| 1 Global symptoms of dyspepsia Show forest plot | 18 | 6172 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.82, 0.94] |
|
| 2 Global symptoms of dyspepsia by duration of treatment Show forest plot | 17 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 2.1 2 weeks' therapy | 4 | 1169 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.70, 0.87] |
| 2.2 4 weeks' therapy | 9 | 2425 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.76, 1.03] |
| 2.3 8 weeks' therapy | 4 | 2447 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.86, 0.98] |
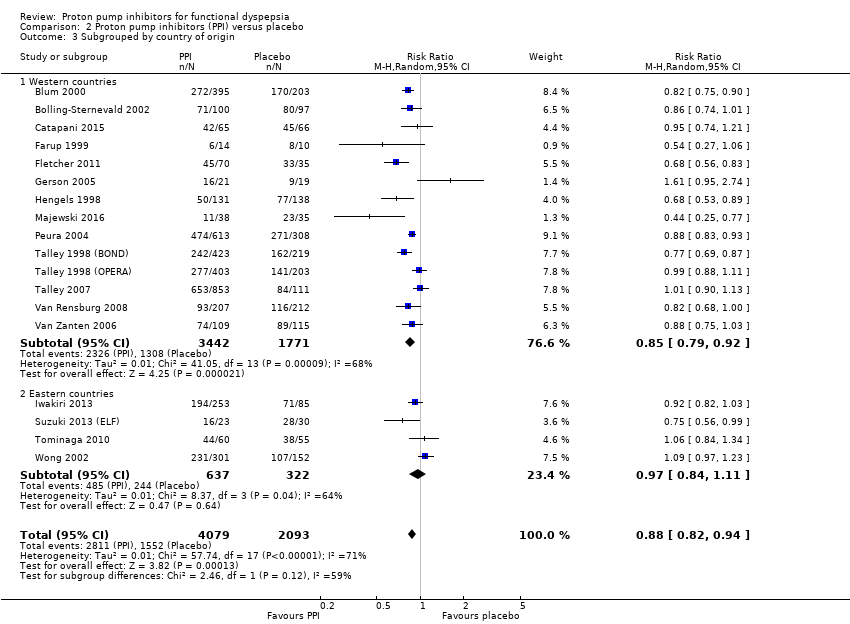
| 3 Subgrouped by country of origin Show forest plot | 18 | 6172 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.82, 0.94] |
|
| 3.1 Western countries | 14 | 5213 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.79, 0.92] |
| 3.2 Eastern countries | 4 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.84, 1.11] |
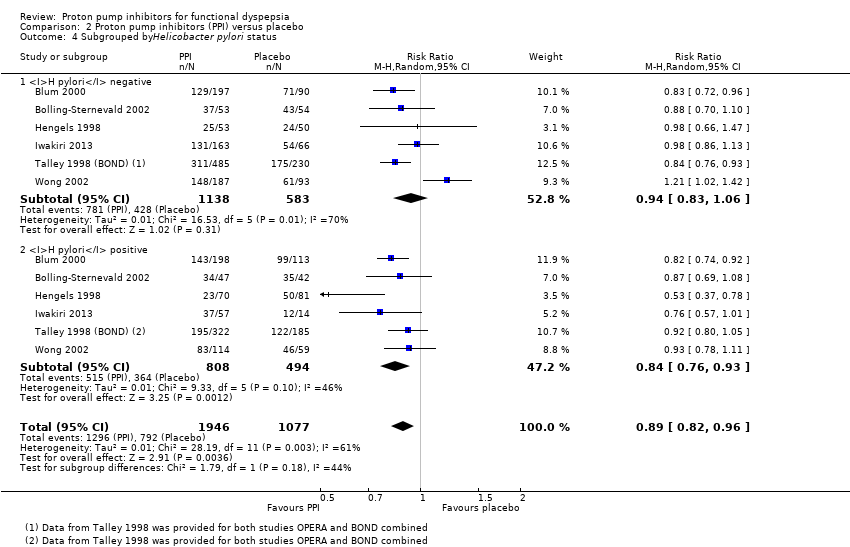
| 4 Subgrouped byHelicobacter pylori status Show forest plot | 6 | 3023 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.82, 0.96] |
|
| 4.1 H pylori negative | 6 | 1721 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.83, 1.06] |
| 4.2 H pylori positive | 6 | 1302 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.76, 0.93] |
| 5 Subgroup by PPI subtype Show forest plot | 18 | 6172 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.82, 0.94] |
|
| 5.1 Omeprazole vs placebo | 7 | 2238 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.98] |
| 5.2 Esomeprazole vs placebo | 3 | 1261 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.65, 1.09] |
| 5.3 Lansoprazole vs placebo | 5 | 1801 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.97] |
| 5.4 Pantoprazole vs placebo | 1 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.68, 1.00] |
