بررسی رژیمهای مختلف درمانی سولفات منیزیم برای توکولیز در زنان با لیبر نارس
چکیده
پیشینه
سولفات منیزیم (magnesium sulphate) برای جلوگیری از لیبر نارس به منظور پیشگیری از زایمان زودرس استفاده شده است. هیچ اجماعی درباره پروفایل بیخطری رژیمهای مختلف درمانی با توجه به دوز، دوره مصرف، روش و چارچوب زمانبندی به کارگیری این رژیمها وجود ندارد.
اهداف
ارزیابی اثربخشی و بیخطری رژیمهای مختلف سولفات منیزیم، زمانی که به عنوان شیوه درمانی تک عاملی توکولیتیک (single agent tocolytic therapy) در طول دوران بارداری استفاده میشوند.
روشهای جستوجو
ما پایگاه ثبت کارآزماییهای گروه بارداری و زایمان در کاکرین (Cochrane Pregnancy and Childbirth Group's Trials Register)؛ (30 سپتامبر 2015) و فهرست منابع مطالعات بازیابی شده را جستوجو کردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی شدهای که به مقایسه رژیمهای مختلف سولفات منیزیم، زمانی که به عنوان یک شیوه درمانی توکولیز تکعاملی در طول دوران بارداری در زنان با لیبر نارس استفاده شده بودند، پرداخته بودند. کارآزماییهای شبه‐تصادفیسازی شده برای ورود به مرور واجد شرایط بودند اما هیچ موردی شناسایی نشد. کارآزماییهای متقاطع و خوشهای برای ورود به مرور واجد شرایط نبودند. پیامدهای سلامت در سطح مادر، نوزاد/کودک و خدمات سلامت در نظر گرفته شدند.
مداخله: سولفات منیزیم داخل وریدی یا خوراکی، به تنهایی برای توکولیز (tocolysis)
مقایسه: رژیمهای سولفات منیزیم با دوزهای مختلف، تجویز شده به تنهایی برای توکولیز
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم به ارزیابی واجد شرایط بودن و کیفیت کارآزمایی و استخراج دادهها پرداختند.
نتایج اصلی
سه کارآزمایی شامل 360 زن و نوزادان ایشان برای ورود به این مرور واجد شرایط شناسایی شدند. دو کارآزمایی به دلیل تولید تصادفی توالی و پنهانسازی تخصیص، دارای خطر پائین سوگیری (bias) ارزیابی شدند. خطر سوگیری سومین کارآزمایی برای این حیطهها نامشخص ارزیابی شد اما هیچ دادهای برای هیچ یک از پیامدهایی که در این مرور بررسی شدند، گزارش نکرد. هیچ یک از کارآزماییها در مجموع به لحاظ کیفیت در سطح بالا ارزیابی نشدند.
سولفات منیزیم داخل وریدی مطابق با رژیمهای با دوز پائین (شروع با دوز 4 گرم به عنوان پایه، سپس 2 گرم/ساعت به صورت اینفیوژن پیوسته و/یا به صورت افزایشی تا 1 گرم/ساعت به صورت ساعتی تا زمان توکولیز موفقیتآمیز یا درمان ناموفق)، یا رژیمهای با دوز بالا (شروع با دوز 4 گرم به عنوان پایه، سپس 5 گرم/ساعت به صورت اینفیوژن پیوسته و به صورت افزایشی تا 1 گرم/ساعت به صورت ساعتی تا زمان توکولیز موفق یا درمان ناموفق، یا 6 گرم به عنوان دوز پایه، سپس تا 2 گرم/ساعت به صورت اینفیوژن پیوسته و به صورت افزایشی تا 1 گرم/ساعت به صورت ساعتی تا زمان توکولیز موفق یا درمان ناموفق) به کار گرفته شدند.
هیچ تفاوتی از نظر پیامد اولیه مرگومیر جنینی (fetal)، نوزاد تازه متولد شده (neonatal) و کودک (infant) میان رژیمهای سولفات منیزیم با دوز بالا در مقایسه با رژیمهای سولفات منیزیم با دوز پائین وجود نداشت (خطر نسبی (RR): 0.43؛ 95% فاصله اطمینان (CI): 1.56 تا 0.12؛ یک کارآزمایی؛ 100 نوزاد). کیفیت شواهد مربوط به مرگومیر جنین، نوزاد تازه متولد شده و کودک با استفاده از رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) در سطح بسیار پائین در نظر گرفته شدند. هیچ دادهای در رابطه با هیچ یک از سایر پیامدهای سلامت اولیه مربوط به مادر و نوزاد گزارش نشدند (تولد کمتر از 48 ساعت بعد از ورود به کارآزمایی؛ پیامدهای جدی ترکیبی مربوط به نوزاد؛ پیامدهای جدی ترکیبی مربوط به مادر).
تفاوتهای آشکاری از نظر پیامدهای سلامت ثانویه مربوط به نوزاد شامل مرگومیر جنینی، مرگومیر نوزاد تازه متولد شده، و نرخ بروز هیپوکلسیمی (hypocalcaemia)،اوستئوپنی (osteopenia) یا شکستگی، و پیامدهای سلامت ثانویه مربوط به مادر شامل زایمان سزارین، ادم ریوی (pulmonary edema)، و عوارض جانبی گزارش شده از سوی مادر، میان رژیمهای سولفات منیزیم با دوز بالا در مقایسه با رژیمهای سولفات منیزیم با دوز پائین وجود نداشت. ادم ریوی در دو مورد از زنانی که رژیم سولفات منیزیم با دوز بالا دریافت کرده بودند گزارش شده بود، اما در هیچ یک از زنانی که رژیم سولفات منیزیم با دوز پائین دریافت کرده بودند گزارش نشده بود.
