Diferentes regímenes terapéuticos de sulfato de magnesio para la tocólisis en pacientes en trabajo de parto prematuro
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011200.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 diciembre 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Helen McNamara wrote the first draft of the review, with Julie Brown and Caroline Crowther making comments and contributing to subsequent drafts. Helen McNamara and Julie Brown assessed each study for inclusion and risk of bias, and extracted data for meta‐analysis. Caroline Crowther acted as the third author, resolving discrepancies where they arose.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Health and Medical Research Council, Australia.
Declarations of interest
Helen C McNamara: none known
Caroline A Crowther: none known
Julie Brown: none known
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Dec 14 | Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour | Review | Helen C McNamara, Caroline A Crowther, Julie Brown | |
| 2014 Jul 07 | Different treatment regimens for magnesium sulphate for tocolysis in women in preterm labour for improving health outcomes | Protocol | Helen C McNamara, Julie Brown, Caroline A Crowther | |
Differences between protocol and review
Birth less than 48 hours after trial entry is no longer included as both a primary infant outcome and a secondary infant outcome. It is listed as a primary infant outcome only.
We have used the GRADE approach to assess the quality of the body of evidence and as detailed in 'summary of findings Table for the main comparison'.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Bone Diseases, Metabolic [epidemiology];
- Fetal Death;
- Fractures, Bone [epidemiology];
- Hypocalcemia [epidemiology];
- Infant Death;
- Injections, Intravenous;
- Magnesium Sulfate [*administration & dosage, adverse effects];
- Obstetric Labor, Premature [*drug therapy];
- Perinatal Death;
- Premature Birth [*prevention & control];
- Randomized Controlled Trials as Topic;
- Tocolysis [*methods];
- Tocolytic Agents [*administration & dosage, adverse effects];
Medical Subject Headings Check Words
Female; Humans; Infant; Infant, Newborn; Pregnancy;
PICO
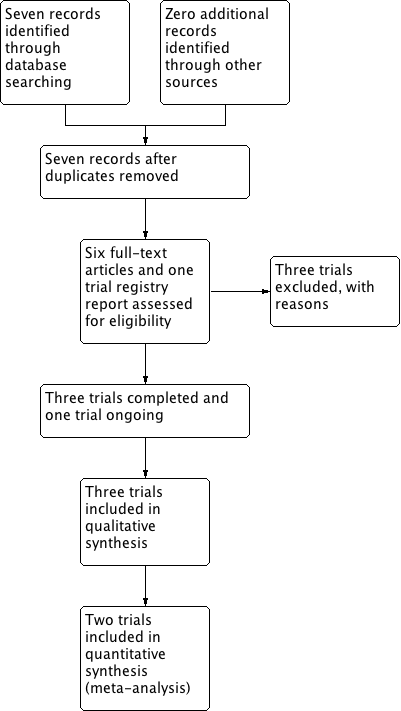
Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
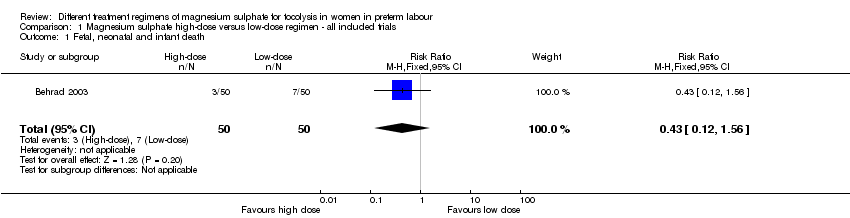
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 1 Fetal, neonatal and infant death.
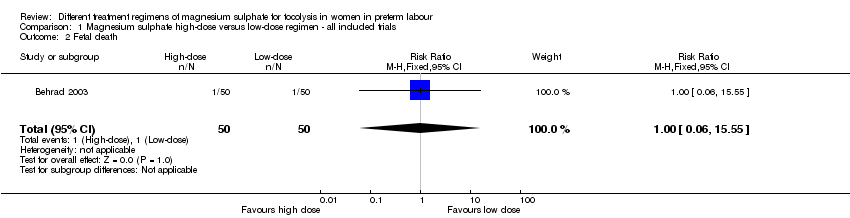
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 2 Fetal death.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 3 Neonatal death.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 4 Respiratory distress syndrome.
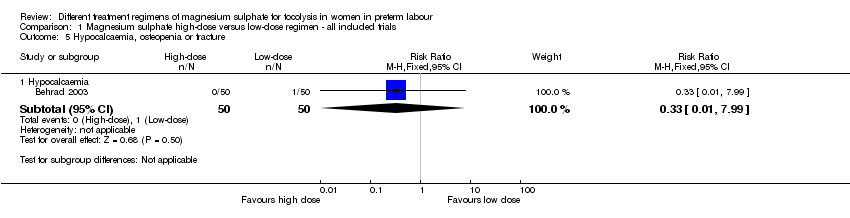
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 5 Hypocalcaemia, osteopenia or fracture.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 6 Mode of birth.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 7 Pulmonary oedema.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 8 Self‐reported adverse effects.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 9 Admission to neonatal intensive care unit.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 10 Length of stay neonatal intensive care unit (days).
| Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour | ||||||
| Patient or population: Women in preterm labour | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with low‐dose (as defined by the trialist) magnesium sulphate | Risk with high‐dose (as defined by the trialist) magnesium sulphate | |||||
| Fetal, neonatal and infant death | Study population | RR 0.43 | 100 | ⊕⊝⊝⊝ | ||
| 140 per 1000 | 60 per 1000 | |||||
| Respiratory distress syndrome | Study population | RR 0.31 | 100 | ⊕⊕⊝⊝ | ||
| 260 per 1000 | 81 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence is based on a single trial 2 Evidence of wide confidence intervals crossing the line of no effect 3 No evidence of blinding of participants, personnel or outcome assessors | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fetal, neonatal and infant death Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.56] |
| 2 Fetal death Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.55] |
| 3 Neonatal death Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.57] |
| 4 Respiratory distress syndrome Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.11, 0.88] |
| 5 Hypocalcaemia, osteopenia or fracture Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Hypocalcaemia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 6 Mode of birth Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Caesarean birth | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.37] |
| 7 Pulmonary oedema Show forest plot | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.49 [0.22, 92.03] |
| 8 Self‐reported adverse effects Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Flushing | 2 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.89, 3.03] |
| 8.2 Headache | 2 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.95, 3.31] |
| 8.3 Nausea | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.73, 2.20] |
| 9 Admission to neonatal intensive care unit Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.19, 1.12] |
| 10 Length of stay neonatal intensive care unit (days) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐5.48, ‐0.72] |

