Subkutane schnell wirkende Insulinanaloga gegen diabetische Ketoazidose
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel randomised controlled trial Randomisation ratio: 1:1 Superiority design | |
| Participants | Inclusion criteria: DKA, blood glucose > 300 mg/dL, pH < 7.3, and/or bicarbonate < 15 mmol/L, and > ++ ketonuria Exclusion criteria: surgery, use of glucocorticoid or immunosuppressive agents Diagnostic criteria: ADA criteria for DKA Causes of DKA (n) ‐ (subcutaneous insulin/intravenous insulin):
| |
| Interventions | Number of study centres: 1 Treatment before study: not stated Group 1: s.c. insulin lispro. 0.15 IU/kg every 2 h until blood glucose < 250 mg/dL, then every 4 h for the next 24 h (n = 30) Group 2: i.v. regular insulin. 0.1 IU/kg/h, continuous infusion until blood glucose < 250 mg/dL, and then 0.15 IU/kg subcutaneously every 4 h for 24 h (n = 30) | |
| Outcomes | Composite outcome measures reported: no | |
| Study details | Run‐in period: no Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Non‐commercial funding: Fundacao de Amparo à Pesquisa do Estado de Sao Paulo grant (FAPESP 00/09682‐7) Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "... to compare the efficacy of a subcutaneous fast‐acting analog (lispro) with continuous intravenous regular insulin (CIRI) in the treatment of pediatric DKA" | |
| Notes | Study authors randomised episodes of DKA, not participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Of the 60 DKA episodes, 30 were randomised to treatment with a subcutaneous fast‐acting insulin analog (lispro) and the other 30 were randomised to treatment with CIRI" Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants and personnel (performance bias) | High risk | Comment: participants and personnel were probably unblinded |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: study personnel/participants probably not blinded, but outcome measurement unlikely to be influenced by the lack of blinding |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: study personnel/participants probably not blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: study personnel/participants probably not blinded, but outcome measurement unlikely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: no detailed information, but outcome measurement unlikely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: no detailed information, but outcome measurement unlikely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detailed information |
| Incomplete outcome data (attrition bias) | Low risk | Comment: reasons for dropouts explained |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: DKA occurrences randomised, not participants (unclear which participants had only 1 DKA) |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: DKA occurrences randomised, not participants (unclear which participants had only 1 DKA) |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: DKA occurrences randomised, not participants (unclear which participants had only 1 DKA) |
| Selective reporting (reporting bias) | Unclear risk | Comment: possible outcome reporting bias for time to resolution of DKA (see Appendix 6) |
| Other bias | Low risk | Comment: none detected |
| Methods | Parallel randomised controlled trial Randomisation ratio: 1:1 Superiority design | |
| Participants | Inclusion criteria: DKA (mild or moderate only), serum blood glucose > 250 mg/dL, arterial pH < 7.3, bicarbonate < 15 mmol/L, beta‐hydroxybutyrate > 1.6 mmol/L, ketonuria Exclusion criteria: plasma glucose > 600 mg/dL, pH < 7.0, bicarbonate < 10 mmol/L, persistent hypotension, hypothermia, severe concomitant illness Diagnostic criteria: ADA criteria for DKA Causes of DKA: new onset diabetes (3 subcutaneous insulin lispro/2 intravenous regular insulin) | |
| Interventions | Number of study centres: 1 Treatment before study: not stated | |
