Antiadhezijska terapija nakon operativne histeroskopije u svrhu liječenja ženske subfertilnosti
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel‐group randomised controlled trial. Single centre, Obstetrics and Gynecology Department, King Saud University, Riyadh, Saudi Arabia. Protocol approved by IRB: yes. Unclear whether statistical power calculation done (query not answered). Unclear about funding and conflicts of interest (query not answered). | |
| Participants | Number recruited: not stated. Number randomly assigned: 28 women. Number excluded after randomisation: 4 women. Number analysed: 24 women. Women with infertility, adverse pregnancy outcomes (diagnosed with intrauterine septum by HSG, sonohysterography, hysteroscopy or a combination of these), or both. Inclusion and exclusion criteria: ill defined. Some women (1 in intervention group; 3 in control group) not trying to conceive after treatment, indicating poor definition of inclusion and exclusion criteria. Mean age and range (years): 29 (23‐38) years in intervention group; 32 (22‐40) years in control group. Study duration: not reported (query not answered). Number of subfertile women: 3 in intervention group; 2 in control group; most women had history of adverse pregnancy outcomes (miscarriage or preterm delivery). | |
| Interventions | Paediatric Foley catheter balloon for 5 days (intervention: n = 13) vs no catheter/balloon (control: n = 15) Cervix dilated to 10 mm, and all uterine septa divided using 26 French (9 mm diameter) resectoscope and a 30‐degree lens (Karl Storz, Tuttlingen, Germany) with monopolar electrode utilising 1.5% glycine as distension medium via an electronic fluid management system (Endomat, Karl Storz, Tuttlingen, Germany) and 120 Watts low‐voltage (cutting current mode) waveform delivered by an ICC 350 Erbe electrosurgical unit (Erbe, Tuttlingen, Germany). Resectoscopic metroplasty carried out using a Collin (Karl Storz, Tuttlingen, Germany) monopolar knife electrode at 90 degrees. All women had general anaesthesia and concomitant laparoscopy and treatment of pelvic pathology including adhesiolysis or reduction/excision of endometriosis, or both, when indicated using a CO2 laser or electrosurgery, or both. No‐one received preoperative endometrial thinning, antibiotic prophylaxis or adjuvant postoperative hormonal therapy. No specific timing was used to perform the surgery with regards to the menstrual cycle. Although reported that 2 women in intervention group and 1 in control group conceived after ART, whether other fertility treatments were offered and how these cotreatments were distributed among comparison groups (query not answered) remained unclear. | |
| Outcomes | Length of residual septum: measured by HSG 12 weeks after operative hysteroscopy. First‐trimester loss, second‐trimester loss, preterm delivery, term delivery, ectopic pregnancy: measured at 12‐18 months after operative hysteroscopy. | |
| Notes | No distinction between primary and secondary outcomes. Whether reproductive outcomes were measured at 1 or > 1 time points unclear; variation in time points at which reproductive outcomes measured was 6 months. Some women (1 in intervention group; 3 in control group) were not trying to conceive after treatment; they should have been excluded from analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on a computer generated list of numbers (unconcealed)." Comment: probably done. |
| Allocation concealment (selection bias) | High risk | Quote: "Randomization was based on a computer generated list of numbers (unconcealed)." Comment: no allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "After ethics approval and informed consent, 28 women were randomized in the operating room into having a no. 14 paediatric Foley catheter/balloon for five days (N = 13) versus no catheter/balloon (N = 15) following resectoscopic septum division. The Foley balloon was inflated with 5 mL of normal saline solution." Quote: "All patients were discharged the same day, and the patients with the Foley catheter/balloon were instructed to cut with scissors the end of the catheter at 5 days at home and remove the catheter themselves." Comment: physicians and personnel not blinded to intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "They were also instructed to avoid pregnancy until their first assessment in 3 months by HSG, and they were reassessed at 6 and 12 to 18 months for pregnancy outcomes." Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "They were also instructed to avoid pregnancy until their first assessment in 3 months by HSG, and they were reassessed at 6 and 12 to 18 months for pregnancy outcomes." Quote: "We could not be certain that the < 1 cm septum, reported by the radiologist, in the balloon group was a recurrence or incomplete division at the time of metroplasty, but in the intention‐to‐treat (ITT) analysis, we considered this cavity as normal." Comment: no blinding of outcome assessors reported; unclear who did the assessment (query not answered). |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "We could not be certain that the < 1 cm septum, reported by the radiologist, in the balloon group was a recurrence or incomplete division at the time of metroplasty but in the intention‐to‐treat (ITT) analysis, we considered this cavity as normal." Comment: no incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | High risk | Quote: "Fertility and pregnancy outcomes at 12 to 18 months post metroplasty are shown in Table 4." Comment: reproductive outcomes measured over considerable time period rather than at 1 predefined time point. Unclear whether more measurements were taken at 18 months in 1 of the comparison groups. Comment: although it reported that 2 women in intervention group and 1 in control group conceived after ART, whether other fertility treatments were provided and how these cotreatments were distributed among comparison groups was unclear. Some women (1 in intervention group; 3 in control group) were not trying to conceive after treatment; they should have been excluded from final analysis because conducting an ITT on the basis of poor inclusion and exclusion criteria can increase risk of bias. Comment: according to Table 1 of publication, mean age (range) in intervention was 29 (23‐38) years and control was 32 (22‐40) years with P = 0.59. Mean age difference should not be considered clinically irrelevant. We judged that some evidence suggested baseline imbalance between comparison groups. Comment: high risk of selection, performance and detection bias. |
| Methods | Parallel‐group randomised controlled trial. Single centre, Hysteroscopic Unit at the University of Naples Frederico II, Naples, Italy. Protocol approved by IRB: yes. Unclear whether statistical power calculation was done (query not answered). Funding and conflicts of interest not reported (query not answered). | |
| Participants | Number recruited: 92 women. Number randomly assigned: 92 women. Number lost to follow‐up: 8 women. Number analysed: 84 women. Inclusion criterion:
Exclusion criteria:
Study duration: 15 months (June 2001 to September 2002). Mean age (± SD): 30.1 (± 3.5) years. Number of subfertile women: 18 in intervention group; 16 in control group. | |
| Interventions | ACP gel (intervention: n = 46) vs no application of ACP gel (control: n = 46) Intervention group: received intrauterine application of 10 mL of ACP gel (Hyalobarrier Gel; Baxter, Pisa, Italy) under hysteroscopic view after operative hysteroscopy. Control group: only received hysteroscopic resection of IUAs. Diagnostic hysteroscopy performed with a 3.5‐mm instrument (Gynecare Versascope; Gynecare, Ethicon Inc., Somerville, NJ, USA) with normal saline solution (sodium chloride 0.9%) used as distension medium. Operative hysteroscopy was performed with a rigid resectoscope (Karl Storz, Tuttlingen, Germany) with a 12‐degree fore‐oblique telescope and a hook‐shaped monopolar electrode. Women in both groups received oral antibiotics (cefixime 400 mg/day) (Cefixoral; Menarini, Firenze, Italy) for 3 days after surgery. | |
| Outcomes | Incidence of de novo adhesions, mean adhesion score and severity of adhesions according to the 1988 AFS classification system; all outcomes measured after 3 months. | |
| Notes | Individual data on subfertile women not presented separately (query not answered). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Following diagnostic hysteroscopy, patients were randomized into two groups: group A (N = 46), the treatment group, and group B (N = 46), the control group, using a computer‐generated randomisation list." Comment: probably done, as the same team of investigators published data from similar randomised trial. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method not described (query not answered). |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Ultrasound scans were performed in each patient from group A immediately after ACP gel application and after 24, 48 and 72 hours. The gel‐related hyperechoic thickness that seemed to separate endometrial walls was the mean evaluated parameter." Comment: no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Both the initial diagnostic hysteroscopy and the 3‐month follow‐up diagnostic hysteroscopy were performed by the same operator (G.A.). G.A. evaluated the adhesion score for each patient and was blind for patients' randomized allocation, whilst operative hysteroscopies and application of ACP gel were performed by a different operator (M.G.)." Comment: method not described (query not answered). |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Eight women (three from group A [intervention] and five from group B [control]) did not attend for follow‐up hysteroscopy." Comment: unlikely to cause substantial attrition bias. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | Low risk | Comment: no evidence of imbalance in baseline participant characteristics ‐ no cotreatment. |
| Methods | Parallel‐group randomised controlled trial with 3 comparison groups. Single centre, Department of Obstetrics and Gynecology of the Ain Shams Medical School, Cairo, Egypt. Protocol approved by IRB: yes. No statistical power calculation (query clarified by Dr Mohamed Amer). No external funding and no conflicts of interest (query clarified by Dr Mohamed Amer). | |
| Participants | Number recruited: 45 women. Number randomly assigned: 45 women. Number lost to follow‐up: 2 women. Number analysed: 43 women Inclusion criteria:
Exclusion criteria:
Study duration: 62 months (from June 2004 to August 2009) Median age (range): 30.4 (26‐40) years. | |
| Interventions | Intrauterine balloon without amniotic graft (group 1; n=15) vs intrauterine balloon with fresh amnion (group 2; n=15) vs intrauterine balloon with dried amnion (group 3; n=15) 2 × misoprostol 200 mg tablets inserted vaginally the night before operation to facilitate cervical dilation. Operative hysteroscopy performed under general anaesthesia in follicular phase of menstrual cycle; however, for women with amenorrhoea, no special time was chosen. Simultaneous laparoscopy performed in women with infertility if they had not undergone a laparoscopy before, in women with previous complications of hysteroscopy such as uterine perforation and in women in whom uterine perforation occurred during the present procedure. Hysterometry with uterine sounding was followed by lysis of IUAs using 5‐French pointed tip semirigid scissors in 5‐mm rigid clinic hysteroscope, based on a 2.9‐mm telescope (Karl Storz GmbH & Co. KB). In women with thick fibrous adhesions, adhesiolysis performed using 9‐mm working element along with sheath and 4‐mm 30‐degree telescope (Karl Storz GmbH & Co. KB) equipped with a hysteroscopic monopolar knife (Collin operating knife) after cervical dilation to Hegar 9. Visualised adhesions incised with 50‐ to 100‐W cutting current, adjusted according to visual tissue effects, from an isolated electrosurgical generator (Valleylab SSE2L; Valleylab, Inc., Boulder, CO, USA). Glycine 1.5% (Glycocolle 1.5%; Aguettant Laboratory, Lyon, France) used as distension medium, with intrauterine pressure 120‐150 mmHg, automatically controlled using a Hamou Hysteromat (Karl Storz GmbH & Co. KB) with termination of procedure if fluid deficit exceeded 1 L. Freeze‐dried amniotic membrane hydrated using normal saline solution in a pan for 10 minutes before use. Previously prepared fresh amniotic graft was washed several times with sterile normal saline solution before application. Amniotic graft was cut to form a 5 × 5‐cm piece. This was spread on the balloon end of an 8‐French paediatric Foley catheter, so that the epithelial or basement membrane surface would be on top facing outwards, where the inflated balloon acts as a mould for the amnion. The catheter tip with the amnion on its surface was then introduced into inside of uterine cavity with aid of straight artery forceps. Balloon inflated with 3 mL to 5 mL of saline solution. A loose knot was made in catheter stem, which was then slipped upwards to just below the inflated balloon, then was tightened with aid of artery forceps, and catheter stem was cut with scissors just below knot after catheter stem was stretched so that balloon with graft on its surface was kept intrauterine. In women with a patulous cervix that would not keep the inflated balloon inside uterus, a cervical cerclage using braided polyester tape (Matrix Health Care SAE, Ameco, Egypt) was applied; it was removed later with the balloon. Postoperatively, ethinyl oestradiol 50 μg/day tablets (Laboratoires Cassenne, Puteaux, France) administered for 50 days. 2 weeks postoperatively, balloon was removed transcervically with crocodile forceps and with participant under paracervical anaesthesia (lidocaine 2%, 6 mL, plus atropine 0.5 mg in the same syringe), as an outpatient procedure without cervical dilation. In women who had cervical cerclage, tape was removed at time of balloon extraction. Second‐look hysteroscopy performed 2‐4 months postoperatively by independent observer blinded to method. Outcome measures included improvement in adhesion grade, improvement in menstruation, increased uterine length at sounding and complications. Subsequently, follow‐up provided via direct contact or telephone every 3 months for a mean (range) of 28 (6‐60) months for menstrual pattern and fertility. | |
| Outcomes | Ongoing pregnancy rate, clinical pregnancy rate, adhesion score, duration of menstruation, improvement in menstruation, uterine length, uterine length increase, adhesion score improvement; some outcomes (improvement in adhesion grade, improvement in menstruation, increased uterine length at sounding and complications) assessed 2‐4 months after surgery, whereas other outcomes assessed via direct contact or telephone every 3 months for a mean (range) of 28 (6‐60) months for menstrual pattern and fertility. | |
