Medikamentöse Behandlung von Antipsychotika‐bedingter Verstopfung
Appendices
Appendix 1. Summary Table: PHARMACOLOGICAL TREATMENT FOR CONSTIPATION
TABLE 1: PHARMACOLOGICAL TREATMENT FOR CONSTIPATION
| TREATMENT | USUAL ADULT DOSE | MECHANISM OF ACTION | SIDE EFFECTS |
| Bulk‐forming laxatives | |||
| Psyllium | Up to 1 tablespoon 3 times/day | Increase colonic residue, producing a stretching reflex eventually stimulating colonic peristalsis | Hard to ingest, colonic atony, faecal impaction, obstruction |
| Methylcellulose | Up to 1 tablespoon 3 times/day | ||
| Calcium polycarbophil | 2 to 4 tabs/day | ||
| Wheat dextrin | 1 to 6 caplets daily (3 to 18 g fibre) | ||
| Emollients (softeners) | |||
| Docusate sodium | 100 mg 2 times/day | Lower the surface tension of the stool, allowing water to enter more easily | Skin rash |
| Lubricants | |||
| Arachis oil, mineral oil | Coats the surface of the stool and prevents water reabsorption into the GI tract | Depletion of fat soluble vitamins | |
| Osmotic agents | |||
| Polyethylene glycol | 8.5 to 34 g in 240 mL liquids | Exerts an osmotic effect on water and electrolytes, which are retained within the GI tract, leading to modification of stool consistency and increased faecal bulk | Nausea, bloating, cramping |
| Lactulose | 15 to 30 mL every other day, or 2 times/day | Abdominal bloating, flatulence, galactosaemia | |
| Sorbitol | 120 mL of 25% solution 1 time/day | Abdominal bloating, flatulence | |
| Glycerine | 3 g suppository 1 time/day | Rectal irritation, can be distressing | |
| Magnesium citrate | 200 mL 1 time/day | Magnesium toxicity (with renal insufficiency) | |
| Magnesium sulphate | 15 g 1 time/day | ||
| Stimulant laxatives | |||
| Bisacodyl | 10 to 30 mg orally 1 time/day | Increase intestinal motility and secretion via fluid and electrolyte accumulation in the distal ileum and colon. Also believed to stimulate sensory nerve ending of the colonic mucosa | Gastric irritation |
| 10 mg suppository 1 time/day | |||
| Senna | 2 to 4 tabs/day | Melanosis coli (pigmentation of colonic mucosa) | |
| Other agents | |||
| Lubiprostone | 24 mcgs 2 times/day | Bicyclic fatty acid which acts locally on chloride channels, enhancing chloride‐rich intestinal fluid secretion | Headache, nausea, diarrhoea, foetal loss reported in animal studies |
