Neuromuskuläre elektrische Stimulation (NMES) zur Behandlung des femoropatellaren Schmerzsyndroms
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial Trial protocol registration: not reported | |
| Participants | Country: Turkey Setting: Hacettepe University, School of Physiotherapy and Rehabilitation, and Department of Orthopaedics and Traumatology, Ankara Data collection period: not reported Inclusion criteria: anterior knee pain (longer than 2 months), positive patellar compression test, age between 15 and 45 years, negative findings in the clinical examination of knee ligaments, bursae, menisci, synovial plicae, hamstring, quadriceps, and patellar tendons Exclusion criteria: history or clinical evidence of patellofemoral dislocation, subluxation, or severe osteoarthritis, X‐rays showing lateral displacement of the patella Mean duration of symptoms: 15.74 ± 9.31 months 62.5% of bilateral complaints, but only the most symptomatic knee was treated Study participants: 44 people with patellofemoral pain assigned and 42 assessed
Mean age (SD): 39.0 (9.6) years Gender (number of women/men): 31/13 | |
| Interventions | Comparison: NMES + other intervention (exercise) versus no NMES + same other intervention Treatment duration: 6 weeks Details of interventions:
| |
| Outcomes | Outcomes analysed in the study and used in this review:
Follow‐up assessments: at 6 weeks (at end of treatment) | |
| Notes | Description of condition: patellofemoral pain syndrome The trial authors provided additional information on random sequence generation, allocation concealment, and blinding (participants, personnel, and assessors) via email (10 March 2015). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The subjects were randomly introduced into either HVPGS and exercise, or only exercise (control group)" Authors' reply: "At the time of study, it was feasible for us to use coin tossing technique to assign patients to either HVPGS and exercise group, or only exercise (control group) in consecutively." |
| Allocation concealment (selection bias) | Unclear risk | Available information did not permit judgement. Authors' reply: "Assigning patients to intervention groups was done by the first author with coin tossing technique to prevent second and third author/researcher from influencing concealing the allocation sequence in the study." |
| Blinding of participants and personnel (performance bias) | High risk | Available information did not permit judgement. Authors' reply: "Patients were also blinded to their intervention groups. Exercise programs were applied by physiotherapist (third researcher/author)" However, since no sham/placebo was used, it is unlikely that the blinding was kept. |
| Blinding of outcome assessment (detection bias) | Low risk | Available information did not permit judgement. Authors reply: "Pre and post treatment muscle strength and VAS measurements were evaluated by the same physiotherapist (second researcher) who is blind from the patient’s group" |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: “Two patients of the HVPGS group did not complete the study and their data results were excluded” (4.5% dropout) No reasons were provided. We are uncertain of the potential effect of these missing data. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. It is not clear if the results included all expected outcomes (e.g. retropatellar pain during activities: steps up and down and squatting). This study did not consider adverse event as an outcome. |
| Other bias | Low risk | The study appears to be free of other sources of bias. Baseline characteristics were balanced between groups. |
| Methods | Randomised controlled trial Trial protocol registration: not reported | |
| Participants | Country: Austria Setting: Department of Physical Medicine and Rehabilitation, Wilhelminenspital Vienna and Core Unit for Medical Statistics and Informatics, Section of Clinical Biometrics, Medical University of Vienna Data collection period: between June 2003 and August 2005 Inclusion criteria: bilateral anterior knee pain for 6 to 120 months and at least 3 of the following 4 clinical criteria: pain associated with prolonged sitting with bended knees, descending stairs, kneeling and squatting, or sports activities Exclusion criteria: clinical evidence of patellar dislocation or subluxation, periarticular bursitis or tendonitis, ligamentous instability, or intra‐articular pathology. Before beginning therapy, all participants were thoroughly clinically examined. Those who did not reveal any obvious reason for a systemic disorder like patellar or lower‐extremity alignment problems or benign joint hypermobility syndrome were not excluded. To rule out osteoarthritic changes or hypoplastic femoral trochlea, radiographs were performed. Pregnancy, a history of knee surgery, or oral or intra‐articular administration of drugs within the last 3 months Mean duration of symptoms: 14 months (6 to 24) Only bilateral complaints (but did not clarify which knee was treated and assessed) Study participants: 38 people with patellofemoral pain assigned and 29 assessed
Mean age (SD): 25.4 (6.7) years Gender (number of women/men): 24/14 | |
| Interventions | Comparison: NMES + other intervention (exercise) versus no NMES + same other intervention Treatment duration: 12 weeks Details of interventions:
| |
| Outcomes | Outcomes analysed in the study and used in this review:
Follow‐up assessments: at 12 weeks (at end of treatment) and 1 year | |
| Notes | Description of condition: patellofemoral pain syndrome The trial authors provided additional information on random sequence generation, allocation concealment and blinding (participants, personnel, and assessors) via email (16 March 2015). Additionally, the trial authors provided information regarding which knee was treated and assessed (9 August 2016). Authors' reply: "EMS was applied on both knees" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors' reply: "Random allocation of the patients to the 2 treatment groups was performed by using shuffled sealed envelopes” |
| Allocation concealment (selection bias) | Unclear risk | Authors' reply: "With sealed envelopes" The authors did not mention if the envelopes were opaque. |
| Blinding of participants and personnel (performance bias) | High risk | Participants: probably not done because the interventions were different between groups. Authors' reply: No measures were used to ensure blinding. |
| Blinding of outcome assessment (detection bias) | High risk | Available information did not permit judgement. Authors' reply: No measures were used to ensure blinding. |