| Notes | * Correspondence with authors on 4 January 2015. Dear Dr. Jan Bosteels, Thanks for your e‐mail and being interested in intrauterine adhesions management. 1. The first study is a pilot study and not a randomized study (Amer MI, Abd‐El‐Maeboud KH. Amnion graft following hysteroscopic lysis of intrauterine adhesions J Obstet Gynaecol Res 2006; 32(6): 559‐66). 2. I confirm that these two studies are different and no patients in the second study were involved in the first study. 3. It was a single‐blinded; only the first surgeon knew if the graft was used or not and which type; also the patient, but the assessor, did not know which group of patients he is assessing. 4. Analyses were conducted using commercially available software (SPSS for Windows, release 15.0; SPSS, Inc., Chicago, IL). All P values refer to 2‐tailed tests of significance, with P <0.05 considered significant. Data are given as count and percentage for categorical variables. Groups were compared using the c2 test and Fisher's exact test for categorized variables. For comparison of menstruation, uterine length and adhesion score, the Kruskal‐Wallis test was used. Data are given as median (interquartile range [IQR]; 25th to 75th percentile). Pairwise comparison was performed using the Mann‐Whitney test with Bonferroni correction. The critical level of significance was <0.02). 5. There was no funding for the present study. 6. There was no conflict of interest. 7. To my knowledge, I do not know that there are new anti‐adhesion therapy following operative hysteroscopy. With my best wishes. Dr. Mohamed I Amer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized preoperatively using a computer‐generated randomisation sheet into 3 groups of 15 women each." Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation to any group was concealed in an opaque envelope, which was opened at the time of operation." Comment: probably done. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "It was a single blinded, only the first surgeon that know if the graft used or not and which type also the patient, but the assessor did not know which group of patients he is assessing" (query clarified by Dr Mohamed Amer). Comment: method of blinding of participants and personnel not described. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "This was a pilot, randomized, comparative study with blinded independent evaluation of changes in adhesion grade, menstruation, uterine length, number of operations needed to achieve a functional uterine cavity, reproductive outcome, and complications." Quote: "A second‐look hysteroscopy was performed 2 to 4 months postoperative by an independent observer blinded to the method." Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "This was a pilot, randomized, comparative study with blinded independent evaluation of changes in adhesion grade, menstruation, uterine length, number of operations needed to achieve a functional uterine cavity, reproductive outcome, and complications." Quote: "A second‐look hysteroscopy was performed 2 to 4 months postoperative by an independent observer blinded to the method." Quote: "It was single blinded ‐ only the first surgeon knew if the graft was used or not and which type, also the patient, but the assessor did not know which group of patients he was assessing" (query clarified by Dr Mohamed Amer). Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Of the 45 patients included in the study, 2 were lost to follow‐up (1 each in groups 1 and 2) and were excluded from analysis." Comment: unlikely to cause attrition bias. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | High risk | Baseline imbalance in participant characteristics concerning caesarean section likely, as cause of IUAs. Quote: "Simultaneous laparoscopy was performed in women with infertility if they did not undergo laparoscopy before, in those with previous complications of hysteroscopy such as uterine perforation or if uterine perforation occurred during the present procedure." Comment: cotreatment by laparoscopy and distribution in numbers among comparison groups not stated. Quote: "All pregnancies were spontaneous except 3 that were achieved after in vitro fertilization (IVF). One pregnancy was terminated at 7 weeks' gestation because of a blighted ovum. Two patients underwent IVF treatment twice, but did not conceive. The other patients could not afford the cost of IVF." Comment: cotreatment with IVF in some women, resulting in 3 pregnancies; no available data on distribution of cotreatment among the 3 comparison groups. Potential for performance bias. |
| Methods | Parallel‐group randomised controlled trial. Single centre, national referral university hospital in Tehran, Iran. Protocol approved by IRB: not reported (query not answered). Unclear whether statistical power calculation done (query not answered). Funding and conflicts of interest not reported (query not answered). | |
| Participants | Number recruited: 59 women. Number excluded before randomisation: 13 women (9 women had abnormal findings at workup; 4 women excluded because angle between cervix and corpus could not be corrected). Number randomly assigned: 46 women. Number lost to follow‐up: 0 women. Number analysed: 46 women. Women with subfertility (15 women) and habitual abortion (44 women) with fundal defect on HSG. Underwent workup that included sperm analysis, assessment for infectious diseases (toxoplasmosis, Listeria monocytogenes, Mycoplasma hominis, syphilis), karyotyping, hormone profile (thyroxine, tri‐iodothyronine, thyroid‐stimulating hormone, T3 resin uptake, prolactin) and mid‐luteal progesterone assay. The 50 women whose examinations were normal and in whom diagnosis of septate uterus was confirmed by laparoscopy participated. Study duration: start and end dates not reported. Age: 26.7 ± 6.5 years in intervention group; 28.4 ± 4.5 years in control; note: not reported whether these numbers are means or medians with SDs. | |
| Interventions | Oestrogen (intervention: n = 23) vs no oestrogen (control: n =23) All women underwent hysteroscopic incision of septum with mini‐scissors by 1 surgeon who was unaware of treatment group. Ampicillin 1 g injected 1 hour before operations performed under general endotracheal anaesthesia. Distending medium 5% dextrose in water. Blood pressure cuff wrapped around plastic bottle to raise pressure of medium. Procedures performed with 7‐mm hysteroscope under laparoscopic guidance. Intervention group: conjugated oestrogen 1.25 mg/day 30 days beginning on day of operation. For last 7 days, they also took medroxyprogesterone acetate tablet 2 × 5‐mg/day. Control group: no hormone. Neither group used a splint. | |
| Outcomes | Difference between ratios of length of septum to length of uterus in HSGs obtained preoperatively and postoperatively, directly measured on HSG on cessation of menstruation 1 month after procedure. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomized into two groups of 23 women each." Comment: method not stated (query not answered). |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All septal incisions were performed by one surgeon, who was unaware of the group to which a patient had been assigned." Comment: method not stated (query not answered). |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: unequivocal outcome; no live birth or pregnancy rates reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The patients were randomized into two groups of 23 women each. Group 1 [intervention] received conjugated oestrogen 1.25 mg/d 30 days beginning on the day of the operation. For the last 7 days, they also took medroxyprogesterone acetate two 5‐mg tablets/d. Group 2 [control] received no hormone." Comment: unclear whether placebo pills used to blind participants and personnel (query not answered). |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: unequivocal outcome; no live birth or pregnancy rates reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Directly on cessation of menstruation 1 month after the procedure, HSG was done and the results were compared with those of the preoperative HSG." Comment: outcome assessors not identified in report, method of blinding not reported (query not answered). |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Four were omitted from the analysis because the angle between the cervix and the uterine corpus could not be corrected, as shown by HSG." Comment: 4/50 (8%) women were excluded; distribution among comparison groups not reported (query not answered). |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | Low risk | No evidence of baseline imbalance in participant characteristics. |
| Methods | Parallel‐group randomised controlled trial. Single centre, Department of Obstetrics and Gynecology of the University of Bologna, Bologna, Italy. Protocol approved by IRB: yes. No statistical power calculation for all outcomes (query clarified by Dr Pierandrea De Iaco). No external funding and no conflicts of interest (query clarified by Dr Pierandrea De Iaco). | |
| Participants | Number recruited: 60 women. Number randomly assigned: 60 women. Number lost to follow‐up: 20 women. Number analysed: 40 women. Quote: "Women were eligible for inclusion if they were undergoing endometrial ablation or hysteroscopic removal of submucosal fibroids, endometrial polyps, septate uterus or intrauterine synechiae." Comment: source population not adequately described in numbers and characteristics. Quote: "Despite this, newly induced synechiae were less severe in the Hyalobarrier gel treated patients, thus reducing the risk of pregnancy morbidity and improving the outcomes of hysteroscopic surgery." Comment: not mentioned whether women were infertile, and if so, how many; some subfertile women might have been included. Study duration: 36 months: 1998 to 2001 (query clarified by Dr Pierandrea De Iaco). Age: 18‐65 years. | |
| Interventions | Application of Hyalobarrier gel (intervention: n = 18 women analysed) vs no adhesion prevention (control: n = 22 women analysed) Number of women randomly assigned to each group not reported and not clarified by study authors. Intervention group: gel applied with 20‐cm cannula with 5‐mm diameter to cover entire uterine cavity. Mean (± SD) volume 10.5 ± 5.5 mL Hyalobarrier gel (range 5 to 20 mL) applied in uterine cavity. Control group: no adhesion prevention measures. An 8‐mm hysteroscopic resectoscope (Storz, Tuttlingen, Germany) with electrosurgical tips used. In all cases, sorbitol‐mannitol (Clear‐Flex, Baxter SA, Lessines, Belgium) used as distension medium; fluid intake and output continuously monitored (Hysteromat, Storz). Second‐look hysteroscopy undertaken 9 weeks after initial procedure by blinded investigator after insertion into uterine cavity with a 5‐mm hysteroscope (Storz) with CO2 distension. | |
| Outcomes | Incidence of de novo adhesions and severity of adhesions according to ASRM* modified scoring system: all outcomes measured after 9 weeks. | |
| Notes | *ASRM modified scoring system distinguishes only between stage I (mild) and stage II (severe) adhesions (different from the AFS 1988 classification system for IUAs). Correspondence with authors on 9 December 2014. Dear Dr. Jan Bosteels I have to admit that I have some difficulties in finding the data you are asking about research details. Anyway, these are my answers: 1. no statistical power had been used before the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After completion of the surgical procedure, the patients who met the inclusion criteria were randomly assigned either to the treatment with Hyalobarrier gel or to the control group, according to a computer‐generated randomisation schedule." Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomly allocated using a random table" (query clarified by Dr Pierandrea De Iaco). Comment: method of allocation concealment not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Dr. De Iaco performed the hysteroscopic surgery, while Dr. Muzzupapa performed the second‐look hysteroscopy without knowing the group of treatment" (query clarified by Dr Pierandrea De Iaco). Comment: method of blinding of participants and personnel not described. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Second look hysteroscopy was undertaken nine weeks after the initial procedure by a blinded investigator after insertion in the uterine cavity of a 5 mm hysteroscope (Storz) with CO2 distension." Quote: "Dr. De Iaco performed the hysteroscopic surgery, while Dr. Muzzupapa performed the second‐look hysteroscopy without knowing the group of treatment" (query clarified by Dr Pierandrea De Iaco). Comment: probably done. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Sixty patients aged from 18 to 65 years old were enrolled in the study and written, informed consent was obtained from each patient." Quote: "A total of 40 patients attended the postoperative diagnostic hysteroscopy, 18 in the intervention and 22 in the control group." Comment: loss to follow‐up of 20/60 enrolled participants, very likely to cause substantial attrition bias. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | High risk | Quote: "In conclusion, the authors recognize that the data reported lack statistical significance given the small sample size of the population evaluated. Despite this, newly induced synechiae were less severe in the Hyalobarrier gel treated patients, thus reducing the risk of pregnancy morbidity and improving the outcomes of hysteroscopic surgery." Comment: our own recalculation demonstrated that differences were not statistically significant; primary study authors' conclusions were not based on results. Baseline characteristics in both comparison groups not explicitly presented; P values not given. |
| Methods | Parallel‐group randomised controlled trial. Single centre, Hysteroscopic Unit of the University of Naples Frederico II, Naples, Italy. Protocol approved by IRB: yes. Statistical power calculation for primary outcome of incidence of de novo adhesions (query clarified by Dr Attilio DiSpiezio Sardo). No external funding and no conflicts of interest (query clarified by Dr Attilio DiSpiezio Sardo). | |
| Participants | Number recruited: 136 women. Number excluded before randomisation: 26 women (8 women declined after explanation of study protocol; 18 women excluded because they were unwilling to undergo surgery). Number randomly assigned: 110 women. Number lost to follow‐up: 0 women. Number excluded after randomisation: 24 women. In intervention group, 11/55 women, and in control group, 13/55 women, treated with endometrial ablation for resistant dysfunctional bleeding; these 24 participants were excluded from analyses, as endometrial ablation/resection is not indicated as a fertility‐enhancing surgical intervention. This judgement was subjected to several sensitivity analyses. Number analysed: 86 women. Premenopausal women diagnosed at clinic diagnostic hysteroscopy (n = 136) with single or multiple lesions suitable for surgical treatment or with resistant dysfunctional uterine bleeding requiring endometrial ablation invited to participate. Of 26 women who declined, 8 declined after explanation of study protocol, and 18 were excluded because they were unwilling to undergo surgery. Between September 2008 and June 2009, 110 premenopausal women were enrolled in study. Exclusion criteria:
Number of subfertile women with or without abnormal uterine bleeding: 12 in intervention group; 9 in control group; not possible to obtain individual outcome data for this small subgroup of subfertile women for IPD analysis (query clarified by Dr Attilio DiSpiezio Sardo). Duration of study: 10 months: September 2008 to June 2009. Mean age (± SD) in intervention group: 37 (± 3.1) years. Mean age (± SD) in control group: 36 (± 2.9) years. | |