در یک کارآزمایی با دوزهای بالا و پائین سولفات منیزیم برای توکولیز شامل 100 نوزاد، خطر سندرم زجر تنفسی (respiratory distress syndrome) با استفاده از رژیم سولفات منیزیم با دوز بالا در مقایسه با رژیم سولفات منیزیم با دوز پائین، کمتر بود (RR: 0.31؛ 95% CI؛ 0.88 تا 0.11؛ یک کارآزمایی؛ 100 نوزاد). کیفیت شواهد مربوط به سندرم زجر تنفسی با استفاده از رویکرد GRADE در سطح پائین قضاوت شد. هیچ تفاوتی در نرخ پذیرش در واحد مراقبتهای ویژه نوزادان مشاهده نشد. با وجود این، در رابطه با آن دسته از کودکانی که پذیرفته شده بودند، میان رژیم سولفات منیزیم با دوز بالا با کاهش در طول دوره بستری در بخش مراقبتهای ویژه نوزادان، در مقایسه با رژیم سولفات منیزیم با دوز پائین، رابطه وجود داشت (میانگین تفاوت (MD)؛ 3.10‐ روز؛ 95% فاصله اطمینان؛ 5.48‐ تا 0.72‐).
هیچ دادهای در رابطه با اکثر پیامدهای ثانویهمان به دست نیاوردیم.
نتیجهگیریهای نویسندگان
دادههای محدودی (سه مطالعه؛ با دادههای به دست آمده از فقط دو مطالعه) در دسترس هستند که به مقایسه رژیمها با دوزهای مختلفی از سولفات منیزیم به عنوان درمان توکولیتیک تکعاملی برای پیشگیری از زایمان زودرس پرداخته بودند. هیچ شواهدی مبنی بر بررسی طول دوره درمان، چارچوب زمانبندی درمان و نقش تکرار دوزها وجود ندارد.
تصمیمات برای کاهش رتبه داده شده (downgrading decisions) در رابطه با پیامد اولیه مربوط به مرگومیر جنین، نوزاد تازه متولد شده و کودک در مرور ما مبتنی بر فواصل اطمینان زیاد (عبور از خط عدم تاثیر)، عدم کورسازی و تعداد محدود مطالعات بودهاند. هیچ نوع دادهای در رابطه با هیچ یک از سایر پیامدهای مهم ما در دسترس نبود: تولد کمتر از 48 ساعت بعد از ورود به کارآزمایی؛ پیامد جدی ترکیبی مربوط به کودک؛ پیامد جدی ترکیبی مربوط به مادر. دادهها با توجه به حجم و پیامدهای گزارش شده، محدود هستند. فقط هشت مورد از 45 پیامد سلامت از پیش تعیین شده مربوط به مادر و کودک مرور ما در مطالعات وارد شده گزارش شده بودند. هیچ پیامد طولانی‐مدتی گزارش نشده بود. تصمیمات کاهش رتبه داده شده در رابطه با شواهد مربوط به خطر دیسترس تنفسی مبنی بر فواصل اطمینان زیاد (عبور از خط عدم تاثیر) و عدم کورسازی بودهاند.
پارهای از شواهد به دست آمده از یک مطالعه واحد وجود دارد که نشان میدهد طول دوره بستری در واحد مراقبتهای ویژه نوزادان و خطر سندرم زجر تنفسی به دنبال استفاده از رژیم سولفات منیزیم با دوز بالا، در مقایسه با رژیم سولفات منیزیم با دوز پائین کاهش مییابد. با وجود این، با توجه به اینکه شواهد از یک مطالعه واحد به دست آمده (با حجم نمونه کوچک)، بهتر است این دادهها با احتیاط تفسیر شوند.
نشان داده شده که سولفات منیزیم در طیف گستردهای از محیطهای زایمانی دارای مزیت هستند، اگرچه استفاده از این ماده برای توکولیز توصیه نشده است. برای لحاظ کردن شواهد ناموجود در رابطه با دوز بهینه (دوز پایه (loading) و دوز نگهدارنده (maintenance))، طول دوره درمانی، چارچوب زمانبندی درمان و نقش تکرار دوزها به لحاظ اثربخشی و بیخطری برای مادران و کودکان ایشان، در محیطهای بالینی، جایی که مزایای سلامت پایهریزی شده و به اثبات میرسند، به کارآزماییهای بیشتری در آینده نیاز است. بررسی مستمر رژیمهای مختلف با توجه به پیامدهای مهم سلامت مورد نیاز است.
PICO
خلاصه به زبان ساده
رژیمهای مختلف سولفات منیزیم تجویز شده برای مادران به منظور پیشگیری از لیبر نارس
کودکانی که زودهنگام ‐ پیش از هفته 37 تخمین زده شده بارداری ‐ به دنیا میآیند، در معرض خطر بالای مرگومیر یا ابتلا به ناخوشی جدی قرار دارند، به ویژه اگر آنها بسیار پیش از موعد مقرر به دنیا بیایند. داروهای متنوعی برای تلاش به منظور ایجاد توقف تولد زودتر از موعد مقرر کودکان تجویز شدهاند. سولفات منیزیم (magnesium sulphate) یکی از داروهایی است که زمانی که زنان دچار درد زایمان میشوند، تجویز میشوند.
اگرچه در حال حاضر نشان داده شده که سولفات منیزیم به پیشگیری از تولد پیش از موعد کودکان کمک نمیکند، اگر قرار بر آن است که این دارو برای مادران با لیبر نارس استفاده شود، مهم است که ایمنترین و بهترین روش تجویز سولفات منیزیم شناخته شود. نگرانیهای خاصی درباره دوزهای بالای سولفات منیزیم برای زنان با لیبر نارس، شامل افزایش خطر مرگومیر کودکان، مطرح شدهاند. (نشان داده شده که سولفات منیزیم به پیشگیری و درمان اکلامپسی (eclampsia) در زنان با فشار خون بالا در طول دوران بارداری کمک میکند، و در زنانی که در معرض خطر زایمان زودرس قرار دارند، استفاده از دوزهای پائین این دارو میتواند از مغز کودک محافظت کرده و پیامدهای طولانی‐مدت مربوط به نوزاد را بهبود دهد. این موارد استفاده در سایر مرورهای کاکرین پوشش داده شدهاند.)
این مرور، سه کارآزمایی (شامل 360 زن و نوزادان ایشان) را شناسایی کرد، اما یک کارآزمایی هیچ نوع داده مرتبط با این مرور را ارائه نکرده بود. کارآزماییها کوچک بودند و دارای خطر سوگیری (bias) پائین یا نامشخص ارزیابی شدند. کارآزماییها بسیاری از پیامدهای مرتبط با این مرور را گزارش نکرده بودند. ما شواهد محدودی به دست آوردیم مبنی بر اینکه وقتی از سولفات منیزیم برای مادران با لیبر نارس استفاده میشود، تفاوت در دوزهای تجویزی (دوز بالا در برابر دوز پائین) هیچ تاثیری روی تعداد مرگومیر کودکان نمیگذاشتند (شواهد با کیفیت بسیار پائین). هیچ دادهای برای ارزیابی سایر پیامدهای مهم وجود نداشتند: تولد کمتر از 48 ساعت بعد از ورود به کارآزمایی، یا بروز پیامدهای جدی برای مادران یا کودکان ایشان.
