Frühe palliative Versorgung für Erwachsene mit fortgeschrittener Krebserkrankung
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel‐group randomised controlled trial (RCT) | |
| Participants | Country and regions: USA, rural area; Norris Cotton Cancer Center (NCCC) and Dartmouth College in Lebanon, New Hampshire, and affiliated outreach clinics, Veterans Administration Medical Center (VAMC), in White River Junction, Vermont Recruitment: identification by research assistants at NCCC and VAMC tumour boards (gastrointestinal, genitourinary, breast, and thoracic cancer management meetings) and review of clinicians' clinic schedules Inclusion criteria: 18 years of age or older; life‐limiting cancer (prognosis approximately 1 year); and within 8 to 12 weeks of a new diagnosis of gastrointestinal tract (unresectable stage III or IV), lung (stage IIIB or IV non–small cell or extensive small cell), genitourinary tract (stage IV, prostate cancers limited to persons with hormone refractory), or breast (stage IV and visceral crisis, lung or liver metastasis, oestrogen receptor (ER) negative, human epidermal growth factor receptor 2 (Her 2 neu) positive)) cancer Exclusion criteria: impaired cognition (< 17 on the Adult Lifestyles and Function Interview‐Mini Mental State Exam); and Axis I psychiatric disorder (schizophrenia, bipolar disorder) or active substance use disorder Number of participants enrolled (survival outcomes sample): N = 322 (161 intervention and 161 control) randomised Participant characteristics (patient outcomes sample): N = 279 (87% returned baseline questionnaires); mean age (intervention/control in years): 65.4/65.2; male gender (intervention/control in %): 62.1/58.2; married or living with partner (intervention/control in %): 73.1/67.2; education < 9 years (intervention/control in %): 11.7/14.9; Caucasian (intervention/control in %): 98.6/98.5; employed (intervention/control in %): 20.0/16.4; live in rural setting (intervention/control in %): 52.4/60.5; Karnofsky Performance Status (intervention/control mean): 78.4/77.4; differences between intervention and control not statistically significant Deaths at end of study (intervention/control in N (%)): 112 (69.6)/119 (73.9); differences between intervention and control not statistically significant Number of caregivers enrolled (intervention/control in N): 116/104 | |
| Interventions | Name: educational and care management palliative care intervention for persons with advanced cancer and a caregiver compared with care as usual (project ENABLE II) Service base: outpatient palliative care Intervention condition (n = 161): case management and educational approach with a manualised, telephone‐based format carried out by 2 advanced practice nurses with palliative care specialty training; 4 initial structured educational and problem‐solving telephone sessions on a weekly basis (education manual: Charting your Course: An Intervention for People and Families Living With Cancer) and at least monthly telephone follow‐up sessions thereafter until the participant died or the study ended; problem‐solving management on the basis of systematic distress assessment using the Distress Thermometer with a cut‐off > 3; when concerns were identified, participants were encouraged to contact the oncology or palliative care clinical teams; monthly medical appointments for participants and their caregivers (attendance in person or by toll‐free conference call) led by a palliative care physician and nurse practitioner, biweekly study team meetings to review audiotaped educational sessions with regards to difficult patient management issues Control condition (n = 161): free access to all oncology and supportive services without restrictions, including referral to the institution's interdisciplinary palliative care service for symptom and supportive care, free access to an advanced illness co‐ordinated care program (Advanced Illness Care Committee, AICC) that provided consultation to oncology staff for inpatients with life‐limiting illness at the VAMC (including prognosis and goals of care assessment, pain and symptom management, advance care planning, referral to hospice) | |
| Outcomes | Primary endpoints: patient‐reported quality of life (Functional Assessment of Chronic Illness Therapy‐Palliative Care, FACIT‐Pal), symptom intensity (Edmonton Symptom Assessment Scale, ESAS), and resource use (number of days in the hospital, number of days in intensive care unit, and number of emergency department visits) Secondary endpoints: mood status (Center for Epidemiological Studies‐Depression scale, CES‐D), survival, caregiver burden (Montgomery Borgatta Caregiver Burden Scale), quality of care (After Death Bereaved Family Member Interview, ADI) Assessment points: baseline/T0: after randomisation; T1: 1 month after baseline; then every 3 months until the participant died or the study was completed (31 December 2007) | |
| Notes | Funding source: National Cancer Institute (NCI), USA Declarations of interest among primary researchers: no financial disclosures reported. Dr Bakitas was a recipient of a Department of Defense Clinical Nurse Researcher award, an American Cancer Society Doctoral scholarship, and a postdoctoral fellowship at Yale University School of Nursing (National Institutes of Health/National Institute of Nursing Research grant T32NR008346). This study was supported by National Cancer Institute grant R01 CA101704 Power considerations: At study completion, final enrolment was 322 owing to slower accrual than was projected in the initial power calculation (target sample size of 400, 80% power for scores on FACIT‐Pal, ESAS, and CES‐D based on a t test comparing treatment groups with respect to the last observed value at a 2‐sided alpha of .01). Reduced sample size and power might have increased the probability of type II error | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from main publication: "Participants were randomised equally into either the intervention or the usual care group using computer‐generated random numbers" Judgement: probably done, as investigators consistently describe the use of random sequences |
| Allocation concealment (selection bias) | High risk | Quote from main publication: "Referring clinicians were neither informed nor formally blinded to participant assignment" Judgement: probably not done |
| Blinding of participants (performance bias) | High risk | Preregistration on clinicaltrials.gov (NCT00253383); says it was an open‐label trial Judgement: not done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Original publication does not explicitly address blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: N = 113 completers in intervention group vs N = 105 completers in control group (Fisher's exact test with 2‐tailed P = 0.40) |
| Selective reporting (reporting bias) | Low risk | Judgement: All outcomes from clinicaltrials.gov registration listed and reported in publications |
| Other bias | Low risk | None detected |
| Methods | Parallel‐group randomised controlled trial (RCT) with fast‐track/delayed‐intervention design | |
| Participants | Country and regions: USA, Norris Cotton Cancer Center and Dartmouth College in Lebanon, New Hampshire, Veterans Administration (VA) medical centre in White River Junction, Vermont Recruitment: 29 months, identification by research assistants/research co‐ordinators reviewing all outpatient clinicians’ schedules and tumour board lists Inclusion criteria: able to speak and understand English; over the age of 18; new diagnosis, recurrence, or progression of advanced stage cancer within approximately 60 days of the date the patient was informed of the diagnosis by his/her oncology clinician; estimated survival of 2 years or less; diagnosed advanced stage solid tumour such as one of the following: • lung cancer: stage IIIB or IV non‐small cell, or extensive stage small cell • breast cancer: stage IV with poor prognostic indicators including but not limited to > 2 cytotoxic regimens for metastatic breast cancer (MBC) or diagnosis of MBC ≤ 12 months since completion of adjuvant or neo‐adjuvant treatment, or triple negative disease (ER/PR and HER‐) or parenchymal brain mets and/or carcinomatous meningitis gastrointestinal cancers: unresectable stage III or IV • genitourinary cancers: stage IV (for prostate cancer inclusion is limited to persons with hormone refractory prostate cancer) • brain cancer: unresectable; grade IV • melanoma: stage IV • haematological malignancies: leukaemia (e.g. acute myeloid leukaemia, acute lymphoblastic leukaemia, chronic myeloid leukaemia, chronic lymphoblastic leukaemia) with advanced stage, treatment refractory, poor prognosis cell type or chromosomal abnormalities, “older age”; lymphoma with stage IV, treatment‐refractory Hodgkin’s disease or non‐Hodgkin’s lymphoma; multiple myeloma with elevated β2‐microglobulin, albumin < 3.5 g/dL, plasma cell labelling index > 1%, CRP > 6 µg/mL, elevated lactate dehydrogenase, plasmablastic morphology, abnormal chromosome 13 Exclusion criteria: dementia or significant confusion (impaired cognitive status as indicated by a score ≤ 3 on the Callahan 6‐item cognitive screening tool); Axis I psychiatric diagnosis of severe mental illness (DSM‐IV) (e.g. schizophrenia, bipolar disorder, active substance use disorder); patients were not excluded if they had not identified a caregiver; 4. prior involvement with palliative care service within the past year; minimum predicted survival < 12 weeks (3 months) Number of participants enrolled: N = 207 (104 intervention and 103 control) randomised Patient characteristics: N = 207; mean age (intervention/control in years): 64.0/64.6; male gender (intervention/control in %): 53.9/51.5; married or living with partner (intervention/control in %): 73.1/69.7; education < 9 years (intervention/control in %): 7.7/2.9; Caucasian (intervention/control in %): 98.1/95.2; employed (intervention/control in %): 24.0/23.3; live in rural setting (intervention/control in %): 59.6/58.3; Karnofsky Performance Status (intervention/control mean): 80.6/81.5; intervention group with significantly less education, higher weekly alcoholic beverage use, and higher clinical trial enrolment Deaths at end of study (intervention/control in N (%)): 50 (48.1)/59 (57.3); difference between intervention and control not statistically significant Number of caregivers enrolled (intervention/control in N): 63/61 | |
| Interventions | Name: early vs. later palliative cancer care: clinical and biobehavioural outcomes (project ENABLE III). All participants received usual oncology care directed by a medical oncologist and consisting of anticancer and symptom control treatments and consultation with oncology and supportive care specialists, including a clinical palliative care team, which was provided whenever requested, regardless of group assignment Service base: outpatient palliative care Intervention condition (n = 104): ENABLE telehealth concurrent palliative care model within 30 to 60 days of being informed of an advancer cancer diagnosis, cancer recurrence, or progression: initial in‐person, standardised outpatient palliative care consultation by a board‐certified palliative care clinician and 6 structured weekly telephone coaching sessions by an advanced practice nurse using a manualised curriculum (Charting Your Course: An Intervention for Patients With Advanced Cancer); sessions 1 to 3 focussed on problem solving, symptom management, self‐care, identification and co‐ordination of local resources, communication, decision making, and advance care planning; sessions 4 to 6 comprised Outlook, a life‐review approach that encourages participants to frame advanced illness challenges as personal growth opportunities; after the 6 Charting Your Course sessions, monthly follow‐up calls reinforced prior content and identified new challenges or care co‐ordination issues; study principal investigator reviewed all palliative care consultation notes and digitally recorded nurse coach sessions for protocol adherence; principal investigator also met with nurse coaches weekly to review and provide feedback on difficult cases Control condition (n = 103): ENABLE telehealth concurrent palliative care model 3 months after being informed of an advancer cancer diagnosis, cancer recurrence, or progression | |
| Outcomes | Primary endpoints: patient‐reported quality of life (FACIT‐Pal) and Trial Outcome Index (TOI), symptom impact (QUAL‐E), mood (CES‐D), 1‐year and overall survival, resource use (patient‐reported hospital and intensive care unit days and emergency department visits, decedents' data for the period between last patient‐reported assessment and death, chemotherapy use in last 14 days and location of death via medical record review) Secondary endpoints: caregiver burden, location of death Assessment points: baseline/T0: before randomisation; T1: 3 months from enrolment; T2: 6 months from enrolment; T3: 12 months from enrolment; in terminal decline joint modelling: T1: 12 months before death; T2: 6 months before death; T3: 3 months before death | |
| Notes | Funding source: grant no. R01NR011871‐01 from the National Institute for Nursing Research; Cancer and Leukemia Group B Foundation Clinical Scholar Award; Foundation for Declarations of interest among primary researchers: Mark T. Hegel reported research funding from Johnson & Johnson. Remaining study authors reported no relationships to disclose Power considerations: At planned study completion date (15 March 2013), final enrolment was 207 because of slower than anticipated accrual. On the basis of final sample size, 3‐month detectable differences were 7.7 points for FACIT‐Pal and 3.2 points for CES‐D and thus was larger than projected in the initial power calculation (target sample size of 360, 80% power to detect a 6‐point difference in FACIT‐Pal and 2.5‐point difference in CES‐D based on a t test comparing 3‐month group differences at a 2‐sided alpha of .05). Reduced sample size and power might have impeded detection of differences (type II error) in patient‐reported outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment was on a one‐to‐one basis using computer‐generated randomly permuted treatment assignments with randomly assigned block sizes of two and four stratified by disease (six categories) and enrolment site (four clinics)" Judgement: probably done, as investigators consistently describe the use of random sequences |
