Oznaczanie stężenia antygenu rakowo‐płodowego (CEA) we krwi w wykrywaniu wznowy raka jelita grubego
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | |||
| Patient sampling | Country Poland Study design Retrospective casenote review Setting Hospital Dates of data collection N/R Population (n) 965 Inclusion criteria Patients after radical surgery in whom prognosis following a possible second operation was good Exclusion criteria Non‐radical surgery or concomitant disease making survival of a second operation unlikely Participants included (n) 340 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A ‐ D Perioperative Investigations done to ensure no residual disease Endoscopic polypectomy Chemotherapy/radiotherapy? Radical Recurrences (n) 112 Site of recurrences Liver 44, Local 32, Lung 7, Disseminated 12, other 6, 2 sites 11 | ||
| Index tests | CEA timing CEA 3, 6, 12 months, then once a year up to 5 years CEA technique N/R CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R | ||
| Target condition and reference standard(s) | Follow‐up schedule Follow‐up visits at 3, 6, 12 months, then once a year up to 5 years. Follow‐up schedule included patient’s history and physical examination, measurement of CEA serum concentration and classic colonoscopy | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Italy Study design Prospective Setting Hospital Dates of data collection N/R Population (n) 66 Inclusion criteria Rectal cancer treated for cure Exclusion criteria N/R Participants included (n) 66 | ||
| Patient characteristics and setting | Age range 62.3 yrs (mean) Smoking status N/R Site of primary tumour rectum Stage of primary tumour 6 Stage A, 32 Stage B, 28 Stage C Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 33 Site of recurrences Local 10, Metastatic 25 (Lungs 3, peritoneum 10,bones 2,liver 21, multiple 8) | ||
| Index tests | CEA timing 3‐monthly CEA CEA technique CEA was analysed using a direct radioimmunologic method (CEA‐PR; Sorin Biomedica) CEA threshold 3 µg/L Definition of positive Any elevation of 1 of the antigen levels greater than the limit defined by the between assay coefficient of variation (calculated on the basis of 2 standard deviations) was defined as significant, and the assay was repeated after 10 days. Which CEA value (s) used? Repeated value. | ||
| Target condition and reference standard(s) | Follow‐up schedule 3‐monthly to 60 months: blood CEA, TPA, CA19.9 and clinical exam. 6, 18, 30, 42, 54 months: USS Abdomen, CXR, Barium Enema. 12, 24, 36, 48, 60 months: colonoscopy, CT body. 6, 18, 30, 42 months: Bone scan. Reference standard Abdominal or total body CT, a chest x‐ray examination, a bone scan, an endoscopy, and a clinical examination were performed. An exploratory laparotomy was performed when all three markers were elevated, even if recurrence was not confirmed by total body CT scan and clinical examinations | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Yes | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country USA Study Design Prospective Setting Department of Surgery and Oncology, Mayo Clinic and Mayo Foundation Dates of data collection 1976 ‐ 1986 Population (n) 149 Inclusion criteria Resection of Dukes' B2 or C colorectal carcinoma was followed from the time of operation until the time of tumour recurrence or writing the published paper Exclusion criteria N/R Participants included (n) 149 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colon Stage of primary tumour Dukes B or C Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Some got radiotherapy, chemotherapy, and/or immunotherapy. Numbers not specified Recurrences (n) 34 Site of recurrences Liver metastasis 14, Chest 6, Pelvic disease 12 | ||
| Index tests | CEA Timing At least every 15 week CEA technique N/R CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R | ||
| Target condition and reference standard(s) | Follow‐up schedule At least every 15 weeks a complete history was taken and physical examination was carried out. A CXR was obtained, and laboratory determinations included complete blood count, alkaline phosphatase, SGOT, SGPT, and CEA. LDH and proctoscopic examinations were done every 6 months. A BE and liver scanning were done annually Reference standard Additional tests including CT, laparoscopy, liver biopsy, and abdominal exploration were ordered as indicated by the history, physical examination, or positive laboratory results. All recurrent tumours were documented histologically | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Country Norway Study Design Prospective Setting Hospital Dates of data collection 1976 ‐ 1979 Population (n) 244 Inclusion criteria colorectal cancer resection operated for cure Exclusion criteria Did not survive resection, residual disease, resection margins not clear, pre‐ and post‐op CEA determination Participants included (n) 164 | ||
| Patient characteristics and setting | Age range N/R Smoking status Some, but not quantified Site of primary tumour Colorectal Stage of primary tumour Dukes A 58, B 76, C 48, D 50, unknown 12 Perioperative Investigations done to ensure no residual disease Clear resection margins, no residual disease Chemotherapy / Radiotherapy? 22 Dukes B ‐ C randomised to 5‐year follow‐up; 21 had preoperative external radiation Recurrences (n) 47 Site of recurrences Liver 12, Lungs 10, Local 8, Local and distant 5, carcinomatosis 6, multiple 6 | ||
| Index tests | CEA timing 3, 6, 12, 18, 24, then yearly CEA technique Roche Ria test‐ repeat if raised, if repeat raised then test as described in follow‐up CEA threshold 3.5 µg/L Definition of positive Transient Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule 3, 6, 12, 18, 24 months then yearly CEA, clinical, biochemical, immunological (immunoglobulins and complement). CXR. Colonoscopy 6, 18 months. DCBE 1, 3, 5 years. "complimentary radiographic, scintographic, ultrasonographic added if indicated." Reference standard If nothing found investigating for increased CEA, then a second‐look operation was performed (laparotomy and biopsy) in 23, liver imaging 8, autopsy 5, clinical course 6 | ||
| Flow and timing | Timing of CEA vs reference standard (days) as per follow‐up schedule | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Was the index test repeated prior to the reference standard? | Yes | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Country Sweden Study design Prospective study Setting Hospital Dates of data collection N/R Population (n) 163 Inclusion Criteria Curative operation for colorectal cancer Exclusion Criteria Advanced age, moving away, death 3 months postop Participants Included (n) 139 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour N/R Perioperative Investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 50 Site of recurrences N/R | ||
| Index tests | CEA timing Blood tests 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 60 months post‐1977‐ blood tests 3, 6, 12, 18, 24, 30, 36, 42, 48, 60 months CEA technique Direct radio immunoassay method developed at the Department of Nuclear Medicine, Malmo General Hospital CEA threshold 3 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Follow‐up schedule Until 1977: Follow‐up exam and rectoscopy 3, 6, 9, 12, 15, 18 ,21, 24, 26, 42, 48, 60 months. Double contrast enema 3, 12, 24, 36, 48, 60 months. CXR and blood tests 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 60 months. From 1977: Physical exam and rectoscopy 3, 12, 24, 36, 48, 60 months. Double contrast enema 3, 12, 24, 36, 48, 60 months. CXR and blood tests 3, 6, 12, 18, 24, 30, 36, 42, 48, 60 months | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Finland Study Design Retrospective Setting Hospital Dates of data collection N/R Population (n) 354 Inclusion criteria Curative surgery, but unclear Exclusion criteria Palliative, followed up elsewhere, no preoperative serum samples, no serum at the time of recurrence Participants included (n) 102 | ||
| Patient characteristics and setting | Age range 29 ‐ 88 yrs Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A ‐ D (16 Dukes A, 45 Dukes B, 34 Dukes C, and 7 Dukes D) Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 40 Site of recurrences Local 17, Liver 10, Various 13 | ||
| Index tests | CEA timing N/R CEA technique CEA was measured with a time‐resolved immunofluorometric assay (AutoDELFIA®; Wallac, Turku, Finland). The detection limit of the assay is 0.2 µg/L, and the inter‐assay coefficient of variation is 3% in the concentration range 3 – 90 µg/L (total CV 4%) CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Reference standard Clinical follow‐up | ||
| Flow and timing | Timing of CEA vs reference standard (days) Unclear | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country Uruguay Study design Retrospective casenote review. Setting Hospital Dates of data collection 1985 ‐ 1998 Population (n) 209 Inclusion criteria Histologically proven colorectal carcinoma, 3 postoperative CEA measurements, minimum period of follow‐up 24 months Exclusion criteria Postoperative death and Stage IV (unless radical resection of synchronous liver metastases), no preop CEA Participants Included (n) 142 | ||
| Patient characteristics and setting | Age range 30 ‐ 91 yrs Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour TNM staging system: 32 patients had Stage I, 57 had Stage II, 86 had Stage III, and 27 had Stage IV disease Perioperative Investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 52 Site of recurrences N/R | ||
| Index tests | CEA timing CEA 3‐monthly for 24 months, 4‐monthly for yrs 3 ‐ 4, and once per year after this (strict adherence in only 42 patients) CEA technique Serum concentrations of CEA were determined by a standard commercially‐available immunoenzymatic assay CEA threshold 5 µg/L Definition of positive 2 consecutive values above 5 regarded abnormal; repeated at 2 ‐ 4 weeks Which CEA value (s) used? Repeated value at the time of recurrence | ||
| Target condition and reference standard(s) | Follow‐up schedule CEA 3‐monthly for 24 months, 4‐monthly for yrs 3 ‐ 4, and once per year after this (strict adherence in only 42 patients). Clinical follow‐up, rectoscopy and/or colonoscopy at 1 yr and 3 yrs Reference standard USS/MRI/CT indicated on basis of raised CEA or clinical suspicion. CEA second‐look surgery never used | ||
| Flow and timing | Timing of CEA vs reference standard (days) as per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Yes | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country USA Study design Prospective Setting Hospital Dates of data collection starting in 1978 Population (n) N/R Inclusion criteria Resection for curable adenocarcinoma of the colon or rectum Exclusion criteria Dukes D Participants included (n) 65 | ||
| Patient characteristics and setting | Age range 67 yrs mean Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour 8 Dukes A tumours, 34 had Dukes B tumours, and 20 had Dukes C tumours Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Some got radio/chemotherapy Recurrences (n) 23 Site of recurrences N/R | ||
| Index tests | CEA timing 3‐monthly in yr 1, then 6‐monthly to year 5 CEA technique N/R CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule 3‐monthly in year 1, then 6‐monthly to year 5: clinical history, examination, FOBT, LFT, CEA. 6‐monthly CXR, CT abdomen, total colonoscopy. BE at 6 and 12 months then annually Reference standard A positive finding on any test prompted additional confirmatory tests, including laparotomy, thoracotomy,or percutaneous CT‐directed biopsy | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Sweden Study design Prospective Setting Hospital Dates of data collection 1998 ‐ 1990 Population (n) 151 Inclusion criteria Surgery with curative intent with 5 years follow‐up Exclusion criteria N/R Participants Included (n) 132 | ||
| Patient characteristics and setting | Age range 27 ‐ 75 Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Duke A 11, B 76, C 43,D 1, Undefined 1 Perioperative investigations done to ensure no residual disease Not specified Chemotherapy/radiotherapy? N/R Recurrences (n) 39 Site of recurrences N/R | ||
| Index tests | CEA timing Monthly during year 1 and then at 18 and 24 months CEA technique Delfia® test kits (Wallac Oy, Turku, Finland). The accuracy of the assays was assessed by analysis of 2 control samples in each assay and by measurement of the coefficient of variation by duplicate analyses of the samples CEA threshold 5.6 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule Monthly outpatient clinic visit during year 1, serum tests monthly during year 1, then 18 and 24 months. Clinical examinations at 1 year and 2 year with CXR, Sigmoidoscopy, BE, and CT Liver. Reference standard Radiologic and/or endoscopic investigations at surgery or post mortem | ||
