医学和饮食干预预防儿童尿路结石复发
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Design: randomized trial. Setting/country: single center/Turkey. Dates when study was conducted: not reported. | |
| Participants | Inclusion criteria: children with calcium‐containing idiopathic nephrolithiasis and normal renal morphology following initial treatment with shockwave lithotripsy. Exclusion criteria: children with anatomic abnormalities, previous stone surgery or urinary tract infection, renal tubular acidosis, renal functional disorders, cystinuria or any other evident metabolic abnormality (primary or secondary hyperoxaluria, hyperparathyroidism, etc.). Total number of participants randomly assigned: 125 (58 boys, 38 girls). Experimental group:
Control group:
| |
| Interventions | Experimental group:
Control group:
Follow‐up (mean): 24.4 months. | |
| Outcomes |
| |
| Notes | Funding source and conflicts of interests: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method unspecified. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method unspecified. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and researchers not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Participants and researchers not blinded. |
| Incomplete outcome data (attrition bias) | High risk | High (23%) attrition rate in study cohort. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for review; therefore, risk of bias from selective reporting was unclear. |
| Other bias | Low risk | Apparently free of other problems that could put it at a risk of bias. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Wrong population (not all children included in the cohort had a primary stone event). | |
| Wrong study design (non‐randomized). | |
| Wrong population (children with small stone, not recurrent). | |
| Wrong intervention (duration of intervention < 12 months). | |
| Wrong study design (single arm). | |
| Wrong study design (single arm). | |
| Wrong study design (non‐randomized retrospective). | |
| Wrong study design (non‐randomized). | |
| Wrong intervention (duration of intervention < 12 months). |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone recurrence and regrowth Show forest plot | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.06, 0.60] |
| Analysis 1.1  Comparison 1 Potassium citrate versus no treatment, Outcome 1 Stone recurrence and regrowth. | ||||
| 2 Adverse events Show forest plot | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 13.00 [0.75, 224.53] |
| Analysis 1.2  Comparison 1 Potassium citrate versus no treatment, Outcome 2 Adverse events. | ||||

Risk of bias summary: review authors' judgments about each risk of bias item for each included study (single study).

Study flow diagram.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies (single study).
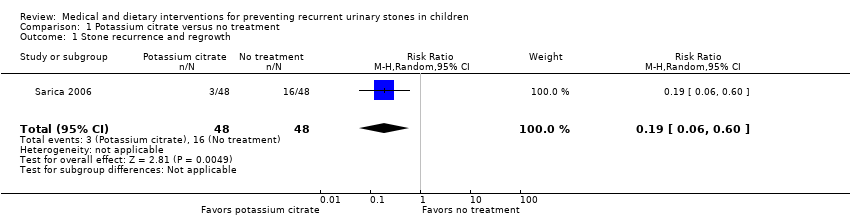
Comparison 1 Potassium citrate versus no treatment, Outcome 1 Stone recurrence and regrowth.

Comparison 1 Potassium citrate versus no treatment, Outcome 2 Adverse events.
| Patient or population: children with idiopathic urinary calculi treated with shockwave lithotripsy | |||||
| Outcomes | No of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with no intervention | Risk difference with medical or dietary interventions | ||||
| Proportion of participants who developed a new urinary stone | 96 | ⊕⊕⊝⊝ | RR 0.19 | Study population | |
| 333 per 1000 | 270 fewer per 1000 | ||||
| Proportion of participants with adverse events while undergoing intervention follow‐up: mean 24.4 months | 96 | ⊕⊝⊝⊝ | RR 13.00 | Study population | |
| ‐ | ‐ | ||||
| Proportion of participants undergoing retreatment for urinary stones | no information found | NA | NA | NA | NA |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; NA: not applicable (since no information found). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by two levels for study limitations: almost all domains were unclear or high risk of bias. 2Downgraded by two levels for imprecision: very rare event resulting in very wide confidence interval. 3No event in control arm. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone recurrence and regrowth Show forest plot | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.06, 0.60] |
| 2 Adverse events Show forest plot | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 13.00 [0.75, 224.53] |