| Allocation concealment (selection bias) | Unclear risk | Original publication does not explicitly address allocation concealment Judgement: unclear risk of bias |
| Blinding of participants (performance bias) | High risk | Preregistration on clinicaltrials.gov (NCT01245621) says it was an open‐label trial that blinded only outcome assessors Judgement: not done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from main publication: "Data collectors were blinded to participant group" Judgement: probably done |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: N = 59 completers in intervention group vs N = 54 completers in control group (Fisher's exact test with 2‐tailed P = 0.58) |
| Selective reporting (reporting bias) | Low risk | Judgement: Inventory of Complicated Grief‐Short Form (ICG‐SF) and Quality of Death and Dying (QODD) as outcomes from clinicaltrials.gov registration not reported in publications so far. Publication does not differentiate between primary and secondary outcomes. However, all key outcomes have been reported. We made a close‐call decision favouring low risk at this point against unclear risk of bias |
| Other bias | Low risk | None detected |
| Methods | Parallel group cluster‐randomised controlled trial (cRCT) | |
| Participants | Country and regions: Italy, 21 centres Recruitment: 29 months, patients with advanced and/or metastatic pancreatic cancer Inclusion criteria: diagnosis of inoperable locally advanced and/or metastatic pancreatic; cancer for a maximum of 8 weeks before enrolment; age ≥ 18 years; Eastern Cooperative Oncology Group Exclusion criteria: patients who were already receiving PC; patients who had received prior chemotherapy for metastatic or advanced disease; patients who had participated in a clinical trial Number of participants enrolled: N = 186 (89 intervention and 97 control) Participant characteristics: N = 186; median age (intervention/control in years): 66/67; male gender (intervention/control in %): 61.5/52.8; married or living with partner (intervention/control in %): 76.9/78.6; ECOG performance status 0, 1, 2 (intervention/control in %): 56.7, 37.1, 6.2/56.2, 39.3, 4.5; differences between intervention and control not statistically significant with respect to age, martial status, and performance status Diseases (intervention/control in %): metastatic pancreatic cancer 100/100 Deaths at end of study (intervention/control in N (%)): 19 (19.6)/16 (17.8); differences between intervention and control not statistically significant Withdrawals/other drop‐outs (intervention/control in N): 33/24; differences between intervention and control not statistically significant | |
| Interventions | Name: standard cancer care plus on‐demand early palliative care or standard cancer care plus systematic early palliative care (interventional arm) Service base: 21 Italian centres Intervention condition (n = 89): "Patients assigned to the interventional arm had an appointment scheduled with a PC specialist who had a predefined checklist of issues to be addressed during the consultation. The use of the checklist by the individual researcher was not monitored from the outside, but reported by the researcher himself. The checklist of topics to be discussed during the visit of PC is the same used by Temel [4] and is reported in the original protocol. Patients met a member of the PC team within 2 weeks of enrolment and were seen thereafter every 2 to 4 weeks until death. In both arms, availability between appointments not scheduled in the protocol, but according to the clinical and organisational solutions, was present in every centre. Moreover, every researcher could have adjunctive routine tools of assessment, not considered in the present study. Palliative care appointments and interventions were oriented by general PC guidelines [12]. The full‐time palliative care specialist who regularly saw interventional arm patients could prescribe drugs and request other interventions pertaining to physical, psychological, and spiritual needs. However, recommendations made by the PC expert on decision making processes had to be shared by the oncologist" Control condition (n = 97): "Patients assigned to the standard arm were not scheduled to meet the PC team unless they, their families, or the attending oncologist requested an appointment. After the evaluation period (T1 = 12 +/‐3 weeks from T0), patients were followed by the PC team as needed" | |
| Outcomes | Primary endpoints: health‐related quality of life (Trial Outcome Index, TOI, as sum of scores on the disease‐specific subscale and on physical and functional well‐being subscales of the Functional Assessment of Cancer Therapy‐Hepatobiliary, FACT‐Hep) Secondary endpoints: mood (Hospital Anxiety and Depression Scale, HADS), family satisfaction with end‐of‐life care (FAMCARE), end‐of‐life care aggressiveness (chemotherapy in the last 30 days of life, median duration of hospice admission, death at home or in hospice) Assessment points: baseline/T0: before randomisation; T1: 12 +‐/+ 3 weeks from enrolment | |
| Notes | Funding source: grant no. RF‐2011‐02350971 from the Italian Ministry of Health Declarations of interest among primary researchers: Study authors declared no conflicts of interest Power considerations: At study completion, final enrolment was 186 and was somewhat lower than projected in the initial power calculation (target sample size of 240, 80% power on a t test comparing treatment groups at a 2‐sided alpha of .05, effect size 0.50). Reduced sample size and power might have increased probability of type II error | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomised for a maximum of 8 weeks after diagnosis and before anticancer treatment to one of the two groups on a 1:1 allocation rate. Separate randomisation lists using a permuted block balanced procedure were generated for each participating centre" Judgement: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (reply received from principal investigator): "The random assignment was done by a telephone call to the Biostatistics and Clinical Trials Unit of the coordinating center in Meldola using computer‐generated randomization lists of permutated blocks of varying sizes stratified for participating center. The sequences were concealed from the physicians" Judgement: probably done |
| Blinding of participants (performance bias) | High risk | Quote: "No masking was involved in this open‐label trial" Judgement: not done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Original publication does not explicitly address blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: N = 64 completers in intervention group vs N = 65 completers in control group (Fisher's exact test with 2‐tailed P value at 0.34) |
| Selective reporting (reporting bias) | Low risk | Judgement: all outcomes from clinicaltrials.gov registration listed and reported in publications |
| Other bias | Low risk | None detected |
| Methods | Parallel‐group cluster‐randomised controlled trial (cRCT) | |
| Participants | Country and regions: USA, Smilow Cancer Hospital at Yale/New Haven, Connecticut Recruitment: 29 months, gynaecological, lung, head and neck, and gastrointestinal clinics, patients identified at weekly tumour boards and approached by their oncologist Inclusion criteria: aged 21 years or older; late‐stage cancer diagnosis within 100 days; post biopsy or surgery with additional treatment recommended; at least 1 self‐reported chronic condition Exclusion criteria: not reported Number of participants enrolled: N = 146 (66 intervention and 80 control), gynaecological and lung clinics allocated to intervention group, head and neck and gastrointestinal clinics allocated to control group Participant characteristics: N = 146; age < 65 years (intervention/control in %): 51.5/71.3; age ≥ 65 years (intervention/control in %): 48.5/28.7; male gender (intervention/control in %): 28.8/56.3; married or living with partner (intervention/control in %): 60.6/52.5; education < 9 years (intervention/control in %): 27.3/30.3; employed (intervention/control in %): 30.3/37.5; number of comorbidities between 3 and 12 (intervention/control in %): 63.6/36.2; differences between intervention and control statistically significant with respect to age, gender, and comorbidity Diseases (intervention/control in %): lung cancer 25.3/‐‐; gastrointestinal tract ‐‐/36.3; gynaecological tumour 19.9/‐‐; head and neck tumour 0/18.5 Deaths at end of study (intervention/control in N (%)): 7 (10.6)/3 (3.8); differences between intervention and control not statistically significant Withdrawals/other drop‐outs (intervention/control in N (%)): 23 (34.8)/21 (26.2); differences between intervention and control not statistically significant | |
| Interventions | Name: advanced practice nurse co‐ordinated multi‐disciplinary intervention vs standard cancer care Service base: 4 disease‐specific outpatient clinics Intervention condition (n = 66): 10‐week standardised intervention delivered by different members of each team included monitoring participants' status, providing symptom management, executing complex care procedures, teaching participants and family caregivers, clarifying the illness experience, co‐ordinating care, responding to the family, enhancing quality of life, and collaborating with other providers; clinic advanced practice nurses initially contacted participants within 24 hours, and weekly phone and scheduled in‐person contacts (5 clinic visits and 5 telephone calls); members of each disease‐specific multi‐disciplinary team worked together as a palliative care unit, each member taking on different functions to ensure all components of the intervention were addressed; clinic advanced practice nurse oversaw co‐ordination and implementation Control condition (n = 80): enhanced usual care, i.e. usual multi‐disciplinary care plus a copy of the symptom management toolkit with instructions on its use | |
| Outcomes | Primary endpoints: symptom distress (Symptom Distress Scale, SDS), health distress (4‐item scale developed by the Stanford Patient Education Research Center), depression (Patient Health Questionnaire, PHQ‐9), emotional distress (Emotional Distress Thermometer, EDT), functional status (Enforced Social Dependency Scale, ESDS), self‐rated health (first item of the SF‐12) Secondary endpoints: anxiety (7‐item Hospital Anxiety and Depression Scale, HADS‐Anxiety), self‐efficacy (Self‐Efficacy for Managing Chronic Disease Scale, SEMCD), uncertainty (Mishel Uncertainty in Illness Scale ‐ Community Form, MUIS‐C), quality of life (Functional Assessment of Cancer Therapy ‐ General, FACT‐G) Assessment points: baseline/T0: after randomisation; T1: 1 month from enrolment; T2: 3 months from enrolment | |
| Notes | Funding source: NIH/NINR grant R01NR011872 Declarations of interest among primary researchers: Apart from funding, no further study author disclosure statements were made Power considerations: No a priori sample size calculation was provided. Small sample size and power might have increased probability of type II error | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from main publication: "Randomization was done using the ranuni function in conjunction with the rank procedure in statistical software SAS (SAS version 9.2 for Windows; SAS Institute Inc., Cary, NC)" Judgement: probably done |
| Allocation concealment (selection bias) | Unclear risk | Original publication does not explicitly address allocation concealment. Judgement: unclear risk of bias |
| Blinding of participants (performance bias) | High risk | Preregistration on clinicaltrials.gov (NCT01272024) says it was an open‐label trial. Judgement: unclear risk of bias |
| Blinding of outcome assessment (detection bias) | Unclear risk | Original publication does not explicitly address blinding of outcome assessment. Judgement: unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: N = 36 completers in intervention group vs N = 56 completers in control group (Fisher's exact test with 2‐tailed P = 0.06). Non‐significant trend for higher attrition in the intervention group. We made a close‐call decision favouring low risk against high risk of bias |
| Selective reporting (reporting bias) | High risk | Uncertainty (MUIS‐C) as single primary outcome in clinicaltrials.gov registration, reported as secondary outcome in publication. Anxiety (HADS‐Anxiety) as single secondary outcome in clinicaltrials.gov registration. Judgement: high risk for reporting bias |
| Other bias | Low risk | Statistically significant differences between arms with respect to age, gender, and comorbidity at baseline. Judgement: Given the baseline imbalance, recruitment bias may potentially be present. We made a close‐call decision favouring low risk for other bias, as high risk for selection bias was already detected |
| Methods | Parallel‐group randomised controlled trial (RCT) | |
