the eye already has scarring from the primary pterygium and its surgical removal. Eyes that are subject to recurrent pterygia may have a predisposition, either biological or environmental, for exuberant tissue response to the causative factors of pterygium. We also had planned to consider a subgroup analysis by comparing trials that enrolled only unilateral cases and those that enrolled bilateral cases, but only two of the included studies reported on the percentage of enrolled participants with bilateral pterygia versus unilateral pterygium, and only one eye was designated the study and was assigned randomly to an intervention group in both these trials (Besharati 2008; Luanratanakorn 2006). Another potential source of heterogeneity among the studies is the type of fixation used for the tissue graft (sutures or glue) and the use of the MMC as an adjuvant to the tissue graft surgery. It is possible that the fixation and use of MMC could have an effect on the recurrence of pterygium. We plan to evaluate this as more information becomes available.
Sensitivity analysis
We performed sensitivity analyses to determine the impact of excluding a study with lower methodological quality due to incomplete outcome data. We also planned to consider sensitivity analyses excluding studies funded by industry and those that were unpublished at the time of this review, however these were not necessary as we did not identify any industry‐funded or unpublished trials eligible for this review.
'Summary of findings' table
The results of our analyses are summarized in the summary of findings Table for the main comparison. The main outcomes presented are recurrence of pterygium at 3 months, recurrence of pterygium at 6 months, clinical improvement, need for repeat surgery, mean change in visual acuity, quality of life and direct and indirect costs. We used GRADE to assess the quality of the evidence (Guyatt 2011). With this approach, we took into account five factors that could affect our confidence in the study results: study limitations, consistency of effect, imprecision, indirectness, and publication bias.
Results
Description of studies
Results of the search
The original electronic searches conducted 23 November 2015 yielded 1841 records, of which we screened 1236 after removing duplicates (Figure 1). Of these, 35 appeared potentially relevant, and we obtained and screened the full‐text reports. After further analysis, we excluded 12 reports of 12 studies, and included 22 reports of 20 randomized controlled trials (RCTs) that compared the surgeries of interest in this review. Additionally, one trial register record was listed under Studies awaiting classification. Our searches of other sources did not identify any potentially eligible trials.
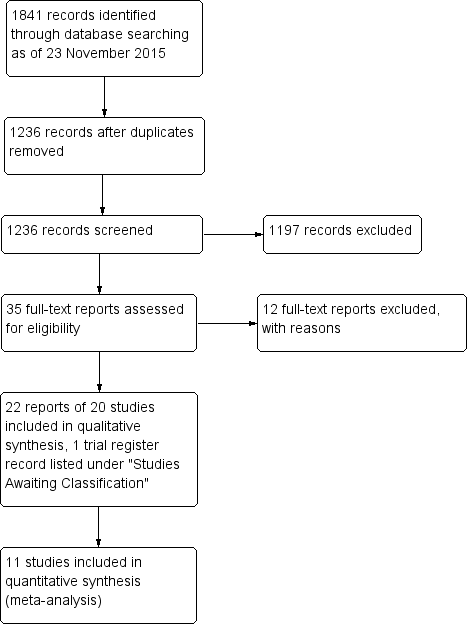
Study flow diagram.
Included studies
We included 20 RCTs in this review (1947 eyes of 1866 participants), published as 22 reports. All of the studies except for one were parallel‐group RCTs (Perry 2000). Perry 2000 was a within‐person RCT, in which each participant had bilateral pterygium, and one eye was randomly assigned to surgery with conjunctival autograft and the other eye was assigned to surgery with amniotic membrane transplant.
Types of participants
We included studies that enrolled participants with primary or recurrent pterygium. Eleven studies restricted enrollment to primary pterygium alone (Chen 2012; Fernández García 2012; Keklikci 2007; Kheirkhah 2011; Küçükerdönmez 2007; Liang 2012; Ozer 2009; Paes 2010; Pérez Parra 2008; Tananuvat 2004; Toker 2016), five studies restricted enrollment to recurrent pterygium alone (Aragonés Cruz 2008; Chen 2009; Katircioglu 2014; Salman 2011; Stangogiannis‐Druya 2004), and three studies included participants with either primary or recurrent pterygium (Besharati 2008; Küçükerdönmez 2007a; Luanratanakorn 2006). Two of the three studies that included participants with both types of pterygium reported outcomes separately by pterygium status (Küçükerdönmez 2007a; Luanratanakorn 2006), and one presented combined results only (Besharati 2008). In one included study, it was unclear whether enrolled participants had primary or recurrent pterygia or both, because participants were reported only as having symptomatic pterygium (Perry 2000). The studies were conducted in eight different countries: one in Brazil, three in China, three in Cuba, one in Egypt, two in Iran, two in Thailand, seven in Turkey, and one in Venezuela.
Types of interventions
The studies analyzed a total of 1947 eyes of 1866 participants. Eight hundred fifty‐eight eyes received conjunctival autograft surgery and 887 eyes received amniotic membrane transplant surgery. Some studies had a third intervention group that we did not include in our meta‐analyses (Chen 2009; Keklikci 2007; Ozer 2009; Salman 2011). Among these third arm intervention groups were 42 participants who received a combination of the two surgeries we assessed (conjunctival autograft plus amniotic membrane transplant) (Chen 2009), 52 participants who were treated with topical mitomycin C over the excision location in lieu of a conjunctival autograft or amniotic membrane transplant (Keklikci 2007 and Salman 2011), and 48 participants who received surgery using the bare sclera technique (pterygia removed and no further treatment) (Ozer 2009).
Types of outcomes
Recurrence of pterygium was the primary outcome in 19 of 20 included studies. Küçükerdönmez 2007 reported on recurrence of pterygium only as a note in their results; the primary outcome of interest in this study was graft vascularization.
Excluded studies
We excluded 12 studies from the review after assessment of full‐text reports. We have reported the excluded studies and reasons for exclusion in the Characteristics of excluded studies table. We excluded two studies because they were not RCTs (Liu 2014; Yan 2010). We excluded four studies because it was unclear whether participants were randomized to the type of surgery they received (Katircioglu 2007; Kim 2008; Ozkurt 2009; Paris 2008). We attempted to contact the authors of these four studies to ask whether their participants were randomized, but received no response, so we chose to exclude them. Two excluded studies had no conjunctival autograft treatment group (Li 2014; Zhang 2014), another was a laboratory study of proteins found in tear films after various surgeries for pterygium (Nava‐Castaneda 2007), and three used a technique that involved a conjunctival flap in addition to a conjunctival autograft, which is an alternative surgical method not analyzed in this review (Lin 2009; Pei 2011; Xia 2008).