| Outcomes | Composite outcome measures reported: no | |
| Study details | Run‐in period: no Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Commercial funding/non‐commercial funding/other funding: no Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "... to evaluate the efficacy and safety of hourly SC insulin lispro administration in the treatment of DKA in comparison with standard IV regular insulin treatment" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "The patients were randomly assigned into two groups" Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants and personnel (performance bias) | High risk | Comment: participants and personnel were probably not blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: study personnel/participants probably not blinded, but outcome measurement unlikely to be influenced by the lack of blinding |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: study personnel/participants probably not blinded, outcome measurement not defined |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: no detailed information, but outcome measurement unlikely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: no detailed information, but outcome measurement unlikely to be influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | Comment: possible outcome reporting bias for time to resolution of DKA (see Appendix 6) |
| Other bias | Low risk | Comment: none detected |
| Methods | Parallel randomised controlled trial Randomisation ratio: 1:1 Superiority design | |
| Participants | Inclusion criteria: DKA (mild or moderate only, ADA criteria) Exclusion criteria: severe DKA and those requiring ICU admission, loss of consciousness, acute myocardial ischaemia, congestive heart failure, end‐stage renal disease, anasarca, pregnancy, serious comorbidities, persistent hypotension Diagnostic criteria: ADA criteria for DKA Causes of DKA (% regular intravenous insulin/subcutaneous insulin lispro)
| |
| Interventions | Number of study centres: 1 Treatment before study: not stated Titration period: no | |
| Outcomes | Composite outcome measures reported: no | |
| Study details | Run‐in period: no Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Commercial funding/non‐commercial funding/other funding: none Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "... to compare the efficacy of insulin lispro subcutaneous 2 hourly in patients of mild to moderate DKA with standard intravenous regular insulin" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "The study patients were randomised in emergency department following a computer generated randomisation table in two groups" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants and personnel (performance bias) | High risk | Quote from publication: "In this prospective, randomised and open trial ..." Comment: participants and personnel were unblinded (open trial) |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from publication: "In this prospective, randomised and open trial ..." Comment: participants and study personnel not blinded, but outcome measurement not likely to be influenced by the lack of blinding |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote from publication: "In this prospective, randomised and open trial ..." Comment: participants and personnel were unblinded (open trial) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote from publication: "In this prospective, randomised and open trial ..." Comment: participants and personnel were unblinded (open trial) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote from publication: "In this prospective, randomised and open trial ..." Comment: participants and personnel were unblinded (open trial) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: no detailed information, outcome not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detailed information |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
