Extracto de Cyclamen europaeum para la sinusitis aguda
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011341.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 11 mayo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Infecciones respiratorias agudas
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Anca Zalmanovici Trestioreanu wrote the protocol, entered data into Review Manager 5, and wrote the review.
Ankur Barua signed off on the protocol and participated with Anca in study selection, quality assessment, and data extraction.
Barak Pertzov participated in data analysis, summary of findings Table for the main comparison, and the interpretation of results.
Declarations of interest
Anca Zalmanovici Trestioreanu: None known.
Ankur Barua: None known.
Barak Pertzov: None known
Acknowledgements
Thanks to the following people for commenting on the draft protocol and draft review and for ongoing assistance: Liz Dooley, Acute Respiratory Infections (ARI) Group Managing Editor, and referees Ann Fonfa, Viviana Rodriguez, Romy Lauche, Mio Hu, Romero Rodriguez, Emma Lake, Julie Gildie, Liz Bickerdike, and Michelle Guppy.
We also wish to thank David Honeyman, Information Specialist, ARI Group, for support with searches. Ekaterina Victorovna Yudina and Liliya Eugenevna Ziganshina translated studies from Russian.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 May 11 | <i>Cyclamen europaeum</i> extract for acute sinusitis | Review | Anca Zalmanovici Trestioreanu, Ankur Barua, Barak Pertzov | |
| 2014 Oct 12 | <i>Cyclamen europaeum</i> extract for acute sinusitis | Protocol | Anca Zalmanovici Trestioreanu, Ankur Barua | |
Differences between protocol and review
We added a post hoc best‐case/worst‐case sensitivity analysis to the review.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO

Study flow diagram.
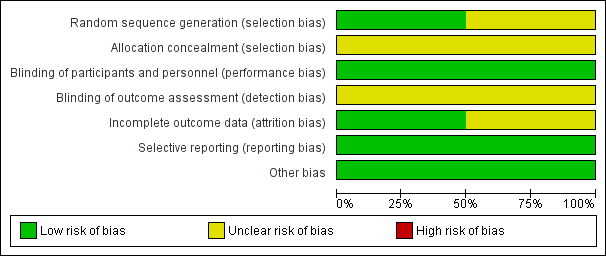
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Cyclamen europaeum versus placebo, Outcome 1 Proportion of participants with any adverse event ‐ intention‐to‐treat.

Comparison 1 Cyclamen europaeum versus placebo, Outcome 2 Proportion of participants with any adverse events ‐ best‐case sensitivity analysis.

Comparison 1 Cyclamen europaeum versus placebo, Outcome 3 Proportion of participants with any adverse event ‐ worst‐case sensitivity analysis.
| Cyclamen europaeum compared to placebo for acute sinusitis | ||||||
| Patient or population: adults with acute sinusitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Cyclamen europaeum | |||||
| Proportion of participants with resolution or improvement of symptoms up to 30 days | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| Proportion of participants with any adverse event | Study population | RR 2.11 | 147 | ⊕⊕⊕⊝ Moderate | We downgraded the quality of the evidence as concealment of allocation to treatment and blinding of outcome assessors were not reported in the studies. Also, 1 study had a small sample size with a high attrition rate. | |
| 240 per 1000 | 506 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| Study ID | Cyclamen europaeum | Placebo |
| 67% total 50% nasal irritation mild/moderate 27% mild epistaxis 4% sneezing 3 discontinued treatment | 29% total 4% nasal irritation 14% mild epistaxis 4% vertigo 2 discontinued treatment | |
| 15.4% total Influenza, throat irritation, migraine, sneezing No serious adverse events Did not discontinue treatment | 12.5% total Headache, ear pain, gastritis, back pain, conjunctival haemorrhage No serious adverse events Did not discontinue treatment |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants with any adverse event ‐ intention‐to‐treat Show forest plot | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.35, 3.29] |
| 2 Proportion of participants with any adverse events ‐ best‐case sensitivity analysis Show forest plot | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.30] |
| 3 Proportion of participants with any adverse event ‐ worst‐case sensitivity analysis Show forest plot | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.49 [2.30, 5.30] |

