母乳对新生儿期后婴儿疼痛的影响
摘要
研究背景
随机对照试验显示,母乳喂养可帮助新生儿手术期间减轻疼痛。我们认为原理是多因素造成的的,包括吮吸,皮肤接触,温暖,摇摆,母亲的声音和气味,以及母乳中可能存在的内源性鸦片剂。
研究目的
为了确定母乳喂养对比无干预措施,安慰剂,亲代控制,皮肤接触,表达母乳,配方牛奶,奶瓶喂养,甜品解决方案(例如:蔗糖或葡萄糖),分心,或其他干预措施等对婴幼儿(新生儿期后(最初28天)到1岁)操作性疼痛的影响。
检索策略
我们检索了以下数据库,时间截止至2016年2月18日:Cochrane对照试验注册库(CENTRAL)(Cochrane图书馆),MEDLINE包含在线和其他非索引引文(OVID),Embase(OVID),PsycINFO(OVID)和CINAHL(EBSCO);the metaRegister of Controlled Trials(mRCT),ClinicalTrials.gov(clinicaltrials.gov),和世界卫生组织国际临床试验注册平台(WHOICTRP)(apps.who.int/trialsearch/)正在进行的试验。
纳入排除标准
我们纳入了随机对照试验和半随机对照试验,包含了出生后28天至12个月的婴儿,并在接受会产生疼痛的针刺操作中进行母乳喂养。对照组包括且不限定于口服水,甜味剂,挤出的母乳或配方奶,不干预,使用安抚奶嘴,固定,拥抱,牵引,表面麻醉剂和皮肤护理等。操作包括且不限定于:皮下或肌内注射,静脉穿刺,静脉插入,脚跟针和手指针。我们不做语言限定。
资料收集与分析
我们使用了Cochrane的标准方法学程序。两名评价作者独立对纳入评价的试验进行筛选,评估偏倚风险并提取数据资料。主要结局指标包括行为或生理指标和综合疼痛评分,以及所纳入研究的作者报告的其他重要临床结果。我们对数据进行合并以获得最相似的结果和至少包含两项研究的数据。我们使用95%的可信区间(CI)的平均差(MD),采用随机效应模型对相同量表进行连续测量。对于在不同尺度上测量的连续结果,我们采用标准化平均差(SMD)和相关的95%的可信区间。对于二分类结果,我们计划通过使用风险比(RR)和95%可信区间(CI)对各组之间的事件进行合并。然而,由于研究报告不充分,我们没有汇集这些事件。我们使用GRADE对证据进行评价,并生成了“结果概要”表。
主要结果
我们纳入10项研究,包括1066名婴儿。所有的研究都是在婴幼儿免疫期间进行的。由于母乳喂养的干预措施不能使用盲法,所以我们将所有研究评估为对参与者和医生施盲的高风险。我们评估了9项研究,结局数据不完整的风险较低。此外,我们将9项研究评估为结局评估盲法的高风险。由于缺乏信息,大多数研究不清楚,我们评估了随机序列的产生,随机分配隐匿和选择性报告的偏倚风险。
主要结局是疼痛。与未治疗,口服水和其他干预如拥抱,口服葡萄糖,局部麻醉,按摩和蒸气冷却剂相比,母乳喂养减少了接种期间的疼痛行为反应(哭泣时间和疼痛评分)。母乳喂养没有持续降低心率等生理指标。我们纳入了六项研究(n=547名婴儿)的哭闹时间数据。与母乳喂养相比,口服水或不治疗使得哭泣时间缩短38秒(MD‐38,95%CI‐50至‐26;P<0.00001)。根据GRADE评估,这一结果的证据质量是中等的,因为大多数婴儿是6个月或更小,并且婴儿在12个月免疫接种期间的结果可能是不同的。我们纳入了五项研究(n=310名婴儿)的疼痛评分数据。母乳喂养与标准疼痛评分降低1.7分相关(SMD‐1.7,95%CI‐2.2至‐1.3);我们认为这些证据质量中等,因为数据主要来自6个月以下的婴儿。只有两项研究(n=186)提供了注射后的心率数据;由于数据不足,我们认为这个证据质量低下。母乳喂养和对照之间没有差异(MD‐3.6,‐23到16)。
10项研究中有4项研究为两臂以上试验。疫苗接种期间,母乳喂养在减少哭闹持续时间或疼痛评分方面比25%右旋糖和局部麻醉膏(EMLA),蒸气冷却剂,母亲拥抱和按摩等更有效。
纳入的研究中没有研究报告了不良事件。
作者结论
我们得出结论,根据本次纳入的10项研究,母乳喂养可能有助于减少新生儿期后婴儿接种疫苗期间的疼痛。母乳喂养持续降低了接种期间和接种后的啼哭时间和复合疼痛评分的行为反应。然而,没有证据表明母乳喂养对生理反应有影响。本评价纳入的研究对象不包括接受其他皮肤破裂手术的住院婴儿。尽管有可能将评估结果外推到这一人群,但是进一步研究对这一人群的有效性,可行性和可接受性是必要的。
PICO
简语概要
母乳喂养是否能够减轻1到12月大婴儿接种疫苗的疼痛?
简要总结
我们发现接种疫苗前以及过程中进行母乳哺乳能够减轻大部分一岁以内婴儿的疼痛。
背景
针常用于婴儿早期疫苗接种和患病时的医疗护理。这些是必不可少的,然而过程是痛苦的。这些对婴儿及他们的父母/照顾者造成痛苦,并可能导致他们以后对针头产生焦虑和恐惧感。母乳喂养减轻了新生儿血液检查带来的疼痛。在可能和可行的情况下进行母乳喂养也会有助于安慰婴儿,减轻新生儿期和整个婴儿期的疼痛感。
研究特点
2016年2月,我们检索了医学文献,研究了1至12个月大的婴儿母乳喂养在用针期间的效果。我们比较了母乳喂养和将婴儿抱着、平躺放或给予水或糖果等方案在减少疼痛(以哭泣时间和疼痛感评分)方面的有效性。我们纳入了10项研究,包含1066名婴儿。所有研究都评估了母乳喂养是否减轻了疫苗接种期间的疼痛。
主要成果
母乳喂养减少了幼小婴儿在接种疫苗期间哭泣的情况。平均而言,母乳喂养的婴儿比没有母乳喂养的婴儿(6项研究;包含547名婴儿;中等质量证据)少哭了38秒,疼痛评分明显降低(5项研究;包含310名婴儿;中等质量证据)。
研究没有报告存在任何伤害(非常低质量的证据)。在接种疫苗期间使用母乳喂养健康婴儿时,我们不能得出有危害风险的结论。
未来方向:如果母亲正在母乳喂养,应该考虑婴儿在接种疫苗期的哪个阶段。需要更多的证据来了解母乳喂养是否对较大的婴儿和在医院期间进行血液检查或输液的婴儿有帮助。
证据质量
对于哭泣时间和疼痛评分的证据质量为中等级别。大多数研究纳入了年龄较小的1至6个月的婴儿。进一步研究纳入12个月以上的年龄较大的婴儿可能会改变我们的结论。此外,研究评估了在接种期间母乳喂养的效果。我们不知道在抽血或静脉输液期间,母乳喂养是否对1至12个月的生病的婴儿有帮助。
Authors' conclusions
Summary of findings
| Breastfeeding compared with other interventions, oral water, or no treatment for pain during vaccination in infants 1 to 12 months | ||||
| Patient or population: Infants 1 to 12 months during vaccination Settings: Diverse community settings Intervention: Breastfeeding Comparison: Other interventions, such as cuddling, sweet solutions, or placebo (oral water) or no treatment | ||||
| Outcomes | No of Participants | Effect Estimates and 95% CI | Quality of the evidence | Comments |
|---|---|---|---|---|
| Cry duration Seconds cry time during procedure or proportion of crying during procedure. Crying was measured during and up to 3 minutes following completion of vaccination. | 547 (6 studies) | MD (seconds) ‐38 ( ‐49.84 to 26.35) | Moderate | Not further downgraded: Breastfeeding consistently resulted in a reduction of crying time. However, as most trials included infants aged 1 to 6 months, further research including older infants up to 12 months of age may have an important impact on our confidence in the estimate of effect and may change the estimate. |
| All pain scores during injection Due to range of pain scores used, data analysed using SMDs. Pain scores were measured during and up to 3 minutes following completion of vaccination. | 310 (5 studies) | SMD ‐1.7 ( ‐2.2 to ‐1.3) | Moderate | Downgraded once: Breastfeeding consistently resulted in a reduction of pain scores. However, only 5 studies and 310 infants were included, and most of the trials included the younger infants aged 1 to 6 months. Further research including older infants up to 12 months of age and larger sample sizes may have an important impact on our confidence in the estimate of effect. |
| NIPS score during injection The NIPS was measured during and up to 3 minutes following completion of vaccination. | 174 (3 studies) | MD ‐1.89 (‐2.55 to ‐1.24) | Low | Not further downgraded: Grade of evidence already taken into account. Only 3 studies and 174 infants were included. Further studies using this pain assessment score may lead to more certainty and have an important impact on our confidence in the estimate of effect. |
| Heart rate after injection Heart rate in the period following completion of the injections was measured in 2 studies. | 186 (2 studies) | MD (beats per minute) ‐3.6 ( ‐23 to 16) | Low | Not further downgraded: Grade of evidence already taken into account. Breastfeeding did not have an effect on heart rate change from baseline. As only 2 studies were included in this outcome, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. |
| Adverse events | No studies reported on adverse events. | ‐ | Very low | No studies reported on outcomes such as coughing or gagging. |
| CI: confidence interval; MD: mean difference; NIPS: Neonatal Infant Pain Scale; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence | ||||
Background