Risk of bias in included studies
No trial was distinguished by having a low risk of bias across all criteria. The overwhelming majority of trials (17 of 20) were marked by having an unclear risk of bias or high risk of bias in five or more of the seven parameters we assessed for bias, categorized into selection, performance, detection, attrition, and reporting biases. The most common assessment was unclear risk of bias, meaning the authors did not describe any or all of the following: methods of selection of participants, allocation of participants to treatment arms, masking of surgical arm from participants and outcome observers, how data attrition was handled, or funding source(s). Attrition bias (incomplete outcome data) was the bias for which the largest number of trials were at highest risk. Figure 2 and Figure 3 show a summary of the risk of bias judgments made by the review authors.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Overall, the majority of included trials were judged to have an unclear risk of selection bias. All trials were reported as randomized, but 16 out of 20 trials did not describe the methods of random sequence generation, and were therefore rated as at unclear risk of bias (Aragonés Cruz 2008; Besharati 2008; Chen 2009; Chen 2012; Fernández García 2012; Katircioglu 2014; Keklikci 2007; Kheirkhah 2011; Liang 2012; Luanratanakorn 2006; Ozer 2009; Pérez Parra 2008; Perry 2000; Salman 2011; Stangogiannis‐Druya 2004; Tananuvat 2004). None of the included trials reported their method of allocation concealment before randomization; we therefore judged each as having unclear risk of this type of selection bias. We judged three studies (two by the same authors) to have a low risk of selection bias as they reported using a random number generator to allocate participants to their surgical group (Küçükerdönmez 2007; Küçükerdönmez 2007a; Toker 2016). Another study that was a published doctoral thesis also reported that the authors used a random number scheme (Paes 2010). In communication with the author, he stated the randomization scheme such that participants were given a number from a random number table by the secretary in the order they arrived. If the sum of digits was an even number, the participants were put into one group; if the sum of digits was an odd number, participants were put into the other group. We judged this technique to be a randomization without allocation concealment and later removed this trial in a sensitivity analysis to see if the study with a lack of allocation concealment had a large effect on our conclusions.
Masking (performance bias and detection bias)
Sixteen studies did not report on whether or not they masked participants and outcomes assessors and were judged to have unclear risk of bias (Aragonés Cruz 2008; Besharati 2008; Chen 2009; Chen 2012; Fernández García 2012; Katircioglu 2014; Keklikci 2007; Kheirkhah 2011; Liang 2012; Ozer 2009; Paes 2010; Pérez Parra 2008; Perry 2000; Salman 2011; Stangogiannis‐Druya 2004; Tananuvat 2004). However, due to the nature of the surgical procedures and the different appearance of the eye in the early postoperative period, both performance and detection biases were inherent to these studies. Surgeons performing the surgery would be able to differentiate between amniotic membrane transplant and conjunctival autograft. For postoperative visits, trained observers (if not a surgeon) could differentiate between amniotic membrane transplant and conjunctival autograft. We judged two studies by the same author as low risk because they reported that both participants and outcomes assessors were masked (Küçükerdönmez 2007; Küçükerdönmez 2007a), and though we feel it was realistic to mask participants, we suspect that efforts at masking outcomes assessors were undermined by the ease with which a trained observer would be able to see whether a conjunctival autograft or amniotic membrane transplant was done, based simply on the fact that the site from which conjunctival autograft was harvested will be visible in the first few weeks to months after surgery. However, we judged these two studies to be at low risk of bias because they attempted to prevent performance and detection bias. One study reported that it was designed as a single‐blind randomized controlled trial, and stated that only outcomes assessor (and not participants or surgeons) were masked (Luanratanakorn 2006). This study was judged as having a high risk of performance bias but a low risk of detection bias. We judged another study, Toker 2016, to have high risk of bias for detection bias after communication with the author revealed that the doctors who did the postoperative examinations were not masked. Though we do believe that since the authors of this trial did not did not attempt to mask the outcomes assessors, this results in a high risk of bias, we also think that masking at this stage is not truly possible, and we are unsure if the knowledge of type of procedure received would affect an assessor's ability to determine whether recurrence, our primary outcome, had occurred.
Incomplete outcome data
We judged eight of the included studies to have low risk of attrition bias because they reported that there was no loss to follow‐up (Chen 2009; Chen 2012; Küçükerdönmez 2007; Küçükerdönmez 2007a; Liang 2012; Ozer 2009; Pérez Parra 2008; Salman 2011). In four studies, there was no mention of loss to follow‐up or missing data, and it was not clear from the tables whether all participants completed the study; we judged these studies to have unclear risk of attrition bias (Aragonés Cruz 2008; Fernández García 2012; Perry 2000; Stangogiannis‐Druya 2004). One study, an abstract reporting a clinical trial that was not linked to a full publication, lacked information about trial methods; another reported that five participants were lost to follow‐up, but still included data from those participants in the analysis (Perry 2000 and Aragonés Cruz 2008, respectively). We judged eight included studies to have a high risk of attrition bias because they reported that participants were lost to follow‐up and that these participants' data were excluded from the analysis (Besharati 2008; Katircioglu 2014; Keklikci 2007; Kheirkhah 2011; Luanratanakorn 2006; Paes 2010; Tananuvat 2004; Toker 2016). This judgment was made because the participants may have been lost to follow‐up due to a complication of the surgery to which they were assigned. It is possible that a participant was lost to follow‐up because of pterygium recurrence (our primary outcome) and chose to have another surgery outside the trial.
Selective reporting
We judged the majority of the RCTs included in this review to be at unclear for risk of reporting bias, as they did not have a protocol available to compare anticipated outcomes to reported outcomes. Two studies reported that they presented a protocol to the human subjects research or ethics committees at their respective institutions, however the authors did not provide a reference or a way to access the protocol and the studies were thus judged to have unclear risk of reporting bias ( Luanratanakorn 2006 and Paes 2010). Additionally, we judged four studies as having a high risk of reporting bias either because the authors reported that they collected data on an outcome that was not reported, or the authors stated that participants were seen at several time points but only the "final" results were reported, not data for each time point (Aragonés Cruz 2008; Besharati 2008; Katircioglu 2014; Tananuvat 2004).
Other potential sources of bias
We judged five studies to have low risk of other biases (Katircioglu 2014; Kheirkhah 2011; Küçükerdönmez 2007; Küçükerdönmez 2007a; Luanratanakorn 2006). In these studies, the funding sources were clearly reported and did not pose a conflict of interest, and there were no concerns about the methodology used during the study. We judged eight studies to have unclear risk of bias, mainly due to not reporting funding, though two were due to questions about the design of the study (Aragonés Cruz 2008; Besharati 2008; Chen 2009; Chen 2012; Keklikci 2007; Ozer 2009; Paes 2010; Perry 2000). We considered no studies to have had high risk of bias due to funding or conflicts of interest, but judged four as high risk because both eyes of some participants were randomized, but the analysis did not take into consideration the non‐independence of eyes (Liang 2012; Pérez Parra 2008; Salman 2011; Tananuvat 2004), and judged two as high risk for unclear study design and a lack of detail about the type of analysis done (Fernández García 2012; Stangogiannis‐Druya 2004).