| Methods | Parallel randomised controlled trial Randomisation ratio: 1:1:1 Superiority design | |
| Participants | Inclusion criteria: "Uncomplicated DKA" defined by a plasma glucose level > 250 mg/dL, serum bicarbonate level < 15 mEq/L, venous pH < 7.3, serum ketone level Exclusion criteria: persistent hypotension after the administration of 1 liter of normal saline (systolic blood pressure < 80 mmHg), acute myocardial ischaemia, end‐stage renal or hepatic failure, anasarca, dementia, or pregnancy Diagnostic criteria: ADA criteria for DKA Causes of DKA (%): poor compliance: 53 (s.c. insulin aspart, every hour)/60 (s.c. insulin aspart, every 2 hours)/60 (i.v. regular insulin); new onset diabetes: 20 (s.c. insulin aspart, every hour)/20 (s.c. insulin aspart, every 2 hours)/13 (i.v. regular insulin) | |
| Interventions | Number of study centres: 1 Treatment before study: not stated Titration period: no | |
| Outcomes | Composite outcome measures reported: no | |
| Study details | Run‐in period: no Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Commercial funding: unrestricted grant from Novo Nordisk; non‐commercial funding: United States Public Health Services/National Institutes of Health grant (RR00211; General Clinical Research Center) Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "We compared the efficacy and safety of aspart insulin given subcutaneously at different time intervals to a standard low‐dose intravenous (IV) infusion protocol of regular insulin in patients with uncomplicated diabetic ketoacidosis (DKA)" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Patients were randomly assigned in the emergency department to receive SC aspart insulin every hour (SC‐1h, n15) or every 2 h (SC‐2h, n15), or to receive IV regular insulin (n15)" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants and personnel (performance bias) | High risk | Quote from publication: "In this prospective, randomised, open trial ..." Comment: participants and personnel were unblinded |
| Blinding of participants and personnel (performance bias) | Low risk | Quote from publication: "In this prospective, randomised, open trial ..." Comment: outcome measurement not likely to be influenced by the lack of blinding |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote from publication: "In this prospective, randomised, open trial ..." Comment: unclear whether outcome was influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote from publication: "In this prospective, randomised, open trial ..." Comment: unclear whether outcome was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: outcome measurement not likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detailed information |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: all randomised participants completed the study |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Unclear risk | Comment: possible sponsor bias (unrestrictive grant from Novo Nordisk) |
| Methods | Parallel randomised controlled trial Randomisation ratio: 1:1 Superiority design | |
| Participants | Inclusion criteria: plasma glucose level > 250 mg/dL, serum bicarbonate level < 15 mEq/L, venous pH < 7.3, serum ketone level, beta‐hydroxybutyrate > 3 mmol/L Exclusion criteria: persistent hypotension after the administration of 1 liter of normal saline (systolic blood pressure < 80 mmHg), acute myocardial ischaemia, heart failure, end‐stage renal disease, anasarca, dementia, or pregnancy Diagnostic criteria: ADA criteria for DKA Causes of DKA (%): poor compliance: 60 (subcutaneous insulin lispro)/70 (intravenous regular insulin) | |
| Interventions | Number of study centres: 1 Treatment before study: not stated Titration period: no | |
| Outcomes | Composite outcome measures reported: no | |