کارآزماییهای وارد شده به مرور، دادههای بسیار کمی در رابطه با سایر پیامدهای مرتبط با این مرور را ارائه کرده بودند (در مجموع، فقط قادر بودیم هشت مورد از 45 پیامدی را که قصد بررسی آنها را داشتیم، ارزیابی کنیم.)
یک کارآزمایی نشان داده بود که نرخ بروز سندرم زجر تنفسی (respiratory distress syndrome) در نوزادان تازه متولد شده (شواهد با کیفیت پائین)، و طول دوره بستری در واحد مراقبتهای ویژه نوزادان به دنبال استفاده از سولفات منیزیم با دوز بالا کاهش یافته بودند (در مقایسه با کودکانی که در گروه زنانی به دنیا آمده بودند که سولفات منیزیم با دوز پائین دریافت کرده بودند). با این حال، این نتیجه مبتنی بر شواهدی است که از یک مطالعه کوچک به دست آمده و بهتر است با احتیاط تفسیر شود.
نرخ تولد به شیوه سزارین میان زنانی که سولفات منیزیم را با دوز بالا و دوز پائین دریافت کرده بودند، هیچ تفاوتی نداشت. همچنین، هیچ تفاوتی میان گروهها به لحاظ تعداد کودکانی که پیش از تولد یا در طول ماه بعدی مرده بودند، یا تعداد کودکان با سطوح کلسیم پائین در خون ایشان، تراکم استخوان پائین یا شکستگی استخوان، وجود نداشت. فراوانی عوارض جانبی گزارش شده از سوی مادران شامل گُرگرفتگی (flushing)، سردرد (دو کارآزمایی؛ 248 زن)، یا تهوع و استفراغ (یک کارآزمایی؛ 100 زن) میان گروهای دریافت کننده سولفات منیزیم با دوز بالا و پائین تفاوتی نداشت. ادم ریوی در دو مادری که سولفات منیزیم را با دوز بالا دریافت کرده بودند، گزارش شده بود، در مادرانی که سولفات منیزیم با دوز پائین دریافت کرده بودند هیچ موردی گزارش نشده بود.
هیچ یک از کارآزماییها به تفاوتهای میان طول دوره درمان، چارچوب زمانبندی و سایر روشهای تجویز سولفات منیزیم به مادرانی که دچار درد زایمان زودرس میشوند، نپرداخته بودند.
برای لحاظ کردن شواهد ناموجود در رابطه با دوز بهینه، طول دوره درمانی، چارچوب زمانبندی درمان و نقش تکرار دوزها به لحاظ اثربخشی و بیخطری برای مادران و کودکان ایشان، به کارآزماییهای بیشتری در آینده نیاز است.
Authors' conclusions
Summary of findings
| Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour | ||||||
| Patient or population: Women in preterm labour | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with low‐dose (as defined by the trialist) magnesium sulphate | Risk with high‐dose (as defined by the trialist) magnesium sulphate | |||||
| Fetal, neonatal and infant death | Study population | RR 0.43 | 100 | ⊕⊝⊝⊝ | ||
| 140 per 1000 | 60 per 1000 | |||||
| Respiratory distress syndrome | Study population | RR 0.31 | 100 | ⊕⊕⊝⊝ | ||
| 260 per 1000 | 81 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence is based on a single trial 2 Evidence of wide confidence intervals crossing the line of no effect 3 No evidence of blinding of participants, personnel or outcome assessors | ||||||
Background
Description of the condition
Preterm birth, defined as birth prior to 37 weeks' estimated gestation (WHO 1992), is a leading cause of perinatal mortality (Beck 2010). Preterm infants are at significant risk of short‐term and long‐term morbidity (How 2006). The costs to individual families and to the community are great, and the burden on modern healthcare systems is significant. Spontaneous preterm labour contributes to 40% to 50% of all preterm birth (Goldenberg 2008). The prevention of spontaneous preterm labour using tocolysis has been a focus of obstetric research (Tsatsaris 2004).
Description of the intervention
Various pharmacological agents have been used in an attempt to arrest spontaneous preterm labour and therefore prolong pregnancy. Betamimetics (Neilson 2014), calcium channel blockers (Flenady 2014b), magnesium sulphate (Crowther 2014) and oxytocin receptor antagonists (Flenady 2014a) have each been the subject of Cochrane systematic reviews. Other drugs advocated for tocolysis include the prostaglandin inhibitor, indomethacin (Klauser 2012), selective COX‐2 inhibitors, rofecoxib and celecoxib (Borna 2007; McWhorter 2004), progesterone (Borna 2008), nitrates and ethanol. Controversy remains as to which agent is preferable. A meta‐analysis of 55 randomised controlled trials of tocolytic therapy (Haas 2012) found prostaglandin inhibitors and magnesium sulphate to have the highest probability of delaying birth by 48 hours. However, prostaglandin inhibitors and calcium channel blockers were most likely to be the best class of therapy in terms of effectiveness, maternal side‐effect profile and neonatal outcomes. The American College of Obstetricians and Gynecologists has supported ‘first‐line tocolytic treatment with beta‐adrenergic receptor agonists, calcium channel blockers, or non‐steroidal anti‐inflammatories (NSAIDs) for short‐term prolongation of pregnancy’ (ACOG 2012).
Magnesium sulphate when given for tocolysis has not been demonstrated to significantly prolong pregnancy (risk ratio 0.87; 95% confidence interval 0.61 to 1.24, nine trials). Its tocolytic efficacy has been found to be similar to that of calcium channel blockers and betamimetics (Crowther 2014). The side‐effect profile of magnesium sulphate when given for tocolysis is greater than that of other tocolytic agents such as the calcium channel blocker, nifedipine (Lyell 2007).
There has been limited evaluation of the efficacy and safety of alternative treatment regimens of magnesium sulphate given for tocolysis. One systematic review has included treatment regimens given for tocolysis in an evaluation of different antenatal magnesium sulphate regimens with respect to maternal adverse effects (Bain 2013).
