Učinci aflibercepta u liječenju neovaskularne senilne makularne degeneracije
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Study design: parallel‐group randomized controlled trial Number randomly assigned: 1217 total participants (1217 eyes) · 304 in the aflibercept 0.5 mg every 4 weeks group · 304 in the aflibercept 2.0 mg every 4 weeks group · 303 in the aflibercept 2.0 mg every 8 weeks group · 306 in the ranibizumab group Exclusions after randomization: Full analysis: 7 total participants · 3 in the aflibercept 0.5 mg every 4 weeks group, 0 in the aflibercept 2.0 mg every 4 weeks group, 2 in the aflibercept 2.0 mg every 8 weeks group, and 2 in the ranibizumab group Safety analysis: 2 total participants (both in the ranibizumab group) Losses to follow‐up: 103 participants discontinued treatment at 1‐year follow‐up · 30 in the aflibercept 0.5 mg every 4 weeks group · 16 in the aflibercept 2.0 mg every 4 weeks group · 30 in the aflibercept 2.0 mg every 8 weeks group · 27 in the ranibizumab group Number analyzed: Full analysis ‐ 1210 total participants at 1‐year follow‐up · 301 in the aflibercept 0.5 mg every 4 weeks group · 304 in the aflibercept 2.0 mg every 4 weeks group, · 301 in the aflibercept 2.0 mg every 8 weeks group · 304 in the ranibizumab group Safety analysis ‐ 1215 total participants at 1‐year follow‐up · 304 in the aflibercept 0.5 mg every 4 weeks group · 304 in the aflibercept 2.0 mg every 4 weeks group · 303 in the aflibercept 2.0 mg every 8 weeks group · 304 in the ranibizumab group Unit of analysis: individual (1 study eye per participant) How were missing data handled? missing values imputed using last observation carried forward approach Power calculation: none reported | |
| Participants | Country: United States and Canada (154 study sites) Mean age (range not reported): 78 years in the aflibercept 0.5 mg every 4 weeks group, 78 years in the aflibercept 2.0 mg every 4 weeks group, 78 years in the aflibercept 2.0 mg every 8 weeks group, and 78 years in the ranibizumab group Gender: 134 men (44.5%) and 167 women (55.5%) in the aflibercept 0.5 mg every 4 weeks group, 110 men (36.2%) and 194 women (63.8%) in the aflibercept 2.0 mg every 4 weeks group, 123 men (40.9%) and 178 women (59.1%) in the aflibercept 2.0 mg every 8 weeks group, and 132 men (43.4%) and 172 women (56.6%) in the ranibizumab group Inclusion criteria: 50 years of age or older; diagnosed with neovascular AMD in the study eye; active subfoveal CNV lesions of any subtype (12 optic disc areas or smaller) constituting ≥ 50% of total lesion size; BCVA between 73 and 25 Early Treatment Diabetic Retinopathy Study (ETDRS) chart letters (20/40 to 20/320 Snellen equivalent); willingness and ability to return for clinic visits and complete study‐related procedures; ability to provide informed consent Exclusion criteria: prior or concomitant treatment for AMD in the study eye; prior treatment with anti‐VEGF therapy; subretinal hemorrhage or scar or fibrosis constituting > 50% of total lesion size or involving the center of the fovea in the study eye; retinal pigment epithelial tears or rips involving the macula in the study eye; history of other ocular conditions such as vitreous hemorrhage, retinal detachment, macular hole, corneal transplant, corneal dystrophy, diabetic retinopathy, diabetic macular edema, uveitis, scleromalacia; presence of other ocular conditions such as uncontrolled glaucoma, significant media opacities, phakia or pseudophakia with absence of posterior capsule, intraocular inflammation or infection; prior vitrectomy, trabeculectomy, or other filtration surgery or therapy in the study eye Equivalence of baseline characteristics: yes; "Baseline demographics and disease characteristics were evenly balanced among all treatment groups" | |
| Interventions | Intervention 1: intravitreal aflibercept 0.5 mg every 4 weeks Intervention 2: intravitreal aflibercept 2.0 mg every 4 weeks Intervention 3: intravitreal aflibercept 2.0 mg every 8 weeks after 3 initial doses at weeks 0, 4, and 8 (to maintain masking, sham injections were given at the interim 4‐week visits after week 8) Intervention 4: intravitreal ranibizumab 0.5 mg every 4 weeks Length of follow‐up: 1 year for primary end point; dosing for all groups changed to as needed (PRN) after 1 year and follow‐up at 2 years from baseline | |
| Outcomes | Primary outcome, as defined in study reports: "proportion of patients maintaining vision at week 52 (losing < 15 letters on Early Treatment Diabetic Retinopathy Study [ETDRS] chart)" Secondary outcomes, as defined in study reports: change in BCVA, proportion gaining ≥ 15 letters, change in total National Eye Institute 25‐Item Visual Function Questionnaire (NEI‐VFQ‐25) score, change in CNV area on fluorescein angiography, retinal thickness and persistent fluid as assessed by OCT, mean number of intravitreal injections, adverse events Intervals at which outcomes assessed: every 4 weeks through 96 weeks; week 1 after first treatment for safety assessment; weeks 12, 24, 36, and 52 for the NEI‐VFQ‐25 assessment | |
