Goma de fibrina versus suturas para el injerto autólogo de conjuntiva en la cirugía primaria para el pterigión
Resumen
Antecedentes
El pterigión, un crecimiento de la conjuntiva por encima de la córnea, es una enfermedad progresiva que, en los estadios avanzados, lleva a la deficiencia visual, la restricción de la motilidad ocular, la inflamación crónica y preocupaciones estéticas. La extracción quirúrgica es el tratamiento de elección, pero la recurrencia puede ser un problema. Actualmente la opción mejor quirúrgica en cuanto a la recurrencia es el injerto autólogo de conjuntiva. Hasta la fecha, las suturas o la goma de fibrina son los métodos quirúrgicos más frecuentes para adherir los injertos autólogos de conjuntiva a la esclerótica. Cada método presenta sus propias ventajas y desventajas. Las suturas requieren considerable habilidad por parte del cirujano y pueden asociarse con un tiempo quirúrgico prolongado, malestar posoperatorio y complicaciones relacionadas con la sutura, mientras que la goma de fibrina puede disminuir el tiempo quirúrgico, mejorar el bienestar posoperatorio y evitar problemas relacionados con la sutura.
Objetivos
Evaluar la efectividad de la goma de fibrina comparada con las suturas en el injerto autólogo de conjuntiva para el tratamiento quirúrgico del pterigión.
Métodos de búsqueda
Se realizaron búsquedas en CENTRAL (que contiene el registro de ensayos del Grupo Cochrane de Trastornos de los Ojos y la Visión [Cochrane Eyes and Vision Group]) (2016, número 9), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (enero 1946 hasta octubre 2016), Embase (enero 1980 hasta octubre 2016), el ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), y en la World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). No se aplicó ninguna restricción de fecha ni de idioma en las búsquedas electrónicas de ensayos. Se buscó por última vez en las bases de datos electrónicas el 14 de octubre 2016.
Criterios de selección
Se incluyeron ensayos controlados aleatorios (ECA) en cualquier ámbito, en que se comparara la goma de fibrina con las suturas para el tratamiento de pacientes con pterigión.
Obtención y análisis de los datos
Dos autores de la revisión examinaron de forma independiente los resultados de la búsqueda, evaluaron la calidad de los ensayos y extrajeron los datos mediante procedimientos metodológicos estándar previstos por Cochrane. El resultado del primario fue la recurrencia del pterigión, definido como el nuevo crecimiento del tejido del área de la escisión a través del limbo sobre la córnea. Los resultados secundarios fueron el tiempo quirúrgico y la tasa de complicaciones. La certeza de las pruebas se evaluó mediante GRADE.
Resultados principales
Se incluyeron 14 ECA realizados en Brasil, China, Egipto, India, Malasia, Nueva Zelanda, Filipinas, Arabia Saudita, Suecia y Turquía. Los ensayos fueron publicados entre 2004 y 2016 y se evaluaron como una combinación riesgo de sesgo poco claro y bajo, y tres estudios con alto riesgo de sesgo de deserción. Sólo se reclutaron adultos en estos estudios.
El uso de goma de fibrina para el injerto autólogo de conjuntiva puede resultar en menos recurrencia del pterigión en comparación con las suturas (cociente de riesgos [CR] 0,47; IC del 95%: 0,27 a 0,82; 762 ojos, 12 ECA; pruebas de baja confiabilidad). Si el pterigión reaparece después de cerca de 10 de cada 100 intervenciones con suturas, entonces el uso de la goma de fibrina puede dar lugar a cerca de 5 casos menos de recurrencia de cada 100 intervenciones (IC del 95%: 2 casos menos a 7 casos menos). El uso de la goma de fibrina puede resultar en más complicaciones en comparación con las suturas (CR 1,92; IC del 95%: 1,22 a 3,02; 11 ECA, 673 ojos, pruebas de baja confiabilidad). Las complicaciones más comunes informadas fueron: dehiscencia del injerto, retracción del injerto y granuloma. En promedio, el uso de la goma de fibrina puede significar que la intervención quirúrgica sea más rápida en comparación con las suturas (diferencia de medias [DM] ‐17,01 minutos; IC del 95%: ‐20,56 a ‐13,46), nueve ECA, 614 ojos, pruebas de baja confiabilidad).
Conclusiones de los autores
Los metanálisis, realizados en pacientes con pterigión ingresados o en consultorios externos, muestran que la goma de fibrina puede dar lugar a menos recurrencia y puede tomar menos tiempo que las suturas para fijar el injerto conjuntival durante la intervención quirúrgica. Hubo pruebas de baja confiabilidad que sugieren una proporción mayor de complicaciones en el grupo de goma de fibrina.
PICO
Resumen en términos sencillos
Goma de fibrina versus suturas para el injerto autólogo de conjuntiva en la cirugía primaria para el pterigión
¿Cuál es el objetivo de esta revisión?