Alternatively, magnesium sulphate is used widely in other obstetric and perinatal contexts. Magnesium sulphate has an established role in the prevention and treatment of eclampsia (Duley 2010a; Duley 2010b; Duley 2010c), and in fetal neuroprotection for women at risk of preterm birth (Doyle 2009). In each setting, the efficacy and safety of different treatment regimens have been examined in Cochrane systematic reviews (Duley 2010d and Bain 2012 respectively).
Clinical trials have evaluated magnesium sulphate administered for both acute tocolysis (Crowther 2014) and maintenance tocolysis (Han 2013). Treatment regimens have differed with respect to dose, route, duration, and timing of administration (James 2010).
Dosing of magnesium sulphate when given for tocolysis has varied between clinical trials. Low‐dose regimens have typically included a loading dose of 4 g followed by an infusion of 2 g per hour. Early trials limited maximum dosing according to total dose administered in 24 hours (usually 10 g to 12 g). More aggressive regimens have included loading doses of 6 g (Glock 1993; Haghighi 1999; Larmon 1999; Morales 1993; Schorr 1997), and maintenance infusion rates with incremental rate increases (usually by 0.5 g at 30‐ or 60‐minute intervals) until cessation of contractions is achieved or a maximum hourly rate of up to 5 g is reached. The current obstetric standard for use in preterm labour indicates administration of intravenous magnesium sulphate with a loading dose of 4 to 6 g, followed by a maintenance infusion of 2 g per hour until uterine quiescence is established (James 2010).
Timing of administration has varied between treatment regimens. Loading doses have been given either as a bolus, over 15 minutes or over 30 minutes. Duration of therapy has extended from six hours to 24 hours, or until tocolysis has been achieved.
Different routes of administration have been evaluated. Oral magnesium therapy has been trialled for maintenance tocolysis either according to study protocol or when it has been established that preterm labour has been arrested (Han 2013). Intramuscular magnesium therapy has been limited in its use for tocolysis by the potential for adverse reaction at the injection site (Bain 2013).
Monitoring of magnesium sulphate therapy has been performed clinically or by evaluating serum magnesium levels. Magnesium sulphate toxicity can allow appropriate administration of calcium gluconate as an antidote (Tsatsaris 2004). Concerns have been raised over the utility of serum monitoring (Lurie 2004): monitoring is resource‐expensive; and serum levels increase slowly and unpredictably after intramuscular administration.
Clinical adverse side effects of magnesium sulphate have been evaluated extensively. For the mother, the most common adverse side effect reported after drug administration is flushing. Other maternal adverse side effects include gastrointestinal disturbance, muscle weakness, thirst, headache, drowsiness and confusion. Serious cardiorespiratory events including pulmonary oedema and cardiac or respiratory arrest have been reported (James 2010).
Common neonatal side effects include increased time to established respiration, lethargy and poor sucking (Klauser 2012). An association between the administration of high‐dose magnesium sulphate and an increased total death rate (fetal, neonatal and infant) has been reported (Mittendorf 1997).
Additionally, it has been suggested that prolonged administration of magnesium sulphate affects fetal calcium metabolism, increasing the risk of hypocalcaemia (Nassar 2006). In May 2013, the US Food and Drug Administration (FDA) issued a statement warning against prolonged (more than five to seven days), continuous administration of magnesium sulphate for the prevention of preterm birth given the risk of fetal hypocalcaemia, osteopenia and fractures (FDA 2013).
While magnesium sulphate has been deemed to be safe when used for the prevention and treatment of eclampsia (FDA 2013), and for fetal neuroprotection in the setting of preterm birth (Doyle 2009), many clinicians and researchers have cautioned against use of magnesium sulphate for tocolysis (Grimes 2006). Tocolytic regimens typically involve high‐dose and prolonged intravenous administration of magnesium sulphate (Pryde 2009). While low‐dose regimens of magnesium sulphate offer fetal neuroprotection (Doyle 2009), high‐dose regimens have been associated with an increased fetal, neonatal and infant death rate (Mittendorf 1997) and fetal osteopenia (Nassar 2006). It has been suggested that this may reflect a dose‐response relationship and a potential 'therapeutic window' (Pryde 2009). Further delineation of this dose‐response relationship is required.
How the intervention might work
Magnesium sulphate was first described for use as a tocolytic agent in 1977 (Steer 1977). An association between magnesium sulphate and uterine quiescence has long been established (Abarbanel 1945; Hall 1957). However, the mechanism of action of magnesium sulphate tocolysis remains incompletely defined. Evidence suggests the tocolytic effect of magnesium has both an intracellular and extracellular component (Lurie 2004). It is suggested that magnesium alters calcium uptake, binding and distribution in uterine smooth muscle cells, thereby reducing the frequency of cell depolarisation and inhibiting myometrial contraction (Lewis 2005). More recent evidence suggests the tocolytic effect of magnesium might have an anti‐inflammatory component (Dowling 2012; Tam Tam 2011).
Why it is important to do this review
Magnesium sulphate has been trialled as a tocolytic agent in the context of threatened preterm labour for the prevention of preterm birth. Controversy exists regarding the benefits of magnesium sulphate therapy when given for tocolysis (Grimes 2006). There has been limited evaluation of the efficacy and safety of alternative regimens of magnesium sulphate when given for tocolysis. In order to determine the optimal dose, duration, route and timing of administration, evaluation of different regimens of magnesium sulphate given for tocolysis is warranted. Given its widespread availability and use in other obstetric contexts, further review of the safety profile of magnesium sulphate is of value.
Objectives
This review assesses the efficacy and safety of alternative magnesium sulphate regimens when used as single agent tocolytic therapy during pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised controlled trials comparing different magnesium sulphate regimens when administered to women as the only tocolytic therapy in the setting of threatened preterm labour were included. Quasi‐randomised trials were eligible for inclusion but none were identified. Cross‐over and cluster‐randomised trials were not eligible for inclusion. Conference abstracts were included.
Types of participants
Women thought to be in preterm labour with a singleton or multiple pregnancy and administered magnesium sulphate for the prevention of preterm birth were included. Women who had a planned preterm birth or preterm induction of labour were not included.
