Pruebas para la detección del estrabismo en niños de uno a seis años de edad en la comunidad
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011221.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 06 noviembre 2017see what's new
- Tipo:
-
- Diagnostic
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud ocular y de la visión
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All authors helped to draft the protocol. Sarah Hull and Vijay Tailor reviewed the abstracts provided by the search as well as full articles which potentially met our inclusion criteria. They extracted relevant data, wrote the Results section and updated this article from protocol to full review. Annegret Dahlmann‐Noor provided a third opinion on study inclusion where required. Gianni Virgili reviewed the analysis and provided critical review of findings. Jugnoo Rahi and Christine Schmucker critically reviewed the final version of this article.
Sources of support
Internal sources
-
NIHR Biomedical Research Centre, UK.
The contributions by Annegret Dahlmann‐Noor to this review were supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
External sources
-
National Institute for Health Research (NIHR), UK.
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Declarations of interest
The authors have no interests to declare.
Acknowledgements
Cochrane Eyes and Vision (CEV) created and executed the electronic search strategies. We wish to thank Mrs Angela Coleman, Head Orthoptist at Moorfields Eye Centre at Bedford Hospital, for the critical review of the protocol; and Tess Garretty, Helen Griffiths and Fiona Rowe for external peer review comments on the protocol and review. We thank Anupa Shah, Managing Editor for CEV for her assistance throughout the review process.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Nov 06 | Tests for detecting strabismus in children aged 1 to 6 years in the community | Review | Sarah Hull, Vijay Tailor, Sara Balduzzi, Jugnoo Rahi, Christine Schmucker, Gianni Virgili, Annegret Dahlmann‐Noor | |
| 2014 Jul 23 | Tests for detecting strabismus in children age 1 to 6 years in the community | Protocol | Vijay Tailor, Sara Balduzzi, Sarah Hull, Jugnoo Rahi, Christine Schmucker, Gianni Virgili, Annegret Dahlmann‐Noor | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Child; Child, Preschool; Humans; Infant;

Study flow diagram.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
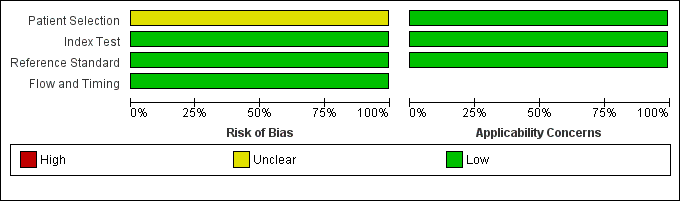
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies

Forest plot of 1 Photoscreener.
| Accuracy of a photoscreener to detect strabismus in the community | ||||||
| Patient/population: children aged 1 to 6 years old Setting: school Index test: plusoptix S04 photoscreener Target condition: constant and intermittent manifest strabismus Reference standard: cover test at distance and near | ||||||
| Number of studies | Number of participants | Number affected by target condition | Sensitivity of test (95% CI) | Specificity of test (95% CI) | Risk of bias based on QUADAS‐2 domains | Comments |
| 271 | 13 | 0.46 (0.19 to 0.75) | 0.97 (0.94 to 0.99) | Unclear risk | Low participation rate of 25% | |
| CI: confidence intervals | ||||||
| Study ID | Arthur 2009 |
| Clinical features and settings | Previous testing and results: unknown. Referral route/selection: all who were screened offered gold standard examination. |
| Participants | Sample size: 306 screened (1343 invited to study (consents sent: this may have introduced selection bias from concerned parents being more likely to return consent forms), 387 returned, 45 excluded as consents too late, 7 excluded for document errors, 28 absent on day of screening, 1 uncooperative), 275 gold standard exam (14 declined, 11 unable to attend within time frame, 6 uncontactable) of which 271 data interpretable for both index and reference (3 photographs unusable, 1 did not complete exam). Socio‐demographic items: 98% 4 to 5 years of age, gender and ethnicity not given, no ocular abnormalities (i.e. media opacities, which would affect test results/technical failure rates). Geographic region: Limestone school district, Ontario, Canada. |
| Study design | Selection: all patients with data available on both index and reference tests as single group. |
| Target condition | Constant and intermittent manifest strabismus (esotropia, exotropia, vertical tropia, microtropia), prevalence of the target condition in the sample: 13 (of 271). |
| Reference standard | Test definition and description: monocular visual acuity with occlusion glasses (crowded Keeler logMAR letter matching test/Crowded Kay pictures/Cardiff cards), cover test at distance and near, ocular movements and convergence, binocular single vision assessment (20D base‐out prism test and/or stereopsis) and red reflex test. Standards: discharged if VA 0.2 logMAR or better, binocular single vision at distance and near and no suspected ocular pathology, 6‐ to 12‐week review and re‐check if borderline, cycloplegic refraction/dilated examination all others. |
