干预措施治疗获得性脑损伤所致的眼球运动障碍
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Brain Injuries] explode all trees
#2 brain near/2 injur*
#3 ABI or TBI or non TB
#4 #1 or #2 or #3
#5 MeSH descriptor: [Eye] explode all trees
#6 MeSH descriptor: [Visually Impaired Persons] explode all trees
#7 MeSH descriptor: [Ocular Physiological Processes] explode all trees
#8 MeSH descriptor: [Diagnostic Techniques, Ophthalmological] explode all trees
#9 MeSH descriptor: [Optometry] explode all trees
#10 MeSH descriptor: [Orthoptics] explode all trees
#11 MeSH descriptor: [Eye Diseases] this term only
#12 MeSH descriptor: [Vision Disorders] this term only
#13 MeSH descriptor: [Eye Manifestations] this term only
#14 MeSH descriptor: [Blindness] this term only
#15 MeSH descriptor: [Diplopia] explode all trees
#16 MeSH descriptor: [Vision, Binocular] this term only
#17 MeSH descriptor: [Vision, Monocular] this term only
#18 MeSH descriptor: [Visual Acuity] explode all trees
#19 MeSH descriptor: [Visual Fields] this term only
#20 MeSH descriptor: [Vision, Low] this term only
#21 MeSH descriptor: [Visual Field Tests] explode all trees
#22 MeSH descriptor: [Ophthalmology] this term only
#23 MeSH descriptor: [Vision Screening] this term only
#24 MeSH descriptor: [Eye Diseases, Hereditary] explode all trees
#25 MeSH descriptor: [Ocular Motility Disorders] explode all trees
#26 MeSH descriptor: [Optic Nerve Diseases] explode all trees
#27 MeSH descriptor: [Orbital Diseases] explode all trees
#28 MeSH descriptor: [Pupil Disorders] explode all trees
#29 MeSH descriptor: [Refractive Errors] explode all trees
#30 MeSH descriptor: [Blindness, Cortical] explode all trees
#31 MeSH descriptor: [Hemianopsia] explode all trees
#32 MeSH descriptor: [Scotoma] this term only
#33 MeSH descriptor: [Abducens Nerve] this term only
#34 MeSH descriptor: [Oculomotor Nerve] this term only
#35 MeSH descriptor: [Trochlear Nerve] this term only
#36 "smooth pursuit" or saccades or saccadic or "depth perception" or stereopsis or gaze disorder* or ophthalm* or optic nerve*
#37 (downbeat or upbeat or vertical) near/3 nystagmus
#38 gaze* near/2 (deficit* or pals* or disorder*)
#39 ocular near/2 (muscle* or align*)
#40 esotropi* or exotropi* or hypertropi* or hypotropi* or cyclotropi*
#41 intranuclear ophthalmoplegia or parinaud's syndrome or weber's syndrome or skew deviation or conjugate deviation
#42 (visual* or vision or eye or eyes or eyesight or sight) near/3 (problem* or disorder* or impair* or disabilit* or loss or disease* or defect* or manifestation* or screening or test* or examination*)
#43 reading near/2 (difficult* or impair*)
#44 hemianop* or blindness or low vision or refractive errors or scotoma or diplopia or optometr* or ocular or orthoptic*
#45 oscillopsia or visual tracking or fresnel prism*
#46 III or IV or VI or third or fourth or sixth near/3 nerve palsy
#47 (#5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #41 or #42 or #44 or #45 or #46)
#48 #4 and #47
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt.
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. exp animals/
10. exp humans/
11. 9 not (9 and 10)
12. 8 not 11
13. exp brain injuries/
14. (brain adj2 injur$).tw.
15. (ABI or TBI or non TBI).tw.
16. or/13‐15
17. exp Eye/
18. exp Visually Impaired Persons/
19. exp Ocular Physiological Processes/
20. exp Diagnostic Techniques, Ophthalmological/
21. exp Optometry/
22. exp Orthoptics/
23. exp Eye Diseases/
24. exp Vision Disorders/
25. exp Eye Manifestations/
26. exp Blindness/
27. exp Diplopia/
28. Vision, Binocular/
29. Vision, Monocular/
30. exp Visual Acuity/
31. Visual Fields/
32. Vision, Low/
33. exp Visual Field Tests/
34. Ophthalmology/
35. Vision Screening/
36. Eye Diseases, Hereditary/
37. exp Ocular Motility Disorders/
38. exp Optic Nerve Diseases/
39. Enophthalmos/
40. exp Pupil Disorders/
41. exp Refractive Errors/
42. Blindness, Cortical/
43. exp Hemianopsia/
44. Scotoma/
45. Abducens Nerve/
46. Oculomotor Nerve/
47. Trochlear Nerve/
48. (smooth pursuit or saccades or saccadic or depth perception or stereopsis or gaze disorder$ or ophthalm$ or optic nerve$).tw.