Types of interventions
Intervention: intravenous or oral magnesium sulphate given alone for tocolysis.
Comparison: alternative dosing regimens of magnesium sulphate given alone for tocolysis.
Trials examining treatment regimens including magnesium sulphate co‐administered with alternative tocolytic agents were not included.
Types of outcome measures
Clinically relevant outcomes for trials of tocolysis for inhibiting preterm labour have been pre‐specified following consultation with the editors and authors of the individual reviews.
Consensus was reached on a set of six ‘core’ outcomes, which are highlighted below. These have been included in all tocolysis reviews. In addition to these core outcomes, individual teams may include other outcomes as necessary.
Primary outcomes
For the infant/child
-
Fetal, neonatal and infant death
-
Birth less than 48 hours after trial entry
-
Composite serious infant outcome (defined as death or chronic lung disease [oxygen requirement at 28 days of life or later]; intraventricular haemorrhage (IVH) [grade three or four] or periventricular leucomalacia (PVL); major neurosensory disability (defined as any of legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay/intellectual impairment [defined as developmental quotient or intelligence quotient less than two standard deviations below the mean])
For the mother
-
Composite serious maternal outcome (defined as maternal death, cardiac arrest, respiratory arrest or admission to intensive care unit)
Secondary outcomes
For the infant
-
Fetal death
-
Neonatal death
-
Preterm birth (less than 37 weeks)
-
Very preterm birth (less than 34 weeks)
-
Extremely preterm birth (less than 28 weeks)
-
Interval between trial entry and birth
-
Gestational age at birth
-
Apgar score less than seven at five minutes
-
Active resuscitation at birth (assisted ventilation via an endotracheal tube)
-
Use of respiratory support (mechanical ventilation or continuous positive airways pressure)
-
Air leak syndrome
-
Respiratory distress syndrome
-
Chronic lung disease (need for supplemental oxygen at 28 days of life or later)
-
Use of postnatal corticosteroids
-
IVH
-
Grade 3 or 4 IVH
-
PVL
-
Necrotising enterocolitis
-
Proven neonatal infection
-
Hypocalcaemia, osteopenia, or fracture(s)
For the child
-
Cerebral palsy (mild, moderate or severe, evaluated separately)
-
Developmental delay or intellectual impairment (defined as developmental quotient or intelligence quotient less than two standard deviations below the mean)
-
Legal blindness
-
Sensorineural deafness requiring hearing aids
For the mother
-
Death
-
Cardiac arrest
-
Respiratory arrest
-
Antepartum haemorrhage
-
Postpartum haemorrhage
-
Need for blood transfusion
-
Mode of delivery
-
Any adverse effect(s) of therapy
-
Hypotension
-
Tachycardia
-
Reduced or absent tendon reflexes
-
Hypocalcaemia
-
Pulmonary oedema
-
Self‐reported adverse effects or symptoms attributed to magnesium sulphate therapy (including discomfort at the infusion site, blurred vision, dizziness, headache, mouth dryness, muscle weakness, nausea or vomiting, drowsiness, sweating, flushing, other)
-
Discontinuation of therapy
-
Satisfaction with therapy
Health services outcomes
-
Admission to intensive care unit for the mother
-
Length of postnatal hospitalisation for the mother
-
Admission to neonatal intensive care unit for the infant
-
Length of stay in neonatal intensive care unit for the infant
-
Length of neonatal hospitalisation for the infant
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 September 2015).
For full search methods used to populate the PCG Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly: the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full texts of all relevant trial reports identified through the searching activities described above are reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Trials Search Co‐ordinator searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that review authors then fully account for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third author. The data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving the third author.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review had been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes had been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there was risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes. We selected up to a maximum of seven outcomes for the mother and the infant.
For the infant/child
-
Fetal, neonatal and infant death
-
Birth less than 48 hours after trial entry
-
Composite serious infant outcome (defined as death or chronic lung disease [oxygen requirement at 28 days of life or later]; IVH [grade three or four] or PVL; major neurosensory disability (defined as any of legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay/intellectual impairment [defined as developmental quotient or intelligence quotient less than two standard deviations below the mean])
-
Respiratory distress syndrome
For the mother
-
Composite serious maternal outcome (defined as maternal death, cardiac arrest, respiratory arrest or admission to intensive care unit)
Assessment of the quality of the evidence using the GRADE approach
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons.
-
Fetal, neonatal and infant death
-
Birth less than 48 hours after trial entry
-
Composite serious infant outcome
-
Composite serious maternal outcome
-
Respiratory distress syndrome
The GRADEpro Guideline Development Tool was used to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Women are the unit of analysis for maternal outcomes. For trials involving multiple pregnancies, we planned that the unit of analysis would be per infant for fetal, neonatal and child outcomes. As infants from multiple pregnancies are not independent, we planned to use cluster‐trial methods in the analyses, where the data allowed and where multiples made up a substantial proportion of the trial population, to account for non‐independence of variables (Gates 2004). Multiple pregnancies did not make up a substantial proportion of the trial population in each included study. Additionally, the presentation of data in the included studies did not allow use of cluster‐trial methods in the analyses. In future updates, if identified studies include sufficient multiple pregnancy data, cluster‐trial methods will be used for analyses to account for non‐independence of variables.
In future updates, where a trial has multiple arms (more than two comparisons) and is included in the same meta‐analysis, the number of events and the sample size from one of the groups will be divided by the number of arms compared in the trial. This will ensure that no participants are double counted. If a subgroup analysis is being undertaken then the overall summary statistic will not be reported.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number of participants randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as present if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. In future updates, if there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
Where random‐effects analyses are used, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We planned to perform comparisons for different types of regimens; including differences in route of administration, loading and maintenance doses, duration of treatment, timing of treatment and the possibility of repeat dosing. Given the data available in included studies was limited, we performed a comparison of different regimens with respect to dose (high‐dose versus low‐dose). If we identify studies including data that allow further comparisons to be made, these will be included in future updates.
We did not identify substantial heterogeneity. However, if we identify substantial heterogeneity in future updates, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if so, use random‐effects analysis to produce it.
Maternal and pregnancy characteristics are likely to affect health outcomes of both mother and child.