| 5.5 Rabeprazole vs placebo | 2 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 6 Subgrouped by 24‐hour pH study Show forest plot | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.73, 1.47] |
|
| 6.1 Abnormal 24‐hour pH test (> 4% pH < 4) | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.70, 1.19] |
| 6.2 Normal 24‐hour pH test (< 4% pH < 4) | 2 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.49, 3.29] |
| 7 Subgrouped by Rome III dyspepsia subtypes Show forest plot | 2 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.03] |
|
| 7.1 Epigastric pain syndrome | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.76, 1.28] |
| 7.2 Postprandial distress syndrome | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.77, 1.03] |
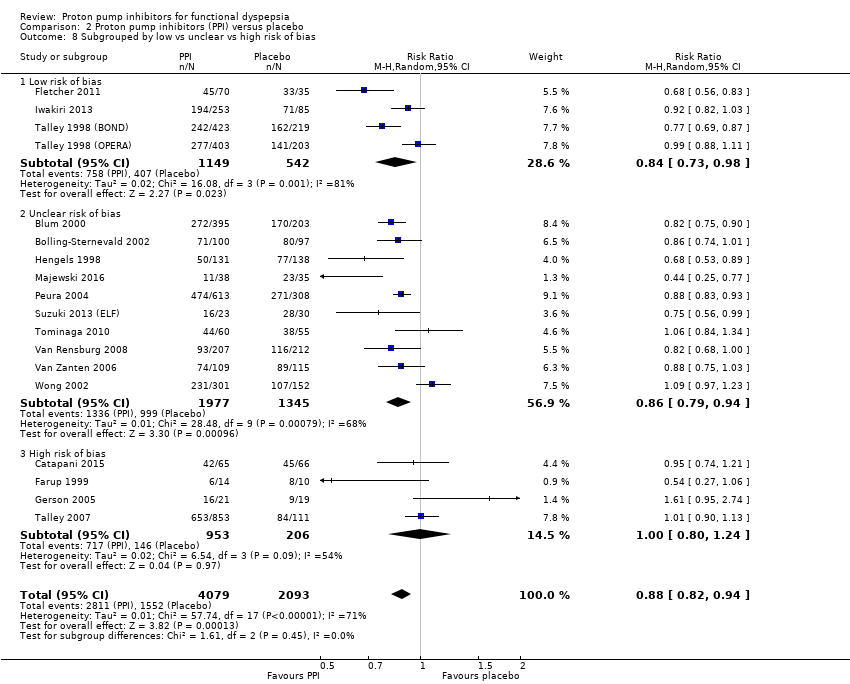
| 8 Subgrouped by low vs unclear vs high risk of bias Show forest plot | 18 | 6172 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.82, 0.94] |
|
| 8.1 Low risk of bias | 4 | 1691 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.98] |
| 8.2 Unclear risk of bias | 10 | 3322 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.79, 0.94] |
| 8.3 High risk of bias | 4 | 1159 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.80, 1.24] |
| 9 Subgrouped by publication type Show forest plot | 18 | 6172 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.82, 0.94] |
|
| 9.1 Full text | 15 | 5657 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.81, 0.94] |
| 9.2 Abstract | 3 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.68, 1.15] |
| 10 Quality of life Show forest plot | 3 | 1630 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.09, 0.11] |
|
| 10.1 Psychological General Well‐Being Index | 2 | 1177 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.09, 0.15] |
| 10.2 36‐item Short Form | 1 | 453 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.25, 0.14] |
| 11 Adverse events Show forest plot | 6 | 2693 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.73, 1.33] |
|