Cirurgia para câncer de mama metastático
Resumo
Introdução
O câncer de mama metastático não é uma doença curável, mas as mulheres com doença metastática estão vivendo por mais tempo. Em outros tipos de câncer metastático, a cirurgia para remover o tumor primário está associada a aumento da sobrevida. A cirurgia de mama não é um tratamento padrão para doença metastática, porém vários estudos retrospectivos recentes sugeriram que a cirurgia de mama poderia aumentar a sobrevida das mulheres. Estes estudos possuem limitações metodológicas, incluindo viés de seleção. Uma revisão sistemática que mapeia todos os ensaios clínicos randomizados que abordam os benefícios e os danos potenciais da cirurgia mamária seria importante para responder a esta pergunta.
Objetivos
Avaliar os efeitos da cirurgia mamária em mulheres com câncer de mama metastático.
Métodos de busca
Realizamos pesquisas utilizando os termos MeSH 'breast neoplasms', 'mastectomy', e 'analysis, survival' nas bases de dados Cochrane Breast Cancer Specialised Register, CENTRAL, MEDLINE (via PubMed) e Embase (via Ovid) em 22 de fevereiro 2016. Também buscamos no ClinicalTrials.gov e na WHO International Clinical Trials Registry Platform. Realizamos uma pesquisa adicional nos resumos dos congressos da American Society of Clinical Oncology (ASCO) até julho de 2016, que incluiu verificação de referências, pesquisa de citações e contato com autores de estudos para identificar pesquisas adicionais.
Critério de seleção
Os critérios de inclusão foram ensaios clínicos randomizados de mulheres com câncer de mama metastático no diagnóstico inicial, comparando cirurgia de mama mais terapia sistêmica versus terapia sistêmica isolada. Os desfechos primários foram sobrevida geral e qualidade de vida. Os desfechos secundários foram sobrevida livre de progressão da doença (controle local e a distância), sobrevida específica do câncer de mama e toxicidade da terapia local.
Coleta dos dados e análises
Dois revisores realizaram, de forma independente, a seleção dos estudos, a extração de dados e a avaliação do "risco de viés" (usando a ferramenta Risco de viés da Cochrane) e um terceiro autor de revisão verificou a avaliação. Utilizamos a ferramenta GRADE para avaliar a qualidade do conjunto de evidências. Utilizamos a razão de risco (RR) para medir o efeito do tratamento para desfechos dicotômicos e hazard ratio (HR) para os desfechos do tipo “tempo para o evento”. Calculamos intervalos de confiança (IC) de 95% para essas medidas. Utilizamos o modelo de efeitos randômicos para as análises, uma vez que esperávamos heterogeneidade clínica ou metodológica, ou ambas, entre os estudos incluídos.
Principais resultados
Incluímos dois estudos, com 624 mulheres no total. Ainda não está claro se a cirurgia de mama melhora a sobrevida global, pois a qualidade da evidência foi muito baixa para este desfecho (HR 0,83, IC 95% 0,53 a 1,31, dois estudos, 624 mulheres). Os dois estudos não relataram dados sobre qualidade de vida. A cirurgia de mama pode melhorar a sobrevida livre de progressão local (HR 0,22, IC 95% 0,08 a 0,57, dois estudos, 607 mulheres, evidência de baixa qualidade), mas provavelmente pode piorar a sobrevida livre de progressão a distância (HR 1,42, IC 95% 1,08 a 1,86; um estudo, 350 mulheres, evidência de qualidade moderada). Os dois estudos incluídos não avaliaram a sobrevida específica relacionada ao câncer de mama. A toxicidade da terapia local foi avaliada por meio da mortalidade após 30 dias, que foi semelhante entre os dois grupos (RR 0,99, IC 95% 0,14 a 6,90, um estudo, 274 mulheres, evidência de baixa qualidade).
Conclusão dos autores
Com base nas evidências de dois ensaios clínicos randomizados, não é possível fazer conclusões definitivas sobre os benefícios e riscos da cirurgia mamária associada ao tratamento sistêmico para mulheres diagnosticadas com câncer de mama metastático. Até que os ensaios clínicos em andamento sejam finalizados, a decisão de realizar cirurgia de mama nessas mulheres deve ser individualizada e compartilhada entre o médico e o paciente, considerando riscos, benefícios e custos potenciais de cada intervenção.
PICO
Resumo para leigos
Cirurgia para câncer de mama metastático (que se espalhou pelo corpo)
Pergunta clínica
Em mulheres com câncer de mama metastático (quando o câncer se espalhou para outras partes do corpo), qual é a eficácia da cirurgia mamária combinada com o tratamento clínico (como quimioterapia e terapia hormonal) em comparação com o tratamento clínico sozinho? Cirurgia mamária pode ser uma mastectomia (remoção da mama total, incluindo mamilo e aréola), ou a remoção do tumor e tecido mamário ao redor, mas preservando o mamilo e a aréola.
Introdução
O câncer de mama metastático é considerado uma doença incurável e com prognóstico ruim, embora algumas mulheres possam viver por muitos anos. É tradicionalmente tratado apenas com tratamento clínico (quimioterapia ou terapia hormonal). Acredita‐se que a cirurgia de mama seja paliativa e realizada apenas para aliviar sintomas, como sangramento local, infecção ou dor. Com o desenvolvimento de novos medicamentos, as mulheres com câncer de mama metastático passaram a viver por mais tempo e a cirurgia de mama pode beneficiar esse grupo de mulheres. Os dados retrospectivos sugerem que a cirurgia de mama poderia melhorar a sobrevida de mulheres com câncer de mama metastático. Porém, estudos do tipo retrospectivo são diferentes de ensaios controlados randomizados, e portanto são mais propensos a sofrer vieses.
Características dos estudos
Esta revisão está atualizada até fevereiro de 2016. Incluímos apenas ensaios clínicos randomizados (considerados o melhor tipo de estudo científico para responder a perguntas sobre tratamento) que compararam a sobrevida de mulheres com câncer de mama metastático submetidas a cirurgia de mama combinada com o tratamento clínico com a sobrevida das mulheres que receberam somente o tratamento clínico. Identificamos e incluímos dois ensaios clínicos randomizados, envolvendo um total de 624 mulheres: 311 foram submetidas a cirurgia de mama mais tratamento clínico e 313 mulheres receberam apenas tratamento clínico.