Infants and children require needle‐related painful procedures for scheduled childhood immunisations (PHA Canada 2014), as well as medical procedures performed for diagnostic and treatment purposes during the course of childhood illnesses (Johnston 2011). Such procedures are known to be painful, causing distress at the time of the procedure and for many children, anxiety and fear during subsequent needle‐related procedures (Schechter 2007; Taddio 2007; Taddio 2009; Wright 2009), and altered pain responses later in life (Taddio 2005). Fear of the pain associated with immunisations, with subsequent fear of needles, has been shown to be one of the reasons why parents do not complete their infants' recommended immunisation schedule (Mills 2005; Schechter 2007; Taddio 2007; Taddio 2009; Wright 2009). It is therefore imperative that effective pain management strategies be consistently used for infants and children in diverse settings where needle‐related painful procedures are performed.
Recently conducted systematic reviews of pain management strategies in the newborn period demonstrated that breastfeeding, in Shah 2012, and sweet solutions of sucrose, in Stevens 2013, and glucose, in Bueno 2013, reduced behavioural responses and composite pain scores during painful procedures. In addition, systematic reviews of sweet‐tasting solutions beyond the neonatal period up to one year of age demonstrated analgesic effects during needle‐related painful procedures when compared to water or no treatment (Harrison 2010a; Kassab 2012). There is a need for this evidence relating to analgesic effects of breastfeeding beyond the newborn period to be systematically reviewed on an ongoing basis to critically evaluate the effectiveness of this intervention in infants up to one year of age.
Description of the condition
Studies of pain management strategies used during commonly performed needle‐related procedures show inconsistent use of recommended interventions (Harrison 2013; Johnston 2011; Stevens 2011; Taddio 2007). However, it is known that untreated or poorly treated procedural pain has negative effects, including infant and parental distress at the time of the procedure, with the risk of longer‐term fear of needles (Schechter 2007; Taddio 1995; Taddio 2007; Taddio 2009; Wright 2009).
Description of the intervention
Breastfeeding commenced prior to the painful procedure, and continuing throughout the procedure until completion. High‐quality evidence from randomised controlled trials (RCTs) and systematic reviews supports the role of breastfeeding in reducing procedural pain during the neonatal period (first 28 days of life). Shah and colleagues conducted a systematic review of 20 RCTs or quasi‐RCTs evaluating breastfeeding (10 studies) or supplemental breast milk placed on the tongue or given through gastric tube (10 studies) during heel lance or venipuncture in newborn infants (Shah 2012). The authors concluded that breastfeeding effectively reduced behavioural responses, including crying duration and total crying time, facial expressions and pain scores, as well the physiological response of heart rate, compared to positioning (swaddled and nursed in a crib), holding by the mother, placebo, or no intervention.
How the intervention might work
Several elements are postulated to contribute to the analgesic effects of breastfeeding. These include skin‐to‐skin contact, sight, sound and smell of the mother, sucking, distraction, pleasant and slightly sweet taste, and intake of naturally occurring endorphins that are present in breast milk (Blass 1995; Blass 1997; Shah 2012). As detailed in Shah 2012, breast milk also contains higher concentrations of tryptophan compared to formula milk (Heine 1999). Tryptophan is a precursor to melatonin, which in animal studies has been shown to increase concentrations of beta‐endorphin (Barrett 2000), a naturally occurring endorphin that is assumed to be one of the mechanisms responsible for the analgesic effects of breast milk. However, small volumes of expressed breast milk do not result in analgesia. Expressed breast milk given in small quantities failed to consistently reduce physiological or behavioural pain indicators or composite pain scores (Shah 2012). This discrepancy may be due to the contribution of multiple factors influencing analgesia during breastfeeding other than taste alone, including maternal contact, skin‐to‐skin contact, familiar smell, heart rate and body movement, sucking, and intake of naturally occurring endorphins present in the breast milk (Harrison 2010b; Zanardo 2001). As breast milk contains around 7% lactose, which is the least sweet of the sugars (sucrose > fructose > glucose > lactose) (Blass 1992), the mildly sweet taste most likely contributes little to analgesia in isolation (e.g. delivered by oral syringe or via pacifier). Bottle feeding larger volumes of either breast milk or formula milk also removes the multiple analgesic factors associated with being held skin‐to‐skin. Evidence of the analgesic effects of breastfeeding in newborn infants is demonstrated in a publicly accessible YouTube video (Harrison 2016).
Why it is important to do this review
Although a systematic review of breastfeeding newborn infants during painful procedures was shown to effectively reduce pain (Shah 2012), and a systematic review of multiple strategies to reduce immunisation pain, which included breastfeeding, also demonstrated analgesic effects of breastfeeding (Shah 2009), to date there is no published systematic review that exclusively focuses on the effectiveness of breastfeeding for pain management during painful procedures beyond the neonatal period. This review is therefore important to further establish the effectiveness of breastfeeding in infants up to one year of age. This strategy has universal applicability for breastfeeding mothers, as it requires no additional cost, no special equipment, and no special preparation or storage.