We will carry out planned subgroup analyses, where sufficient data are available, based on:
-
significant factor(s) contributing to or precipitating preterm labour: including presence or absence of preterm prelabour rupture of membranes;
-
multiple versus singleton pregnancy;
-
gestational age at time of randomisation and treatment: less than 28 weeks', 28 weeks' to less than 34 weeks', 34 weeks' to less than 37 weeks' or 37 weeks' or more;
-
use of antenatal corticosteroids for fetal lung maturation: in 50% or more of the study population or in less than 50% of the study population.
Subgroup analysis will be restricted to the review's primary outcomes.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014), and we will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
In future updates, sensitivity analysis will be performed where necessary to investigate the effect of trial quality, as defined by allocation concealment and other 'Risk of bias' components, by excluding studies determined as 'high risk of bias' for these components. Sensitivity analyses will be restricted to primary outcomes examined.
Results
Description of studies
Results of the search
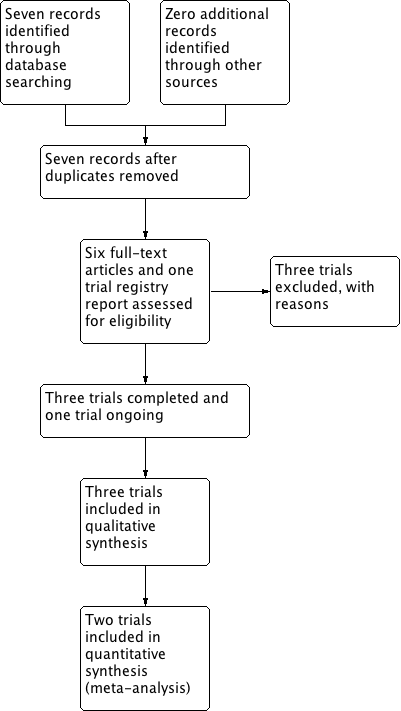
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register retrieved seven reports relating to seven studies (see:Figure 1). We included three studies (Behrad 2003; Soguk 2004; Terrone 2000) and excluded three studies (Martin 1990; Martin 1998; Zygmunt 2003). One study is ongoing (Namazi 2013).

Study flow diagram.
Included studies
Three trials (involving 360 women) comparing different treatment regimens of magnesium sulphate given for tocolysis were included in the review (Behrad 2003; Soguk 2004; Terrone 2000), of which only two trials reported clinically meaningful data (Behrad 2003; Terrone 2000). The third trial did not report data for any of the outcomes examined in this review (Soguk 2004).
The trials were conducted in Iran (Behrad 2003), Turkey (Soguk 2004), and the United States of America (Terrone 2000).
Women with both singleton and twin pregnancies were included in the trials. One trial included twin gestations in 3% of cases (5/148) (Terrone 2000). A second trial included twin gestations in 4% of cases (4/100) (Behrad 2003). Gestational age at recruitment was similar in two trials at 24 to 35 weeks' and 24 to 34 weeks' respectively (Behrad 2003; Terrone 2000). One trial recruited women from 28 to 34 weeks' gestation (Soguk 2004).
The treatment regimens of magnesium sulphate given for tocolysis varied. In the trial conducted by Terrone, all women received a fixed loading dose of 4 g, followed by a continuous infusion of 2 g/hour in the low‐dose group and 5 g/hour in the high‐dose group. In both the low‐dose and high‐dose groups, where women continued to exhibit signs of labour, the infusion rate was increased on an hourly basis by 1 g/hour until successful tocolysis or treatment failure (Terrone 2000).
In the trial conducted by Behrad, women received a loading dose of 4 g in the low‐dose group and 6 g in the high‐dose group. All women then received a continuous infusion of 2 g/hour. In the high‐dose group, for women with continued contractions or cervical dilatation or effacement, the maintenance dose was increased on an hourly basis by 1 g/hour until successful tocolysis or treatment failure (Behrad 2003).
In the trial conducted by Soguk, women in the low‐dose group did not receive a loading dose. A maintenance dose of magnesium sulphate was administered via continuous infusion at the rate of 1 g/hour. Women in the high‐dose group ('standard dose group') received a loading dose of 4 g followed by a continuous infusion of 2 g/hour (Soguk 2004).
In two trials, women received corticosteroids for accelerating fetal lung maturation and prophylactic antibiotics where Group B streptococcus had been identified antenatally. Alternative tocolytic therapies and maintenance tocolytic therapies were not used in either trial (Behrad 2003; Terrone 2000).
One study examining different treatment regimens of intravenous magnesium sulphate in preterm labour pain management was ongoing at the time of completion of this review (Namazi 2013). See Characteristics of ongoing studies.
Excluded studies
Three trials were excluded because they did not compare different treatment regimens of magnesium sulphate given as single agent tocolytic therapy during pregnancy.
Two trials compared preparations of oral magnesium gluconate and magnesium chloride (Martin 1990; Martin 1998). A third trial compared different preparations of magnesium sulphate (ready to use infusion solution versus infusion solution concentrate diluted in carrier solution) administered according to the same treatment regimen (Zygmunt 2003).
Risk of bias in included studies
The summary of risk of bias can be referred to in Figure 2; and Figure 3.
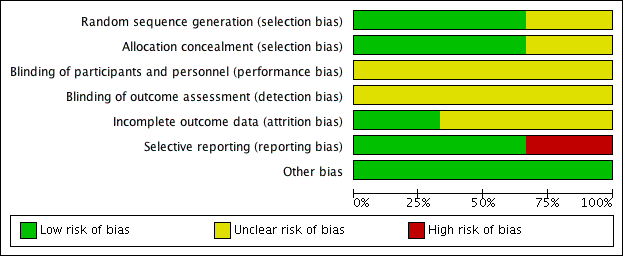
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
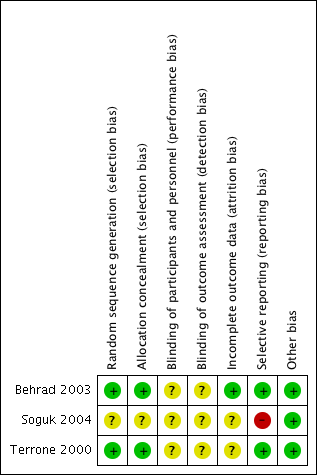
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation using computer‐generated random number tables was reported in two trials (Behrad 2003; Terrone 2000), and these were assessed as low risk of bias. The third trial reported that the participants were randomly assigned but gave no further details and this trial was assessed as unclear risk of bias (Soguk 2004).