| Incomplete outcome data (attrition bias) | High risk | Some participants did not complete the study (5.2% dropout at the end of the treatment and 19% dropout in the long term), and it is unclear how the authors dealt with these missing data. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. It is not clear if the results included all expected outcomes. This study did not consider adverse event as an outcome. |
| Other bias | Unclear risk | The study appears to be free of other sources of bias. However, although baseline characteristics were balanced between groups, muscle strength before training was greater in the NMES group. |
| Methods | Randomised controlled trial Trial protocol registration: not reported | |
| Participants | Country: United Kingdom Setting: Centre for Rehabilitation Science, Manchester Royal Infirmary, Manchester Data collection period: not reported Inclusion criteria: atraumatic peripatellar pain (greater than 6 months and not longer than 3 years); patellofemoral pain was provoked by 1 of the following alone or in combination: prolonged sitting, deep squatting, kneeling, ascending or descending stairs; quadriceps cross‐sectional area differences between affected and unaffected limb greater than 4% Exclusion criteria: epilepsy, cancer, cardiac pacemaker, suspected heart problem, recent surgery (not including arthroscopy). In order to exclude abnormal foot and ankle pronation as the cause of patellofemoral pain, the participants were screened by kinetic gait analysis to detect abnormal values of mediolateral force. Pain from the lumbar spine and hip joint, severe leg length discrepancy, knee ligament, quadriceps tendon, and meniscal pathologies, Hoffa’s syndrome, medial plica syndrome, femoral anteversion and tibial torsion Mean duration of symptoms: not reported All unilateral complaints Study participants: 16 people with patellofemoral pain assigned and 14 assessed
Mean age (SD): 29.6 (5.9) years Gender (number of women/men): 12/2 | |
| Interventions | Comparison: NMES (simultaneous mixed frequencies) versus control NMES (sequential mixed frequencies) Treatment duration: 6 weeks Details of interventions:
| |
| Outcomes | Outcomes analysed in the study and used in this review:
Follow‐up assessments: 6 weeks (at end of treatment) | |
| Notes | Description of condition: patellofemoral pain syndrome The trial authors provided additional information on NMES parameters via email (9 March 2015). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated by computer program to either the experimental stimulation or standard stimulation treatment regimes" |
| Allocation concealment (selection bias) | Unclear risk | Available information did not permit judgement. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants: Available information did not permit judgement. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Available information did not permit judgement. |
| Incomplete outcome data (attrition bias) | High risk | 2 participants (12% dropout) did not complete the study and were excluded from the analysis. |
| Selective reporting (reporting bias) | Low risk | No study protocol available. The authors considered only 1 adverse event (muscle fatigue); however, this is 1 of the most important adverse events in clinical practice. |
| Other bias | Unclear risk | Unable to judge because it is unclear if the groups were similar regarding relevant characteristics at baseline (age, gender, level of pain) |
| Methods | Randomised controlled trial Trial protocol registration: not reported | |
| Participants | Country: United Kingdom Setting: Centre for Rehabilitation Science, Manchester Royal Infirmary, Manchester Data collection period: not reported Inclusion criteria: atraumatic peripatellar pain (greater than 6 months and not longer than 3 years), patellofemoral pain was provoked by 1 of the following alone or in combination: prolonged sitting, deep squatting, kneeling, ascending or descending stairs Exclusion criteria: epilepsy, cancer, cardiac pacemaker, suspected heart problem, recent surgery (not including arthroscopy). In order to exclude abnormal foot and ankle pronation as the cause of patellofemoral pain, the participants were screened by kinetic gait analysis to detect abnormal values of mediolateral force. Presence of other lower extremity dysfunction that could account for the knee symptoms Mean duration of symptoms: not reported All unilateral complaints Study participants: 80 people with patellofemoral pain assigned and 74 assessed
Mean age (SD): 35 (11.4) years Gender (number of women/men): 43/31 | |
| Interventions | Comparison: NMES (simultaneous mixed frequencies) versus control NMES (fixed frequency) Treatment duration: 6 weeks Details of interventions:
Stimulation intensity for both groups was the highest comfortably tolerable for all the participants. | |
| Outcomes | Outcomes analysed in the study and used in this review:
Follow‐up assessments: 6 weeks (at end of treatment) | |
| Notes | Description of condition: patellofemoral pain syndrome The trial authors provided additional information on NMES parameters via email (9 March 2015). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by consulting 4 computer‐generated randomization lists, 1 for each of the 4 stratified groups." |
| Allocation concealment (selection bias) | Unclear risk | Available information did not permit judgement. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants: Available information did not permit judgement. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The lead investigator who examined and measured the patients was not part of the randomisation process, thus ensuring blindness to the stimulator allocation" |
| Incomplete outcome data (attrition bias) | High risk | Some participants did not complete the study (12% dropout), and their data were excluded from the analysis. Losses not balanced between groups. |
| Selective reporting (reporting bias) | Low risk | No study protocol available. The authors considered only 1 adverse event (muscle fatigue); however, this is 1 of the most important adverse events in clinical practice. |
| Other bias | Low risk | The study appears to be free of other sources of bias. Baseline characteristics were balanced between groups. |
| Methods | Double‐blinded, randomised sham‐controlled trial Trial protocol registration: not reported | |