| Participants | Country and regions: Australia, Department of Medical Oncology, Royal Prince Alfred Hospital (RPAH) Camperdown, New South Wales Recruitment: 22 months, no additional details reported Inclusion criteria: newly detected incurable metastatic cancer (just diagnosed or relapsed with metastatic disease after previous adjuvant chemotherapy); life expectancy < 12 months (oncologist estimate of patient's likely survival time) Exclusion criteria: previous contact with palliative care Number of participants enrolled: N = 120 (60 intervention and 60 control) randomised Participant characteristics: N = 107; mean age (intervention/control in years): 63/64; male gender (intervention/control in %): 53/43; married or living with partner (intervention/control in %): 67/68; education ≤ 10 years (intervention/control in %): 38/53; oncologist estimate of participant's likely survival time (intervention/control in %): 4‐12 weeks 2/0, 3‐6 months 15/10, 6‐12 months 55/50, > 12 months 18/33, not stated 10/7; intervention group with significantly more recent initial diagnosis and significantly more participants with likely survival time > 12 months Diseases (intervention/control in %): lung cancer 20/18; gastrointestinal tract 33/40; breast 8/20; other gynaecological tumour 18/13; prostate 0/3; other tumour 20/5; differences between intervention and control not statistically significant Deaths at end of study (intervention/control in N (%)): 39 (65)/31 (51.7); differences between intervention and control not statistically significant Withdrawals/other drop‐outs (intervention/control in N (%)): 36 (60.0)/37 (61.7); differences between intervention and control not statistically significant | |
| Interventions | Name: early contact with a palliative care nurse consultant with ongoing oncologist care vs oncologist care alone Service base: outpatient palliative care Intervention condition (n = 60): meeting with a palliative care nurse consultant member of the hospital palliative care team, who outlined available palliative care services including advice about symptom control, offered to arrange review by a palliative care physician, and provided contact details for the palliative care service; palliative care nurse offered to telephone the participant monthly to check on his or her well‐being, or, if the participant preferred, provided contact details for use by participant; standard oncological care given consistent with the oncologist’s recommendation | |
| Outcomes | Primary endpoints: symptom severity (Rotterdam Symptom Checklist, RSC), quality of life (McGill Quality of Life Questionnaire, MQOL), degree of perceived support (Supportive Secondary endpoints: hospital medical records including end‐of‐life experiences, number of lines of chemotherapy, place of death Assessment points: baseline/T0: after randomisation; T1: 1 month from enrolment; T2: 3 months from enrolment; T3: 6 months from enrolment; T4: 9 months from enrolment; T5: 12 months from enrolment | |
| Notes | Funding source: National Health & Medical Research Council strategic palliative care research grant no. 219141 Declarations of interest among primary researchers: Apart from funding, no additional study author disclosure statements were made Power considerations: At study completion, final enrolment was 120 owing to slower accrual than was projected in the initial power calculation (target sample size of 150, 80% power on a t test comparing treatment groups at a 2‐sided alpha of .05, effect size 0.50). Reduced sample size and power might have increased probability of type II error | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from main publication: "For allocation of the participants, a computer‐generated list of random numbers was used, and allocation was concealed using sequentially numbered, opaque sealed envelopes. No stratification was made for oncologist or cancer diagnosis" Judgement: probably done |
| Allocation concealment (selection bias) | Low risk | Quote from main publication: "allocation was concealed using sequentially numbered, opaque sealed envelopes" Judgement: probably done |
| Blinding of participants (performance bias) | High risk | Registration in the Australian New Zealand Clinical Trial Registry (ACTRN12611001137987) says it was an open‐label trial. Judgement: not done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Original publication does not explicitly address blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | High risk | Judgement: N = 21 completers in intervention group vs N = 29 completers in control group (Fisher's exact test with 1‐tailed P = 0.19). However, we assumed high risk of bias due to high loss of follow‐up across both groups |
| Selective reporting (reporting bias) | Unclear risk | Judgement: Outcomes from Australian New Zealand Clinical Trials Registry (ANZCTR) listed and reported in publications. However, trial registration was conducted after recruitment of participants. We made a close‐call decision favouring unclear risk against high risk of bias |
| Other bias | Unclear risk | Quote: "Most baseline characteristics were adequately balanced across the two study groups (Table 1), however there were differences between the groups in the time since initial cancer diagnosis (mean of 29 versus 34 months in the early referral and standard care groups respectively), and the oncologists’ estimate of likely survival (i.e. 11 versus 20 patients with estimates of > 12 months likely survival in the early referral and standard care groups respectively). Therefore, these variables were controlled for in subsequent analyses. There were no remarkable baseline differences on the patient reported outcome measures between the groups" Judgement: Given the baseline imbalance, recruitment bias may potentially be present. However, accounting for imbalance in statistical analysis did not change results. Thus, we made a close‐call decision favouring unclear risk against high risk of bias |
| Methods | Parallel‐group randomised controlled trial (RCT) | |
| Participants | Country and regions: USA, Massachusetts General Hospital, Boston, Massachusetts Recruitment: 38 months; patients who presented to the outpatient thoracic oncology clinic were invited by their medical oncologists; all medical oncologists in the clinic agreed to approach, recruit, and obtain consent from their patients; physicians were encouraged, but were not required, to offer participation to all eligible patients; no additional screening or recruitment measures were used Inclusion criteria: pathologically confirmed metastatic non‐small cell lung cancer; diagnosis within previous 8 weeks; Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2; able to read and respond to questions in English Exclusion criteria: patients already receiving care from the palliative care service Number of participants enrolled: N = 151 (77 intervention and 74 control) Participant characteristics: N = 151; mean age (intervention/control in years): 64.9/65.0; male gender (intervention/control in %): 51/45; married or living with partner (intervention/control in %): 61/62; Caucasian (intervention/control in %): 95/100; differences between intervention and control not statistically significant Diseases (intervention/control in %): non‐small cell lung cancer 100/100; presence of brain metastases 26/31; receipt of initial chemotherapy as part of a clinical trial 27/21; never smoked or smoked ≤ 10 packs/y 22/24; illness perception of curable cancer 32/31; differences between intervention and control not statistically significant Deaths at end of study (intervention/control in N (%)): 10 (13.0)/17 (23.0); differences not statistically significant (P = 0.14) Withdrawals/other drop‐outs (intervention/control in N(%)): 7 (9.1)/10 (13.5); differences not statistically significant | |
| Interventions | Name: early palliative care integrated with standard oncological care as compared with standard oncological care alone. All participants continued to receive routine oncological care throughout the study period Service base: outpatient palliative care Intervention condition (n = 77): "Patients who were assigned to early palliative care met with a member of the palliative care team, which consisted of board‐certified palliative care physicians and advanced‐practice nurses, within 3 weeks after enrolment and at least monthly thereafter in the outpatient setting until death. Additional visits with the palliative care service were scheduled at the discretion of the patient, oncologist, or palliative care provider. General guidelines for the palliative care visits in the ambulatory setting were adapted from the National Consensus Project for Quality Palliative Care and were included in the study protocol. Using a template in the electronic medical record, palliative care clinicians documented the care they provided according to these guidelines. Specific attention was paid to assessing physical and psychosocial symptoms, establishing goals of care, assisting with decision making regarding treatment, and coordinating care on the basis of the individual needs of the patient" Control condition (n = 74): "Patients who were randomly assigned to standard care were not scheduled to meet with the palliative care service unless a meeting was requested by the patient, the family, or the oncologist; those who were referred to the service did not cross over to the palliative care group or follow the specified palliative care protocol" | |
| Outcomes | Primary endpoints: quality of life (Trial Outcome Index, TOI, as sum of scores on the Lung Cancer Subscale and on physical and functional well‐being subscales of the Functional Assessment of Cancer Therapy‐Lung, FACT‐L) Secondary endpoints: mood (Hospital Anxiety and Depression Scale, HADS; Patient Health Questionnaire 9, PHQ‐9), healthcare use and end‐of‐life care (anticancer therapy, medication prescriptions, referral to hospice, hospital admissions, emergency department visits, date and location of death), aggressive care, participants' resuscitation preferences Assessment points: baseline/T0: before randomisation; T1: 3 months from enrolment | |
| Notes | Funding source: American Society of Clinical Oncology Career Development Award and philanthropic gifts Declarations of interest among primary researchers: Apart from funding, no additional study author disclosure statements were made Power considerations: Primary outcome was change in score on the TOI from baseline to 12 weeks. Study authors estimated that with 120 participants, the study would have 80% power to detect a significant between‐group difference in the change in TOI score from baseline to 12 weeks, with a medium effect size of 0.5, SD.24. Protocol was amended in August 2008 to allow for enrolment of 30 additional participants to compensate for loss of any participants to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to a reply received from the principal investigator, computer‐generated random sequence generation with no stratification was applied. Judgement: probably done |
| Allocation concealment (selection bias) | High risk | According to a reply received from the principal investigator, no allocation concealment. Judgment: not done |
| Blinding of participants (performance bias) | High risk | Preregistration on clinicaltrials.gov (NCT01038271) says it was an open‐label trial. Judgement: not done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Original publication does not explicitly address blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: N = 60 completers in intervention group vs N = 47 completers in control group (Fisher's exact test with 2‐tailed P value at 0.07). Trend toward higher attrition in the control group |
| Selective reporting (reporting bias) | Low risk | Judgement: All outcomes from clinicaltrials.gov registration listed and reported in publications |
| Other bias | Low risk | None detected |
| Methods | Study design: parallel‐group cluster‐randomised controlled trial (cRCT) | |
| Participants | Country and regions: Canada, Princess Margaret Cancer Centre, University Health Network Toronto, Ontario Recruitment: 51 months, daily screening of participating oncology clinics by research personnel Inclusion criteria: aged 18 years or older; stage IV cancer (refractory to hormonal therapy as additional criterion for breast or prostate cancer, patients with stage III cancer and poor clinical prognosis were included at the discretion of the oncologist); estimated survival of 6‐24 months (assessed by main oncologist); ECOG performance status of 0, 1, or 2; completed baseline measures Exclusion criteria: insufficient English literacy; inability to pass the cognitive screening test (Short Orientation‐Memory‐Concentration Test score < 20 or > 10 errors) Number of participants enrolled: N = 461 (228 intervention and 233 control), 12 clinics allocated to intervention and control groups, respectively Participant characteristics: N = 461; mean age (intervention/control in years): 61.2/60.2; male gender (intervention/control in %): 40.4/46.4; married or living with partner (intervention/control in %): 68.4/71.7; education < 9 years (intervention/control in %): 8.0/10.3; employed (intervention/control in %): 19.7/25.3; differences between intervention and control not statistically significant Diseases (intervention/control in %): lung cancer 24.1/19.7; gastrointestinal tract 32.5/27.9; genitourinary 11.8/21.9; breast 18.0/13.3; other gynaecological tumour 13.6/17.2; control group with significantly larger number of participants with genitourinary cancers .02 Deaths at end of study (intervention/control in N (%)): 26 (11.4)/44 (18.9); differences statistically significant at P = 0.02 Withdrawals/other drop‐outs (intervention/control in N (%)): 52 (22.8)/53 (22.7); differences between intervention and control not statistically significant | |