Effects of interventions
Recurrence of pterygium
Of the 20 studies, 13 specified the times from surgery to recurrences. The primary outcome of this review was recurrence between three and six months. As some studies reported data at exactly three months and six months, and others reported data monthly, we have included any report of recurrence between two months and four months in our "three month" section and any report of recurrence between five months and seven months in our "six month" section. Six studies reported recurrence at three months after surgery (Besharati 2008; Keklikci 2007; Kheirkhah 2011; Paes 2010; Tananuvat 2004; Toker 2016), and 10 studies reported recurrence at six months after surgery (Besharati 2008; Fernández García 2012; Kheirkhah 2011; Küçükerdönmez 2007a; Luanratanakorn 2006; Paes 2010; Salman 2011; Stangogiannis‐Druya 2004; Tananuvat 2004; Toker 2016). One study reported recurrence only at 12 months after surgery (Liang 2012), and one reported recurrence only at 24 months after surgery (Chen 2012). As these time points were not one of our specified outcomes, we did not present these data individually.
Recurrence at three months after surgery
Recurrence of pterygium at three months after surgery ranged from 0% to 16.7% in the conjunctival autograft groups and 4.76% to 26.9% in the amniotic membrane transplant groups. These groups included participants with both primary and recurrent pterygia. In a meta‐analysis of data from the six studies reporting this outcome, the risk of recurrence was statistically equivalent between the two groups (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.43 to 1.77). Therefore, we are uncertain as to which surgery resulted in a lower risk of recurrence at three months (Figure 4, Analysis 1.1). We graded the quality of the evidence for this outcome as very low due to imprecision in the estimate, inconsistencies among the individual study results and a lack of allocation concealment in the included studies. A grade of very low means the true effect may be substantially different from the estimate of effect.

Forest plot of comparison: 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), outcome: 1.1 Recurrence of pterygium at 3 months.
In a subgroup analysis, we separated from the group four studies that included only participants with primary pterygium. In this subgroup, there was also no statistically significant difference between risk of recurrence of pterygium between participants treated with conjunctival autograft and participants treated with amniotic membrane transplant. The risk ratio was 0.92, but the 95% confidence interval crossed the null value (0.37 to 2.30) (Figure 4, Analysis 1.1). Findings from the two analyses support no difference in recurrence after 3 months.
Recurrence at six months after surgery
Recurrence of pterygium at six months after surgery ranged from 3.33% to 16.7% in the conjunctival autograft groups and 2.6% to 42.3% in the amniotic membrane transplant groups. Though there was some statistical heterogeneity among the studies that included participants with primary pterygia (I2 = 60%), we elected to perform a meta‐analysis because we did not judge the methodological heterogeneity to be too great to combine the data. The statistical heterogeneity was attributable to one larger study that included 228 participants and reported that the risk of recurrence of pterygium was higher in the conjunctival autograft group compared to the amniotic membrane transplant group (RR 2.32, 95% CI 1.02 to 5.15). The nine other studies included in this meta‐analysis favored the conjunctival autograft group, reporting a lower risk of recurrence with this surgery compared to the amniotic membrane transplant group. However, it should be noted that only two of these were statistically significantly in favor of the conjunctival autograft surgery. Our meta‐analysis favored the conjunctival autograft group, finding that this group had a 47% lower risk of recurrence compared with the amniotic membrane transplant group (RR 0.53, 95% CI 0.33 to 0.85) (Figure 5, Analysis 1.2). We graded the quality of evidence for this outcome as moderate due to concerns about allocation concealment and evidence of possible attrition bias.

Forest plot of comparison: 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), outcome: 1.2 Recurrence of pterygium at 6 months.
For this outcome, we performed a subgroup analysis on data for participants with primary pterygium and participants with recurrent pterygium. In the analysis of participants with primary pterygium, there was also no difference in recurrence of pterygium at six months between the conjunctival autograft group and the amniotic membrane transplant group (RR 0.58, 95% CI 0.27 to 1.27) (Figure 5, Analysis 1.2). However, for participants who had recurrent pterygium, there was a 55% reduction in risk of recurrence for participants who received conjunctival autograft surgery compared with participants who received amniotic membrane transplant surgery (RR 0.45, 95% CI 0.21 to 0.99) (Figure 5, Analysis 1.2). Thus, participants with recurrent pterygia who received conjunctival autograft had recurrence of pterygium six months after surgery less often than did participants who received AMT for their recurrent pterygia.
Studies reporting recurrence overall or recurrence at non‐specific time points
Seven studies reported on recurrence over the course of follow‐up but did not assess recurrence at specific time points (Aragonés Cruz 2008; Chen 2009; Katircioglu 2014; Küçükerdönmez 2007; Ozer 2009; Pérez Parra 2008; Perry 2000). These studies reported the mean follow‐up time for each arm and the number of participants with recurrence during the study. We have summarized these results below.
-
Aragonés Cruz 2008 reported an overall risk of recurrence of 2 out of 28 (7.1%) participants in the limbal conjunctival autograft group compared with 3 out of 28 participants (10.7%) in the amniotic membrane transplant group, representing a non‐statistically significant difference in risk of recurrence between the two study arms (RR 0.67, 95% CI 0.12 to 3.69).
-
Chen 2009 analyzed pterygium recurrence between 12 and 24 months after surgery. The risks of recurrence were similar in the two surgeries: 9 out of 40 (22.5% of participants) in the limbal conjunctival autograft group versus 10 out of 45 (22.2%) in the amniotic membrane transplant group. These risks of recurrence were not statistically significantly different (RR 1.01, 95% CI 0.46 to 2.24).
-
Katircioglu 2014 summarized recurrence of pterygium at time of last follow‐up, which was 27.2 ± 20.8 months (range 12 to 94 months) overall. The mean follow‐up time was 25.9 ± 24.4 months in the conjunctival autograft group and 28.8 ± 15.7 months in the amniotic membrane transplant group. Recurrence was defined as fibrovascular tissue growth onto the cornea, as described by Prabhasawat 1997. Four out of 30 participants (13.4%) in the conjunctival autograft group had recurrence of pterygium, and 2 out of 25 participants (8%) in the amniotic membrane transplant group had recurrence of pterygium, a difference that was not statistically significant (RR 1.67, 95% CI 0.33 to 8.36).
-
Küçükerdönmez 2007 had a mean follow‐up of 13.66 ± 5.23 months in the limbal conjunctival autograft group (range 6 to 24 months) and 14.40 ± 3.25 in the amniotic membrane transplant group (range 6 to 26 months). They reported that "during the study" no pterygium recurrence was observed in either group.
-
Ozer 2009 reported recurrence "during the study period." Average follow‐up for participants who received conjunctival limbal autograft was 69.91 ± 12.41 months (range 59 to 82), and among this group, 11 out of 63 had recurrence of pterygium (14.29%). Average follow‐up time for participants in the amniotic membrane transplant group was 61.43 ± 9.83 months (range 53 to 74), and 12 of 52 had recurrence of pterygium during this time (23.08%). There was no statistically significant difference in risk of recurrence between the two groups (RR 0.76, 95% CI 0.36 to 1.57).
-
Pérez Parra 2008 reported one case of recurrence in the conjunctival autograft group at five months, and this represented 2.2% recurrence in this surgical group. The authors reported three cases of recurrent pterygium in the amniotic membrane transplant group (6.6%), but they did not report the time points at which these recurrences occurred. The risk of recurrence in this study was not significantly different between the two surgical groups (RR 0.33, 95% CI 0.36 to 3.08).