| Misoprotol | 200 mcgs 2 times/day | Prostaglandin E analogue | Diarrhoea, abdominal pain, nausea, flatulence, dyspepsia, vomiting, headache, dizziness, foetal loss |
| Tegaserod | 6 mg 2 times/day | Partial 5‐HT4 agonist and stimulator of GI motility and secretion. Also decreases visceral sensitivity | Cardiovascular ischaemic events |
| Colchicine | 0.6 to 1mg 1 time/day | Anti‐inflammatory alkaloid agent used to treat gout. Known to induce diarrhoea in higher doses (mechanism unknown). Significantly decreases mean colonic transit time | Abdominal discomfort, diarrhoea, nausea, vomiting, myopathy, neuropathy |
| Cisapride | 5 to 10 mg 3 times/day | Prokinetic. 5‐HT receptor agonist which reduces GI transit time | Prolongation of QT interval, ventricular arrhythmia, cardiovascular events |
| Metoclopramide | 5 to 10 mg 3 times/day | Prokinetic benzamide. 5‐HT3 receptor antagonist, 5‐HT4 agonist. Increases GI peristalsis | Extrapyramidal symptoms (acute dystonic reaction), restlessness, drowsiness, delirium, headache, hypo/hypertension, hyperprolactinaemia |
| Renzapride | 2 mg/day to 2 mg 2 times/day | 5‐HT3 receptor antagonist, 5‐HT4 agonist. Increases GI peristalsis | Unknown, but early trials demonstrated no significant side effects |
| Prucalopride | 2 mg/day | Dihydrobenzofurancarboxamide derivative with selective high‐affinity for 5HT4 receptor agonist. Increases GI peristalsis | Headache and abdominal pain |
| Linaclotide | 75‐600 mcg/day | Guanylate cyclase C receptor agonist. Activates chloride and bicarbonate secretion in the gut and may reduce visceral hypersensitivity | Diarrhoea |
| Orlistat | 120 mg 3 times/day | GI lipase inhibitor. Unabsorbed fat in GI tract digested by bacteria promoting bacteria | Weight loss, faecal urgency, faecal incontinence, abdominal pain, tooth/gingival disorder |
| Elobixibat | 10 mg/day | Ileal bile acid transporter inhibitor. Induces colonic secretion and accelerates transit | Abdominal cramps and diarrhoea |
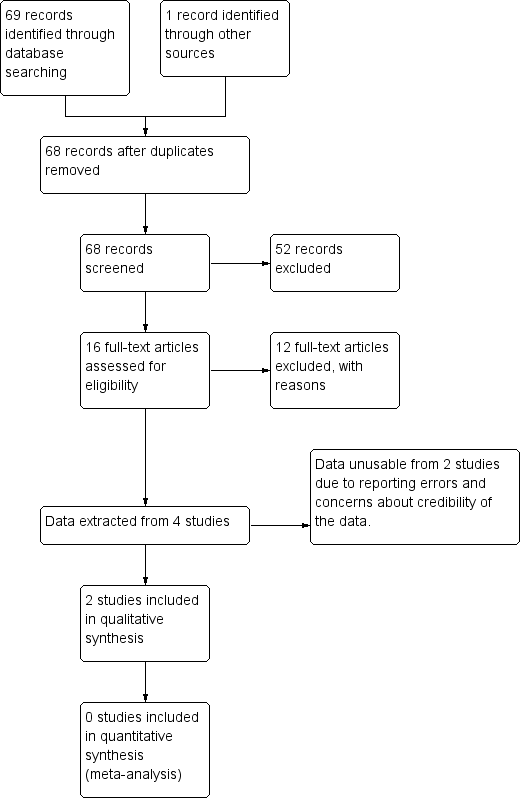
Study flow diagram.
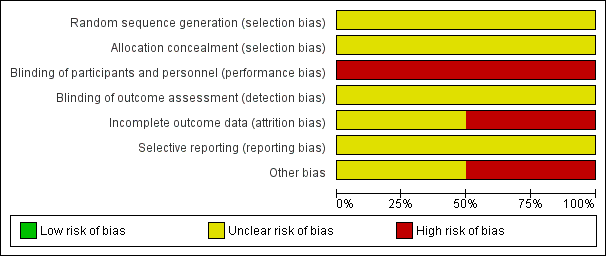
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
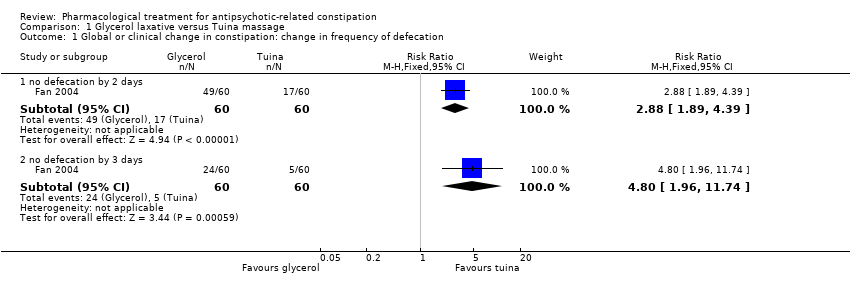
Comparison 1 Glycerol laxative versus Tuina massage, Outcome 1 Global or clinical change in constipation: change in frequency of defecation.