| Notes | Type of study reports: published journal articles; clinical trial registration Funding sources: "Sponsored by Regeneron Pharmaceuticals, Inc, Tarrytown, New York, and Bayer HealthCare, Berlin Germany. The sponsors participated in the design and conduct of the study, analysis of the data, and preparation of the manuscript" Disclosures of interest: "J.S.H. is a consultant to and has received research funding from Alimera, Allergan, Fovea, Genentech, Genzyme, GlaxoSmithKline, Neovista, and Regeneron Pharmaceuticals. He has also received travel support from Regeneron Pharmaceuticals. D.M.B. is a consultant to Alimera, Allergan, Bayer, Genentech/Roche, Novartis, Regeneron Pharmaceuticals, and Thrombogenics and has received research funding from Alcon, Alimera, Allergan, Eli Lilly, Genentech, GlaxoSmithKline, Novartis, Regeneron Pharmaceuticals, and Thrombogenics. He has also received travel support from Regeneron Pharmaceuticals and lecture fees from Genentech. V.C. is a consultant to Alimera and Bayer and has received research funding from Alcon, Allergan, Bayer, Novartis, and Pfizer. He is an advisory board member for Allergan and Novartis and has also received travel support from Bayer. J.‐F.K. is a consultant to Alcon, Bayer, and Thea and an advisory board member for Allergan, Bayer, and Novartis. He has received travel support from Regeneron Pharmaceuticals. P.K.K. is a consultant to Bayer, Genentech, Novartis, and Regeneron Pharmaceuticals. He has received research funding from Regeneron Pharmaceuticals. Q.D.N. is a consultant to Bausch & Lomb and Santen and has received research funding from Genentech, Novartis, and Pfizer. B.K. has received travel support from Bayer. A.H. is a consultant to Alcon, Allergan, Centocor, Johnson & Johnson, Neovista, Merck, Ophthotech, Oraya, Paloma, P.R.N., Q.L.T., Regeneron Pharmaceuticals, and Thrombogenics. He has received research funding and lecture fees from Alcon, Allergan, Genentech, Neovista, Ophthotech, Oraya, P.R.N., Q.L.T., Regeneron Pharmaceuticals, and Second Sight. Y.O. is a consultant to Alcon and Bayer and has received travel support from Bayer. G.D.Y., N.S., R.V., A.J.B., and Y.S. are employees of Regeneron Pharmaceuticals. M.A., G.G., B.S., and R.S. are employees of Bayer HealthCare. C.S.’s institution has received payments from the Medical University of Vienna for data monitoring/reviewing and statistical analysis. U.S.‐E. is a consultant to Alcon, Allergan, Bayer HealthCare, and Novartis, and an advisory board member for Alcon and Novartis. She has received travel support from Bayer HealthCare and lecture fees from Bayer HealthCare and Novartis" Study period: July 2007 to September 2010 Subgroup analyses: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of random sequence generation was unclear. “Consecutively enrolled patients were assigned to treatment groups on the basis of a predetermined central randomization scheme with balanced allocation, managed by an interactive voice response system” |
| Allocation concealment (selection bias) | Low risk | Central randomization: “Consecutively enrolled patients were assigned to treatment groups on the basis of a predetermined central randomization scheme with balanced allocation, managed by an interactive voice response system” |
| Masking of participants and personnel (performance bias) | Low risk | “Patients were masked as to treatments. An unmasked investigator also was responsible for the receipt, tracking, preparation, destruction, and administration of study drug, as well as safety assessments both pre‐ and post‐dose...All other study site personnel were masked to treatment assignment by separating study records or masked packaging” |
| Masking of outcome assessment (detection bias) | Low risk | “A separate masked physician assessed adverse events and supervised the masked assessment of efficacy. All other study site personnel were masked to treatment assignment by separating study records or masked packaging. Optical coherence tomography technicians and visual acuity examiners remained masked relative to treatment assignment” |
| Incomplete outcome data (attrition bias) | Low risk | A full analysis set and a per protocol set were reported. Last observation carried forward (LOCF) approach was used to impute missing values; 91.1% to 96.4% of participants per treatment group completed 52 weeks of follow‐up |
| Selective reporting (reporting bias) | Low risk | The study was registered at clinicaltrials.gov; intended outcomes were reported |
| Other bias | High risk | Many authors are employees of, consultants to, or have received research funding from Regeneron Pharmaceuticals, which manufactures aflibercept and participated in the design of the trial, collected and analyzed data, and prepared the study reports |