-
Perry 2000 reported on a group of eight participants with bilateral pterygium who randomly received conjunctival autograft in one eye and amniotic membrane transplant in the other eye. Throughout the study follow‐up (mean 12.5 months, range 9 to 22 months), none of the eyes that received amniotic membrane transplant had pterygium recurrence, but two of the eyes (25%) that received conjunctival autograft had recurrence.
Sensitivity analysis
We performed a sensitivity analysis on the recurrence of pterygium outcomes in which we removed one study from the meta‐analysis. Paes 2010 was a doctoral thesis rather than a peer‐reviewed journal article. We had planned to perform a sensitivity analysis to exclude studies at high risk of bias and those that were unpublished at the time of the review, and this study met both criteria. In communication with the author, it appeared that the study was randomized, however allocation was not concealed. The author described the randomization method as assigning each participant a number when he/she arrived, then allocating participants to conjunctival autograft or amniotic membrane transplant depending on whether the sum of the digits of the random number the participant received was even or odd. In the sensitivity analysis excluding this study, the risk of recurrence of pterygium at three months' postsurgery in the conjunctival autograft group was still not statistically different from the risk in the amniotic membrane transplant group (RR 0.62, 95% CI 0.30 to 1.27) (Table 1). In the analysis of pterygium recurrence at six months after surgery excluding the Paes 2010 study, the risk of recurrence was lower in the conjunctival autograft group compared with the amniotic membrane transplant group (RR 0.43, 95% CI 0.30 to 0.62). Participants in the conjunctival autograft group had a statistically significantly 57% lower risk of recurrence compared with the amniotic membrane transplant group (Table 1).
| Estimates Including Paes 2010 Study | Estimates Not Including Paes 2010 Study | |||
| Outcome | Number of Studies (Participants) | Risk Ratio (M‐H, Random, 95% CI) | Number of Studies (Participants) | Risk Ratio (M‐H, Random, 95% CI) |
| Recurrence of pterygium at 3 months | 6 (538) | 0.87 [0.43, 1.77] | 5 (310) | 0.62 [0.30, 1.27] |
| Participants with primary pterygium | 5 (488) | 0.92 [0.37, 2.30] | 4 (260) | 0.62 [0.24, 1.60] |
| Participants with primary or recurrent pterygium | 1 (50) | 0.62 [0.21, 1.85] | 1 (50) | 0.62 [0.21, 1.85] |
| Recurrence of pterygium at 6 months | 10 (1,021) | 0.53 [0.33, 0.85] | 9 (793) | 0.43 [0.30, 0.62] |
| Participants with primary pterygium | 7 (815) | 0.58 [0.27, 1.27] | 6 (587) | 0.43 [0.27, 0.69] |
| Participants with recurrent pterygium | 3 (96) | 0.45 [0.21, 0.99] | 3 (96) | 0.45 [0.21, 0.99] |
| Participants with primary or recurrent pterygium | 2 (110) | 0.38 [0.15, 0.95] | 2 (110) | 0.38 [0.15, 0.95] |
Proportion of participants with clinical improvement
Keklikci 2007 was the only study that specifically reported on clinical improvement, citing the risk of non‐recurrence as 93.8% for participants in the conjunctival limbal autograft group and 93.3% in the amniotic membrane transplant group at three months after surgery. None of the other studies reported on clinical improvement per se, and though it is easy to derive the risk of non‐recurrence by taking the inverse of the risk of recurrence, non‐recurrence is not necessarily equivalent to clinical improvement, so we opted against this calculation or inclusion of "clinical improvement" as thus defined. Another way to report clinical improvement would be to describe and compare the participants' symptoms before and after surgery, but no studies did so.
Proportion of participants requiring repeat surgery for pterygium
One study mentioned the need for repeat surgery, but the context was in reference to adverse events and not as a planned outcome (Keklikci 2007). It was noted that in the amniotic membrane graft group, suture lysis was detected in one case (3.33%) on the third day postoperatively and that amniotic membrane revision was performed. There was no information about the need for any repeat surgery in the conjunctival autograft group. Another study reported that two participants (one each from the conjunctival autograft and amniotic membrane transplant groups) had repeat surgery, but the time point during follow‐up at which the surgery was required was not reported (Toker 2016).
Mean change in visual acuity from baseline to follow‐up points
No studies reported the mean change in visual acuity, but one trial described visual acuity over the study period (Katircioglu 2014). This study included only participants with recurrent pterygium. The authors compared baseline and postoperative logMAR but did not provide a specific time point at which the mean change in visual acuity was assessed. The mean baseline logMAR in participants of the conjunctival autograft group (n = 30) was 0.2 ± 1.4, and the mean baseline logMAR in the amniotic membrane transplant group (n = 25) was 0.2 ± 1.0. The mean postoperative logMAR in the conjunctival autograft group was 0.1 ± 1.3 and in the amniotic membrane transplant group was 0.1 ± 1.2. There was no difference in the visual acuity postoperatively (mean difference 0.00, 95% CI ‐0.66 to 0.66).
Tananuvat 2004 did not report measures of visual acuity, but the authors noted that "no eye had a loss of uncorrected visual acuity greater than 1 line at the last visit."
Quality of life measures
None of the included studies reported on quality of life measures.
Direct and indirect costs
No study examined direct or indirect costs of conjunctival autografting or amniotic membrane transplant.
Adverse events
Thirteen studies reported adverse events associated with conjunctival autograft surgery and amniotic membrane transplant surgery (Aragonés Cruz 2008; Besharati 2008; Chen 2012; Katircioglu 2014; Kheirkhah 2011; Küçükerdönmez 2007; Liang 2012; Luanratanakorn 2006; Pérez Parra 2008; Perry 2000; Salman 2011; Tananuvat 2004; Toker 2016), however only one study included incidences of two adverse events of highest interest, diplopia and induced astigmatism. Besharati 2008 reported on diplopia at three months and six months. Two participants in the amniotic membrane transplant group developed diplopia by six months versus no participants in the conjunctival autograft group, and one participant in the amniotic membrane transplant group had restriction of eye movement. It is unclear whether the participant with restriction of eye movement also had diplopia.
Other adverse events that occurred in more than one study were granuloma and pyogenic granuloma and increased intraocular pressure. Table 2 shows a summary of all adverse events reported in the included studies. None of the included studies reported that any participants had developed induced astigmatism; only one paper analyzed change in visual acuity, describing no difference between conjunctival autograft and amniotic membrane transplant. Adverse events that were reported in multiple studies are summarized in Figure 6 (Analysis 1.3).