Objectives
We sought to determine the effect of breastfeeding on procedural pain in infants beyond the neonatal period (first 28 days of life) up to one year of age compared to no intervention, placebo, parental holding, skin‐to‐skin contact, expressed breast milk, formula milk, bottle feeding, sweet‐tasting solutions (e.g. sucrose or glucose), distraction, or other interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐RCTs as defined by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Types of participants
Infants undergoing a painful procedure who are 28 days postnatal age (corrected for prematurity, 37 weeks plus 28 days) up to aged 12 months, including infants receiving their 12‐month vaccination. The procedures included, but were not limited to: subcutaneous or intramuscular injection, venipuncture, intravenous line insertion, heel lance, and finger lance.
Types of interventions
Breastfeeding during a painful procedure, with or without additional interventions such as topical anaesthetic agents or sweet solutions. We did not classify expressed breast milk delivered by methods such as a dropper, syringe, spoon, or bottle as breastfeeding, but did include such delivery methods as comparators in control or other treatment arms.
Comparators included oral administration of: water, sweet‐tasting solutions (such as sucrose or glucose), expressed breast or formula milk, no intervention, use of pacifiers, positioning, cuddling, distraction, topical anaesthetics, and skin‐to‐skin care, also referred to as kangaroo care.
We included all settings where breastfeeding was evaluated for pain reduction during painful procedures. These included inpatient hospital units, emergency departments, and outpatient or community settings.
Types of outcome measures
Primary outcomes
The primary outcome was pain, as assessed by at least one of the following.
-
Behavioural indicators such as:
-
cry variables (duration of crying, expressed in total seconds of crying or proportion of duration of crying during painful procedure or following completion of painful procedure (recovery period));
-
facial expressions (grimace); or
-
body posture and movements.
-
-
Physiological responses such as heart rate, heart rate variability, respiratory rate, transcutaneous oxygen (TcPO2), transcutaneous carbon dioxide (TcPCO2), oxygen saturation (SpO2), or other measures such as skin conductance or biomedical markers (e.g. serum, salivary or urinary cortisol).
-
Composite pain measures: unidimensional or multidimensional (including a combination of behavioural, physiological, and contextual indicators).
Secondary outcomes
-
Other clinically important outcomes reported by authors of included studies (not prespecified).
-
Any adverse effects reported by authors (e.g. choking, gagging, spitting, coughing).
Timing of measurements and aggregation of data will vary from study to study, but common times of measurement include:
-
baseline prior to delivery of intervention/control;
-
upon commencement of procedure;
-
15, 30, 60, 120, 180 seconds following commencement of procedure;
-
throughout entire duration of procedure; and
-
up to 10 minutes following completion of procedure.
Search methods for identification of studies
Electronic searches
We searched the following databases.
-
Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Library (Issue 1 of 12, 2016).
-
MEDLINE including In‐Process & Other Non‐Indexed Citations (OVID) (1946 to 18 February 2016).
-
Embase (OVID) (1947 to 18 February 2016).
-
PsycINFO (OVID) (1806 to 18 February 2016).
-
CINAHL (EBSCO) (searched 18 February 2016).
Our librarian (MS), who is highly qualified in systematic review searching, developed the MEDLINE search strategy, which another librarian peer reviewed using the Peer Review of Electronic Search Strategies (PRESS) standard (McGowan 2010). We adapted the MEDLINE search strategy for the other databases (see Appendix 1, Appendix 2, Appendix 3, Appendix 4, Appendix 5). We limited study design to controlled trials only in MEDLINE and Embase. We applied no language restrictions. We included studies irrespective of their publication status. In addition, we reviewed eligible studies for cited references.
Searching other resources
As per Cochrane Pain, Palliative and Supportive Care (PaPaS) Review Group recommendations, we also searched the metaRegister of controlled trials (mRCT), ClinicalTrials.gov (clinicaltrials.gov), and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/) for ongoing trials. In addition, we checked reference lists of reviews and retrieved articles; relevant recent neonatal, paediatric, and pain journals; and paediatric pain conference proceedings.
Data collection and analysis
Selection of studies
Two review authors (JR and MB) independently screened abstracts to identify potentially eligible studies found via electronic searching. Any conflicts were resolved through a consensus process, with a third review author (DH), if required. We retrieved full‐text articles of all potentially relevant abstracts, which two review authors (JR and MB) independently assessed for inclusion. We resolved all discrepancies over the full texts through a consensus process or by consulting a third review author (DH) if required. We included a PRISMA study flow diagram to document the screening process (Liberati 2009), as recommended in Part 2, Section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Data extraction and management
We extracted the following data from each study: study design, setting, age of infants, overall sample size, sample size per group, number of groups, painful procedure, and outcomes and adverse events per groups as reported by the authors. Two review authors (JR and MB or JR and CL) independently extracted data from the studies using a standardised data extraction form. Differences were resolved through a consensus process or by consulting a third review author (DH) when necessary.
Assessment of risk of bias in included studies
We used standard methods of Cochrane to assess the potential risk of bias using the Cochrane 'Risk of bias' classification tool (Higgins 2011b).
Two review authors (JR and MB) independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion or by consulting a third review author (DH), if necessary (Higgins 2011a). The review authors were not blinded to authors or institutions. We assessed the following for each study.
Selection bias (random sequence generation and allocation concealment)
Random sequence generation
For each included study, we categorised the risk of selection bias as:
-
low risk: adequate (any truly random process, e.g. random number table, computer random number generator);
-
high risk: inadequate (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); or
-
unclear risk: no or unclear information provided.
Allocation concealment
The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. For each included study, we categorised the risk of bias regarding allocation concealment as:
-
low risk: adequate (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
-
high risk: inadequate (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth); or
-
unclear risk: no or unclear information provided.
Blinding of participants, personnel, and outcome assessors (performance and detection bias)
Blinding of participants and personnel (performance bias)
Due to the nature of the intervention of breastfeeding, blinding of participants and personnel was not possible. Nevertheless, for each included study, we categorised the methods used to blind study participants and personnel to knowledge of which intervention a participant received as:
-
low risk: adequate for personnel (a placebo that could not be distinguished from the active solution was used in the control group);
-
high risk: inadequate (participants or personnel were aware of group assignment); or
-
unclear risk: no or unclear information provided.
Blinding of outcome assessment (detection bias)
For each included study, we categorised the methods used to blind outcome assessors to knowledge of which intervention a participant received (if the study population consisted of young children unable to verbalise, they were considered to pose no risk of revealing group assignment). We assessed blinding separately for different outcomes or classes of outcomes. We categorised the methods used with regards to detection bias as:
-
low risk: adequate (study states that pain assessment from audio data or physiological data download was blinded and describes the method used to achieve blinding);
-
high risk: inadequate (assessors at follow‐up were aware of group assignment); or
-
unclear risk: no or unclear information provided.
Incomplete outcome data
Incomplete outcome data (attrition bias)
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), whether reasons for attrition or exclusion were reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we planned to re‐include missing data in the analyses. We categorised the methods with respect to attrition bias as:
-
low risk: adequate (less than 10% missing data);
-
high risk: inadequate (more than 10% missing data); or
-
unclear risk: no or unclear information provided.
Selective outcome reporting
Selective outcome reporting (reporting bias)
For each included study, we described how we investigated the risk of selective outcome reporting bias and what we found. We assessed the methods as:
-
low risk: adequate (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported, as per the study protocol);
-
high risk: inadequate (where not all of the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported, as per the study protocol); or
-
unclear risk: no or unclear information provided (the study protocol was not available).
Other bias
Other potential sources of bias
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
-
low risk (no concerns of other bias raised);
-
high risk (concerns raised about multiple examinations of the data before completion of data collection; differences in the number of participants enrolled between the abstract and final publications of the paper); or
-
unclear (concerns raised about potential sources of bias that could not be verified by contacting the authors).