| Flow and timing | Timing of CEA vs reference standard (days) as per follow‐up schedule | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Italy Study design Retrospective Setting Hospital Dates of data collection N/R Population (n) 87 Inclusion criteria Preoperative CEA test > 6, operated in with end‐to‐end anastomosis Exclusion criteria N/R Participants included (n) 35 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A 3, B 26, C 6 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 10 Site of recurrences N/R | ||
| Index tests | CEA timing 3 monthly CEA technique CEA radioimmunoassay direct method CEA threshold 6 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule CEA and colonoscopy every 3 months Reference standard Second look surgery if not clear from CEA + colonoscopy. | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Germany Study design Prospective Setting Hospital Dates of data collection 1984 ‐ 1986 Population (n) 48 Inclusion criteria radical surgery Exclusion criteria No exclusion criteria were defined; results from all 48 participants are included in the study Participants Included (n) 48 | ||
| Patient characteristics and setting | Age range Study does not describe age bands; median age is 64 Smoking status N/R Site of primary tumour Rectum Stage of primary tumour Dukes A 9, Dukes B 16, Dukes C1 19, Dukes C2 4 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 5 Site of recurrences 5 local rectal recurrences | ||
| Index tests | CEA timing 6 weeks, (then 3‐monthly); main text of the study only mentions that CEA was measured postoperatively; ?Only once; it is not clear if there was a sequence of measurements. Table 4 looks more like a one‐off CEA technique Unknown CEA threshold 2.5 µg/L Definition of positive Unclear Which CEA value (s) used? Probably 4 ‐ 6 weeks postoperatively | ||
| Target condition and reference standard(s) | Follow‐up schedule 4 ‐ 6 weeks postoperatively, then 3‐monthly clinical examination, CT, and CEA Reference standard 4 patients underwent CT guided biopsy but at unknown stage | ||
| Flow and timing | Timing of CEA vs reference standard (days) Unclear | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Italy Study design Retrospective Setting Hospital Dates of data collection 1979 ‐ 1983 Population (n) 64 Inclusion criteria Potentially curative surgery Exclusion criteria Died or demonstrated recurrence before 1982 (introduction of TPA and CA19‐9 assays) Participants Included (n) 52 | ||
| Patient characteristics and setting | Age range 40 ‐ 77 Smoking status 1 smoker Site of primary tumour Colorectal Stage of primary tumour Dukes A: 28, B 17, C19 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 10 Site of recurrences N/R | ||
| Index tests | CEA timing As per protocol, then repeated within 2 weeks considered positive CEA technique Double antibody method (CEA‐PR, Sorin Biomedica) CEA threshold 20 (95% control group) Definition of positive 2 consecutive samples Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Follow‐up schedule CEA + TPA + CA19‐9, clinical exam at 3, 7, 14 days then 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 54, 60 months. Blood count at 3, 6, 12, 18, 24, 26, 48, 60 months. Liver USS at 3, 6, 18, 30, 36, 42, 48, 54, 60 months. CXR 3, 6, 12, 18, 24, 30, 36, 48, 60 months. DCBE at 18, 42, 60 months. Colonoscopy 6, 12, 24, 36, 48, 60 months. APCT 12, 24 months. Random perineal percutaneous needle biopsy (rectal cancer) 6, 12, 18, 24, 36, 48, 60 | ||
| Flow and timing | Timing of CEA vs reference standard (days) Sensitivity uses CEA at the time of recurrence, specificity uses CEA over threshold at any time during follow‐up | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Sweden Study design Prospective Setting Hospital Dates of data collection N/R Population (n) 190 Inclusion criteria Curative resection, age able to attend follow‐up Exclusion criteria Moved from area, died of intercurrent illness, did not follow the schedule Participants included (n) 167 | ||
| Patient characteristics and setting | Age range 55 ‐ 74 Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A 24, B 89, C 77 Perioperative Investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 47 Site of recurrences Liver 18, anastomotic 4, perineal 7, lungs 4, skin 5, multiple organs 8, skeleton 1 | ||
| Index tests | CEA timing CEA every second month during the first 2 years and every third month thereafter CEA technique Radioimmunoassay CEA threshold Abnormal blood values (CEA used same method as Colleen et al 1979 "the reference value was calculated from serum sampled from 89 apparently healthy persons aged 25 to 69 years. It was 10+/‐ 2.5 ug/l (mean+/‐S.D)") or a rise of CEA levels within the normal range of more than 50% Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Follow‐up schedule CEA, ESR, haemoglobin, ALP, glutamyltranspeptidase (GGT), orosomucoid, alpha‐antitrypsin, and haptoglobin every second month during the first 2 years and every third month thereafter. Physical exam and rectoscopy 3, 6, 9, 12, 18, 24, 36, 48, 60 months. DCBE and CXR 12, 36, 60 months Reference standard CXR, CT liver, CT perineum, endoscopic investigation of anastomosis, DCBE, angiography and bone scintography in selected cases | ||
| Flow and timing | Timing of CEA vs reference standard (days) if abnormal CEA detected reference standard triggered | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Japan Study design Retrospective Setting Hospital Dates of data collection 1990 ‐ 2000 Population (n) 680 Inclusion criteria Curative resection, dukes C Exclusion criteria Multiple cancers, insufficient examinations, persistent post‐op CEA, and SCC, randomised to pretest probability group Participants Included (n) 174 | ||
| Patient characteristics and setting | Age range 60.6 ± 11.1 Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour All Dukes C‐ Stage 1 18, 2 59, 3 232, 4 39 Perioperative investigations done to ensure no residual disease Persistent CEA elevation excluded Chemotherapy/radiotherapy? No Recurrences (n) 51 Site of recurrences N/R | ||
| Index tests | CEA timing 3‐monthly CEA technique N/R CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Follow‐up schedule All patients were followed for more than 5 years or until death with routine serum CEA examination every 3 months. USS and/or CT and CXR examinations were performed every 3 ‐ 6 months Reference standard Additional imaging was performed in patients with elevated postoperative CEA levels to determine whether recurrence was present | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Japan Study design Retrospective Setting Hospital Dates of data collection 1990 ‐ 2004 Population (n) 488 Inclusion criteria Stage II or III curative resection Exclusion criteria Patients with squamous cell, carcinoma, more than one cancer, or insufficient follow‐up Participants Included (n) Stage II: 167 Stage III: 136 | ||
| Patient characteristics and setting | Age range Stage II: 68.3 ± 10.5 (38 – 92) Stage III: 63.4 ± 9.4 (44 – 88) Smoking status N/R Site of primary tumour Stage II: Colon 112, rectum 55 Stage III: Colon 89, rectum 47 Stage of primary tumour Stage II: Depth T1 0, 2 0, 3 142, 4 23 Stage III: Depth T1 3, 2 89, 3 32, 4 12 Perioperative investigations done to ensure no residual disease Not specified Chemotherapy/radiotherapy? No Recurrences (n) Stage II: 23 Stage III: 51 Site of recurrences N/R | ||
| Index tests | CEA timing Unclear CEA technique N/R CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R | ||
| Target condition and reference standard(s) | Follow‐up schedule All patients underwent routine serum CEA assays and radiological examination | ||
| Flow and timing | Timing of CEA vs reference standard (days) unclear | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country UK Study design Prospective Setting Hospital Dates of data collection N/R Population (n) 663 Inclusion criteria Radical surgery for colorectal cancer Exclusion criteria SCC anus, tumours in the appendix. 6 were lost to clinical follow‐up and 5 others were removed from the trial. Removal followed the development of unassociated conditions such as alcoholic cirrhosis which interfered with the interpretation of a significant CEA rise (3 patients) and in 2 patients the onset of psychiatric illness made the use of cancer chemotherapy inadvisable Participants Included (n) 626 | ||
| Patient characteristics and setting | Age range 59 Smoking status Unknown Site of primary tumour 290 rectum, 373 colon Stage of primary tumour A in 38, B in 377 and C in 248 Perioperative investigations done to ensure no residual disease Not specified Chemotherapy/radiotherapy? Patients with at least 2 progressively rising CEA values of > 35 ngml‐1 but no other definite evidence of recurrent malignancy were randomised in a prospective trial of cytotoxic therapy Recurrences (n) 171 Site of recurrences N/R | ||
| Index tests | CEA Timing At each follow‐up visit CEA Technique CEA was measured in the unextracted serum by a double antibody radio‐immunoassay as developed by Egan et al. (1972) and adapted by Laurence et al. (1972). The inter‐ and intra‐assay variation of the method was found to be < 10%. An upper limit of 15 µg/L will include 99% of a normal population and in the present study a level of > 20 µg/L was regarded as abnormal CEA threshold 20 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule 3‐monthly for for first 2 postoperative years, then 6 ‐ 12‐monthly depending on the surgeon. Full clinical examination including sigmoidoscopy was performed Reference standard recurrence was primarily made on the basis of symptoms and signs of disease confirmed by other investigations when indicated (e.g. liver scan, bone scan, biopsy). Thorough clinical examination including sigmoidoscopy. If this indicated recurrent malignancy, confirmatory investigations were ordered and management was initiated appropriate to the results. When clinical examination failed to reveal malignancy, the subsequent course of events depended on the degree of elevation of the CEA. If the level was >20ngml‐1 but <35ngml‐ 1, the test was repeated at monthly intervals until it fell below 20ngml‐1 or rose above 35ngml‐1. All patients with levels >35ngml‐1 and no clinical evidence of recurrence had a further CEA estimation, full blood count, erythrocyte sedimentation rate, liver function tests, barium enema, chest X‐ray and isotope and/or ultrasound liver scan, together with bone scan and colonoscopy where indicated. If recurrence was diagnosed from the results of these | ||
| Flow and timing | Timing of CEA vs reference standard (days) Raised CEAs were recalled to clinic within 2 months of the date of the first sample for clinical exam and sigmoidoscopoy. If no recurrence found intensified frequency of testing whilst in the 20 ‐ 35 range. If > 35 but no signs of recurrence, then chemotherapy | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Unclear | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country UK Study design Retrospective Setting Hospital Dates of data collection 1996 ‐ 2000 Population (n) 150 Inclusion criteria Curative surgery for colorectal cancer Exclusion criteria Palliative patients, non‐operative patients, 11 who developed metastases or recurrences within 3 months of surgery, persistently elevated CEA postoperatively (deemed non‐curative resection) Participants Included (n) 139 | ||
| Patient characteristics and setting | Age range 22 ‐ 87 Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A 10, B 82, C 47 Perioperative investigations done to ensure no residual disease Development of metastases or recurrence within 3 months of surgery, persistently elevated CEA postoperatively Chemotherapy/radiotherapy? No Recurrences (n) 46 Site of recurrences N/R | ||
| Index tests | CEA timing Postoperatively 3‐monthly for 2 yrs, then 6‐monthly to 5 yrs. The CEA measurements for each patient were analysed twice, once looking for a small rise in CEA and again looking for a CEA value that rose above the traditional normal limit (10 µg/L) CEA technique Bayer immunoassay, which at the levels in this study has an error rate of 2.3% CEA threshold 10 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Follow‐up schedule 6‐monthly CT for 2 years, plus CEA 3‐monthly for 2 years, then 6‐monthly to 5 years | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Norway Study design Propsective Setting Hosptial + primary care Dates of data collection N/R Population (n) 93 Inclusion criteria Radical treatment for colorectal cancer Exclusion criteria Palliative, new cancers, no CEA monitoring Participants included (n) 51 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colon 49, rectal 44 Stage of primary tumour Dukes A 28, B 27, C 21, palliative 17 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 15 Site of recurrences N/R | ||