El objetivo de esta revisión Cochrane era determinar si es mejor usar la goma de fibrina o las suturas (puntos) cuando se opera un pterigión (crecimiento no deseado de tejido en la parte frontal del ojo). La operación incluye el reemplazo del pterigión con un trozo de tejido de otra parte del ojo (injerto autólogo). Los investigadores Cochrane recopilaron y analizan todos los estudios pertinentes para responder a esta pregunta y encontraron 14 estudios.
Mensajes clave
Cuando se realiza el injerto, el uso de goma de fibrina puede dar lugar a menos probabilidades de recurrencia del pterigión. Además, la operación puede llevar menos tiempo. La goma de fibrina puede asociarse con más complicaciones, como rotura del injerto, reducción del injerto y desarrollo de una zona de inflamación (granuloma).
¿Qué se estudió en la revisión?
En ocasiones, un trozo de tejido puede crecer en el frente del ojo, y si adquiere un tamaño suficiente puede afectar la visión. Este tejido se conoce como pterigión. Los pacientes que residen en lugares cálidos, polvorientos, con alta luz solar tienen mayor probabilidad de contraer un pterigión. Un pterigión puede ser incómodo y causar picazón, y puede afectar la apariencia del ojo.
Los médicos pueden retirar este tejido y reemplazarlo con tejido de otra parte del cuerpo, por lo general, de otra parte de la conjuntiva (que cubre la parte blanca del ojo). Este procedimiento se conoce como injerto o injerto autólogo.
Los investigadores Cochrane consideraron dos métodos diferentes de fijación de este injerto durante la intervención para el pterigión: con goma de fibrina o con puntos.
¿Cuáles son los principales resultados de la revisión?
Los autores de la revisión encontraron 14 estudios pertinentes. Los estudios fueron de Brasil, China, Egipto, India, Malasia, Nueva Zelanda, Filipinas, Arabia Saudita, Suecia y Turquía. Estos estudios compararon la goma de fibrina con los puntos en pacientes con pterigión removido y un injerto del tejido de la conjuntiva.
El uso de goma de fibrina durante la intervención quirúrgica para el pterigión puede dar lugar a menos casos de recurrencia en comparación con las suturas (pruebas de baja confiabilidad). Puede llevar menos tiempo una operación de pterigión e injerto con goma de fibrina (pruebas de baja confiabilidad). Pueden ser más probables las complicaciones con la goma de fibrina, como rotura del injerto, reducción del injerto o desarrollo de una zona de inflamación (granuloma).
¿Cuán actualizada está esta revisión?
Los investigadores Cochrane buscaron estudios que se habían publicado hasta el 14 octubre 2016.
Authors' conclusions
Summary of findings
| Fibrin glue compared with sutures for primary pterygium | ||||||
| Patient or population: individuals with pterygium Settings: hospital or outpatients Intervention: fibrin glue Comparison: sutures | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of eyes | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sutures | Fibrin glue | |||||
| Recurrence of pterygium (follow‐up 2 to 24 months) | 100 per 1000 | 47 per 1000 | RR 0.47 (0.27 to 0.82) | 762 (12 RCTs) | ⊕⊕⊝⊝ | Large variability in duration of follow‐up |
| Occurrence of complication | 70 per 1000 | 134 per 1000 | RR 1.92 (1.22 to 3.02) | 673 (11 RCTs) | ⊕⊕⊝⊝ | |
| Operating time | The mean operating time ranged across control groups from | The mean operating time in the intervention groups was17.0 minutes less (from 20.6 minutes less to 13.5 minutes less) | 614 (9 RCTs) | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded 1 level for risk of bias due to study limitations, in particular generation of allocation sequence, allocation concealment, and incomplete outcome data. | ||||||
Background
Primary pterygium is one of the most common eye diseases, affecting a large percentage of the population, especially those living between the Tropics of Cancer and Capricorn. Effectively the incidence is high in geographic areas with high ultraviolet radiation (Moran 1984) or hot, dry, windy, dusty and smoky environments (Nakaishi 1997; Norn 1991), however hereditary factors also play a role (Anguria 2014; Romano 2016). The estimated prevalence of pterygium is variable, ranging from 0.7% to 31%.
Surgical removal is the treatment of choice. For many years, the surgical management of pterygium involved a simple excision of the excessive tissue mass overlying the cornea and the adjacent sclera, leaving a wide area of bare sclera. However, recurrence of the pterygium was unacceptably high, in as many as 89% of cases (Cameron 1965; Youngson 1972). To improve surgical results, two strategies have been adopted: the destructive approach, which enhances the effect of excision by radiation and chemotherapy (mitomycin C (MMC), thiotepa, 5‐fluorouracil, beta‐irradiation) and the reconstructive approach, namely transplantation of various tissue grafts (conjunctival autograft, amniotic membrane transplantation, mucous membrane graft, conjunctival limbal transplantation). Recurrences after conjunctival autograft vary from 0% to 39% (Cano‐Parra 1995; Chen 1995; Hirst 2009; Jaros 1988; Kenyon 1985; Lewallen 1989; Sebban 1991a; Sebban 1991b) and from 0% to 70% after adjunctive chemotherapy. Most studies have demonstrated that conjunctival autograft and MMC application are highly successful and equally effective (0% to 38% recurrence rate) (Cano‐Parra 1995; Chen 1995; Hayasaka 1988; Mahar 1993; Singh 1988). However, severe complications may occur following MMC therapy, such as melting of the conjunctiva and sclera, and even perforation (Dunn 1991; Mackenzie 1991; Rubinfeld 1992). Currently several studies have demonstrated that conjunctival autograft is the best method to avoid recurrence (Al Fayez 2002; Chen 1995; Kenyon 1985; Prabhasawat 1997). To date the most common surgical methods of attaching conjunctival autografts to the sclera are through suturing or fibrin glue. Each one presents its own advantages and disadvantages. Sutures require major ability of the surgeon and can be associated with a prolonged operation time, postoperative discomfort and suture‐related complications, whereas fibrin glue gives a decreased operation time, improves postoperative comfort and avoids suture‐related problems. However currently the results of efficacy of fibrin glue and sutures in pterygium surgery are not completely consistent.