| Interventions | Name: early intervention in patients with advanced cancer by a palliative care team vs standard cancer care Service base: outpatient clinics, hospital service, home care Intervention condition (n = 228): multi‐disciplinary approach to care addressing physical, psychological, social, and spiritual needs Outpatient clinics: palliative care physician and nurse; routine visits once monthly and more often if necessary; routine structured symptom assessment in clinic during every visit by palliative care nurse and physician; routine psychosocial assessment in clinic and discussion of goals of care, of participant and family support needs, and of participant and family coping and psychological distress; discussion of advance care planning according to participant and family readiness; routine telephone follow‐up by palliative care nurse after each visit; more often as needed by palliative care nurse and physician; 24‐hour on‐call service explained during first visit, provided by specialised palliative care physicians Hospital service: direct access to palliative care unit for symptom management; primary care by trained palliative care nurses and physicians; formal 10‐day training for staff at opening of palliative care unit and continued education by palliative care unit advanced practice nurse; follow‐up by palliative care team when admitted to non‐palliative care unit service at University Health Network. Home care: community care access centre services explained and offered during first visit, reassessed at each visit; routine communication with family physician and community care access centre; home palliative care physician was explained during first visit and was offered with ECOG performance status ≥ 3 or when participant requested Control condition (n = 233): approach to care mainly via addressing physical needs Outpatient clinics: oncologist and oncology nurses; visits ad hoc and mainly based on chemotherapy or radiation schedule; no structured symptom assessment; no routine psychological assessment; follow‐up as needed and conducted by oncology nurse, rare access to oncologist; access to 24‐hour on‐call service (oncology resident or clinical associate). Hospital service: no access to palliative care unit; admission to oncology ward or medical ward (via emergency department for urgent care); primary care by oncology nurses and clinical associates; no formal palliative care training; no follow‐up by palliative care team. Home care: community care access centre services ad hoc; generally no home care referral until referral to palliative care team; rarely an ad hoc communication with family physician and community care access centre; no home palliative care physician | |
| Outcomes | Primary endpoints: participant‐reported quality of life (Functional Assessment of Chronic Illness Therapy‐Spiritual Well‐Being, FACIT‐Sp and Quality of Life at the End of Life, QUAL‐E) Secondary endpoints: symptom impact (Edmonton Symptom Assessment System, ESAS), participant interaction with nurses and doctors (Cancer Rehabilitation Evaluation System Medical Interaction Subscale, CARES‐MIS), satisfaction with care (family satisfaction with advanced cancer care, FAMCARE‐P16) Assessment points: baseline/T0: after randomisation; T1: 1 month from enrolment; T2: 2 months from enrolment; T3: 3 months from enrolment; T4: 4 months from enrolment | |
| Notes | Funding source: Canadian Cancer Society, Ontario Ministry of Health and Long Term Care Declarations of interest among primary researchers: Study authors declared no competing interests Caregiver assessment: data from caregivers collected for an exploratory substudy, publication pending Power considerations: Initial sample size estimation showed that 380 participants (190 per group) would provide 80% power at the 2‐sided 5% level of significance to detect a between‐group difference in FACIT‐Sp of 0.45 SD (medium effect size) by the primary endpoint of 3 months. Sample size was recalculated in 2008, on the basis of observed SD (from aggregated baseline data of 245 participants), intracluster correlation coefficient, attrition, and adherence. Revised sample size was 450 participants (225 per group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from main publication: "Randomisation was done by the statistical team at Western University (London, ON, Canada) using a computer‐generated sequence, was in a 1:1 ratio, and was stratified by clinic size and tumour site [...]" Judgement: probably done |
| Allocation concealment (selection bias) | High risk | Quote from main publication: "There was also selection bias, which is common in cluster‐randomised studies because of randomisation of clusters before consent of individuals. A larger number of patients declined participation in the intervention group because of lack of symptoms" Judgement: probably not done |
| Blinding of participants (performance bias) | Low risk | Quote from main publication: "Although complete masking of interventions was not possible, patients provided written informed consent to participate in their own study group, without being informed of the existence of another group. This form of masking is common in cluster randomised trials, and avoids potential bias from patients in the control group requesting the intervention or otherwise altering their behaviour" Judgement: probably done |
| Blinding of outcome assessment (detection bias) | High risk | Quote from main publication: "Oncologists and investigators were aware of assignment" Judgement: probably not done |
| Incomplete outcome data (attrition bias) | Low risk | Judgement: N = 131 completers in intervention group vs N = 155 completers in control group (Fisher's exact test with 2‐tailed P value at 0.05). By conventional criteria, higher attrition in the intervention group of borderline significance. We made a close‐call decision favouring low risk against high risk of bias |
| Selective reporting (reporting bias) | Low risk | Judgement: Functional Assessment of Cancer Therapy‐General (FACT‐G) as primary outcome in clinicaltrials.gov registration, FACIT‐Sp (includes FACT‐G) reported as primary outcome in publications so far. Secondary outcomes Caregiver Quality of Life Index‐Cancer (CQOL‐C) and SF‐36 not reported in publications so far. However, all key outcomes have been reported |
| Other bias | Low risk | Tendency for higher outcome measure scores (for FACIT‐Sp at P = 0.03; for ESAS at P < 0.001; for FAMCARE‐P16 at P < 0.001) in intervention group at baseline. Larger number of participants with genitourinary cancers in the control group at baseline. No loss of clusters reported Judgement: Given the baseline imbalance, recruitment bias may potentially be present. We made a close‐call decision favouring low risk for other bias, as high risk for selection bias was already detected |
g/dL: grams per decilitre
µg/mL: microgram per millilitre
N = number of participants
packs/y: packs per year
PC: palliative care
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Psychosocial intervention focussing on dyadic coping. No genuine multi‐dimensional palliative care approach | |
| No outcome measurement regarding symptom intensity or quality of life | |
| Psychotherapy only. No genuine multi‐dimensional palliative care approach | |
| Discussions of the benefits of hospice and advanced directives, led by a single nurse only. No genuine multi‐dimensional palliative care approach | |
| Non‐randomisation, instead quasi‐experimental trial with sequential enrolment of patients into control and intervention groups | |
| Study followed a conventional palliative care approach. No genuine early palliative care intent | |
| ED‐initiated palliative care consultation. No genuine early palliative care intent | |
| Aerobic exercise, resistance or respiratory training only. No genuine multi‐dimensional palliative care approach | |
| Portion of patient sample in the terminal phase. Study followed a conventional palliative care approach. No genuine early palliative care intent | |
| Randomised prospective trial comparing "no immediate treatment" with single‐ and multiple‐agent chemotherapy. However, study did not follow a proactive palliative care intent as characteristic for early palliative care | |
| Psychosocial intervention applying narrative interviews. No genuine multi‐dimensional palliative care approach | |
| Portion of patient sample in the terminal phase. Study followed a conventional palliative care approach. No genuine early palliative care intent | |
| Investigators withdrew the study before enrolment | |
| Study followed a conventional palliative care approach. No genuine early palliative care intent | |
| Patient sample had a life expectancy of 1 to 5 years and varying diseases. No genuine early palliative care intent | |
| No explicit focus on physical domain/symptom control. No genuine early palliative care intent | |
| Portion of patient sample in the terminal phase. Study followed a conventional palliative care approach. No genuine early palliative care intent | |
| Psychosocial intervention only. No genuine multi‐dimensional palliative care approach | |
| Ongoing implementation study in which general practitioners were randomised. We do not consider this study to be a clinical trial on a patient population | |
| Psychosocial intervention only. No genuine multi‐dimensional palliative care approach | |
| Telephone follow‐up intervention for postoperative patients with colorectal cancer. Most patients with prognosis longer than 24 months. No genuine early palliative care intent |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT |
| Participants | Patients with newly diagnosed NSCLC have a high symptom burden, poor quality of life, and a prognosis less than 1 year |
| Interventions | Early palliative care integrated with standard oncological care |
| Outcomes | Primary outcome measures: quality of life at 12 weeks assessed with the ESAS |
| Notes | Abstract with results published. We found no preregistration entry for this study |
| Methods | RCT |
| Participants | Patients in contact with oncology departments who had cancer stage IV according to the ‘TNM’ (tumour, node, metastases) classification or cancer in the central nervous system grade 3 or 4, were at least 18 years of age, lived in the area of one of the participating specialised palliative care centres, and had not had contact with an SPC during the previous year received a screening questionnaire. If, according to their answers on the questionnaire, patients had a palliative need and 4 additional symptoms (see definition below), they were informed about the study and were invited to participate. Patients were excluded from the study if they could not understand Danish well enough to complete a questionnaire or were considered incapable of complying with the study protocol |
| Interventions | Experimental condition: specialised palliative care Control condition: standard care |
| Outcomes | All randomised participants are assessed at baseline (the screening); after a 3‐week follow‐up period; and after an 8‐week follow‐up period. The primary outcome is estimated as the difference between intervention and control groups in the change from baseline to the weighted mean of the 3‐ and 8‐week follow‐up measured as area under the curve for the EORTC QLQ‐C30 scale score that constitutes the primary need. The primary need is defined as the palliative need having the highest intensity at baseline according to the EORTC QLQ‐C30. Secondary outcomes, estimated in the same way, are remaining symptoms and problems measured by the EORTC QLQ‐C30 (14 scales); anxiety and depression measured by the HADS; participants' evaluation of treatment and care provided by the healthcare system and measured by FAMCARE‐P16; survival; and economical consequences per week from the start of the study to minimum 3 months after the end of the intervention |
| Notes | Study completed. Protocol published |
| Methods | Non‐randomised parallel assignment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental condition: group II (palliative care intervention); participants receive an individualized interdisciplinary palliative care intervention combining patient‐centred teaching principles and concepts that are learner‐entered (builds on strengths, interests, and needs of the learner), knowledge‐entered (teacher is proficient in the content being taught), assessment‐entered (learners are given an opportunity to test their understanding and receive feedback), and community‐entered (opportunities are available for continued learning and support); patients undergo 4 teaching sessions (based on patient‐entered teaching principles and concepts) that focus on physical, psychological, social, and spiritual well‐being, respectively, once a week in weeks 3‐6; patients then receive 4 follow‐up phone calls in weeks 9‐21 to clarify questions or review concerns from teaching sessions and to co‐ordinate follow‐up resources as needed Control condition: group I (standard care); participants receive standard care; participants complete questionnaires at baseline and at 6, 12, 24, 36, and 52 weeks to evaluate quality of life (QOL), symptoms, psychological distress, and geriatric assessments |
| Outcomes | Primary outcome measures: overall quality of life and psychological distress at 6 months; symptom control at 6 months; geriatric assessment outcomes (OARS (Older Americans' Resources and Services) Instrumental Activities of Daily Living, MOS (Medical Outcomes Study) Activities of Daily Living, MOS Social Activities Limitation Scale, HADS scores, and Karnofsky performance scale); resource use (chart audits) |
| Notes | Study completed and results published. |
| Methods | Multi‐site RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental condition: "intervention arm dyads received a copy of The Home Care Guide for Cancer. Each book chapter addresses a problem Control condition: "usual care" |
| Outcomes | Primary outcome measures: City of Hope (COH) quality of life (QOL) instruments for patients or caregivers and the social problem solving inventory revised |