49. (ocular adj2 (muscle$ or align$)).tw.
50. (esotropi$ or exotropi$ or hypertropi$ or hypotropi$ or cyclotropi$).tw.
51. (intranuclear ophthalmoplegia or parinaud's syndrome or weber's syndrome or skew deviation or conjugate deviation).tw.
52. ((visual$ or vision or eye or eyes or eyesight or sight) adj3 (problem$ or disorder$ or impair$ or disabilit$ or loss or disease$ or defect$ or manifestation$ or screening or test$ or examination$)).tw.
53. (reading adj2 (difficult$ or impair$)).tw.
54. (hemianop$ or blindness or low vision or refractive errors or scotoma or diplopia or optometr$ or ocular or orthoptic$).tw.
55. (oscillopsia or visual tracking or fresnel prism$).tw.
56. or/17‐55
57. 12 and 16 and 56
58. ((downbeat or upbeat or vertical) adj3 nystagmus).tw.
59. (gaze$ adj2 (deficit$ or pals$ or disorder$)).tw.
60. ((III or IV or VI or third or fourth or sixth) adj3 nerve pals$).tw.
61. or/58‐60
62. 12 and 61
63. 57 or 62
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp randomized controlled trial/
2. exp randomization/
3. exp double blind procedure/
4. exp single blind procedure/
5. random$.tw.
6. or/1‐5
7. (animal or animal experiment).sh.
8. human.sh.
9. 7 and 8
10. 7 not 9
11. 6 not 10
12. exp clinical trial/
13. (clin$ adj3 trial$).tw.
14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
15. exp placebo/
16. placebo$.tw.
17. random$.tw.
18. exp experimental design/
19. exp crossover procedure/
20. exp control group/
21. exp latin square design/
22. or/12‐21
23. 22 not 10
24. 23 not 11
25. exp comparative study/
26. exp evaluation/
27. exp prospective study/
28. (control$ or prospectiv$ or volunteer$).tw.
29. or/25‐28
30. 29 not 10
31. 30 not (11 or 23)
32. 11 or 24 or 31
33. exp brain injury/
34. (brain adj2 injur$).tw.
35. (ABI or TBI or non TBI).tw.
36. or/33‐35
37. eye/
38. visual system function/
39. visual system examination/
40. optometry/
41. orthoptics/
42. eye disease/
43. visual disorder/
44. exp eye movement disorder/
45. exp visual impairment/
46. exp vision/
47. perimetry/
48. ophthalmology/
49. exp optic nerve disease/
50. exp cranial nerve/
51. (smooth pursuit or saccades or saccadic or depth perception or stereopsis or gaze disorder$ or ophthalm$ or optic nerve$).tw.
52. (ocular adj2 (muscle$ or align$)).tw.
53. (esotropi$ or exotropi$ or hypertropi$ or hypotropi$ or cyclotropi$).tw.
54. (intranuclear ophthalmoplegia or parinaud's syndrome or weber's syndrome or skew deviation or conjugate deviation).tw.
55. ((visual$ or vision or eye or eyes or eyesight or sight) adj3 (problem$ or disorder$ or impair$ or disabilit$ or loss or disease$ or defect$ or manifestation$ or screening or test$ or examination$)).tw.
56. (reading adj2 (difficult$ or impair$)).tw.
57. (hemianop$ or blindness or low vision or refractive errors or scotoma or diplopia or optometr$ or ocular or orthoptic$).tw.
58. (oscillopsia or visual tracking or fresnel prism$).tw.
59. or/37‐58
60. 32 and 36 and 59
61. ((downbeat or upbeat or vertical) adj3 nystagmus).tw.
62. (gaze$ adj2 (deficit$ or pals$ or disorder$)).tw.
63. ((III or IV or VI or third or fourth or sixth) adj3 nerve pals$).tw.