| Index tests | CEA timing Postoperatively, then at 3 ‐ 4‐monthly intervals CEA technique N/R CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R More data available? N/R | ||
| Target condition and reference standard(s) | Follow‐up schedule Postoperatively, then at 3 ‐ 4 monthly intervals, rising CEA resulted in further investigation, general clinical investigations, angiography of the liver, resection. No fixed schedule | ||
| Flow and timing | Timing of CEA vs reference standard (days) CEA triggered investigation | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Unclear | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country USA Study design Retrospective Setting Hospital Dates of data collection N/R Population (n) 97 Inclusion criteria Colorectal cancer Exclusion criteria N/R Participants Included (n) 97 | ||
| Patient characteristics and setting | Age range 65 mean (39 ‐ 89) Smoking status Unknown Site of primary tumour Colon 56, rectum 41 Stage of primary tumour Dukes A 10, B 42, C 34, D 6 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? 5 chemo, 5 immuno Recurrences (n) 20 Site of recurrences 7 liver, 13 non‐liver | ||
| Index tests | CEA timing At 6‐week intervals postoperatively CEA technique N/R CEA threshold 2.5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Follow‐up schedule CEA is done preoperatively and at six week intervals postoperatively. In addition, patients are evaluated postoperatively at 6 to 8 week intervals by physical examination and the usual laboratory and radiological tests, and where indicated, suspicions of recurrence and/or metastasis are documented histologically for the most part. Reference standard "suspicions of recurrence and/or metastasis are documented histologically for the most part". | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country Greece Study design Prospective Setting Hospital Dates of data collection 1991 ‐ 1999 Population (n) N/R Inclusion criteria Histologically proven colorectal cancer, no detectable liver metastasis, curative surgery for colorectal cancer Exclusion criteria Confirmed liver metastasis, peritoneal carcinomatosis, ascites, emergency surgery for obstruction or perforation, smokers, obstructive biliary disease or biliary surgery, or refused consent Participants Included (n) 73 | ||
| Patient characteristics and setting | Age range 64.2 (SD: 9.7) Smoking status Non‐smokers Site of primary tumour Colorectal Stage of primary tumour Stage I 14, II 37, III 22 Perioperative investigations done to ensure no residual disease Pre‐op abdominal CT, intraoperative liver palpation to exclude liver metastases Chemotherapy/radiotherapy? 22 patients with stage III cancer had adjuvant chemo Recurrences (n) 10 Site of recurrences N/R | ||
| Index tests | CEA timing 3‐monthly to 3 yrs, the 6‐monthly to 5 yrs CEA technique Monoclonal antibody technique, using a solid‐phase 2‐site mouse monoclonal antibody radioimmunoassay kit CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Follow‐up schedule Every 3 months for the first 3 years and every 6 months thereafter: clinical examination routine biochemical analysis, CXR, and CT. | ||
| Flow and timing | Timing of CEA vs reference standard (days) Simultaneous, per protocol. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Japan Study design Prospective Setting Hospital Dates of data collection 1977 ‐ 79 Population (n) N/R Inclusion criteria Surgically treated for adenocarcinoma of the colon or rectum with curative intent Exclusion criteria Incomplete CEA dataset Participants Included (n) 129 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A,B,C Perioperative investigations done to ensure no residual disease Not specified Chemotherapy/radiotherapy? No Recurrences (n) 32 Site of recurrences N/R | ||
| Index tests | CEA timing Unclear CEA technique RIA kit by Dynabot CEA threshold 2.5 and 5 µg/L Definition of positive N/R Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Follow‐up schedule N/R | ||
| Flow and timing | Timing of CEA vs reference standard (days) Unclear | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Unclear | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country Korea Study design Retrospective Setting Hospital Dates of data collection 2005 ‐ 2009 Population (n) N/R Inclusion criteria Radical resection Exclusion criteria Patients with stage 0, I or IV cancer, insufficient follow‐up (less than 3 years), abnormal CEA in the first measurement after surgery (checked within three months after surgery), history of other cancers and/or history of preoperative concurrent chemoradiation therapy were excluded Participants Included (n) 336 | ||
| Patient characteristics and setting | Age range Stage 111: 29 ‐ 81, Stage 11: 33 ‐ 83 Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Stage II 189, Stage III 147 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 79 Site of recurrences | ||
| Index tests | CEA timing CEA levels were assayed with a 3‐month interval for the first 2 years and every 6 months thereafter CEA technique Immunoassay method (ADIVA Centaur XP immunoassay system, Siemen AG, Erlangen, Germany) CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule CEA levels were assayed with a 3‐month interval for the first 2 years and every 6 months thereafter. Chest CT and abdomino‐pelvic CT were performed with a 6‐month interval for the first 2 years and every year thereafter Reference standard The diagnosis of a tumour recurrence was confirmed by biopsy and radiologic evidence | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country USA Study design Retrospective casenote review. Setting Hospital Dates of data collection 1971 ‐ 1974 Population (n) 144 Inclusion criteria Surgically confirmed adenocarcinoma of colon or rectum Exclusion criteria N/R Participants Included (n) 49 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour N/R Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 22 Site of recurrences N/R | ||
| Index tests | CEA timing Not clear CEA technique Hansens radioimmunoassay CEA threshold 2.5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule N/R | ||
| Flow and timing | Timing of CEA vs reference standard (days) N/R | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Unclear | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Country Japan Study design Cross‐sectional with follow‐up of cases Setting Hospital Dates of data collection 1986 ‐ 1990 Population (n) 194 Inclusion criteria Unclear Exclusion criteria Cases undergoing operation later, benign colorectal disease. Participants Included (n) 77 | ||
| Patient characteristics and setting | Age range 32 ‐ 83 Smoking status Unknown Site of primary tumour Colorectal Stage of primary tumour Unknown Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Follow‐up schedule N/R Recurrences (n) 34 Site of recurrences N/R | ||
| Index tests | CEA timing N/R CEA technique N/R CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Reference standard Unclear | ||
| Flow and timing | Timing of CEA vs reference standard (days) N/R | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country Norway Study design Prospective cohort with retrospective sampling Setting Hospital Dates of data collection 1996 ‐ 1999 Population (n) 314 Inclusion criteria Surgically treated for adenocarcinoma of the colon or rectum with curative intent, age < 75 yrs, national guidelines followed Exclusion criteria Not systematically followed up for 5 years or until recurrence, incomplete CEA dataset. Dukes D Participants included (n) 153 | ||
| Patient characteristics and setting | Age range < 75 Smoking status N/R Site of primary tumour Colon 102, rectum 50 Stage of primary tumour Dukes A 31, B 79, C 42 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 37 Site of recurrences N/R | ||
| Index tests | CEA timing CEA 3, 6, 9, 12, 18, 24, 30, 36, 42, 48, 54, 60 months CEA technique Immunoassay kit from Abbot diagnostic IL, USA CEA threshold 4 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Follow‐up schedule CEA 3, 6, 9, 12, 18, 24, 30, 36, 42, 48, 54, 60 months. USS Liver & CXR 6, 12, 18, 24, 30, 36, 42, 48, 54, 60 months. Colonoscopy 12, 60 months. Reference standard Biopsy and/or imaging studies to confirm recurrence, or disease‐free interval of 60 months without proof of recurrence. | ||
| Flow and timing | Timing of CEA vs reference standard (days) not specified if different from protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Italy Study design Retrospective Setting Hospital Dates of data collection N/R Population (n) 364 Inclusion criteria Radical surgery for colorectal cancer CEA measured postoperatively Exclusion criteria N/R Participants included (n) 239 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour N/R Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 45 Site of recurrences hepatic 18, non‐hepatic 22, mixed 5 | ||
| Index tests | CEA timing CEA monitoring, conducted every 3 months for years 1, 2, and 3, every 6 months for years 4 and 5, then yearly up to year 10 CEA technique The antigen was determined using the radioimmunoassay method. CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R | ||
| Target condition and reference standard(s) | Follow‐up schedule CEA monitoring, conducted every 3 months for years 1, 2, and 3, every 6 months for years 4 and 5, then yearly up to year 10. | ||
| Flow and timing | Timing of CEA vs reference standard (days) N/R | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country USA Study design Retrospective Setting Hospital Dates of data collection 1981 ‐ 1985 Population (n) N/R Inclusion criteria Newly diagnosed colorectal cancer undergoing operative resection for cure (Astler Coller A,B,C) Exclusion criteria Metastatic disease and synchronous cancers Participants Included (n) 285 | ||
| Patient characteristics and setting | Age range 66.8 (range, 31 ‐ 96) Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Astler‐Coller Stage A 39, B1 57, B2 109, C1 15, C2 60 Perioperative investigations done to ensure no residual disease Intraoperative criteria for curative resection included absence of gross residual disease Chemotherapy/radiotherapy? No Recurrences (n) 66 Site of recurrences N/R | ||
| Index tests | CEA timing 2‐monthly for 2 years, 3‐monthly for year 3, 6‐monthly for years 4 ‐ 5, annually afterwards. A repeat CEA was performed in patients who had an abnormal rise CEA technique Abbott CEA threshold 5 µg/L Definition of positive 2 consecutive samples Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Follow‐up schedule 2 monthly for 2 years, 3 monthly for year 3, 6 monthly for years 4 and 5, annually afterwards. A detailed history and physical examination was performed, and CEA levels were monitored at each encounter. Reference standard Two successive CEA elevations were investigated with diagnostic imaging and / or endoscopy when indicated. | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Country Italy Study design retrospective Setting Hospital Dates of data collection 1974 ‐ 1976 Population (n) 204 Inclusion criteria Large bowel malignancies, radical resection Exclusion criteria N/R Participants Included (n) 198 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Large intestine Stage of primary tumour Dukes A ‐ B 11, C1 39, C2 30, CH (liver involvement) 32 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Yes Recurrences (n) 62 Site of recurrences N/R | ||
| Index tests | CEA timing N/R CEA technique N/R CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R More data available? N/R | ||
| Target condition and reference standard(s) | Follow‐up schedule N/R | ||
| Flow and timing | Timing of CEA vs reference standard (days) Unclear | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country Switzerland Study design Retrospective Setting Hospital Dates of data collection 1977 ‐ 1978 Population (n) 200 Inclusion criteria Histologically confirmed diagnosis of adenocarcinoma of colon or rectum Exclusion criteria Incomplete tumour resection Participants Included (n) 66 | ||
| Patient characteristics and setting | Age range 65 Smoking status 12 patients who had CEA levels fluctuating around the normal limit of 5 ng/ml during the last 2 or 3 years without a definite rise of CEA levels and also without clinical evidence of tumour relapse. Among them were 6 heavy smokers Site of primary tumour Colorectal Stage of primary tumour Dukes ABCD Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? 2 of the recurrences were reported to have chemo Recurrences (n) 19 Site of recurrences N/R | ||