| Participants | Country: United States Setting: laboratory (single NMES session) Data collection period: not described Inclusion criteria: age between 15 and 65, atraumatic knee pain (greater than 3 months), pain with more than 2 of the following activities: jumping, kneeling, prolonged sitting, quadriceps contraction, running, squatting, or stair climbing or when pressure was placed on the patella. Participants were required to score less than 85 of 100 on the Anterior Knee Pain Scale. Exclusion criteria: previous knee surgery, ligamentous instability, meniscal injury, or other sources of anterior knee pain, such as patellar tendinitis, bursitis, or patella subluxation. Contraindications to electrical stimulation: implanted biomedical devices, history of neuropathy, muscular abnormality, hypersensitivity to electrical stimulation, or active infection where the electrodes would be placed. Mean duration of symptoms: not reported People who presented bilateral complaints were included, but only the most symptomatic knee was treated. Study participants: 22 people with patellofemoral pain assigned and assessed
Mean age (SD): 26.0 (7.9) years Gender (number of women/men): 15/7 | |
| Interventions | Comparison: NMES versus placebo Treatment duration: single session (15‐minute treatment) Details of interventions:
At the end of the intervention, the PENS electrodes were removed, and the participants were instructed to perform 2 functional movements. Outcome data were collected for both tasks.
| |
| Outcomes | Outcomes analysed in the study and used in this review:
Follow‐up assessments: immediately at the end of the single‐session treatment (after completing the single‐leg squat and lateral step‐down) | |
| Notes | Description of condition: patellofemoral pain The trial authors provided additional information on random sequence generation, allocation concealment, and blinding (participants, personnel, and assessors) via email (12 August 2016). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Available information did not permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "One researcher concealed treatment interventions in envelopes, which were randomly allocated to participants before enrolment." The authors did not mention if the envelopes were sealed and opaque. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "sham controlled laboratory study" Since the authors did not exclude people who had received previous NMES therapy, it is difficult to affirm that the blinding was effective. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "At the conclusion of the 15‐minute treatment, electrodes were removed, the blinded researcher left the laboratory, and the primary researcher returned to the laboratory to conduct post‐intervention assessments" It is not clear if the outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | High risk | No study protocol available. It is unclear if the results included all expected outcomes. This study did not consider adverse event as an outcome. Another report of this study that included a subgroup of 15 females presented the same baseline characteristics (age, height, mass, anterior knee pain score, and VAS pain knee) as those for the 22 participants in the study. |
| Other bias | Unclear risk | Although the baseline characteristics were balanced between groups, we are uncertain whether these are correct. The pain scores (1.9 in both groups) were below the threshold ("more than 2") for inclusion in the trial. |
| Methods | Randomised controlled trial Trial protocol registration: not reported | |
| Participants | Country: Switzerland Setting: Physical Medicine and Rehabilitation, Sion Hospital, Switzerland and Orthopaedic Hospital of Lausanne Data collection period: not reported Inclusion criteria: non‐traumatic retropatellar painful chondropathy, without radiological lesion, with or without Wiberg patellar dysplasia type I or II Exclusion criteria: Wiberg dysplasia type III Mean duration of symptoms: not reported % of bilateral complaints not reported. Study participants: 120 people with patellofemoral pain assigned and 94 assessed
Mean age (SD): 26.4 (11.2) years | |
| Interventions | Comparison: NMES versus exercise (isokinetic) versus exercise (isometric) Treatment duration: 4 weeks Details of co‐interventions:
| |
| Outcomes | Outcomes analysed in the study and used in this review:
Follow‐up assessments: 4 weeks (at end of treatment) | |
| Notes | Description of condition: retropatellar chondropathy It was not possible to obtain additional data on random sequence generation, allocation concealment, and blinding (participants, personnel, and assessors) after attempt to contact authors by email (9 March 2015). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Available information did not permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Available information did not permit judgement. |
| Blinding of participants and personnel (performance bias) | High risk | Participants: probably not done because the interventions were different between groups. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The evaluation of the isokinetic strength and Arpège criteria was performed by a neutral observer. This investigator does not belong to the rehabilitation team" |
| Incomplete outcome data (attrition bias) | High risk | Some participants did not complete the study (22% dropout) and it is unclear how the authors dealt with these missing data. Losses imbalanced between the groups. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. It is unclear if the results included all expected outcomes. This study did not consider adverse event as an outcome. |
| Other bias | Unclear risk | This study provided no information on the side(s) affected, and it is unclear which knee was treated. |
| Methods | Randomised controlled trial Trial protocol registration: not reported | |
| Participants | Country: Turkey Setting: Faculty of Medicine, Department of Sports Medicine, Hacettepe University, Ankara Data collection period: not reported Inclusion criteria: pain longer than 6 months, presence of retropatellar pain, crepitation and pain in patellar grinding, age between 18 to 40 years, no abnormalities on magnetic resonance imaging Exclusion criteria: history or clinical evidence of patellofemoral dislocation, subluxation, or osteoarthritis, presence in the clinical examination of injury or dysfunction to the knee ligaments, bursae, menisci, and synovial plicae, history of lower extremity surgery, radiographic evidence of osteoarthritis in any compartments of the knee joint. Mean duration of symptoms: not reported All unilateral complaints Study participants: 30 participants with patellofemoral pain assigned and assessed