64. or/61‐63
65. 32 and 64
66. 60 or 65
Appendix 4. CINAHL EBSCO search strategy
S56 S12 AND S55
S55 S16 AND S54
S54 S51 or S52 or S53
S53 S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51
S52 S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40
S51 S17 or S18 or S19 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30
S50 nerve palsy
S49 oscillopsia or visual tracking or fresnel prism*
S48 hemianop* or blindness or low vision or refractive errors or scotoma or diplopia or optometr* or ocular or orthoptic*
S47 reading N2 (difficult* or impair*)
S46 (visual* or vision or eye or eyes or eyesight or sight) N5 (problem* or disorder* or impair* or disabilit* or loss or disease* or defect* or manifestation* or screening or test* or examination*)
S45 intranuclear ophthalmoplegia or parinaud's syndrome or weber's syndrome or skew deviation or conjugate deviation
S44 esotropi* or exotropi* or hypertropi* or hypotropi* or cyclotropi*
S43 ocular N2 (muscle* or align*)
S42 gaze* N2 (deficit* or palsy or disorder*)
S41 nystagmus or smooth pursuit or saccades or depth perception or stereopsis or ophthalmol* or optic nerve
S40 (MH "Trochlear Nerve") OR (MH "Trochlear Nerve Diseases")
S39 (MH "Abducens Nerve Diseases+") OR (MM "Abducens Nerve")
S38 (MM "Refractive Errors+")
S37 (MH "Pupil Disorders+")
S36 (MH "Orbital Diseases+")
S35 (MH "Optic Nerve Diseases+")
S34 (MM "Ocular Motility Disorders")
S33 (MM "Eye Diseases, Hereditary")
S32 (MM "Ophthalmology")
S31 (MH "Vision Tests+")
S30 (MH "Visual Perception+")
S29 (MM "Visual Fields")
S28 (MH "Visual Acuity")
S27 (MH "Depth Perception")
S26 (MH "Vision, Subnormal")
S25 (MM "Diplopia")
S24 (MH "Blindness+")
S23 (MH "Eye Manifestations+")
S22 (MH "Vision Disorders+")
S21 (MH "Eye Diseases+")
S20 (MM "Optometry")
S19 (MH "Diagnosis, Eye+")
S18 (MH "Rehabilitation of Vision Impaired+")
S17 (MH "Eye+")
S16 S13 or S14 or S15
S15 ABI or TBI or non TBI
S14 brain N2 injur*
S13 (MH "Brain Injuries+")
S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11
S11 TX allocat* random*
S10 (MM "Quantitative Studies")
S9 (MM "Placebos")
S8 TX placebo*
S7 TX random* allocat*
S6 (MM "Random Assignment")
S5 TX randomi* control* trial*
S4 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) )
S3 TX clinic* n1 trial*
S2 PT Clinical trial
S1 (MH "Clinical Trials+")
Appendix 5. AMED Ovid search strategy
1. "Randomized controlled trials"/
2. prospective studies/
3. single blind method/
4. random$.tw.
5. placebo$.tw.
6. trial$.tw.
7. groups.tw.
8. ((singl$ or doubl$) adj3 (blind$ or mask$)).tw.
9. or/1‐8
10. brain injuries/
11. (brain adj2 injur$).tw.
12. (ABI or TBI or non TBI).tw.
13. or/10‐12
14. eye/
15. Blindness/
16. Vision disorders/
17. Vision/
18. Ocular Motility Disorders/
19. Eye Movements/
20. Optic nerve/
21. Refractive Errors/
22. Cranial nerves/
23. (smooth pursuit or saccades or saccadic or depth perception or stereopsis or gaze disorder$ or ophthalm$ or optic nerve$).tw.
24. (ocular adj2 (muscle$ or align$)).tw.
25. (esotropi$ or exotropi$ or hypertropi$ or hypotropi$ or cyclotropi$).tw.
26. (intranuclear ophthalmoplegia or parinaud's syndrome or weber's syndrome or skew deviation or conjugate deviation).tw.
27. ((visual$ or vision or eye or eyes or eyesight or sight) adj3 (problem$ or disorder$ or impair$ or disabilit$ or loss or disease$ or defect$ or manifestation$ or screening or test$ or examination$)).tw.
28. (reading adj2 (difficult$ or impair$)).tw.
29. (hemianop$ or blindness or low vision or refractive errors or scotoma or diplopia or optometr$ or ocular or orthoptic$).tw.
30. (oscillopsia or visual tracking or fresnel prism$).tw.