Principais resultados
Os autores da revisão não têm certeza se a cirurgia de mama melhora a sobrevida global, pois a qualidade da evidência foi avaliada como muito baixa. Os estudos incluídos não relataram qualquer informação relacionada à qualidade de vida. A cirurgia de mama pode melhorar o controle da doença local, mas provavelmente pode piorar o controle da doença em locais distantes (metástase). Os dois estudos incluídos não mediram a sobrevida específica relacionada ao câncer de mama. A toxicidade da terapia local pareceu ser a mesma nos dois grupos comparados.
O que isto significa?
Não é possível fazer conclusões definitivas sobre os benefícios da cirurgia mamária associada ao tratamento clínico para mulheres com câncer de mama metastático. A decisão de realizar cirurgia em tais casos deve ser individualizada e compartilhada entre o médico e a paciente, considerando os potenciais riscos e benefícios envolvidos nesta escolha. A inclusão dos resultados de ensaios em andamento na próxima atualização desta revisão ajudará a diminuir as incertezas existentes.
Authors' conclusions
Summary of findings
| Breast surgery plus systemic treatment compared to systemic treatment for metastatic breast cancer | ||||||
| Patient or population: metastatic breast cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with systemic treatment | Risk with breast surgery plus systemic treatment | |||||
| Overall survival at 2 years Follow‐up: range 23 months to 40 months | Study population | HR 0.83 | 624 | ⊕⊝⊝⊝ | The estimates for the control group are based upon an average of the estimates from Badwe 2015 and Soran 2016. | |
| 511 per 1000 | 448 per 1000 | |||||
| Quality of life | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Local PFS at 2 years Follow‐up: range 23 months to 40 months | Study population | HR 0.22 | 607 | ⊕⊕⊝⊝ | The estimates for the control group are based upon an average of the estimates from Badwe 2015 and Soran 2016. | |
| 500 per 1000 | 141 per 1000 | |||||
| Distant PFS at 2 years Follow‐up: 23 months | Study population | HR 1.42 | 350 | ⊕⊕⊕⊝ | The estimates for the control group are based upon the estimates from Badwe 2015. | |
| 548 per 1000 | 676 per 1000 | |||||
| Breast cancer‐specific survival | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Toxicity from local therapy Follow‐up: 40 months | Study population | RR 0.99 | 274 | ⊕⊕⊝⊝ | The estimates for the control group are based upon the estimates from Soran 2016. | |
| 15 per 1000 | 15 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1In Soran 2016, trial random sequence generation and allocation concealment were unclear. Downgraded one level. | ||||||
Background
Description of the condition
Breast cancer is one of the most frequently occurring cancers in women. An estimated 1.67 million new cases of breast cancer are diagnosed worldwide each year, accounting for 25% of all diagnosed cancers (GLOBOCAN 2012). Between 20% and 30% of women with breast cancer will develop synchronous or metachronous distant metastases, leading to an estimated 400,000 to 500,000 deaths per year worldwide (Caudle 2012). At the moment of their initial diagnosis, 3.5% of all women with breast cancer in the United States already have distant metastatic disease, and this percentage is higher in low‐ and middle‐income countries (Khan 2002). This means that every year approximately 50,000 women will receive an initial diagnosis of metastatic breast cancer (Ly 2010).
Although metastatic breast cancer is considered to be an incurable disease with a poor prognosis, women with this diagnosis now live longer, and the survival rate at five years has increased from 10% in 1970 to about 40% in women treated after 1995 (Giordano 2004). Women with metastatic breast cancer treated between 1995 and 2002 had an 18% lower risk of death than women treated between 1985 and 1994 (Ernst 2007). Median overall survival has improved from 20 months (1988 to 1991) to 26 months (2007 to 2011) in another series (Thomas 2015). Increased survival also increases the risk of local symptoms in women who have not undergone breast surgery to remove the primary tumour.
The treatment of breast cancer can also have a negative impact on a woman's quality of life and is associated with fears such as the future development of the disease in their daughters, job loss, and decreased sexual desire (Ferrel 1997). One study that evaluated the quality of life of women with metastatic breast cancer showed that they gave greater importance to treatments that prolonged disease‐free survival than treatments that prolonged survival (Hurvitz 2013). Although most breast cancer survivors rate their quality of life as being positive, they often complain about adaptation and psychosocial problems rather than physical deficits (Sales 2001). A multidisciplinary team should become familiar with these issues in order to offer a better rehabilitation programme for these women.
Description of the intervention
Historically, women diagnosed with metastatic breast cancer were not treated surgically and received only systemic therapy (Bermas 2009). Over the last decade, these women have been living longer. Surgical resection of the primary tumour was believed to be palliative and performed only to relieve symptoms such as bleeding, infection, or pain. This therapeutic approach was based on the premise that local treatment in metastatic breast cancer did not improve overall survival. However, in women with metastatic renal cell carcinoma, two prospective randomised controlled trials showed that local surgery plus systemic therapy led to longer survival rates than systemic therapy alone (Flanigan 2001; Mickisch 2001). Another recent study showed that local surgery was beneficial for people with metastatic colorectal cancer (Anwar 2012). This has led to the hypothesis that local treatment in metastatic breast cancer can also improve survival.
In recent years, elective surgical resection of the primary breast tumour before the onset of local symptoms has become more popular. Morrogh and colleagues reported a significant increase in the number of women with metastatic breast cancer having breast surgery between 2000 and 2005 compared with 1995 and 2000 (Morrogh 2008). Another study using the American Surveillance, Epidemiology, and End Results (SEER) database showed a decrease in breast surgery performed on women with metastatic breast cancer from 67.8% in 1988 to 25.1% in 2011 (Thomas 2015).
The intervention consists of the surgical removal of the breast tumour, either by conservative (lumpectomy) or radical surgery (mastectomy). The surgical treatment is complemented with the evaluation of axillary disease through the technique of sentinel lymph node or lymphadenectomy. Thoracic radiotherapy may be part of locoregional treatment.
How the intervention might work
Cancer cells can migrate to other organs, initiate cell division, and alter the tumour microenvironment favouring the growth of metastatic foci. Because the primary breast tumour can be a reservoir of cancer stem cells, its removal can decrease the probability of developing new metastatic sites (Bermas 2009). Moreover, the primary tumour can secrete growth factors such as tumour‐growth factor (TGF)‐β, which can send signals that favour implantation and growth of metastatic sites (Karnoub 2007). Studies in animal models suggest that resection of the primary breast tumour in mice can restore immunocompetence of the host (Danna 2004). Another experimental study in mice showed that after resection of the primary tumour there was a reduction in the growth of metastatic tumours, suggesting that the removal of the primary tumour is not only a local phenomenon (Fisher 1989).
