停止使用长效β2受体激动剂(LABA)治疗哮喘通过LABA加吸入皮质类固醇治疗得到良好控制的儿童
Referencias
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Wrong comparison ‐ not stepping down LABA | |
| Wrong population ‐ adults | |
| Wrong comparison ‐ not stopping LABA | |
| Wrong comparison ‐ not stopping LABA | |
| Wrong comparison ‐ not stopping LABA | |
| Wrong comparison ‐ ICS dose was higher in the step‐down group | |
| Wrong population ‐ adults | |
| Wrong comparison ‐ not stopping LABA Analysis of healthcare utilisation and costs of stepping down LABA | |
| Wrong population ‐ adults | |
| Wrong study design ‐ cross‐over study | |
| Wrong comparison ‐ not stopping LABA | |
| Wrong comparison ‐ not stopping LABA | |
| Wrong population ‐ adults | |
| Wrong population ‐ adults | |
| Wrong comparison ‐ not stopping LABA | |
| Wrong comparison ‐ not stopping LABA using symptom scores with or without PD20 methacholine to adjust treatment | |
| Wrong comparison ‐ no clear step‐down strategy for LABA | |
| Wrong population ‐ adults (ongoing trial) | |
| Wrong study design ‐ single‐group observational study The effect of stepping down from LABA/ICS combination therapy to ICS monotherapy on exercise and mannitol challenge tests in asthmatic children | |
| Wrong comparison ‐ not stopping LABA | |
| Wrong comparison ‐ not stopping LABA | |
| Wrong comparison ‐ not stopping LABA | |
| Wrong population ‐ adults | |
| Wrong comparison ‐ ICS stepped down | |
| Wrong comparison ‐ two step‐down groups | |
| Wrong population ‐ adults | |
| Wrong comparison ‐ ICS stepped down |
Characteristics of ongoing studies [ordered by study ID]
Ir a:
| Trial name or title | Long‐acting Beta Agonist Step Down Study (LASST) |
| Methods | 56‐week, multicentre, blinded, randomised, double‐masked parallel group comparative effectiveness study of approaches to stepping down therapy for people with well‐controlled asthma treated with combination ICS and LABA. |
| Participants | Inclusion Criteria: males and females aged 12‐80 years with well‐controlled asthma on moderate dose ICS/LABA based on an Asthma Control Test score ≥ 20, absence of unscheduled visits or use of rescue prednisone for 4 weeks prior to enrolment and a pre‐bronchodilator FEV1 ≥ 70% predicted Exclusion Criteria: chronic oral steroid therapy, hospitalisation or urgent care visit within 4 weeks of the screening visit, lung disease other than asthma including COPD, bronchiectasis, sarcoidosis, or other lung disease. Less than 10 pack/yr of tobacco use and abstinence, post‐bronchodilator FEV1 < 70% predicted, near fatal asthma (intubation or ICU admission for asthma) within 2 yrs of enrolment, high risk of near fatal or fatal asthma, history of known premature birth less than 33 weeks or any significant level of respiratory care including prolonged oxygen administration or mechanical ventilation during the neonatal period, unstable cardiac disease (decompensated congestive heart failure, unstable angina, recent myocardial infarction, atrial fibrillation, supraventricular or ventricular tachycardia, congenital heart disease, or severe uncontrolled hypertension), other major chronic illnesses, drug allergies, pregnancy, lactation |
| Interventions | Stepping down from fluticasone/salmeterol Diskus 250/50 mcg twice a day to fluticasone Diskus 250 mcg twice a day without salmeterol |
| Outcomes | Primary: Rate of treatment failures (decline in peak flow or FEV1, increased need for beta agonists, requirement for non‐scheduled medical care for asthma symptoms, or prednisone taper) Secondary: Pulmonary function (morning peak expiratory flow, pre‐bronchodilator FEV1), episodes of poor asthma control defined by unscheduled medical care, hospitalisation, use of oral corticosteroids and/or increased use of rescue medications and/or decrease of 30% or more in morning peak expiratory flow rate |
| Starting date | March 2012 |
| Contact information | Joy Saams, Registered Nurse |
| Notes | Estimated enrolment: 450. Estimated study completion date: June 2015 May be able to obtain data for children and adolescents separately from the adult participants |
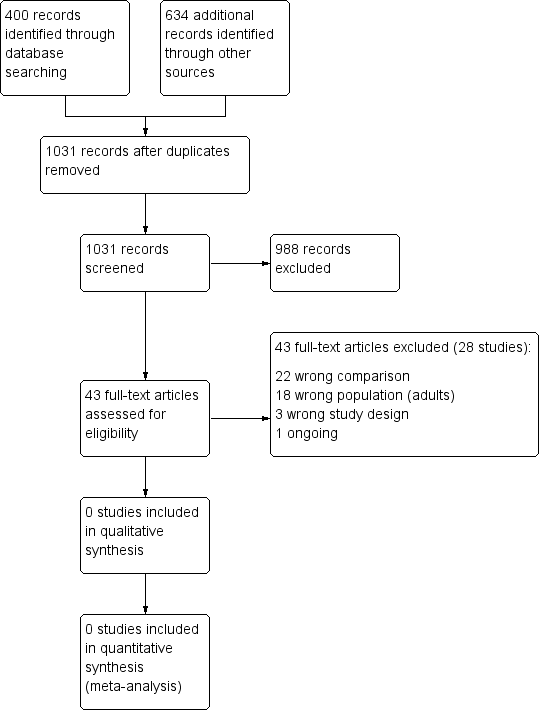
Results of the search and study selection process
| Stopping long‐acting beta2‐agonists (LABA) compared with continuing inhaled corticosteroids (ICS) + LABA for children with well‐controlled asthma | ||
| Patient or population: children (aged 18 years or younger) whose asthma is well controlled on combination ICS + LABA Settings: outpatient Intervention: stopping LABA Comparison: continuing ICS + LABA | ||
| Outcomes | No of Participants | Comments |
| Exacerbations requiring systemic corticosteroids | 0 (0) | No studies met the inclusion criteria for the review |
| Asthma control (validated scales) | 0 (0) | |
| Serious adverse events (all cause) | 0 (0) | |
| Quality of life (validated scales) | 0 (0) | |
| Exacerbations requiring hospitalisation or emergency department visit | 0 (0) | |
| Adverse events (all cause) | 0 (0) | |
| Withdrawals | 0 (0) | |