The aim of this review is to summarise and compare outcomes of tissue glue and sutures that are currently used to attach conjunctival autografts in pterygium surgery.
Description of the condition
Primary pterygium is a fibrovascular wing of tissue extending onto the cornea, generally situated on the nasal side. Before it enters the optical zone, an advancing pterygium can cause localised flattening of the horizontal meridian of the cornea to the leading apex (Bedrossian 1960; Fong 1998; Gasser 2016; Hansen 1980; Holladay 1985; Lin 1998; Oldenburg 1990; Starck 1991; Stern 1998; Tomidokoro 1999; Walland 1994), often resulting in with‐the‐rule astigmatism (Lin 1997). The pterygium is conventionally divided into three parts of a triangle: a 'head', a 'neck' and a 'body'. The former is the apex of the triangle that points towards the cornea and it is connected to the 'body' by the 'neck'. The pathogenesis of pterygium is still not fully understood. Recent studies have shown alterations of limbal stem cells along the affected pterygial area. UV‐B causes mutations at the limbal edge, resulting in apoptosis and altering the production of various growth factors. Indications for surgery include visual impairment (Bedrossian 1960; Oldenburg 1990; Starck 1991), restriction of ocular motility, chronic inflammation and cosmetic concerns. Many procedures have been suggested, with or without adjunctive techniques that aim to lower the recurrence rate (Akarsu 2003; Avisar 2003; Chapman‐Smith 1992; Dadeya 2002; Mackenzie 1991).
Description of the intervention
The goal of surgical treatment is the excision of the lesion, the prevention of its recurrence and the restoration of the integrity of the ocular surface. Several different techniques have been described (Haik 1962; Kenyon 1985; King 1950; Prabhasawat 1997; Singh 1988; Vastine 1982). Briefly, the operation is performed under peribulbar anaesthesia. The eye is prepped and draped in the standard fashion and a lid speculum is inserted. When removing the pterygium, the first step involves dissection and excision of the head off the cornea. This allows for exposure of the underlying fibrovascular tissue that can then be accurately removed by dissection up to the insertion of the medial rectus muscle (if the pterygium is situated on nasal side, as usually happens). It is important in this phase to preserve as much surrounding conjunctival tissue as possible. Abnormal scar tissue on the corneal and scleral surface is also removed. The scleral bed is then measured and a very thin piece of autologous conjunctiva, 1 mm larger than this area, is excised from the superior bulbar conjunctiva. Care is taken not to include Tenon's tissue when dissecting the graft, in order to avoid postoperative oedema and graft retraction. The conjunctival graft is secured to the sclera, taking care to maintain the polarity of the tissue, with interrupted 7.0 to 8.0 Vicryl or 10.0 nylon sutures, or episclerally with fibrin glue added either sequentially or simultaneously with a double‐barrel syringe. This review will consider all types of sutures and glues currently used for conjunctival autograft.
How the intervention might work
The use of natural substances such as fibrin may have significant advantages over traditional suturing techniques (Calson 1987). Fibrin glue is a blood‐derived product that consists of two biologic components: fibrinogen and thrombin. When the components are mixed and fibrinogen is activated by thrombin, an adhesive fibrin network is formed in 30 seconds. Within days, the two components of the fibrin glue are digested, but in the meantime a strong network has been formed between the transplant and the underlying sclera. In the last 10 years, fibrin adhesives have been used to close cataract incisions (Henrick 1987), to attach soft tissue in oculoplastic surgery (Gosain 2002), to attach conjunctiva in strabismus (Dadeya 2001; Mohan 2003; Spierer 1997), to treat leaking blebs (Wright 1998) in glaucoma surgery (O'Sullivan 1996), and to close macular holes in retinal surgeries (Tilanus 1995). Recent studies have demonstrated that the use of fibrin glue in pterygium surgery might be an ideal alternative to suturing because it shortens the time of the surgical procedure, is easy to use and is associated with less postoperative inflammation, discomfort and recurrence.