Retrospective data suggest that the surgical removal of the primary tumour may improve overall survival in metastatic breast cancer (Blanchard 2008; Fields 2007; Gnerlich 2008; Khan 2002; Rapiti 2006). Achieving negative margins when surgery is used to treat the primary tumour appears to be an important prognostic marker for survival (Khan 2002; Rapiti 2006). Some subgroups of women with oligometastatic disease appear to experience benefits with breast surgery (Di Lascio 2014; Rastogi 2014). Since improvements in systemic therapy have prolonged the survival of women with metastatic breast cancer, surgery for the primary disease may reduce the risk of symptomatic local disease and potentially increase survival. The potential benefits of breast surgery for metastatic breast cancer need to be balanced against both perioperative complications and costs (McNeely 2012).
Why it is important to do this review
According to a meta‐analysis of retrospective studies, women with metastatic breast cancer who underwent surgical resection of the primary tumour had better overall survival compared with those who did not (Petrelli 2012; Ruiterkamp 2010). In another meta‐analysis, women who underwent breast surgery had a better survival rate at three years than women who did not have surgery (Harris 2013). However, all of these studies included in the meta‐analyses were retrospective and had the typical limitations of this design, including selection and performance bias. Surgeons also tended to perform surgical resection in women with metastatic disease who had a better prognosis, that is usually younger women with a good performance status and oligometastatic disease, who would have survived longer anyway.
Randomised controlled trials (RCTs) are the ideal study design to reduce uncertainties and clarify whether surgery is beneficial for women diagnosed with metastatic breast cancer. There are several ongoing trials evaluating breast surgery for metastatic breast cancer, which are including women with metastatic breast cancer and randomising women to either breast surgery combined with systemic treatment or systemic treatment alone. The results of some of these trials (e.g. NCT00193778 and NCT00557986) are expected to become available in the coming years. A systematic review including RCTs would provide a comprehensive summary of the current evidence on breast surgery for women with metastatic breast cancer.
Objectives
To assess the effect of breast surgery on women with metastatic breast cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). We considered studies reported as full text, published as abstract only, and unpublished data.
Types of participants
Women with metastatic breast cancer at initial diagnosis: TNM (tumour, lymph nodes, metastases) stage IV (Sobin 2002). This includes when breast cancer has spread beyond the breast, chest wall, and regional nodes. We applied no restrictions regarding age or histological type. If a study contained a subset of eligible participants, we would include them in the review as long as we could extract the relevant results.
Types of interventions
-
Intervention group: breast surgery of the primary tumour, in addition to systemic therapy (chemotherapy, endocrine therapy, immunotherapy or biological agents).
Breast surgery included lumpectomy (tumour resection with safety margin) or mastectomy (removal of all breast tissue). Management of the axilla could include sentinel node biopsy with clinically negative axilla, and axillary dissection (levels I or II only, or levels I, II, and III axillary clearance) with clinically compromised axilla or positive sentinel lymph node.
Systemic therapy included chemotherapy, endocrine therapy, immunotherapy or biological agents, and other novel therapies. Any sequencing of therapies was permitted in both arms. Surgery could have been performed before or after systemic therapy.
Radiotherapy routinely performed as part of locoregional breast cancer treatment was acceptable. Radiotherapy could have been performed in the breast or chest wall, and supraclavicular fossa and ipsilateral axilla, according to institutional guidelines. This could have included a boost using electron interstitial therapy or external beam and new techniques. Radiation therapy for pain relief or palliation was also allowed, in local or distant disease.
-
Comparison group: systemic therapy without breast surgery.
In the control group, breast surgery was not performed. We allowed resection of the primary tumour only for palliative treatment, such as bleeding, ulceration, infection, pain, or local progression.
If co‐interventions such as chemotherapy were used, they needed to be applied equally to each study group.
Types of outcome measures
We included studies that assessed at least one of the outcomes listed below.
Primary outcomes
-
Overall survival, defined as the time from date randomised to date of death (any cause).
-
Quality of life, assessed through validated questionnaires such as the European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐BR23, EORTC QLQ‐BR23, and BREAST‐Q, BREAST‐Q, questionnaires or others described in the clinical trial.
Secondary outcomes
-
Progression‐free survival (PFS), defined as the time from randomisation to the date of objective tumour progression or death. We analysed local and distant tumour progression (local control and distant control) as below:
-
local PFS, defined as the time from randomisation to local recurrence in the intervention group and as local tumour progression in the control group;
-
distant PFS, defined as the time from randomisation to progression outside local site and lymph nodes.
-
-
Breast cancer‐specific survival, defined as the time from randomisation to breast cancer death. Women with death due to other causes than breast cancer were censored.
-
Toxicity from local therapy, including local and systemic complications.
We assessed the time‐to‐event outcomes (overall survival, PFS, and breast cancer‐specific survival) at the end of the follow‐up period. We assessed the outcomes quality of life and toxicity from local therapy at short‐ (up to six months), intermediate‐ (from six to 24 months), and long‐term (more than 24 months).
Search methods for identification of studies
See: Breast Cancer Group for search methods used in reviews.
There were no language restrictions for the studies included in this systematic review. We undertook full translations of all non‐English language papers using local resources.
Electronic searches
We searched the following databases on the 22 February 2016:
-
The Cochrane Breast Cancer Group (CBCG) Specialised Register. Details of search strategies used by the CBCG for the identification of studies and the procedures to code references are outlined in the CBCG's module (onlinelibrary.wiley.com/o/cochrane/clabout/articles/BREASTCA/frame.html). Trials with the keywords 'advanced breast cancer', 'metastatic breast cancer', 'breast surgery', 'breast‐conserving surgery', 'mastectomy', 'lumpectomy', 'segmentectomy', were extracted and considered for inclusion in the review.
-
The Cochrane Central Register of Controlled Trials (CENTRAL, Issue 1, 2016). See Appendix 1.
-
MEDLINE (via PubMed). See Appendix 2.
-
Embase. See Appendix 3 (via Embase.com for first search) and Appendix 4 (via OvidSP for top‐up search).
-
The World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/Default.aspx) for all prospectively registered and ongoing trials. See Appendix 5.
-
ClinicalTrials.gov (clinicaltrials.gov/). See Appendix 6.
Searching other resources
We also searched for additional studies in the reference lists of identified trials and reviews. We obtained a full copy of each article reporting a potentially eligible trial.
We contacted the authors of the primary studies to seek unpublished data or additional information about the outcomes of interest.
We conducted an additional search in the American Society of Clinical Oncology (ASCO) conference proceedings in July 2016.