| Methods | Study design: parallel‐group randomized controlled trial Number randomly assigned: 1240 total participants (1240 eyes) · 311 in the aflibercept 0.5 mg every 4 weeks group · 313 in the aflibercept 2.0 mg every 4 weeks group · 313 in the aflibercept 2.0 mg every 8 weeks group · 303 in the ranibizumab group Exclusions after randomization: Full analysis ‐ 38 total participants: · 15 in the aflibercept 0.5 mg every 4 weeks group · 4 in the aflibercept 2.0 mg every 4 weeks group · 7 in the aflibercept 2.0 mg every 8 weeks group · 12 in the ranibizumab group Safety analysis ‐ 36 total participants: · 14 in the aflibercept 0.5 mg every 4 weeks group · 4 in the aflibercept 2.0 mg every 4 weeks group · 6 in the aflibercept 2.0 mg every 8 weeks group · 12 in the ranibizumab group Losses to follow‐up: 148 participants discontinued treatment at 1‐year follow‐up · 45 in the aflibercept 0.5 mg every 4 weeks group · 37 in the aflibercept 2.0 mg every 4 weeks group · 33 in the aflibercept 2.0 mg every 8 weeks group · 33 in the ranibizumab group Number analyzed: Full analysis ‐ 1202 total participants at 1‐year follow‐up · 296 in the aflibercept 0.5 mg every 4 weeks group · 309 in the aflibercept 2.0 mg every 4 weeks group · 306 in the aflibercept 2.0 mg every 8 weeks group · 291 in the ranibizumab group Safety analysis ‐ 1204 total participants at 1‐year follow‐up · 297 in the aflibercept 0.5 mg every 4 weeks group · 309 in the aflibercept 2.0 mg every 4 weeks group · 307 in the aflibercept 2.0 mg every 8 weeks group · 291 in the ranibizumab group Unit of analysis: individual (1 study eye per participant) How were missing data handled? missing values imputed using last observation carried forward approach Power calculation: none reported | |
| Participants | Country: Argentina; Australia; Austria; Brazil; Belgium; Colombia; Czech Republic; France; Germany; Hungary; India; Israel; Italy; Japan; Latvia; Mexico; Netherlands; Poland; Portugal; South Korea; Singapore; Slovakia; Spain; Sweden; Switzerland; United Kingdom (172 study sites) Mean age (range not reported): 75 years in the aflibercept 0.5 mg every 4 weeks group, 74 years in the aflibercept 2.0 mg every 4 weeks group, 74 years in the aflibercept 2.0 mg every 8 weeks group, and 73 years in the ranibizumab group Gender: 149 men (50.3%) and 147 women (49.7%) in the aflibercept 0.5 mg every 4 weeks group, 133 men (43.0%) and 176 women (57.0%) in the aflibercept 2.0 mg every 4 weeks group, 131 men (42.8%) and 175 women (57.2%) in the aflibercept 2.0 mg every 8 weeks group, and 122 men (41.9%) and 169 women (58.1%) in the ranibizumab group Inclusion criteria: 50 years or older; diagnosed with neovascular AMD in the study eye; active subfoveal CNV lesions of any subtype (12 optic disc areas or fewer) constituting ≥ 50% of total lesion size; BCVA between 73 and 25 Early Treatment Diabetic Retinopathy Study (ETDRS) chart letters (20/40 to 20/320 Snellen equivalent); willingness and ability to return for clinic visits and complete study‐related procedures; ability to provide informed consent Exclusion criteria: prior or concomitant treatment for AMD in the study eye; prior treatment with anti‐VEGF therapy; subretinal hemorrhage or scar or fibrosis constituting > 50% of total lesion size or involving the center of the fovea in the study eye; retinal pigment epithelial tears or rips involving the macula in the study eye; history of other ocular conditions such as vitreous hemorrhage, retinal detachment, macular hole, corneal transplant, corneal dystrophy, diabetic retinopathy, diabetic macular edema, uveitis, scleromalacia; presence of other ocular conditions such as uncontrolled glaucoma, significant media opacities, phakia or pseudophakia with absence of posterior capsule, intraocular inflammation or infection; prior vitrectomy, trabeculectomy, or other filtration surgery or therapy in the study eye Equivalence of baseline characteristics: yes; "Baseline demographics and disease characteristics were evenly balanced among all treatment groups" | |
| Interventions | Intervention 1: intravitreal aflibercept 0.5 mg every 4 weeks Intervention 2: intravitreal aflibercept 2.0 mg every 4 weeks Intervention 3: intravitreal aflibercept 2.0 mg every 8 weeks after 3 initial doses at weeks 0, 4, and 8 (to maintain masking, sham injections were given at the interim 4‐week visits after week 8) Intervention 4: intravitreal ranibizumab 0.5 mg every 4 weeks Length of follow‐up: 1 year for primary end point; dosing for all groups changed to as needed (PRN) after 1 year and follow‐up at 2 years from baseline | |
| Outcomes | Primary outcome, as defined in study reports: "proportion of patients maintaining vision at week 52 (losing < 15 letters on Early Treatment Diabetic Retinopathy Study [ETDRS] chart)" Secondary outcomes, as defined in study reports: change in BCVA and anatomic measures, proportion gaining ≥ 15 letters, change in total National Eye Institute 25‐Item Visual Function Questionnaire (NEI‐VFQ‐25) score, change in CNV area on fluorescein angiography, retinal thickness and persistent fluid as assessed by OCT, mean number of intravitreal injections, adverse events Intervals at which outcomes assessed: every 4 weeks through 96 weeks; week 1 after first treatment for safety assessment; weeks 12, 24, 36, and 52 for the NEI‐VFQ‐25 assessment | |