| Index tests | CEA timing 3‐monthly CEA technique The radioimmunoassay of CEA was performed according to the method of Goldz as modified by Mach el al. The major modification was that duplicates of 1 ml of plasma (10 ml of blood was collected in tubes containing 33 mg of dry E.D.T.A. K3) instead of 5 ml of serum, were extracted in perchloric acid. The sensitivity of the test is 1 µg/L. The normal value determined in 90 nonsmoking blood bank donors, unselected for age and sex, ranged between 0 to 3.5 µg/L. Our CEA assay is similar to the Hansen method,'but our numerical values are slightly higher and should be divided by a factor of 1.5 in order to make a direct comparison. CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow up schedule N/R | ||
| Flow and timing | Timing of CEA vs reference standard (days) N/R | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country UK Study design Prospective Setting Hospital Dates of data collection Approx 1970 ‐ 1973 Population (n) N/R Inclusion criteria Surgically resected colorectal carcinoma (a) Their operations were considered to be clinically curative. (b) Pathological staging showed the carcinoma to fall into Dukes (1950) A, B, or C category. (c) The participants had been followed up for at least 12 months and most for 24 months either after the operation or after the first plasma CEA assay Exclusion criteria Inadequate follow‐up time or because the plasma CEA values had risen temporarily to or remained at levels between 20 and 40 µg/L Participants included (n) 220 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Duke ABC Perioperative investigations done to ensure no residual disease Unclear Chemotherapy/radiotherapy? N/R Recurrences (n) 53 Site of recurrences Liver 31, lung 3, peritoneum and pelvis 17, bones 2, local 6, skin 2 | ||
| Index tests | CEA timing 3 monthly CEA technique Double‐antibody radioimmunoassay CEA threshold 40 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule N/R Reference standard Recurrence of tumour was detected clinically or by radioisotope scanning or other radiographic techniques. | ||
| Flow and timing | Timing of CEA vs reference standard (days) Reference standard triggered by a rise in CEA | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Country Italy Study design Prospective Setting Hospital Dates of data collection N/R Population (n) N/R Inclusion criteria Histologically confirmed adenocarcinoma submitted for resection (included ± pre‐op measurements) Exclusion criteria Heavy smokers (> 15 cigarettes/day) and patients with known, or suspected alcoholic hepatitis Participants included (n) 69 | ||
| Patient characteristics and setting | Age range 60.2 ± 11.6 yrs Smoking status Excluded Site of primary tumour Colorectal Stage of primary tumour Dukes A 5, B 18, C 14, D 2. Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 24 Site of recurrences N/R | ||
| Index tests | CEA timing The 4th and 14th day after surgery. Subsequent blood samples were taken at regular intervals (every 2 ‐ 3 months) in the following 12 ‐ 20 months. Moreover, an increased CEA value was always confirmed by repeated assays of the same sample, and by assaying an additional sample obtained from the same patient CEA technique Radioimmunoassay (RIA), using commercial EAK kits (purchased through SORIN Biomedica, Saluggia, Italy) CEA threshold 10 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule All patients had a blood sample taken for CEA assay preoperatively, then at the 4th and 14th day after surgery. Subsequent blood samples were taken at regular intervals (every 2‐3 months) in the following 12‐20 months with follow‐up examinations; the complete work‐up of the patients included physical examination, chest standard X‐ray, recto‐sigmoidoscopy, liver scan, hemogram and liver function tests; barium enema and bone scan were performed when indicated. | ||
| Flow and timing | Timing of CEA vs reference standard (days) not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Country Australia Study design Prospective RCT Setting Hospital Dates of data collection 1984 ‐ 1990 Population (n) 328 Inclusion criteria curative resection of colorectal cancers Exclusion criteria Patients with metastatic disease at presentation and those who for geographic or medical reasons were not able to be followed were excluded from the trial. Less than two years follow‐up completed (16 patients: 10 died of unrelated causes; 6 withdrew consent or were lost to follow‐up) and failure to obtain CEA levels (one patient). Participants Included (n) 311 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes ABC Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 98 Site of recurrences N/R | ||
| Index tests | CEA timing Patients entered into both arms of the study had serum CEA levels measured for 5 consecutive years: every 3 months for the first 2 years, then every 6 months for the next 3 years CEA technique Enzyme immunoassay method (Abbott Laboratories, North Chicago, IL) CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule Standard follow up: Clinical review plus CEA, Liver function, and fecal occult blood ‐ 3 monthly til 2 years, 6 monthly til 5 years. CXR, Liver CT, Colonoscopy at 0 and 5 years; Aggressive follow up: As for standard follow‐up plus CXR , Liver CT and Colonoscopy annually Reference standard Radiology, histology | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Scotland Study design Retrospective notes review Setting Hospital Dates of data collection 1988 ‐ 1992 Population (n) 265 Inclusion criteria Patients who underwent a resection, with curative intent. Exclusion criteria Patients were excluded where, on inspection of the patients' notes, it was found that primary surgery was palliative, follow‐up was incomplete or there were fewer than 1 preoperative and 2 postoperative carcinoembryonic antigen level estimations Participants included (n) 125 | ||
| Patient characteristics and setting | Age range 69 (41 ‐ 90) Smoking status Unknown Site of primary tumour Colorectal Stage of primary tumour Dukes A 10, B 27, C 38, D 22, unknown 27 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 53 Site of recurrences N/R | ||
| Index tests | CEA timing Not clear CEA technique Using international standard International Reference Preparation 73/601, National Institute for Biological Standards and Control CEA threshold 10 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule History is recorded and clinical examination (including rectal examination and rigid sigmoidoscopy), faecal occult blood test and estimation of carcinoembryonic antigen level are undertaken Reference standard The presence of recurrent disease is confirmed by clinical examination, colonoscopy, biopsy, chest radiography, ultrasonography, computerized axial tomography scanning and laparotomy. | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol, CEA triggers reference standard. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Country USA Study design Prospective Setting Hospital Dates of data collection 1978 ‐ 1983 Population (n) 400 Inclusion criteria post‐colorectal cancer resection Exclusion criteria N/R Participants included (n) 400 | ||
| Patient characteristics and setting | Age range 58 (18 ‐ 84) Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes A 17, B1 91, B2 31, C1 119, C2 122, D 6, unknown 6 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 130 Site of recurrences Liver 49, Anastomosis site or mesentery of bowel 26, peritoneum 7, pelvis 6, para‐aortic nodes 2, mesentric nodes 2, multiple 7, other 28, no disease found 3 | ||
| Index tests | CEA timing CEA performed every 2 months for the first 2 years, and then every 4 months for the next 3 years. To rule out laboratory variations, a repeat CEA value was required to confirm an abnormal CEA elevation CEA technique N/R CEA threshold 2.5 µg/L Definition of positive Abnormal repeated Which CEA value (s) used? Unclear | ||
| Target condition and reference standard(s) | Follow‐up schedule Patients were evaluated postoperatively with each surgeon's customary follow‐up procedures and frequency of CEA determinations. Reference standard Second‐look surgery was performed on any potentially resectable recurrent cancer discovered by physical examination or symptoms of bowel or ureteral obstruction, gastrointestinal bleeding, or findings from rectal, vaginal, or colostomy examinations. In addition, second‐look surgery was done when a persistently rising CEA value was detected. Before the second‐look procedure was performed a careful physical examination complemented by chest roentgenogram, bone and brain scans, and appropriate gastrointestinal and genitourinary roentgenograms was done to rule out the possibility of unresectable metastases. A computerized axial tomography (CAT) scan of the abdomen was not required, but was considered appropriate for institutions with that capability. | ||
| Flow and timing | Timing of CEA vs reference standard (days) not specified | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country India Study design Retrospective Setting Hospital Dates of data collection N/R Population (n) 73 Inclusion criteria Histologically proven postoperative CRC resection undergoing PET/CT and conventional imaging to detect suspected recurrence triggered by a rising CEA Exclusion criteria N/R Participants included (n) 73 | ||
| Patient characteristics and setting | Age range 25 ‐ 80 Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour N/R Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 38 Site of recurrences N/R | ||
| Index tests | CEA timing Within 7 ‐ 10 days of imaging CEA technique Electro‐chemiluminescent immunoassay CEA threshold 3 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At point of recurrence | ||
| Target condition and reference standard(s) | Reference standard PET/CT | ||
| Flow and timing | Timing of CEA vs reference standard (days) within 7‐10 days of CEA | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Japan Study design Prospective Setting Hospital Dates of data collection N/R Population (n) N/R Inclusion criteria Surgically treated for adenocarcinoma of the colon or rectum with curative intent and CEA measurements Exclusion criteria incomplete CEA dataset Participants included (n) 66 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Stage I ‐ V Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Follow‐up schedule N/R Recurrences (n) 20 Site of recurrences N/R | ||
| Index tests | CEA timing CEA 1 month CEA technique RIA kit by Dynabot CEA threshold 2.5 µg/L Definition of positive N/R Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Reference standard N/R | ||
| Flow and timing | Timing of CEA vs reference standard (days) Unclear | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Unclear | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country Spain Study design Retrospective Setting Hospital Dates of data collection 2007 ‐ 2011 Population (n) 54 Inclusion criteria Referred to the Dept of Nuclear Medicine for FDG PET‐CT with suspected CRC recurrence following surgical resection and posterior histological confirmation Exclusion criteria Not possible to follow up, mixed malignancy of the salivary gland Participants Included (n) 47 | ||
| Patient characteristics and setting | Age range 63 (32 ‐ 87) Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour N/R Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? 38 chemo, 9 chemo and radio Recurrences (n) 34 Site of recurrences N/R | ||
| Index tests | CEA timing CEA used as a marker of suspected recurrence or measured when recurrence suspected by CT CEA technique Radioimmunoanalysis CEA threshold 10 µg/L Definition of positive 1 elevated value Which CEA value (s) used? Single measurement taken at point of recurrence | ||
| Target condition and reference standard(s) | Reference standard Histopathology or Clinical evolution, FDG PET‐CT | ||
| Flow and timing | Timing of CEA vs reference standard (days) CEA prior to Referral; no more clear than this | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Sweden Study design RCT Setting Hospital Dates of data collection 1983 ‐ 1986 Population (n) 107 Inclusion criteria Resection with curative intent, recruited to follow‐up group Exclusion criteria Patients operated with local excision or having demonstrable distant metastases were excluded, as were patients in whom age or severe illness was considered to preclude treatment of recurrent disease. Other exclusion criteria were: Inability to cooperate, ulcerative colitis, Crohn's disease, familial polyposis, and incomplete colonoscopy together with uncertain findings at the barium enema examination Participants Included (n) 53 | ||
| Patient characteristics and setting | Age range 65.7 (40.6 ‐ 83.3) Smoking status N/R Site of primary tumour Rectum 19, colon 34 Stage of primary tumour Dukes A 10, B 21, C 22 Perioperative investigations done to ensure no residual disease Preoperative investigation included barium enema, pulmonary x‐ray, and blood tests for liver function test, carcinoembryonic antigen and colonoscopy Chemotherapy/radiotherapy? N/R Recurrences (n) 17 Site of recurrences Local 11, liver 3, lung 3, peritoneum 2, ovary 1 | ||