Mean age (SD): 42.7 (10.0) years Gender (number of women/men): only women were included. | |
| Interventions | Comparison: NMES + other intervention (exercise + taping) versus no NMES + same other intervention Treatment duration: 6 weeks Treatment setting: outpatient rehabilitation programme Details of interventions:
| |
| Outcomes | Outcomes analysed in the study and used in this review:
Follow‐up assessments: short term (6 weeks) | |
| Notes | Description of condition: patellofemoral pain syndrome This trial had 3 treatment arms. Data from 1 group (NMES alone) were not included in this review. It was not possible to obtain additional data on random sequence generation, allocation concealment, and blinding (participants, personnel, and assessors) after attempt to contact the authors by email (10 March 2015). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were randomly allocated into three groups by the second author who was blinded in measurements and assessments” The sentence above is unclear about the method used. |
| Allocation concealment (selection bias) | Unclear risk | Available information did not permit judgement. |
| Blinding of participants and personnel (performance bias) | High risk | Participants: probably not done because the interventions were different between groups. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “The patients were randomly allocated into three groups by the second author who was blinded in measurements and assessments” |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All patients completed the rehabilitation program and all assessment procedures" |
| Selective reporting (reporting bias) | High risk | No study protocol available. It is unclear if the results included all expected outcomes. This study did not consider adverse event as an outcome. The conclusions for pain were contradicted by the data, raising doubts regarding their reliability. |
| Other bias | High risk | Baseline characteristics were balanced between groups, except for pain, which was significantly lower in the control group for all 3 functional activities. |
| Methods | Randomised controlled trial Trial protocol registration: not reported | |
| Participants | Country: Turkey Setting: Hacettepe University, School of Physiotherapy and Rehabilitation, Sports Physiotherapy and Gulhane Military Medical Academy, Department of Orthopaedics and Traumatology, Ankara Data collection period: not reported Inclusion criteria: unilateral patellofemoral pain lasting more than 1 month Exclusion criteria: history or clinical findings of patellar dislocation, meniscal or ligamentous injury, synovial plicae, knee surgery or trauma Mean duration of symptoms: 1.8 years (range 1 month to 5 years) All unilateral complaints Study participants: 40 people with patellofemoral pain assigned and assessed
Mean age (SD): 32.9 (7.3) years Gender (number of women/men): no information | |
| Interventions | Comparison: NMES + other intervention (exercise, taping, and ice) versus no NMES + same other intervention Treatment duration: 3 weeks (total of 15 sessions) Details of interventions:
| |
| Outcomes | Outcomes analysed in the study and used in this review:
Follow‐up assessments: short term (3 weeks) | |
| Notes | Description of condition: patellofemoral pain syndrome This trial had 4 treatment arms. Data from 2 groups (NMES, ice, medial patellar glide and exercises; ice and home exercises) were not included in this review. The trial authors provided additional information on NMES parameters and exercise programme via email, confirming that "same exercises were given to the all groups" (12 March 2015). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Available information did not permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Available information did not permit judgement. |
| Blinding of participants and personnel (performance bias) | High risk | Participants: probably not done because the interventions were different between groups. |
| Blinding of outcome assessment (detection bias) | High risk | Available information did not permit judgement, but seems unlikely given the nature of the intervention. |
| Incomplete outcome data (attrition bias) | Unclear risk | It is not clear if there were losses. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. It is unclear if the results included all expected outcomes. This study did not consider adverse event as an outcome. |
| Other bias | Low risk | The study appears to be free of other sources of bias. Baseline characteristics were balanced between groups. |
EMG: electromyography
HVPGS: high‐voltage pulsed galvanic simulation
mA: milliamp
NMES: neuromuscular electrical stimulation
PENS: patterned electrical neuromuscular stimulation
SD: standard deviation
VAS: visual analogue scale
VMO: vastus medialis oblique
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The purpose of this randomised controlled trial was to investigate the effects of electromyographic biofeedback treatment in people with patellofemoral pain (biofeedback group versus control group). No neuromuscular electrical stimulation intervention. | |
| This was not a randomised or quasi‐randomised controlled trial. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Rehabilitation with patterned electrical neuromuscular stimulation for patients with patellofemoral pain (PENS for PFP) |
| Methods | Randomised controlled trial |
| Participants | Country: United States Setting: University of Virginia, Charlottesville Data collection period: this study was recruiting participants (verified May 2015) Inclusion criteria: insidious onset of symptoms, presence of peripatellar or retropatellar knee pain during at least 2 of the following functional activities: stair ascent or descent, running, kneeling, squatting, prolonged sitting, jumping; pain for more than 3 months (> 3/10 on VAS); 85 or less on the Anterior Knee Pain Scale Exclusion criteria: previous knee surgery; internal derangement; ligamentous instability, other sources of anterior knee pain (patella tendonitis, Osgood Schlatter, knee plica, etc.), neurological involvement, any biomedical device; muscular abnormalities; currently pregnant; hypersensitivity to electrical stimulation; active infection over the site of the electrode placement Study participants: people with patellofemoral pain, ages between 15 and 40 years, both genders Estimated sample: 32 participants |
| Interventions | Comparison: NMES versus placebo Treatment duration: single session (15‐minute treatment) Details of interventions:
|
| Outcomes | Data collection was planned for 4 weeks.