Data collection and analysis
Selection of studies
Two review authors (GT and BSM) independently screened the titles and abstracts to identify potentially relevant RCTs. We then obtained full‐text articles of all potentially relevant citations. Any disagreements regarding RCT selection were resolved by consulting a third review author (RR). There were no restrictions regarding language or reporting status. We summarised the excluded studies in the Characteristics of excluded studies table. The entire study selection process was reported according to PRISMA guidelines (Moher 2009).
Data extraction and management
Two review authors (GT and BSM) independently conducted full data extraction, consulting a third review author (RR) to help resolve any disagreements. We contacted the authors from primary studies to seek unpublished data or additional information about the outcomes of interest (Badwe 2015; Soran 2016). We developed and piloted the data extraction forms, which included the following information from individual studies:
-
publication details;
-
study design, study setting, inclusion/exclusion criteria;
-
participant population (e.g. age, type of surgical procedure, type of tumour);
-
details of intervention;
-
outcome measures;
-
withdrawals, length and method of follow‐up, and the number of participants followed up.
When available, we pooled quantitative data and carried out the analyses using Review Manager 5 software (RevMan 2012).
In the case of studies with more than one publication, we extracted data from all publications and used them to fill in the same data extraction form, since the unit of analysis was the study rather than the publication. The primary reference was the first publication.
Assessment of risk of bias in included studies
We assessed risk of bias using Cochrane's 'Risk of bias' tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Two review authors (GT and BSM) rated the studies on all seven domains of the 'Risk of bias' tool, consulting a third review author (MRT or RR) to help resolve disagreements. For each domain, we classified the study as having a low, unclear, or high risk of bias. We evaluated the following seven domains:
-
random sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessors;
-
incomplete outcome data;
-
selective reporting;
-
other bias.
We took into account the risk of bias in the Authors' conclusions.
Measures of treatment effect
We reported time‐to‐event outcomes such as overall survival, PFS, and breast cancer‐specific survival as hazard ratios (HR) with 95% confidence interval (CI). If necessary, we indirectly estimated HRs using the methods described by Parmar and colleagues (Parmar 1998). We performed intention‐to‐treat analyses.
We intended to report continuous outcomes such as quality of life as mean differences (MD) with 95% CIs. We planned to use standardised mean differences (SMD) if continuous outcomes were reported in different scales.
We reported dichotomous outcomes such as toxicity as risk ratios (RR).
In cases where it was not possible to pool data through meta‐analysis, we presented outcome data narratively.
In an ASCO consensus meeting, the members considered that relevant improvements in median overall survival of at least 20% are necessary to define a clinically meaningful improvement in outcome. They considered HR < 0.8, corresponding to an improvement in median overall survival within a range of 2.5 to 6 months, as the minimum incremental improvement over standard therapy that would define a clinically meaningful outcome (Ellis 2014). We used this definition in the review.
Unit of analysis issues
The unit of analysis was the individual participant, rather than surgical unit, hospital, or centre.
Dealing with missing data
Where data were missing or unsuitable for analyses (e.g. intention‐to‐treat data were not available), we contacted the study authors to request further information. If data could not be obtained, we did not include the study in the meta‐analysis and discussed these results in the review.
Assessment of heterogeneity
First, we inspected heterogeneity graphically using forest plots displaying effects of individual studies with 95% CIs.
If appropriate, we assessed heterogeneity between studies using the Chi2 statistic (considering a P value < 0.10 as significant). We also used the I2 statistic as an approximate guide to interpret the magnitude of heterogeneity: an I2 value between 30% and 60% was indicative of moderate heterogeneity, while values greater than 50% were considered substantial heterogeneity (Higgins 2011).
We investigated the following factors as potential causes of heterogeneity in the included studies, using the framework described below.
-
Clinical heterogeneity: related to study location and setting, full characteristics of participants, comorbidity, and treatments that women may be receiving at trial entry. We considered how outcomes were measured, the definition of outcomes, and how they were recorded.
-
Methodological heterogeneity: related to randomisation process and overall methodological quality of primary studies.
Assessment of reporting biases
We planned to create a funnel plot to explore the risk of reporting bias in the case of a meta‐analysis including at least 10 trials. However, none of the meta‐analyses achieved this number of studies. We also planned to perform exploratory analyses to investigate possible reasons for visual asymmetry of funnel plot (chance, publication bias, and true heterogeneity).
Data synthesis
We synthesised data using Review Manager 5 software (RevMan 2012). To estimate the effect size, we planned to use the MD or SMD for continuous outcomes. We used the HR for time‐to‐event outcomes and RR for dichotomous outcomes. We used the inverse variance method to estimate the combined effect size for the outcomes. We used the random‐effects model by default, as we expected clinical or methodological heterogeneity, or both, among the included studies. Where the data were too diverse for combining effect sizes in a meaningful or valid manner, we presented the results of individual studies in table and graphical format and used a narrative approach to summarise data.
'Summary of findings' table
We created a 'Summary of findings' table (summary of findings Table for the main comparison) using the following outcomes: overall survival, quality of life, local PFS, distant PFS, breast cancer‐specific survival, and toxicity from local therapy.
We used the five GRADE criteria (risk of bias, inconsistency, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome stated above. We followed the methods presented in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro software (GRADEpro 2014). We explained each judgement to downgrade or upgrade the quality of evidence in the footnotes.
Subgroup analysis and investigation of heterogeneity
As we expected there to be a small number of published studies, we anticipated that subgroup analyses would not be feasible. Whenever possible, we considered the following subgroup analyses:
-
age > 55 years or < 55 years;
-
oestrogen receptor (ER) status (Lang 2013; Neuman 2010; Perez‐Fidalgo 2011; Petrelli 2012; Samiee 2012);
-
HER2 status (Neuman 2010; Samiee 2012);
-
only bone metastases (Rapiti 2006; Rhu 2015);
-
radiotherapy at primary site or not (Bourgier 2009; Le Scodan 2009).
We based the choice of these planned subgroups on published evidence that the intervention effect could be modified by these characteristics.
Sensitivity analysis
We intended to conduct the following sensitivity analyses:
-
by excluding trials at high risk of bias in more than four of the seven domains;
-
by removing studies with eligibility criteria that differed markedly from most of the included studies;
-
by excluding studies in which it was necessary to re‐estimate HRs and CIs using other accepted methodologies;
-
by excluding studies that used any imputation methods for missing data
The sensitivity analysis was not possible due to the small number of studies, but will be considered for future updates of this review.