| Index tests | CEA timing 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 60 months CEA technique Not specified CEA threshold N/R Definition of positive N/R Which CEA value (s) used? N/R | ||
| Target condition and reference standard(s) | Follow‐up schedule Physical examination, Rigid Proctosigmoidoscopy, Blood tests ‐ CEA, ALP, GGT, Faecal Heamoglobin, CXR: 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 60 months. Endoscopic control of the anastomosis: 9, 21, 42 months. Colonoscopy: 3, 15, 30, 60 months. CT Pelvis: 3, 6, 12, 18, 24 months. Reference standard CT/ Endoscopy/colonoscopy | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol, immediate diagnostic work‐up did not reveal the site of recurrence in 4 asymptomatic patients with raised CEA levels; in these patients the time interval between elevation of CEA and symptoms of tumour recurrence varied between 0.2 and 4.7 (median 0.5) years | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Japan Study design Retrospective Setting Hospital Dates of data collection 2002 ‐ 2005 Population (n) 138 Inclusion criteria Curative resection, stage 0 – III according to the General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum, and Anus, 7th edition, 2006, no residuals Exclusion criteria History of another malignancy before or after the operation, lost to follow‐up Participants Included (n) 97 | ||
| Patient characteristics and setting | Age range 70 (37 ‐ 86) Smoking status Chronic benign disease or smoking in 46 cases Site of primary tumour 32 right colon, 32 left colon, 30 rectum, 3 multiple Stage of primary tumour 0 in 8, I in 12, II in 37, and III in 40 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Yes, but not described Recurrences (n) 22 Site of recurrences | ||
| Index tests | CEA timing Every 1 – 3 months during the initial 6 months after the operation, every 3 – 6 months from 6 months to 2 years, and every 6 – 12 months during 2 – 5 years after the operation CEA technique CEA, a latex immunoassay, Mitsubishi Chemical Ltd., Japan CEA threshold 5 µg/L Definition of positive N/R Which CEA value (s) used? N/R | ||
| Target condition and reference standard(s) | Follow‐up schedule the follow‐up schedule of the tumour markers and physical examination after the operation were: every 1 – 3 months during the initial 6 months after the operation, every 3 – 6 months from 6 months to 2 years, and every 6 – 12 months during 2 – 5 years after the operation. Radiological examinations including abdominal ultrasonography, computed tomography (CT), chest X‐ray, gastrointestinal series, and/or endoscopic evaluation were performed every 6 – 12 months during the follow‐up period. Marker evaluations and physical/radiological examinations were performed at shorter‐term intervals than those described above in patients with suspected recurrence, those undergoing chemotherapy, or in those demonstrating marker elevations. Reference standard radiological examinations / histology | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol or reference standard triggered by rise in CEA | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Korea Study design Prospective Setting Hospital Dates of data collection N/R Population (n) 1707 Inclusion criteria curative resection for colorectal cancer followed by surveillance programme Exclusion criteria Patients with synchronous metastatic disease or patients undergoing palliative resection, and those with carcinoma in situ, inflammatory bowel disease, familial adenomatous polyposis or pathology other than adenocarcinoma were excluded, as were patients with T1 cancer treated by endoscopic mucosal resection or transanal excision. In addition, patients with chronic obstructive lung disease, chronic liver disease, peptic ulcer, and diabetes were excluded. Participants Included (n) 1263 | ||
| Patient characteristics and setting | Age range 61 (21 ‐ 90) Smoking status N/R Site of primary tumour Colon 631, rectum 632 Stage of primary tumour I 212, II 514, III 537 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Yes, but not specified Recurrences (n) 291 Site of recurrences N/R | ||
| Index tests | CEA timing per schedule CEA technique N/R CEA threshold 7 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All, although at point of recurrence for 18.8% | ||
| Target condition and reference standard(s) | Follow‐up schedule 2‐ or 3‐ month intervals for the first 2 years and at 6‐month intervals thereafter. At each visit, CEA levels are assayed, a full history is obtained, and a physical examination is per‐ formed. A serum CEA assay is performed with at least a 2‐ week interval after the administration of chemotherapy. Colonoscopy is performed within 6 months to 1 year following surgery, and every 3years thereafter. Chest radiographs and abdominopelvic computed tomography (CT) are performed 6 months postoperatively and then at yearly intervals. Unscheduled CT or positron emission tomography (PET) scans were performed on patients with increased serum CEA concentrations or patients who were symptomatic. Reference standard diagnosis of a tumour recurrence was confirmed by biopsy or examination of the resected specimen. Other‐ wise, tumour recurrence was documented from the first clinical or radiologic sign of disease that showed an unrelenting course leading to tumour progression and/or death. The criteria for establishment of recurrent disease included histologic confirmation, palpable disease, or radiographic evidence of disease with subsequent clinical progression and supportive biochemical data, particularly an increased CEA level. | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Country China Study design Retrospective comparative diagnostic accuracy study Setting Hospital Dates of data collection 2006 ‐ 2012 Population (n) 128 Inclusion criteria Colorectal cancer with full response to primary surgery ± chemo, undergoing FDG‐PET/CT for either elevated CEA levels or in patients with a suspicion of recurrence without CEA rise Exclusion criteria Unstable, severe DM, severe illness, 1 or more additional tumours, unable to remain supine for 30 mins Participants Included (n) 96 | ||
| Patient characteristics and setting | Age range 61 (34 ‐ 85) Smoking status N/R Site of primary tumour Colon 53, rectum 42 Stage of primary tumour 0 in 1, I 15, II 31, III39, IV 9, unknown 1 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Yes, but not specified Recurrences (n) 63 Site of recurrences N/R | ||
| Index tests | CEA timing 3‐monthly CEA technique N/R CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At time of recurrence | ||
| Target condition and reference standard(s) | Reference standard FDG‐PET/CT +/‐ histology | ||
| Flow and timing | Timing of CEA vs reference standard (days) Detection of recurrent lesions within 6 months of the FDG‐PET scan/CEA ± histology | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Italy Study design Retrospective Setting Hospital Dates of data collection 1975 ‐ 1990 Population (n) 431 Inclusion criteria Curative resection Exclusion criteria N/R Participants Included (n) 336 | ||
| Patient characteristics and setting | Age range 21 ‐ 92 Smoking status N/R Site of primary tumour Colon 247, rectum 184 Stage of primary tumour Dukes A 40, B 186, C 107, D 72 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 136 Site of recurrences 50 local recurrences, 136 distant recurrences | ||
| Index tests | CEA timing N/R CEA technique N/R CEA threshold N/R Definition of positive Unclear Which CEA value (s) used? Unclear | ||
| Target condition and reference standard(s) | Reference standard N/R | ||
| Flow and timing | Timing of CEA vs reference standard (days) N/R | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Is the same method and instrument used for all CEA measurements? | Unclear | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country Germany Study design Prospective Setting Hospital Dates of data collection 1994 ‐ 1998 Population (n) 100 Inclusion Criteria Patients undergoing a whole‐body PET scan for suspected relapse after curative resection of histologically confirmed colorectal cancer and who caused a “diagnostic problem”. The “diagnostic problems” of the patients that led to a PET scan were (1) staging of rest of the body in patients with known recurrence (n = 30); (2) suspected recurrence (n = 32); (3) increasing CEA level (n = 13); (4) unclear finding on pelvic CT (n = 7); and (5) confirmation of liver metastases (n = 12) and lung metastases (n = 6). Exclusion Criteria No CEA evaluation, uncontrolled DM, or acute inflammation Participants Included (n) 98 | ||
| Patient characteristics and setting | Age range 62 (32 ‐ 80) Smoking status N/R Site of primary tumour Rectal 52, sigmoid 12, colon 22, lung or liver metastases 9, peritoneum 1 Stage of primary tumour I 8, II 25, III 46, IV 21 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? Chemo/immunotherapy 25 Recurrences (n) 58 Site of recurrences N/R | ||
| Index tests | CEA timing N/R CEA technique Liaison Kit (Byk‐Sangtec, Diet‐ zenbach, Germany) CEA threshold 3 µg/L Definition of positive 1 elevated value Which CEA value (s) used? At point of recurrence | ||
| Target condition and reference standard(s) | Follow‐up schedule Followed up with the department’s established follow‐up program. The indication for a whole body PET scan was given for patient s with suspected relapse after curative resection of colorectal cancer and who caused a “diagnostic problem” Reference standard FDG‐PET/CT | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country USA Study design RCT Setting Hospital Dates of data collection 1975 ‐ 1980 Population (n) 770 Inclusion criteria B2 C colon or rectal cancer, 2 treatment arms: GITSG protocol 7175 was designed to evaluate adjuvant therapy (chemotherapy, radiotherapy, both, and none) following curative resection of Dukes' B2,C1,or C2 rectal carcinoma. Protocol 6175 was the study of the potential benefit of adjuvant therapy (chemotherapy, immunotherapy, both, and none) following clinically curative resection of Dukes' B2, C1, or C2 colon cancers. Exclusion criteria CEA not recorded post‐op Participants Included (n) 734 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Rectal 191, colon 543 Stage of primary tumour N/R Perioperative investigations done to ensure no residual disease CEA < 5 Chemotherapy/radiotherapy? Yes, but not described Recurrences (n) 149 Site of recurrences Colon | ||
| Index tests | CEA timing On active treatment arms CEA values during and after treatment were to be obtained monthly during the first 3 months,every 3 months for the remainder of the first year, and every six months from then on. For control arms were to have CEA values obtained before operation, 1 week after operation, and at weeks 5, 10, 15, 25 after operation,and every 15 weeks thereafter CEA technique Hansen Z‐gel technique. Interassay comparisons among the institutions and intra‐assay analysis performed in the GITSG CEA reference laboratory at the Mallory Gastrointestinal Institute (Boston, Massachusetts) showed excellent reproducibility and acceptable variation among the various laboratories CEA threshold 2.5 µg/L Definition of positive Maximum level of CEA Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule Patients in both protocols were scheduled for regular clinic visits every 5 weeks during the first 6 months after surgery and every 15 weeks for the remainder of the first year. Physical examination, complete blood count, and liver function tests were performed at each visit. Liver/ spleen scan,chest posterio‐anterior,and lateral roentgenograms were obtained every 6 months. Sigmoidoscopic examination and large‐bowel, contrast roentgenograms were performed every year.Histologic evidence of tumor was the fundamental criterion for recurrence. However,roentgenographic evidence was acceptable in cases of lung or bony metastases. In the rectal‐cancer adjuvant study, liver metastases were also accepted on the basis of liver scan, and local recurrence was accepted on the basis of perineal pain occurring acutely after a pain‐free interval. Reference standard Histology, XR for bony or lung mets, liver scan for liver mets in rectal study, or perineal pain occurring acutely after a pain‐free interval. | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | Yes | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country Taiwan Study design prospective Setting Hospital Dates of data collection 1995 ‐ 2007 Population (n) N/A Inclusion criteria (1) Prior curative resection for histology‐proven primary adenocarcinoma of the colorectum between 1995 and 2002, (2) availability of serial serum samples from before the operation and from after the surgery, and (3) follow‐up with a definitive clinical outcome Exclusion criteria (1) synchronous or metachronous extracolonic cancers, (2) having neoadjuvant therapy for rectal cancer, and (3) fewer than 3 follow‐up samples available for s‐p53Ab analysis Participants Included (n) 305 | ||