Two trials reported allocation concealment using sealed numbered opaque envelopes (low risk of bias) (Terrone 2000; Behrad 2003). No details for allocation concealment were reported by one trial (unclear risk of bias) (Soguk 2004).
Blinding
No information was given on blinding of participants and research personnel in any of the included trials (Behrad 2003; Soguk 2004; Terrone 2000). Consequently, all three studies were assessed as being at unclear risk for performance and detection bias.
Incomplete outcome data
In one trial, 12 out of 160 women (eight in the low‐dose group and four in the high‐dose group) were excluded from analysis because of treatment failure and subsequent birth. The authors reported that the reason for exclusion of these women was that the time to successful tocolysis could not be assessed (Terrone 2000).
Losses to follow‐up were not reported for any of the included trials (Behrad 2003; Soguk 2004; Terrone 2000).
Selective reporting
There was no obvious evidence of selective reporting in two of the trials (Behrad 2003; Terrone 2000). One trial did not pre‐specify any of the outcomes reported (high risk of bias) (Soguk 2004).
Other potential sources of bias
There was no evidence of other bias.
Effects of interventions
Magnesium sulphate high‐dose regimen versus magnesium sulphate low‐dose regimen
Primary outcomes
For the Infant/child
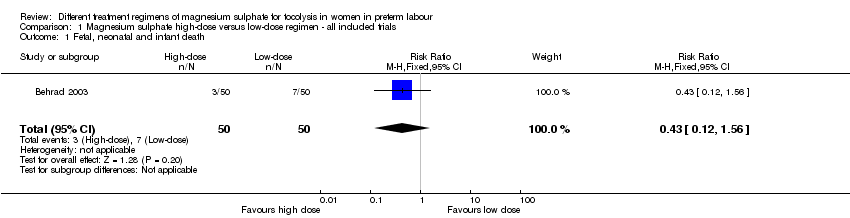
Fetal, neonatal and infant death was reported in one trial involving 100 women (Behrad 2003). No difference in the risk of fetal, neonatal and infant death for high‐dose compared with low‐dose magnesium sulphate was seen in this trial (risk ratio (RR) 0.43, 95% confidence interval (CI) 0.12 to 1.56; one trial, 100 infants) (Analysis 1.1). Using the GRADE approach the quality of the evidence was judged to be VERY LOW.
No data were reported for birth less than 48 hours after trial entry or for composite serious infant outcome in any of the included trials.
For the mother
None of the included trials reported data for composite serious maternal outcome.
Secondary outcomes
For the infant/child
Data from one trial (Behrad 2003, reporting data on 100 infants) were available for four of this review's secondary outcomes.
No differences were seen in the rate of fetal death (RR 1.00, 95% CI 0.06 to 15.55; Analysis 1.2), neonatal death (RR 0.33, 95% CI 0.07 to 1.57; Analysis 1.3), or rate of hypocalcaemia, osteopenia or fracture (RR 0.33, 95% CI 0.01 to 7.99; Analysis 1.5). The risk of respiratory distress syndrome was lower with use of high‐dose magnesium sulphate compared with low‐dose magnesium sulphate (RR 0.31, 95% CI 0.11 to 0.88; one trial, 100 infants) (Analysis 1.4). Using the GRADE approach the evidence was considered to be LOW quality.
No data were reported for preterm birth (< 37 weeks'), very preterm birth (< 34 weeks'), extremely preterm birth (< 28 weeks'), birth < 48 hours after trial entry, interval between trial entry and birth, gestational age at birth, Apgar score less than seven at five minutes, active resuscitation at birth, use of respiratory support, air leak syndrome, chronic lung disease, use of postnatal corticosteroids, intraventricular haemorrhage (IVH), Grade 3 or 4 IVH, periventricular leucomalacia (PVL), necrotising enterocolitis, proven neonatal infection, cerebral palsy, developmental delay, legal blindness or sensorineural deafness.
For the mother
Maternal data were reported in two trials (Behrad 2003; Terrone 2000).
There was no difference in the rate of caesarean birth in women given a high‐dose regimen compared with those given a low‐dose regimen of magnesium sulphate (RR 0.90, 95% CI 0.59 to 1.37; two trials, 248 women; Analysis 1.6). Pulmonary oedema was reported in two women receiving high‐dose treatment. There were no cases of pulmonary oedema reported in women receiving low‐dose treatment (RR 4.49, 95% CI 0.22 to 92.03; one trial, 148 women; Analysis 1.7) (Terrone 2000).
There were no differences in the frequency of self‐reported adverse effects including flushing (average RR 1.64, 95% CI 0.89 to 3.03; two trials, 248 women; I2 = 60%, Tau2 = 0.12; Analysis 1.8), headache (average RR 1.78, 95% CI 0.95 to 3.31; two trials, 248 women; Analysis 1.8), or nausea and vomiting (average RR 1.27, 95% CI 0.73 to 2.20; one trial, 100 women; Analysis 1.8).
No data were reported for maternal death, cardiac arrest, respiratory arrest, antepartum haemorrhage, postpartum haemorrhage, need for blood transfusion, any adverse effects of therapy, hypotension, tachycardia, reduced/absent tendon reflexes, hypocalcaemia, discontinuation of therapy or satisfaction with therapy.
Health services outcomes
Minimal data were reported for health services outcomes. One trial examined the rate of admission to the neonatal intensive care unit and found no difference in those treated with a high‐dose magnesium sulphate regimen compared with a low‐dose regimen (RR 0.46, 95% CI 0.19 to 1.12; one trial, 100 infants; Analysis 1.9). A high‐dose regimen of magnesium sulphate was associated with a reduction in length of stay in the neonatal intensive care unit (mean difference (MD) ‐3.10 days, 95% CI ‐5.48 to ‐0.72; one study, 100 infants; Analysis 1.10). No data were reported for maternal admission to the intensive care unit, length of postnatal hospitalisation or length of neonatal hospitalisation.