Results
Description of studies
Results of the search
Our search strategy identified 4644 records, and two additional trials were identified through manual search. After removing duplicates, we screened the titles and abstracts of 4409 records, excluding 4399 records and selecting 10 for full‐text reading. We excluded two of these 10 records: one was an observational study and one was terminated due to lack of accrual and no further funding (NCT01906112; Ruiterkamp 2012). Four studies were ongoing trials (NCT01015625; NCT01242800; NCT02125630; UMIN000005586). We therefore included four records reporting the results of two RCTs in the review. Both RCTs were included in the quantitative and qualitative syntheses of this review (Badwe 2015; Soran 2016). The flow diagram of the process of study identification and selection is presented in Figure 1.

Study flow diagram.
Included studies
The two included studies have the same participants (women with metastatic breast cancer), the same intervention (breast surgery), and the same main outcome (overall survival).
The main difference between the studies was that the Indian study included only women who had responded to systemic therapy (Badwe 2015), while the Turkish study included women with metastatic breast cancer without previous treatment (Soran 2016). Soran 2016 randomised women to upfront breast surgery followed by systemic therapy versus systemic therapy, while Badwe 2015 enrolled women who had responded to first‐line anthracycline‐based chemotherapy and randomised them to breast surgery or continuing medical therapy. By excluding those women who did not respond to chemotherapy, Badwe 2015 excluded the cases of worse prognosis, which did not occur in Soran 2016.
Another important difference between the two studies was the fact that most HER2‐positive women were not treated with anti‐HER2 therapy in Badwe 2015. The fact that only 9 out of 107 women with HER2 overexpression received HER2‐targeted treatment could have influenced the results. Moreover, eight of the nine women who received anti‐HER2 therapy did so only after disease progression (Badwe 2015). In Soran 2016, all women with HER2 overexpression received trastuzumab. The benefits of anti‐HER2 therapy on response rate, PFS, and overall survival are well known since 2001 (Slamon 2001). The combination of trastuzumab and chemotherapy has changed the aggressive natural history of metastatic breast cancer with HER2 overexpression (Dawood 2009).
Another important fact is that the groups were not well balanced in Soran 2016. The group undergoing breast surgery had a larger proportion of women who had tumours that were ER‐positive and HER2‐negative, were younger than 55 years of age, and had single bone metastases compared to the systemic therapy‐alone group. This may have improved the prognosis of the breast surgery group and influenced the results. On the other hand, the two treatment groups in Badwe 2015 were well balanced.
Additional details of the two included RCTs are presented in the Characteristics of included studies table.
Design
Both studies were superiority randomised clinical trials with parallel design (Badwe 2015; Soran 2016).
Sample sizes
To estimate sample sizes, the trial authors considered the following factors.
-
Badwe 2015 considered an improvement in overall survival from 18 to 24 months for breast cancer surgery. Using these premises and considering a P < 0.05 and 80% power, 350 women would be necessary. A total of 716 women were recruited, and 691 women were eligible for systemic chemotherapy. The number of women responding to chemotherapy was 415, and over 25 women were eligible for endocrine therapy. Of the 440 eligible women, 90 were not suitable for surgery or declined to participate. A total of 350 women were randomised: 173 women underwent breast surgery, and 177 women did not.
-
Soran 2016 based the calculations on an expected difference in overall survival between the two groups of 18% (based on previous retrospective studies), a 10% dropout rate including lost to follow‐up, an alfa = 0.05, and a 90% power. This resulted in a total of 271 women. A total of 312 women were recruited, of whom 19 were excluded. Out of 293 eligible women, 19 withdrew or were lost to follow‐up. Of the 274 available women, 138 had breast surgery and systemic treatment, and 136 received systemic treatment alone.
Participants
The two studies evaluated a total of 624 women with metastatic breast cancer. The average age of the women was 49 years. There were 426 women with ER‐positive tumours; 200 women with ER‐negative tumours; 192 women with HER2‐positive tumours; 421 with HER2‐negative tumours; and 226 women with bone‐only metastases.
Badwe 2015 included women with metastatic breast cancer with objective response to first‐line chemotherapy (> or = 50% clinical response). Women were stratified by site of metastases (visceral only, bone only, or visceral plus bone), number of metastases (≤ 3 or > 3), and hormone receptor expression sensitivity (ER‐ or progesterone receptor (PR)‐positive or ER‐ and PR‐negative), and then randomised to receive breast surgery plus further systemic treatment or systemic treatment alone.
Soran 2016 enrolled treatment‐naïve women with resectable primary tumour and randomly assigned women to upfront surgery followed by systemic therapy or systemic therapy alone.
Interventions
Surgery
The intervention was the surgical resection of the tumour with safety margins through mastectomy or conservative surgery. The assessment of axillary involvement was performed at the same time as the breast surgery. Soran 2016 performed sentinel lymph node biopsy in women without clinical disease in the lymph nodes and performed lymphadenectomy in women with proven (previous axillary biopsy or positive sentinel node) or clinically affected armpit. In Badwe 2015, all women underwent axillary lymph node dissection and additional supraclavicular lymph node dissection for suspected disease.
Radiotherapy
Postoperative radiotherapy was performed in all women who underwent breast‐conserving surgery in both studies. In Badwe 2015, postoperative radiation was given to women who underwent mastectomy with pre‐chemotherapy tumours over 5 cm or skin or chest wall involvement or axillary lymph node‐positive disease. Conventional external beam radiotherapy was delivered to the chest wall with or without the supraclavicular fossa at a dose of 45 Gy, 20 fractions over 4 weeks. Women undergoing breast‐conserving surgery received whole‐breast radiotherapy in one of two ways: 45 Gy, 25 fractions over 5 weeks with a tumour bed boost of 15 Gy, 6 fractions over 1 week; or 50 Gy, 25 fractions over 5 weeks with a tumour bed boost of 15 Gy, 6 fractions over 1 week in locally advanced cancers (Badwe 2015). In Soran 2016, radiotherapy was given to women undergoing mastectomy depending on the extent of the disease and following each institutional guideline. Radiation therapy fields, dosing, and schedule were not described (Soran 2016).
Chemotherapy and targeted therapy
Most women in both studies received anthracycline‐based chemotherapy.
In Badwe 2015, women underwent chemotherapy before randomisation, and only those who had clinical response greater than or equal to 50% were included. Chemotherapy comprised six cycles of anthracycline‐based or eight cycles of a sequential anthracycline‐taxane regimen or six cycles of concurrent anthracycline‐taxane chemotherapy. About 95% of women received anthracycline‐based combination chemotherapy. Anti‐HER2 treatment was available to a small number of women; just 9 of 107 with HER2‐positive tumours received HER2 target treatment (Badwe 2015).
In Soran 2016, all women received upfront surgery followed by chemotherapy or upfront chemotherapy. Approximately 80% of women received anthracycline‐based chemotherapy. Trastuzumab was available to all HER2‐positive women (Soran 2016).
