Alemtuzumab bei Multipler Sklerose
Referencias
References to studies included in this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Main interventions
Comparator
All participants received 1 g of intravenous methylprednisolone for 3 days at baseline and at months 12 and 24, coinciding with infusion cycles as premedication for those receiving alemtuzumab. Some participants also received antihistamines or antipyretics at the investigators' discretion | |
| Outcomes | Primary outcome measure
Secondary outcome measures
All adverse events with an onset up to 36 months were reported. In addition, all serious adverse events and autoimmune‐associated disorders occurring before 1 March 2008, were listed. A subsequent adverse event of Burkitt's lymphoma not associated with Epstein–Barr virus (EBV) was also included in this report. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were randomly assigned in a 1:1:1 with the use of the Pocock and Simon minimisation algorithm. |
| Allocation concealment (selection bias) | Unclear risk | No information available to allow a judgement |
| Blinding of participants and personnel (performance bias) | High risk | Both study drugs have adverse effects that precluded masking of participants and treating clinicians to treatment assignment |
| Blinding of outcome EDSS assessment | Low risk | "EDSS scores were determined quarterly in a blinded fashion by a neurologist who also adjudicated possible relapses". |
| Blinding of outcome assessment (detection bias) | Low risk | "MRI scans were performed annually and interpreted by a neuroradiologist who was unaware of assignments to study groups". |
| Blinding of safety outcome assessment | High risk | "Safety was assessed quarterly by the treating neurologist, who was aware of study‐group assignment". |
| Incomplete outcome data (attrition bias) | Low risk | Number and reasons of withdrawals were similar in the interventions and control arms. |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | — |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Main interventions
After a protocol amendment in January 2009, alemtuzumab participants received oral aciclovir 200 mg twice daily during alemtuzumab infusion and for 28 days thereafter as prophylaxis against herpes infection. Comparator
Participants in both groups received 1 g per day of intravenous methylprednisolone on 3 consecutive days at baseline and at month 12. Concomitant treatment with an antipyretic or antihistamine drug was allowed, at the discretion of the treating neurologist. | |
| Outcomes | Primary outcome measures
Secondary outcome measure (24 months)
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomly allocated patients using an interactive voice response system" |
| Allocation concealment (selection bias) | Low risk | Use of an interactive voice response system |
| Blinding of participants and personnel (performance bias) | High risk | "Both study drugs have adverse effects that precluded masking of patients and treating clinicians to treatment assignment, and subcutaneous interferon beta 1a was available only in proprietary prefilled syringes that could not effectively be duplicated for placebo" |
| Blinding of outcome EDSS assessment | High risk | "In the absence of a masked rater, unmasked raters could submit EDSS assessments" |
| Blinding of outcome assessment (detection bias) | Low risk | "Stringent clinical and MRI rater masking, and adjudication of relapses by a committee comprising six independent and masked neurologists" |
| Blinding of safety outcome assessment | Low risk | "Stringent clinical and MRI rater masking, and adjudication of relapses by a committee comprising six independent and masked neurologists" |
| Incomplete outcome data (attrition bias) | High risk | Only participants who received at least one dose of study drugs were included in the efficacy and safety analyses according to treatment assignment (modified ITT analysis) |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | |
| Methods |