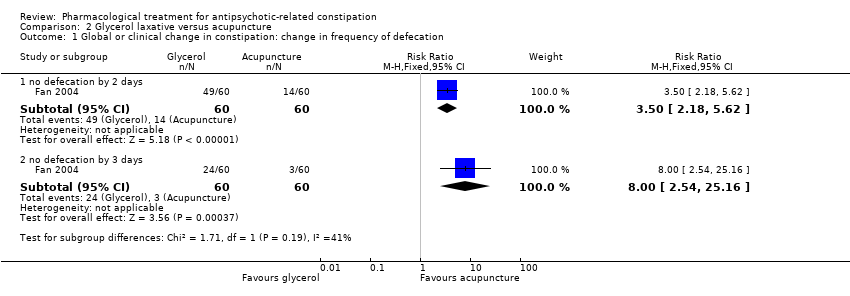
Comparison 2 Glycerol laxative versus acupuncture, Outcome 1 Global or clinical change in constipation: change in frequency of defecation.
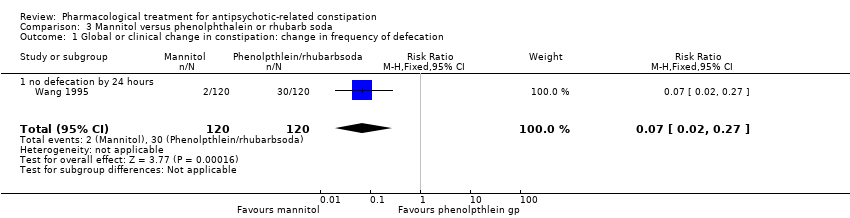
Comparison 3 Mannitol versus phenolphthalein or rhubarb soda, Outcome 1 Global or clinical change in constipation: change in frequency of defecation.
| Methods | Allocation: centralised, randomised, sequence generation described. Blinding: participants, personnel recruiting and assigning participants, and assessors. Blinding can be tested by asking participants and raters to guess the assigned treatment. Study duration: 12 weeks. Setting: Inpatients and outpatients |
| Participants | Diagnosis: antipsychotic‐related constipation or antipsychotic induced gastrointestinal hypomotility N = sample size obtained through power calculation* Age: any Sex: both |
| Intervention | Any of the interventions listed in Appendix 1 compared against any other intervention or placebo, |
| Outcomes | Primary Outcomes 1. Global or clinical change in constipation 1.1 Change in the frequency of defecation (e.g. complete spontaneous bowel movements per week) Secondary outcomes
|
| Notes | * Size of study with sufficient power to detect a approximate 10% difference between the two groups for the primary outcome with 80% certainty. |
| Glycerol laxative versus tuina massage | ||||||
| Patient or population: antipsychotic‐related constipation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with Tuina | Risk with Glycerol | |||||
| 1. Global or clinical change in constipation (a) as defined by the study Still constipated (no defecation) at 2 days | Study population | RR 2.88 | 120 | ⊕⊝⊝⊝ | ||
| 283 per 1000 | 816 per 1000 | |||||
| Global or clinical change in constipation (a) as defined by the study Still constipated (no defecation) at 3 days | Study population | RR 4.80 | 120 | ⊕⊝⊝⊝ | ||
| 83 per 1000 | 400 per 1000 | |||||
| (b) Change in the frequency of defecation | No studies reported these important outcomes | |||||
| (c) Change in straining at defecation | ||||||
| (d) Change in the frequency of lumpy or hard stools | ||||||
| (e) Change in the frequency of manual manoeuvres to facilitate defecation | ||||||
| 2. Need for rescue medication | ||||||
| 3. Presence of antipsychotic‐related constipation complications such as bowel obstruction | ||||||
| 4. Quality of life (changed to any extent) | ||||||
| 5. Adverse events | ||||||
| 6. Leaving the study early | ||||||
| 7. Economic costs | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Randomisation and allocation concealment methods unclear. Management of incomplete outcome data unclear. Blinding unlikely to have occurred ‐ rated as very serious ‐ downgraded by 2. 2 No validated method used for measuring constipation. Unclear how reported defecation was assessed (e.g. stool chart, participant recall from memory). No recording of any of the other ROME constipation symptoms (e.g. straining, stool consistency, manual manoeuvres) ‐ rated as serious ‐ downgraded by 1. | ||||||
| Glycerol laxative versus acupuncture | ||||||
| Patient or population: antipsychotic‐related constipation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with acupuncture | Risk with glycerol laxative | |||||
| 1. Global or clinical change in constipation (a) as defined by the study Still constipated (no defecation) at 2 days | Study population | RR 3.50 | 120 | ⊕⊝⊝⊝ | ||
| 233 per 1000 | 817 per 1000 | |||||
| Still constipated (no defecation) at 3 days | Study population | RR 8.00 | 120 | ⊕⊝⊝⊝ | ||
| 50 per 1000 | 400 per 1000 | |||||
| (b) Change in the frequency of defecation | No studies reported these important outcomes | |||||
| (c) Change in straining at defecation | ||||||
| (d) Change in the frequency of lumpy or hard stools | ||||||