Why it is important to do this review
The use of sutures in pterygium surgery is associated with postoperative inflammation, discomfort and complications related to the sutures themselves, such as granuloma formation, suture abscesses, buttonholes, tissue necrosis, and giant papillary conjunctivitis (Sridhar 2002; Tan 1997; Ti 2000). This systematic review will compare the results obtained with sutures to those with fibrin glue in conjunctival autografting procedures for the treatment of pterygium, which present advantages related to surgical skills, postoperative complications and patient's discomfort. This information is of interest not only to ophthalmic surgeons and ophthalmologists, but also to other healthcare professionals and patients.
Objectives
To assess the effectiveness of fibrin glue compared to sutures in conjunctival autografting for the surgical treatment of pterygium.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in any setting (hospital or outpatient basis), as planned in the protocol (Romano 2014).
Types of participants
Participants in the trials were people with advancing pterygium, especially people with visual impairment, restriction of ocular motility, chronic inflammation and cosmetic concerns. We excluded trials which recruited participants with connective tissue disease, previous ocular surgery and with other ocular disease.
Types of interventions
We included trials that compared fibrin glue (Tisseel or Full Link or Beriplast P or Quixil) to sutures (Vicryl 8/0 or Vicryl 7/0 or nylon 10‐0).
Types of outcome measures
Primary outcomes
-
Recurrence of pterygium: any re‐growth of tissue from the area of excision across the limbus onto the cornea, calculated by the frequency of recurrences at six months after surgery. If the study's follow‐up was longer, we considered the last follow‐up.
Secondary outcomes
-
Surgical time: the time spent to complete the surgery. We considered the mean and standard deviation.
-
Complication rate: the occurrence of at least one of the following major complications such as dehiscence, displacement or loss of the autograft, infection, haemorrhage, oedema, fibrosis, retraction and other indications that required special treatment.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 9), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to October 2016), Embase (January 1980 to October 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 14 October 2016.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), Embase (Appendix 3), ISRCTN (Appendix 4), ClinicalTrials.gov (Appendix 5) and the ICTRP (Appendix 6).
Searching other resources
We also searched the reference lists of all potentially relevant trials to identify further reports of studies.
Data collection and analysis
Selection of studies
Two review authors (VR and LC) independently assessed the search results to see if they met the inclusion criteria. We specified reasons for exclusion of studies.
The process for selecting studies for inclusion in a review was in accordance with the following procedures, as described in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
-
Merge search results using reference management software (ProCite) and remove duplicate records of the same report.
-
Examine titles and abstracts to remove obviously irrelevant reports.
-
Retrieve the full text of potentially relevant reports.
-
Link together multiple reports of the same study.
-
Examine full‐text reports for compliance of the studies with the eligibility criteria.
-
Correspond with investigators, where appropriate, to clarify study eligibility.
-
Make final decisions on study inclusion and proceed to data collection.
We planned to resolve any disagreements that arose through discussion.
Data extraction and management
Two review authors (VR and LC) independently extracted data from the selected trials using a standardised data extraction form, as suggested in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We considered items related to source, eligibility, methods, participants, interventions, outcomes and results. Further information can be found in Appendix 7.
Assessment of risk of bias in included studies
Each review author independently assessed the risk of bias of each trial using a simple form and followed the domain‐based evaluation as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We compared the assessment results and discussed any discrepancies between ourselves. We aimed to achieve agreement on the final assessment for each criteria by discussion.
We assessed each domain as 'low risk of bias', 'unclear risk of bias' or 'high risk of bias'. We evaluated the following domains.
-
Randomisation sequence generation
-
Allocation concealment
-
Blinding (masking) of outcome assessors
-
Incomplete outcome data
-
Selective outcome reporting
-
Free of other bias (e.g. baseline imbalance, early stopping)
A detailed list of items considered and measures of assessment is provided in Appendix 8.
Measures of treatment effect
We used Review Manager 5.3 (RevMan) (RevMan 2014) to analyse the data.
-
We have treated the recurrence of pterygium and complication rate as dichotomous variables and have calculated them using the risk ratio (RR) with 95% confidence intervals (CI).
-
We have treated the surgical time as a continuous variable and calculated it using the mean difference (MD) with 95% CI.
In accordance with Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011) we checked for skewed data by calculating the observed mean minus the lowest possible value and dividing this by the standard deviation. A ratio of less than 2 suggested skewness; if the ratio was less than 1 there was strong evidence of a skewed distribution. When the data were skewed, we presented results on a log scale as geometric means and ratios.
Unit of analysis issues
Trials may be parallel group (people randomised to treatment) or within‐person (eyes randomly allocated to treatment). The following table summarises the possible designs and issues in the analysis.
| Type of study | Number of eyes enrolled | Number of eyes reported | Analysis | |
| 1 | Parallel group | One | One | No unit of analysis issue. Criteria for selection of study eye should be specified |
| 2 | Parallel group | Two | One | No unit of analysis issue. Criteria for choice of eye should be specified in the trial protocol and could include worst, best, average of two eyes, right eye, left eye |
| 3 | Parallel group | Two | Two | Ideally effect estimates should be adjusted for within‐person correlation |
| 4 | Within‐person | Two | Two | Ideally study should do a pair‐matched analysis |
We documented the type of study design and approach to the analysis of people and eyes. In the case of categories 3 and 4, which may represent unit of analysis issues, we contacted study investigators for further clarification or data, as needed.