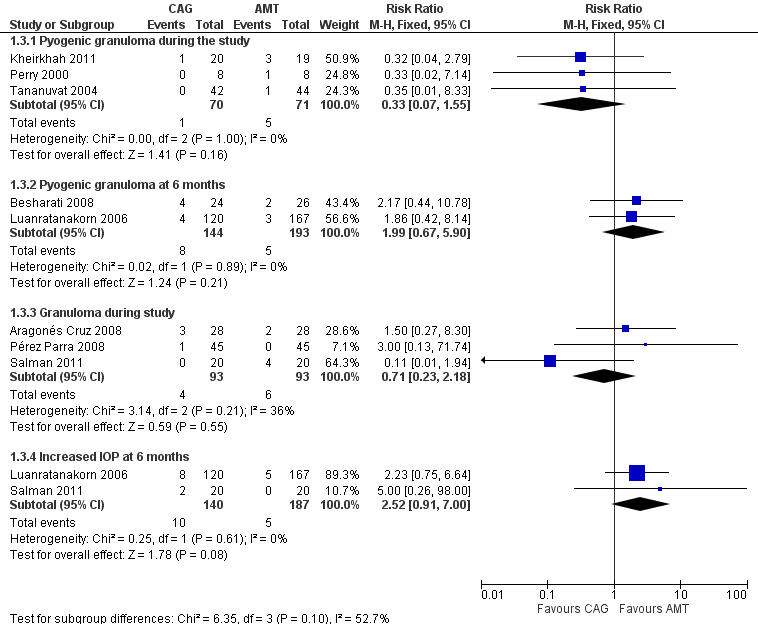
Forest plot of comparison: 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), outcome: 1.3 Adverse events.
| Event | Time point | Number of studies (reference) | Conjunctival autograft, n (%) | Amniotic membrane transplant, n (%) | Risk ratio (95% CI) |
| Chemosis | 6 months | 1 (Salman 2011) | 4 (20.0) | 2 (10.0) | 2.00 (0.41 to 9.71) |
| Conjunctival contraction | 1 month | 1 (Besharati 2008) | 3 (12.5) | 4 (15.4) | 0.81 (0.20 to 3.26) |
| 3 months | 1 (Besharati 2008) | 1 (4.2) | 3 (11.5) | 0.36 (0.04 to 3.24) | |
| 6 months | 1 (Besharati 2008) | 2 (8.5) | 5 (19.2) | 0.43 (0.09 to 2.03) | |
| Conjunctival inflammation ‐ grade 1 to 3 | During study | 1 (Kheirkhah 2011) | 3 (15) | 16 (84.2) | 0.18 (0.06 to 0.51) |
| Corneal scar | 1 month | 1 (Besharati 2008) | 16 (66.7) | 16 (61.5) | 1.08 (0.72 to 1.64) |
| 3 months | 1 (Besharati 2008) | 16 (66.7) | 16 (61.5) | 1.08 (0.72 to 1.64) | |
| 6 months | 1 (Besharati 2008) | 16 (66.7) | 16 (61.5) | 1.08 (0.72 to 1.64) | |
| Conjunctivitis | Overall | 0 (0) | 1 (3.6) | 0.33 (0.01 to 7.85) | |
| Diplopia | 3 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| 6 months | 1 (Besharati 2008) | 0 (0) | 2 (7.7) | 0.22 (0.01 to 4.28) | |
| Epithelial defect (lasting more than 5 days) | During study | 1 (Katircioglu 2014) | 1 (3.3) | 0 (0) | 0.67 (0.20 to 2.22) |
| Eye movement restriction | 3 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| 6 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) | |
| Eyelid edema and conjunctival hyperemia edema | 12 months | 1 (Liang 2012) | 8 (9.9) | 12 (23.1) | 0.43 (0.19 to 0.98) |
| Foreign body sensation or discomfort | 12 months | 1 (Liang 2012) | 11 (13.6) | 17 (32.7) | 0.42 (0.21 to 0.81) |
| Graft dehiscence/suture dehiscence | 1 month | 1 (Besharati 2008) | 3 (12.5) | 1 (3.8) | 3.25 (0.36 to 29.16) |
| 3 months | 1 (Besharati 2008) | 3 (12.5) | 1 (3.8) | 3.25 (0.36 to 29.16) | |
| 6 months | 1 (Besharati 2008) | 3 (12.5) | 1 (3.8) | 3.25 (0.36 to 29.16) | |
| During study | 1 (Toker 2016) | 2 (5.4) | 2 (5.6) | 0.97 (0.14 to 6.54) | |
| Overall | 0 (0) | 1 (3.6) | 0.33 (0.01 to 7.85) | ||
| Graft reaction | During study | 1 (7.1) | 0 (0) | 2.80 (0.12 to 63.20) | |
| Granuloma | During study | 4 (4.3) | 6 (6.5) | 0.71 (0.23 to 2.18) | |
| Increased intraocular pressure | 6 months | 8 (6.7) | 5 (3.0) | 2.52 (0.91 to 7.00) | |
| During study | 1 (Tananuvat 2004) | 5 (11.9) | 4 (9.1) | 1.31 (0.38 to 4.55) | |
| Infection | 1 month | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| Inflammation | During study | 1 (Pérez Parra 2008) | 2 (4.4) | 0 (0) | 5.00 (0.25 to 101.31) |
| Graft edema | During study | 0 (0) | 2 (15.4) | 0.19 (0.01 to 3.56) | |
| Other defect taking > 10 days to heal | During study | 1 (Katircioglu 2014) | 4 (13.4) | 5 (20) | 2.52 (0.11 to 59.18) |
| Pyogenic granuloma | 1 month | 1 (Besharati 2008) | 4 (16.7) | 2 (7.7) | 2.17 (0.44 to 10.78) |
| 3 months | 1 (Besharati 2008) | 4 (16.7) | 2 (7.7) | 2.17 (0.44 to 10.78) | |
| 6 months | 8 (5.6) | 5 (2.6) | 1.99 (0.67 to 5.90) | ||
| During study | 1 (1.3) | 5 (7.0) | 0.33 (0.07 to 1.55) | ||
| Severe pain | During study | 1 (Katircioglu 2014) | 4 (13.4) | 2 (8.0) | 1.67 (0.33 to 8.36) |
| Subconjunctival hemorrhage | Overall | 4 (14.2) | 0 (0) | 9.00 (0.51 to 159.70) | |
| Superficial punctate keratitis | Overall | 3 (10.7) | 3 (10.7) | 1.00 (0.22 to 4.54) | |
| 2 years | 1 (Chen 2012) | 1 (2.2) | 2 (4.4) | 0.50 (0.05 to 5.32) | |
| Symblepharon | 6 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| 2 years | 1 (Chen 2012) | 1 (2.2) | 1 (2.2) | 1.00 (0.06 to 15.50) | |
| During study | 1 (Perry 2000) | 1 (12.5) | 0 (0) | 3.00 (0.14 to 64.26) | |
| Wound healing | 2 years | 1 (Chen 2012) | 3 (6.7) | 3 (6.7) | 1.00 (0.21 to 4.69) |
CI: confidence interval
Discussion
Summary of main results
In this review, we report outcome data from 20 randomized controlled trials that compared conjunctival autograft versus amniotic membrane transplant for the treatment of pterygium. We could include eleven studies in a meta‐analysis for our primary outcome, recurrence of pterygium after the surgery, assessed at three and six months after surgery. At three months, there was no statistically significant difference in risk of recurrence between the two procedures in studies of primary pterygia or studies of both recurrent and primary pterygia. At six months, without regard to primary or recurrent nature of the pterygia, the conjunctival autograft group had a 47% lower risk of recurrence compared with the amniotic membrane transplant group. Subgroup analysis showed that at six months after surgery, there was no difference in recurrence between the two procedures performed for primary pterygia; however, there was a 55% reduction in risk of recurrence with conjunctival autograft compared with amniotic membrane transplant for recurrent pterygia. After removing a study that lacked allocation concealment, there was still no difference in risk of recurrence between the two surgeries at three months. The meta‐analysis favored conjunctival autograft at six months for participants with primary and recurrent pterygia. Other than the graft material being different (conjunctiva vs. amniotic membrane), there are no inherent technical differences between the two types of surgery. Seven studies reported recurrence at non‐specific time points during the study. These studies showed no difference in the risk of pterygium recurrence when comparing conjunctival autograft with amniotic membrane transplant.