|
| Starting date | 4 May 2015 |
| Contact information | Neal Glaviano, MEd, ATC University of Virginia, Charlottesville, Virginia, United States 22902, 434‐924‐6184; email: [email protected] |
| Notes | A related laboratory study testing the same intervention from the same team is available (Glaviano 2016). A check on the status of this trial on 1 May 2017 found that "The recruitment status of the study is unknown. The completion data has passed and the status has not been verified in more than two years." |
NMES: neuromuscular electrical stimulation
PENS: patterned electrical neuromuscular stimulation
VAS: visual analogue scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knee pain during activities (end of the treatment, single 15‐minute NMES session): VAS scale: 0 to 10; higher score = worse pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1 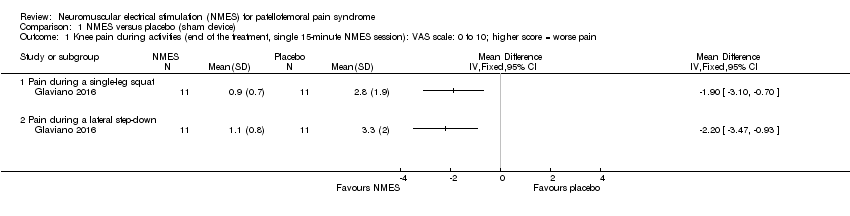 Comparison 1 NMES versus placebo (sham device), Outcome 1 Knee pain during activities (end of the treatment, single 15‐minute NMES session): VAS scale: 0 to 10; higher score = worse pain. | ||||
| 1.1 Pain during a single‐leg squat | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain during a lateral step‐down | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knee pain (end of the treatment, 3 to 12 weeks): VAS scale: 0 to 10; higher score = worse pain Show forest plot | 3 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐1.63 [‐2.23, ‐1.02] |
| Analysis 2.1  Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 1 Knee pain (end of the treatment, 3 to 12 weeks): VAS scale: 0 to 10; higher score = worse pain. | ||||
| 2 Knee pain during activities (end of the treatment, 6 weeks): VAS scale: 0 to 10; higher score = worse pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 2 Knee pain during activities (end of the treatment, 6 weeks): VAS scale: 0 to 10; higher score = worse pain. | ||||
| 2.1 Pain during step‐down | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Pain during step‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Pain during squat | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Knee function (end of the treatment, 3 and 6 weeks): Cincinnati Knee Rating System and LEFS; higher score = better function Show forest plot | 2 | 70 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐0.11, 0.84] |
| Analysis 2.3 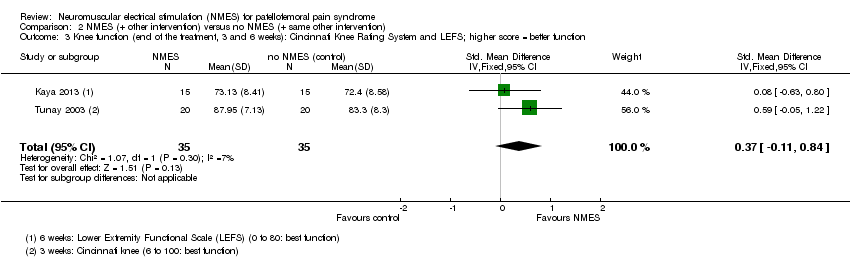 Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 3 Knee function (end of the treatment, 3 and 6 weeks): Cincinnati Knee Rating System and LEFS; higher score = better function. | ||||
| 4 Change score for KPS (end of treatment, 12 weeks) (0 to 100; higher score = better function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 4 Change score for KPS (end of treatment, 12 weeks) (0 to 100; higher score = better function). | ||||
| 5 Good or normal muscle strength (end of the treatment, 6 weeks): grades 4 to 5 Lovett’s manual muscle scale Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5 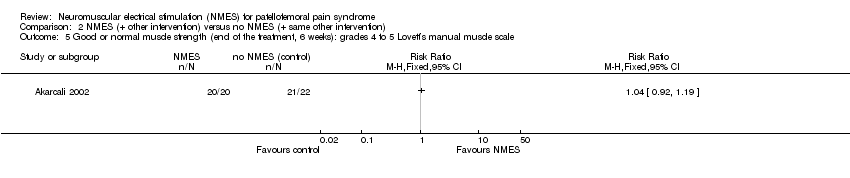 Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 5 Good or normal muscle strength (end of the treatment, 6 weeks): grades 4 to 5 Lovett’s manual muscle scale. | ||||
| 6 Muscle strength (end of the treatment, 12 weeks): isokinetic dynamometer (N) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6 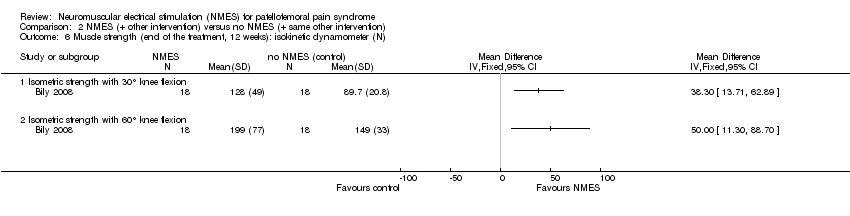 Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 6 Muscle strength (end of the treatment, 12 weeks): isokinetic dynamometer (N). | ||||