| |
| Participants | Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Main Interventions
Alemtuzumab was administered in 2 annual cycles, once at the beginning of the study and again 1 year later
Comparator
| |
| Outcomes | Primary outcome measure
Secondary outcome measures
Co‐primary endpoints were relapse rate and time to 6‐month sustained accumulation of disability, comparing alemtuzumab 12 mg and interferon beta‐1a in all participants who received at least one dose of study drug. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomly allocated patients with an interactive voice response system in a 2:2:1 scheme" |
| Allocation concealment (selection bias) | Low risk | Use of an interactive voice response system |
| Blinding of participants and personnel (performance bias) | High risk | "Because both study drugs had adverse effects that precluded double‐blinding, and interferon beta 1a proprietary syringes could not effectively be duplicated for placebo" |
| Blinding of outcome EDSS assessment | High risk | "In the absence of a masked rater, unmasked raters could submit EDSS assessments" |
| Blinding of outcome assessment (detection bias) | Low risk | "Raters were masked to treatment‐group assignment" |
| Blinding of safety outcome assessment | Low risk | "Raters were masked to treatment‐group assignment" |
| Incomplete outcome data (attrition bias) | Unclear risk | — |
| Selective reporting (reporting bias) | High risk | Efficacy outcomes for alemtuzumab 24 mg were not provided. The results from some previously planned outcomes were not provided (i.e. quality of life) |
| Other bias | Low risk | |
EDSS: Expanded Disability Status Scale
ITT: intention‐to treat
IV: intravenous
MRI: magnetic resonance imaging
MS: multiple sclerosis
SC: subcutaneous
VAS: visual analogue scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse‐free survival Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 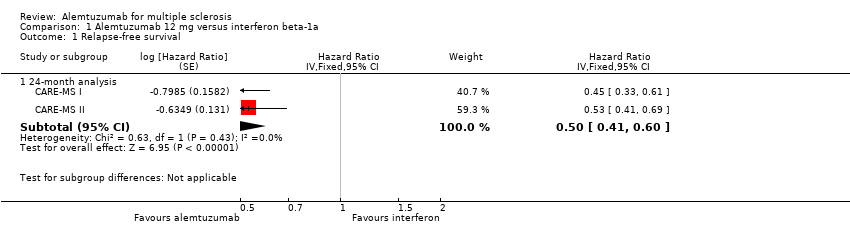 Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 1 Relapse‐free survival. | ||||
| 1.1 24‐month analysis | 2 | Hazard Ratio (Fixed, 95% CI) | 0.50 [0.41, 0.60] | |
| 2 Sustained disease progression‐free survival Show forest plot | 3 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 2 Sustained disease progression‐free survival. | ||||
| 2.1 24‐month analysis | 2 | Hazard Ratio (Fixed, 95% CI) | 0.62 [0.44, 0.87] | |
| 2.2 36‐month analysis | 1 | Hazard Ratio (Fixed, 95% CI) | 0.25 [0.11, 0.57] | |
| 3 Number of participants with at least one adverse event Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 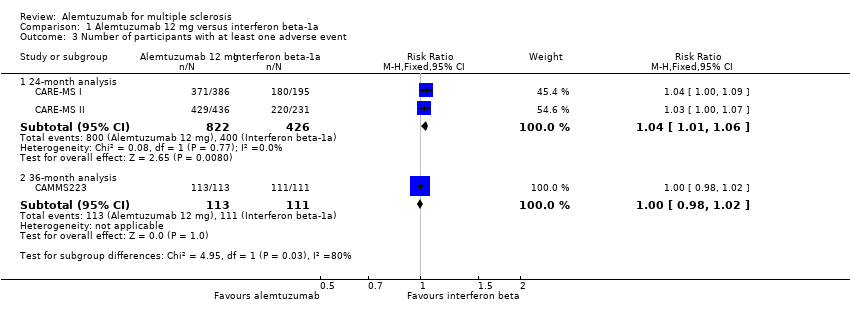 Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 3 Number of participants with at least one adverse event. | ||||
| 3.1 24‐month analysis | 2 | 1248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [1.01, 1.06] |
| 3.2 36‐month analysis | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.98, 1.02] |
| 4 Change in EDSS score Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.4 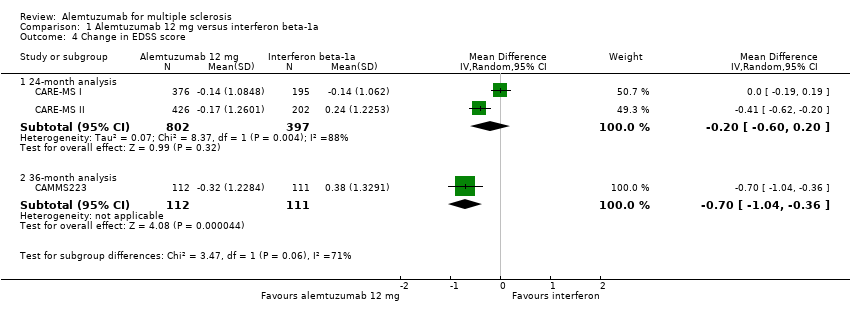 Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 4 Change in EDSS score. | ||||
| 4.1 24‐month analysis | 2 | 1199 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 4.2 36‐month analysis | 1 | 223 | Mean Difference (IV, Random, 95% CI) | ‐0.7 [‐1.04, ‐0.36] |
| 5 Number of participants with new or enlarging T2‐hyperintense lesions Show forest plot | 2 | 1238 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.91] |
| Analysis 1.5  Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 5 Number of participants with new or enlarging T2‐hyperintense lesions. | ||||
| 6 Number of dropouts Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6 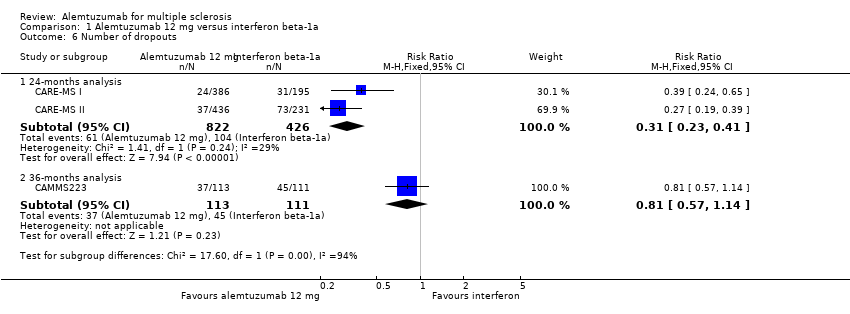 Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 6 Number of dropouts. | ||||
| 6.1 24‐months analysis | 2 | 1248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.23, 0.41] |
| 6.2 36‐months analysis | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.14] |