| Interventions | Intercoat gel (intervention: n = 55) vs no gel (control: n = 55) Intervention group: after surgery, women underwent intrauterine application of 10 mL Intercoat gel under hysteroscopic guidance through inflow channel of resectoscope while operator gradually moved resectoscope from fundus of uterus back to external uterine ostium to apply gel throughout cavity and cervical canal. Procedure considered complete when, under hysteroscopic visualisation, gel seemed to have replaced all liquid medium, and cavity appeared completely filled by gel from tubal ostia to external uterine orifice. Control group: hysteroscopic surgery alone. Clinic diagnostic hysteroscopy performed with 5‐mm continuous‐flow hysteroscope with oval profile, a 30‐degree fore‐oblique telescope and a 5‐F operating channel (Karl Storz GmbH & Co. KG, Tuttlingen, Germany). Sodium chloride 0.9% solution used as distension medium and administered through electronic system of irrigation/aspiration (Endomat; Karl Storz GmbH & Co. KG). Operative hysteroscopy performed with rigid 27‐F resectoscope with 30‐degree fore‐oblique telescope with various bipolar loops and a bipolar energy source (Versapoint; Gynecare, division of Ethicon, Inc.). Sodium chloride 0.9% solution used as distension medium. Administration of antibiotics not reported. | |
| Outcomes | Incidence of de novo adhesions, severity of adhesions according to 1988 AFS classification system and improvement of degree of patency of internal uterine ostium; all outcomes measured after 4 weeks (during early proliferating phase of following menstrual cycle). | |
| Notes | * Correspondence with authors on 27 December 2014: 1. Which method was used for a statistical power calculation before the trial? Our primary outcome was measured by the incidence of de novo IUA. On the basis of data previously published by our group [Guida M, Acunzo G, Di Spiezio Sardo A, Bifulco G, Piccoli R, Pellicano M, Cerrota G, Cirillo D, Nappi C. Effectiveness of auto‐cross‐linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic adhesiolysis: a prospective randomized, controlled study. Hum Reprod 2004;19:1461‐1464; Acunzo G, Guida M, Pellicano M, Tommaselli GA, Di Spiezio Sardo A, Bifulco G, Cirillo D, Taylor A, Nappi C. Effectiveness of auto‐cross‐linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic adhesiolysis: a prospective randomized, controlled study. Hum Reprod 2003;18:1918‐1921], we expected the incidence of adhesions at follow‐up in patients undergoing hysteroscopic procedures with the application of the gel to be 10%, and without to be 28%, respectively. These figures are consistent with current literature, which shows a mean incidence of IUA of 25% after common resectoscopic procedures (polypectomy, myomectomy and metroplasty) if adjusted by taking into account that our study was meant to include more adhesiogenic procedures such as endometrial ablation. For the probability of a type 1 statistical error to be less than 0.05, we calculated that a sample of 55 patients per group would provide 80% of statistical power. 2. Was there any funding for the present study? Was there any conflict of interest? The study was not funded by an external source. All authors had no conflict of interest regarding this study at that time. 3. Is it possible to provide the outcome data of the infertile women included in this study to be able to analyse them on an individual level? Unfortunately it is not possible. However the infertile patients were only a small proportion (12 Group 1 [intervention]; 9 Group 2 [control]). 4. Which method was used to conceal the allocation to one of the two interventions? The allocation sequence was concealed from the researchers (S.M, B.M, S.M.) who enrolled and assessed the participants and attached a sequentially numbered, opaque, sealed and stapled envelope containing the allocated treatment to the clinical record of the patient after having signed the informed consent. The envelope was opened immediately after the surgical removal of the intrauterine removal of the removal of the intrauterine lesion, in order for the surgeon (A.D.S.S.) to either inject the gel (group 1 [intervention]) or not (group 2 [control]). Patients were blinded to the procedure until the end of the study. This single‐blind study design was adopted to reduce bias derived from the patient's knowledge of which procedure she underwent. 5. How were the study participants, the treating physicians and the outcome assessors blinded? Who did the outcome assessments? Finally, are you aware of any ongoing research on anti‐adhesion therapy following operative hysteroscopy? Patients were blinded since they underwent operative hysteroscopy in general anaesthesia or loco‐regional anaesthesia (they were awake but couldn't see the monitor) and were kept blinded until the three months follow‐up visit. The treating physician (A.D.S.S.) was blinded until removal of the intrauterine lesion or after endometrial ablation, when he was informed whether to inject or not the intrauterine gel. The assessor (M.G.) was blinded since he performed the baseline and the follow‐up hysteroscopies and did not participate to the operative hysteroscopies, so he was completely unaware of the allocation of patients. This single‐blind study design was adopted to reduce bias derived from the patient's knowledge of which procedure she underwent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After diagnostic hysteroscopy, patients were randomized via computer‐generated randomisation list into group 1 (treatment group: operative hysteroscopy plus intrauterine application of Intercoat gel; N = 55) and group 2 (control group: operative hysteroscopy alone; N = 55)." Comment: probably done, as the same team of investigators has published data on a similar randomised trial |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was concealed from the researchers (S.M, B.M, S.M.) who enrolled and assessed the participants and attached a sequentially numbered, opaque, sealed, and stapled envelope containing the allocated treatment to the clinical record of the patient after having signed the informed consent" (query clarified by Dr Attilio DiSpiezio Sardo). Comment: probably done. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Patients were blinded since they underwent operative hysteroscopy in general anaesthesia or loco‐regional anaesthesia (they were awake but couldn't see the monitor) and were kept blinded until the three months follow‐up visit" (query clarified by Dr Attilio DiSpiezio Sardo). Quote: "The envelope was opened immediately after the surgical removal of the intrauterine removal of the removal of the intrauterine lesion, in order for the surgeon (A.D.S.S.) to either inject the gel (group 1) or not (group 2)" (query clarified by Dr Attilio DiSpiezio Sardo). Comment: personnel not blinded; participants blinded (query clarified by Dr Attilio DiSpiezio Sardo). |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Both the initial and follow‐up diagnostic hysteroscopy were performed by the same surgeon (M.G.), who, blinded to patients' randomized allocation, also evaluated the rate and severity of adhesions in each patient." Quote: "The assessor (M.G.) was blinded since he performed the baseline and the follow‐up hysteroscopy and did not participate to the operative hysteroscopy, so he was completely unaware of the allocation of patients" (query clarified by Dr Attilio DiSpiezio Sardo). Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Intention‐to‐treat was the analysis method used; however, there were no deviations from random allocation." Comment: probably done; unlikely to cause attrition bias. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | Low risk | Comment: no evidence of imbalance in baseline participant characteristics; no cotreatment. |
| Methods | Parallel‐group randomised controlled trial. Single centre, Hallym University Kangdong Sacred Heart Hospital, Seoul, South Korea. Protocol approved by IRB: not reported; no contact possible due to absence of contact details. Statistical power calculation not reported; no contact possible due to absence of contact details. External funding and conflicts of interest not reported; no contact possible due to absence of contact details. | |
| Participants | Number recruited: 64 women. Number randomly assigned: 64 women. Number excluded: 2 women, reason for exclusion not reported. Number lost to follow‐up: 0 women. Number analysed: 62 women. Inclusion criterion:
Exclusion criteria:
Proportion of women with infertility: unclear if infertile women were included or excluded. Study duration of study: 10 months. Mean age (range): 28 (22‐43) years. Mean age in intervention group: 26 years. Mean age in control group: 31 years. | |
| Interventions | HA/CMC gel (intervention: n = 32) vs saline (control: n = 30) Intervention group: after intrauterine surgery, 10 mL of HA + CMC applied on uterine cavity. Control group: 10 mL of saline applied. After surgery, antibiotics injected for 1 day, and then oral antibiotics administered for 3 days. Women who underwent dilatation and curettage were discharged on 1st postoperative day, and women who underwent hysteroscopy were discharged on 2nd postoperative. | |
| Outcomes | Frequency and severity of IUAs compared by microhysteroscopy on 4th postoperative week, severity of IUAs classified in accordance with AFS 1988 guidelines. | |
| Notes | No contact data of the primary study authors reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized to 32 patients of study group (group A) and 32 patients of control group (group B) each." Comment: method not described; unclear if stratified randomisation was used; no contact possible. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described; no contact possible. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "After intrauterine surgery, in group A [intervention], 10ml of Hyaluronic acid + Sodium Carboxymethyl Cellulose (HA + CMC) was applied on uterine cavity, and in group B [control], 10ml of saline was applied." Comment: surgeons not blinded; easy to distinguish saline from gel. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "After intrauterine surgery, in group A, 10ml of Hyaluronic acid + Sodium Carboxymethyl Cellulose (HA + CMC) was applied on uterine cavity, and in group B, 10ml of saline was applied." Comment: surgeons not blinded; easy to distinguish saline from gel. |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported if gynaecologists who performed second‐look procedure 4 weeks after surgery were blinded or not; no contact possible. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported if gynaecologists who performed second‐look procedure 4 weeks after surgery were blinded or not; no contact possible. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "In total 64 patients, 62 patients were followed up postoperatively. Group A was 32 patients, Group B was 30 patients, and 2 patients were excluded during study." Comment: reasons for postrandomisation exclusion not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | High risk | Quote: "See table of baseline characteristics Age in years group A [intervention]: 26 Age in years group B [control]: 31 Parity in group A: 0.8 Parity in group B: 1.5 Abortion in group A: 1.0 Abortion in group B: 1.8." Comment: high risk of selection bias. Quote: "As a result of transvaginal sonography, intrauterine adhesion was observed at 4 patients (13%) out of 32 patients in group A and had mild intrauterine adhesions." Comment: unclear if micro‐hysteroscopy or transvaginal ultrasound used for outcome assessment of IUAs. High risk of information bias. |
| Methods | Parallel‐group randomised controlled trial. Single centre, gynaecologic endoscopy unit of a tertiary care medical centre in Zerifin, Israel. Protocol approved by IRB: yes. Post hoc statistical power calculation; non‐inferiority design. No external funding and no conflicts of interest (query clarified by Dr Moty Pansky). | |
| Participants | Number recruited: 110 women. Number excluded before randomisation: 58 women (14 did not meet inclusion criteria; 37 declined to participate; 7 excluded for other reasons). Number randomly assigned: 52 women. Number lost to follow‐up: 11 women. Number analysed: 41 women. Women who underwent hysteroscopic surgery because of suspected RPOC between September 2009 and June 2012 invited to participate in study, and enrollees gave signed informed consent. Inclusion criteria:
Study duration: 34 months; September 2009 to June 2012. Mean age (± SD) in intervention group: 29.5 (± 5.1) years. Mean age (± SD) in control group: 31.4 (± 6.5) years. Quote: "The study didn't include women with primary subfertility" (query clarified by Dr Moty Pansky). Comment: only women with confirmed fertility included in study. | |
| Interventions | Oxiplex gel (intervention: n = 21) vs no gel (control: n = 20) All hysteroscopic procedures performed under general anaesthesia. Pelvic bimanual examination performed under anaesthesia, and findings recorded in the medical records. Uterus considered enlarged when uterine fundus was palpated above pelvic brim. Sodium chloride 0.9% solution used as distension medium. Suspected RPOC removed via blunt dissection, with 4‐mm loop resectoscope (Stryker Corp., Kalamazoo, MI, USA) as a curette and under direct hysteroscopic view. All specimens sent for pathological analysis. Intervention group: after completion of hysteroscopic dissection, Oxiplex gel inserted into uterine cavity, up to complete filling of the cavity or up to 10 mL gel, whichever occurred first. All women discharged from the hospital several hours after procedure. Control group: no gel. Both intervention and control groups received sequential hormonal treatment (oestradiol valerate 2 mg/day for 11 days, followed by oestradiol valerate 2 mg/day + norgestrel 0.5 mg/day for 10 days) and antibiotic therapy (amoxicillin‐clavulanic acid, 875 mg, twice daily for 7 days). All women underwent diagnostic clinic hysteroscopy at 6‐8 weeks after operative procedure, performed by a surgeon blinded to treatment group. | |
| Outcomes | Intraoperative and postoperative complication rates, incidence of moderate or severe adhesions and pregnancy defined as a positive heartbeat (query clarified by Dr Moty Pansky). Comment: primary and secondary outcomes not determined. | |