| Notes | Type of study reports: published journal articles; clinical trial registration Funding sources: "Sponsored by Regeneron Pharmaceuticals, Inc, Tarrytown, New York, and Bayer HealthCare, Berlin Germany. The sponsors participated in the design and conduct of the study, analysis of the data, and preparation of the manuscript" Disclosures of interest: "J.S.H. is a consultant to and has received research funding from Alimera, Allergan, Fovea, Genentech, Genzyme, GlaxoSmithKline, Neovista, and Regeneron Pharmaceuticals. He has also received travel support from Regeneron Pharmaceuticals. D.M.B. is a consultant to Alimera, Allergan, Bayer, Genentech/Roche, Novartis, Regeneron Pharmaceuticals, and Thrombogenics and has received research funding from Alcon, Alimera, Allergan, Eli Lilly, Genentech, GlaxoSmithKline, Novartis, Regeneron Pharmaceuticals, and Thrombogenics. He has also received travel support from Regeneron Pharmaceuticals and lecture fees from Genentech. V.C. is a consultant to Alimera and Bayer and has received research funding from Alcon, Allergan, Bayer, Novartis, and Pfizer. He is an advisory board member for Allergan and Novartis and has also received travel support from Bayer. J.‐F.K. is a consultant to Alcon, Bayer, and Thea and an advisory board member for Allergan, Bayer, and Novartis. He has received travel support from Regeneron Pharmaceuticals. P.K.K. is a consultant to Bayer, Genentech, Novartis, and Regeneron Pharmaceuticals. He has received research funding from Regeneron Pharmaceuticals. Q.D.N. is a consultant to Bausch & Lomb and Santen and has received research funding from Genentech, Novartis, and Pfizer. B.K. has received travel support from Bayer. A.H. is a consultant to Alcon, Allergan, Centocor, Johnson & Johnson, Neovista, Merck, Ophthotech, Oraya, Paloma, P.R.N., Q.L.T., Regeneron Pharmaceuticals, and Thrombogenics. He has received research funding and lecture fees from Alcon, Allergan, Genentech, Neovista, Ophthotech, Oraya, P.R.N., Q.L.T., Regeneron Pharmaceuticals, and Second Sight. Y.O. is a consultant to Alcon and Bayer and has received travel support from Bayer. G.D.Y., N.S., R.V., A.J.B., and Y.S. are employees of Regeneron Pharmaceuticals. M.A., G.G., B.S., and R.S. are employees of Bayer HealthCare. C.S.’s institution has received payments from the Medical University of Vienna for data monitoring/reviewing and statistical analysis. U.S.‐E. is a consultant to Alcon, Allergan, Bayer HealthCare, and Novartis, and an advisory board member for Alcon and Novartis. She has received travel support from Bayer HealthCare and lecture fees from Bayer HealthCare and Novartis" Study period: March 2008 to September 2010 Subgroup analyses: yes; Japanese subgroup | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of random sequence generation was unclear. “Consecutively enrolled patients were assigned to treatment groups on the basis of a predetermined central randomization scheme with balanced allocation, managed by an interactive voice response system” |
| Allocation concealment (selection bias) | Low risk | Central randomization: “Consecutively enrolled patients were assigned to treatment groups on the basis of a predetermined central randomization scheme with balanced allocation, managed by an interactive voice response system” |
| Masking of participants and personnel (performance bias) | Low risk | “Patients were masked as to treatments. An unmasked investigator also was responsible for the receipt, tracking, preparation, destruction, and administration of study drug, as well as safety assessments both pre‐ and post‐dose...All other study site personnel were masked to treatment assignment by separating study records or masked packaging” |
| Masking of outcome assessment (detection bias) | Low risk | “A separate masked physician assessed adverse events and supervised the masked assessment of efficacy. All other study site personnel were masked to treatment assignment by separating study records or masked packaging. Optical coherence tomography technicians and visual acuity examiners remained masked relative to treatment assignment” |
| Incomplete outcome data (attrition bias) | Low risk | A full analysis set and a per protocol set were reported. Last observation carried forward (LOCF) approach was used to impute missing values; 88.1% to 91.1% of participants per treatment group completed 52 weeks of follow‐up |
| Selective reporting (reporting bias) | Low risk | Study was registered at clinicaltrials.gov; intended outcomes were reported |
| Other bias | High risk | Many authors are employees of, consultants to, or have received research funding from Regeneron Pharmaceuticals, which manufactures aflibercept and participated in the design of the trial, collected and analyzed data, and prepared the study reports |
AMD: age‐related macular degeneration.