| Index tests | plusoptiX S04 photoscreener, co‐axial camera, handheld at 1 m. |
| Follow‐up | How many participants were lost to follow‐up: 31. |
| Notes | Sources of funding: none declared. Anything else of relevance: low participation rate (25%). |
| Study ID | First author, year of publication |
| Clinical features and settings | Previous testing and results. Referral route/selection. |
| Participants | Sample size. Socio‐demographic items: age, gender, ethnicity, frequency of ocular abnormalities (i.e. media opacities, which would affect test results/technical failure rates), geographic region. |
| Study design | Selection: as single group/as separate group with/without target condition. |
| Target condition | Constant and intermittent manifest strabismus (esotropia, exotropia, vertical tropia, microtropia), including the prevalence of the target condition in the sample. |
| Reference standard | Test definition and description, i.e. cover test; 'comprehensive eye examination' (visual acuity, cover test, cycloplegic refraction). |
| Index tests | Test definition and description. |
| Follow‐up | How many participants were lost to follow‐up: unknown. |
| Notes | Sources of funding. Abbreviations. Anything else of relevance. |
| Domain | Yes | No | Unclear |
| PATIENT SELECTION Describe methods of patient selection: Describe included patients (prior testing, presentation, intended use of index test and setting) | |||
| Was a consecutive or random sample of patients enrolled? | Consecutive sampling or random sampling of children according to inclusion criteria. | Non‐random sampling or sampling based on volunteering or referral. | Unclear whether consecutive or random sampling used. |
| Was a case‐control design avoided? | Yes for all studies since case‐control studies are excluded unless nested in cohort studies. | N/A | N/A |
| Did the study avoid inappropriate exclusions? | Exclusions are detailed and felt to be appropriate (systemic disease causing strabismus). Children with known strabismus can be excluded. | Inappropriate exclusions are reported e.g. of children in whom strabismus has been suspected in primary care but not confirmed by trained professionals. | Exclusions are not detailed (pending contact with study authors). |
| Risk of bias: could the selection of patients have introduced bias? | ‐ | ‐ | ‐ |
| Concerns regarding applicability: are there concerns that the included patients do not match the review question? | Inclusion of children in community settings, such as school or screening settings, with no previous diagnosis of any eye disease. | Inclusion of children over the age of 6 years, referred to clinical settings, referred to eye professionals for suspect eye disease, or assessed in commercial settings on a volunteer basis; or previous diagnosis of failed screening test or strabismus. | Unclear inclusion criteria. |
| INDEX TEST Describe the index test and how it was conducted and interpreted | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Test performed "blinded" or "independently and without knowledge of" reference standard results are sufficient and full details of the blinding procedure are not required; or clear temporal pattern to the order of testing that precludes the need for formal blinding. | Reference standard results available to those who conducted or interpreted the index tests. | Unclear whether results are interpreted independently. |
| If a threshold was used, was it pre‐specified? | Many included index tests are based on continuous measures (e.g. eye deviation, stereopsis, refractive error, visual acuity); the study authors declare that the selected cut‐off used to dichotomise data was specified a priori, or a protocol is available with this information. | A study is classified at higher risk of bias if the authors define the optimal cut‐off post hoc based on their own study data. | No information on pre‐selection of index test cut‐off values. |
| Risk of bias: could the conduct or interpretation of the index test have introduced bias? | ‐ | ‐ | ‐ |
| Concerns regarding applicability: are there concerns that the index test, its conduct, or interpretation differ from the review question? | Tests used and testing procedure clearly reported and tests executed by personnel with sufficient training. | Tests used are not validated or study personnel is insufficiently trained. | Unclear tests (e.g. stereopsis‐based tests but does not mention if a validated test is used) or unclear study personnel profile, background and training. |
| REFERENCE STANDARD Describe the reference standard and how it was conducted and interpreted | |||