| Notes | Quote: "Because this was a pilot study using a non‐inferiority design, post hoc power analysis was performed. This calculation showed that the power for detection of a statistically significant difference in rates of intrauterine adhesions between the 2 groups was 24%." Comment: study was substantially underpowered for the outcome of incidence of moderate or severe IUAs. * Correspondence with authors on 19 January 2015: 1. The first citation is an interim analysis that included 30 women, and was presented at AAGL [American Association of Gynecologic Laparoscopists] on 2011. The second citation is the final analysis that was published in JMIG [Journal of Minimally Invasive Gynecology] 2014 and included 52 women. The study population of the second citation includes all 30 women from the first one and 22 additional women. 2. Allocation was based on a computer‐generated randomisation scheme that was prepared in advance by the study coordinator. Sealed envelopes containing allocation were opened only following consent by the treating physician. The study coordinator documented the allocation on a password protected computer. 3. The control group received NS [normal saline] at the end of the procedure. The participants didn't know which group they were allocated to, nor did the outcome assessors. Naturally, the treating physician at time of procedure was aware of the treatment. Treating physicians' identity was documented and the study coordinator made sure that different physicians performed the treatment and the assessment per patient. 4. The gel was provided by J&J [Johnson & Johnson]. There was no funding for the study. There was no conflict of interest. 5. The study didn't include women with primary subfertility. 6. This was a pilot study designed to assess safety, hence there was no distinction between primary and secondary outcomes. 7. Pregnancy was defined as a positive heartbeat. 8. We are not aware of any ongoing research on anti‐adhesion therapy following operative hysteroscopy." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study entrants, in blocks of 12, were randomly allocated via a computer‐generated randomisation schedule, using institutional computer software, to treatment with (study group) or without (control group) Oxiplex gel." Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was based on a computer generated randomisation scheme that was prepared in advance by the study coordinator. Sealed envelopes containing allocation were opened only following consent by the treating physician. The study coordinator documented the allocation on a password protected computer" (query clarified by Dr Moty Pansky). Comment: probably done. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Different surgeons performed the operative hysteroscopy and the follow‐up diagnostic hysteroscopy. Both the patients and the surgeons who performed the follow‐up studies were unaware of patient group assignment." Quote: "The participants didn't know which group they were allocated to, nor did the outcome assessors. Naturally, the treating physician at time of procedure was aware of the treatment. Treating physicians' identity was documented and the study coordinator made sure that different physicians performed the treatment and the assessment per patient" (query clarified by Dr Moty Pansky). Comment: participants probably blinded, as they were under general anaesthesia, but treating physicians not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Different surgeons performed the operative hysteroscopy and the follow‐up diagnostic hysteroscopy." Quote: "All patients underwent diagnostic office hysteroscopy at 6 to 8 weeks after the operative procedure, performed by a surgeon who was blinded to the treatment group." Quote: "The participants didn't know which group they were allocated to, nor did the outcome assessors. Naturally, the treating physician at time of procedure was aware of the treatment. Treating physicians' identity was documented and the study coordinator made sure that different physicians performed the treatment and the assessment per patient" (query clarified by Dr Moty Pansky). Comment: probably done. |
| Incomplete outcome data (attrition bias) | High risk | Quote from the figure 1 CONSORT flow diagram of the study report: "In the intervention group five women were excluded from analysis after randomisation: the intervention was discontinued but no further clarification was given." Quote from the figure 1 CONSORT flow diagram of the study report: "In the control group six women were excluded from analysis after randomisation: lost to follow‐up (3) and discontinuation of the intervention (3) without further clarification." Comment: likely to cause attrition bias. |
| Selective reporting (reporting bias) | High risk | Comment: at high risk of selective outcome reporting, as live birth rates not reported for a study from September 2009 to June 2012, and publication of the final study report in 2014. |
| Other bias | High risk | Quote: "Patients with a diagnosis of adhesions (AFS grade 1) were offered an additional procedure for adhesiolysis." Quote: "At follow‐up hysteroscopy, 3 patients in the control group (14%) had AFS stage 2 or 3 (moderate to severe) intrauterine adhesions, compared with 1 woman in the study group (4%), who had AFS stage 3 intrauterine adhesions (P = 0.30)." Comment: imbalance between groups for a cointervention. |
| Methods | Parallel‐group randomised controlled trial. Single centre, Department of Minimally Invasive Gynecologic Center, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, China. Protocol approved by IRB: yes. Study protocol registered as NCT02496052 in ClinicalTrials.gov. Statistical power calculation reported; sample size determined based on findings of a pilot study. External funding: supported by grants from Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (Grant No. ZYLX201406), Capital Health Research and Development of Special (Grant No. 2014‐1‐2112) and National Science and Technology Infrastructure Program (Grant No. 2014BAI05B03). Conflicts of interest reported: authors had no conflicts of interest. | |
| Participants | Number recruited: 88 women. Number randomly assigned: 88 women. Number excluded: 2 women; 1 per treatment arm; reason: protocol violation. Number lost to follow‐up: 6 women. Intervention group: 3 women lost to follow‐up; 2 not undergo second hysteroscopy and 1 had incomplete data collection for defaulted follow‐up. Control group: 3 women lost to follow‐up; 3 did not undergo second hysteroscopy. Number analysed: 80 women. Consecutive series of women who fulfilled the recruitment criteria were invited to participate in the study until the enrolment target was met. All women had severe IUAs confirmed by outpatient diagnostic hysteroscopy and AFS IUA score ≥ 8. Inclusion criteria:
Exclusion criteria:
Precise proportion of women with infertility not reported in this mixed population of women with infertility or ≥ 1 spontaneous miscarriage. Study duration: 12 months. Mean age (± SD) in intervention group: 29.6 (± 3.7) years. Mean age (± SD) in control group: 30.8 (± 3.7) years. | |
| Interventions | Freeze‐dried amnion graft using a modified Foley catheter balloon as a scaffold (intervention: n = 40) vs Foley catheter balloon without amniotic grafting (control: n = 40) Hysteroscopic adhesiolysis performed under general anaesthesia by 1 experienced hysteroscopic operator. 2 × misoprostol 200 μg tablets administered vaginally the night before surgery for cervical priming. A bipolar resectoscope with a 9‐mm sheath and a 4‐mm 12‐degree telescope (Olympus Optical Company, Tokyo, Japan) used after cervical dilation with a 10 Hegar cervix dilator. Ultrasonographic guidance routinely used during procedure. Laparoscopy used to inspect pelvis and rule out pathology, such as endometriosis, and to verify tubal patency at end of hysteroscopic surgery. Normal saline used as distention medium and delivered through automated hysteroscopic distension pump at 260 mL/minute, under 100 mmHg of intrauterine pressure. Once location, extent and severity of IUAs had been assessed, they were resected using a needle or loop diathermy with electrosurgical generator voltage set at 320 W for the cutting mode and 160 W for the coagulation mode. Fluid volume recorded using modified automated fluid management system. Operating surgeon assessed when complete adhesiolysis had been achieved for all participants during surgery, and this was verified using normal panoramic view of uterine cavity under direct hysteroscopic visualisation; adhesiolysis characterised by adequate uterine cavity, no evidence of IUA and visible bilateral uterine horn, with or without tubal ostium. Following surgery, a 20 Foley catheter, with tip distal to balloon cut away, used as a scaffold for insertion of the amnion graft into uterine cavity. Intervention: balloon portion of Foley catheter covered with sterilised freeze‐dried amnion graft (Jiangxi Rui Ji Biotechnology, Jiangxi, China) and hydrated in sterile normal saline for 10 minutes before use. Size of each amnion graft 30 × 20 mm. 2 amnion grafts applied to Foley catheter, with epithelial amnion membrane surface facing outwards. Foley catheter was inserted into uterine cavity under ultrasonographic guidance. Balloon was initially inflated with 8‐10 mL of normal saline for 2‐3 minutes to ensure that amnion graft fully adhered to uterine cavity. Afterwards, 3‐5 mL of normal saline solution was withdrawn, leaving a mean of 5 mL within balloon. Control: protocol for insertion of Foley catheter and inflation of balloon was same as that used in intervention group; however, amnion grafting was not used. All images were digitally captured for further review and comparison using an integrated operating room (Karl Storz, Tuttlingen, Germany). Foley catheter remained in place for 1 week, after which time balloon was deflated and catheter removed as an outpatient procedure. All participants treated with daily dose of intravenous cefmetazole sodium 2 g for 7 days until the Foley catheter was removed. They also received cyclical postoperative therapy with oestrogens and progestogens as standard. Hormone therapy comprised oral oestradiol valerate 4 mg, which was administered daily for 21 days, with the addition of oral dydrogesterone 20 mg daily on days 12‐21 of menstrual cycle. | |
| Outcomes | Primary outcome: AFS IUA score at follow‐up hysteroscopy 3 months after surgery. Secondary outcomes: changes in menstruation measured by PBAC score, IUA reformation rate, pregnancy rate. Follow‐up of the secondary outcomes conducted via direct contact or telephone contact every 3 months to assess menstrual pattern and reproductive outcomes. Total duration of follow‐up: 6‐12 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before surgery, the participants were randomly assigned to either the amnion group or the control group in a 1:1 ratio using a computer‐generated randomisation sheet." Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Group allocation was concealed using sealed opaque envelopes that were opened at the time of operation by the coordinator." Comment: probably done‐unclear if sequentially numbered opaque sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Group assignment was not masked; however, the surgeons who performed the follow‐up hysteroscopy were blinded to both randomisation and allocation." Comment: surgeons and personnel not blinded, unclear if participants were blinded or not; query not answered. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Group assignment was not masked; however, the surgeons who performed the follow‐up hysteroscopy were blinded to both randomisation and allocation." Comment: surgeons and personnel not blinded, unclear if participants were blinded or not; query not answered. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Group assignment was not masked; however, the surgeons who performed the follow‐up hysteroscopy were blinded to both randomisation and allocation." Comment: outcome assessors blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Group assignment was not masked; however, the surgeons who performed the follow‐up hysteroscopy were blinded to both randomisation and allocation." Comment: outcome assessors blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Of the 88 women recruited, 80 were included in the final analysis (40 in each group)." Comment: intervention group: 4 women excluded or lost to follow‐up; 2 did not undergo second hysteroscopy, 1 protocol violation and 1 incomplete data collection for defaulted follow‐up. Control group: 4 women excluded or lost to follow‐up; 3 did not undergo second hysteroscopy and 1 protocol violation. |
| Selective reporting (reporting bias) | High risk | Quote: "The primary efficacy outcome was the AFS IUA score at follow‐up hysteroscopy. This outpatient procedure was performed under local anaesthesia at 3 months after surgery using 4.5‐mm continuous perfusion hysteroscopy (30°) with settings, intrauterine pressure, and irrigation rates similar to those used during the initial hysteroscopic surgery. The extent and severity of any reformed IUAs was recorded. Lesions were scored according to the AFS system." Secondary outcomes were changes in menstruation, which were evaluated according to PBAC score, IUA reformation rate and pregnancy rate." Comment: in study protocol registered as NCT02496052 all secondary outcomes mentioned in the published study report were not predefined. |
| Other bias | High risk | Quote: "During the follow‐up period, nine women in the amnion group achieved pregnancy; six of these pregnancies occurred naturally, whereas three occurred following in vitro fertilization and embryo transfer. Spontaneous abortion during the first trimester was reported among three of the nine pregnancies in the amnion group. The remaining six pregnancies were ongoing at the time of final follow‐up (two pregnancies at <12 weeks and four pregnancies at >24 weeks). Seven pregnancies were reported in the control group: five had occurred naturally and two had occurred following in vitro fertilization and embryo transfer. Among these seven pregnancies, four spontaneous abortions were reported during the first trimester, whereas the remaining three pregnancies were ongoing (>18 weeks) at the time of final follow‐up. As shown in Table 1, the pregnancy rate in the amnion group (23%) was not statistically different from that the control group (18%)." Comment: it is unclear if all women of this mixed population infertility/spontaneous miscarriage were trying to conceive. Proportions of women treated with IVF/embryo transfer vs natural conception not reported; query not answered. Quote: "Laparoscopy was used to inspect the pelvis and rule out pathology, such as endometriosis, and to verify tubal patency at the end of the hysteroscopic surgery." Comment: differences in proportions of cotreatment with laparoscopy not reported; query not answered. Quote: "Secondary outcomes were changes in menstruation, which were evaluated according to pictorial blood‐loss assessment chart (PBAC) score the IUA reformation rate, and the pregnancy rate. Follow‐up was conducted via direct contact or telephone contact every 3 months to assess menstrual pattern and reproductive outcomes. The total duration of follow‐up was 6‐12 months." Comment: at high risk of detection bias if not all women were followed up until 12 months given that there was no fixed endpoint to measure secondary outcomes. |
| Methods | Parallel‐group randomised controlled trial after stratification according to type of pathology. Single centre, Hysteroscopic Unit of University of Naples Frederico II, Naples, Italy. Protocol approved by IRB: yes. Statistical power calculation for primary outcome of incidence of de novo adhesions (query clarified by Dr Attilio DiSpiezio Sardo). No external funding and no conflicts of interest (query clarified by Dr Attilio DiSpiezio Sardo). | |
| Participants | Number recruited: 164 women. Number excluded before randomisation: 26 women (18 refused to undergo operative hysteroscopy; 8 refused to participate after explanation of the study protocol). Number randomly assigned: 138 women. Number lost to follow‐up: 6 women. Number analysed: 132 women. All participants with surgically remediable single lesions (myomas, polyps and uterine septa, subgroups I‐III) at diagnostic hysteroscopy were invited to participate. Between September 2002 and June 2003, 164 women met the study's inclusion criteria and were invited to participate. Of these, 26 did not participate: 18 refused to undergo operative hysteroscopy, and 8 refused after explanation of the study protocol. Inclusion criterion:
Exclusion criteria:
Study duration: 10 months (September 2002 to June 2003). Mean age (± SD) in intervention group: 37 (± 3.2) years. Mean age (± SD) in control group: 36 (± 2.8) years. Number of subfertile participants and individual outcome data not available for further IPD analysis (query clarified by Dr Attilio DiSpiezio Sardo). | |