| 6.1 Isometric strength with 30° knee flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Isometric strength with 60° knee flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knee function (end of the treatment, 4 weeks): Arpège function scale: 0 to 18; higher score = better function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 NMES versus exercise, Outcome 1 Knee function (end of the treatment, 4 weeks): Arpège function scale: 0 to 18; higher score = better function. | ||||
| 2 Muscle strength (end of the treatment, 4 weeks): isokinetic dynamometer (Nm) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 NMES versus exercise, Outcome 2 Muscle strength (end of the treatment, 4 weeks): isokinetic dynamometer (Nm). | ||||
| 2.1 Isokinetic dynamometer at 30°/s | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Isokinetic dynamometer at 300°/s | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knee pain (end of the treatment, 6 weeks): VAS scale: 0 to 10; higher score = worse pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1 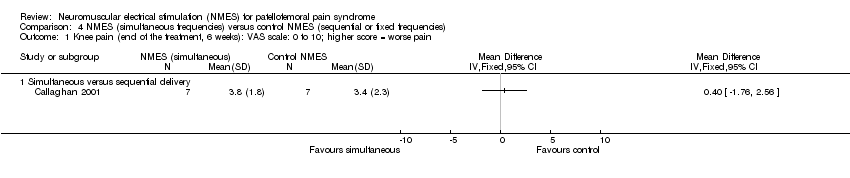 Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 1 Knee pain (end of the treatment, 6 weeks): VAS scale: 0 to 10; higher score = worse pain. | ||||
| 1.1 Simultaneous versus sequential delivery | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Knee function (end of the treatment, 6 weeks): KPS: 0 to 100 scale; higher score = better function Show forest plot | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐6.79, 4.47] |
| Analysis 4.2  Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 2 Knee function (end of the treatment, 6 weeks): KPS: 0 to 100 scale; higher score = better function. | ||||
| 2.1 Simultaneous versus sequential delivery | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐5.90 [‐16.14, 4.34] |
| 2.2 Simultaneous versus fixed delivery | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐5.84, 7.64] |
| 3 Functional performance (end of the treatment, 6 weeks): number of steps up and down until pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 3 Functional performance (end of the treatment, 6 weeks): number of steps up and down until pain. | ||||
| 3.1 Simultaneous versus sequential delivery | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Quadriceps isometric muscle strength (end of the treatment, 6 weeks): dynamometer at 90°/s (Nm) Show forest plot | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐16.24, 13.94] |
| Analysis 4.4  Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 4 Quadriceps isometric muscle strength (end of the treatment, 6 weeks): dynamometer at 90°/s (Nm). | ||||
| 4.1 Simultaneous versus sequential delivery | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [‐25.83, 32.63] |
| 4.2 Simultaneous versus fixed delivery | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐20.42, 14.82] |
| 5 Quadriceps isokinetic muscle strength (end of the treatment, 6 weeks): dynamometer at 90°/s (Nm) Show forest plot | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐7.28 [‐24.45, 9.89] |
| Analysis 4.5  Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 5 Quadriceps isokinetic muscle strength (end of the treatment, 6 weeks): dynamometer at 90°/s (Nm). | ||||
| 5.1 Simultaneous versus sequential delivery | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [‐30.70, 42.90] |
| 5.2 Simultaneous versus fixed delivery | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐30.41, 8.41] |

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
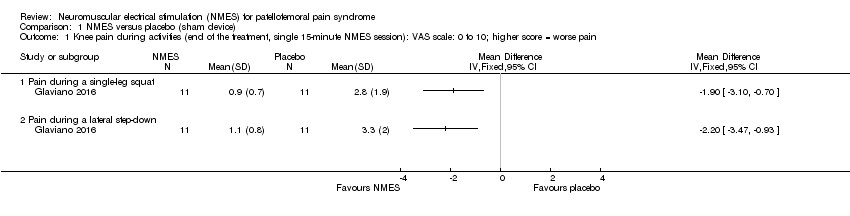
Comparison 1 NMES versus placebo (sham device), Outcome 1 Knee pain during activities (end of the treatment, single 15‐minute NMES session): VAS scale: 0 to 10; higher score = worse pain.

Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 1 Knee pain (end of the treatment, 3 to 12 weeks): VAS scale: 0 to 10; higher score = worse pain.

Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 2 Knee pain during activities (end of the treatment, 6 weeks): VAS scale: 0 to 10; higher score = worse pain.

Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 3 Knee function (end of the treatment, 3 and 6 weeks): Cincinnati Knee Rating System and LEFS; higher score = better function.
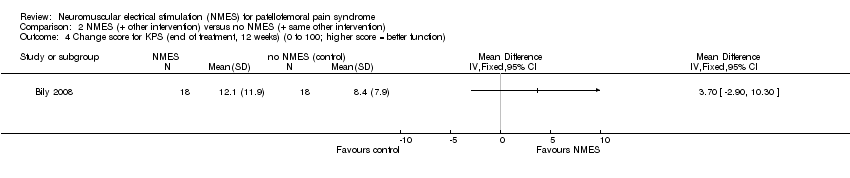
Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 4 Change score for KPS (end of treatment, 12 weeks) (0 to 100; higher score = better function).

Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 5 Good or normal muscle strength (end of the treatment, 6 weeks): grades 4 to 5 Lovett’s manual muscle scale.

Comparison 2 NMES (+ other intervention) versus no NMES (+ same other intervention), Outcome 6 Muscle strength (end of the treatment, 12 weeks): isokinetic dynamometer (N).

Comparison 3 NMES versus exercise, Outcome 1 Knee function (end of the treatment, 4 weeks): Arpège function scale: 0 to 18; higher score = better function.

Comparison 3 NMES versus exercise, Outcome 2 Muscle strength (end of the treatment, 4 weeks): isokinetic dynamometer (Nm).
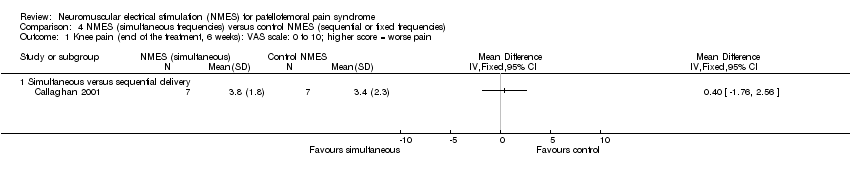
Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 1 Knee pain (end of the treatment, 6 weeks): VAS scale: 0 to 10; higher score = worse pain.

Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 2 Knee function (end of the treatment, 6 weeks): KPS: 0 to 100 scale; higher score = better function.
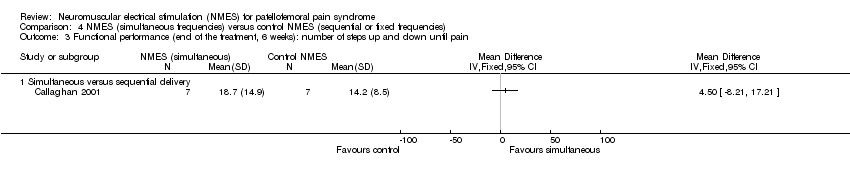
Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 3 Functional performance (end of the treatment, 6 weeks): number of steps up and down until pain.

Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 4 Quadriceps isometric muscle strength (end of the treatment, 6 weeks): dynamometer at 90°/s (Nm).

Comparison 4 NMES (simultaneous frequencies) versus control NMES (sequential or fixed frequencies), Outcome 5 Quadriceps isokinetic muscle strength (end of the treatment, 6 weeks): dynamometer at 90°/s (Nm).