31. or/14‐30
32. 9 and 13 and 31
33. ((downbeat or upbeat or vertical) adj3 nystagmus).tw.
34. (gaze$ adj2 (deficit$ or pals$ or disorder$)).tw.
35. ((III or IV or VI or third or fourth or sixth) adj3 nerve palsy).tw.
36. or/33‐35
37. 9 and 36
38 32 or 37
Appendix 6. PsychINFO Ovid search strategy
1. exp Treatment Effectiveness Evaluation/
2. exp Clinical Trials/
3. exp Placebo/
4. placebo$.tw.
5. randomly.tw.
6. randomi#ed.tw.
7. trial$.tw.
8. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).tw.
9. (factorial$ or allocat$ or assign$ or volunteer$).tw.
10. (crossover$ or cross over$).tw.
11. (quasi adj (experimental or random$)).tw.
12. (control$ adj3 (trial$ or study or studies or group$)).tw.
13. or/1‐12
14. exp Brain Damage/
15. exp Traumatic Brain Injury/
16. (ABI or TBI or non TB).tw.
17. exp Head Injuries/
18. or/14‐17
19. exp Eye Disorders/
20. exp Eye Fixation/
21. exp "Pupil (Eye)"/
22. exp Vision Disorders/
23. exp Vision/
24. exp Blind/
25. exp Optometry/
26. exp Strabismus/
27. exp Amblyopia/
28. exp Binocular Vision/
29. exp Eye Convergence/
30. exp Color Perception/
31. exp Visual Acuity/
32. exp Visual Field/
33. exp Ophthalmology/
34. exp Ophthalmologic Examination/
35. exp Hemianopia/
36. exp Abducens Nerve/
37. exp Cranial Nerves/
38. (smooth pursuit or saccades or saccadic or depth perception or stereopsis or gaze disorder$ or ophthalm$ or optic nerve$).tw.
39. (ocular adj2 (muscle$ or align$)).tw.
40. (esotropi$ or exotropi$ or hypertropi$ or hypotropi$ or cyclotropi$).tw.
41. (intranuclear ophthalmoplegia or parinaud's syndrome or weber's syndrome or skew deviation or conjugate deviation).tw.
42. ((visual$ or vision or eye or eyes or eyesight or sight) adj3 (problem$ or disorder$ or impair$ or disabilit$ or loss or disease$ or defect$ or manifestation$ or screening or test$ or examination$)).tw.
43. (reading adj2 (difficult$ or impair$)).tw.
44. (hemianop$ or blindness or low vision or refractive errors or scotoma or diplopia or optometr$ or ocular or orthoptic$).tw.
45. (oscillopsia or visual tracking or fresnel prism$).tw.
46. or/19‐45
47. 13 and 18 and 46
48. ((downbeat or upbeat or vertical) adj3 nystagmus).tw.
49. (gaze$ adj2 (deficit$ or pals$ or disorder$)).tw.
50. ((III or IV or VI or third or fourth or sixth) adj3 nerve pals$).tw.
51. or/48‐50
52. 13 and 51
53. 47 or 52
Appendix 7. Dissertations and Theses (PQDT) database
Keywords (dissertation topic) = brain injury
Appendix 8. PsycBITE database
PsycBITE was searched using the following options from the search interface:
Neurological Group = Traumatic Brain Injury (TBI) /Head Injury
Method = Randomised Controlled Trials
Target area = Literacy/Numeracy OR Visual Field Loss OR Community Re‐entry
Appendix 9. ISRCTN search strategy
brain Injury
nystagmus
gaze deficit
nerve palsy
Appendix 10. ClinicalTrials.gov search strategy
brain Injury OR nystagmus OR gaze deficit OR nerve palsy
Appendix 11. Health Services Research Projects in Progress
brain Injury
nystagmus
gaze deficit
nerve palsy
Appendix 12. National Eye Institute Clinical Studies Database
brain Injury
nystagmus
gaze deficit
nerve palsy
Appendix 13. WHO ICTRP search strategy
brain Injury
nystagmus
gaze deficit
nerve palsy
Appendix 14. Standardised headings for included studies table
| Headings in table in RevMan | Proposed subheadings | |
| Methods | Study design |
|
| Eyes |
| |
| Participants | Country | |
| Setting | ||
| Number of participants | ||
| Number of men | ||
| Number of women | ||
| Average age | ||
| Age range | ||
| Ethnic group | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Eye movement disorders | Description of the type of eye movement disorder (III, IV, and VI cranial nerve palsy, reduced fixation, gaze holding, gaze palsy, saccadic problems, smooth pursuit problems, strabismus, nystagmus, reduced convergence or divergence, conjugate deviation, skew deviation), the deviation of eye movement (horizontal, vertical, torsional), and the severity of eye movement disorder (slight, small, moderate, marked; paralysis, paresis; monocular, binocular). | |