| Interventions | ACP gel (intervention: n = 69) vs no treatment (control: n = 69) After diagnostic hysteroscopy and after written consent form was signed, women from each pathology subgroup (submucous myomas, endometrial polyps, septa) were randomly assigned to 2 groups using a computer‐generated randomisation list. Intervention group: intrauterine application of 10 mL of ACP gel (Hyalobarrier Gel; Baxter, Pisa, Italy) under hysteroscopic view after operative hysteroscopy. Control group: hysteroscopic surgery alone. Diagnostic hysteroscopy performed with a 3.5‐mm instrument (Gynecare Versascope; Gynecare, Ethicon Inc., Somerville, NJ, USA) and sodium chloride 0.9% solution as distension medium. Operative hysteroscopy performed using a rigid resectoscope (Karl Storz, Tuttlingen, Germany) with 12‐degree fore‐oblique telescope with hook‐shaped monopolar electrode. Both groups received oral antibiotics (cefixime 400 mg/day) (Cefixoral; Menarini, Firenze, Italy) for 3 days after surgery. | |
| Outcomes | Incidence of de novo adhesions, mean adhesion score and severity of adhesions according to 1988 AFS classification system; all outcomes measured after 3 months. | |
| Notes | * Correspondence with authors on 27 December 2014: 1. Which method was used for a statistical power calculation before the trial? Primary outcome was the incidence of adhesion formation at three month follow‐up in the two groups (hysteroscopy plus gel vs. hysteroscopy only). We assumed that difference between the two groups in term of de novo intrauterine adhesion formation would be 15% with an incidence of de novo adhesion formation in the hysteroscopy only group of 25% (Taskin et al. J Am Assoc Gynecol Laparosc 2000; 7: 351‐354). For the probability of a type I error to be less than .05, we calculated that a sample of 136 patients (68 per group) would provide 80% statistical power. In the study, 138 patients were enrolled and unfortunately, 6 dropped out, leaving 67 patients in the hysteroscopy plus gel group and 65 in the hysteroscopy only group. For this reason, 80% power of the study using the per‐protocol sample size analysis was not reached. Nevertheless, the post‐hoc power analysis revealed that the study reached an 80% power. 2. Was there any funding for the present study? Was there any conflict of interest? The study was not funded by an external source. All authors had no conflict of interest regarding this study at that time. 3. Is it possible to provide the outcome data of the infertile women included in this study to be able to analyse them separately? Unfortunately it is not possible. 4. Which method was used to conceal the allocation to one of the two interventions? The allocation sequence was concealed from the researchers (G.A., G.B., R.P., M.P.), who enrolled and assessed the participants and attached a sequentially numbered, opaque, sealed, and stapled envelope containing the allocated treatment to the clinical record of the patient after having signed the informed consent. The envelope was opened immediately after the surgical removal of the intrauterine removal of the removal of the intrauterine lesion, in order for the surgeon (M.G.) to either inject the gel (group A) or not (group B). Patients were blinded to the procedure until the end of the study. This single‐blind study design was adopted to reduce bias derived from the patient's knowledge of which procedure she underwent. 5. How were the outcome assessors blinded? Finally, are you aware of any ongoing research on anti‐adhesion therapy following operative hysteroscopy? The researcher who assessed the de novo formation of intrauterine adhesion (G.A.) was the one who performed the baseline diagnostic hysteroscopy and, successively, performed the 3 month follow‐up hysteroscopy. He did not participate to any of the operative hysteroscopies, when the patients were allocated to group A or B and, thus, he was completely unaware to which group the patients were allocated. We are not aware of any ongoing research on anti‐adhesion therapy following operative hysteroscopy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After diagnostic hysteroscopy and after the written consent form was signed, patients from each pathology subgroup (submucous myomas, endometrial polyps, septa) were randomized into two groups, group A (treatment [intervention] group) (N = 69) and group B (control group) (N = 69), using a computer‐generated randomisation list." Comment: probably done, as the same team of investigators published data on a similar randomised trial. |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was concealed from the researchers (G.A., G.B., R.P., M.P.), who enrolled and assessed the participants and attached a sequentially numbered, opaque, sealed, and stapled envelope containing the allocated treatment to the clinical record of the patient after having signed the informed consent" (query clarified by Dr Attilio DiSpiezio Sardo). Comment: probably done. |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The envelope was opened immediately after the surgical removal of the intrauterine removal of the removal of the intrauterine lesion, in order for the surgeon (M.G.) to either inject the gel (group A) or not (group B). Patients were blinded to the procedure until the end of the study. This single blind study design was adopted to reduce bias derived from the patient's knowledge of which procedure she underwent" (query clarified by Dr Attilio DiSpiezio Sardo). Comment: personnel not blinded; participants blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Both the initial diagnostic hysteroscopy and the follow‐up diagnostic hysteroscopy were performed by the same operator (G.A.). G.A. evaluated the adhesion score for each patient and was blind for patients' randomized allocation, whilst operative hysteroscopies and application of ACP gel were performed by a different operator (M.G.)." Quote: "The researcher who assessed the de novo formation of intrauterine adhesion (G.A.) was the one who performed the baseline diagnostic hysteroscopy and, successively, performed the 3 month follow‐up hysteroscopy. He did not participate to any of the operative hysteroscopies, when the patients were allocated to group A [intervention] or B [control] and, thus, he was completely unaware to which group the patients were allocated" (query clarified by Dr Attilio DiSpiezio Sardo). Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Six women (two from group A [intervention] and four from group B [control]) did not attend for follow‐up hysteroscopy." Comment: unlikely to cause substantial attrition bias. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting when abstract, methods and results were compared. |
| Other bias | Low risk | Comment: no evidence of imbalance in baseline participant characteristics; no cotreatment. |
| Methods | Parallel‐group randomised controlled trial. Single centre, tertiary medical centre, Shin Kong Wu Ho‐Su Memorial Hospital, Taipei, Taiwan. Protocol approved by IRB: yes. Study protocol registered as NCT01167296 in ClinicalTrials.gov. Statistical power calculation done before start of trial. No conflicts of interest declared by study authors. External funding not reported. | |
| Participants | Number recruited: 68 women. Number excluded before randomisation: 6 women (5 refused to participate; 1 had history of PID). Number randomly assigned: 62 women. Number lost to follow‐up: 2 women. Number analysed: 60 women. Inclusion criterion:
Exclusion criteria:
Study duration: 8 months; trial recruited from July 2010 to April 2011. Mean age (± SD) in intervention group: 33.4 (± 4.8) years. Mean age (± SD) in control group: 35.4 years (± 7.2) years. Unclear whether participants had subfertility, and if so, how many (query not clarified by study authors). | |
| Interventions | Balloon uterine stent (intervention: n = 31) vs no stent (control: n = 31) Randomisation based on a 1:1 computer‐generated scheme in balanced blocks of 4. Randomisation codes sealed in sequentially numbered opaque envelopes by study co‐ordinator. Immediately before surgery, co‐ordinator opened envelope and assigned participants to receive balloon uterine stent insertion (intervention) or not (control). Intervention group: uterine stent present for 30 days after surgery. Endometrium swabbed before and 30 days after surgery, and stent removed and sent for bacterial culture. Control group: endometrial swabbing done before and 30 days after surgery, but no stent was inserted. Co‐ordinator, participants and gynaecologists were not blinded to intervention after assignment. Per routine practice, women self‐administered misoprostol 400 μg (Cytotec; Pharmacia) into vagina 24 hours and 12 hours before surgery to prime cervix. After anaesthesia, perineum and vagina disinfected and draped. Cervix and vagina subsequently thoroughly disinfected with povidone‐iodine, as in vaginal surgery. Applicator swab (Copan Venturi Transystem; Copan Italia) then inserted into uterine cavity, with care taken to avoid contact with vaginal wall. Whole endometrium swabbed from fundus to cervix. Applicator swab placed in a transport tube and sent to laboratory immediately for bacterial culture. Operative hysteroscopies performed with 22‐F resectoscope (Karl Storz) and 5% glucose solution for uterine distension and irrigation. For women in intervention group, stent was inserted into uterine cavity at conclusion of hysteroscopy, and balloon inflated with 8 mL sterile water. Postoperatively, women were prescribed 3 days of diclofenac (Cataflam; Novartis Farma) for pain relief. Prophylactic antibiotics were not given. 1 surgeon performed all operative procedures and swabbing. Women instructed to return if any symptoms of PID developed 30 days after surgery, all participants returned to hospital for bacterial culture and second‐look hysteroscopy. After disinfection of vagina and cervix with povidone‐iodine, endometrium was swabbed. For intervention group, after balloon was deflated, stent was removed carefully without touching the vaginal wall. Balloon was cut from stem and placed in a sterile jar. Then endometrium was swabbed and balloon and swab sent to laboratory immediately for bacterial culture. After cultures were collected, all participants underwent second‐look hysteroscopy for assessment of endometrium. | |
| Outcomes | Primary outcome: incidence of bacterial colonisation of the uterus. Secondary outcomes: pain intensity on VAS scale used to record worst pain score from 3 days to 30 days following surgery; species of colonising bacteria. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on a 1:1 computer generated scheme in balanced blocks of four." Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization codes were sealed in sequentially numbered opaque envelopes by the study coordinator." Comment: probably done. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The coordinator, patients, and gynaecologists were not blinded to intervention after assignment." Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The coordinator, patients, and gynaecologists were not blinded to intervention after assignment." Comment: no blinding of participants, personnel and outcome assessors. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The coordinator, patients, and gynaecologists were not blinded to intervention after assignment." Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "The coordinator, patients, and gynaecologists were not blinded to intervention after assignment." Comment: no blinding of participants, personnel and outcome assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "A total of 62 women were included in the study, and 31 women were assigned to each group. The balloon uterine stent fell out after a week in one woman in the stent group, and one woman in the control group was lost to follow‐up. Both of these patients were excluded from analysis. Data for 60 women were analysed." Comment: unlikely to cause substantial attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Main outcome measure(s): The primary outcome was the incidence of bacterial colonization of the uterus. Secondary outcomes were pain intensity and species of colonizing bacteria." Quote: "All second‐look hysteroscopies revealed a normal endometrium. No woman had IUAs." Comment: according to registered protocol, predefined outcomes were:
Published report stated in results section that no participant had IUAs at second‐look hysteroscopy, but this important finding was not explicitly stated in abstract. |
| Other bias | High risk | Number of participants with IUAs twice as high in intervention group (17/31) vs control group (10/31). Comment: imbalance in baseline characteristics between comparison groups. |
| Methods | Parallel‐group randomised controlled trial. Single centre, university referral centre, Reproductive Medicine Centre of the Sir Run Run Shaw Hospital, Hangzhou, China. Protocol approved by IRB: yes. Study protocol registered as ISRCTN69690272 in ISRCTN Registry. Statistical power calculation done before start of trial. No conflicts of interest declared by authors. External funding: National Science Foundation of China (81270657), Zhejiang Public Welfare Technology Application Research Project (2013C33236), and Zhejiang Key Science and Technology Innovation Team Project (2011R50013‐26). | |
| Participants | Number recruited: 207 women. Number excluded before randomisation: 6 women (3 surgical complications; 3 declined to participate). Number randomly assigned: 201 women. Number excluded after randomisation: 5 women (1 intervention group; 4 control group) with reason: protocol violation. Number lost to follow‐up: 34 women (16 intervention group; 18 control group) with reason: no second‐look hysteroscopy in time. Number analysed: 162 women (82 intervention group, 80 control group). Inclusion criteria:
Exclusion criteria:
Mean age (± SD) in intervention group: 29.7 (± 4.3) years. Mean age (± SD) in control group: 30.1 (± 5.1) years. Proportion of women with infertility in intervention group: 21/82 (26%). Proportion of women with infertility in control group: 18/80 (22%). Primary vs secondary infertility: not reported. Study duration: 20 months. | |
| Interventions | Intrauterine balloon (intervention: n = 82) vs IUD (control: n = 80) Hysteroscopic adhesiolysis carried out by 1 of 2 experienced hysteroscopic surgeons with use of 4.5‐mm rigid hysteroscope (Storz) with 5% mannitol perfusion under 100 mmHg pressure. Procedure performed under general anaesthesia in a day surgery unit. Ultrasonographic guidance routinely used; in some cases, laparoscopy was also performed either in exceptionally difficult cases or when there was a need to inspect pelvic organs to rule out pathology such as endometriosis or to verify tubal patency. Once the extent and severity of uterine adhesion had been assessed, adhesions were divided with use of hysteroscopic scissors until normal uterine anatomy was achieved. Intervention group: immediately following operative hysteroscopy specially designed intrauterine balloon (Cook Medical) inflated with 3‐5 mL normal saline fitted into uterine cavity. Control group: immediately following operative hysteroscopy heart‐shaped copper IUD (Yantai Contraceptive Instrument) with thread knitted tail fitted into uterine cavity. Both devices were removed after 1 week in outpatient department. All participants were treated with oral cefuroxime combined with metronidazole for 7 days. In all cases, hormone therapy was also begun from the day of operation, consisting of oestradiol valerate 6 mg/day for 21 days, with medroxyprogesterone acetate 6 mg/day for the last 7 days of the oestrogen therapy. After withdrawal bleed, hormone therapy repeated for another cycle. Second‐look hysteroscopy carried out in early proliferating phase, 1‐2 months after initial operation. After assessment of extent and severity of any reformed adhesions, hysteroscopic adhesiolysis was also carried out at time of second‐look procedure, if adhesions had recurred. | |
