Različiti načini liječenja žena magnezijevim sulfatom kao terapijom za inhibiciju kontrakcija maternice u svrhu odgode prijevremenog poroda
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial. | |
| Participants | Setting: Imam Reza’s Hospital, Iran. Participants: women (n = 100; 50 in the low‐dose group, 50 in the high‐dose group) between 24‐35 weeks' gestation with spontaneous preterm labour. Singleton and twin pregnancies were included. Definition of preterm labour: uterine contractions of more than 4 contractions per 20 minutes plus 1 of: cervical dilatation of at least 1 cm but less than 5 cm diameter, cervical effacement ≥ 80%, and/or progressive cervical dilatation and effacement. Exclusion criteria: higher‐order multiple gestations, rupture of membranes, non‐reassuring fetal assessment (abnormalities of the fetal heart rate pattern), evidence of intrauterine infection (temperature ≥ 38°C, leucocytosis, uterine tenderness, malodorous discharge), vaginal bleeding, patients with a history of diabetes mellitus, myasthenia gravis, any other neuromuscular diseases, impaired renal function (serum creatinine > 1.2 mg/dL), hypotension (mean arterial pressure < 70 mm Hg), maternal bradycardia (heart rate < 60 beats per minute), atrioventricular block, inability or refusal to provide informed consent. | |
| Interventions | All women
Low‐dose group
High‐dose group
| |
| Outcomes | Primary
Secondary
| |
| Notes | Mean age of women: 23.8 ± 5.2 years in the low‐dose group; 24 ± 4.4 years in the high‐dose group. Twin gestations: 3/50 in the low‐dose group; 1/50 in the high‐dose group. Approval for this study was granted from the institutional review board of the University of Mashhad Medical Sciences. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by computer‐generated number allocation. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details were given regarding blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details were given regarding blinding. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selecting reporting. |
| Other bias | Low risk | No obvious risk of other bias. |
| Methods | Randomised controlled trial. | |
| Participants | Setting: Sekai Tahir Burak Women Health Education and Research Hospital, Ankara, Turkey. Participants: women (n = 100; 50 in the low‐dose group, 50 in the high‐dose group) between 28‐34 weeks' gestation with preterm labour that was unresponsive to intravenous fluid therapy and sedation. Definition of preterm labour: not stated. Exclusion criteria: not stated. | |
| Interventions | Low‐dose group
High‐dose group ('standard dose group')
| |
| Outcomes | Primary
Secondary
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants received low‐dose or standard dose therapy 'randomly'. |
| Allocation concealment (selection bias) | Unclear risk | No information was given on allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information was given on blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information was given on blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to make the judgement. |
| Selective reporting (reporting bias) | High risk | No outcomes were listed a priori. It is unclear if the outcomes reported were the pre‐specified primary and secondary outcomes. |
| Other bias | Low risk | No obvious risk of other bias. |
| Methods | Randomised controlled trial. | |
| Participants | Setting: University of Mississippi Medical Center, Mississippi, USA. Participants: women (n = 160; 78 in the low‐dose group, 82 in the high‐dose group) between 24‐34 weeks' gestation with spontaneous preterm labour. Singleton and twin pregnancies were included. Definition of preterm labour: advancement seen on cervical examination with uterine contractions while the patient was admitted to the triage unit OR dilatation of 2 cm and effacement of 80% with ≥ 6 uterine contractions per hour. Exclusion criteria: higher‐order multiple gestations, rupture of membranes, non reassuring fetal assessment, evidence of intrauterine infection, treatment with any tocolytic agent before maternal transport, women who could not tolerate high doses of magnesium sulphate (for example, women with renal failure), inability or refusal to provide informed consent. | |