| Acquired brain injury | Description of type of brain injury, natural history, side of brain injury. | |
| Interventions | Intervention Comparator | We provide a description of interventions given to each treatment group including, if relevant, the duration, intensity, frequency, or dose. We classify the type of intervention as restitution, compensation, pharmacological or substitution, type of brain injury, type of eye movement disorder, and the type of control as no treatment, placebo, control, or standard care. We document the professional background of the person providing the intervention (e.g. ophthalmologist, orthoptist). |
| Outcomes | List | We document the primary and secondary outcomes relevant to this review as listed in the Types of outcome measures section. Specifically, we document measurements showing change in angle of deviation and/or extent of eye movement range, measurement of binocular single vision, documentation of participant‐reported symptoms, documentation of questionnaires and adverse events. If a study has used a number of different methods of measuring the same outcome (e.g. prisms and degrees for measurement of ocular deviation), we note each method to be used for any subsequent analysis. |
| Notes | Date conducted | Indicating specific dates of recruitment of participants mm/yr to mm/yr |
| Sources of funding | ||
| Declaration of interest | Indicating any declarations of interest among the primary researchers | |
| Other | We record any important confounding variables. If a study includes more than two intervention groups, we also record the method of including these groups in any subsequent analysis. |

Study flow diagram
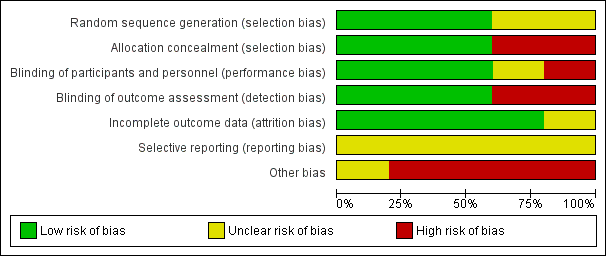
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
| Botulinum toxin versus observation in people with sixth nerve palsy | ||||||
| Participant or population: people with sixth nerve palsy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with observation | Risk with botulinum toxin | |||||
| Improvement in ocular motility (ocular alignment ≤ 10 prism dioptres). Follow‐up to 4 months | 800 per 1,000 | 952 per 1,000 | RR 1.19 | 47 | ⊕⊕⊝⊝ | |
| Achievement of binocular single vision (fusion and stereopsis present). Follow‐up to 4 months | 800 per 1,000 | 952 per 1,000 | RR 1.19 | 47 | ⊕⊕⊝⊝ | |
| Improvement in functional ability | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Adverse events. Follow‐up to 4 months | In the injection group only, there were 2/22 (9%) cases of transient ptosis and 4/22 (18%) with transient vertical deviation, with a total complication rate of 24% per injection and 27% per participant. All adverse events recovered within the follow‐up time period of 6 months with no lasting adverse effects. | 47 (1 RCT) | ⊕⊕⊝⊝ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias (investigators were aware of the randomisation and it was not possible to mask investigators or participants to the allocation and there was variable follow‐up between groups) and downgraded one level for imprecision (confidence intervals include 1, no effect). | ||||||
| Pharmacological treatments (Gabapentin / Baclofen / 3,4‐DAP / 4‐AP) for people with acquired nystagmus | |||
| Participant or population: people with acquired nystagmus | |||
| Comparison | Main findings | № of participants | Certainty of the evidence |
| Gabapentin up to 900 mg/day) versus baclofen (up to 30 mg/day). Follow‐up 2 weeks | Gabapentin may work better than baclofen in improving ocular motility and reducing participant‐reported symptoms (oscillopsia). These effects may be different in pendular and jerk nystagmus but there was no formal subgroup analysis so it is unclear if the difference between the two types of nystagmus was a chance finding. Quality of life was not reported but ten participants with pendular nystagmus chose to continue treatment with gabapentin and one with baclofen. Two participants with jerk nystagmus chose to continue treatment with gabapentin and one with baclofen. Drug intolerance was reported in one person for gabapentin and four participants for baclofen. Increased ataxia was reported in three participants for gabapentin and two participants for baclofen. | 21 | ⊕⊝⊝⊝ |