| Patient characteristics and setting | Age range 20 ‐ 90 Smoking status N/R Site of primary tumour Colon 95, rectum 101, both 4 Stage of primary tumour I 45, II 130, III 130. Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 76 Site of recurrences locoregional 7, intra‐abdominal or retro‐peritoneal 18, hepatic 29, pulmonary 17, brain or bone 9 | ||
| Index tests | CEA timing The CEA test was defined as positive if 2 consecutive postoperative CEA values were greater than 5 µg/L or the elevated preoperative CEA values had not returned to the normal level (5 µg/L) after surgery CEA technique Abbott Architect 2000 (Abbott Laboratories, Abbott Park, IL, USA) CEA threshold 5 µg/L Definition of positive The CEA test was defined as positive if 2 consecutive postoperative CEA values were greater than 5 µg/L or the elevated preoperative CEA values did not returned to the normal level (5 µg/L) after surgery Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule All cases were followed up at the outpatient department every 3 – 6 months until death or until December 2007. All the patients were followed according to the hospital guidelines of care. Briefly, all patients underwent a follow‐up protocol of an outpatient visits every 3 – 6 months. The follow‐up included physical examination and carcinoembryonic antigen tests as well as chest X‐ray, abdominal sonography or abdominal computer‐assisted tomography scan, and colonoscopy every 1 – 3 years after operation. Reference standard Relapse confirmed by histology or by an imaging study | ||
| Flow and timing | Timing of CEA vs reference standard (days) Triggered by positive CEA | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | Unclear | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Country UK Study design Prospective study after some retrospective sampling Setting Hospital Dates of data collection 1973 ‐ 1978 Population (n) 520 Inclusion criteria curative resection Exclusion criteria Dukes D, no follow‐up information available, signs of malignancy on first postoperative examination, malignancy of other sites during follow‐up Participants Included (n) 468 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour N/R Stage of primary tumour A 94, B 226, C 128, unknown 20 Perioperative investigations done to ensure no residual disease First postoperative exam Chemotherapy/radiotherapy? Not stated Recurrences (n) 108 Site of recurrences N/R | ||
| Index tests | CEA timing At each follow‐up visit CEA technique Assayed by a double‐antibody radioimmunoassay system CEA threshold 40 µg/L Definition of positive 1 elevated value Which CEA value (s) used? all | ||
| Target condition and reference standard(s) | Follow‐up schedule The follow‐up procedure for each patient complied with the normal clinical practice for the hospital concerned and, in addition, at each follow up examination a specimen of plasma was taken for CEA determination. At least 6mly. Reference standard Variable | ||
| Flow and timing | Timing of CEA vs reference standard (days) Very variable | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
| Study characteristics | |||
| Patient sampling | Country Spain Study design Retrospective Setting Hospital Dates of data collection 1988 ‐ 1993 Population (n) N/R Inclusion Criteria Colorectal cancer, curative surgery for Dukes C disease. Exclusion Criteria Dukes A, B, D Participants Included (n) 60 | ||
| Patient characteristics and setting | Age range < 5 preop 60.9 (34 ‐ 85) + > 5 preop 64.9 (47 ‐ 83) Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes C = 60 Perioperative investigations done to ensure no residual disease No Chemotherapy/radiotherapy? N/R Recurrences (n) 21 Site of recurrences Hepatic 9, locoregional 6, combined 3, pulmonary 3 | ||
| Index tests | CEA timing As follow‐up schedule CEA technique Enzyme‐linked immunoassay CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule Physical examination and CEA 3 monthly for 2 years, then 6 monthly up to 5 years. USS abdomen twice a year. CT if CEA increased Reference standard CT if CEA increased | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Country France Study design Prospective cohort study Setting Hospital Dates of data collection 1976 ‐ 1979 Population (n) 91 Inclusion criteria Operated on with curative intent for colorectal cancer Exclusion criteria Conditions which could affect B2 microglobulin level: altered renal function (creatinine > 88.4 umol/l); liver disease: chronic active cirrhosis, primary biliary cirrhosis, acute hepatitis. Metastasis or Dukes D cancers. Patients whose CEA had not returned to normal within 3 months of the operation. | ||
| Patient characteristics and setting | Participants included (n) 91 Age range 33 ‐ 80 Smoking status N/R Site of primary tumour Colon 65, rectum 26 Stage of primary tumour Dukes A&B = 50; C = 41 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? No Recurrences (n) 43 Site of recurrences 12 rectum, 31 colon | ||
| Index tests | CEA timing Every 3 months CEA technique Radioimmunoassay (sorin) CEA threshold 20 µg/L Definition of positive N/R Which CEA value (s) used? N/R | ||
| Target condition and reference standard(s) | Follow‐up schedule CEA & B2m every 3 months for at least 2 years. Clinical and laboratory monitoring was ensured by the same physician during the first two years post‐op in a pre‐established protocol with a barium enema and / or an endoscopy during the first two years enema. CXR and Liver USS annually. Further investigations if indicated (CT chest, bone scan) | ||
| Flow and timing | Timing of CEA vs reference standard (days) Yearly CXR and liver USS; enema and/or endoscopy done at least once in the 2 year follow‐up | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | Unclear | ||
| Was the the timing between index test(s) and reference standard ascertainable? | Unclear | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Country Study design Retrospective Setting Hospital Dates of data collection 1981 ‐ 1986 Population (n) 352 Inclusion criteria Operated for histologically proven colorectal cancer Exclusion criteria No preoperative CEA or lost to follow‐up, Dukes A, or Dukes D Participants Included (n) 272 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour Dukes B 160, C 112 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 27 Site of recurrences N/R | ||
| Index tests | CEA timing Blood samples for CEA measurement were taken a few days before operation and about 1 month after operation and afterward at intervals of 3 ‐ 4 months, combined with physical examination CEA technique Radioimmunoassay kit manufactured by Abbott Laboratory (Chicago, IL, USA) CEA threshold 5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule Blood samples for CEA measurement were taken a. few days before operation and about one month after operation and afterward at intervals of three to four months, combined with physical examination. Other procedures such as colonoscopy, liver sonography, and chest x‐ray were performed annually, Reference standard In the cases where we suspected recurrence the patient underwent additional abdominal computed tomography, bone scanning, or other diagnostic procedures. | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country UK Study design Retrospective Setting Hospital Dates of data collection 1974 ‐ 1976 Population (n) 148 Inclusion criteria Apparently curative surgery for adenocarcinoma of the colon and rectum without evidence of metastatic disease Exclusion criteria N/R Participants Included (n) 148 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colorectal Stage of primary tumour N/R Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 36 Site of recurrences Local 17, local + liver 2, local + bone 2, local + metachronous primary 1, liver 8, bone 5, lung 2 | ||
| Index tests | CEA timing Each follow‐up visit, 2 consecutive raised CEA triggered investigation for recurrence CEA technique CEA levels were assayed by a double antibody radioimmunoassay on unextracted serum CEA threshold 25 µg/L Definition of positive 2 consecutively elevated values Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule CEA at 3 ‐ 6 months intervals post‐operative for up to 56 months or until death. Reference standard If CEA positive then CXR, Liver scan, and bone scan. If these are negative, additional BE and/or colonoscopy. | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
| Study characteristics | |||
| Patient sampling | Country Japan Study design Prospective Setting Hospital Dates of data collection 1999 ‐ 2003 Population (n) 266 Inclusion criteria Curative resection for colorectal cancer, TNM stages I ‐ III, postoperative examinations according to the follow‐up schedule Exclusion criteria Inappropriate follow‐up Participants Included (n) 227 | ||
| Patient characteristics and setting | Age range 65.2 (± 10.8) years Smoking status N/R Site of primary tumour Colon 138, rectum 89 Stage of primary tumour I 34, II 94, III 99 Perioperative investigations done to ensure no residual disease N/R Chemotherapy/radiotherapy? N/R Recurrences (n) 62 Site of recurrences N/R | ||
| Index tests | CEA timing 3 months for the first 3 years and every 6 months during years 4 and 5 CEA technique Latex immunoassay, Mitsubishi Chemical Ltd, Japan CEA threshold 4.5 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule History was taken and a physical examination and measurement of tumor markers were performed every 3 months for the first 3 years and every 6 months during years 4 and 5. Chest X‐ ray and abdominal computed tomography (CT) were done every 6 months for 5 years, and colonoscopy was performed at 1 and 3 years after surgery. Patients were observed until 5 years after surgery or until recurrence was confirmed. Reference standard Recurrence was confirmed histologically or radiologically | ||
| Flow and timing | Timing of CEA vs reference standard (days) per protocol | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
| Study characteristics | |||
| Patient sampling | Country China Study design Retrospective observational study Setting Teaching hospital in Shanghai Dates of data collection May 1988 ‐ March 1990 Population (n) 216 Inclusion criteria Primary colorectal cancer having curative surgery in the teaching hospital or other hospitals Exclusion Criteria N/R Participants Included (n) 182 | ||
| Patient characteristics and setting | Age range N/R Smoking status N/R Site of primary tumour Colorectal cancer 121, colon cancer 95 Stage of primary tumour Only reported Dukes stage data for the 28 before‐ surgery cases (Table 1) Perioperative investigations done to ensure no residual disease N/R Chemotherapy/ radiotherapy? N/R Recurrences (n) 66 Site of recurrences N/R | ||
| Index tests | CEA timing N/R CEA technique RIA CEA threshold 15 µg/L Definition of positive 1 elevated value Which CEA value (s) used? All | ||
| Target condition and reference standard(s) | Follow‐up schedule CEA first measured at 6 weeks after curative surgery; then every 3 months, plus liver ultrasound test and basic health check. Reference standard Positive CEA and CA‐19‐9 triggers ultrasound and CT or colonoscopy | ||
| Flow and timing | Timing of CEA vs reference standard (days) N/R | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All CEA thresholds | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Is the same method and instrument used for all CEA measurements? | Yes | ||
| Is there an estimation of reproducibility of the method, for example the % coefficient of variation at specific concentrations? | No | ||
| Is there an indication of method accuracy, for example, is there evidence of participation in an external quality assessment and proficiency testing scheme? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Was the index test repeated prior to the reference standard? | No | ||
| Was the the timing between index test(s) and reference standard ascertainable? | No | ||
| Did all patients receive a reference standard? | No | ||
| High | |||
ACBE= air contrast barium enema
ALP: alkaline phosphatase
APCT: abdominopelvic computed tomography
BE: barium enema
CT: computed tomography
CXR: chest xray
DCBE: double contrast barium enema
DM: diabetes mellitus
ESR: erythrocyte sedimentation rate
FOBT: faecal occult blood test
LDH: lactate dehydrogenase
LFT: latex fixation test
MRI: magnetic resonance imaging
N/R: not reported
RIA: radioimmunoassay
SCC: squamous cell carcinoma.