Only one study reported on change in visual acuity from baseline to follow‐up points; there was no difference in this outcome between conjunctival autograft and amniotic membrane transplant. Only one study reported "clinical improvement" (defined by the authors as the non‐recurrence risk), and another study reported the need for repeat surgery. No studies compared participants' symptoms before and after surgery. Non‐recurrence is not equivalent to clinical improvement, because clinical improvement implies a favorable change in patients' quality of vision or quality of life. Clinical improvement might also imply that the patient would choose to have pterygium surgery again if presented with the option.
We found no difference in adverse events between conjunctival autograft and amniotic membrane transplant groups. However, "adverse events" often were not prespecified or described in sufficient detail. Many "adverse events" listed in study reports were non‐specific and could be expected in the typical postoperative course of most ophthalmic procedures, including pterygium surgery: "conjunctival edema," "conjunctivitis," "conjunctival inflammation," "corneal scar," punctate epithelial erosions, eyelid edema, pain. Other "adverse events" mentioned in the studies were too vague, such as "graft reaction." Adverse events specific to the surgeries evaluated in this review are symblepharon, pyogenic granuloma, and diplopia and/or restriction of eye movement.
None of the included studies evaluated the participant‐reported outcome of vision‐related quality of life. Additionally, none of the included studies reported on the differences in the direct or indirect costs of the interventions.
Overall completeness and applicability of evidence
All included studies evaluated recurrence of pterygium, our primary outcome; however, only eleven reported recurrence at specific time points that were useful in our meta‐analysis. Though there was some statistical heterogeneity among the studies, the types of participants included and the techniques for conjunctival autograft and amniotic membrane transplant were similar. Some studies included participants with primary pterygia, others participants with recurrent pterygia, while some studies included participants with either primary or recurrent pterygia. As results from separate analyses of primary versus recurrent pterygia were similar, we believe the applicability of the evidence to the average pterygium patient to be high. The results from separate analyses of primary versus recurrent pterygium from patient populations of different latitudes and different ethnicities were similar, barring one study (Paes 2010) that had opposing results.The evidence supports that conjunctival autograft in combination with surgical excision of the pterygium confers a lower risk of pterygium recurrence than does surgical excision with amniotic membrane transplant six months after surgery.
We took special consideration in assessing the risk of detection bias. As noted, a surgeon or trained observer could easily distinguish which surgery had been performed on an eye by noting whether there were two surgical sites (conjunctival autograft surgery) or only one surgical site (amniotic membrane transplant). Bias may be present if the same person who performed the surgery was also an outcomes assessor. It is possible that the surgeon who performed amniotic membrane transplant would want to believe that his/her surgery had a successful outcome and may not want to admit if there is recurrence. When surgeons and outcomes assessors are separate individuals, there is a lower risk of these inherent biases because recurrence is an objective outcome. Still, it is possible that even a masked observer would be able to determine which procedure was used because at three months after surgery, the area from which the conjunctiva autograft was harvested will not look normal compared with amniotic membrane transplant, where there is no part of the surgical eye from which tissue was removed. If an outcomes assessor were to see two surgical sites on the eye that were healing, he/she would be able to tell that the participant received conjunctival autograft surgery rather than amniotic membrane transplant. We cannot say whether the ability of a theoretically masked outcome observer to practically tell which of the two procedures was performed would bias their decision on recurrence, and because so many of the included studies were unclear on who observed outcomes (the surgeon versus a different assessor), the best we could do while evaluating these studies was to keep these inherent biases in mind and consider the fact that there was a risk for performance and detection bias.
The time frame of this evidence is clinically applicable and biologically appropriate. Like other types of corneal procedures (for example corneal transplantation, laser in‐situ keratomileusis, photorefractive keratectomy), extensive wound healing and remodeling occur after either type of pterygium surgery. Therefore, outcomes at six months are more clinically applicable than outcomes at three months, at which time tissue is still undergoing remodeling and outcomes are not stable. One study, Liang 2012, used conjunctival hyperemia and edema in the first few months after surgery as an outcome measure, with which we and other authors did not agree. Edema and hyperemia commonly occur after uncomplicated pterygium surgery and other types of eye surgery and are thus non‐specific outcomes. They have not been shown to be associated with recurrence of pterygium.
The relative beneficial effect of conjunctival autograft over amniotic membrane transplant in preventing further recurrence of recurrent pterygia is clinically important given that recurrent pterygia are more fibrovascular and adherent to underlying tissue than are primary pterygia. There may also be fibrotic scarring with recurrent pterygium, making it more difficult for the surgeon to resect the pterygia. No study reported whether the primary surgery in cases of recurrent pterygium was performed with conjunctival autograft or amniotic membrane transplant. The beneficial effect of conjunctival autograft is clinically significant because if the first surgery also was done with an autograft, there may not be enough tissue to harvest for a second autograft. Another advantage of conjunctival autograft over amniotic membrane transplant is that though donors of amniotic membrane are serologically tested for transmissible diseases, including hepatitis B, hepatitis C, and human immunodeficiency virus, there is always a risk of microbial transmission (for example prion disease) when using biological products.
Few data have been reported on adverse events associated with these two procedures; moreover, there was wide variation in terms of what constituted an "adverse event" in these studies. The limited data available suggest no difference in adverse events between conjunctival autograft and amniotic membrane transplant. However, more data must be collected on well‐defined and prespecified adverse events to ascertain whether a difference in adverse events exists between the two procedures.
A source of heterogeneity among our included studies was the fixation technique used by the surgeons in each study. It is possible that using sutures versus using glue to fix the tissue graft over the location of pterygium removal may have an effect on the risk of recurrence. We chose to combine studies based on recurrent versus primary pterygia and by outcome time points, but in doing so, we did not take into account the fixation technique. Estimates of the risk ratio for recurrence may have been different if we had also completed a subgroup analysis by fixation technique. The recurrence could additionally have been affected by the combination of fixation technique plus the use of adjuvant MMC. These surgical techniques should be evaluated as more information becomes available.
Quality of the evidence
The quality of evidence in favor of conjunctival autograft is moderate. The majority of trials did not describe methods for randomization and allocation or for masking of participants and outcomes assessors, leading to assessments of unclear risk of bias. Seven of 10 studies had a high level of attrition bias, the most common reason being excluding the data of participants lost to follow‐up. In our summary of findings Table for the main comparison, we have used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria to report the level of evidence for the outcomes included in this study. For the primary outcome of recurrence at three months, we graded the quality of evidence as very low because there was a lack of description in the methods of the individual studies, the estimate was imprecise, the results of the individual studies were inconsistent (ranging from 0.4 to 0.2). We graded the primary outcome of recurrence at six months as moderate, because there was a lack of description in the methods of the studies, but this was not subject to the same imprecision or inconsistencies as seen in the data on recurrence at three months.