| Notes | Study completed. Results published. We contacted study authors for explicit information on palliative care intent. The reply to this study author request is pending |
| Methods | Non‐randomised parallel assignment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental condition: phase 2 intervention GROUP II (palliative care intervention); participants receive an individualised interdisciplinary palliative care intervention comprising learner‐centred, knowledge‐centred, assessment‐centred, and community‐centred concepts; participants undergo 4 teaching sessions, focussed on physical, psychological, social, and spiritual well‐being, once weekly in weeks 3‐6; participants then receive 4 follow‐up phone calls in weeks 9, 13, 17, and 21 Control condition: no Intervention; phase I usual care GROUP I (usual care); participants receive standard care |
| Outcomes | Primary outcome measures: overall quality of life and psychological distress at 6 months; symptom control at 6 months; geriatric assessment outcomes (OARS (Older Americans' Resources and Services) Instrumental Activities of Daily Living, MOS (Medical Outcomes Study) Activities of Daily Living, MOS Social Activities Limitation Scale, Hospital Anxiety and Depression Scale scores, and Karnofsky performance scale); resource use (chart audits) |
| Notes | Study completed. Publication in preparation according to study authors |
| Methods | RCT |
| Participants | Inclusion criteria: At least 1 of the following
And also all of the following inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental condition: palliative home care nursing group; in addition to standard home care nursing, families will receive 6 home visits from a research nurse with at least 1 year specialised palliative care experience; during the first 2‐hour visit, a family assessment is obtained that identifies family roles, resources, and coping strategies; the first home visit takes place no later than 1 week after randomisation; visits continue every third week up to 16 weeks, each visit with a duration of 1.5 hours; at every visit, the EORTC‐QLQ‐C30 patient‐administered questionnaire is used to identify the nature, frequency, and intensity of the patient's physical and psychosocial problems Control condition: standard home care nursing group; patients continue to receive standard home care nursing; they can contact municipality services for visitation to home care nursing if they feel that additional home care is needed, or if they do not yet receive this service and feel they need home care nursing |
| Outcomes | Primary outcome measures: participant‐reported health‐related quality of life (EORTC QLQ‐C30 in relation to the global health status scale) at baseline, week 9, week 16, and week 24 |
| Notes | Study completed. According to the principal investigator, publications are in preparation |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental condition 1: early palliative care; a first medical consult at the palliative care service will be scheduled after 2 to 3 weeks from study inclusion and every 3 to 4 weeks thereafter Experimental condition 2: psychosocial plus early palliative care; 5 weekly sessions of a brief psychosocial intervention based on cognitive‐behavioural therapy plus early palliative care; regarding early palliative care, a first medical consult at the palliative care service will be scheduled after 2 to 3 weeks from study inclusion and every 3 to 4 weeks thereafter Control condition: no Intervention; standard oncological care |
| Outcomes | Primary outcome measures: change from baseline in depression symptoms (HADS, PHQ‐9) at day 90; change from baseline in satisfaction with care on the FAMCARE‐patient scale at days 45, 90, 120, and 180; descriptive results about feasibility of the study |
| Notes | Last updated on ClinicalTrials.gov on 12 February 2017: "This study has been terminated. Planned interim analysis did not show the expected benefit of intervention A over B (effect size <0.2)." |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental condition:
Control condition: Standard transplant oncology care with participant enrolment and caregiver enrolment (within 72 hours of participant enrolment) and longitudinal data collection (participants and family caregivers) |
| Outcomes | Primary outcome measures: change in FACT‐BMT score at 2 weeks, comparison of changes in quality of life (FACT‐BMT) scores from baseline to week 2 (day+5 for autologous, day+8 for myeloablative or reduced intensity allogeneic HSCT) between study arms by the 2‐sample t‐test |
| Notes | Study completed. Abstract with results published. Last updated on ClinicalTrials.gov on 28 January 2017: "This study is ongoing, but not recruiting participants." |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental condition: early palliative care; participants receive standard care with early palliative care Control condition: no intervention; participants receive standard of care |
| Outcomes | Primary outcome measures: quality of life (FACT) at baseline and at 12 weeks |
| Notes | Study completed and results published. |
| Methods | RCT |
| Participants | All patients defined as having high risk of gynaecological malignancies (< 30% 5‐year predicted survival) |
| Interventions | Referred to palliative care consultation within 8 weeks of tumour board registration for primary occurrence or recurrence |
| Outcomes | Primary outcome measure: increase in palliative consultation from historical 50% as reported at the tertiary care institution |
| Notes | Study completed. Abstracts with results published, |
EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire
EORTC QLQ‐C15‐Pal: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire for Palliative Cancer Care Patients
ESAS: Edmonton Symptom Assessment System
FACT: Functional Assessment of Cancer Therapy
FACT‐BMT: Functional Assessment of Cancer Therapy ‐ Bone Marrow Transplant
FAMCARE: Family Satisfaction with Advanced Cancer Care Questionnaire
HADS: Hospital Anxiety and Depression Scale
HSCT: Haematopoietic stem cell transplantation
MGH: Massachusetts General Hospital (MGH)
NSCLC: non‐small cell lung cancer
PHQ‐9: Patient Health Questionnaire
RCT: randomised controlled trial
SF‐36: Short Form (36) Health Survey
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Improving communication and quality of life (QOL) at the end of life: a randomised controlled trial of a multifocal communication intervention for patients with advanced incurable cancer, carers and doctors |
| Methods | Parallel‐group RCT |
| Participants | Patients are eligible if they have been given the diagnosis of any type of cancer, and their medical oncologist believes they have a life expectancy of between 2 and 12 months. Caregivers are eligible if they are identified as the primary, informal providers of care to a patient participating in the study. Patients and caregivers must read and speak English well enough to be interviewed and to complete questionnaires without the aid of an interpreter, must be over the age of 18 years, and must be capable of giving informed consent. Patients and caregivers will be excluded if they do not speak English or have significant psychological morbidity or cognitive impairment |
| Interventions | Guided by the self‐determination theory of health‐behaviour change, the communication support programme pairs a purpose‐designed Question Prompt List (an evidence‐based list of questions participants/caregivers can ask clinicians) with nurse‐led exploration of Question Prompt List content, communication challenges, participant values and concerns, and the value of early discussion of end‐of‐life issues. Oncologists are also cued to endorse participant and caregiver questions and use of the QPL. Behavioural and self‐report data will be collected from participants/caregivers approximately quarterly for up to 2.5 years, or until participant death, after which participant medical records will be examined. Analyses will examine the impact of the intervention on participants' and caregivers’ participation in medical consultations, their self‐efficacy in medical encounters, quality‐of‐life, end‐of‐life care receipt, and quality‐of‐death indicators |
| Outcomes | Patients: demographic details, communication self‐efficacy (Perceived Efficacy in Patient Physician Interactions Scale), quality of life (FACT‐G, and the McGill Quality of Life Scale), preferences for information and involvement in decisions about care as well as achievement of preferences for information and involvement in decisions (Degner Control Preference Scale, Cassileth Information Styles Questionnaire), hopes for treatment, preferences for future interventions, acceptance of disease (Peace, Equanimity and Acceptance in the Cancer Experience Scale), understanding of prognosis, doctor’s communication skills and manner Caregiver: demographic details, communication self‐efficacy (adapted version of the Perceived Efficacy in Patient Physician Interactions Scale), quality of life (SF‐36), preferences for information and involvement in decisions about patient care (adapted Degner Control Preference Scale, Cassileth Information Styles Questionnaire), achievement of preferences for information and involvement in decisions about care, understanding of participants' hopes for treatment, understanding of participants' preferences for future interventions, understanding of participants' prognosis, Quality of Death and Dying Scale. |
| Starting date | April 2010 |
| Contact information | Prof Phyllis Butow Centre for Medical Psychology & Evidence‐based Decision‐making School of Psychology Brennan MacCallum Building (A18) University of Sydney NSW 2006 Australia E‐mail: [email protected] |
| Notes | Recruiting. Protocol published. |
| Trial name or title | A study to assess the feasibility of introducing early palliative care in ambulatory patients with advanced lung cancer |
| Methods | Feasibility study |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental condition: pain and symptom management and participant counselling; participants meet the palliative care team on the day of referral, then every month thereafter for 6 months |
| Outcomes | Primary outcome measures: More than 60% of referred patients have met the palliative care team; more than 50% of referred patients have completed the EORTC QLQ‐30 and EORTC QLQ ‐ LC13 (lung‐ cancer) and ESAS Secondary outcome measures: symptom burden (ESAS), quality of life (EORTC QLQ 30 and EORTC QLQ ‐ LC13) |
| Starting date | September 2013 |
| Contact information | Dr Jayita Deodhar Associate Professor Tata Memorial Hospital Dr. E. Borges Road Parel Mumbai Mumbai, Maharastra, 400012 India E‐mail: [email protected] |
| Notes | Last updated on CTRI on 22 May 2017: "open to recruitment" |
| Trial name or title | Effect of early integration of specialized palliative care into standard oncologic treatment on the quality of life of patients with advanced head and neck cancers: a randomized controlled trial |
| Methods | Randomised, parallel‐group trial |
| Participants | Inclusion criteria:
Exclusion criteria:
Target sample size is N = 180 |
| Interventions | Experimental condition: early palliative care arm: Participant will receive specialist palliative care along with standard oncological treatment; participants in this arm will consult palliative care team along with parent oncology team; follow‐up visit will be provided as per need; chemotherapeutic drugs will be given according to disease status as per institutional protocol Control condition: standard arm: Participants in this arm will receive standard oncological treatment; follow‐up visits will be decided by the oncologist as per treatment protocol; chemotherapeutic drugs will be given according to disease status as per institutional protocol |
| Outcomes | Primary outcome measures: change in quality of life, measured by FACIT‐H&N at 3 months after initial visit |
| Starting date | March 2016 |
| Contact information | M.A. Muckaden Room 132, Department of Palliative Medicine, Ground Floor, Main Building, Tata Memorial Hospital, Parel (E), Mumbai 400012 Mumbai, MAHARASHTRA India |
| Notes | Last updated on CTRI on 22 May 2017: "not yet recruiting" |
| Trial name or title | Early palliative care – health services research and implementation of sustainable changes |
| Methods | Feasibility study |
| Participants | Early palliative care services provided in this study are aimed at patients with advanced metastatic cancer that is unresponsive to curative treatments (ICD 10 C 1–80 + ICD 10 C 78–79). In all participating comprehensive cancer centres, patients will be identified by the tumour boards at each centre. As soon as the diagnostic process has been concluded and treatment has started (i.e. within the first 8 weeks after diagnosis), patients will be referred to the PC physician Target sample size is N = 2000 |
| Interventions | In the main study phase, participants with metastatic cancer will routinely be offered a consultation with the palliative care physician within 8 weeks of diagnosis. This initial consultation has multiple objectives. First, this meeting serves to provide information regarding the value and accessibility of specialist palliative care. The palliative care physician will explain to participants that interdisciplinary cancer treatment ensures that all meaningful treatment options will continue to be available (“fight against cancer”), but that high priority will be placed on quality of life also. For quality of life needs, specialist palliative care services will be available to participants, alongside treatment from the primary cancer specialist |