SGOT: serum glutamic oxaloacetic transaminase
SGPT: serum glutamate pyruvate transaminase
TNM: primary tumour, regional nodes, metastasis
TPA: tissue plasminogen activator
µg/L = micrograms per litre
USS = ultrasound scan
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| 2 x 2 data not ascertainable | |
| Only CEA positive | |
| Only CEA positive | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Stomach and colorectal cancer combined | |
| Liver metastases only | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| Alternative analysis ‐ median CEA | |
| n < 30 | |
| Alternative analysis ‐ slope | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| Alternative analysis ‐ doubling time | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| n < 30 | |
| Case‐control study | |
| Alternative analysis ‐ slope | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Pre‐operative CEA | |
| Only CEA positive cases | |
| Pre‐operative CEA | |
| Only CEA positive cases | |
| 2 x 2 data not ascertainable | |
| Alternative analysis | |
| 2 x 2 data not ascertainable | |
| Only CEA negative cases | |
| 2 x 2 data not ascertainable | |
| n<30 | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| Alter n a tive analysis ‐ economic | |
| Only cases of recurrence | |
| Only CEA positive cases | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Not follow‐up for recurrence ‐ prognostic value of postoperative CEA | |
| Not curative resection | |
| Not follow‐up for recurrence ‐ includes patients with suspicion of recurrence on CT | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Preoperative CEA | |
| n < 30 | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| Duplicated dataset | |
| n < 30 | |
| 2 x 2 data not ascertainable | |
| Alteranative analysis ‐ modelling | |
| Not curative surgery | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| Duplicate dataset | |
| Case‐control study | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| n < 30 | |
| 2 x 2 data not ascertainable | |
| Only CEA negative cases | |
| 2 x 2 data not ascertainable | |
| Unable to locate full text | |
| Unable to locate full text | |
| Alternative analysis ‐ doubling time | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| Not follow‐up ‐ portal CEA sampling | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Not follow‐up for recurrence ‐ prognostic value of postoperative CEA | |
| Not follow‐up for recurrence ‐ prognostic value of postoperative CEA | |
| Alternative analysis ‐ doubling time | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| Case‐control study | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Duplicate dataset | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| Not follow‐up for recurrence ‐ prognostic value of postoperative CEA | |
| Only cases of recurrence | |
| Not follow‐up for recurrence ‐ prognostic value of postoperative CEA | |
| Case‐control study | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Duplicate dataset | |
| Only CEA positive case | |
| Only CEA positive case | |
| Only CEA positive case | |
| Not follow‐up for recurrence ‐ prognostic value of postoperative CEA | |
| 2 x 2 data not ascertainable | |
| Unable to locate full text | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| Alternative analysis ‐ nomogram | |
| Only cases of recurrence | |
| Alternative analysis ‐ nomogram | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| n<30 | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| Review article | |
| Review article | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Only CEA positive cases | |
| 2 x 2 data not ascertainable | |
| Only CEA positive cases | |
| Only cases of recurrence | |
| Unable to translate | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Unable to locate | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| n < 30 | |
| Only cases of recurrence | |
| Duplicate dataset | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| Case‐control stu dy | |
| Review article | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Only CEA positive cases | |
| Only cases of recurrence | |
| Only CEA negative cases | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Not follow‐up for recurrence ‐ prognostic value of postoperative CEA | |
| 2 x 2 data not ascertainable | |
| Only CEA positive cases | |
| Only CEA positive cases | |
| Alternative analysis ‐ CEA trend | |
| Only CEA positive cases | |
| Alternative analysis ‐ slope | |
| Alternative analysis ‐ slope | |
| Only cases of recurrence | |
| Only CEA positive cases | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Only CEA positive cases | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Non‐curative surgery | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| Liver metastases only | |
| Liver metastases only | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| Not follow‐up for recurrence ‐ prognostic value of postoperative CEA | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| Only cases of recurrence | |
| Preoperative CEA | |
| Preoperative CEA | |
| Only cases of recurrence | |
| Unable to locate | |
| Only cases of recurrence | |
| Only cases of recurrence | |
| 2 x 2 data not ascertainable | |
| Alternative analysis ‐ trend | |
| 2 x 2 data not ascertainable |
Data
Presented below are all the data for all of the tests entered into the review.
| Test | No. of studies | No. of participants |
| 1 CEA ‐ all thresholds Show forest plot | 52 | 9717 |
| Test 1  CEA ‐ all thresholds. | ||
| 2 CEA at 2.5µg/L Show forest plot | 7 | 1515 |
| Test 2  CEA at 2.5µg/L. | ||
| 3 CEA at 5µg/L Show forest plot | 23 | 4585 |
| Test 3  CEA at 5µg/L. | ||
| 4 CEA at 10µg/L Show forest plot | 7 | 1607 |
| Test 4  CEA at 10µg/L. | ||

PRISMA flow diagram: results of the search for studies evaluating the diagnostic accuracy of blood CEA to detect recurrent colorectal cancer in patients following curative resection.
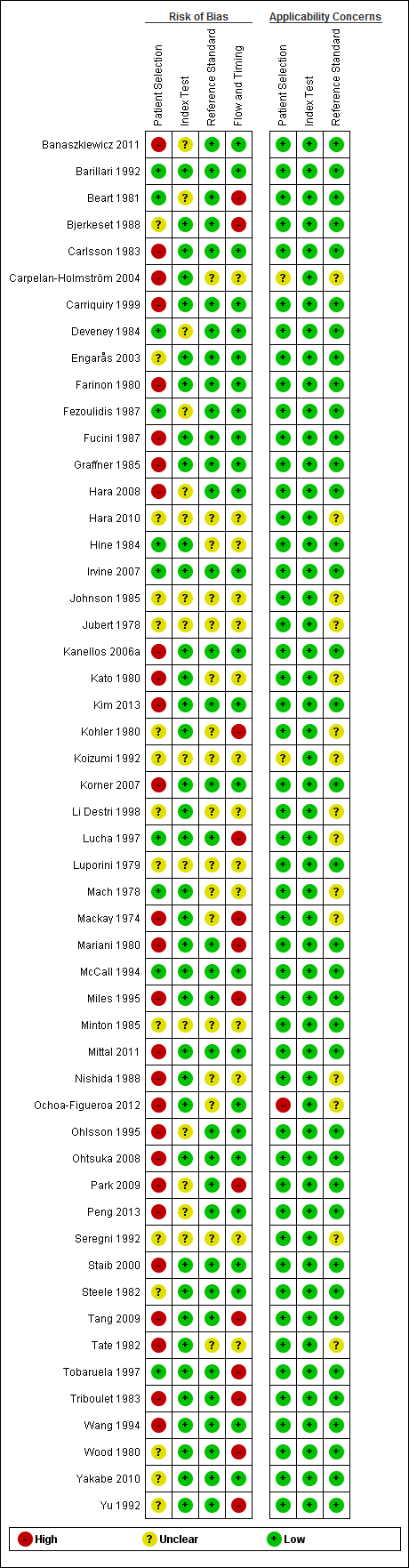
QUADAS‐2 risk of bias and applicability concerns summary including review authors' judgements about each domain for each included study

QUADAS‐2 risk of bias and applicability concerns graph including review authors' judgements about each domain presented as percentages across included studies
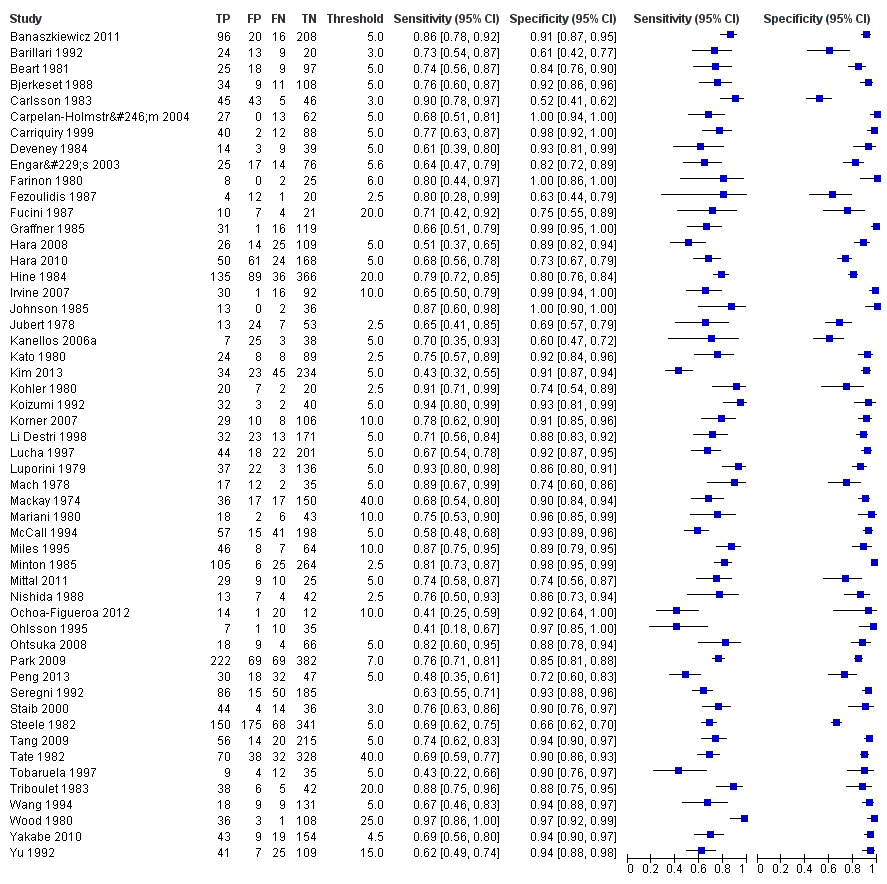
Forest plot for all 52 included studies for the threshold reported closest to 5 µg/L
TP = true positive; FP = false positive; FN = false negative; TN = true negative
The blue square depicts the sensitivity and specificity for each study and the horizontal line represents the corresponding 95% confidence interval for these estimates.