| Study details | Run‐in period: no Study terminated before regular end (for benefit/because of adverse events): no | |
| Publication details | Language of publication: English Commercial funding: unrestricted grant from Eli Lilly; non‐commercial funding: United States Public Health Services grant (RR00211) Publication status: peer‐reviewed journal | |
| Stated aim for study | Quote from publication: "To compare the efficacy and safety of subcutaneous insulin lispro with that of low‐dose continuous intravenous regular insulin in the treatment of patients with uncomplicated diabetic ketoacidosis" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Patients were assigned in the emergency department to receive subcutaneous insulin lispro or intravenous regular insulin following a computer‐generated randomisation table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants and personnel (performance bias) | High risk | Quote from publication: "open trial" Comment: participants and personnel were unblinded (open trial) |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: study personnel not blinded, but outcome measurement not likely to be influenced by the lack of blinding of outcome assessment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: unclear whether outcome was influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote from publication: "open trial" Comment: participants and personnel were unblinded (open trial) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: no information on blinding of outcome assessment, but outcome measurement not likely to be influenced |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: no detailed information |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomised participants completed the study |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Unclear risk | Comment: possible sponsor bias (unrestrictive grant from Eli Lilly) |
Note: where the judgement is 'unclear risk' and the description is blank, the trial did not report that particular outcome
ADA: American Diabetes Association; DKA: diabetic ketoacidosis; ICU: intensive care unit; i.v.: intravenous; s.c.: subcutaneous
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Intervention not relevant (insulin therapy was by the intramuscular route) | |
| Not an RCT (case series) | |
| Not aimed at treating DKA (well‐controlled insulin‐dependent diabetes mellitus) | |
| Intervention not relevant (regular insulin by various routes) | |
| Intervention not relevant (s.c. administration of long‐acting insulins) | |
| Not aimed at treating DKA | |
| Intervention not relevant (s.c. and i.v. infusion of regular insulin) | |
| Intervention not relevant (insulin levemir) | |
| Intervention not relevant (fluid therapy) | |
| Intervention not relevant (s.c. insulin glargine) | |
| Not aimed at treating DKA | |
| Not an RCT (review article) | |
| Intervention not relevant (i.v. regular or i.v. glulisine insulin) | |
| Not an RCT (review article) | |
| Not aimed at treating DKA | |
| Not an RCT (case report) |
DKA: diabetic ketoacidosis; i.v.: intravenous; RCT: randomised controlled trial: s.c.: subcutaneous
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial |
| Participants | 80 people with DKA |
| Interventions | Group 1: regular insulin infusion (n = 20) Group 2: s.c. rapid‐acting insulin analogue aspart every 2 hours (n = 20) Group 3: s.c. rapid‐acting insulin analogue aspart every hour (n = 20) Group 4: rapid‐acting insulin analogue by subcutaneous insulin pump (n = 20) |
| Outcomes | Time to resolution of DKA |
| Study details | Intervention model: factorial design Masking: not stated Primary purpose: treatment |
| Publication details | Conference abstract |
| Stated aim of study | To look for technical simplification and economic efficiency in the treatment of DKA with s.c. use of rapid‐acting insulin analogue and to compare its use with regular i.v. insulin treatment |