| Outcomes | Early palliative care will be considered feasible if 75% of all eligible patients (i.e. adult patients with the diagnosis of an incurable, metastatic cancer [ICD 10 C 1–80 + ICD 10 C 78–79]) are referred to a palliative care physician at their centre at least once within 8 weeks of the initial diagnosis. Participants' quality of life and symptom burden will be assessed at the initial palliative care consultation on the POS, the EORTC QLQ‐C30, and the HADS. In both preliminary and main study phases, follow‐up assessment will be conducted at 12 and 24 weeks with these 3 instruments. Family/caregivers of the participant will be asked to assess the participant's situation by filling out the Quality of Dying and Death questionnaire. |
| Starting date | October 2014 |
| Contact information | Dr Cornelia Meffert Department of Palliative Care, Comprehensive Cancer Center University Medical Center Freiburg Robert‐Koch‐Str. 3 79106 Freiburg Germany E‐mail: cornelia.meffert@uniklinik‐freiburg.de |
| Notes | Protocol published. Last updated on DRKS on 22 May 2017: "recruiting" |
| Trial name or title | SPECIAL: Standard or palliative care in advanced lung cancer ‐ does early referral of patients with metastatic non‐small cell lung cancer to UK specialist palliative care services make a difference in their quality of life or survival? |
| Methods | Phase III randomised controlled trial with integral feasibility stage (non‐randomised) |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Arm A: standard of care (i.e. standard referral to specialised palliative care, if participant is willing) |
| Outcomes | Primary outcome measures: Global Health Status Score at 3 months after study entry, quality‐adjusted survival time over 6 months Secondary outcome measures: overall survival, anxiety/depression, pain, health economics, quality of life, memory and cognitive ability, Modified Glasgow Prognostic Score |
| Starting date | September 2015 |
| Contact information | Sam H. Ahmedzai c/o Trial Co‐ordinator Cancer Research (UK) Clinical Trials Unit School of Cancer Sciences University of Birmingham Edgbaston B15 2TT Birmingham United Kingdom E‐mail: [email protected] |
| Notes | Last updated on ISRCTN on 28 November 2016: "recruiting completed" |
| Trial name or title | A multicentre non‐blinded randomised controlled trial to assess the impact of regular early specialist symptom control treatment on quality of life in malignant mesothelioma (RESPECT‐MESO) |
| Methods | Multi‐centre non‐blinded, randomised controlled, parallel‐group trial |
| Participants | Inclusion criteria:
Exclusion criteria:
Target sample size is N = 174 |
| Interventions | Experimental condition: regular early SSCT: In the regular early SSCT group, participants will be seen within 3 weeks of randomisation by the specialised palliative care team (regardless of, and in addition to, all other treatments being offered). The initial meeting will consist of an approximately 1‐hour consultation with a member of the specialist palliative care team. This may be a Consultant or Specialist Palliative Care Clinical Nurse Specialist. Participants then will continue to be seen regularly on at least a 4‐weekly basis (regardless of other treatments, interventions, and symptoms) by a member of the specialist palliative care team, with consultations lasting approximately 30 minutes. These monthly reviews will continue until end of trial or participant death. Control condition: "standard therapy" |
| Outcomes | Primary outcome measures: global quality of life at 12 weeks post randomisation Secondary outcome measures:
Added 21/08/2014:
|
| Starting date | January 2014 |
| Contact information | Portsmouth Hospitals NHS Trust |
| Notes | Protocol published. Last updated on ISRCTN on 17 October 2016: "completed" |
| Trial name or title | Randomized controlled trials for the effect of early management on PAin and DEpression in patients with PancreatoBiliary cancer (EPADE‐PB) |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
Target sample size is N = 288 |
| Interventions | Experimental condition: early palliative care; interventions consisted of the following: nursing assessment of pain and depression mood; pain control‐based NCCN guideline; depression control by psychoeducation and/or consultation of psychiatrist specialist; participant education Control condition: usual oncological care; participants randomly assigned to usual oncological care were not scheduled to meet with the palliative care service unless a meeting was requested by the participant, the family, or the oncologist; those who were referred to the service did not cross over to the early palliative care group or follow the specified palliative care protocol |
| Outcomes | Primary outcome measures: reduction in pain score (BPI) at 1 month and every 3 months up to 1 year; reduction in depression score (CES‐D) at 1 month and every 3 months up to 1 year |
| Starting date | April 2012 |
| Contact information | WooJin Lee, MD National Cancer Center Goyang, 410‐769, Gyeonggi‐do Republic of Korea E‐mail: [email protected] |
| Notes | Last updated on ClinicalTrials.gov on 3 October 2016: "This study is currently recruiting participants." |
| Trial name or title | Integration of palliative care for cancer patients on phase I trials |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental condition: early PCI; participants receive part I of the PCI comprising quantitative surveys, comprehensive palliative care assessment by research nurses, and goals of care discussions beginning before administration of the first dose of phase I treatment; participants then receive part II of the PCI comprising recommendations from the interdisciplinary team, participant educational sessions, and supportive care referrals following the first dose of phase I treatment and completed within 1 month of the first treatment Control condition: delayed PCI; participants receive usual care until 12 weeks post treatment initiation; participants then receive both part I and part II of the PCI |
| Outcomes | Primary outcome measures: change in overall quality of life scores (FACT‐G and FACIT‐Sp) at 12 weeks; change in psychological distress (NCCN Distress Thermometer) at 12 weeks; satisfaction with communication (FAMCARE) at 12 weeks; participants' symptom intensity and symptom interference with daily activities (Psychological Patient‐Reported Outcomes Version of the Common Terminology Criteria for Adverse Events) at 4 and 12 weeks; total numbers of supportive care referrals (social work, dietician, chaplaincy, psychologist/psychiatrist) at 12 weeks; total numbers of unscheduled outpatient encounters and inpatient admissions at 12 weeks; total number of hospice referrals at 12 weeks; retention in the phase I trial at 12 weeks; participant satisfaction with the PCI at 12 weeks |
| Starting date | September 2014 |
| Contact information | Betty Ferrell, PhD, MA, FAAN, FPCN City of Hope ‐ Main Campus (Duarte) United States E‐mail: [email protected] |
| Notes | Last updated on ClinicalTrials.gov on 31 May 2017: "This study is currently recruiting participants." |
| Trial name or title | Effect of early palliative care on quality of life of patients with advanced cancer: a randomised controlled trial |
| Methods | RCT |
| Participants | Inclusion criteria: Patients with life‐limiting cancer (prognosis of approximately 1 year) are eligible if:
Exclusion criteria:
Target sample size is N = 186 |
| Interventions | Experimental condition: early palliative care; interventional palliative care after diagnosis and once a month Control condition: standard care; participants will receive standard oncological care |
| Outcomes | Primary outcome measures: quality of life of the participant and his or her family caregiver at baseline, at 12 weeks, and 6‐weekly after 12 weeks (EORTC‐QLQ C30, McGill QOL, SF‐36) |
| Starting date | April 2013 |
| Contact information | Gaëlle Vanbutsele, MSc, Clinical Psychologist Doctoral Researcher End‐of‐Life Care Research Group Vrije Universiteit Brussel & Ghent University UZ Gent De Pintelaan 185, 9000 Gent Belgium E‐mail: [email protected] |
| Notes | Abstract published. Last updated on ClinicalTrials.gov on 1 July 2016: "This study is ongoing, but not recruiting participants." |
| Trial name or title | A pilot trial of an embedded collaborative model of supportive care for pancreatic cancer |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
Target sample size is N = 60 |
| Interventions | Experimental condition: supportive care intervention; monthly (minimum) participant and caregiver visits with a supportive care physician, embedded within their standard oncological care (through collaboration with oncology providers) Control condition: usual care; participants will receive standard oncology care from their oncology providers |
| Outcomes | Primary outcome measures: trial feasibility; acceptability of intervention participation at 3 months (+/‐ 3 weeks); perceived effectiveness at 3 months (+/‐ 3 weeks) Secondary outcome measures: change in participant quality of life (FACT‐Hep) at 3 months (+/‐ 3 weeks); participant healthcare utilisation (numbers and types of chemotherapy regimens, frequency and timing of chemotherapy regimens, number and length (days) of hospital admissions, number and length (days) of intensive care unit admissions, number of emergency department visits, frequency and timing (days before death) of hospice use, place of death) |
| Starting date | July 2013 |
| Contact information | Yael Schenker, MD, MAS Assistant Professor of Medicine University of Pittsburgh Cancer Institute (UPCI) Hillman Cancer Center Pittsburgh, Pennsylvania, 15232 United States E‐mail: [email protected] |
| Notes | Last updated on ClinicalTrials.gov on 10 May 2016: "This study has been completed." |
| Trial name or title | A structured early palliative care intervention for patients with advanced cancer ‐ a randomized controlled trial with a nested qualitative study (SENS trial) |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
Target sample size is N = 150 |
| Interventions | Experimental condition: structured approach intervention with the SENS model based on the bio‐psycho‐social‐spiritual model of care and WHO definitions of palliative care, as well as NCCN Practice Guidelines for Palliative Care. It supports assessment of areas and complexity of concerns from the participant perspective, determines the priority and structures the support needed. Intervention is provided by palliative care physicians and nurses collaboratively. It is utilised as baseline assessment and afterwards is integrated into each routine oncology care outpatient and inpatient visit. Depending on the goals, it may be applied between routine visits. In addition, participants will receive usual oncology care throughout the study period Control condition: Participants in the usual care group will receive routine oncology care throughout the study. This incorporates a routine assessment according to the standard Swiss Group for Clinical Cancer Research (SAKK) protocol, which assesses overall symptoms. Participants are not seen by nurses during a routine visit to the outpatient clinic unless they need a blood withdrawal or any intravenous or subcutaneous treatment. Only nursing staff in the palliative care unit is familiar with using the SENS‐assessment instrument. Participants assigned to usual care may meet with the palliative care service on request according to established practice |
| Outcomes | Primary outcome measures: distress over 6 months (NCCN Distress Thermometer) |
| Starting date | December 2013 |
| Contact information | Steffen Eychmueller, MD University Center for Palliative Care Bern University Hospital SWAN Haus CH‐3010 Bern Switzerland E‐mail: [email protected] |
| Notes | Last updated on ClinicalTrials.gov on 27 September 2016: "This study is currently recruiting participants." |
| Trial name or title | Impact of early palliative care on quality of life and survival of patients with non‐small‐cell metastatic lung cancer in Northern France |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
Target sample size is N = 144 |
| Interventions | Experimental condition: multidisciplinary palliative care monthly consultations with a doctor, a nurse, a psychologist, and possibility a physical therapist and a chaplain, in addition to standard onco‐pneumological care Control condition: participant supported by the oncological respiratory service for treatment of disease by chemotherapy and for treatment of complications |
| Outcomes | Primary outcome measures: quality of life (Trial Outcome Index) at 12 weeks |
| Starting date | October 2014 |
| Contact information | Licia Touzet, MD University Hospital Lille 59000 Lille France E‐mail: licia.touzet@chru‐lille.fr |
| Notes | Last updated on ClinicalTrials.gov on 18 May 2017: "This study is currently recruiting participants." |
| Trial name or title | A randomized, controlled phase III study of integrated, specialized palliative rehabilitation for patients with newly diagnosed non‐resectable cancer |
| Methods | RCT |
| Participants | Inclusion criteria: Participants must:
Exclusion criteria:
Target sample size is N = 300 |
| Interventions | Experimental condition: 150 participants will receive standard oncology treatment alongside a 12‐week specialised palliative rehabilitation programme Control condition: 150 participants will receive standard oncology treatment |
| Outcomes | Primary outcome measures: effect of the intervention on "The Primary Problem" chosen by the participant (EORTC‐QLQ‐C30 that correlates with "the primary problem" of the participant) at 6 and 12 weeks |
| Starting date | November 2014 |
| Contact information | Lars Henrik Jensen, MD, PhD Department of Oncology VejleHospital DK‐7100 Vejle Denmark E‐mail: [email protected] |
| Notes | Last updated on ClinicalTrials.gov on 31 May 2017: "This study is currently recruiting participants." |
| Trial name or title | Early integrated supportive care study for gastrointestinal cancer patients |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