Size
Size ‐ based on number of participants in each study arm
According to the Cochrane PaPaS Review Group guidance on sample size, for each included study we evaluated risk of bias based on number of participants included in the study as:
-
low risk (≥ 200 participants per arm);
-
high risk (< 50 participants per arm); or
-
unclear risk (50 to 199 participants per arm).
We integrated risk of bias results into the analyses wherever possible in the results of this review.
Measures of treatment effect
We used the statistical package Review Manager 5 provided by Cochrane (RevMan 2014). For continuous outcomes, we extracted mean scores and their standard deviations in the treatment and control groups. For dichotomous outcomes, we collected the number of events per comparison group.
We pooled data for the most comparable outcomes and where data from at least two studies could be included. This included data for cry duration, standardised mean pain scores, Neonatal Infant Pain Scale (NIPS) pain scores, and heart rate following the procedure.
Unit of analysis issues
The unit of analysis was the infant receiving breastfeeding or control. When multiple intervention groups were included, the primary comparison was breastfeeding compared to the control group, or group receiving 'no intervention'.
For cross‐over trials, we used the data from the first intervention infants received and treated the study as a RCT.
Dealing with missing data
We presented the data in narrative form only if the data were not in a format suitable for use in Review Manager 5. We attempted to contact study authors if data were missing or required clarification, requesting data in a format that could be used for inclusion in meta‐analyses, and obtained the necessary information to complete a 'Risk of bias' assessment.
Assessment of heterogeneity
We explored heterogeneity using the I2 statistic (Higgins 2011a), and visually inspected forest plots for heterogenous estimates of effect. We graded the degree of heterogeneity as none, low, moderate, and high for values of < 25%, 25% to 49%, 50% to 74%, and ≥ 75%, respectively (GRADEpro 2015). We explored possible causes of heterogeneity using sensitivity analysis if applicable.
We used the I2 statistic for between‐study heterogeneity to assess the appropriateness of pooling data from studies for meta‐analyses, employing random‐effects models. We used GRADEpro to present a summary of findings table in this review (summary of findings Table 1; GRADEpro 2015).
Assessment of reporting biases
We planned to use Review Manager 5 funnel plots to investigate small‐study effects. However, no single meta‐analysis included at least 10 trials (Higgins 2011b; Sterne 2011).
Data synthesis
We pooled data for the most comparable outcomes and where data from at least two studies could be included using mean difference (MD) with 95% confidence interval (CI), employing a random‐effects model for continuous outcomes measured on the same scales. For continuous outcomes measured on different scales, we pooled standardised mean differences (SMDs) and associated 95% CIs.
For dichotomous outcomes, we had planned to pool events between groups across studies using risk ratios (RRs) and 95% CIs, risk differences (RDs), and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH). However, there were no data reported as dichotomous outcomes that we were able to pool.
Quality of the evidence
Two review authors (MB and JR) independently rated the quality of all outcomes (behavioural responses (crying time, pain scores) and physiological outcomes of heart rate and oxygen saturation). The first review author (DH) reviewed and finalised all decisions with MB and JR. We used the GRADE system to rank the quality of the evidence employing GRADEpro GDT software and the guidelines provided in Section 12.2 of the CochraneHandbook for Systematic Reviews of Interventions (GRADEPro GDT 2015).
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome. The GRADE system uses the following criteria for assigning grade of evidence.
-
High: further research is very unlikely to change our confidence in the estimate of effect.
-
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
-
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
-
Very low: any estimate of effect is very uncertain.
Summary of findings table
We included a summary of findings table table to present the main findings in a transparent and simple tabular format. In particular, we included key information concerning the quality of the evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes of crying time, pain scores, and heart rate.
Subgroup analysis and investigation of heterogeneity
If, as we expected, the majority of eligible trials were related to the procedure of vaccination, we planned to report data by age group, and perform subgroup analyses according to the early‐childhood immunisation schedules of 2, 4, 6, and 12 months' age groups, if possible. For trials using breastfeeding plus another intervention, we subgrouped these into breastfeeding alone and breastfeeding plus another intervention. We performed subgroup analyses on different comparisons (i.e. no treatment, water, sucrose).
Sensitivity analysis
We included all studies regardless of their risk of bias, and presented a narrative discussion of the potential influence of the risk of bias.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
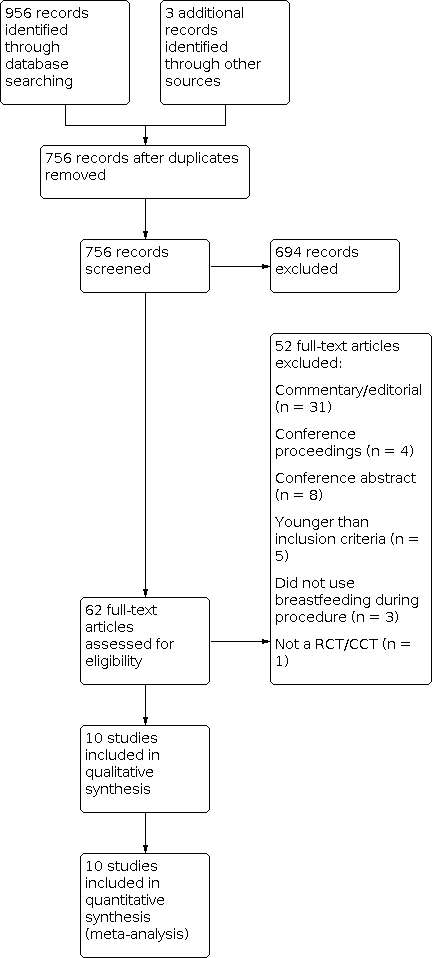
Our initial search of the literature retrieved 756 records after duplicates were removed. Two review authors (JR and MB) screened the 756 titles and abstracts and identified 62 potentially eligible studies. We excluded 52 studies, primarily because the paper was a commentary, editorial, or conference abstract only. We finally included a total of 10 studies with 1066 infants (Figure 1). There were no disagreements between the two review authors that required a third review author to reach a consensus.
Study flow diagram.
Included studies
Details of the included studies are summarised in Characteristics of included studies.
All 10 included studies evaluated breastfeeding during vaccination injections. Nine studies evaluated breastfeeding during a single vaccination episode; one evaluated breastfeeding during two episodes of vaccination, at 2 and again at 4 months (Barr unpublished). Of the 10 included studies, eight were RCTs (Barr unpublished; Boroumandfar 2013; Dilli 2009; Efe 2007; Esfahani 2013; Goswami 2013; Gupta 2013; Taavoni 2009a), and two were quasi‐RCTs (Razek 2009; Thomas 2011). All 10 studies involved intramuscular injections including HepB (hepatitis B) (Boroumandfar 2013; Dilli 2009; Esfahani 2013), DPT (diphtheria, tetanus, pertussis) (Barr unpublished; Boroumandfar 2013; Dilli 2009; Efe 2007; Esfahani 2013; Goswami 2013; Gupta 2013; Taavoni 2009a; Thomas 2011), DTaP‐IPV (diptheria, tetanus, pertussis, polio) (Dilli 2009), Haemophilus influenzae type b (Dilli 2009), MMR (measles, mumps, rubella) (Esfahani 2013), bacillus Calmette‐Guérin (Dilli 2009), and one study did not identify the type of vaccination (Razek 2009).