| Notes | No outcome data reported in abstract (authors contacted by email; no reply received) |
| Methods | Randomised controlled trial |
| Participants | Adults with DKA |
| Interventions | Group 1: s.c. insulin aspart every 2 hours Group 2: i.v. regular insulin Group 3: i.v. insulin aspart (NovoLog) |
| Outcomes | Hours to resolution of ketoacidosis as defined as beta‐hydroxybutyrate < 0.6 Hours to achieve blood glucose less than 200 mg/dL |
| Study details | Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Publication details | Study start date: January 2005 Study completion date: December 2007 Primary completion date: July 2007 (final data collection date for primary outcome measure) Study not yet published (authors contacted by email; no reply received yet) |
| Stated aim of study | To determine whether insulin administered by a subcutaneous injection is effective in the treatment of a diabetic crisis and to determine whether it is useful to monitor beta‐hydroxybutyrate during treatment of a diabetic crisis |
| Notes | Responsible party: David Baldwin, MD. Rush University Medical Center Chicago, Illinois, United States, 60612 Registered in ClinicalTrials.gov. Study completed, no results published |
DKA: diabetic ketoacidosis; i.v.: intravenous; s.c.: subcutaneous
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to resolution of diabetic ketoacidosis Show forest plot | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.64, 0.90] |
| Analysis 1.1  Comparison 1 Insulin lispro versus regular insulin, Outcome 1 Time to resolution of diabetic ketoacidosis. | ||||
| 2 All‐cause mortality Show forest plot | 4 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.2 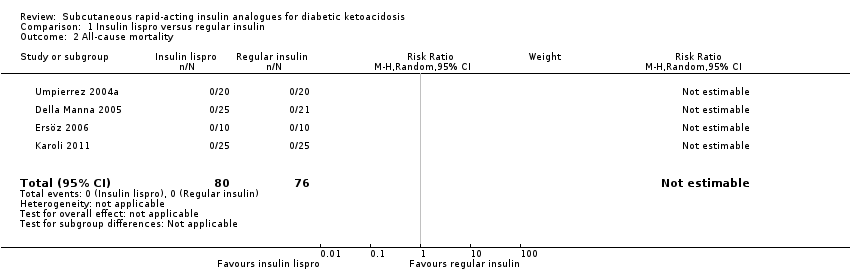 Comparison 1 Insulin lispro versus regular insulin, Outcome 2 All‐cause mortality. | ||||
| 3 Hypoglycaemic episodes Show forest plot | 4 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.23, 1.52] |
| Analysis 1.3  Comparison 1 Insulin lispro versus regular insulin, Outcome 3 Hypoglycaemic episodes. | ||||
| 3.1 Adults | 3 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.11, 3.94] |
| 3.2 Children | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.18, 1.72] |
| 4 Length of hospital stay Show forest plot | 2 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.97, 0.22] |
| Analysis 1.4  Comparison 1 Insulin lispro versus regular insulin, Outcome 4 Length of hospital stay. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to resolution of diabetic ketoacidosis Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1 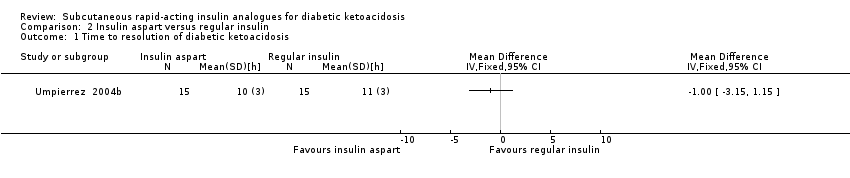 Comparison 2 Insulin aspart versus regular insulin, Outcome 1 Time to resolution of diabetic ketoacidosis. | ||||
| 2 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Insulin aspart versus regular insulin, Outcome 2 All‐cause mortality. | ||||
| 3 Hypoglycaemic episodes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Insulin aspart versus regular insulin, Outcome 3 Hypoglycaemic episodes. | ||||
| 4 Length of hospital stay Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Insulin aspart versus regular insulin, Outcome 4 Length of hospital stay. | ||||
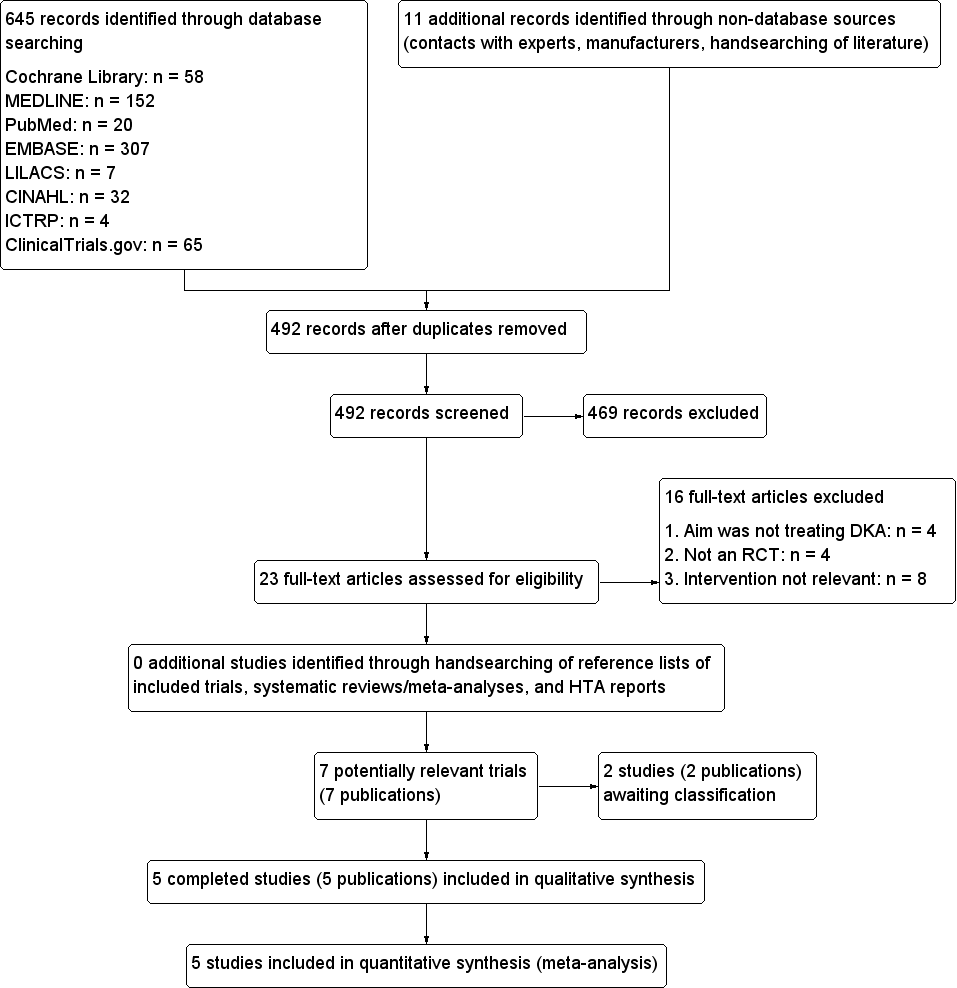
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials (blank cells indicate that the particular outcome was not measured in some trials).

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial (blank cells indicate that the trial did not measure that particular outcome).
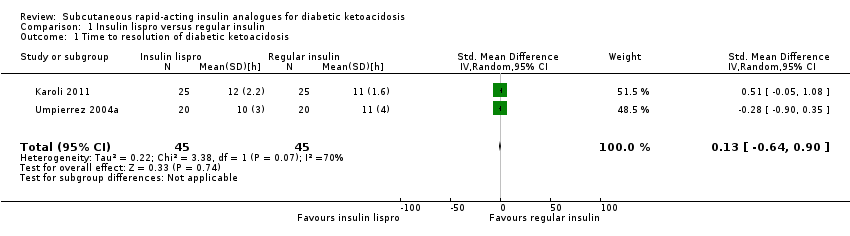
Comparison 1 Insulin lispro versus regular insulin, Outcome 1 Time to resolution of diabetic ketoacidosis.

Comparison 1 Insulin lispro versus regular insulin, Outcome 2 All‐cause mortality.

Comparison 1 Insulin lispro versus regular insulin, Outcome 3 Hypoglycaemic episodes.