Target sample size is N = 152 |
| Interventions | Experimental condition: early palliative care; during first oncology appointment, participants in the intervention arm will self‐report to the study team any symptoms related to their cancer or treatment; scores at or above a defined benchmark will be seen by Pain and Symptom Management/Palliative Care team members during or immediately after their oncology appointment; participants will be asked to self‐report symptoms once a month following recruitment for 4 months Control condition: standard care; during first oncology appointment, participants in the control arm will self‐report to the study team any symptoms related to their cancer or treatment; self‐reports will be collected but will not be shared with the Pain and Symptom Management/Palliative Care Team, and participants will continue with their oncology appointment as per standard procedure; participants will be asked to self‐report symptoms once a month following recruitment for 4 months |
| Outcomes | Primary outcome measure: total symptom distress score at 4 months after recruitment (modified ESAS) |
| Starting date | February 2015 |
| Contact information | Pippa Hawley, MD Head Palliative Care Physician British Columbia Cancer Agency Vancouver, British Columbia, V5Z 4E6 Canada E‐mail: [email protected] |
| Notes | Last updated on ClinicalTrials.gov on 14 April 2015: "The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than two years." |
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | The study intervention consists of early integration of palliative care services into standard oncology care provided in an outpatient setting for participants with advanced lung and non‐colorectal gastrointestinal malignancies who are not being treated with curative intent. Palliative care services provided to participants randomised to the intervention will be provided by board‐certified physicians and/or advanced practice nurses and will focus on the following areas: developing and maintaining the therapeutic relationship with participants and family caregivers; assessing and treating participant symptoms; providing support and reinforcement for coping with advanced cancer among participants and family caregivers; assessing and enhancing prognostic awareness and illness understanding in participants and family caregivers; assisting with treatment decision making; and (6) assisting with end‐of‐life care planning |
| Outcomes | Primary outcome measure: change in FACT‐G scores from baseline to 12 weeks |
| Starting date | April 2015 |
| Contact information | Jennifer S. Temel, MD Massachusetts General Hospital, 55 Fruit St. United States E‐mail: [email protected] |
| Notes | Last updated on ClinicalTrials.gov on 19 April 2017: "This study is ongoing, but not recruiting participants." |
| Trial name or title | Evaluation of the implementation of an early integrated palliative care program in the esophageal cancer population |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
Target sample size is N = 700 |
| Interventions | Early palliative care (not further specified) |
| Outcomes | Primary outcome measure: quality of life |
| Starting date | October 2015 |
| Contact information | Christian J Finley, MD, MPH, FRCSC McMaster University 50 Charlton Avenue East, T‐2105 Canada E‐mail: [email protected] |
| Notes | Last updated on ClinicalTrials.gov on 15 March 2016: "This study is currently recruiting participants." |
| Trial name or title | Early palliative care in patients with acute leukaemia (Pablo Hemato) |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
Target sample size is N = 40 |
| Interventions | Experimental condition: Participants will be seen by palliative care team at least once a month until the 12th week, more often if needed. Symptoms and suffering will be assessed by a multi‐disciplinary palliative specialist team of physician, nurse, and psychologist. Physical, psychological, social, and existential suffering will be addressed Control condition: "usual medical follow‐up" |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | November 2015 |
| Contact information | Marilène FILBET, PU‐PH Centre Hospitalier Lyon Sud Pierre Bénite, France, 69495 E‐Mail: marilene.filbet@chu‐lyon.fr |
| Notes | Last updated on ClinicalTrials.gov on 11 December 2015: "This study is currently recruiting participants." |
| Trial name or title | A primary palliative care intervention for patients with advanced cancer (CONNECT) |
| Methods | Cluster‐randomised controlled trial (cRCT) |
| Participants | Inclusion criteria: Participants will be patients with advanced cancer receiving care at a participating clinic; their caregivers; their oncology staff nurses, oncologists, and practice managers. They will be adults (≥ 21 years old); with metastatic solid tumours; the oncologist "would not be surprised if the patient died in the next year"; Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2; planning to receive ongoing care from a participating oncologist and willing to be seen at least monthly Exclusion criteria: Inability to read and respond to questions in English; cognitive impairment or inability to consent to treatment, as determined by the patient's oncologist; inability to complete baseline interview; ECOG PS of 3 (capable of limited self‐care; confined to bed or chair > 50% of waking hours) or 4 (cannot carry on any self‐care; totally confined to bed or chair); haematological malignancy Target sample size is N = 1486 |
| Interventions | Experimental condition: CONNECT CONNECT is a primary palliative care ‐ care management intervention led by existing oncology nurses. CONNECT is deployed through a series of nurse‐led encounters occurring before or after regularly scheduled oncology clinic visits. Based on best practices in palliative oncology care, the first visit focusses on establishing rapport, addressing symptom needs, and choosing a surrogate decision maker. Subsequent visits include additional focus on treatment preferences and future goals. CONNECT visits are guided by participant‐reported outcomes. During every CONNECT encounter, the nurse will work with participants and caregivers to complete and update individualized shared care plans. After every CONNECT visit, the nurse will discuss participants' symptoms, preferences, and goals with their oncologists via a mandatory check‐in session and will conduct a follow‐up call with the participant and/or caregiver. Control condition: Usual care control. At clinics randomised to usual care, enrolled participants and caregivers will continue to receive supportive oncology care according to usual practice. |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | April 2016 |
| Contact information | Yael Schenker, MD, MAS Assistant Professor of Medicine University of Pittsburgh Cancer Institute (UPCI) Hillman Cancer Center Pittsburgh, Pennsylvania, 15232 United States E‐mail: [email protected] |
| Notes | Last updated on ClinicalTrials.gov on 7 December 2016: "This study is currently recruiting participants." |
| Trial name or title | Palliative and oncology care model In breast cancer |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
Target sample size is N = 120 |
| Interventions | Experimental condition: Participants randomised to the intervention will receive collaborative care from palliative care and oncology for the remainder of their illness. The initial 5 visits with palliative care will be conducted in accordance with study‐specific clinical practice guidelines and will occur at least monthly Control condition: Participants randomised to oncology care alone will continue to receive routine care identical to what they would have received if they had not participated in the trial. Participants or their oncologists can request palliative care consultation at any point in time |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | May 2016 |
| Contact information | Jennifer Temel, MD Massachusetts General Hospital Bosten, Massachusetts, United States, 02115 E‐Mail: [email protected] |
| Notes | Last updated on ClinicalTrials.gov on 15 April 2017: "This study is currently recruiting participants." |
| Trial name or title | Early palliative care in patients with metastatic upper gastrointestinal cancers treated with first‐line chemotherapy (EPIC‐1511) |
| Methods | RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
Target sample size is N = 558 |
| Interventions | Sham comparator: Arm A: chemotherapy alone. The medical oncologists (or gastroenterologist physicians) are in charge of the participant for chemotherapy administration, and for management of symptoms related to the disease and/or the treatment, in accordance with professional practices. If needed (at any time), a palliative consultation visit could be performed. Interventions include the EORTC‐QLQ‐C30 questionnaire for assessment of quality of life and HADS score for anxiety and depression assessment Experimental: Arm B: chemotherapy + early palliative care. Standard oncology care as for arm A plus early palliative consultation visits. Interventions include EORTC‐QLQ‐C30 questionnaire for assessment of quality of life and HADS score for anxiety and depression assessment. Early palliative care visits: A palliative consultation visit is a visit done by a palliative care physician. Any visits done by other professionals ARE NOT palliative consultation visits. Five palliative consultation visits are scheduled in this arm |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | August 2016 |
| Contact information | Ariette Da Silva, MD Antoine Adenis, MD, PhD Centre Oscar Lambret 3 Rue Frédéric Combemale, 59000 Lille, France E‐Mail: a‐dasilva@o‐lambret.fr |
| Notes | Last updated on ClinicalTrials.gov on 10 January 2017: "This study is currently recruiting participants." |
BPI: Brief Pain Inventory
CES‐D: Center for Epidemiological Studies‐Depression Scale
ECOG: Eastern Cooperative Oncology Group Performance Status
EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire
ESAS: Edmonton Symptom Assessment System
FACIT‐Pal: Functional Assessment of Chronic Illness Therapy ‐ Palliative Care
FACIT‐Sp: Functional Assessment of Chronic Illness Therapy ‐ Spirituality
FACT‐Breast: Functional Assessment of Cancer Therapy ‐ Breast
FACT‐E: Functional Assessment of Cancer Therapy ‐ Esophagus
FACT‐G: Functional Assessment of Cancer Therapy ‐ General
FACT‐Hep: Functional Assessment of Cancer Therapy ‐ Hepatobiliary
FACT‐H&N: Functional Assessment of Cancer Therapy ‐ Head and Neck
FACT‐Leu: Functional Assessment of Cancer Therapy – Leukemia
FAMCARE: Family Satisfaction with Advanced Cancer Care Questionnaire
HADS: Hospital Anxiety and Depression Scale
NCCN: National Comprehensive Cancer Network
NSCLC: non‐small cell lung cancer
PCI: palliative care intervention
PHQ‐9: Patient Health Questionnaire
RCT: randomised controlled trial
SSCT: specialist symptom control treatment
SF‐36: Short Form (36) Health Survey
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Health‐related quality of life Show forest plot | 7 | 1028 | Std. Mean Difference (Random, 95% CI) | 0.27 [0.15, 0.38] |
| Analysis 1.1  Comparison 1 Early palliative care vs standard oncological care, Outcome 1 Health‐related quality of life. | ||||
| 1.1 Co‐ordinated care model | 3 | 485 | Std. Mean Difference (Random, 95% CI) | 0.21 [0.03, 0.39] |
| 1.2 Integrated care model | 4 | 543 | Std. Mean Difference (Random, 95% CI) | 0.31 [0.15, 0.46] |
| 2 Depression Show forest plot | 5 | 762 | Std. Mean Difference (Random, 95% CI) | ‐0.11 [‐0.26, 0.03] |
| Analysis 1.2  Comparison 1 Early palliative care vs standard oncological care, Outcome 2 Depression. | ||||
| 2.1 Co‐ordinated care model | 3 | 526 | Std. Mean Difference (Random, 95% CI) | ‐0.06 [‐0.23, 0.12] |
| 2.2 Integrated care model | 2 | 236 | Std. Mean Difference (Random, 95% CI) | ‐0.24 [‐0.50, 0.02] |
| 3 Survival Show forest plot | 4 | 800 | Hazard Ratio (Random, 95% CI) | 0.85 [0.56, 1.28] |
| Analysis 1.3 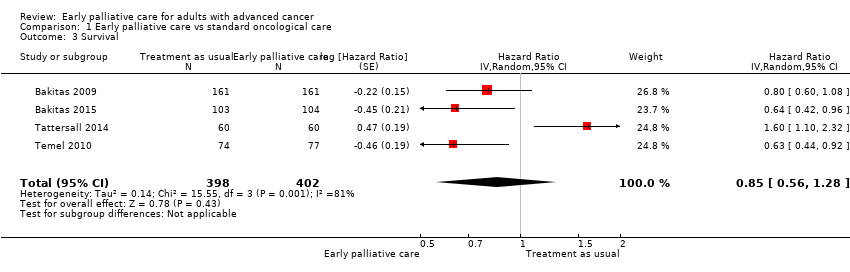 Comparison 1 Early palliative care vs standard oncological care, Outcome 3 Survival. | ||||
| 4 Symptom intensity Show forest plot | 7 | 1054 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.35, ‐0.10] |
| Analysis 1.4  Comparison 1 Early palliative care vs standard oncological care, Outcome 4 Symptom intensity. | ||||
| 4.1 Co‐ordinated care model | 3 | 492 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.41, ‐0.04] |
| 4.2 Integrated care model | 4 | 562 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.43, ‐0.04] |
| 5 Health‐related quality of life (sensitivity analysis for study design including RCTs only) Show forest plot | 5 | 696 | Std. Mean Difference (Random, 95% CI) | 0.29 [0.14, 0.44] |
| Analysis 1.5  Comparison 1 Early palliative care vs standard oncological care, Outcome 5 Health‐related quality of life (sensitivity analysis for study design including RCTs only). | ||||
| 6 Symptom intensity (sensitivity analysis for study design including RCTs only) Show forest plot | 5 | 696 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.43, ‐0.13] |
| Analysis 1.6  Comparison 1 Early palliative care vs standard oncological care, Outcome 6 Symptom intensity (sensitivity analysis for study design including RCTs only). | ||||
Screening process diagram (as recommended by the PRISMA statement).
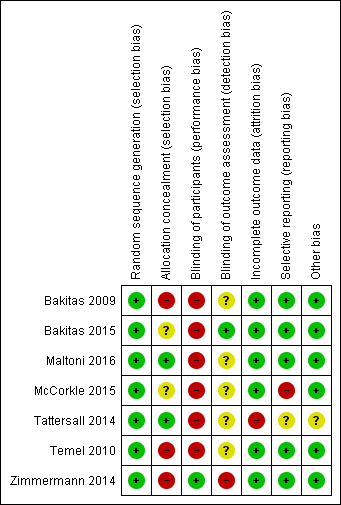
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
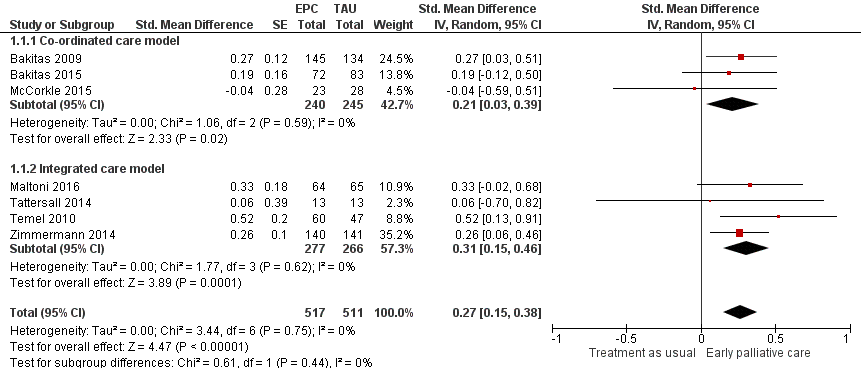
Forest plot of comparison: 1 Health‐related quality of life, outcome: 1.1 Health‐related quality of life.

Forest plot of comparison: 1 Early palliative care vs TAU, outcome: 1.2 Survival.

Forest plot of comparison: 1 Early palliative care vs standard oncological care, outcome: 1.2 Depression.