| Interventions | All women
Low‐dose group
High‐dose group
| |
| Outcomes | Primary
Secondary
| |
| Notes | Mean age of women: 24 ± 5.1 years in the low‐dose group; 24 ± 4.8 years in the high‐dose group. Twin gestations: 2/70 in the low‐dose group; 3/78 in the high‐dose group. Approval for this study was granted from the institutional review board of The University of Mississippi Medical Centre. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number allocation, assigned by selection of the next numbered envelope. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information was given on blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information was given on blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | 12 women excluded from analysis due to treatment failure and subsequent birth (8 in the low‐dose group and 4 in the high‐dose group). No losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selective reporting. |
| Other bias | Low risk | No obvious risk of other bias. |
°C: degrees Celsius
cm: centimetre(s)
g: gram(s)
g/h: gram(s) per hour
mg: milligram(s)
mg/dL: milligrams per decilitre
mm Hg: millimetres of mercury
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Randomised controlled trial. Setting: University of Mississippi Medical Center, Mississippi, USA. Participants: women (n = 44; 23 magnesium gluconate, 21 magnesium chloride) with preterm labour. Definition of preterm labour: not stated. Intervention: oral magnesium gluconate compared with oral magnesium chloride given for maintenance tocolysis after the successful arrest of labour with parenteral magnesium sulphate. Reason for exclusion This study did not examine different treatment regimens of parenteral magnesium sulphate as single agent tocolytic therapy for the arrest of preterm labour. Rather, it examined the use of different oral magnesium preparations for maintenance tocolysis given after the successful arrest of labour with parenteral magnesium sulphate. | |
| Randomised controlled trial. Setting: University of Mississippi Medical Center, Mississippi, USA. Participants: women (n = 47; 25 magnesium gluconate, 22 magnesium chloride) with preterm labour. Definition of preterm labour: presence of regular uterine contractions with a change in cervical exam. Intervention: oral magnesium gluconate compared with oral magnesium chloride given for maintenance tocolysis after the successful arrest of labour with parenteral magnesium sulphate. Reason for exclusion This study did not examine different treatment regimens of parenteral magnesium sulphate as single agent tocolytic therapy for the arrest of preterm labour. Rather, it examined the use of different oral magnesium preparations for maintenance tocolysis given after the successful arrest of labour with parenteral magnesium sulphate. | |
| Open‐label, randomised, parallel‐group, actively controlled study. Setting: three study centres in Germany (Hessian, Giessen, Heidelberg). Participants: women (n = 46; 23 in treatment group) with preterm labour with indication for single agent tocolysis therapy with magnesium sulphate. Definition of preterm labour: not stated. Intervention: loading dose of 4 g magnesium sulphate administered over 30 minutes. Maintenance dose of 1‐2 g/h magnesium sulphate up to 21 days via (1) ready to use infusion solution with 24 g magnesium sulphate per 500 mL OR (2) infusion solution concentrate, diluted in carrier solution before administration 20 g/500 mL. Reason for exclusion This study did not examine different treatment regimens of parenteral magnesium sulphate as single agent tocolytic therapy for the arrest of preterm labour. All women were given the same treatment regimen using two different preparations of magnesium sulphate (ready to use infusion solution versus infusion solution concentrate diluted in carrier solution). |