| Outcomes | Primary outcomes: adhesion reformation, measured by second‐look hysteroscopy 1‐2 months after surgery. Power calculation done before start of trial; reduction in adhesion scores, measured by second‐look hysteroscopy 1‐2 months after surgery. Severity and extent of IUAs scored according to AFS 1988 classification. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After the completion of hysteroscopic adhesiolysis, recruited patients were randomized to one of the two treatment groups by computer‐generated numbers…" Comment: probably done; low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. Comment: unclear risk of bias; query not answered. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of surgeons impossible since balloon and IUD were easily recognised as being different. Blinding of participants not reported but device removed after 1 week at the outpatient department. Comment: probably no blinding of participants and personnel. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of surgeons impossible since balloon and IUD were easily recognised as being different. Blinding of participants not reported but device removed after 1 week at the outpatient department. Comment: probably no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The surgeon who later performed the second‐look hysteroscopy was blinded to the randomisation." Comment: probably done; low risk of bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The surgeon who later performed the second‐look hysteroscopy was blinded to the randomisation." Comment: probably done; low risk of bias. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "There were 39 women who were subsequently excluded from the study for the following reasons. In the balloon group, 1 woman was excluded because of protocol violation, in addition to 16 withdrawals because they did not proceed to second‐look hysteroscopy within the specified time frame. In the IUD group, 4 women were excluded because of protocol violation, in addition to 18 withdrawals because they did not proceed to second‐look hysteroscopy within the specified time frame." Comment: proportion of women lost follow‐up 34/201 (17%) women; high risk of attrition bias. |
| Selective reporting (reporting bias) | High risk | According to study protocol registered as ISRCTN69690272, secondary outcome was pregnancy rate after surgery. Not reported in study report. Comment: at high risk of selective outcome reporting since duration of study was 20 months and no data reported in final review of secondary outcome predefined in registered study protocol. |
| Other bias | Low risk | Quote: "No difference in baseline characteristics. Co‐treatment with antibiotics and hormone therapy in both comparison groups." Comment: no evidence for other potential sources of bias. |
| Methods | Parallel‐group randomised controlled trial. Single centre, Department of Obstetrics and Gynecology, All India Institute of Medical Sciences, New Delhi, India. Protocol approved by IRB: yes. No statistical power calculation (query clarified by Dr Murali Subbaiah). No funding (query clarified by Dr Murali Subbaiah). No conflict of interest (query clarified by Dr Murali Subbaiah). | |
| Participants | Number recruited: 100 women. Number excluded before randomisation: 10 women. Number randomly assigned: 90 women. Number lost to follow‐up: 5 women did not attend for second‐look hysteroscopy and were excluded from analysis of second‐look hysteroscopy findings; 2 women did attend for second‐look hysteroscopy but were lost to follow‐up for assessment of reproductive outcome. Number analysed: 85 women for second‐look hysteroscopy findings; 83 women for reproductive outcomes. Inclusion criteria:
Exclusion criteria:
90 original participants aged 20‐35 years with history of infertility (n = 31) or abortion (n = 59); of these, 40 had first‐trimester and 19 had second‐trimester spontaneous abortions. Study duration: 12 months; January 2011 to December 2011. Mean duration of infertility (± SD) in intervention group: 5.9 (± 1.8) years. Mean age (± SD) in intervention group: 28.7 (± 4.8) years. Mean duration of infertility (± SD) in control group: 6.2 (± 1.1) years. Mean age (± SD) in control group: 27.3 (± 3.9) years. Comment: mixed population of primary/secondary subfertility and miscarriage. Clarified by Dr Murali Subbaiah, quoting: "only 30 infertile patients were included ‐ the rest had abortions." | |
| Interventions | Oestrogen therapy (intervention: n = 42) vs placebo (control: n = 43) Hysteroscopic resection of septum under general anaesthesia by single operator in early proliferating phase of menstrual cycle. Operative hysteroscopy by rigid resectoscope (Karl Storz Endoskope, Germany) with 30‐degree telescope, equipped with a hysteroscopic monopolar (Collin's) knife. Cutting current set at 60 Watts. After 10‐mm cervical dilation achieved using Hegar's dilator, uterine cavity distended by glycine solution (1.5%). Intervention group: after septal resection, oestradiol valerate 2 mg once daily for 30 days. Control group: folic acid 5 mg tablet for 30 days. Second‐look hysteroscopy performed by same operator after 2 months to check for residual septum and uterine cavity adhesions. Performed as an outpatient procedure with a 4‐mm, 30‐degree angled lens. | |
| Outcomes | IUAs at second‐look hysteroscopy after 2 months, classified according to AFS classification; remnant septum defined as septum > 1 cm at second‐look hysteroscopy after 2 months; pregnancy, ongoing pregnancy and miscarriage measured after contact by telephone on a 3‐month basis during 12‐ to 24‐month period of follow‐up. | |
| Notes | Answers to queries on 6 December 2014: "Respected Sir, I would like to apologize for the delay in response. This was a small study and only 30 infertile patients were included (The rest had abortions). Fertility outcome after septal resection in infertile women was not separately analysed (Numbers are too small and the period of follow up is also less). Power calculation was not done for this study. There was no funding or conflict of interest. The two groups were coded as A and B and were concealed in separate covers. A third person who was not involved in the study was asked to choose one of the concealed covers randomly, and this was assigned. The investigators and patients were blinded to treatment allotment. I am not aware of any ongoing research on anti‐adhesion therapy following operative hysteroscopy. Yours sincerely, Dr. Murali Subbaiah" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were prospectively randomized into two groups, group A (treatment group) (N = 45) and group B (control group) (N = 45), using a computer‐generated randomisation list." Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: "The investigators and patients were blinded to treatment allotment." Comment: "The two groups were coded as A and B and were concealed in separate covers. A third person who was not involved in the study was asked to choose one of the concealed covers randomly, and this was assigned. The investigators and patients were blinded to treatment allotment" (method clarified by Dr Murali Subbaiah). |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The investigators and patients were blinded to treatment allotment." Quote: "After septal resection, the treatment group received 2 mg of oestradiol valerate, once daily for 30 days; in the control group, folic acid tablet (5 mg) was given as a placebo for 30 days." Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: unequivocal outcome. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The investigators and patients were blinded to treatment allotment." Quote: "After septal resection, the treatment group received 2 mg of oestradiol valerate, once daily for 30 days; in the control group, folic acid tablet (5 mg) was given as a placebo for 30 days." Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Five women (three from group A and two from group B) did not attend for follow‐up hysteroscopy and were excluded from the study. Further, two patients (one from each group) were lost to follow up." Comment: no ITT analysis, but numbers of women excluded after randomisation or lost to follow‐up and reasons were balanced between comparison groups. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective reporting. |
| Other bias | Low risk | No evidence of baseline imbalance. |
| Methods | Parallel‐group randomised controlled trial. Single centre, university referral centre, 1.a. Clinica Ostetrica e Ginecologica "L. Mangiagalli" dell Università di Milano, Milan, Italy. Ethical board/IRB approval: Council of the Institute of the First Obstetrics and Gynecologic Department of the Università degli Studi, Milan (query clarified by Paolo Vercellini). Study protocol registered in a clinical trial register: not registered (query clarified by Paolo Vercellini). Statistical power calculation before start of the trial: pilot study without preplanned power calculation (query clarified by Paolo Vercellini). Conflicts of interest: none (query clarified by Paolo Vercellini). External funding: investigator‐driven non‐commercial study (query clarified by Paolo Vercellini). | |
| Participants | Number recruited: 20 women. Number randomly assigned: 20 women. Number excluded after randomisation: 0 women. Number lost to follow‐up: 0 women. Number analysed: 20 women (intervention: IUD + hormone treatment: n = 10; control: no IUD or hormone treatment: n = 10). Inclusion criteria:
Exclusion criteria: not reported. Mean age (range): 29 (25‐36) years. Proportion of women with infertility: all (query clarified by Paolo Vercellini). Primary vs secondary infertility: not applicable. Study duration: 24 months; January 1986 to December 1987. | |
| Interventions | IUD + hormone treatment (intervention: n = 10) vs no additional treatment (control: n = 10) Hysteroscopic incision in uterine septum scheduled for the early proliferating phase of the menstrual cycle. Participants allocated randomly to 2 groups. Intervention group: IUD (ML CU 205, Multilan S.A., Pfäffikon, Switzerland) inserted postoperatively + conjugated oestrogen 1.25 mg twice daily for 30 days + medroxyprogesterone acetate 10 mg/day on days 26‐30. Follow‐up HSG scheduled after withdrawal bleeding and IUD removal. Control group: no other therapeutic measures. In both groups a follow‐up HSG was scheduled after the first spontaneous menstrual period with repeat hysteroscopy in the next cycle in the case of abnormal HSG findings. | |
| Outcomes | Main outcomes: residual fundal notch ≥ 5 cm, incidence of IUAs. No prioritisation of outcomes reported. | |
| Notes | * Correspondence with authors on 17 March 2017: Dear Professor Bosteels, 1. Can you describe the method used to randomly allocate the study participants to one of both treatment groups? Computer generated randomisation list. 2. Can you describe the method that you used to make the surgeons unaware of the treatment allocation? Did you use sequentially numbered opaque sealed envelopes? Did you phone to a central randomisation trial office? Other method? Sequentially numbered opaque sealed envelops. 3. Were the outcome assessor who evaluated the HSG or did the second look hysteroscopy in case of abnormal HSG the same surgeons that performed the septum resection? Yes, they were the same surgeons that performed the septum resection. 4. Do you have any baseline characteristics data of both comparison groups e.g. mean age of women in either group, length of septum, etc…? Unfortunately I am unable to retrieve these data. The study was completed almost 30 years ago. 5. Two women had their IUD removed early in the intervention group and in 1 woman in the control group a balloon catheter was left in situ for 24 hours because of bleeding. What was the outcome regarding normality of the cavity in these 3 women? See reply to point 4. 6. Is it correct that this is a single centre study conducted at 1.a. Clinica Ostetrica e Ginecologica "L. Mangiagalli” dell Università di Milano? Yes, it is correct. 7. Were the study participants all women of proven fertility or did the study also include women with infertility with two miscarriages? Do you have data on the proportions of infertile women in both comparison groups? The study participants were all fertile. 8. Was there IRB/Ethical committee approval for this clinical trial? Yes, the study was approved by the Council of the Institute of the First Obstetrics and Gynecologic Department of the Università degli Studi, Milan. 9. Was the study funded by a research grant or was it an investigator‐driven non‐ commercial study? It was an investigator‐driven non‐commercial study. 10. Was a power calculation done before the conduct of the study? It was a pilot study and no pre‐planned power calculation was performed. Thank you for your interest in our study and best wishes for your work. Paolo Vercellini Department of Clinical Sciences and Community Health, Università degli Studi di Milano and Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Via Commenda 12, 20122 Milan, Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were allocated randomly to two groups." Comment: computer‐generated randomisation list (query clarified by Paolo Vercellini). |
| Allocation concealment (selection bias) | Low risk | Quote: "method of allocation concealment not reported." Comment: sequentially numbered opaque sealed envelops (query clarified by Paolo Vercellini). |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "An IUD (ML CU 205, Multilan S.A., Pfäffikon, Switzerland) was inserted postoperatively in the ten women in group I; they also received conjugated estrogens, 1.25 mg twice daily for 30 days, with medroxyprogesterone acetate, 10 mg/d on days 26‐30. The ten women in group II were followed without other therapeutic measures." Quote: "Follow‐up HSG was scheduled for after withdrawal bleeding and IUD removal in group I and after the first spontaneous menstrual period in group II, with repeat hysteroscopy in the next cycle in the case of abnormal HSG findings." Comment: neither physicians nor participants were blinded. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "An IUD (ML CU 205, Multilan S.A., Pfäffikon, Switzerland) was inserted postoperatively in the ten women in group I; they also received conjugated estrogens, 1.25 mg twice daily for 30 days, with medroxyprogesterone acetate, 10 mg/d on days 26‐30. The ten women in group II were followed without other therapeutic measures." Quote: "Follow‐up HSG was scheduled for after withdrawal bleeding and IUD removal in group I and after the first spontaneous menstrual period in group II, with repeat hysteroscopy in the next cycle in the case of abnormal HSG findings." Comment: neither physicians nor participants were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Follow‐up HSG was scheduled for after withdrawal bleeding and IUD removal in group I and after the first spontaneous menstrual period in group II, with repeat hysteroscopy in the next cycle in the case of abnormal HSG findings." Comment: outcome assessors who evaluated HSG or did second‐look hysteroscopy in case of abnormal HSG were same surgeons who performed septum resection (query clarified by Paolo Vercellini). |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Follow‐up HSG was scheduled for after withdrawal bleeding and IUD removal in group I and after the first spontaneous menstrual period in group II, with repeat hysteroscopy in the next cycle in the case of abnormal HSG findings." Comment: outcome assessors who evaluated HSG or did second‐look hysteroscopy in case of abnormal HSG were same surgeons who performed septum resection (query clarified by Paolo Vercellini). |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "From January 1986 to December 1987 we studied 20 women aged 25‐36 years (mean, 29) with two or more unexplained spontaneous abortions, a double uterine cavity at hysterosalpingography (HSG) and ultrasonographic evidence of a normal uterine fundus with a half‐full bladder (Ansaldo 920 real‐time scanner with 3.5‐MHz convex transducer)." Quote: "At follow‐up HSG, five group I women had a regular uterine cavity and five a residual fundal notch ≥ 1 cm. In group II four had a normal uterine cavity and six a residual fundal notch ≥ 1 cm. No IUAs were detected in any of the patients." Comment: no exclusion; no loss‐to‐follow‐up. |