Dealing with missing data
We assessed the percentages of dropouts overall for each included trial and per each randomisation arm and we evaluated whether an intention‐to‐treat (ITT) analysis was performed or could be performed, from the available published information.
In order to allow us to undertake an ITT analysis, we sought data from the trial authors on the number of participants by treatment group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow‐up.
When additional data were needed, we contacted the corresponding author of each study by email in order to access further information. When missing data were not available, we limited our analysis to the available data.
Assessment of heterogeneity
We used the I2 statistic to measure statistical heterogeneity among the trials in each analysis. The I2 statistic describes the percentage of total variation across trials that is due to heterogeneity rather than sampling error (Higgins 2003). We considered there to be substantial statistical heterogeneity if the I2 statistic was greater than 50% (Deeks 2011; Higgins 2003; Higgins 2011b). However, we considered the importance of the I2 value by taking into consideration the magnitude and direction of effects and the strength of evidence for heterogeneity (e.g. P values from the Chi2 test, or confidence intervals for I2). Where possible, we explored clinical heterogeneity according to study setting and characteristics of participants).
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' in Appendix 8), we attempted to contact study authors and asked them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis. The assessment focused on the primary outcome and, among secondary outcomes, on complication rate.
We also assessed publication bias (the publication or non‐publication of research findings, depending on the nature and direction of the results) by using a funnel plot to graphically illustrate variability between trials. If asymmetry was detected, we explored causes other than publication bias. We produced a funnel plot if 10 or more RCTs were included.
Data synthesis
We used the random‐effects model and used the fixed‐effect model as a sensitivity analysis for evaluating the possible bias effects of smaller studies, or when there were fewer than three studies.
Subgroup analysis and investigation of heterogeneity
We did not plan to conduct subgroup analysis.
Sensitivity analysis
If a sufficient number of trials was identified, we planned to conduct a sensitivity analysis excluding low‐quality studies (Higgins 2011b). Because the possibility of masking in these trials was unlikely, we used only items in the domain of randomisation (selection bias), incomplete outcome data (attrition bias), selective outcome reporting, and other bias.
'Summary of findings' table
We have included a 'Summary of findings' table (GRADEpro 2014) to give a concise overview and synthesis of the volume and certainty of the evidence for these comparisons.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 174 references (Figure 1). The Cochrane Information Specialist removed 87 duplicate records and we screened the remaining 87 reports. We rejected 70 records after reading the abstracts and obtained the full‐text reports of 17 references for further assessment. We identified 14 studies which met the inclusion criteria, see Characteristics of included studies and excluded one study (CTRI/2013/06/00376). There are two ongoing studies for which results are not currently available, see Characteristics of ongoing studies, If we are able to access the results for these studies, we will include them in further updates of this review.

Study flow diagram
Included studies
We included clinical trials which used randomisation. The sample size achieved and analysed was 811 eyes for the recurrence rate, 722 eyes for complication rate and 614 eyes for operating time. The RCTs were conducted in Brazil, China, Egypt, India, Malaysia, New Zealand, Philippines, Saudi Arabia, Sweden and Turkey. The clinical trials were conducted in academic or hospital settings. All the trials included adult participants where there was evidence of visual impairment, restriction of ocular motility, chronic inflammation and cosmetic concerns. All the RCTs compared fibrin glue to suture using the same technique for pterygium excision. The follow‐up range was between 6 months and 24 months. In particular, two studies reported results at 24 months, nine at 12 months, five at 6 months, and one between 9 and 13 months. Not all included studies reported all outcomes evaluated in this meta‐analysis; Al‐Fayez 2008 and Ozdamar 2008 did not report operating time (see Characteristics of included studies for further details).
Excluded studies
We excluded CTRI/2013/06/00376 as this study was a randomised trial that compared autologous blood versus fibrin glue but there was no comparison with sutures.
Risk of bias in included studies

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Generation of allocation sequence
In seven studies (Al‐Fayez 2008; Hall 2009; Jaih 2006; Karalezli 2008; Ozdamar 2008; Rubin 2011; Yuksel 2010) investigators stated that the participants were randomly assigned to interventions and control, but the method used to achieve randomisation was not explicit; thus we judged this domain as 'unclear risk of bias'. The others studies reported how they generated the allocation sequence (e.g. computer‐generated, centrally‐randomised, tossing a coin) and we have judged this domain as 'low risk of bias'.
Allocation concealment
We made a judgement of 'low risk of bias' for this domain in three studies with central randomisation or with measures aimed to reduce intraobserver bias and minimise the influence of the known surgical technique (Hall 2009; Karalezli 2008; Ratnalingam 2010). In the remaining studies it was unclear if adequate measures were taken to ensure that investigators were unaware of the upcoming assignment. Therefore we judged this domain as 'unclear risk of bias' for these studies.