g: gram(s)
g/h: gram(s) per hour
mL: millilitre(s)
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Comparison of maintenance therapy and continuous intravenous therapy with magnesium sulphate in preterm labour pain management at 24‐36 weeks' gestation: a randomized controlled trial. |
| Methods | Single‐arm, single‐blinded, randomised controlled trial. |
| Participants | Setting: Shariati Hospital, Bandar Abbas, Hormozgan, Iran. Participants: women (n = 70) with preterm labour pains, between 24‐36 weeks’ gestation. Inclusion criteria: presence of ≥ 4 uterine contractions during 20 minutes, cervical dilatation less than 4 cm, effacement less than 80%. Exclusion criteria: co‐existing medical disease (including diabetes mellitus, asthma, cardiovascular disease), pre‐eclampsia, uterine bleeding, rupture of membranes, placenta decolman, intrauterine infection, urinary tract infection, fetal anomalies, previous preterm labour pain, lack of decreasing uterine contractions during first 2 hours after administration of magnesium sulphate, patient requiring administration of magnesium sulphate after 24 hours. |
| Interventions | Low‐dose group (Group A)
High‐dose group (Group B)
|
| Outcomes | Primary
Secondary
|
| Starting date | 2013‐03‐20. |
| Contact information | Seyed Shojaeddin Namazi. |
| Notes | Approval for this study was granted from the Student Research Committee, Hormozgan University of Medical Sciences. |
cc: cubic centimetre(s)
cm: centimetre(s)
g: gram(s)
g/h: gram(s) per hour
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fetal, neonatal and infant death Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.56] |
| Analysis 1.1 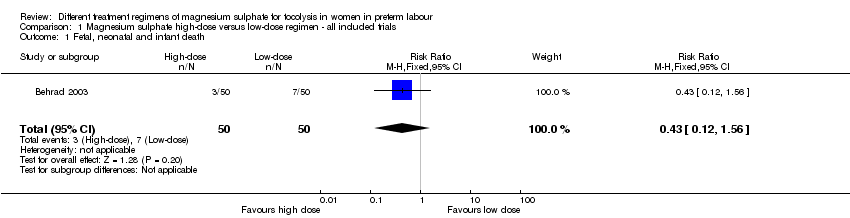 Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 1 Fetal, neonatal and infant death. | ||||
| 2 Fetal death Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.55] |
| Analysis 1.2  Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 2 Fetal death. | ||||
| 3 Neonatal death Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.57] |
| Analysis 1.3  Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 3 Neonatal death. | ||||
| 4 Respiratory distress syndrome Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.11, 0.88] |
| Analysis 1.4 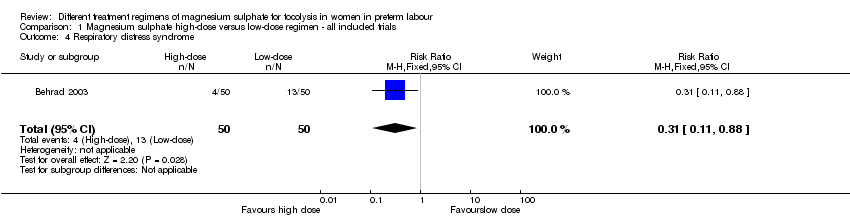 Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 4 Respiratory distress syndrome. | ||||
| 5 Hypocalcaemia, osteopenia or fracture Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5 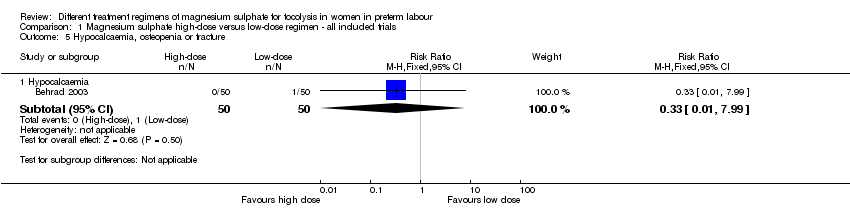 Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 5 Hypocalcaemia, osteopenia or fracture. | ||||
| 5.1 Hypocalcaemia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 6 Mode of birth Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6 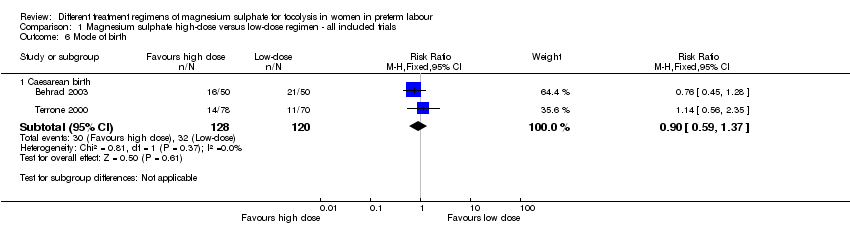 Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 6 Mode of birth. | ||||
| 6.1 Caesarean birth | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.37] |
| 7 Pulmonary oedema Show forest plot | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.49 [0.22, 92.03] |
| Analysis 1.7  Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 7 Pulmonary oedema. | ||||
| 8 Self‐reported adverse effects Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 8 Self‐reported adverse effects. | ||||
| 8.1 Flushing | 2 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.89, 3.03] |