BCVA: best‐corrected visual acuity.
CNV: choroidal neovascularization.
ETDRS: Early Treatment Diabetic Retinopathy Study.
NEI‐VFQ‐25: National Eye Institute 25‐Item Visual Function Questionnaire.
OCT: optical coherence tomography.
VEGF: vascular endothelial growth factor.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Study administered aflibercept and placebo via an intravenous injection. This method of administration is not used in clinical practice | |
| Not a randomized controlled trial; uses data from other trials to create a cost‐utility model comparing aflibercept vs other AMD drugs | |
| Not a randomized controlled trial | |
| Included participants who had been previously treated with other anti‐VEGF medications (not treatment‐naive); reported only outcomes for 4 weeks | |
| Not a randomized controlled trial |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA in ETDRS letters at 1 year Show forest plot | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐1.47, 1.17] |
| Analysis 1.1 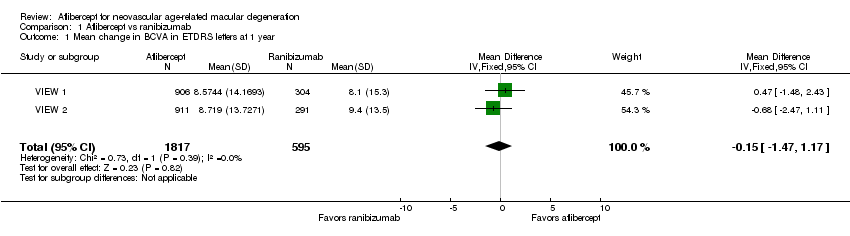 Comparison 1 Aflibercept vs ranibizumab, Outcome 1 Mean change in BCVA in ETDRS letters at 1 year. | ||||
| 2 Gain of ≥ 15 letters of BVCA at 1 year Show forest plot | 2 | 2412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.85, 1.11] |
| Analysis 1.2  Comparison 1 Aflibercept vs ranibizumab, Outcome 2 Gain of ≥ 15 letters of BVCA at 1 year. | ||||
| 3 Loss of ≥ 15 letters of BVCA at 1 year Show forest plot | 2 | 2412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.30] |
| Analysis 1.3  Comparison 1 Aflibercept vs ranibizumab, Outcome 3 Loss of ≥ 15 letters of BVCA at 1 year. | ||||
| 4 Absence of fluid on optical coherence tomography (OCT) at 1 year Show forest plot | 2 | 2291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.98, 1.14] |
| Analysis 1.4  Comparison 1 Aflibercept vs ranibizumab, Outcome 4 Absence of fluid on optical coherence tomography (OCT) at 1 year. | ||||
| 5 Mean change in size of the choroidal neovascularization at 1 year Show forest plot | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.78, 0.29] |
| Analysis 1.5  Comparison 1 Aflibercept vs ranibizumab, Outcome 5 Mean change in size of the choroidal neovascularization at 1 year. | ||||
| 6 Mean change in central retinal thickness at 1 year Show forest plot | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐4.94 [‐15.48, 5.61] |