Blinding
We did not assess masking of participants or personnel to the intervention, as it is difficult to mask participants or personnel (clinicians for example) to the intervention. We evaluated the masking of outcome assessor to treatment allocation. However, as trials used two different types of intervention (sutures or glue), masking of outcome assessors could have been problematic, unless assessment was performed by someone not involved in the study.
All the included trials were reported as open‐label studies; however, masking probably had limited importance for more objective outcomes (such as some of the primary outcomes), because the risk of ascertainment bias was limited, but not for all outcomes. We made a judgement of 'unclear risk of bias' for nine studies (Al‐Fayez 2008; Elwan 2014; Jaih 2006; Jiang 2008; Koranyi 2004; Ozdamar 2008; Sharma 2015; Uy 2005; Yuksel 2010), and of 'low risk of bias' for the remaining studies that stated that particular attention was paid to limiting potential judgement biases.
Incomplete outcome data
Seven trials clarified the number of and reasons for dropouts (Hall 2009; Jiang 2008; Karalezli 2008; Koranyi 2004; Sati 2014; Sharma 2015; Uy 2005), and we made a judgement of 'low risk of bias' for this domain. Four trials did not provide sufficient information to permit a judgement (Al‐Fayez 2008; Elwan 2014; Jaih 2006; Ozdamar 2008). Three trials (Ratnalingam 2010; Rubin 2011; Yuksel 2010), judged at 'high risk of bias', were not on ITT basis and did not provide information on reasons and distribution of withdrawal.
Selective reporting
There was no evidence of selective outcome reporting in the large majority of included trials and it appeared that the outcomes reported were comparable to those specified in the methods section of the reports. Two studies, however, did not provide sufficient information to provide a judgement.
Publication bias was assessed by visual inspection of funnel plots (Figure 4; Figure 5. Funnel plots had a symmetric appearance for all the outcomes analysed

Funnel plot of comparison: 1 Fibrin glue versus suture, outcome: 1.1 Recurrence of pterygium.

Funnel plot of comparison: 1 Fibrin glue versus suture, outcome: 1.2 Occurrence of 1 or more complications.
Effects of interventions
See: Summary of findings for the main comparison
See summary of findings Table for the main comparison.
We conducted three meta‐analyses for recurrence of pterygium (primary outcome), surgical time and complication rate (secondary outcomes). The meta‐analyses for recurrence of pterygium shows that the recurrence of pterygium is less in the fibrin glue group (Analysis 1.1) (low‐certainty evidence). Analysing 762 procedures, there were 15 recurrences in the fibrin glue group and 41 in the suture group with a risk ratio of 0.47. Instead, taking into account 673 procedures and looking at the complication rate there was evidence of a higher rate of complications in the fibrin glue group (Analysis 1.2) (low‐certainty evidence), while our analysis reports a significant reduction in surgical time using a fibrin glue (Analysis 1.3) (low‐certainty evidence). The main results of the meta‐analyses (effect estimate and 95% confidence intervals (CIs)) are respectively: risk ratio (RR) 0.47; 95% CI 0.27 to 0.82 for recurrence of pterygium; RR 1.92; 95% CI 1.22 to 3.02 for complication rate, and mean difference ‐17.01; 95% CI ‐20.56 to ‐13.46 for operating time.
In sensitivity analysis, the exclusion of three studies at high risk of bias (Ratnalingam 2010; Rubin 2011; Yuksel 2010) had marginal impact on effect estimates: RR 0.54; 95% CI 0.27 to 1.08 for recurrence of pterygium (10 studies, 542 eyes; Analysis 2.1); RR 2.16; 95% CI 1.32 to 3.51 for complication rate (9 studies, 489 eyes; Analysis 2.2); and mean difference ‐15.59; 95% CI ‐19.90 to ‐11.28 for operating time (6 studies, 372 eyes; Analysis 2.3). For all the comparisons, we used a random‐effects model.
Sensitivity analysis using a fixed‐effect model showed similar estimates.
There were no reports on any adverse event due to fibrin glue or suture use in the studies analysed.
Discussion
Conjunctival autograft transplantation is the most effective surgical procedure to prevent recurrence of pterygium and ensure a white cosmetic conjunctiva, with no persistent symptoms. However there is still no clear evidence to suggest whether the preferred option to fix the conjunctival autograft is fibrin glue or sutures. We conducted three meta‐analyses to answer this question in terms of recurrence of pterygium, complication rate and surgical time.
Summary of main results
Pterygium recurrence is the most common complication after its removal; its etiopathogenesis is multifactorial. Our meta‐analysis points out the benefits of using fibrin glues in terms of recurrence rate (RR 0.47; 95% CI 0.27 to 0.82). Exclusion of studies at high risk of bias led to a reduction of the effect size for the outcome recurrence of pterygium, though a trend towards a reduction of recurrences was still evident (RR 0.54; 95% CI 0.27 to 1.08). The reasons could be attributed to reduced postoperative inflammation (Kheirkhah 2008) and an immediate adherence of the graft, which plays a crucial role inhibiting fibroblast ingrowth, encouraging earlier vascularisation of the graft and reducing the recurrence (Zauberman 1988). Instead this adherence is not achieved by sutures, because they can only fix the edges of the graft, having no direct apposition of the graft in proximity to the underlying episclera. Also the different tensile strength between sutures and fibrin glue could have a determinant role.