Endocrine therapy
Endocrine therapy was available to all ER‐positive breast cancer patients in both studies. In Badwe 2015, women with ER‐ or PR‐positive tumours received standard endocrine therapy after locoregional treatment. The treatment consisted of tamoxifen 20 mg per day for premenopausal women and an aromatase inhibitor (2.5 mg letrozole per day or 1 mg anastrozole per day) or tamoxifen for postmenopausal women, until disease progression. In the intervention group, premenopausal women with ER‐ or PR‐positive tumours who continued to have menstrual cycles after chemotherapy, had bilateral oophorectomy at the time of the surgical removal of their primary tumour. In the group without breast surgery, women with ER‐ or PR‐positive tumours who continued to have menstrual cycles after chemotherapy also underwent bilateral oophorectomy followed by hormone therapy treatment as previously described (Badwe 2015). Soran 2016 did not specify which endocrine therapy was used.
Follow‐up
The median follow up was 23 months in Badwe 2015 and 40 months in Soran 2016. The number of deaths precluded mature data for overall survival.
Excluded studies
We excluded two studies; the reasons for exclusion are presented in the Characteristics of excluded studies table.
Risk of bias in included studies
We used Cochrane’s tool for assessing risk of bias as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). See the 'Risk of bias' summary in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Selection bias
The two studies were described as randomised clinical trials. Badwe 2015 used a computerised stratified randomisation, and allocation concealment was ensured by a central office. These procedures minimised the risk of selection bias in this study. Soran 2016 described verbatim "a phase III randomised controlled trial". Despite several attempts to contact the authors, we were unable to obtain further explanation. We judged Soran 2016 as having an unclear risk of selection bias.
Blinding
Performance and detection bias
Blinding was not feasible in either study due to the surgical procedure planned in the interventional arm. The lack of blinding of the outcome assessor may have influenced the results for subjective outcomes (high risk of bias) but not for overall survival (low risk of bias).
Incomplete outcome data
Attrition bias
In both studies, there was no information about missing/censored data, and we classified them as having an unclear risk of bias for this domain.
Selective reporting
Reporting bias
The study protocols were available for both studies, and the primary outcomes were fully reported. The secondary outcomes have not been reported so far, but we did not consider this as a potential source of reporting bias.
Other potential sources of bias
There was an imbalance between groups in Soran 2016. In the group undergoing breast surgery, there was a higher proportion of women who were younger than 55 years, had ER‐positive and HER2‐negative tumours, and had single bone metastases. It is likely that these differences between the two groups could have influenced the result. We therefore considered Soran 2016 as having a high risk of bias for this domain.
Effects of interventions
Overall survival
As the quality of evidence was judged as very low, it is uncertain whether breast surgery plus systemic treatment improves overall survival compared to the systemic treatment alone (HR 0.83, 95% CI 0.53 to 1.31; 2 studies; 624 women; I2 = 82%; very low‐quality evidence, downgraded due to study limitations, inconsistency, and imprecision) (Analysis 1.1; Figure 3). The estimated number of deaths was 448 per 1000 participants (ranging from 318 to 608 deaths per 1000 participants) in the breast surgery plus systemic treatment group and 511 per 1000 participants in the systemic treatment‐alone group (summary of findings Table for the main comparison).
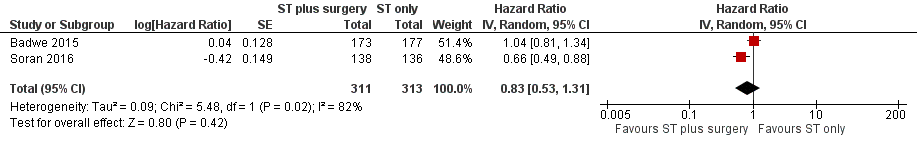
Forest plot of comparison: 1 Systemic treatment plus surgery versus systemic treatment, outcome: 1.1 Overall survival.
Overall survival subgroup analysis
Refer to 'Overall survival ‐ subgroup analyses' (Table 1).
| Overall survival subgroup analysis | Number of studies | N | HR | Lower CI | Upper CI | P value |
| HER2‐positive | 2 | 192 | 0.90 | 0.60 | 1.35 | NS |
| HER2‐negative | 2 | 421 | 0.85 | 0.67 | 1.08 | NS |
| ER positive | 2 | 426 | 0.79 | 0.61 | 1.03 | NS |
| ER negative | 2 | 200 | 1.01 | 0.73 | 1.40 | NS |
| Bone‐only metastasis | 2 | 226 | 0.91 | 0.49 | 1.69 | NS |
CI: confidence interval
ER: oestrogen receptor
HR: hazard ratio
NS: not significant
HER2 status
For both HER2‐positive and ‐negative subgroups, the results were consistent with the main analysis:
-
HER2‐positive: HR 0.85 (95% CI 0.48 to 1.50; 2 studies; 192 women; I2 = 39%; Analysis 1.2);
-
HER2‐negative: HR 0.84 (95% CI 0.50 to 1.40; 2 studies; 421 women; I2 = 79%; Analysis 1.2).
There was no evidence of a difference in overall survival between HER2‐positive and HER2‐negative subgroups (Chi2 = 0.00, df = 1 (P = 0.97), I2 = 0%).
Oestrogen receptor status
For both ER‐positive and ‐negative subgroups, the results were consistent with the main analysis:
-
ER‐positive: HR 0.83 (95% CI 0.48 to 1.42; 2 studies; 426 women; I2 = 74%; Analysis 1.3);
-
ER‐negative: HR 1.04 (95% CI 0.70 to 1.55; 2 studies; 200 women; I2 = 19%; Analysis 1.3).
There was no evidence of a difference in overall survival between ER‐positive and ER‐negative subgroups (Chi2 = 0.44, df = 1 (P = 0.51), I2 = 0%).
Only bone metastasis
For the subgroup of women with bone metastasis, there was no difference between the interventions: HR 0.91 (95% CI 0.49 to 1.69; 2 studies; 226 women; I2 = 70%; Analysis 1.4).
Radiotherapy or no radiotherapy at the primary site
Not measured in included studies.
Quality of life
The included studies did not report this outcome. Quality of life was described as an outcome in the protocols of both studies; we expect these results to be published soon.