| Analysis 1.6  Comparison 1 Aflibercept vs ranibizumab, Outcome 6 Mean change in central retinal thickness at 1 year. | ||||
| 7 Mean change in vision‐related quality‐of‐life scores at 1 year Show forest plot | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.71, 0.93] |
| Analysis 1.7 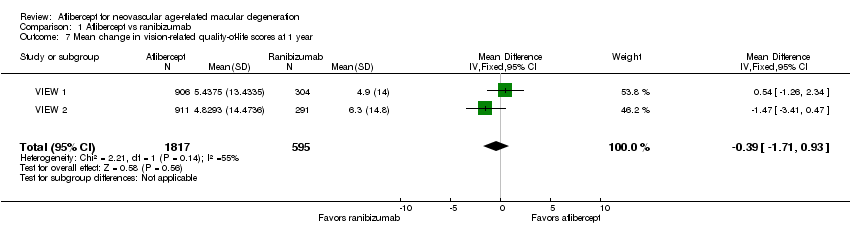 Comparison 1 Aflibercept vs ranibizumab, Outcome 7 Mean change in vision‐related quality‐of‐life scores at 1 year. | ||||
| 8 Adverse events ‐ arterial thrombotic events at 1 year Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8 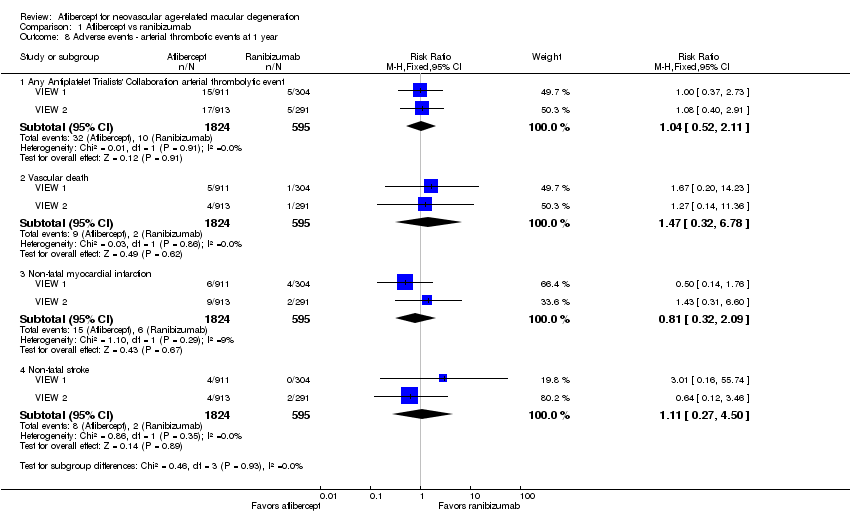 Comparison 1 Aflibercept vs ranibizumab, Outcome 8 Adverse events ‐ arterial thrombotic events at 1 year. | ||||
| 8.1 Any Antiplatelet Trialists' Collaboration arterial thrombolytic event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.52, 2.11] |
| 8.2 Vascular death | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.32, 6.78] |
| 8.3 Non‐fatal myocardial infarction | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.32, 2.09] |
| 8.4 Non‐fatal stroke | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.27, 4.50] |
| 9 Adverse events ‐ serious systemic events at 1 year Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9 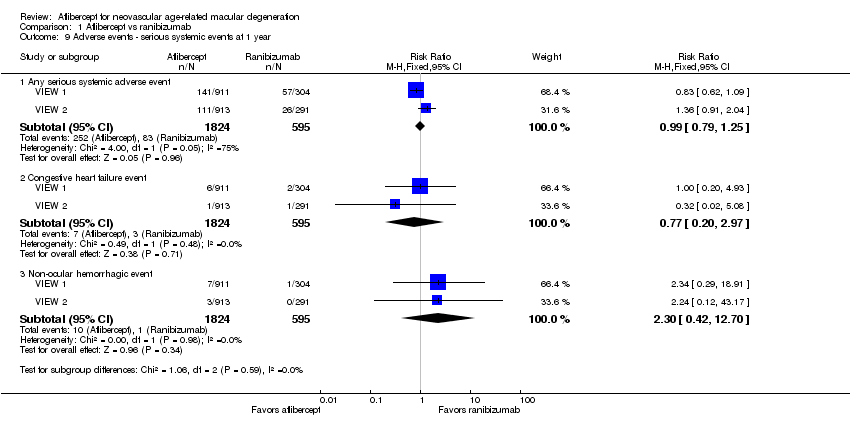 Comparison 1 Aflibercept vs ranibizumab, Outcome 9 Adverse events ‐ serious systemic events at 1 year. | ||||
| 9.1 Any serious systemic adverse event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.79, 1.25] |
| 9.2 Congestive heart failure event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.20, 2.97] |
| 9.3 Non‐ocular hemorrhagic event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.42, 12.70] |
| 10 Adverse events ‐ serious ocular events at 1 year Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10 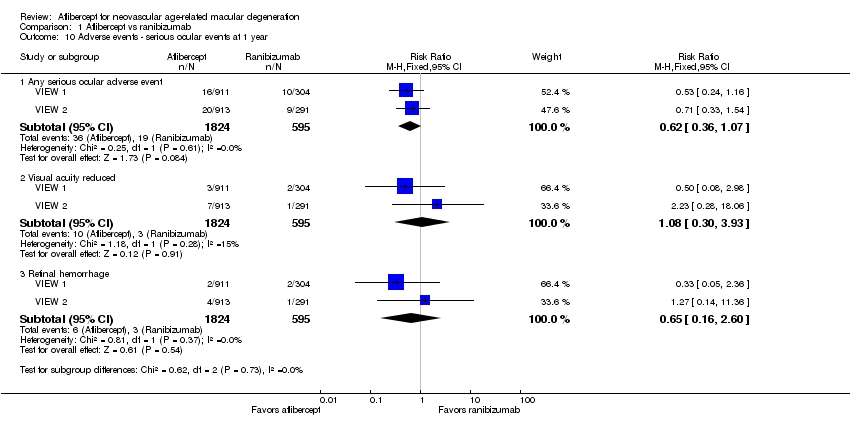 Comparison 1 Aflibercept vs ranibizumab, Outcome 10 Adverse events ‐ serious ocular events at 1 year. | ||||
| 10.1 Any serious ocular adverse event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.36, 1.07] |
| 10.2 Visual acuity reduced | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.30, 3.93] |
| 10.3 Retinal hemorrhage | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.16, 2.60] |

Study flow diagram.