The success rate also depends on surgical time, and its increment is directly associated with an increase in postoperative inflammation. The operating time is also correlated to the surgeon's skills. In fact Ti 2000 attributed the variability of success rate of sutured conjunctival autograft to learning curves and different skill levels. The results highlight the benefits of fibrin glue in terms of operating time. Using fibrin glue with conjunctival autograft transplantation shortens the surgical time, and makes the procedure easier with minimal manipulation of the graft and therefore less inflammation. Consequently, better results may be more consistently achieved despite differences in surgical expertise.
Our results report a higher complication rate in the fibrin glue group compared to the suture group (Table 1). It must be noted that the complication rates especially depend on graft preparation, graft manipulation, surgical experience and participant selection. In the fibrin group graft dehiscence is the most common complication (Srinivasan 2007), however it is usually associated with eye trauma or a person rubbing their eyes. A meticulous graft preparation (thin donor conjunctival autograft and free of Tenon's capsule) improves the success rate of graft uptake. Foroutan 2011 reported 13.33% cases of graft dehiscence and attributed this to a low concentration of thrombin and fibrinogen in autologous glue compared to a commercial preparation. Also the graft loss in the case of fibrin glue is a common complication or consequence of dehiscence. The precautions suggested by experts to avoid it are to ensure that the conjunctival autograft and conjunctiva are properly adhered; that fibrin glue is cleared from the ocular surface; and that no Tenon’s capsule remains between the graft and the conjunctiva. Graft retraction is the most common complication in the suture group. Tan 1999 attributed it to sub‐conjunctival fibrosis and suggested a meticulous dissection of sub‐epithelial graft tissue. Different authors (Malik 2012; de Wit 2010) postulated that no direct tension on the free edges, which occurs using fibrin glue, results in reduced stimulus for sub‐conjunctival scar formation; apposition of the eyelids to the bulbar conjunctiva provides a natural biological dressing, and confers a unique wound‐healing environment and a smooth, frictionless surface. The eyelids are able to provide compression and a vascular bed with immune capability in close proximity to the injury site. It must also be noted that intraoperative complications are not very common. However, there are some intraoperative advantages using fibrin glue such as the possibility of still using grafts with buttonholes, and the ability to be more efficient with unco‐operative patients who persistently move their eyes. In these people, the suturing process can be very difficult. We believe that complications, such as graft dehiscence, should be expected but they can be minimised: maintaining a dry scleral bed prior to applying the tissue glue and graft; educating the patient; avoiding eye rubbing postoperatively; and patient selection may reduce this risk.
| Complications | Fibrin glue | Suture |
| Conjunctival cyst | 2/372 | 0/439 |
| Dellen | 1/372 | 3/439 |
| Graft dehiscence | 7/372 | 2/439 |
| Graft loss | 3/372 | 0/439 |
| Graft overlying the limbus | 0/372 | 1/439 |
| Graft retraction | 7/372 | 6/439 |
| Granuloma | 4/372 | 11/439 |
| Subconjunctival haemorrhage | 3/372 | 0/439 |
Overall completeness and applicability of evidence
There was variability across studies related to different race of participants, the use of different kinds of sutures and fibrin glue, and different length of follow‐up and in particular some follow‐up time was short.
Certainty of the evidence
We judged the evidence for all three outcomes to be low‐certainty. We downgraded for risk of bias as we judged many studies at unclear risk of selection and detection bias and some at high risk of attrition bias. We downgraded recurrence and complications outcomes for imprecision as there were few events. We downgraded the operating time outcome for inconsistency as there was high statistical heterogeneity.
Potential biases in the review process
As far as we are aware we have minimised potential biases in the review process. We have followed the methods set out in the published protocol (Romano 2014). The only amendment was to add in a summary of findings table and GRADE assessment as required by new Cochrane standards.
Agreements and disagreements with other studies or reviews
Other retrospective studies, such as the large cohort reported by Koranyi 2005 where they studied 461 procedures with follow‐up ranging from 6 to 12 months reported a recurrence rate of 5.3% for the fibrin glue group and 13.5% in the suture group. They also found all the recurrences occurred within six months after surgery. Marticorena 2006 and Uy 2002 suggested that the use of fibrin glue reduced postoperative discomfort. A previous meta‐analysis of pterygium recurrence after surgery concluded that simple bare sclera resection alone is associated with six times higher odds of pterygium recurrence if a conjunctival autograft was not used and 25 times higher odds of recurrence if mitomycin C was not used. The authors recommended avoiding simple bare sclera excision (Sanchez‐Thorin 1998). However, the use of MMC can be associated with sight‐threatening complications such as corneoscleral melt, cataract, uveitis, secondary glaucoma, and symblepharon (Amano 2000; Hayasaka 1988; Rubinfeld 1992).