The studies included infants aged 0 to 12 months. For the one study that included infants under the age of one month (Dilli 2009), we obtained study data from the first author and only included data for infants over the age of one month in this review. Study sample sizes, based on the number of participants included in this review, ranged from 40 to 144 in total. Four studies reported a sample size calculation (Barr unpublished; Goswami 2013; Gupta 2013; Taavoni 2009a). Barr unpublished estimated their sample size using cry data from previous immunisation studies (effect size of 0.40, type I and type II error rates of 5% and 10%); Goswami 2013 based their sample size on their primary outcome of cry duration (90% power and significance of 0.05); Gupta 2013 based their sample size on a previously conducted pilot study (90% power and significance of 0.05); Taavoni 2009a based their sample size on a 95% confidence interval and 90% power.
All studies involved an intervention group with the mother initiating breastfeeding prior to the immunisation procedure and continuing breastfeeding during the immunisation procedure. The breastfeeding group in one study also received 1 g of topical anaesthetic cream (EMLA Cream), applied topically on the injection site 60 minutes before the immunisation (Gupta 2013). All studies included a comparison group where the infant received no pain treatment. Four studies included other comparator groups: 2 mL of 25% dextrose (Goswami 2013); 1 g EMLA Cream plus 2 mL oral distilled water (Gupta 2013); massage therapy (Esfahani 2013); and topical vapocoolant spray (Boroumandfar 2013).
Six studies measured cry duration (Barr unpublished; Dilli 2009; Efe 2007; Goswami 2013; Gupta 2013; Razek 2009), and two studies measured latency of onset of cry (Goswami 2013; Gupta 2013). Eight studies included at least one validated pain scale as an outcome measurement, including the Neonatal Infant Pain Scale (NIPS) (Boroumandfar 2013; Dilli 2009; Esfahani 2013; Razek 2009; Thomas 2011), Neonatal Facial Coding System (NFCS) (Barr unpublished), Modified Facial Coding System (MFCS) (Goswami 2013; Gupta 2013), Modified Behavioral Pain Scale (MBPS) (Taavoni 2009a), and Wong‐Baker FACES Pain Rating Scale (Razek 2009). Three studies measured physiological responses (Barr unpublished; Efe 2007; Razek 2009). Razek 2009 reported heart rate before and after the injection. Efe 2007 reported heart rate and oxygen saturation during and after the injection (Efe 2007). One study also measured facial flushing before, during, and after the injection and salivary cortisol level before and after the injection (Barr unpublished).
Excluded studies
Of the 62 titles/abstracts identified as potentially eligible for inclusion, we excluded 52. Thirty‐one of these were commentaries, newsletters, or editorials, and four were conference proceedings with no relevant abstracts identified. Of the remaining 17 excluded studies, eight were reported as conference abstracts only (Barr 2001; Gradin 2003; Singal 2004; Taavoni 2009b; Taavoni 2010; Taavoni 2011; Taavoni 2012; Taavoni 2013), five studied populations younger than our inclusion criteria (Gray 2002; Hashemi 2016; Modarres 2014; Shendurnikar 2005; Uga 2008), three did not use breastfeeding during the painful procedure (Achema 2011; Jebreili 2015; Sahebihag 2011), and one was not a RCT or quasi‐RCT (Otero López 2014).
Risk of bias in included studies

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We rated five studies as at low risk of bias for random sequence generation that used methods such as shuffling envelopes (Efe 2007), use of a random number table (Esfahani 2013; Taavoni 2009a), or computer‐generated random numbers (Goswami 2013; Gupta 2013). We assessed one study as being at high risk of bias since it was a quasi‐experimental study and thus did not involve randomisation of participants (Razek 2009). We rated four studies in which the specific randomisation method was not described as unclear (Barr unpublished; Boroumandfar 2013; Dilli 2009; Thomas 2011).
Allocation concealment
We rated two studies as being at low risk of bias for allocation concealment as they both used the SNOSE method (serially numbered, opaque, sealed envelopes) (Goswami 2013; Gupta 2013). We rated the remaining eight studies as unclear, as not enough information was provided to assess how allocation was concealed (Barr unpublished; Boroumandfar 2013; Dilli 2009; Efe 2007; Esfahani 2013; Razek 2009; Taavoni 2009a; Thomas 2011).
Blinding
Blinding of participants and personnel
We rated all studies as at high risk for blinding of participants and personnel due to the nature of using breastfeeding as a pain management intervention; there is no practical way for the mothers, healthcare professionals performing the vaccinations, and research staff present during the procedure to be blinded to whether the infant is breastfeeding or not.
Blinding of outcome assessment
We rated only one study as at low risk of bias for blinding of outcome assessment; the study used audio recording only, thus ensuring that the research personnel coding the cry time were not able to see if the infant was being breastfed or not (Efe 2007). The remaining nine studies either completed the outcome assessment at the time of the procedure (Boroumandfar 2013; Dilli 2009; Esfahani 2013; Razek 2009; Taavoni 2009a; Thomas 2011), or used video recording (Barr unpublished; Goswami 2013; Gupta 2013), and were therefore assessed as being at high risk of bias due to the inability to blind outcome assessors to breastfeeding.
Incomplete outcome data
We rated nine studies as at low risk of bias for incomplete outcome data, as they either reported that all randomised infants were included in the results (Efe 2007; Goswami 2013; Gupta 2013; Razek 2009; Taavoni 2009a; Thomas 2011), or more than 90% of participants completed the study (Barr unpublished; Dilli 2009; Esfahani 2013). In one study, there was no information on how many infants were randomised and completed the study per group, and was thus rated as unclear (Boroumandfar 2013).
Selective reporting
We rated one study as at high risk of bias that presented the outcome data in a way that was inconsistent with the recommended use of the validated pain scales being used (Razek 2009). We rated eight studies as being at unclear risk of bias related to selective reporting: seven studies did not have a study protocol registered in the trial registries searched (metaRegister of Controlled Trials, ClinicalTrials.gov (clinicaltrials.gov), and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/)), therefore there was insufficient information to judge (Barr unpublished; Boroumandfar 2013; Dilli 2009; Efe 2007; Esfahani 2013; Goswami 2013; Taavoni 2009a; Thomas 2011), and the remaining study had a protocol available, but it was registered retrospectively (Gupta 2013).
Other potential sources of bias
Sample size
We rated nine studies as at high risk of bias due to fewer than 50 infants being enrolled in each arm (Barr unpublished; Boroumandfar 2013; Dilli 2009; Efe 2007; Esfahani 2013; Goswami 2013; Gupta 2013; Taavoni 2009a; Thomas 2011). One study had between 50 and 199 infants enrolled in each arm and was thus rated as at unclear risk of bias (Razek 2009). It should be noted that although Barr unpublished, Goswami 2013, Gupta 2013, and Taavoni 2009a were rated as at high risk of bias for sample size based on the Cochrane PaPaS guidance on sample size, these four studies reported performing sample size calculations. It should also be noted that Dilli 2009 included 162 infants between the ages of 0 and 6 months in their breastfeeding study (77 in breastfeeding group, 85 in control group), but because we only included the data for infants between the aged of 1 and 6 months, the reduced number per group led us to rate the study as at high risk of bias for sample size.
Other
We rated most studies as at low risk for other sources of bias (Barr unpublished; Boroumandfar 2013; Efe 2007; Esfahani 2013; Goswami 2013; Gupta 2013; Taavoni 2009a; Thomas 2011). One study, Razek 2009, included use of a pain assessment tool (Wong‐Baker FACES), which is considered as self report (Wong 1999), yet was reported by nursing staff. This tool is not validated for the infant population and was therefore rated as at high risk of bias. We rated one study as at unclear risk of bias due to demographic data (including information on number of injections and type of injection) being presented for the whole group only, rather than by intervention and control group (Dilli 2009).