Potential biases in the review process
This review was performed to Cochrane standards, and we attempted to avoid bias by using systematic methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Agreements and disagreements with other studies or reviews
A literature review and meta‐analysis published in 2012 reported that conjunctival autograft had a lower recurrence risk compared to amniotic membrane transplant for primary pterygium treatment (Li 2012). This review included both randomized controlled trials and cohort studies and reported a hazard ratio instead of a risk ratio. They found that the recurrence of conjunctival autograft compared with amniotic membrane transplant was 0.30 (95% CI 0.16 to 0.59) for primary and 0.22 (95% CI 0.02 to 2.37) for recurrent pterygium.
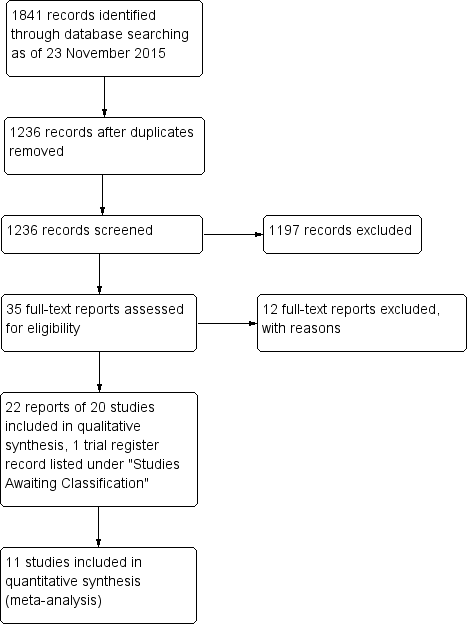
Study flow diagram.
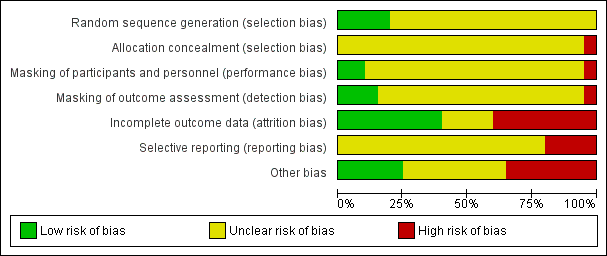
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Forest plot of comparison: 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), outcome: 1.1 Recurrence of pterygium at 3 months.
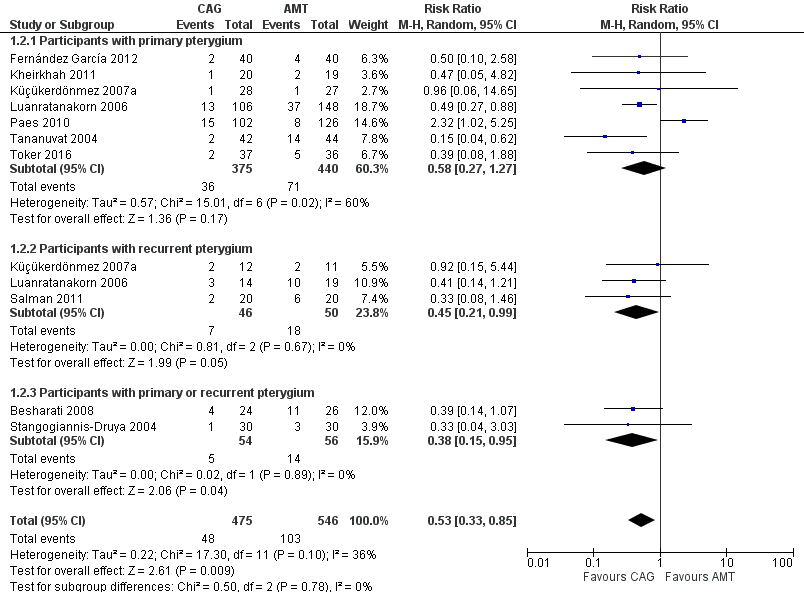
Forest plot of comparison: 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), outcome: 1.2 Recurrence of pterygium at 6 months.

Forest plot of comparison: 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), outcome: 1.3 Adverse events.

Comparison 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), Outcome 1 Recurrence of pterygium at 3 months.

Comparison 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), Outcome 2 Recurrence of pterygium at 6 months.

Comparison 1 Conjunctival autograft (CAG) versus amniotic membrane transplant (AMT), Outcome 3 Adverse events.
| Conjunctival autograft compared to amniotic membrane transplant for pterygium | ||||||
| Patient or population: people with primary or recurrent pterygium | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of eyes | Quality of the evidence | Comment | |
| Risk with amniotic membrane transplant | Risk with conjunctival autograft | |||||
| Recurrence of pterygium | Study population | RR 0.87 (0.43 to 1.77) | 538 | ⊕⊝⊝⊝ | ||
| 89 per 1000 | 77 per 1000 | |||||
| Recurrence of pterygium | Study population | RR 0.53 (0.33 to 0.85) | 1021 | ⊕⊕⊕⊝ | ||
| 189 per 1000 | 100 per 1000 | |||||
| Clinical improvement (non‐recurrence risk) follow‐up: 3 months | See comment | One study reported the risk of non‐recurrence as 93.8% for participants in the conjunctival limbal autograft group and 93.3% in the amniotic membrane transplant group at 3 months after surgery | ||||
| Need for repeat surgery | See comment | 2 studies reported the need for repeat surgery but did not provide time points. In 1 study, 1 participant in the amniotic membrane transplant group developed suture lysis, and amniotic membrane revision was performed. In the other study, 1 participant in each surgical group had surgery again | ||||
| Mean change in visual acuity | See comment | No study reported mean change. 1 study reported the logMAR at baseline and postoperatively, and there was no difference (mean difference 0.00, 95% CI ‐0.66 to 0.66) | ||||
| Quality of life | None of the included studies reported on quality of life measures after the 2 surgeries | |||||
| Direct and indirect costs | None of the included studies reported on direct or indirect costs after the 2 surgeries | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Allocation concealment unclear in every study and evidence of possible attrition bias. | ||||||
| Estimates Including Paes 2010 Study | Estimates Not Including Paes 2010 Study | |||
| Outcome | Number of Studies (Participants) | Risk Ratio (M‐H, Random, 95% CI) | Number of Studies (Participants) | Risk Ratio (M‐H, Random, 95% CI) |
| Recurrence of pterygium at 3 months | 6 (538) | 0.87 [0.43, 1.77] | 5 (310) | 0.62 [0.30, 1.27] |
| Participants with primary pterygium | 5 (488) | 0.92 [0.37, 2.30] | 4 (260) | 0.62 [0.24, 1.60] |
| Participants with primary or recurrent pterygium | 1 (50) | 0.62 [0.21, 1.85] | 1 (50) | 0.62 [0.21, 1.85] |
| Recurrence of pterygium at 6 months | 10 (1,021) | 0.53 [0.33, 0.85] | 9 (793) | 0.43 [0.30, 0.62] |
| Participants with primary pterygium | 7 (815) | 0.58 [0.27, 1.27] | 6 (587) | 0.43 [0.27, 0.69] |
| Participants with recurrent pterygium | 3 (96) | 0.45 [0.21, 0.99] | 3 (96) | 0.45 [0.21, 0.99] |
| Participants with primary or recurrent pterygium | 2 (110) | 0.38 [0.15, 0.95] | 2 (110) | 0.38 [0.15, 0.95] |
| Event | Time point | Number of studies (reference) | Conjunctival autograft, n (%) | Amniotic membrane transplant, n (%) | Risk ratio (95% CI) |
| Chemosis | 6 months | 1 (Salman 2011) | 4 (20.0) | 2 (10.0) | 2.00 (0.41 to 9.71) |
| Conjunctival contraction | 1 month | 1 (Besharati 2008) | 3 (12.5) | 4 (15.4) | 0.81 (0.20 to 3.26) |