Progression‐free survival
Local PFS
The two included studies evaluated local PFS involving 607 women, and breast surgery plus systemic treatment may improve local PFS when compared to systemic treatment alone (HR 0.22, 95% CI 0.08 to 0.57; 2 studies; 607 women; I2 = 43%; low‐quality evidence) (Analysis 1.5;Figure 4). The estimated number of events was 141 per 1000 participants (ranging from 54 to 326 events per 1000 participants) in the breast surgery plus systemic treatment group and 500 in 1000 participants in the systemic treatment‐only group. We downgraded the quality of the evidence due to study limitations and inconsistency. When considering the width of the confidence interval, the result is clinically relevant.

Forest plot of comparison: 1 Systemic treatment plus surgery versus systemic treatment, outcome: 1.5 Progression‐free survival.
Distant PFS
Only Badwe 2015 analysed distant PFS. The group receiving breast surgery plus systemic treatment probably had a shorter time to distant PFS compared to the group receiving systemic treatment alone (HR 1.42, 95% CI 1.08 to 1.86; 1 study; 350 women; moderate‐quality evidence; Analysis 1.5; Figure 4). The estimated number of events was 676 per 1000 participants (ranging from 576 to 772 events per 1000 participants) in the breast surgery plus systemic treatment group and 548 in 1000 participants in the systemic treatment‐only group. We downgraded the quality of the evidence due to study limitation (lack of blinding of outcome assessors).
Breast cancer‐specific survival
The included studies did not measure this outcome.
Toxicity from local therapy
Soran 2016 reported that toxicity (assessed by 30‐day mortality) did not appear to differ between the breast surgery plus systemic treatment group and systemic treatment‐only group (RR 0.99, 95% CI 0.14 to 6.90; 1 study; 274 women; low‐quality evidence). We downgraded the quality of the evidence due to study limitation (lack of blinding of outcome assessors).
Discussion
Summary of main results
We are uncertain as to whether breast surgery improves overall survival in women with metastatic breast cancer due to the very low‐quality evidence. Surgery may improve local PFS (low‐quality evidence) and worsen distant PFS (moderate‐quality evidence). We found no data for breast cancer‐specific survival. Toxicity from local therapy (measured by 30‐day mortality) appears to be similar in both groups (low‐quality evidence) based on one study.
Women with metastatic breast cancer are a heterogeneous group of patients with different prognoses. Women with minimal metastatic disease have better survival than those with multiple organs affected by metastases (Badwe 2015). Badwe 2015 included symptomatic women with probably later diagnosis in the natural course of de novo metastatic disease. While 74% of the women included in the Indian study had more than three metastatic sites (Badwe 2015), in the Turkish study this percentage was 40% (Soran 2016). Studies from countries such as the United States and Europe may include women with metastatic disease at an earlier stage, and the effect of breast surgery in these women may be different.
Systemic treatment is effective in the control of breast and metastatic disease with an impact on overall survival (Kiess 2012; Slamon 2001). The large majority of women in both studies received anthracycline‐based chemotherapy: 80% in Soran 2016 and 95% in Badwe 2015. Only 5% of women in Badwe 2015 received anthracycline plus taxane. In the Turkish study (Soran 2016), all women with tumour HER2 overexpression received trastuzumab, while only 8.5% received anti‐HER2 therapy in the Indian study (Badwe 2015); therefore 98 (15%) women included in the meta‐analysis did not receive adequate systemic treatment. The Clinical Evaluation of Pertuzumab and Trastuzumab (CLEOPATRA) study showed survival gain with the use of double blockage (trastuzumab plus pertuzumab) when compared to the use of only one monoclonal antibody, as first‐line therapy (Swain 2015). The non‐use of anti‐HER2 therapy thus reduced the overall survival of these women and also decreased the chance of a possible benefit from breast surgery.
We did not expect that the local treatment would bring a large size benefit to the survival of women with metastatic breast cancer. We make a parallel with the effect of the addition of radiotherapy to the treatment of conservative breast surgery. The first publications already reported that radiotherapy showed a benefit in local disease control, but only the intervention showed a benefit in survival only after a recent meta‐analysis with a large numbers of participants and longer follow‐up (Darby 2011).
Breast surgery was shown to be effective in controlling the local disease and halved the number of deaths due to uncontrolled local disease (6% died of locally uncontrolled disease in the group with no locoregional treatment versus 3% in the group with locoregional treatment) (Badwe 2015). The rate of salvage breast surgery for local progression treatment in the control group was 10.2% (18 out of 177) in Badwe 2015 and 3.6% (5 out of 138) in Soran 2016. It is important to emphasise the low toxicity of local treatment and the patients' point of view that the surgery reduces the risk of the need for a late intervention in the course of the disease. Distant PFS was worse in women undergoing breast surgery. The time that these women were left without systemic therapy due to surgery may have contributed to this outcome.
The overall quality of the evidence is low due to study limitations, inconsistency, and imprecision. It is likely that further research could have an important impact on the effect estimate and will probably change the estimate.
Overall completeness and applicability of evidence
The women included in the Indian study were at an advanced stage of the disease and were mostly symptomatic (Badwe 2015). This patient profile is most often found in countries where the health system and screening programs are not effective. We believe that studies from high‐income countries may include women with less advanced stages of the disease, which could influence the effects of the intervention.
We hope to include data on quality of life in future updates of this review. This outcome is extremely important for decision‐making.
Quality of the evidence
As presented in summary of findings Table for the main comparison, the quality of the body of evidence for each outcome was moderate to very low.
The main reasons for downgrading the quality of evidence were high heterogeneity between studies (inconsistency), wide CIs (imprecision), and the risk of selection bias for one study (risk of bias).
For the outcome overall survival, we downgraded the quality to very low due to: (a) unclear random sequence generation and allocation concealment in Soran 2016; (b) high clinical and statistical heterogeneity between studies (I2 = 82%); and (c) very wide confidence intervals (95% CI 0.53 to 1.31) (GRADEpro 2014).
For the outcome local PFS, we downgraded the quality of the evidence to low due to: (a) unclear random sequence generation and allocation concealment in Soran 2016; and (b) high clinical and statistical heterogeneity between studies (I2 = 42%) (GRADEpro 2014).
For the outcome distant PFS, we downgraded the quality of the evidence to moderate due to study limitations (outcome assessors were not blinded, and this is a subjective outcome) (GRADEpro 2014).
For the outcome toxicity from local therapy, we downgraded the quality of the evidence to low due to: (a) unclear random sequence generation and allocation concealment in Soran 2016; and (b) wide confidence intervals (95% CI 0.14 to 6.9) (GRADEpro 2014).
Additional research with longer follow‐up periods is therefore likely to have an impact on the effect estimates.
Since there are four ongoing studies, it is possible that some of these limitations can be minimised in the update of this review.