Scatter plot of sensitivity versus specificity for all 52 studies, regardless of threshold.
Each box represents the 2 x 2 data extracted from each study, with the width of the boxes being proportional to the inverse standard error of the specificity and the height of the boxes proportional to the inverse standard error of the sensitivity.

Forest plot broken down by threshold: CEA at 2.5µg/L, CEA at 5µg/L, CEA at 10µg/L.
TP = true positive; FP = false positive; FN = false negative; TN = true negative
The blue square depicts the sensitivity and specificity for each study and the horizontal line represents the corresponding 95% confidence intervals for these estimates.

Summary ROC plot of accuracy at a threshold of 2.5 µg/L.
Each box represents the 2 x 2 data extracted from each study. The width of the box is proportional to the number of patients who did not experience recurrence in each study, and the height is proportional to the number of patients that did develop recurrent CRC.
The filled circle is the pooled estimate for sensitivity and specificity and the line running through it is the summary ROC curve.
The smaller dotted ellipse represents the 95% credible region around the summary estimate; the larger dashed ellipse represents the 95% prediction region.

Summary ROC plot of accuracy at a threshold of 5 µg/L.
Each box represents the 2 x 2 data extracted from each study.
The width of the box is proportional to the number of patients who did not experience recurrence in each study, and the height is proportional to the number of patients that did develop recurrent CRC.
The filled circle is the pooled estimate for sensitivity and specificity and the line running through it is the summary ROC curve.
The smaller dotted ellipse represents the 95% credible region around the summary estimate; the larger dashed ellipse represents the 95% prediction region.
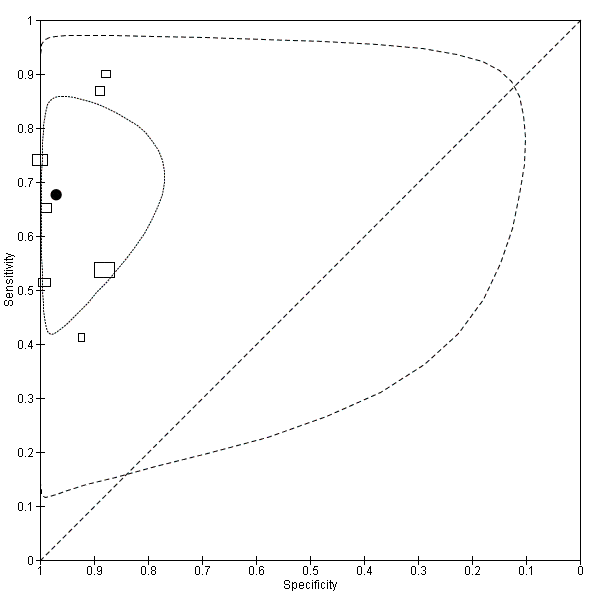
Summary ROC plot of accuracy at a threshold of 10 µg/L.
Each box represents the 2 x 2 data extracted from each study.
The width of the box is proportional to the number of patients who did not experience recurrence in each study, and the height is proportional to the number of patients that did develop recurrent CRC.
The filled circle is the pooled estimate for sensitivity and specificity and the line running through it is the summary ROC curve.
The smaller dotted ellipse represents the 95% credible region around the summary estimate; the larger dashed ellipse represents the 95% prediction region.

CEA ‐ all thresholds.
| Review question: What is the accuracy of single‐measurement blood CEA as a triage test to prompt further investigation for colorectal cancer recurrence after curative resection? | ||||
| Population: adults with no detectable residual disease after curative surgery (with or without adjuvant therapy) | ||||
| Studies: cross‐sectional diagnostic test accuracy studies, cohort studies, and RCTs, reporting 2 x 2 data | ||||
| Index test: Blood carcino‐embryonic antigen (CEA) | ||||
| Reference standard: appropriate¹ imaging, histology, or routine clinical follow‐up | ||||
| Setting: primary or hospital care. | ||||
| Subgroup | Number | Sensitivity (95% CI) | Specificity (95% CI) | Interpretation Assuming a constant incidence of 2%² recurrence at each measurement point, testing 1000 people will have the following outcome depending on the CEA threshold applied |
| 2.5 µg/L | 1515 (7) | 82% (78 to 86) | 80% (59 to 92) | 16 cases of recurrence will be detected and 4 cases will be missed. |
| 5 µg/L | 4585 (23) | 71% (64 to 76) | 88% (84 to 92) | 14 cases of recurrence will be detected and 6 cases will be missed. |
| 10 µg/L | 2341 (7) | 68% (53 to 79) | 97% (90 to 99) | 14 cases of recurrence will be detected and 6 cases will be missed. |
| 1as defined in the Reference standards section of the Methods. | ||||
| Month when CEA measured | per 1000 patients tested at a threshold of 2.5 µg/L | False alarm rate | ||||
| Estimated recurrences¹ | Referrals for raised CEA | Cases of recurrence detected | Cases of recurrence missed | False alarms (cases investigated when cancer not present) | ||
| Follow‐up years 1 and 2: 3‐monthly CEA testing | ||||||
| 3 | 19 | 212 | 16 | 3 | 196 | 92% |
| 6 | 19 | 212 | 16 | 3 | 196 | 92% |
| 9 | 39 | 224 | 32 | 7 | 192 | 86% |
| 12 | 39 | 224 | 32 | 7 | 192 | 86% |
| 15 | 37 | 223 | 30 | 7 | 193 | 87% |
| 18 | 37 | 223 | 30 | 7 | 193 | 87% |
| 21 | 31 | 219 | 25 | 6 | 194 | 89% |
| 24 | 31 | 219 | 25 | 6 | 194 | 89% |
| Follow‐up years 3, 4 and 5: 6‐monthly CEA testing | ||||||
| 30 | 46 | 229 | 38 | 8 | 191 | 83% |
| 36 | 36 | 223 | 30 | 6 | 193 | 87% |
| 42 | 27 | 217 | 22 | 5 | 195 | 90% |
| 48 | 25 | 216 | 21 | 4 | 195 | 90% |
| 54 | 17 | 211 | 14 | 3 | 197 | 93% |
| 60 | 14 | 208 | 11 | 3 | 197 | 95% |
| 1Estimates are based on data reported by Sargent 2007. Three‐monthly data were unavailable, and so constant rates were assumed during each six‐month period for the first two years. Estimates are rounded. | ||||||
| Month when CEA measured | per 1000 patients tested at a threshold of 5 µg/L | False alarm rate | ||||
| Estimated recurrences¹ | Referrals for raised CEA | Cases of recurrence detected | Cases of recurrence missed | False alarms (cases investigated when cancer not present) | ||
| Follow‐up years 1 and 2: 3‐monthly CEA testing | ||||||
| 3 | 19 | 131 | 13 | 6 | 118 | 90% |
| 6 | 19 | 131 | 13 | 6 | 118 | 90% |
| 9 | 39 | 143 | 28 | 11 | 115 | 80% |
| 12 | 39 | 143 | 28 | 11 | 115 | 80% |
| 15 | 37 | 142 | 26 | 11 | 116 | 82% |
| 18 | 37 | 142 | 26 | 11 | 116 | 82% |
| 21 | 31 | 138 | 22 | 9 | 116 | 84% |
| 24 | 31 | 138 | 22 | 9 | 116 | 84% |
| Follow‐up years 3, 4 and 5: 6‐ monthly CEA testing | ||||||
| 30 | 46 | 147 | 33 | 13 | 114 | 78% |
| 36 | 36 | 142 | 26 | 10 | 116 | 82% |
| 42 | 27 | 136 | 19 | 8 | 117 | 86% |
| 48 | 25 | 135 | 18 | 7 | 117 | 87% |
| 54 | 17 | 130 | 12 | 5 | 118 | 91% |
| 60 | 14 | 128 | 10 | 4 | 118 | 92% |
| 1Estimates are based on data reported by Sargent 2007. Three‐monthly data were unavailable, and so constant rates were assumed during each six‐month period for the first two years. Estimates are rounded. | ||||||
| Month when CEA measured | per 1000 patients tested at a threshold of 10 µg/L | False alarm rate | ||||
| Estimated recurrences¹ | Referrals for raised CEA | Cases of recurrence detected | Cases of recurrence missed | False alarms (cases investigated when cancer not present) | ||
| Follow‐up years 1 and 2: 3‐ monthly CEA testing | ||||||
| 3 | 19 | 42 | 13 | 6 | 30 | 70% |
| 6 | 19 | 42 | 13 | 6 | 29 | 70% |
| 9 | 39 | 55 | 27 | 13 | 29 | 52% |
| 12 | 39 | 55 | 27 | 13 | 29 | 52% |
| 15 | 37 | 54 | 25 | 12 | 29 | 53% |
| 18 | 37 | 54 | 25 | 12 | 29 | 53% |
| 21 | 31 | 50 | 21 | 10 | 29 | 58% |
| 24 | 31 | 50 | 21 | 10 | 29 | 58% |
| Follow‐up years 3, 4 and 5: 6‐ monthly CEA testing | ||||||
| 30 | 46 | 60 | 31 | 15 | 29 | 48% |
| 36 | 36 | 53 | 24 | 12 | 29 | 54% |
| 42 | 27 | 48 | 19 | 9 | 29 | 61% |
| 48 | 25 | 46 | 17 | 8 | 29 | 63% |
| 54 | 17 | 41 | 11 | 6 | 30 | 72% |
| 60 | 14 | 39 | 10 | 5 | 30 | 75% |
| 1Estimates are based on data reported by Sargent 2007. Three‐monthly data were unavailable, and so constant rates were assumed during each six‐month period for the first two years. Estimates are rounded. | ||||||
| Test | No. of studies | No. of participants |
| 1 CEA ‐ all thresholds Show forest plot | 52 | 9717 |
| 2 CEA at 2.5µg/L Show forest plot | 7 | 1515 |
| 3 CEA at 5µg/L Show forest plot | 23 | 4585 |
| 4 CEA at 10µg/L Show forest plot | 7 | 1607 |