Effects of interventions
Breastfeeding versus control: cry duration
Six studies comparing breastfeeding (547 infants in total) to control conditions reported cry duration during immunisation. Three studies reported the duration of crying up to a maximum of 3 minutes (Efe 2007; Goswami 2013; Gupta 2013), and two studies reported the period until all crying ceased (Dilli 2009; Razek 2009). One study reported the percentage of time the infant cried during three time periods (Barr unpublished): 60 seconds before the injection; from time of injection to 10 seconds after the injection; and from 11 seconds to 60 seconds after the injection. We obtained the raw data from the study authors. To make the data more consistent with other data in this meta‐analysis, we converted the raw data from Barr unpublished from percentage of time crying to cry time in seconds between time of injection and 60 seconds after the injection. Barr unpublished collected data from the same infants at two months and again at four months. We assumed independence and entered both age groups separately into this meta‐analysis.
Goswami 2013 included three groups (breastfeeding, 25% dextrose, distilled water). For the purpose of meta‐analysis, to ensure the groups were as comparable as possible, we included data for breastfeeding compared to distilled water in Analysis 1.1. Gupta 2013 also included three groups: topical EMLA combined with breastfeeding; topical EMLA combined with water; and topical placebo cream combined with water. For the purpose of meta‐analysis, we included data for topical EMLA combined with breastfeeding and topical EMLA combined with water in Analysis 1.1 to ensure the groups were as comparable as possible.
We pooled data for cry time (in seconds) from these six studies. Analysis 1.1 and Figure 4 show the results of the meta‐analysis. There was a significant reduction in cry time in seconds in the breastfeeding groups compared to the control groups (MD ‐38, 95% CI ‐50 to ‐26; P < 0.00001). However, there was high between‐study heterogeneity (I2 = 85%).

Forest plot of comparison: 1 Breastfeeding versus control, outcome: 1.1 Cry duration.
We judged the quality of the evidence according to the GRADE criteria, for breastfeeding during vaccinations, on the outcome of cry duration, to be moderate. Most of the included infants were younger than 6 months of age, and outcomes may be different for older infants during their 12‐month immunisation. We did not further downgrade the quality of evidence, but consider that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate (summary of findings Table 1).
Breastfeeding versus control: all pain scores
We pooled data for pain scores following immunisation from 5 studies and 310 infants (NIPS ‐ Dilli 2009, Esfahani 2013, Thomas 2011; MFCS ‐ Gupta 2013; MBPS ‐ Taavoni 2009a). Pain scores were collected at more than one time point for Gupta 2013, Taavoni 2009a, and Thomas 2011. The data used in this meta‐analysis was for the closest time point following completion of the procedure. We conducted the meta‐analysis using standardised mean differences (SMD). Results showed a significant reduction in pain scores in the breastfeeding group compared to the control (SMD ‐1.7, 95% CI ‐2.2 to ‐1.3; P < 0.00001) and moderate between‐study heterogeneity (I2 = 69%) (Analysis 1.2; Figure 5).

Forest plot of comparison: 1 Breastfeeding versus control, outcome: 1.2 All pain scores during injection.
We could not include MFCS scores from Goswami 2013 (for breastfeeding and control groups) in this composite pain score meta‐analysis, as data were presented in graphical form only. We contacted the authors requesting raw data but did not receive a response.
We judged the quality of the evidence according to the GRADE criteria, for breastfeeding during vaccinations, on the outcome of combined pain scores, to be moderate. Most of the included infants were younger than 6 months of age, and outcomes may be different for older infants during their 12‐month immunisation.
Breastfeeding versus control: NIPS
Five studies reported NIPS scores during immunisation (Boroumandfar 2013; Dilli 2009; Esfahani 2013; Razek 2009; Thomas 2011). Dilli 2009 included infants between the ages of 0 and 6 months, however they provided us with raw data, and we excluded infants under the age of 1 month from the analysis.
Boroumandfar 2013 presented the data in dichotomous form only (pain = NIPS score greater than 3, or no pain = NIPS score 3 or less), which precluded inclusion in meta‐analyses. We requested data from the authors but did not receive a response; we have therefore reported the data in narrative form only. The authors reported statistically significant differences in frequency of pain between the three groups (breastfeeding, vapocoolant, and control), with the breastfeeding group having significantly lower NIPS scores during the vaccination procedure (P < 0.001).
Razek 2009 presented the frequency of infants with NIPS scores of 0, 1, 2, 3, 4, and "hurts worst". The inclusion of the "hurts worst" category is inconsistent with the NIPS tool, which is a score ranging from 0 to 7. We contacted the authors for clarification and to request raw data but did not receive a response, and therefore excluded this data from our meta‐analysis. Although the published paper did not state whether there was a statistically significant difference in frequency of each NIPS score between the breastfeeding and control groups, 6 of the 60 infants (10%) in the breastfeeding group scored the highest NIPS scores of 5 to 7, whereas 12 of the 60 infants (20%) in the control group scored the highest NIPS scores.
We pooled NIPS scores during immunisation for meta‐analysis from three studies enrolling 174 infants (Dilli 2009; Esfahani 2013; Thomas 2011). Esfahani 2013 included three groups (breastfeeding, massage, and control), but we included only breastfeeding and control in the meta‐analysis. Meta‐analysis results showed a significant reduction in mean NIPS scores in the breastfeeding group compared to control (MD ‐1.9, 95% CI ‐2.5 to ‐1.2; P = 0.04). There was moderate between‐study heterogeneity (I2 = 69%) (Analysis 1.3; Figure 6).

Forest plot of comparison: 1 Breastfeeding versus control, outcome: 1.3 NIPS.
We judged the quality of the evidence according to the GRADE criteria, for breastfeeding during vaccinations, on the outcome of the behavioural pain score NIPS, to be moderate. Most of the included infants were younger than 6 months of age, and outcomes may be different for older infants during their 12‐month immunisation.
Breastfeeding versus control: heart rate
One study measured heart rate during immunisation (Efe 2007). Based on 66 infants, there was no statistically significant difference in mean heart rate. Heart rate following completion of the injections was measured by Efe 2007 (N = 66) and Razek 2009 (N = 120). We pooled data for heart rate following the procedure and found no statistically significant differences (MD ‐4, 95% CI ‐23 to 16; P = 0.03) (Analysis 1.4). There was high heterogeneity (I2 = 78%).
As we could pool results for only two studies, we judged the quality of the evidence according to the GRADE criteria, for breastfeeding during vaccinations, on the outcome of heart rate differences, to be low.
Breastfeeding versus control: oxygen saturation
One study measured oxygen saturation during and after immunisation (Efe 2007). There was no statistically significant difference in mean oxygen saturation during or following completion of the injection.
As only one study measured oxygen saturation, we judged the quality of the evidence to be very low.
Breastfeeding versus control: MBPS
One study of 76 infants reported MBPS scores 5 seconds before the injection and 15 seconds after the injection (Taavoni 2009a). There was no significant difference in MBPS scores for the breastfeeding group 5 seconds before the injection, however breastfeeding resulted in a statistically significant mean reduction of MBPS of 3.8 in the 15 seconds following the injection procedure.
As only one study measured the MBPS, we judged the quality of the evidence to be very low.