| (e) Change in the frequency of manual manoeuvres to facilitate defecation | ||||||
| 2. Need for rescue medication | ||||||
| 3. Presence of antipsychotic‐related constipation complications such as bowel obstruction | ||||||
| 4. Quality of life (changed to any extent) | ||||||
| 5. Adverse events | ||||||
| 6. Leaving the study early | ||||||
| 7. Economic costs | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Randomisation and allocation concealment methods unclear. Management of incomplete outcome data unclear. Blinding unlikely to have occurred ‐ rated as very serious ‐ downgraded by 2. 2 No validated method used for measuring constipation. Unclear how reported defecation was assessed (e.g. stool chart, participant recall from memory). No recording of any of the other ROME constipation symptoms (e.g. straining, stool consistency, manual manoeuvres) ‐ rated as serious ‐ downgraded by 1. | ||||||
| Mannitol versus phenolphthalein or rhubarb soda | ||||||
| Patient or population: antipsychotic‐related constipation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with phenolphthalein or rhubarb soda | Risk with Mannitol | |||||
| 1. Global or clinical change in constipation as defined by the study a) Still constipated (no defecation) at 24 hours | Study population | RR 0.07 | 240 | ⊕⊝⊝⊝ | Results from the phenolphthalein and rhubarb soda groups were combined by the study authors. | |
| 250 per 1000 | 18 per 1000 | |||||
| (b) Change in the frequency of defecation | No studies reported these important outcomes | |||||
| (c) Change in straining at defecation | ||||||
| (d) Change in the frequency of lumpy or hard stools | ||||||
| (e) Change in the frequency of manual manoeuvres to facilitate defecation | ||||||
| 2. Need for rescue medication | ||||||
| 3. Presence of antipsychotic‐related constipation complications such as bowel obstruction | ||||||
| 4. Quality of life (changed to any extent) | ||||||
| 5. Adverse events | 0 per 1000 | 0 per 1000 | Not estimable | 240 (1 RCT) | ‐ | It is highly questionable how well adverse effects were monitored and recorded. The study simply notes "No side‐effects were detected in the two groups after treatment". |
| 6. Leaving the study early | No studies reported these important outcomes | |||||
| 7. Economic costs | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Randomisation and allocation concealment methods unclear. Management of incomplete outcome data unclear. Blinding unlikely to have occurred ‐ rated as very serious ‐ downgraded by 2. 2 No validated method used for measuring constipation. Unclear how reported defecation was assessed (e.g. stool chart, participant recall from memory). No recording of any of the other ROME constipation symptoms (e.g. straining, stool consistency, manual manoeuvres) ‐ rated as serious ‐ downgraded by 1. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global or clinical change in constipation: change in frequency of defecation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 no defecation by 2 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.89, 4.39] |
| 1.2 no defecation by 3 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.8 [1.96, 11.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global or clinical change in constipation: change in frequency of defecation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 no defecation by 2 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.5 [2.18, 5.62] |
| 1.2 no defecation by 3 days | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.0 [2.54, 25.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global or clinical change in constipation: change in frequency of defecation Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.27] |
| 1.1 no defecation by 24 hours | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.27] |