Potential biases in the review process
To minimise the risk of bias, we strictly followed the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions on searching, study selection, data collection, and data analysis (Higgins 2011). One of the strengths of this review is its broad and updated literature search.
The limitations of this review include: (a) no assessment of publication bias through funnel plot analysis because fewer than 10 studies were included in the meta‐analysis; (b) some subgroup analyses were not planned in the protocol phase; and (c) typical limitations of aggregate‐data meta‐analysis, where individual patient data are absent, and subgroup analysis is usually underpowered.
Agreements and disagreements with other studies or reviews
Although thousands of women are diagnosed with metastatic breast cancer annually, to the best of our knowledge, this is the first systematic review with meta‐analysis of randomised trials on the effects of local surgery on these cases.There are a few systematic reviews of observational studies showing overall survival benefit for women with metastatic breast cancer undergoing breast surgery. These studies have the inherent limitations of observational designs and are not the most appropriate type of studies to assess the effectiveness of an intervention. This systematic review is in disagreement with retrospective studies showing benefit in survival for women undergoing breast surgery (Blanchard 2008; Fields 2007; Gnerlich 2008; Khan 2002; Rapiti 2006).

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Systemic treatment plus surgery versus systemic treatment, outcome: 1.1 Overall survival.
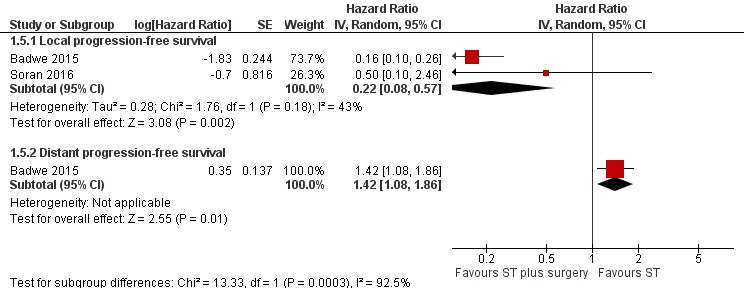
Forest plot of comparison: 1 Systemic treatment plus surgery versus systemic treatment, outcome: 1.5 Progression‐free survival.

Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 1 Overall survival.
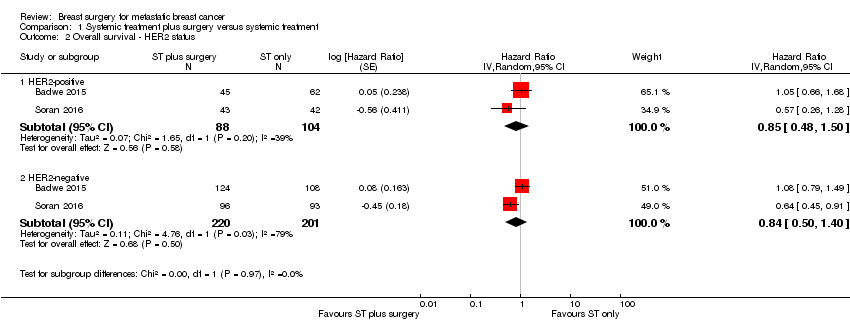
Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 2 Overall survival ‐ HER2 status.

Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 3 Overall survival ‐ ER status.

Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 4 Overall survival ‐ only bone metastasis.

Comparison 1 Systemic treatment plus surgery versus systemic treatment, Outcome 5 Progression‐free survival.
| Breast surgery plus systemic treatment compared to systemic treatment for metastatic breast cancer | ||||||
| Patient or population: metastatic breast cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with systemic treatment | Risk with breast surgery plus systemic treatment | |||||
| Overall survival at 2 years Follow‐up: range 23 months to 40 months | Study population | HR 0.83 | 624 | ⊕⊝⊝⊝ | The estimates for the control group are based upon an average of the estimates from Badwe 2015 and Soran 2016. | |
| 511 per 1000 | 448 per 1000 | |||||
| Quality of life | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Local PFS at 2 years Follow‐up: range 23 months to 40 months | Study population | HR 0.22 | 607 | ⊕⊕⊝⊝ | The estimates for the control group are based upon an average of the estimates from Badwe 2015 and Soran 2016. | |
| 500 per 1000 | 141 per 1000 | |||||
| Distant PFS at 2 years Follow‐up: 23 months | Study population | HR 1.42 | 350 | ⊕⊕⊕⊝ | The estimates for the control group are based upon the estimates from Badwe 2015. | |
| 548 per 1000 | 676 per 1000 | |||||
| Breast cancer‐specific survival | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Toxicity from local therapy Follow‐up: 40 months | Study population | RR 0.99 | 274 | ⊕⊕⊝⊝ | The estimates for the control group are based upon the estimates from Soran 2016. | |
| 15 per 1000 | 15 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1In Soran 2016, trial random sequence generation and allocation concealment were unclear. Downgraded one level. | ||||||
| Overall survival subgroup analysis | Number of studies | N | HR | Lower CI | Upper CI | P value |
| HER2‐positive | 2 | 192 | 0.90 | 0.60 | 1.35 | NS |
| HER2‐negative | 2 | 421 | 0.85 | 0.67 | 1.08 | NS |
| ER positive | 2 | 426 | 0.79 | 0.61 | 1.03 | NS |
| ER negative | 2 | 200 | 1.01 | 0.73 | 1.40 | NS |
| Bone‐only metastasis | 2 | 226 | 0.91 | 0.49 | 1.69 | NS |
| CI: confidence interval | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 2 | 624 | Hazard Ratio (Random, 95% CI) | 0.83 [0.53, 1.31] |
| 2 Overall survival ‐ HER2 status Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 HER2‐positive | 2 | 192 | Hazard Ratio (Random, 95% CI) | 0.85 [0.48, 1.50] |
| 2.2 HER2‐negative | 2 | 421 | Hazard Ratio (Random, 95% CI) | 0.84 [0.50, 1.40] |
| 3 Overall survival ‐ ER status Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 ER‐positive | 2 | 426 | Hazard Ratio (Random, 95% CI) | 0.83 [0.48, 1.42] |
| 3.2 ER‐negative | 2 | 200 | Hazard Ratio (Random, 95% CI) | 1.04 [0.70, 1.55] |
| 4 Overall survival ‐ only bone metastasis Show forest plot | 2 | 226 | Hazard Ratio (Random, 95% CI) | 0.91 [0.49, 1.69] |
| 5 Progression‐free survival Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 5.1 Local progression‐free survival | 2 | Hazard Ratio (Random, 95% CI) | 0.22 [0.08, 0.57] | |
| 5.2 Distant progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | 1.42 [1.08, 1.86] | |