Comparisons of breastfeeding with other interventions
Four studies had more than two study arms (Boroumandfar 2013; Esfahani 2013; Goswami 2013; Gupta 2013). Our summaries of the results for these additional arms are as follows.
Breastfeeding versus 25% dextrose versus water: MFCS and cry duration
Goswami 2013 included three study arms: breastfeeding, water, and 25% dextrose. MFCS data were presented in graphical form only and therefore could not be extracted. The authors reported a significant reduction in MFCS for infants who were breastfed or given 25% dextrose compared to water at 1 minute and 3 minutes after needle insertion. Breastfeeding resulted in a significantly reduced cry time compared to 25% dextrose and compared to water.
Breastfeeding with EMLA versus control with EMLA versus no intervention: MFCS and cry duration
Gupta 2013 reported MFCS scores and crying characteristics for three groups: EMLA combined with breastfeeding, EMLA combined with water, and placebo cream combined with water. The combination of EMLA and breastfeeding resulted in a statistically significant reduction in MFCS pain scores at 1 minute and 3 minutes following the vaccinations compared to EMLA and water. In addition, the combination of breastfeeding and EMLA resulted in significantly shorter crying duration compared to both EMLA and water, and placebo cream and water.
Breastfeeding versus vapocoolant versus water: NIPS scores > 3
Boroumandfar 2013 included three study arms: breastfeeding, water and vapocoolant. They classified NIPS pain scores of greater than 3 as "pain". The number and proportion of babies allocated a score of "pain" were lowest in the breastfeeding group compared to vapocoolant and water (P < 0.001): breastfeeding: n = 17/48 (35%); vapocoolant: n = 36/48 (75%); and control (water): n = 48/48 (100%).
Breastfeeding versus massage with maternal hugging versus maternal hugging only (control): NIPS scores
Esfahani 2013 reported that the mean (standard deviation) of NIPS in the breastfeeding, massage combined with maternal hugging, and maternal hugging only (control) groups were 3.4 (0.83), 3.9 (1.0), and 4.8 (1.1), respectively. The difference between breastfeeding and massage combined with maternal hugging was statistically significant (P = 0.04), and the differences between both interventions compared to the control were statistically significant (breastfeeding compared to control: P < 0.001; massage with maternal hugging compared to control: P = 0.002).
Secondary outcomes and subgroup analyses
None of the included studies reported adverse effects such as choking, gagging, spitting, coughing, aspiration, or cyanosis. Only Dilli 2009 reported the number of infants coming off the breast during the procedure, stating that 4 of the 158 infants (2.5%) failed to complete the study as they did not resume feeding.
We were unable to conduct planned subgroup analyses based on age of the infants at the time of study as data were not reported separately for the different ages. Importantly, however, only two studies included infants up to 12 months of age. Esfahani 2013 included infants at 6 and 12 months of age, and Razek 2009 included infants from 1 month to 12 months of age.
Discussion
Summary of main results
This systematic review of RCTs or quasi‐RCTs evaluating breastfeeding for procedural‐pain reduction in infants aged 1 to 12 months included 10 trials. All trials evaluated breastfeeding during early childhood vaccination, with nine of the 10 evaluating breastfeeding during a single injection. Results showed that breastfeeding was associated with statistically significant reductions in cry duration and composite pain scores (NIPS, NFCS, MFCS, MBPS, Wong‐Baker FACES Pain Rating Scale) compared to no treatment or water. Breastfeeding was not associated with a statistically significant reduction in physiological responses of heart rate or oxygen saturation levels. Breastfeeding was more effective in reducing pain than other interventions including massage combined with maternal hugging, maternal hugging alone, topical vapocoolant, topical anaesthetic cream (EMLA), and 25% dextrose.
Outcome measures differed across the studies, however we were able to pool results for meta‐analysis for crying duration in seconds for six studies. Results showed a statistically significant reduction in cry duration of 38 seconds associated with breastfeeding compared to control. We were also able to conduct a meta‐analysis using standardised mean pain scores, pooled from five studies. Based on a pain score range of 0 to 10, with 10 being the maximum pain score, breastfeeding was associated with a statistically significant reduction in pain scores of ‐1.73.
Only two studies reported physiological responses of heart rate following the vaccination procedure (Efe 2007; Razek 2009). A meta‐analysis of these pooled data for 186 infants showed a non‐significant reduction of 3.6 beats/min, with a wide 95% CI of ‐23.17 to 16.05 beats/min. Only Efe 2007 reported oxygen saturation changes, and showed no differences between study groups.
No study reported any adverse outcomes. In addition, no study reported on acceptability of breastfeeding, from the perspectives of mothers or healthcare providers, and no studies reported on the logistics of facilitating breastfeeding in the clinical settings. Only one study reported on infants failing to continue breastfeeding after the vaccination Dilli 2009, and this applied to only a small number of infants.
Eight of the 10 trials included infants aged 1 to 6 months, with only 2 studies including older infants up to 1 year of age (Esfahani 2013; Razek 2009). We could not conduct any subgroup analyses by age, as the study outcomes were not reported in sufficient detail to be extracted for pooling, or data differed. For example, Razek 2009 reported means and standard deviations of cry duration, and Esfahani 2013 reported means and standard deviations of NIPS scores. However, individual results of these two studies were consistent with the overall results, showing a statistically significant reduction in behavioural pain responses.
Breastfeeding effectively reduces needle‐pain during vaccinations, based on reduced crying and pain scores. If mothers are breastfeeding, then breastfeeding is free during vaccinations. Its use is therefore recommended during early childhood vaccinations. Breastfeeding mothers are encouraged to advocate for their infants and request to breastfeed before and during early childhood vaccinations.
Overall completeness and applicability of evidence
All 10 studies in this systematic review reported that breastfeeding reduced behavioural responses to pain during vaccination injections in infants beyond the newborn period compared to no treatment or control conditions. These consistent findings across diverse settings globally (Iran (n = 3); India (n = 2); Turkey (n = 2), Jordan (n = 1); Canada (n = 1)) highlights the applicability of the evidence and the generalisability of the findings.
Eight of the 10 studies in this review assessed the analgesic effects of breastfeeding prior to and during early childhood vaccination in infants aged 1 to 12 months during single‐vaccination procedures; one study evaluated breastfeeding during multiple injections in a single consultation (Dilli 2009), and one study evaluated effects at two time points, a two‐month and a four‐month vaccination (Barr 2001). We can conclude that the results of this review are applicable to large populations of infants requiring early childhood immunisation. If the mother is breastfeeding, then breastfeeding infants during vaccinations and other non‐urgent painful procedures should be promoted and supported where feasible. Potential benefits of breastfeeding during painful procedures, in addition to pain reduction for the infant, include the possibility that this may further encourage mothers to continue to breastfeed, and empower the mother through her knowledge that she can effectively reduce her baby's distress during a painful procedure. However, if the mother did not establish breastfeeding, or had ceased to breastfeed by the time of early childhood vaccinations, or breastfeeding during vaccinations is simply not possible, sucrose or glucose with or without a pacifier can be recommended for pain treatment (Harrison 2010a; Harrison 2010c).
Quality of the evidence
All studies scored high risk of bias for blinding of participants and personnel, and all studies except for one scored high risk of bias for blinding of outcome assessment, highlighting the challenges in conducting research evaluating breastfeeding for procedural‐pain treatment. Based on the Cochrane PaPaS guidance on sample size, we scored nine of the 10 included studies as high risk of bias, as there were fewer than 50 participants per arm. However, three of these nine studies that included fewer than 50 participants per arm reported their a priori power calculation to inform their sample size.
