Medyczne i dietetyczne interwencje zapobiegające nawracającej kamicy moczowej u dzieci
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| Embase |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
| Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomized sequence. | Low risk of bias: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgment of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: insufficient information about the sequence generation process to permit judgment. | |
| Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. | Low risk of bias: randomization method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomization; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: used an open random allocation schedule (e.g. a list of random numbers); assignment envelopes used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: randomization stated but no information on method used. | |
| Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. | Low risk of bias: no blinding or incomplete blinding, but review authors judged that outcome was not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: no blinding or incomplete blinding, and outcome was likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that blinding could have been broken, and outcome is likely to be influenced by lack of blinding. | |
| Unclear: insufficient information to permit judgment. | |
| Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. | Low risk of bias: no blinding of outcome assessment, but review authors judged that outcome measurement was not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that blinding could have been broken. |
| High risk of bias: no blinding of outcome assessment, and outcome measurement was likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that blinding could have been broken, and outcome measurement was likely to be influenced by lack of blinding. | |
| Unclear: insufficient information to permit judgment. | |
| Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. | Low risk of bias: no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data were imputed using appropriate methods. |
| High risk of bias: reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; 'as‐treated' analysis done with substantial departure of the intervention received from that assigned at randomization; potentially inappropriate application of simple imputation. | |
| Unclear: insufficient information to permit judgment. | |
| Selective reporting Reporting bias due to selective outcome reporting. | Low risk of bias: study protocol was available and all of the study's prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way; study protocol was not available but it was clear that the published reports included all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). |
| High risk of bias: not all of the study's prespecified primary outcomes were reported; ≥ 1 primary outcomes was reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified; ≥ 1 reported primary outcomes were not prespecified (unless clear justification for their reporting was provided, such as an unexpected adverse effect); ≥ 1 outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis; study report failed to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: insufficient information to permit judgment. | |
| Other bias Bias due to problems not covered elsewhere in this table. | Low risk of bias: study appeared free of other sources of bias. |
| High risk of bias: had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; was claimed to have been fraudulent; had some other problem. | |
| Unclear: insufficient information to assess whether an important risk of bias existed; insufficient rationale or evidence that an identified problem would introduce bias. |

Risk of bias summary: review authors' judgments about each risk of bias item for each included study (single study).

Study flow diagram.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies (single study).
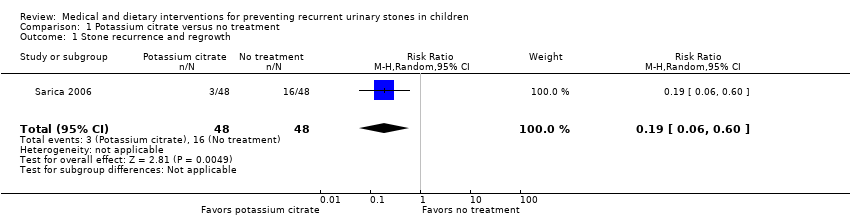
Comparison 1 Potassium citrate versus no treatment, Outcome 1 Stone recurrence and regrowth.

Comparison 1 Potassium citrate versus no treatment, Outcome 2 Adverse events.
| Patient or population: children with idiopathic urinary calculi treated with shockwave lithotripsy | |||||
| Outcomes | No of participants | Quality of the evidence | Relative effect | Anticipated absolute effects* (95% CI) | |
| Risk with no intervention | Risk difference with medical or dietary interventions | ||||
| Proportion of participants who developed a new urinary stone | 96 | ⊕⊕⊝⊝ | RR 0.19 | Study population | |
| 333 per 1000 | 270 fewer per 1000 | ||||
| Proportion of participants with adverse events while undergoing intervention follow‐up: mean 24.4 months | 96 | ⊕⊝⊝⊝ | RR 13.00 | Study population | |
| ‐ | ‐ | ||||
| Proportion of participants undergoing retreatment for urinary stones | no information found | NA | NA | NA | NA |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; NA: not applicable (since no information found). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded by two levels for study limitations: almost all domains were unclear or high risk of bias. 2Downgraded by two levels for imprecision: very rare event resulting in very wide confidence interval. 3No event in control arm. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stone recurrence and regrowth Show forest plot | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.06, 0.60] |
| 2 Adverse events Show forest plot | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 13.00 [0.75, 224.53] |

