Intervenciones médicas y alimentarias para la prevención de los cálculos urinarios recurrentes en niños
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011252.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 09 noviembre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Urología
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Draft the protocol: GG, LB.
Study selection: HM, AK.
Extract data from studies: AK.
Enter data into RevMan: AK.
Carry out the analysis: AK.
Interpret the analysis: AK, LB.
Draft the final review: AK.
Disagreement resolution: LB, GG.
Update the review: AK.
Sources of support
Internal sources
-
Departmental, USA.
External sources
-
No sources of support supplied
Declarations of interest
AK: none known.
GG: none known.
HM: none known.
LB none known.
Acknowledgements
We would like to thank the referees for their comments and feedback during the preparation of this review, and acknowledge Cochrane Urology Group for their support.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Nov 09 | Medical and dietary interventions for preventing recurrent urinary stones in children | Review | Adam Kern, Gwen Grimsby, Helen Mayo, Linda A Baker | |
| 2014 Aug 04 | Medical and dietary interventions for preventing recurrent urinary stones in children | Protocol | Gwen Grimsby, Helen Mayo, Micah A Jacobs, Linda A Baker | |
Differences between protocol and review
This review was based on a published protocol (Grimsby 2014), with differences as described here.
In addition to the previously published methodology, the review included plans for GRADE assessment and for preparation of a 'Summary of Findings' tables using GRADEpro GDT software. GRADE assessment is reported in the review, as well as within the 'Summary of findings' table alongside the reportable primary outcome for the main comparison estimable from the available literature.
Due to a combination of low quality available evidence and overall limited numbers of available studies, only one full prospective randomized study was included. There were no high quality descriptive studies available, only low quality descriptive studies by GRADE criteria; therefore, these descriptive studies were not included as planned.
The Newcastle‐Ottawa scoring scale was not utilized for assessment of risk of bias, as the review included no non‐randomized studies (Wells 2012).
Several data collection and analysis steps were not performed since we found no relevant data. For measurement of treatment effect, only dichotomous outcome information was available from the single included study, so no analysis of continuous outcomes data was performed. Similarly, no special analysis was undertaken for unit of analysis issues, as this was not relevant to the single included study, neither was an assessment of heterogeneity of data undertaken. Likewise, data synthesis, subgroup and sensitivity analysis steps were omitted since only one study met inclusion criteria.
We dropped the secondary outcome of number of retreatment per year predefined in the protocol as it was too similar to the primary outcome of retreatment to add value. However, there was no evidence for either outcome.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Child; Female; Humans; Male;
PICO

Risk of bias summary: review authors' judgments about each risk of bias item for each included study (single study).

Study flow diagram.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies (single study).
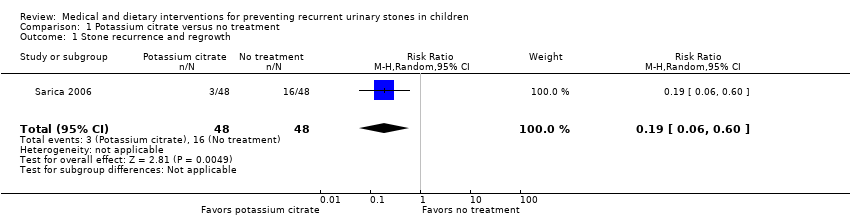
Comparison 1 Potassium citrate versus no treatment, Outcome 1 Stone recurrence and regrowth.

Comparison 1 Potassium citrate versus no treatment, Outcome 2 Adverse events.
| Patient or population: children with idiopathic urinary calculi treated with shockwave lithotripsy | |||||
| Outcomes | No of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with no intervention | Risk difference with medical or dietary interventions | ||||
| Proportion of participants who developed a new urinary stone | 96 | ⊕⊕⊝⊝ | RR 0.19 | Study population | |
| 333 per 1000 | 270 fewer per 1000 | ||||
| Proportion of participants with adverse events while undergoing intervention follow‐up: mean 24.4 months | 96 | ⊕⊝⊝⊝ | RR 13.00 | Study population | |
| ‐ | ‐ | ||||
| Proportion of participants undergoing retreatment for urinary stones | no information found | NA | NA | NA | NA |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; NA: not applicable (since no information found). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by two levels for study limitations: almost all domains were unclear or high risk of bias. 2Downgraded by two levels for imprecision: very rare event resulting in very wide confidence interval. 3No event in control arm. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone recurrence and regrowth Show forest plot | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.06, 0.60] |
| 2 Adverse events Show forest plot | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 13.00 [0.75, 224.53] |