| Neuromuscular electrical stimulation (NMES) plus other intervention (e.g. exercise) versus no NMES plus same other intervention for patellofemoral pain syndrome | ||||||
| Patient or population: people with patellofemoral pain syndrome1 Comparison: no NMES control plus same other active intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No NMES plus same other intervention | NMES plus other intervention (e.g. exercise) | |||||
| Knee pain (short term) | The mean knee pain ranged across control groups from | The mean knee pain in the intervention groups was | MD ‐1.63 (‐2.23 to ‐1.02) | 118 | ⊕⊝⊝⊝ | This difference may not be clinically important since the MCID for VAS (1.5 to 2.0, out of 10 points)5 lies within the 95% CI. |
| Knee pain (long term) | The median pain score in the study control group was 0.4 points (IQR 0.2 to 3.4). | The median pain score in the NMES group was 1.8 points (IQR 0.1 to 3.6). | See comment | 29 | ⊕⊝⊝⊝ | The difference was reported as not statistically significant. |
| Knee function (short term) | The mean knee function in the study control groups was | The mean difference in knee function in the intervention groups was | SMD 0.37 (‐0.11 to 0.84) | 70 | ⊕⊝⊝⊝ | 0.2 SD represents a small difference, 0.5 SD a moderate difference, and 0.8 SD a large difference. However, the mean differences in the 2 trials were small and unlikely to be clinically important (LEFS scale: MD 0.73; Cincinnati score: MD 4.65). |
| Knee function (long term) Kujala Patellofemoral Score (KPS) (0 to 100; higher score = better function). | The median KPS in the study control group was 95 (IQR 85 to 96). | The median KPS in the NMES group was 94 (IQR 88 to 96). | See comment | 29 | ⊕⊝⊝⊝ | The very small difference was reported as not statistically significant. |
| Adverse events ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Where reported, there was a higher percentage of females (63% to 100%). The mean ages of the participants in the four trials ranged from 25 to 39 years. There was a wide duration of symptoms, with the minimum duration of symptoms for trial inclusion ranging from one to six months. | ||||||
| Neuromuscular electrical stimulation (NMES) versus exercise for patellofemoral pain syndrome | ||||||
| Patient or population: people with patellofemoral pain syndrome Comparison: exercise (either isokinetic or isometric; data combined in the analyses) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Exercise | NMES | |||||
| Knee pain (short term: < 3 months) | See comment | See comment | Not estimable | ‐ | See comment | Not estimable |
| Knee pain (longer term: > 3 months) | See comment | See comment | Not estimable | ‐ | See comment | Not estimable |
| Knee function | The mean knee function in the study control group was | The mean knee function in the intervention groups was | MD ‐0.94 (‐2.10 to 0.22) | 94 | ⊕⊝⊝⊝ | |
| Knee function (longer term: > 3 months) | See comment | See comment | Not estimable | ‐ | See comment | Not estimable |
| Adverse events | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1This very demanding schedule is unlikely to be found in clinical practice. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knee pain during activities (end of the treatment, single 15‐minute NMES session): VAS scale: 0 to 10; higher score = worse pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain during a single‐leg squat | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain during a lateral step‐down | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knee pain (end of the treatment, 3 to 12 weeks): VAS scale: 0 to 10; higher score = worse pain Show forest plot | 3 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐1.63 [‐2.23, ‐1.02] |
| 2 Knee pain during activities (end of the treatment, 6 weeks): VAS scale: 0 to 10; higher score = worse pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Pain during step‐down | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Pain during step‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Pain during squat | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Knee function (end of the treatment, 3 and 6 weeks): Cincinnati Knee Rating System and LEFS; higher score = better function Show forest plot | 2 | 70 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐0.11, 0.84] |
| 4 Change score for KPS (end of treatment, 12 weeks) (0 to 100; higher score = better function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Good or normal muscle strength (end of the treatment, 6 weeks): grades 4 to 5 Lovett’s manual muscle scale Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Muscle strength (end of the treatment, 12 weeks): isokinetic dynamometer (N) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Isometric strength with 30° knee flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Isometric strength with 60° knee flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knee function (end of the treatment, 4 weeks): Arpège function scale: 0 to 18; higher score = better function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Muscle strength (end of the treatment, 4 weeks): isokinetic dynamometer (Nm) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Isokinetic dynamometer at 30°/s | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Isokinetic dynamometer at 300°/s | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Knee pain (end of the treatment, 6 weeks): VAS scale: 0 to 10; higher score = worse pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Simultaneous versus sequential delivery | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Knee function (end of the treatment, 6 weeks): KPS: 0 to 100 scale; higher score = better function Show forest plot | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐6.79, 4.47] |
| 2.1 Simultaneous versus sequential delivery | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐5.90 [‐16.14, 4.34] |
| 2.2 Simultaneous versus fixed delivery | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐5.84, 7.64] |
| 3 Functional performance (end of the treatment, 6 weeks): number of steps up and down until pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Simultaneous versus sequential delivery | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Quadriceps isometric muscle strength (end of the treatment, 6 weeks): dynamometer at 90°/s (Nm) Show forest plot | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐16.24, 13.94] |
| 4.1 Simultaneous versus sequential delivery | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [‐25.83, 32.63] |
| 4.2 Simultaneous versus fixed delivery | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐20.42, 14.82] |
| 5 Quadriceps isokinetic muscle strength (end of the treatment, 6 weeks): dynamometer at 90°/s (Nm) Show forest plot | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐7.28 [‐24.45, 9.89] |
| 5.1 Simultaneous versus sequential delivery | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [‐30.70, 42.90] |
| 5.2 Simultaneous versus fixed delivery | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐30.41, 8.41] |