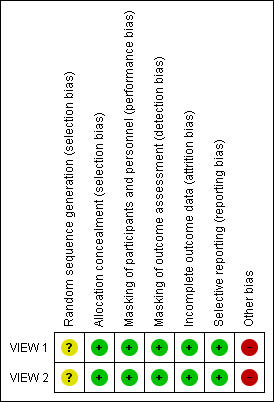
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Aflibercept vs ranibizumab, outcome: 1.1 Mean change in BCVA in ETDRS letters at 1 year.

Forest plot of comparison: 1 Aflibercept vs ranibizumab, outcome: 1.2 Gain of ≥ 15 letters of BVCA at 1 year.

Forest plot of comparison: 1 Aflibercept vs ranibizumab, outcome: 1.4 Absence of fluid on optical coherence tomography (OCT) at 1 year.

Forest plot of comparison: 1 Aflibercept vs ranibizumab, outcome: 1.7 Mean change in vision‐related quality‐of‐life scores at 1 year.
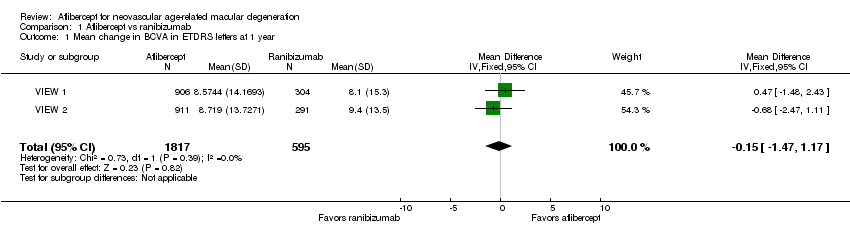
Comparison 1 Aflibercept vs ranibizumab, Outcome 1 Mean change in BCVA in ETDRS letters at 1 year.
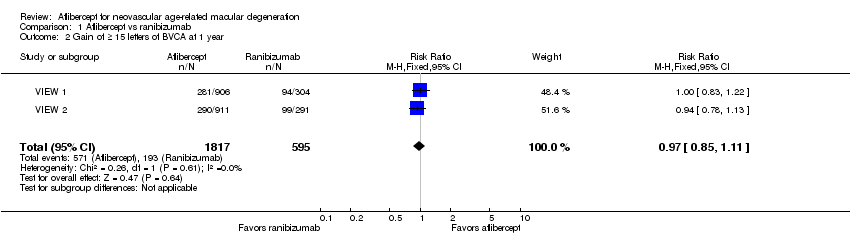
Comparison 1 Aflibercept vs ranibizumab, Outcome 2 Gain of ≥ 15 letters of BVCA at 1 year.

Comparison 1 Aflibercept vs ranibizumab, Outcome 3 Loss of ≥ 15 letters of BVCA at 1 year.
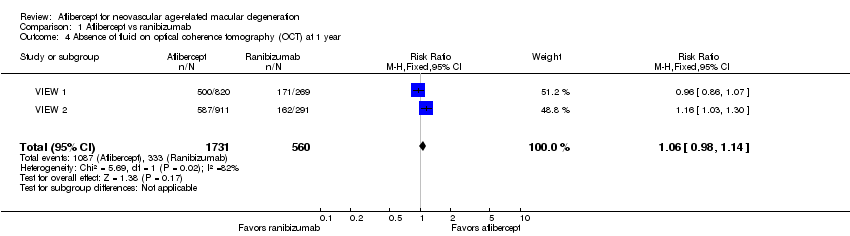
Comparison 1 Aflibercept vs ranibizumab, Outcome 4 Absence of fluid on optical coherence tomography (OCT) at 1 year.

Comparison 1 Aflibercept vs ranibizumab, Outcome 5 Mean change in size of the choroidal neovascularization at 1 year.

Comparison 1 Aflibercept vs ranibizumab, Outcome 6 Mean change in central retinal thickness at 1 year.

Comparison 1 Aflibercept vs ranibizumab, Outcome 7 Mean change in vision‐related quality‐of‐life scores at 1 year.

Comparison 1 Aflibercept vs ranibizumab, Outcome 8 Adverse events ‐ arterial thrombotic events at 1 year.