| Selective reporting (reporting bias) | Low risk | Quote: "No intrauterine adhesions were detected in any of the patients. IUD insertion and hormonal therapy after hysteroscopic metroplasty do not seem to be needed to prevent septal fusion." Comment: no difference between outcomes reported in abstract vs results section. |
| Other bias | Unclear risk | Quote: "In three group I and two group II patients, undue bleeding occurred, mainly from small, traumatized sites in the surrounding endometrium. Postoperative bleeding was observed in two women in group I [intervention] and one in group II [control]; the IUD was removed from the two group I patients 8 and 11 hours postoperatively, and methylergonovine, 0.2 mg intramuscularly, was administered. That was sufficient to arrest the bleeding in those cases whereas in the group II patient we had to insert in the uterine cavity a no. 16 Foley catheter distended with 5 mL of fluid; it remained there for 24 hours. The subsequent course was uneventful." Comment: baseline characteristics not reported. Sensitivity analyses of an ITT analysis vs a per‐protocol analysis not possible since outcomes of these 3 women were not reported. These 3 women should have been excluded since early removal of IUD in intervention group and leaving a balloon catheter in situ for 24 hours could have affected outcomes. Since study was completed in the late 1980s, it is no longer possible to retrieve these data (query clarified by Paolo Vercellini). |
| Methods | Parallel‐group randomised controlled trial. Single centre, university referral centre: Gynecological Minimally Invasive Center, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, China. Ethical board/IRB approval: yes. Study protocol registered in a clinical trial register: not reported; query not answered. Statistical power calculation before start of the trial: not reported; query not answered. Conflicts of interest: not reported; query not answered. External funding: Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (zylx201406); The Capital Health Research and Development of Special (2014‐1‐2112); National Science and Technology Infrastructure Program (2004BAI05B02). | |
| Participants | Number recruited: 57 women. Number randomly assigned: 57 women. Number excluded after randomisation: 0 women. Number lost to follow‐up: 0 women. Number analysed: 57 women (intervention: amniotic scaffold balloon, n = 29; control: Foley's balloon without amniotic membrane: n = 28). 57 women with IUA score ≥ 10 on hysteroscopy selected at the Beijing Maternity Hospital (affiliated to the Beijing Medical University). Inclusion criterion:
Exclusion criterion:
Mean age (± SD) in intervention group: 29 (± 3) years. Mean age (± SD) in control group: 31 (± 3) years. Proportion of women with infertility: all women had infertility related to severe IUAs. Primary vs secondary infertility: not reported. Study duration: recruitment of 12 months; June 2013 to June 2014. Follow‐up: 12‐18 months. Mean (± SD) follow‐up 14.6 (± 2.7) months. | |
| Interventions | Foley balloon catheter wrapped in amniotic membrane (intervention: n = 29) vs Foley balloon without amniotic membrane (control: n = 28) Tracheal intubation combined with intravenous general anaesthesia. Routine cervical priming performed preoperatively. Hysteroscopic adhesiolysis combined with laparoscopy and B‐mode ultrasound. In hysteroscopy, uterine cavity inspected looking for sites and severity of adhesions or anatomical abnormality. Adhesiolysis performed with needle electrodes and loop electrodes until normal morphology of uterine cavity restored, both uterine horns were visible and fallopian tube opening visible or invisible, or both. Emphasis placed on preserving residual endometrium. Amniotic membranes used were obtained from the Jiangxi Ruizeng Biological Engineering Technology Co., Ltd., specifications for the 30 mm × 20 mm dry sterilised biological amniotic membrane. Intervention group: before operation, 2 sheets of amniotic membrane were soaked in 25‐30 °C normal saline for 15‐20 minute to allow for rehydration and wrapped onto surface of a Foley catheter. Cervix dilated using Hegar 12 dilators, following which any fluid/gas was aspirated by the Foley catheter. Subsequently, balloon catheter was inflated with 8‐10 mL saline resulting in amniotic membrane products adhering completely to the uterine wound. After waiting 1‐2 minutes, residual volume of 3‐4 mL was retained in balloon catheter to maintain a separation between uterine walls. Catheter was left in place attached to an external drainage bag for 7 days, and routine antibiotic prophylaxis given. Balloon removed 7 days postoperatively. Control group: treatment as in intervention group except Foley balloon catheter alone had no external wrapping of amniotic membrane. | |
| Outcomes | No prioritisation of the outcomes reported. Menstrual flow changes; 1 month and 3 months postoperatively; IUA score by hysteroscopy performed after 3 cycles. If IUA score ≥ 5, participants were considered to have recurrence of adhesions, thereafter, participants were followed up every 3 months by telephone call or outpatients visits where pregnancy rates were recorded; IUA diagnosis and grading according to 1988 AFS grading method, which is a summation of adhesion score by hysteroscopy and menstrual pattern score by WHO menstrual blood loss chart (PBAC); reformation of IUAs scored by hysteroscopy; pregnancy; not further specified; ongoing pregnancy; spontaneous miscarriage; preterm birth; reformation of IUAs scored by hysteroscopy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "57 IUA patients with IUA score ≥10 on hysteroscopy were selected from June 2013 to June 2014 at the Beijing Maternity Hospital (affiliated to the Capital Medical University). Using the SPSS random number generator, patients were divided into 2 groups." Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported; query not answered. |
| Blinding of participants and personnel (performance bias) | Low risk | Surgeons were not blinded. Unclear if women were blinded to allocated treatment; query not answered. |
| Blinding of participants and personnel (performance bias) | High risk | Surgeons were not blinded. Unclear if women were blinded to allocated treatment; query not answered. |
| Blinding of outcome assessment (detection bias) | Low risk | Unclear if surgeons performing second‐look hysteroscopy were different from surgeons who performed hysteroscopic adhesiolysis; query not answered. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if surgeons performing second‐look hysteroscopy were different from surgeons who performed hysteroscopic adhesiolysis; query not answered. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Registration of study protocol not reported. Data collected as mentioned in methods section were all reported in results section. |
| Other bias | Unclear risk | Quote: "All patients were taking artificial cycle treatment." Comment: all women were cotreated with fertility treatment but unclear if this means that all women were offered same treatment regimen (e.g. all women received clomiphene with or without IUI or gonadotropin treatment with or without IUI or IVF) and that the proportions of different treatment regimens with different fertility prognoses were equally distributed among both groups; query not answered. |
| Methods | Parallel‐group randomised controlled trial. Multicentre, Third Xiangya Hospital of Central South University, Changsha 410013, China, Beijing, China and 2 affiliated hospitals: Hunan Provincial People's Hospital and Hunan First People's Hospital of Chenzhou City, China. Ethical board/IRB approval: yes. Study protocol registered in clinical trial register: not reported; query not answered. Statistical power calculation before start of trial: not reported; query not answered. Conflicts of interest: not reported; query not answered. External funding: not reported; query not answered. | |
| Participants | Number recruited: 120 women. Number randomly assigned: 120 women. Number excluded after randomisation: 0 women. Number lost to follow‐up: 9 women. Number analysed: 111 women (intervention group: n = 55; control group: n = 56). From November 2011 to November 2012, women with IUA from 3 hospitals affiliated with Xiangya Medical College and other hospitals who fulfilled inclusion criteria were included. Inclusion criteria:
Exclusion criteria:
Mean age (± SD) in intervention group: 33 (± 5) years. Mean age (± SD) in control group: 33 (± 5) years. Proportion of women with infertility: not reported; unclear if infertile women were included; query not answered. Primary vs secondary infertility: not reported. Study duration: recruitment of 12 months. | |
| Interventions | Foley balloon catheter + AC HA gel (intervention: n = 55) vs Foley balloon catheter only (control: n = 56) Participants with moderate‐to‐severe IUAs underwent routine hysteroscopic adhesiolysis. After surgery, participants randomly assigned into intervention and control groups according to treatment allocation table. Intervention group: Foley balloon catheter placed in uterine cavity, 3 mL saline injected to inflate balloon to seal mouth of cervix. Then, 2 mL medical self‐cross‐linking sodium hyaluronate gel (product of Changzhou Biarui Biomedical Co., Ltd.) injected from another lumen of Foley balloon catheter to fill uterine cavity and cover surgical wound. Control group: Foley balloon catheters placed in uterine cavity in same manner as intervention group with no self‐cross‐linking sodium hyaluronate gel. According to literature, after 72 hours, Foley balloon catheters were removed from and same routine postoperative treatment given to all participants. At 1 and 3 months, participants attended for clinical follow‐up 3‐7 days after menstrual bleeding had stopped for that cycle. Participant's general condition, symptoms, signs and possible complications checked and recorded. At third month of follow‐up, second‐look hysteroscopy performed and IUAs were graded (light, moderate and severe) according to AFS criteria. | |
| Outcomes | Primary outcome: effectiveness of treatment as seen by recurrence of adhesions on hysteroscopy 3 months after surgery. Treatment success defined as decrease in total AFS score of ≥ 4 points. Formula to calculate rate of treatment success: cases with AFS total score < 4 divided by total number of cases × 100%. Secondary outcomes: comparison of AFS score, including extent of IUAs, adhesion type; menstrual pattern score before and after surgery, and between groups. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After the surgery, the patients were randomly assigned into treatment and control groups according to a treatment allocation table." Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported; query not answered. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The surgeons performing the second hysteroscopy at 3 months were blinded to the choice of treatment of the patient. In addition, patients were also blinded to allocation to treatment or control. Therefore, this study can still be defined as double‐blinded (those assessing treatment response and those receiving treatment did not know of the treatments). Due to the properties of the self‐crosslinking sodium hyaluronate gel material, no gel material with the same properties can be used as a control. In addition, blank control material was not used in order to ensure efficacy of treatment and adherence to medical ethics. As a result, the surgeon performing adhesiolysis was not blinded." Comment: surgeons performing surgery not blinded but participants were blinded. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The surgeons performing the second hysteroscopy at 3 months were blinded to the choice of treatment of the patient. In addition, patients were also blinded to allocation to treatment or control. Therefore, this study can still be defined as double‐blinded (those assessing treatment response and those receiving treatment did not know of the treatments). Due to the properties of the self‐crosslinking sodium hyaluronate gel material, no gel material with the same properties can be used as a control. In addition, blank control material was not used in order to ensure efficacy of treatment and adherence to medical ethics. As a result, the surgeon performing adhesiolysis was not blinded." Comment: surgeons performing surgery not blinded but participants were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The surgeons performing the second hysteroscopy at 3 months were blinded to the choice of treatment of the patient. In addition, patients were also blinded to allocation to treatment or control. Therefore, this study can still be defined as double‐blinded (those assessing treatment response and those receiving treatment did not know of the treatments). Due to the properties of the self‐crosslinking sodium hyaluronate gel material, no gel material with the same properties can be used as a control. In addition, blank control material was not used in order to ensure efficacy of treatment and adherence to medical ethics. As a result, the surgeon performing adhesiolysis was not blinded." Comment: surgeons performing second‐look hysteroscopy were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The surgeons performing the second hysteroscopy at 3 months were blinded to the choice of treatment of the patient. In addition, patients were also blinded to allocation to treatment or control. Therefore, this study can still be defined as double‐blinded (those assessing treatment response and those receiving treatment did not know of the treatments). Due to the properties of the self‐crosslinking sodium hyaluronate gel material, no gel material with the same properties can be used as a control. In addition, blank control material was not used in order to ensure efficacy of treatment and adherence to medical ethics. As a result, the surgeon performing adhesiolysis was not blinded." Comment: surgeons performing second‐look hysteroscopy were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The study period was from November 2011 to November 2012. 120 subjects were randomized into the experimental group and the control group of 60 cases each." Quote: "There were 111 patients who completed follow‐up and met the requirements of statistical analysis, including 55 patients in the trial group and 56 patients in the control group." Comment: 9 participants lost to follow‐up or excluded (5 in intervention and 4 in control group; reasons not reported). |