| 8.2 Headache | 2 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.95, 3.31] |
| 8.3 Nausea | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.73, 2.20] |
| 9 Admission to neonatal intensive care unit Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.19, 1.12] |
| Analysis 1.9 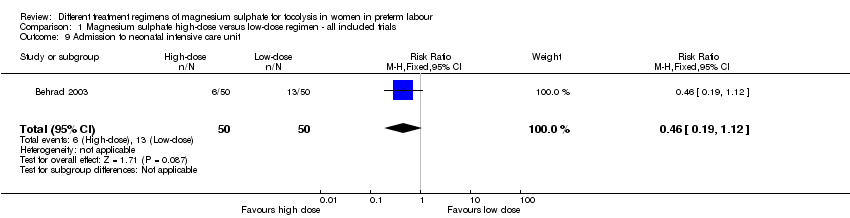 Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 9 Admission to neonatal intensive care unit. | ||||
| 10 Length of stay neonatal intensive care unit (days) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐5.48, ‐0.72] |
| Analysis 1.10  Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 10 Length of stay neonatal intensive care unit (days). | ||||
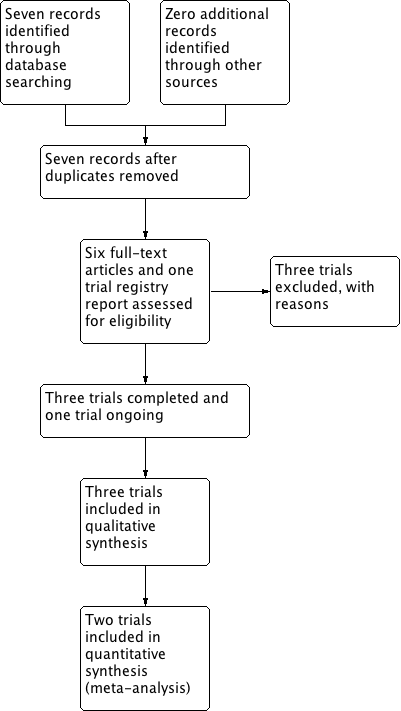
Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
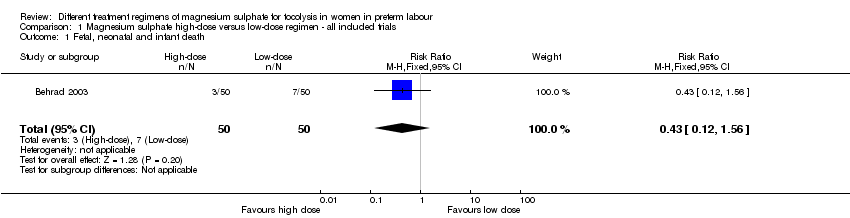
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 1 Fetal, neonatal and infant death.
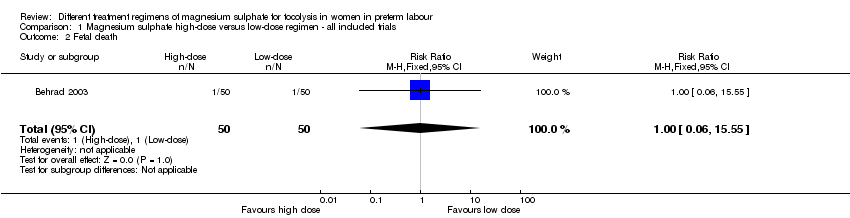
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 2 Fetal death.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 3 Neonatal death.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 4 Respiratory distress syndrome.
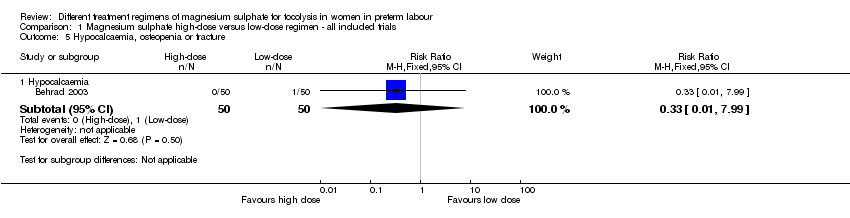
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 5 Hypocalcaemia, osteopenia or fracture.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 6 Mode of birth.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 7 Pulmonary oedema.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 8 Self‐reported adverse effects.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 9 Admission to neonatal intensive care unit.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 10 Length of stay neonatal intensive care unit (days).
| Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour | ||||||
| Patient or population: Women in preterm labour | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with low‐dose (as defined by the trialist) magnesium sulphate | Risk with high‐dose (as defined by the trialist) magnesium sulphate | |||||
| Fetal, neonatal and infant death | Study population | RR 0.43 | 100 | ⊕⊝⊝⊝ | ||
| 140 per 1000 | 60 per 1000 | |||||
| Respiratory distress syndrome | Study population | RR 0.31 | 100 | ⊕⊕⊝⊝ | ||
| 260 per 1000 | 81 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence is based on a single trial 2 Evidence of wide confidence intervals crossing the line of no effect 3 No evidence of blinding of participants, personnel or outcome assessors | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fetal, neonatal and infant death Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.56] |
| 2 Fetal death Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.55] |
| 3 Neonatal death Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.57] |
| 4 Respiratory distress syndrome Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.11, 0.88] |
| 5 Hypocalcaemia, osteopenia or fracture Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Hypocalcaemia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 6 Mode of birth Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Caesarean birth | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.37] |
| 7 Pulmonary oedema Show forest plot | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.49 [0.22, 92.03] |
| 8 Self‐reported adverse effects Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Flushing | 2 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.89, 3.03] |
| 8.2 Headache | 2 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.95, 3.31] |
| 8.3 Nausea | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.73, 2.20] |
| 9 Admission to neonatal intensive care unit Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.19, 1.12] |
| 10 Length of stay neonatal intensive care unit (days) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐5.48, ‐0.72] |