| Is the reference standard likely to correctly classify the target condition? | Cover‒uncover test performed by trained professionals, e.g. ophthalmologists, optometrists, orthoptists. | Complete eye examination with cover‒uncover test used as reference standard but not only the cover‒uncover test used to judge on strabismus (e.g. visual acuity measure also used). | Complete eye examination used but unclear whether cover‐uncover test used. |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Reference standard performed "blinded" or "independently and without knowledge of" index test results are sufficient and full details of the blinding procedure are not required; or clear temporal pattern to the order of testing that precludes the need for formal blinding. | Index test results available to those who conducted the reference standard; or the index test is part of the reference standard (e.g. visual acuity within a compete ophthalmic examination used as reference standard and visual acuity is also the index test analysed ‒ this will be specific of each analysis). | Unclear whether results are interpreted independently. |
| Risk of bias: could the reference standard, its conduct, or its interpretation have introduced bias? |
|
|
|
| Concerns regarding applicability: are there concerns that the target condition as defined by the reference standard does not match the review question? | Cover‒uncover test used and testing procedure executed by personnel with sufficient training. | Cover‒uncover test used by personnel with inappropriate profile or insufficient training. | Unclear study personnel profile, background and training. |
| FLOW AND TIMING Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2×2 table (refer to flow diagram): describe the time interval and any interventions between index test(s) and reference standard | |||
| Was there an appropriate interval between index test(s) and reference standard? | No more than three months between index and reference test execution, and no corrective intervention between assessments. | More than three months between index and reference test execution. | Unclear whether test results are executed within three months. |
| Did all patients receive a reference standard? | The verification rate of index test‐positive children is definitely higher than that of negative children (the opposite is unlikely). | All children receiving the index test are verified with the reference standard. | Unclear whether all children receiving the index test are verified with the reference standard. |
| Did all patients receive the same reference standard? | All children are verified with the cover‒uncover test by trained professionals. | Some children, i.e. positive children, are verified with the cover‒uncover test by specialised personnel, while the others are verified by personnel with lower level of training. | Unclear whether all children are verified with the cover‒uncover test by trained professionals. |
| Were all patients included in the analysis? | The number of children included in the study does not match the number in analyses or children with undefined or borderline test results are excluded. However, children in whom one or more index tests are not performed because they are poorly cooperative can be excluded. | The number of children included in the study does not match the number in analyses and children with undefined or borderline test results are excluded from the analyses. | The number of children analysed, but not that included in the study, are reported; or unclear if there were inappropriate exclusions. |
| Risk of bias: could the patient flow have introduced bias? | ‐ | ‐ | ‐ |
| COMPARATIVE STUDIES (MULTIPLE INDEX TESTS) | |||
| Were all tests performed on all patients, or randomly assigned? | All children received all index tests, or tests were randomly assigned. | Not all children received all index tests and the assignment criterion was opportunistic or non‐random (e.g. depending on test availability or type of professional). | Not all children received all index tests and the assignment criterion was unclear. |
| Could the order in which the index tests were used affect the target condition or the interpretation of the alternative tests? | The order of presentation of the index test was random or alternate to avoid fatigue effects; or clear that no fatigue effect can arise. | Several tests are delivered in a fixed order which can cause children to be less compliant with the second or later test. | Unclear order of test presentation. |
| Test type categories | Tests included | Output measure | Threshold to extract data |
| 1) Tests which identify ocular misalignment | 1.1) Corneal reflections tests: Hirschberg, Krimsky (prism reflection test). | Prism dioptres (PD). | 8 PD for horizontal deviations; 1 PD for vertical deviations (no published threshold identified). |
| 2) Test of binocular function: stereopsis | Stereoacuity tests such as contour and random dot stereotests. | Seconds of arc. | 400 seconds of arc. |
| 3) Tests designed to detect reduced ventral vision | 3.1) Visual acuity tests, e.g. HOTV, LEA symbols, Keeler (previously Glasgow) crowded logMAR, Sonksen crowded logMAR, crowded Kay picture test. 3.2) Suppression tests. | LogMAR or logMAR equivalent. | 0.2 logMAR. |
| 4) Automated refraction devices designed to report ocular misalignment | ‐ | Millimetres of asymmetry or corneal reflections. | No published threshold identified. |
| Test | No. of studies | No. of participants |
| 1 Photoscreener Show forest plot | 1 | 271 |