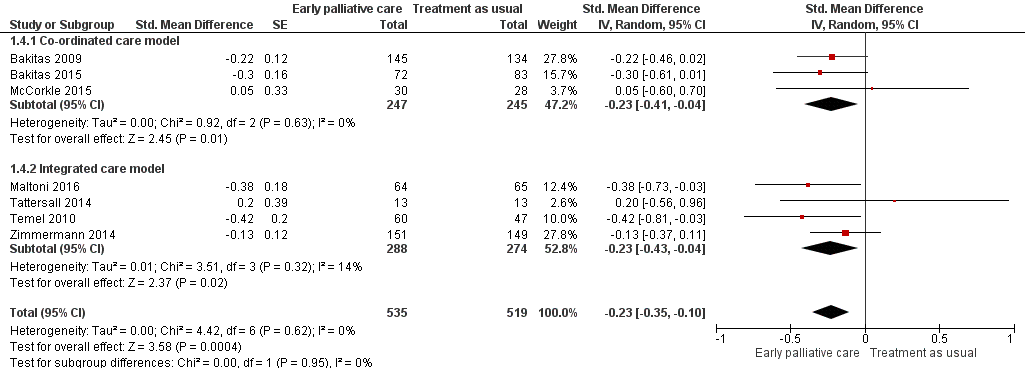
Forest plot of comparison: 1 Early palliative care vs standard oncological care, outcome: 1.4 Symptom intensity.

Forest plot of comparison: 1 Early palliative care vs standard oncological care, outcome: 1.5 Health‐related quality of life (sensitivity analysis for study design including RCTs only).
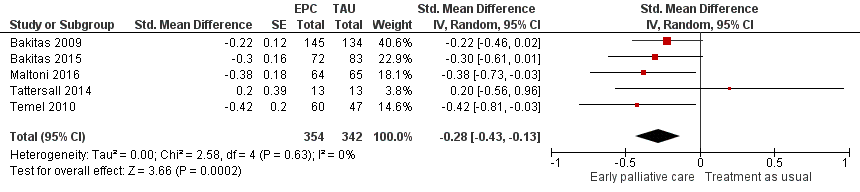
Forest plot of comparison: 1 Early palliative care vs standard oncological care, outcome: 1.6 Symptom intensity (sensitivity analysis for study design including RCTs only).

Comparison 1 Early palliative care vs standard oncological care, Outcome 1 Health‐related quality of life.
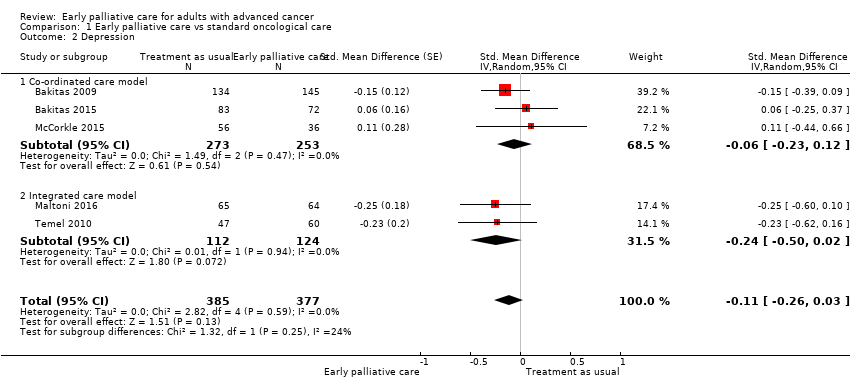
Comparison 1 Early palliative care vs standard oncological care, Outcome 2 Depression.
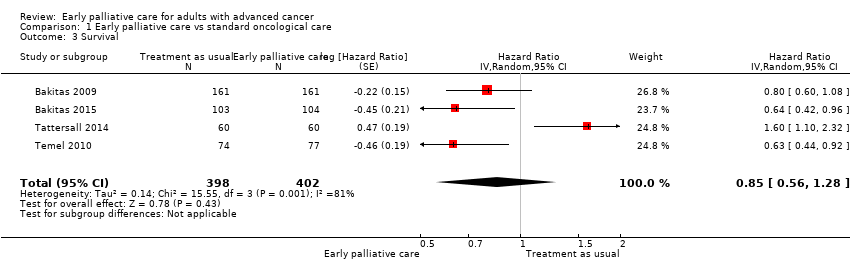
Comparison 1 Early palliative care vs standard oncological care, Outcome 3 Survival.

Comparison 1 Early palliative care vs standard oncological care, Outcome 4 Symptom intensity.
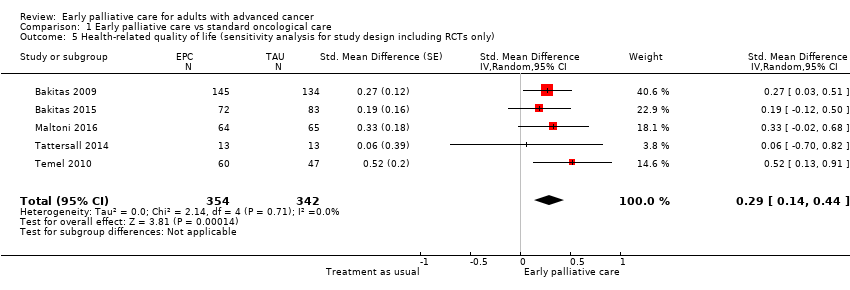
Comparison 1 Early palliative care vs standard oncological care, Outcome 5 Health‐related quality of life (sensitivity analysis for study design including RCTs only).

Comparison 1 Early palliative care vs standard oncological care, Outcome 6 Symptom intensity (sensitivity analysis for study design including RCTs only).
| Clinical question: Should early palliative care be preferred over treatment as usual for improving health‐related quality of life, depression, and symptom intensity in patients with advanced cancer? | ||||||
| Patient or population: patients with advanced cancer Settings: mainly outpatient care in Australia, Canada, Italy, and the USA Comparison: treatment as usual Follow‐up: at 12 weeks or mean difference in repeated measurement results for longitudinal designs | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with treatment as usual | Risk with early palliative care | |||||
| Health‐related quality of life (HRQOL), SD units: measured on FACIT‐Pal, TOI of FACT‐Hep, TOI of FACT‐L, FACT‐G, McGill Quality of Life, FACIT‐Sp. Higher scores indicate better HRQOL. Follow‐up: range 12 weeks to 52 weeks | HRQOL score improved on average 0.27 (95% CI 0.15 to 0.38) SDs more in early palliative care participants than in control participants | ‐ | 1028 | ⊕⊕⊝⊝ | By conventional criteria, an SMD of 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) | |
| Health‐related quality of life (HRQOL), natural units: measured on FACT‐G (from 0 to 108) | Baseline control group mean score at 70.5 pointsa | HRQOL score improved on average 4.59 (95% CI 2.55 to 6.46) points more in early palliative care participants than in control participants | ‐ | 1028 | ⊕⊕⊝⊝ | Calculated by transforming all scales to the FACT‐G in which the minimal clinically important difference is approximately 5 and the SD in the cancer validation sample was 17.0 (Brucker 2005) |
| Survival: estimated with the unadjusted death hazard ratio | Study populationb | HR 0.85, 95% CI 0.56 to 1.28 | 800 | ⊕⊝⊝⊝ | ||
| 61 per 100 | 56 per 100 (41‐71) | |||||
| Depression, SD units: measured on CES‐D, HADS‐D, PHQ‐9. Higher scores indicate higher depressive symptom load. Follow‐up: range 12 weeks to 52 weeks | Depression score improved on average ‐0.11 (95% CI ‐0.26 to 0.03) SDs more in early palliative care participants than in control participants | ‐ | 762 | ⊕⊝⊝⊝ VERY LOW1,2,4 | By conventional criteria, an SMD of 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) | |
| Depression, natural units: measured on CES‐D (from 0 to 60). Higher scores indicate higher depressive symptom load | Baseline control group mean score at 13.8 pointsc | Depressive symptoms score improved on average ‐0.98 (95% CI ‐2.31 to 0.27) points more in early palliative care participants than in control participants | ‐ | 762 | ⊕⊝⊝⊝ VERY LOW1,2,4 | Calculated by transforming all scales to CES‐D and applying an SD of 8.9 from baseline control group score in Bakitas 2009 |
| Symptom intensity, SD units: measured on ESAS, QUAL‐E Symptom Impact Subscale, SDS, RSC, LCS of FACT‐L, HCS of FACT‐Hep. Higher scores indicate higher symptom intensity. Follow‐up: range 12 weeks to 52 weeks | Symptom intensity score improved on average ‐0.23 (95% CI ‐0.35 to ‐0.1) SDs more in early palliative care participants than in control participants | ‐ | 1054 | ⊕⊕⊝⊝ | By conventional criteria, an SMD of 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988) | |
| Symptom intensity, natural units: measured on ESAS (from 0 to 900). Follow‐up: range 12 weeks to 52 weeks | Baseline control group mean score at 286.3 pointsc | Symptom intensity symptoms score improved on average ‐35.4 (95% CI ‐53.9 to ‐15.4) points more in early palliative care participants than in control participants | ‐ | 1054 | ⊕⊕⊝⊝ | Calculated by transforming all scales to the ESAS and applying an SD of 154.0 from baseline control group score in Bakitas 2009 |
| Adverse events | See comment | See comment | Not estimable | 1614 | See comment | Most often, study authors did not address assessment or findings on adverse events in their study publications. However, on request, authors of 6 studies described the tolerability of early palliative care as very good. A single study mentioned adverse events only in the early palliative care group, i.e. higher percentage of participants with severe scores for pain and poor appetite along with higher level of unmet needs (Tattersall 2014) |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) aApproximate average of baseline control group FACT‐G scores across 4 included studies (Bakitas 2009; Bakitas 2015; Maltoni 2016; Temel 2010) b12‐Month follow‐up control group risk in the largest study reporting on survival (Bakitas 2009) cBaseline control group CES‐D score in the largest study reporting on depression (Bakitas 2009) CI: confidence interval; GRADE: Grading of Recommendations Assessment; HR: unadjusted death hazard ratio; SD: standard deviation; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded 2 points owing to very serious limitations in study quality (high risk of bias across studies) 2We decided against downgrading for indirectness, although 2 studies were conducted exclusively in patients with metastatic pancreatic and advanced lung cancer, respectively (Maltoni 2016; Temel 2010). We decided against downgrading for inconsistency, as we did not detect significant heterogeneity 3We decided against downgrading for imprecision, as the optimal information size (OIS) criterion was met, and the 95% confidence interval around the difference in effect between intervention and control excludes zero 4We downgraded 1 point for imprecision, as the optimal information size (OIS) criterion was met, but the 95% confidence interval around the difference in effect between intervention and control includes zero. The 95% confidence interval fails to exclude harm 5We decided against downgrading for important inconsistency (large I2) because we had downgraded by 3 points already 6We decided against downgrading for indirectness, as only a single study was conducted exclusively in patients with advanced lung cancer (Temel 2010) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Health‐related quality of life Show forest plot | 7 | 1028 | Std. Mean Difference (Random, 95% CI) | 0.27 [0.15, 0.38] |
| 1.1 Co‐ordinated care model | 3 | 485 | Std. Mean Difference (Random, 95% CI) | 0.21 [0.03, 0.39] |
| 1.2 Integrated care model | 4 | 543 | Std. Mean Difference (Random, 95% CI) | 0.31 [0.15, 0.46] |
| 2 Depression Show forest plot | 5 | 762 | Std. Mean Difference (Random, 95% CI) | ‐0.11 [‐0.26, 0.03] |
| 2.1 Co‐ordinated care model | 3 | 526 | Std. Mean Difference (Random, 95% CI) | ‐0.06 [‐0.23, 0.12] |
| 2.2 Integrated care model | 2 | 236 | Std. Mean Difference (Random, 95% CI) | ‐0.24 [‐0.50, 0.02] |
| 3 Survival Show forest plot | 4 | 800 | Hazard Ratio (Random, 95% CI) | 0.85 [0.56, 1.28] |
| 4 Symptom intensity Show forest plot | 7 | 1054 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.35, ‐0.10] |
| 4.1 Co‐ordinated care model | 3 | 492 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.41, ‐0.04] |
| 4.2 Integrated care model | 4 | 562 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.43, ‐0.04] |
| 5 Health‐related quality of life (sensitivity analysis for study design including RCTs only) Show forest plot | 5 | 696 | Std. Mean Difference (Random, 95% CI) | 0.29 [0.14, 0.44] |
| 6 Symptom intensity (sensitivity analysis for study design including RCTs only) Show forest plot | 5 | 696 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.43, ‐0.13] |