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Alemtuzumab 12 mg versus interferon beta‐1a, outcome: 1.1 Relapse‐free survival.

Forest plot of comparison: 1 Alemtuzumab 12 mg versus interferon beta‐1a, outcome: 1.2 Sustained disease progression‐free survival.

Forest plot of comparison: 1 Alemtuzumab 12 mg versus interferon beta‐1a, outcome: 1.2 Rate of participants with at least one adverse event.

Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 1 Relapse‐free survival.

Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 2 Sustained disease progression‐free survival.

Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 3 Number of participants with at least one adverse event.

Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 4 Change in EDSS score.

Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 5 Number of participants with new or enlarging T2‐hyperintense lesions.
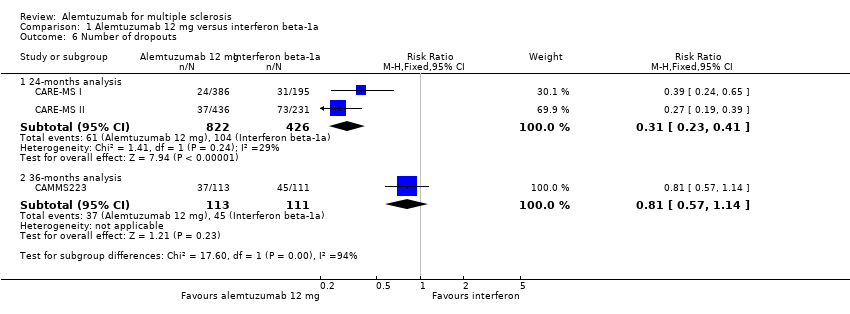
Comparison 1 Alemtuzumab 12 mg versus interferon beta‐1a, Outcome 6 Number of dropouts.
| Alemtuzumab 12 mg compared to interferon beta‐1a for multiple sclerosis | ||||||
| Patient or population: patients with multiple sclerosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Interferon beta‐1a | Alemtuzumab 12 mg | |||||
| Relapse‐free survival | Not estimated | Not estimated | HR 0.50 (0.41 to 0.60) | 1248 | ⊕⊕⊕⊝ | — |
| Sustained disease progression‐free survival | Not estimated | Not estimated | HR 0.62 (0.44 to 0.87) | 1191 | ⊕⊕⊕⊝ | — |
| Number of participants with at least one adverse event | Study population | RR 1.04 | 1248 | ⊕⊕⊕⊝ | — | |
| 94 per 100 | 98 per 100 | |||||
| Moderate | ||||||
| 94 per 100 | 98 per 100 | |||||
| Change in EDSS score | — | The mean change in EDSS score in the intervention groups was | — | 1199 | ⊕⊝⊝⊝ | — |
| Number of participants with new or enlarging T2‐hyperintense lesions | 69 per 100 | 51 per 100 | RR 0.74 | 1238 | ⊕⊕⊕⊝ | — |
| Dropouts | Study population | RR 0.31 | 1248 | ⊕⊕⊝⊝ | — | |
| 24 per 100 | 8 per 100 | |||||
| Moderate | ||||||
| 24 per 100 | 7 per 100 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Participants and personnel were not blinded and this outcome could be affected by this fact. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse‐free survival Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 24‐month analysis | 2 | Hazard Ratio (Fixed, 95% CI) | 0.50 [0.41, 0.60] | |
| 2 Sustained disease progression‐free survival Show forest plot | 3 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 24‐month analysis | 2 | Hazard Ratio (Fixed, 95% CI) | 0.62 [0.44, 0.87] | |
| 2.2 36‐month analysis | 1 | Hazard Ratio (Fixed, 95% CI) | 0.25 [0.11, 0.57] | |
| 3 Number of participants with at least one adverse event Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 24‐month analysis | 2 | 1248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [1.01, 1.06] |
| 3.2 36‐month analysis | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.98, 1.02] |
| 4 Change in EDSS score Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24‐month analysis | 2 | 1199 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 4.2 36‐month analysis | 1 | 223 | Mean Difference (IV, Random, 95% CI) | ‐0.7 [‐1.04, ‐0.36] |
| 5 Number of participants with new or enlarging T2‐hyperintense lesions Show forest plot | 2 | 1238 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.91] |
| 6 Number of dropouts Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 24‐months analysis | 2 | 1248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.23, 0.41] |
| 6.2 36‐months analysis | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.14] |