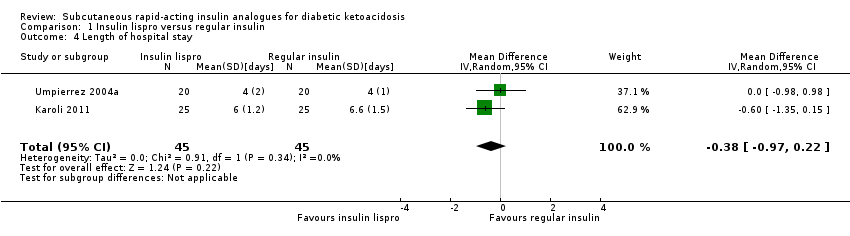
Comparison 1 Insulin lispro versus regular insulin, Outcome 4 Length of hospital stay.

Comparison 2 Insulin aspart versus regular insulin, Outcome 1 Time to resolution of diabetic ketoacidosis.
Comparison 2 Insulin aspart versus regular insulin, Outcome 2 All‐cause mortality.

Comparison 2 Insulin aspart versus regular insulin, Outcome 3 Hypoglycaemic episodes.

Comparison 2 Insulin aspart versus regular insulin, Outcome 4 Length of hospital stay.
| Subcutaneous insulin lispro versus intravenous regular insulin for diabetic ketoacidosis | ||||||
| Patient: participants with diabetic ketoacidosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous regular insulin | Subcutaneous insulin lispro | |||||
| All‐cause mortality (N) Mean hospital stay: 2‐7 days | See comment | See comment | Not estimable | 156 (4) | ⊕⊕⊕⊝ | No deaths reported |
| Hypoglycaemic episodes (N) Mean hospital stay: 2‐7 days | 118 per 1000 | 70 per 1000 | RR 0.59 | 156 (4) | ⊕⊕⊝⊝ | Comparable risk ratios for adults (4 trials) and children (1 trial) |
| Morbidity (N) Mean hospital stay: 2‐7 days | See comment | See comment | Not estimable | 96 (2) | See comment | No cases of cerebral oedema, venous thrombosis, adult respiratory distress syndrome, hyperchloraemic acidosis |
| Adverse events other than hypoglycaemic episodes | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Time to resolution of diabetic ketoacidosis (h) Mean hospital stay: 2‐4 days | The mean time to resolution of diabetic ketoacidosis across the intravenous regular insulin groups was 11 h | The mean time to resolution of diabetic ketoacidosis in the subcutaneous insulin lispro groups was 0.2 h higher (1.7 h lower to 2.1 h higher) | ‐ | 90 (2) | ⊕⊝⊝⊝ | Metabolic acidosis and ketosis took longer to resolve in the subcutaneous insulin lispro group in 1 trial (60 children); no exact data published |
| Patient satisfaction | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Socioeconomic effects: length of hospital stay (days) Mean hospital stay: 4‐7 days | The mean length of hospital stay in the intravenous regular insulin groups ranged between 4 and 6.6 days | The mean length of hospital stay in the subcutaneous insulin lispro groups was 0.4 days shorter (1 day shorter to 0.2 days longer) | ‐ | 90 (2) | ⊕⊕⊝⊝ | US setting: treatment of diabetic ketoacidosis in a non–intensive care setting (step‐down unit or general medicine ward) was associated with a 39% lower hospitalisation charge than was treatment with intravenous regular insulin in the intensive care unit (USD 8801 (SD USD 5549) vs USD 14,429 (SD USD 5243); the average hospitalisation charges per day were USD 3981 (SD USD 1067) for participants treated in an intensive care unit compared with USD 2682 (SD USD 636) for those treated in a non–intensive care setting |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| *Assumed risk was derived from the event rates in the comparator groups. | ||||||
| Subcutaneous insulin aspart versus intravenous regular insulin for diabetic ketoacidosis | ||||||
| Patient: participants with diabetic ketoacidosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous regular insulin | Subcutaneous insulin aspart | |||||
| All‐cause mortality (N) Mean hospital stay: 3‐5 days | See comment | See comment | Not estimable | 45 (1) | ⊕⊕⊝⊝ | No deaths reported |
| Hypoglycaemic episodes (N) Mean hospital stay: 3‐5 days | 67 per 1000 | 67 per 1000 | RR 1.00 | 30 (1) | ⊕⊕⊝⊝ | ‐ |
| Morbidity | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Adverse events other than hypoglycaemic episodes | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Time to resolution of diabetic ketoacidosis (h) Mean hospital stay: 3‐5 days | The mean time to resolution of diabetic ketoacidosis across the intravenous regular insulin groups was 11 h | The mean time to resolution of diabetic ketoacidosis in the subcutaneous insulin aspart group was 1 h lower (3.2 h lower to 1.2 h higher) | ‐ | 30 (1) | ⊕⊝⊝⊝ | ‐ |