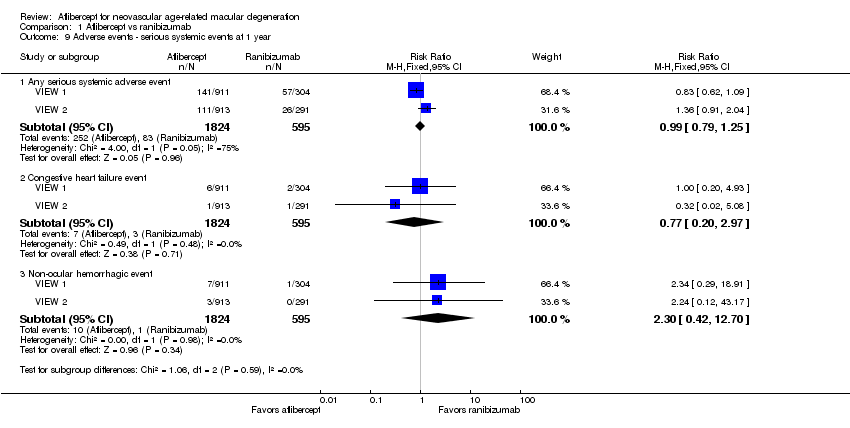
Comparison 1 Aflibercept vs ranibizumab, Outcome 9 Adverse events ‐ serious systemic events at 1 year.

Comparison 1 Aflibercept vs ranibizumab, Outcome 10 Adverse events ‐ serious ocular events at 1 year.
| Aflibercept vs ranibizumab for neovascular age‐related macular degeneration | ||||||
| Patient or population: people with age‐related macular degeneration Settings: clinical centers Intervention: intravitreal injections of aflibercept Comparison: intravitreal injections of ranibizumab | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Ranibizumab | Aflibercept | |||||
| Mean change in BCVA in ETDRS letters at 1 year (number of letters) | Mean change in visual acuity across ranibizumab groups ranged from gains of 8.57 letters to 8.71 letters | Mean change in visual acuity in aflibercept groups was on average 0.15 fewer letters gained (95% CI 1.47 fewer letters to 1.17 more letters) | MD ‐0.15 | 2412 | ⊕⊕⊕⊕ | |
| Gain of ≥ 15 letters of BVCA at 1 year | 324 per 1000 | 314 per 1000 | RR 0.97 | 2412 (2) | ⊕⊕⊕⊕ | |
| Absence of fluid on optical coherence tomography (OCT) at 1 year | 595 per 1000 | 630 per 1000 | RR 1.06 | 2291 (2) | ⊕⊕⊕⊕ | |
| Quality‐of‐life measures at 1 year (National Eye Institute‐Visual Function Questionnaire [NEI‐VFQ]) | Mean improvement in composite NEI‐VQF score ranged across control groups from 4.9 to 6.3 points | Mean improvement in composite NEI‐VQF score in intervention groups was on average 0.39 points lower (95% CI 1.71 points lower to 0.93 points higher) | MD ‐0.39 | 2412 (2) | ⊕⊕⊕⊕ | |
| Adverse events ‐ serious systemic events at 1 year | 139 per 1000 | 138 per 1000 | RR 0.99 (0.79 to 1.25) | 2419 (2) | ⊕⊕⊕⊝ | |
| Adverse events ‐ serious ocular events at 1 year | 32 per 1000 | 20 per 1000 | RR 0.62 (0.36 to 1.07) | 2419 (2) | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (eg, median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The unit of analysis is the individual (one study eye per person). | ||||||
| GRADE Working Group grades of evidence aAdverse events downgraded to moderate quality as the number of events is small (wide confidence intervals) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean change in BCVA in ETDRS letters at 1 year Show forest plot | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐1.47, 1.17] |
| 2 Gain of ≥ 15 letters of BVCA at 1 year Show forest plot | 2 | 2412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.85, 1.11] |
| 3 Loss of ≥ 15 letters of BVCA at 1 year Show forest plot | 2 | 2412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.30] |
| 4 Absence of fluid on optical coherence tomography (OCT) at 1 year Show forest plot | 2 | 2291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.98, 1.14] |
| 5 Mean change in size of the choroidal neovascularization at 1 year Show forest plot | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.78, 0.29] |
| 6 Mean change in central retinal thickness at 1 year Show forest plot | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐4.94 [‐15.48, 5.61] |
| 7 Mean change in vision‐related quality‐of‐life scores at 1 year Show forest plot | 2 | 2412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.71, 0.93] |
| 8 Adverse events ‐ arterial thrombotic events at 1 year Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Any Antiplatelet Trialists' Collaboration arterial thrombolytic event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.52, 2.11] |
| 8.2 Vascular death | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.32, 6.78] |
| 8.3 Non‐fatal myocardial infarction | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.32, 2.09] |
| 8.4 Non‐fatal stroke | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.27, 4.50] |
| 9 Adverse events ‐ serious systemic events at 1 year Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Any serious systemic adverse event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.79, 1.25] |
| 9.2 Congestive heart failure event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.20, 2.97] |
| 9.3 Non‐ocular hemorrhagic event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.42, 12.70] |
| 10 Adverse events ‐ serious ocular events at 1 year Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Any serious ocular adverse event | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.36, 1.07] |
| 10.2 Visual acuity reduced | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.30, 3.93] |
| 10.3 Retinal hemorrhage | 2 | 2419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.16, 2.60] |