Discussion
Summary of main results
Three trials (involving 360 women) comparing different treatment regimens of magnesium sulphate given for tocolysis were included in this review (although one trial did not report data for any of the pre‐specified outcomes examined). There were no differences seen between high‐dose magnesium sulphate regimens compared with low‐dose magnesium sulphate regimens for the primary outcome of fetal, neonatal and infant death. No data were reported for any of the other primary maternal and infant health outcomes (birth less than 48 hours after trial entry; composite serious infant outcome; composite serious maternal outcome). No data were reported for childhood outcomes. The data are limited by volume and the outcomes reported. Only eight of our 45 pre‐specified primary and secondary maternal and infant health outcomes were reported on in the included studies. No long‐term outcomes were reported. No trials were rated to be of high quality overall.
Although the need for admission to a neonatal intensive care unit was not different, for babies that were admitted, a high‐dose regimen of magnesium sulphate was associated with a reduced length of stay compared with a low‐dose regimen. Similarly, there was some evidence to suggest that a high‐dose regimen was associated with a reduced risk of respiratory distress syndrome compared to babies born to women in the low‐dose regimen. However, this evidence was based on a single trial including 100 infants and therefore should be interpreted with caution.
Pulmonary oedema was reported in two women given high‐dose magnesium sulphate, but not in any of the women given low‐dose magnesium sulphate. This difference did not reach significance. There were no differences between regimens for maternal self‐reported adverse effects associated with treatment including flushing, headache or nausea and vomiting.
Overall completeness and applicability of evidence
Despite the fact that magnesium sulphate has been used widely for tocolysis for many years, only three small trials have compared different treatment regimens of magnesium sulphate to determine optimal efficacy and safety when given as single‐agent tocolytic therapy.
Quality of the evidence
Of the three trials included, two trials reported randomisation via the use of numbered envelopes. The third trial reported no details for allocation concealment. There was no evidence of blinding of participants, research staff or outcome assessors in any of the trials. Losses to follow‐up were not reported or unclear. No trials were rated to be of high quality overall.
The quality of the evidence was downgraded (three times) for our primary outcome of fetal, neonatal and infant death, based on wide confidence intervals (crossing the line of no effect), lack of blinding and a limited number of studies. Evidence relating to our secondary outcome of respiratory distress syndrome was downgraded twice due to wide confidence intervals crossing the line of no effect and lack of blinding.
Potential biases in the review process
Given the small number of mothers and infants in the three trials included, these data should be interpreted with caution. One of the three trials did not report on any of the outcomes specified for this review.
Agreements and disagreements with other studies or reviews
There was no evidence of benefit in terms of fetal or neonatal mortality with the use of either high‐ or low‐dose magnesium sulphate treatment regimens. Previously, it has been reported that high‐dose magnesium sulphate therapy may be associated with an increase in the fetal and paediatric death rate (Crowther 2014). In this review, no increased risk was observed when high‐dose treatment regimens were compared to low‐dose treatment regimens.
Magnesium sulphate has been widely used in obstetric contexts as a tocolytic, for neuroprotection of the fetus for mothers at risk of preterm birth, and for the prevention and treatment of eclampsia. To date, a consensus has not been reached regarding the optimal treatment regimen in terms of efficacy and safety in each setting (Bain 2012; Duley 2010d).
Magnesium sulphate has not been shown to prevent or delay preterm birth. However, examination of different treatment regimens with respect to dose, duration and timing of administration may provide useful information with respect to the safety and tolerability of magnesium sulphate when given in pregnancy.
Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 1 Fetal, neonatal and infant death.
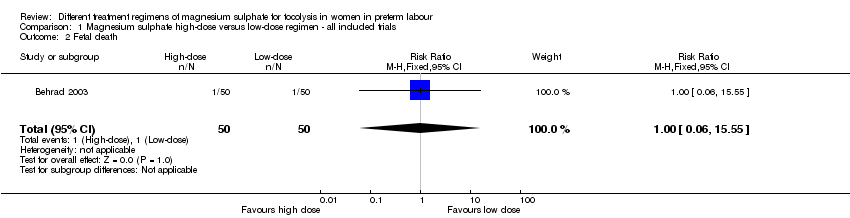
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 2 Fetal death.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 3 Neonatal death.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 4 Respiratory distress syndrome.
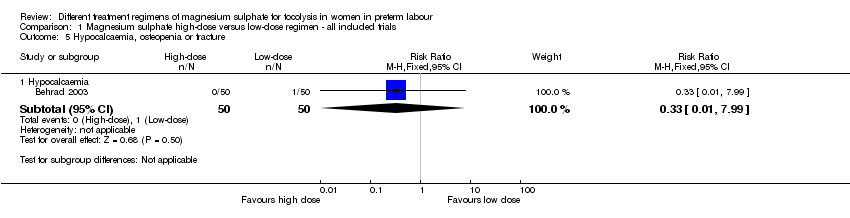
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 5 Hypocalcaemia, osteopenia or fracture.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 6 Mode of birth.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 7 Pulmonary oedema.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 8 Self‐reported adverse effects.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 9 Admission to neonatal intensive care unit.

Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 10 Length of stay neonatal intensive care unit (days).
| Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour | ||||||
| Patient or population: Women in preterm labour | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with low‐dose (as defined by the trialist) magnesium sulphate | Risk with high‐dose (as defined by the trialist) magnesium sulphate | |||||
| Fetal, neonatal and infant death | Study population | RR 0.43 | 100 | ⊕⊝⊝⊝ | ||
| 140 per 1000 | 60 per 1000 | |||||
| Respiratory distress syndrome | Study population | RR 0.31 | 100 | ⊕⊕⊝⊝ | ||
| 260 per 1000 | 81 per 1000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence is based on a single trial 2 Evidence of wide confidence intervals crossing the line of no effect 3 No evidence of blinding of participants, personnel or outcome assessors | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fetal, neonatal and infant death Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.56] |
| 2 Fetal death Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.55] |
| 3 Neonatal death Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.57] |
| 4 Respiratory distress syndrome Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.11, 0.88] |
| 5 Hypocalcaemia, osteopenia or fracture Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Hypocalcaemia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 6 Mode of birth Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Caesarean birth | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.37] |
| 7 Pulmonary oedema Show forest plot | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.49 [0.22, 92.03] |
| 8 Self‐reported adverse effects Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Flushing | 2 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.89, 3.03] |
| 8.2 Headache | 2 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.95, 3.31] |
| 8.3 Nausea | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.73, 2.20] |
| 9 Admission to neonatal intensive care unit Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.19, 1.12] |
| 10 Length of stay neonatal intensive care unit (days) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐5.48, ‐0.72] |