There are some drawbacks to using the fibrin glue technique, mainly the cost. Most surgeons recommend dividing one vial of fibrin glue between six to 10 patients, reducing the costs, but that means that all the patients must be operated on the same day. However, as the use of fibrin glue reduces the risk of recurrence, the cost of a second surgery will probably be avoided. In addition, reducing the surgical time provides, according to previous published data from the USA, a saving of USD 67.50/min (Montgomery 2007) in terms of surgical operating room expense. Therefore, just a reduction in surgical time would make it cost‐effective compared with sutures and without the need to split the use of the glue over a number of cases. Another theoretical drawback is the risk of transmission of infectious agents, such as parvovirus B19 (Morita 2000) and prions (Hino 2000), as fibrin glue is made from blood products. Other concerns include the risk of transmission of blood‐borne diseases and anaphylaxis. However, careful selection of donors and improved viral inactivation techniques has largely minimised this problem. Autologous fibrin could be the solution, however it is yet to be used widely because of the time taken to procure the fibrin and lack of laboratory facilities at all centres.

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Funnel plot of comparison: 1 Fibrin glue versus suture, outcome: 1.1 Recurrence of pterygium.

Funnel plot of comparison: 1 Fibrin glue versus suture, outcome: 1.2 Occurrence of 1 or more complications.

Comparison 1 Fibrin glue versus suture, Outcome 1 Recurrence of pterygium.

Comparison 1 Fibrin glue versus suture, Outcome 2 Occurrence of 1 or more complications.
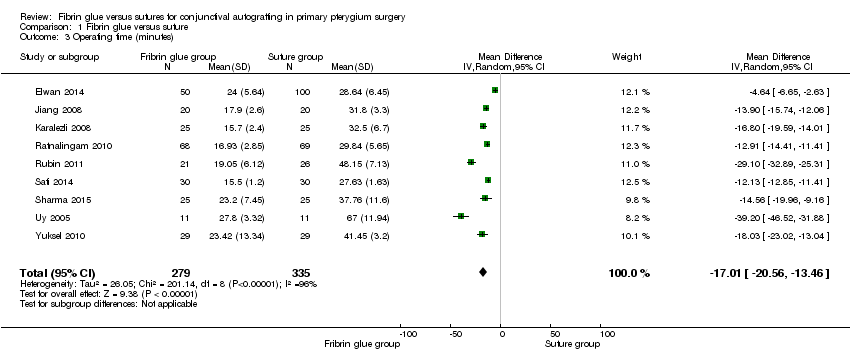
Comparison 1 Fibrin glue versus suture, Outcome 3 Operating time (minutes).

Comparison 2 Fibrin glue versus suture: excluding trials at high risk of bias, Outcome 1 Recurrence of pterygium.

Comparison 2 Fibrin glue versus suture: excluding trials at high risk of bias, Outcome 2 Occurrence of 1 or more complications.

Comparison 2 Fibrin glue versus suture: excluding trials at high risk of bias, Outcome 3 Operating time (minutes).
| Fibrin glue compared with sutures for primary pterygium | ||||||
| Patient or population: individuals with pterygium Settings: hospital or outpatients Intervention: fibrin glue Comparison: sutures | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of eyes | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sutures | Fibrin glue | |||||
| Recurrence of pterygium (follow‐up 2 to 24 months) | 100 per 1000 | 47 per 1000 | RR 0.47 (0.27 to 0.82) | 762 (12 RCTs) | ⊕⊕⊝⊝ | Large variability in duration of follow‐up |
| Occurrence of complication | 70 per 1000 | 134 per 1000 | RR 1.92 (1.22 to 3.02) | 673 (11 RCTs) | ⊕⊕⊝⊝ | |
| Operating time | The mean operating time ranged across control groups from | The mean operating time in the intervention groups was17.0 minutes less (from 20.6 minutes less to 13.5 minutes less) | 614 (9 RCTs) | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded 1 level for risk of bias due to study limitations, in particular generation of allocation sequence, allocation concealment, and incomplete outcome data. | ||||||
| Complications | Fibrin glue | Suture |
| Conjunctival cyst | 2/372 | 0/439 |
| Dellen | 1/372 | 3/439 |
| Graft dehiscence | 7/372 | 2/439 |
| Graft loss | 3/372 | 0/439 |
| Graft overlying the limbus | 0/372 | 1/439 |
| Graft retraction | 7/372 | 6/439 |
| Granuloma | 4/372 | 11/439 |
| Subconjunctival haemorrhage | 3/372 | 0/439 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of pterygium Show forest plot | 12 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.27, 0.82] |
| 2 Occurrence of 1 or more complications Show forest plot | 11 | 673 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [1.22, 3.02] |
| 3 Operating time (minutes) Show forest plot | 9 | 614 | Mean Difference (IV, Random, 95% CI) | ‐17.01 [‐20.56, ‐13.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of pterygium Show forest plot | 10 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.27, 1.08] |
| 2 Occurrence of 1 or more complications Show forest plot | 9 | 489 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.32, 3.51] |
| 3 Operating time (minutes) Show forest plot | 6 | 372 | Mean Difference (IV, Random, 95% CI) | ‐15.59 [‐19.90, ‐11.28] |