| 3 months | 1 (Besharati 2008) | 1 (4.2) | 3 (11.5) | 0.36 (0.04 to 3.24) | |
| 6 months | 1 (Besharati 2008) | 2 (8.5) | 5 (19.2) | 0.43 (0.09 to 2.03) | |
| Conjunctival inflammation ‐ grade 1 to 3 | During study | 1 (Kheirkhah 2011) | 3 (15) | 16 (84.2) | 0.18 (0.06 to 0.51) |
| Corneal scar | 1 month | 1 (Besharati 2008) | 16 (66.7) | 16 (61.5) | 1.08 (0.72 to 1.64) |
| 3 months | 1 (Besharati 2008) | 16 (66.7) | 16 (61.5) | 1.08 (0.72 to 1.64) | |
| 6 months | 1 (Besharati 2008) | 16 (66.7) | 16 (61.5) | 1.08 (0.72 to 1.64) | |
| Conjunctivitis | Overall | 0 (0) | 1 (3.6) | 0.33 (0.01 to 7.85) | |
| Diplopia | 3 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| 6 months | 1 (Besharati 2008) | 0 (0) | 2 (7.7) | 0.22 (0.01 to 4.28) | |
| Epithelial defect (lasting more than 5 days) | During study | 1 (Katircioglu 2014) | 1 (3.3) | 0 (0) | 0.67 (0.20 to 2.22) |
| Eye movement restriction | 3 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| 6 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) | |
| Eyelid edema and conjunctival hyperemia edema | 12 months | 1 (Liang 2012) | 8 (9.9) | 12 (23.1) | 0.43 (0.19 to 0.98) |
| Foreign body sensation or discomfort | 12 months | 1 (Liang 2012) | 11 (13.6) | 17 (32.7) | 0.42 (0.21 to 0.81) |
| Graft dehiscence/suture dehiscence | 1 month | 1 (Besharati 2008) | 3 (12.5) | 1 (3.8) | 3.25 (0.36 to 29.16) |
| 3 months | 1 (Besharati 2008) | 3 (12.5) | 1 (3.8) | 3.25 (0.36 to 29.16) | |
| 6 months | 1 (Besharati 2008) | 3 (12.5) | 1 (3.8) | 3.25 (0.36 to 29.16) | |
| During study | 1 (Toker 2016) | 2 (5.4) | 2 (5.6) | 0.97 (0.14 to 6.54) | |
| Overall | 0 (0) | 1 (3.6) | 0.33 (0.01 to 7.85) | ||
| Graft reaction | During study | 1 (7.1) | 0 (0) | 2.80 (0.12 to 63.20) | |
| Granuloma | During study | 4 (4.3) | 6 (6.5) | 0.71 (0.23 to 2.18) | |
| Increased intraocular pressure | 6 months | 8 (6.7) | 5 (3.0) | 2.52 (0.91 to 7.00) | |
| During study | 1 (Tananuvat 2004) | 5 (11.9) | 4 (9.1) | 1.31 (0.38 to 4.55) | |
| Infection | 1 month | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| Inflammation | During study | 1 (Pérez Parra 2008) | 2 (4.4) | 0 (0) | 5.00 (0.25 to 101.31) |
| Graft edema | During study | 0 (0) | 2 (15.4) | 0.19 (0.01 to 3.56) | |
| Other defect taking > 10 days to heal | During study | 1 (Katircioglu 2014) | 4 (13.4) | 5 (20) | 2.52 (0.11 to 59.18) |
| Pyogenic granuloma | 1 month | 1 (Besharati 2008) | 4 (16.7) | 2 (7.7) | 2.17 (0.44 to 10.78) |
| 3 months | 1 (Besharati 2008) | 4 (16.7) | 2 (7.7) | 2.17 (0.44 to 10.78) | |
| 6 months | 8 (5.6) | 5 (2.6) | 1.99 (0.67 to 5.90) | ||
| During study | 1 (1.3) | 5 (7.0) | 0.33 (0.07 to 1.55) | ||
| Severe pain | During study | 1 (Katircioglu 2014) | 4 (13.4) | 2 (8.0) | 1.67 (0.33 to 8.36) |
| Subconjunctival hemorrhage | Overall | 4 (14.2) | 0 (0) | 9.00 (0.51 to 159.70) | |
| Superficial punctate keratitis | Overall | 3 (10.7) | 3 (10.7) | 1.00 (0.22 to 4.54) | |
| 2 years | 1 (Chen 2012) | 1 (2.2) | 2 (4.4) | 0.50 (0.05 to 5.32) | |
| Symblepharon | 6 months | 1 (Besharati 2008) | 0 (0) | 1 (3.8) | 0.36 (0.02 to 8.43) |
| 2 years | 1 (Chen 2012) | 1 (2.2) | 1 (2.2) | 1.00 (0.06 to 15.50) | |
| During study | 1 (Perry 2000) | 1 (12.5) | 0 (0) | 3.00 (0.14 to 64.26) | |
| Wound healing | 2 years | 1 (Chen 2012) | 3 (6.7) | 3 (6.7) | 1.00 (0.21 to 4.69) |
| CI: confidence interval | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of pterygium at 3 months Show forest plot | 6 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.77] |
| 1.1 Participants with primary pterygium | 5 | 488 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.37, 2.30] |
| 1.2 Participants with primary or recurrent pterygium | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.21, 1.85] |
| 2 Recurrence of pterygium at 6 months Show forest plot | 10 | 1021 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.33, 0.85] |
| 2.1 Participants with primary pterygium | 7 | 815 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.27, 1.27] |
| 2.2 Participants with recurrent pterygium | 3 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.21, 0.99] |
| 2.3 Participants with primary or recurrent pterygium | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 0.95] |
| 3 Adverse events Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Pyogenic granuloma during the study | 3 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.55] |
| 3.2 Pyogenic granuloma at 6 months | 2 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.67, 5.90] |
| 3.3 Granuloma during study | 3 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.18] |
| 3.4 Increased IOP at 6 months | 2 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.91, 7.00] |