Most studies scored low risk of bias for incomplete outcome data and other bias, while results were variable for random sequence generation and allocation concealment. We were unable to identify completed protocols for most studies, therefore we predominantly gave a risk of bias rating of unclear for selective reporting bias.
For behavioural responses of crying time and standardised pain scores, the overall evidence based on GRADE was moderate. As most studies (8 out of 10) included infants in the younger age spectrum, of 1 to 6 months, further research including older infants up to 12 months of age may have an important impact on our confidence in the estimate of effect and may change the estimate. As we could only pool heart rate data for two studies, evidence for physiological outcomes based on GRADE was considered to be low quality, and further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Potential biases in the review process
There were no known potential biases in the review process. We performed extensive searches of the literature with no language restrictions, and attempted to contact authors for additional information where required.
Agreements and disagreements with other studies or reviews
In terms of effects of breastfeeding on behavioural responses to pain, these review findings agree with the systematic review of breastfeeding for procedural pain in newborn infants (Shah 2012), and a recently published systematic review of interventions to reduce vaccine pain that included infants and children (Shah 2015). In all three systematic reviews, breastfeeding resulted in a statistically significant reduction in behavioural parameters and composite pain scores. However, in this current systematic review, there were no consistent effects on physiological parameters of heart rate and oxygen saturation levels. This finding disagrees with the Cochrane systematic review of breastfeeding for procedural pain in neonates (Shah 2012), in which breastfeeding reduced heart rate change from baseline compared to being held by the mother, sucking on a pacifier, sucrose, or simply positioned supine or prone. Both Shah 2012 and this current review included one study that compared breastfeeding with small volumes of sweet solutions. Results of both reviews showed that breastfeeding was associated with reduced behavioural responses to pain compared to sweet solutions. In pain studies, a difference of at least 13% is considered by parents of newborn infants to be clinically important, and a difference of 19% is considered by nurses to be important (Shah 2004). The 1.75 difference in pain scores out of a possible standardised score of 10 is 17.5%, therefore well within the range of what parents consider to be important, and just under what nurses in general consider to be important.
Our findings in this review are also consistent with that of the systematic review of skin‐to‐skin care for procedural pain in neonates (Johnston 2014); both reviews demonstrated that maternal care resulted in a statistically significant difference in composite pain scores immediately and within 90 seconds after completion of the painful procedures compared to no treatment, yet there were inconsistencies in physiological responses of heart rate and oxygen saturation levels. To our knowledge, there are no studies evaluating skin‐to‐skin care in infants beyond the neonatal period, and there are only three studies that included full‐term newborn infants in the systematic review of skin‐to‐skin care for procedural pain in neonates (Johnston 2014). We are therefore unable to make a direct comparison of analgesic effects of breastfeeding and skin‐to‐skin care in the age group of 1 to 12 months.
Adverse outcomes were not reported in any of the trials included in this review, or in the systematic review of breastfeeding for procedural pain in neonates (Shah 2012). This suggests that there are no adverse outcomes such as airway compromise including coughing, choking, gagging, or aspiration. However, the inclusion of adverse events as an outcome measure and clear reporting of the same in future studies of breastfeeding all populations of infants and young children during painful procedures is recommended.
In this review, we identified no studies comparing formula feeding or bottle feeding expressed breast milk with breastfeeding. Shah 2012 included one such study in their systematic review of breastfeeding for procedural pain in neonates (Weissman 2009), which showed that formula feeding was as effective as breastfeeding in reducing behavioural and physiological responses during heel lance.
Breastfeeding is now recommended by the World Health Organization (WHO) for pain management for infants (World Health Organization 2015). Although previous studies have shown that breastfeeding is infrequently used for vaccination pain management (Lisi 2013; Russell 2015; Taddio 2009), the global recommendations made by WHO, alongside the additional evidence of pain management effectiveness provided in this systematic review, may lead to more consistent adoption of this strategy in diverse settings where vaccinations take place.

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Breastfeeding versus control, outcome: 1.1 Cry duration.

Forest plot of comparison: 1 Breastfeeding versus control, outcome: 1.2 All pain scores during injection.

Forest plot of comparison: 1 Breastfeeding versus control, outcome: 1.3 NIPS.

Comparison 1: Breastfeeding versus control, Outcome 1: Cry duration

Comparison 1: Breastfeeding versus control, Outcome 2: All pain scores during injection

Comparison 1: Breastfeeding versus control, Outcome 3: NIPS

Comparison 1: Breastfeeding versus control, Outcome 4: Heart rate after injection
| Breastfeeding compared with other interventions, oral water, or no treatment for pain during vaccination in infants 1 to 12 months | ||||
| Patient or population: Infants 1 to 12 months during vaccination Settings: Diverse community settings Intervention: Breastfeeding Comparison: Other interventions, such as cuddling, sweet solutions, or placebo (oral water) or no treatment | ||||
| Outcomes | No of Participants | Effect Estimates and 95% CI | Quality of the evidence | Comments |
|---|---|---|---|---|
| Cry duration Seconds cry time during procedure or proportion of crying during procedure. Crying was measured during and up to 3 minutes following completion of vaccination. | 547 (6 studies) | MD (seconds) ‐38 ( ‐49.84 to 26.35) | Moderate | Not further downgraded: Breastfeeding consistently resulted in a reduction of crying time. However, as most trials included infants aged 1 to 6 months, further research including older infants up to 12 months of age may have an important impact on our confidence in the estimate of effect and may change the estimate. |
| All pain scores during injection Due to range of pain scores used, data analysed using SMDs. Pain scores were measured during and up to 3 minutes following completion of vaccination. | 310 (5 studies) | SMD ‐1.7 ( ‐2.2 to ‐1.3) | Moderate | Downgraded once: Breastfeeding consistently resulted in a reduction of pain scores. However, only 5 studies and 310 infants were included, and most of the trials included the younger infants aged 1 to 6 months. Further research including older infants up to 12 months of age and larger sample sizes may have an important impact on our confidence in the estimate of effect. |
| NIPS score during injection The NIPS was measured during and up to 3 minutes following completion of vaccination. | 174 (3 studies) | MD ‐1.89 (‐2.55 to ‐1.24) | Low | Not further downgraded: Grade of evidence already taken into account. Only 3 studies and 174 infants were included. Further studies using this pain assessment score may lead to more certainty and have an important impact on our confidence in the estimate of effect. |
| Heart rate after injection Heart rate in the period following completion of the injections was measured in 2 studies. | 186 (2 studies) | MD (beats per minute) ‐3.6 ( ‐23 to 16) | Low | Not further downgraded: Grade of evidence already taken into account. Breastfeeding did not have an effect on heart rate change from baseline. As only 2 studies were included in this outcome, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. |
| Adverse events | No studies reported on adverse events. | ‐ | Very low | No studies reported on outcomes such as coughing or gagging. |
| CI: confidence interval; MD: mean difference; NIPS: Neonatal Infant Pain Scale; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Cry duration Show forest plot | 6 | 547 | Mean Difference (IV, Random, 95% CI) | ‐38.09 [‐49.84, ‐26.35] |
| 1.2 All pain scores during injection Show forest plot | 5 | 310 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐2.20, ‐1.25] |
| 1.3 NIPS Show forest plot | 3 | 174 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐2.55, ‐1.24] |
| 1.4 Heart rate after injection Show forest plot | 2 | 186 | Mean Difference (IV, Random, 95% CI) | ‐3.56 [‐23.17, 16.05] |