| 3,4‐DAP (20 mg, single dose) versus placebo. Assessments made 30 minutes after taking the drug or placebo | 3,4‐DAP may reduce the mean peak slow‐phase velocity in people with downbeat nystagmus. In 10 of the 17 participants, mean peak slow‐phase velocity decreased by more than 50% and these 10 people reported having less oscillopsia. No significant adverse events were reported. Nine participants continued treatment. Three participants reported transient side effects of minor perioral/distal paraesthesia. | 17 | ⊕⊝⊝⊝ |
| 4‐AP (10 mg, single dose) versus 3,4‐DAP (10 mg, single dose) Assessments made at 45 and 90 minutes after taking the drug | 3,4 DAP and 4‐AP may reduce mean slow‐phase velocity in people with downbeat nystagmus. This effect may be stronger with 4‐AP. All participants reported mild paraesthesias with both medications. | 8 | ⊕⊝⊝⊝ |
| GRADE Working Group grades of evidence | |||
| 1 Downgraded two levels for imprecision (due to small number of participants) and one level for serious risk of bias (cross‐over study with analysis that did not permit estimation of effect size). | |||
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved binocular single vision | Secondary: improved symptoms | Secondary: adverse events |
| Lee 1994 | 47, parallel arm RCT 22 ‐ botulinum toxin 25 ‐ observation 6 month follow‐up | 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation | Success: 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation Partial: 3 (12%) ‐ observation Fail: 1 (4.5%) ‐ botulinum toxin 2 (8%) ‐ observation | 21 (95.5%) ‐ botulinum toxin 20 (80%) ‐ observation | 9% ptosis 18% vertical deviation |
| RCT: randomised controlled trial | |||||
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved functional vision | Secondary: improved symptoms | Secondary: adverse events |
| Thiagarajan 2014 | 12, cross‐over RCT 13‐week follow‐up | Baseline 2.1 saccadic ratio reducing to 1.7, P < 0.05 — OM rehabilitation Control group change not reported | Reading rate: Baseline 142 (10) wpm improving to 177 (14). Reading level: Baseline 4.1 (0.7) grade level improving to 6.3 (1.2), P < 0.01 Fixations per 100 words: Baseline 164 (10) improving to 135 (11), P = 0.02 Regressions per 100 words: Baseline 30 (3) improving to 23 (4) Control group changes not reported [means (SEM)] | Improved for OM rehabilitation. Control group changes not reported | Nil reported |
| SEM: standard error mean | |||||
| Study ID | Total participants | Primary: improved ocular motility | Secondary: improved visual acuity | Secondary: improved symptoms | Secondary: adverse events |
| Averbuch‐Heller 1997 | 21, crossover RCT 15 ‐ pendular 6 ‐ jerk 6‐week trial duration | 15 pendular ‐ gabapentin | 15 pendular ‐ gabapentin 1 jerk ‐ gabapentin 1 jerk ‐ baclofen | 6 pendular ‐ gabapentin 1 jerk ‐ gabapentin 1 jerk ‐ baclofen | 1 drug intolerance ‐ gabapentin 4 drug intolerance ‐ baclofen 3 ataxia ‐ gabapentin 2 ataxia ‐ baclofen |
| Kalla 2011 | 8, crossover RCT 8 ‐ downbeat 8‐day trial duration | Baseline ‐6.04; 45 mins ‐1.58; 90 mins ‐1.21 (4‐aminopyridine) Baseline ‐5.68; 45 mins ‐3.29; 90 mins ‐2.96 (3,4‐diaminopyridine) | ‐ | ‐ | All with mild paraesthesia |
| Strupp 2003 | 17, crossover RCT 17 ‐ downbeat 16‐day trial duration | Baseline 7.2 ± 4.2 °/sec reducing to 3.1 ± 2.5 (3,4‐diaminopyridine) Baseline 7.4 ± 4.1 °/sec reducing to 7.3 ± 3.7 (placebo) | ‐ | 10 ‐ reduced symptoms (3,4‐diaminopyridine) 0 ‐ reduced symptoms (placebo) | 3 ‐ mild paraesthesia (3,4‐diaminopyridine) 1 ‐ nausea/headache (3,4‐diaminopyridine) |
| RCT: randomised controlled trial | |||||