| Patient satisfaction | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Socioeconomic effects: length of hospital stay (days) Mean hospital stay: 3‐5 days | The mean length of hospital stay in the intravenous regular insulin group was 4.5 days | The mean length of hospital stay in the subcutaneous insulin aspart group was 1.1 days shorter (3.3 days shorter to 1.1 days longer) | ‐ | 30 (1) | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| *Assumed risk was derived from the event rates in the comparator groups | ||||||
| Intervention(s) and comparator(s) | Sample sizea | Screened/eligible | Randomised | Analysed | Finishing trial | Randomised finishing trial | Follow‐up timeb | |
| Umpierrez 2004a | I: s.c. insulin lispro | Arbitrary estimation of a difference between groups of ≥ 5 hours to determine ketoacidosis as being clinically important; a sample size of 20 participants was needed in each group to provide a power of 0.93, given an alpha level of 0.05, a SD of 4, and a 1:1 inclusion ratio | ‐ | 20 | 20 | 20 | 100 | Mean hospital stay: 4 days |
| C: i.v. regular insulin | 20 | 20 | 20 | 100 | ||||
| total: | 40 | 40 | 40 | 100 | ||||
| Umpierrez 2004b | I1: s.c. insulin aspart, every hour | Arbitrary estimation of a difference between groups of ≥ 4 hours to determine ketoacidosis as being clinically significant. A sample size of 15 participants was needed in each group to provide a power of 0.81, given an alpha error of 0.05 and a SD of 3 | ‐ | 15 | 15 | 15 | 100 | Mean hospital stay: 3.4 days |
| I2: s.c. insulin aspart, every 2 h | 15 | 15 | 15 | 100 | Mean hospital stay: 3.9 days | |||
| C: i.v. regular insulin | 15 | 15 | 15 | 100 | Mean hospital stay: 4.5 days | |||
| total: | 45 | 45 | 45 | 100 | ||||
| Della Manna 2005 | I: s.c. insulin lispro | ‐ | ‐ | 25 | 25 | 25 | 100 | Mean hospital stay: 2‐3 days |
| C: i.v. regular insulin | 21 | 21 | 21 | 100 | ||||
| total: | 46 | 46 | 46 | 100 | ||||
| Ersöz 2006 | I: s.c. insulin lispro | ‐ | ‐ | 10 | 10 | 10 | 100 | ‐ |
| C: i.v. regular insulin | 10 | 10 | 10 | 100 | ||||
| total: | 20 | 20 | 20 | 100 | ||||
| Karoli 2011 | I: s.c. insulin lispro | ‐ | ‐ | 25 | 25 | 25 | 100 | Mean hospital stay: 6 days |
| C: i.v. regular insulin | 25 | 25 | 25 | 100 | Mean hospital stay: 6.6 days | |||
| total: | 50 | 50 | 50 | 100 | ||||
| Grand total | All interventions | 110 | 110 | |||||
| All comparators | 91 | 91 | ||||||
| All interventions and comparators | 201 | 201 | ||||||
| aAccording to power calculation in study publication or report ‐ denotes not reported C: comparator; I: intervention; i.v.: intravenous; s.c.: subcutaneous; SD: standard deviation | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to resolution of diabetic ketoacidosis Show forest plot | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.64, 0.90] |
| 2 All‐cause mortality Show forest plot | 4 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Hypoglycaemic episodes Show forest plot | 4 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.23, 1.52] |
| 3.1 Adults | 3 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.11, 3.94] |
| 3.2 Children | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.18, 1.72] |
| 4 Length of hospital stay Show forest plot | 2 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.97, 0.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to resolution of diabetic ketoacidosis Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Hypoglycaemic episodes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Length of hospital stay Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