| Selective reporting (reporting bias) | Low risk | Results of all predefined endpoints were all reported. |
| Other bias | Low risk | No statistically significant differences in age, weight, height, number of previous pregnancies and preoperative AFS score. No cotreatments. |
ACP: auto‐cross‐linked polysaccharide; AFS: American Fertility Society; ART: assisted reproductive technology; ASRM: American Society for Reproductive Medicine; CMC: carboxymethyl cellulose; β‐hCG: beta‐human chorionic gonadotropin; FSH: follicle‐stimulating hormone; HA: hyaluronic acid; HSG: hysterosalpingography; IPD: individual participant data; IRB: institutional review board; ITT: intention to treat; IUA: intrauterine adhesion; IUD: intrauterine device; IVF: in vitro fertilisation; n: number of women; PBAC: pictorial blood‐loss assessment chart; PID: pelvic inflammatory disease; RPOC: retained products of conception; SD: standard deviation; VAS: visual analogue scale; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Quote: "We performed a randomised non‐inferiority trial involving women undergoing uterine aspiration for induced or spontaneous abortion at 5 to 12 weeks of gestation who desired an IUD. Subjects were randomly assigned (in a 5:6 ratio) to IUD insertion immediately after the procedure or 2 to 6 weeks afterward (delayed insertion). The primary outcome was the rate of IUD expulsion 6 months after IUD insertion." Comment: not answering PICO research question. | |
| Quote: "Effects of aspirin and intrauterine balloon on endometrial repair and reproductive prognosis in patients with severe intrauterine adhesion: a prospective cohort study." Comment: observational study. | |
| Quote: "The use of Hyalobarrier post salpingo‐ovariolysis did not influence follicular development as inferred from the results of the day 21 progesterone and folliculogram on day 10‐12 3‐month postsurgery." Comment: not answering PICO research question. | |
| Intervention: hysteroscopic adhesiolysis followed by collagen scaffold loaded with autologous bone marrow stem cell treatment. Study design: observational; case series. Comment: observational study. | |
| Intervention: hysteroscopic adhesiolysis followed by collagen scaffold loaded with umbilical cord blood‐derived mesenchymal stem cell treatment. Study design: observational; case series. Comment: observational study. | |
| Quote: "OBJECTIVE: To assess the safety and efficacy of the Intergel adhesion prevention solution, a 0.5% ferric hyaluronate gel, in reducing adhesions in patients undergoing peritoneal cavity surgery by laparotomy with a planned second‐look laparoscopy. DESIGN: Randomized, third‐party blinded, placebo‐controlled, parallel group. SETTING: Eleven centres in the United States, and five centres in Europe. PATIENT(S): Women aged 18‐46 years who wanted to retain their fertility. INTERVENTION(S): Patients received 300 mL of Intergel solution (N = 143) or lactated Ringer's solution (N = 138) as an intraperitoneal instillate at the completion of surgery. MAIN OUTCOME MEASURE(S): At second‐look laparoscopy 6‐12 weeks later, the presence of adhesions was evaluated at 24 abdominal sites." Comment: not answering PICO research question. | |
| Quote: "The exclusion criteria were women who planned to use an intrauterine device for contraception during the study period; (...); women who were pregnant or who planned pregnancy during the study period (...)." Comment: excluded women with subfertility. | |
| Quote: "This randomised controlled blind prospective study is undertaken to evaluate the safety and efficacy of Seprafilm™ ‐ a novel bioresorbable membrane of chemically modified hyaluronic acid and carboxymethylcellulose ‐ in prevention and reduction of postoperative endometrial and endocervical synechiae formation after general suction evacuation or curettage for incomplete, missed, and recurrent abortion." Comment: not answering PICO research question. | |
| Quote: "A retrospective analysis was carried out to explore the clinical data of 120 cases of severe IUA patients who were treated in Woman and Infant Hospital of Zhengzhou and The Third Affiliated Hospital of Zhengzhou University during the period between January 2010 and December 2013." Comment: observational study. | |
| Quote: "The main objective of this study is to describe the level of expression of the biological factors involved in the formation of adhesions (Transforming growth factor beta, Activin A, inhibin) at the time of a first diagnostic hysteroscopy among women with synechia, another intracavitary disease or no intracavitary disease"; "Study design: observational model: cohort; time perspective: prospective." Comment: observational study. | |
| Quote: "Consented patients, who had at least one previous suction or abrasive (blunt or sharp) curettage for a miscarriage in the history, visiting the outpatient clinic with a miscarriage and planned for curettage, will be included in the study. The ultrasound is a key in the diagnosis of miscarriage; at least one recent ultrasound examination (made within 7 days before randomisation) is required for inclusion. The maximum gestational age at inclusion is 14 weeks." Comment: not answering PICO research question. | |
| Quote: "We randomized patients sequentially, according to their entry into the study, after the study started." Comment: quasi‐randomised study. | |
| Quote: "A statistician allotted the participants to their postsurgical treatment groups according to their application numbers." Comment: quasi‐randomised study. | |
| Quote: "This randomised controlled blind prospective study is undertaken to evaluate the safety and efficacy of Seprafilm™ ‐ a novel bioresorbable membrane of chemically modified hyaluronic acid and carboxymethylcellulose ‐ in prevention and reduction of postoperative endometrial and endocervical synechiae formation after general suction evacuation or curettage for incomplete, missed, and recurrent abortion." Quote: "Endometrial synechiae formation was evaluated with the use of hysterosalpingography (HSG) in patients of all groups without pregnancy success 8 months after the intervention." Comment: not answering PICO research question. | |
| Quote: "OBJECTIVE: To evaluate the role of prophylactic estrogen administration on preventing intrauterine adhesion formation following D&C [dilatation and curettage]." Comment: not answering PICO research question. |
IUD: intrauterine device; PICO: population, intervention, comparator, outcome.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Parallel‐group randomised controlled trial. Single centre, referral centre, the Netherlands. |
| Participants | 110 women undergoing hysteroscopic adhesiolysis for intrauterine adhesions. |
| Interventions | IUD, Cu‐IUD flexi‐T with copper removed, inserted in uterine cavity in both groups. Intervention: hormone treatment with schedule of oestrogen + norethisterone. Control: no hormone treatment. |
| Outcomes | Primary outcome: recurrence of IUAs. Secondary outcomes: pregnancy, restoration of menstrual flow and endometrial thickness. |
| Notes | Study results and conclusions presented as an abstract at an ESGE meeting. Authors are preparing a publication in a peer‐reviewed journal. |
ESGE: European Society for Gynaecological Endoscopy; IUA: intrauterine adhesion; IUD: intrauterine device.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Safety Study of Use of Hyaluronic Acid Gel to Prevent Intrauterine Adhesions in Hysteroscopic Surgery. |
| Methods | Allocation: randomised. Endpoint classification: safety study. Intervention model: parallel assignment. Masking: single‐blind (participant). Primary purpose: prevention. |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: hyaluronic acid gel. Control: no hyaluronic acid gel. |
| Outcomes | Primary outcome: participant satisfaction following gel application. |
| Starting date | November 2011. Estimated recruitment: 10 women. |
| Contact information | Ariel Revel, MD. Hadassah Medical Organization, Israel. Telephone: 97226777111 ext 76389. e‐mail: [email protected]. |
| Notes | 7 May 2017: overall status: not yet recruiting. The completion date has passed and the status has been last updated on 2 November 2011. |
| Trial name or title | Efficiency of INTERCOAT (Oxiplex/AP Gel) in Preventing Intrauterine Adhesion Formation in Hysteroscopic Surgery. |
| Methods | Allocation: randomised. Endpoint classification: efficacy study. Intervention model: parallel assignment. Masking: double‐blind (participant, carer). Primary purpose: prevention. |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: injection of Intercoat into the uterine cavity at the end of hysteroscopy. Control: no injection of Intercoat. |
| Outcomes | Not provided. |
| Starting date | December 2012. Status on 7 May 2017: still recruiting the estimated sample size of 130 women. |
| Contact information | Moran Paz, MD. Carmel Medical Center, Israel. Telephone: 972‐4‐8250637. e‐mail: [email protected]. |
| Notes | Estimated primary completion date according to ClinicalTrials.gov was March 2016. Last update received 30 July 2015. |
PID: pelvic inflammatory disease.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 2 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.42, 2.12] |
| Analysis 1.1 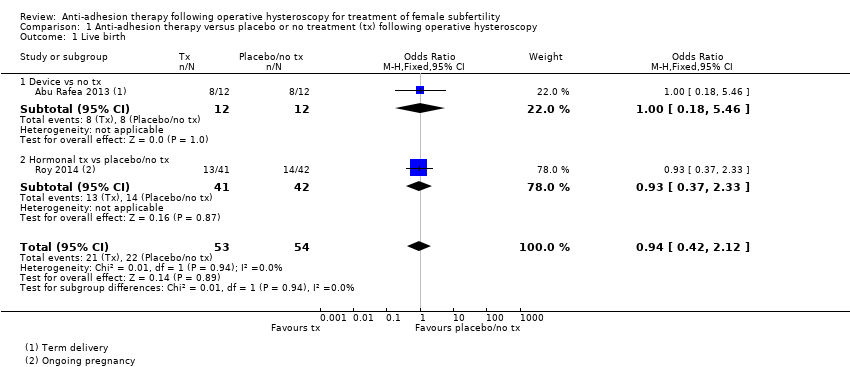 Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 1 Live birth. | ||||
| 1.1 Device vs no tx | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.18, 5.46] |
| 1.2 Hormonal tx vs placebo/no tx | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.37, 2.33] |
| 2 Clinical pregnancy Show forest plot | 2 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.37, 2.01] |
| Analysis 1.2  Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 2 Clinical pregnancy. | ||||
| 2.1 Device vs no tx | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 18.08] |
| 2.2 Hormonal tx vs placebo | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.35, 2.06] |
| 3 Miscarriage Show forest plot | 2 | 54 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.18, 2.57] |
| Analysis 1.3  Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 3 Miscarriage. | ||||
| 3.1 Device vs no tx | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 4.00] |
| 3.2 Hormonal tx vs placebo | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.10, 5.01] |
| 4 Presence of intrauterine adhesions at second‐look hysteroscopy Show forest plot | 8 | 560 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.21, 0.60] |
| Analysis 1.4 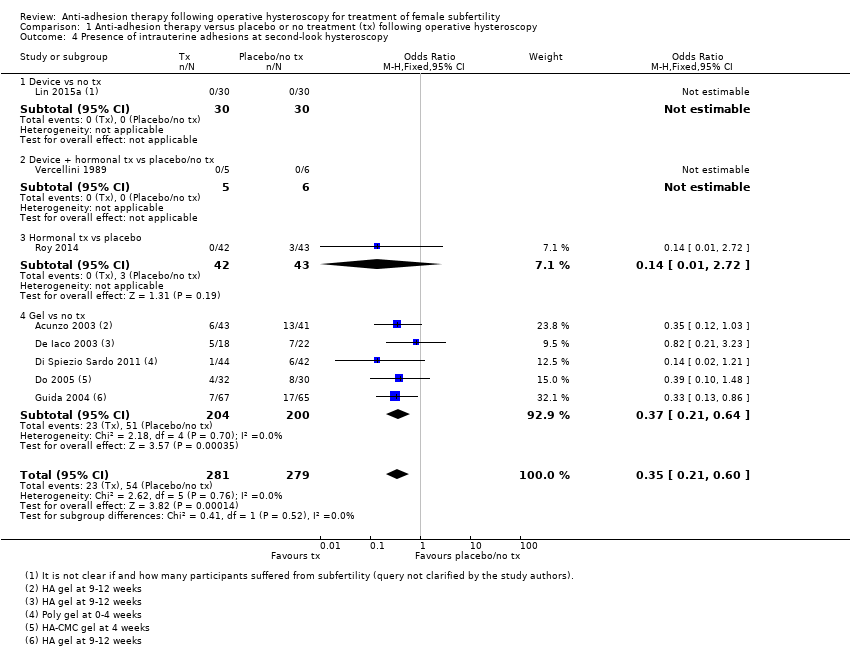 Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 4 Presence of intrauterine adhesions at second‐look hysteroscopy. | ||||
| 4.1 Device vs no tx | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Device + hormonal tx vs placebo/no tx | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Hormonal tx vs placebo | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.72] |
| 4.4 Gel vs no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.21, 0.64] |
| 5 Mean adhesion scores at second‐look hysteroscopy in women not treated for intrauterine adhesions Show forest plot | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐1.64, ‐1.29] |
| Analysis 1.5  Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 5 Mean adhesion scores at second‐look hysteroscopy in women not treated for intrauterine adhesions. | ||||
| 5.1 Gel vs no tx | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐1.64, ‐1.29] |
| 6 Mean adhesion scores at second‐look hysteroscopy in women treated for intrauterine adhesions Show forest plot | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐3.3 [‐3.37, ‐3.23] |
| Analysis 1.6 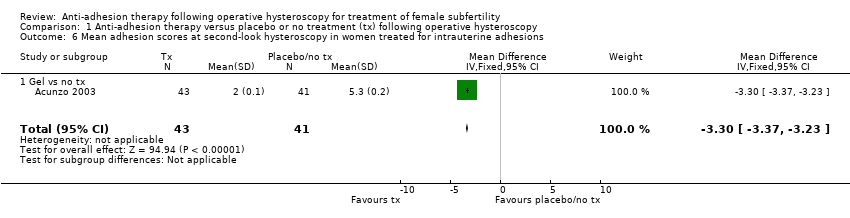 Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 6 Mean adhesion scores at second‐look hysteroscopy in women treated for intrauterine adhesions. | ||||
| 6.1 Gel vs no tx | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐3.3 [‐3.37, ‐3.23] |
| 7 Mild adhesions at second‐look hysteroscopy Show forest plot | 6 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.68, 2.61] |
| Analysis 1.7 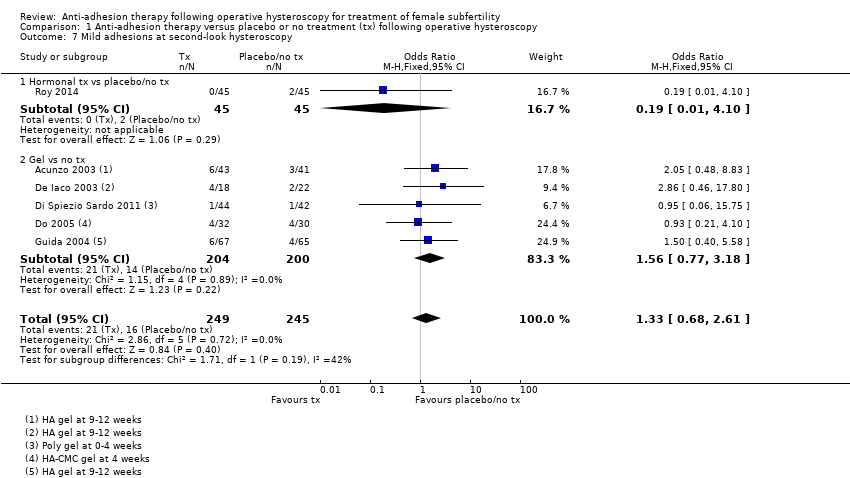 Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 7 Mild adhesions at second‐look hysteroscopy. | ||||
| 7.1 Hormonal tx vs placebo/no tx | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.10] |
| 7.2 Gel vs no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.77, 3.18] |
| 8 Moderate or severe adhesions at second‐look hysteroscopy Show forest plot | 6 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.03, 0.24] |
| Analysis 1.8 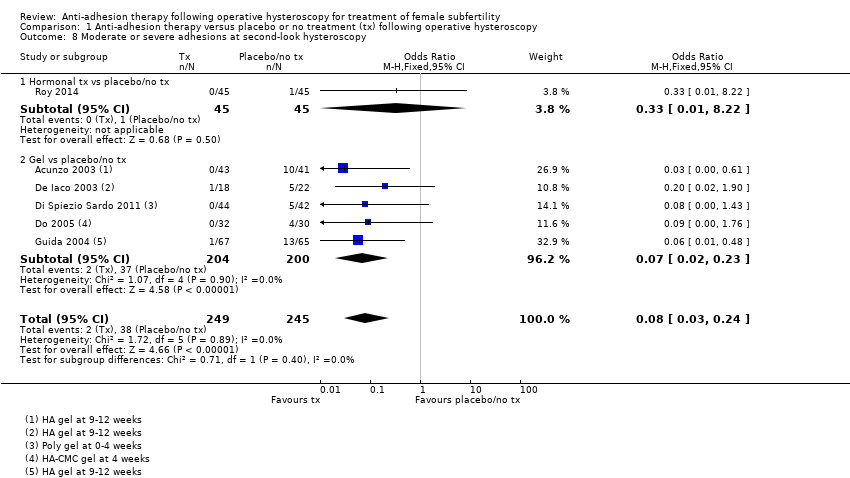 Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 8 Moderate or severe adhesions at second‐look hysteroscopy. | ||||
| 8.1 Hormonal tx vs placebo/no tx | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.22] |
| 8.2 Gel vs placebo/no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.57, 3.83] |
| Analysis 2.1 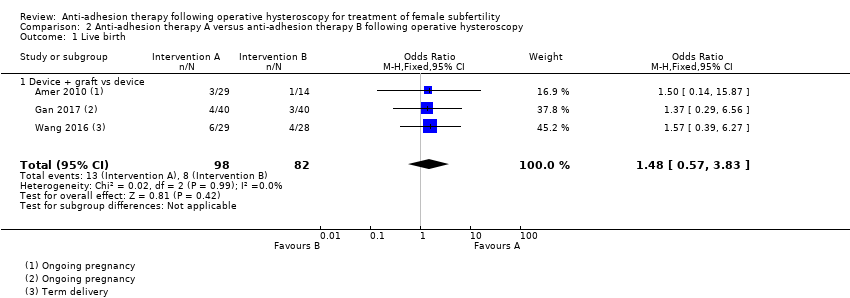 Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 1 Live birth. | ||||
| 1.1 Device + graft vs device | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.57, 3.83] |
| 2 Clinical pregnancy Show forest plot | 4 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.89, 3.33] |
| Analysis 2.2 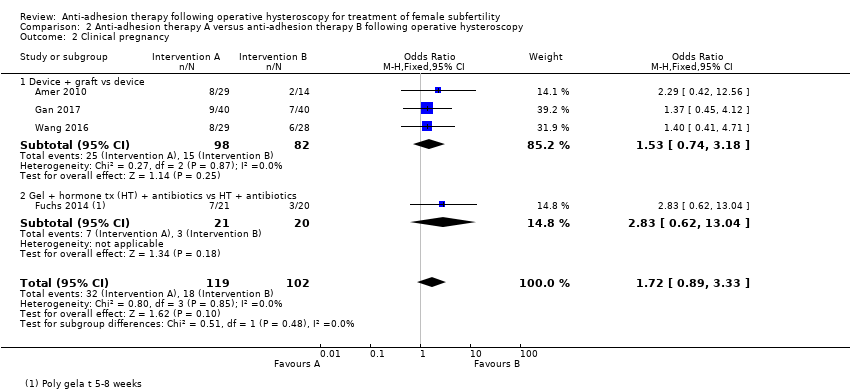 Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 2 Clinical pregnancy. | ||||
| 2.1 Device + graft vs device | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.74, 3.18] |
| 2.2 Gel + hormone tx (HT) + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.62, 13.04] |
| 3 Miscarriage Show forest plot | 3 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.20, 3.19] |
| Analysis 2.3 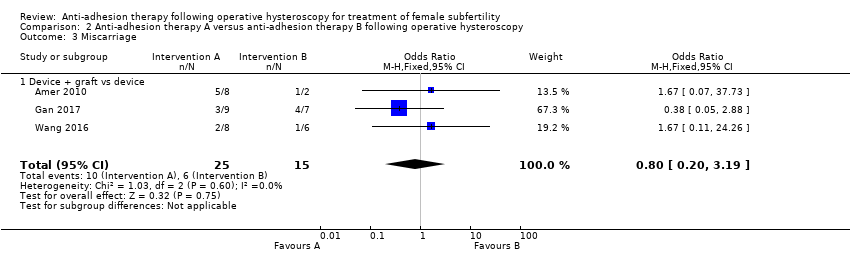 Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 3 Miscarriage. | ||||
| 3.1 Device + graft vs device | 3 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.20, 3.19] |
| 4 Presence of intrauterine adhesions at second‐look hysteroscopy Show forest plot | 5 | 451 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.36, 0.83] |
| Analysis 2.4 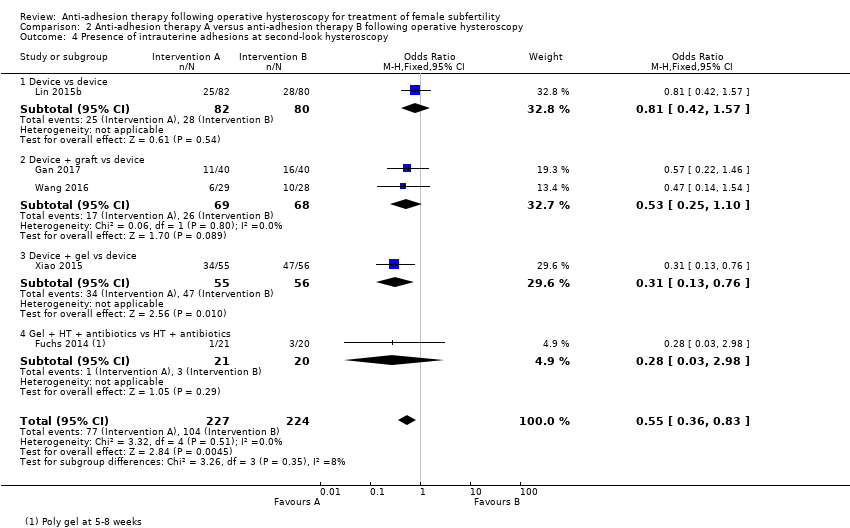 Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 4 Presence of intrauterine adhesions at second‐look hysteroscopy. | ||||
| 4.1 Device vs device | 1 | 162 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.42, 1.57] |
| 4.2 Device + graft vs device | 2 | 137 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.10] |
| 4.3 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.13, 0.76] |
| 4.4 Gel + HT + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.98] |
| 5 Mean adhesion scores in women treated for intrauterine adhesions Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.5 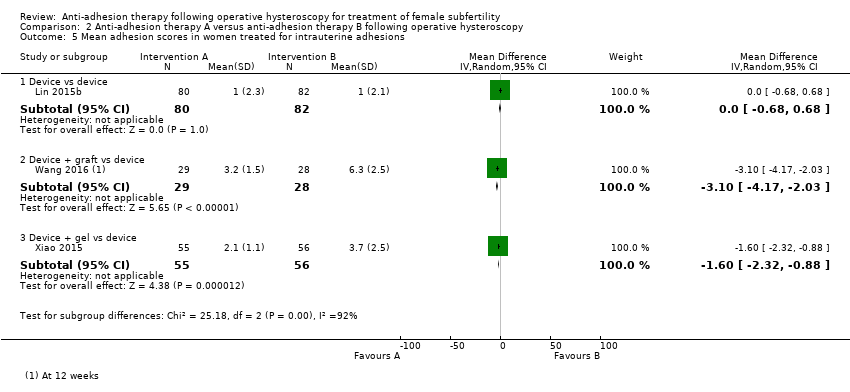 Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 5 Mean adhesion scores in women treated for intrauterine adhesions. | ||||
| 5.1 Device vs device | 1 | 162 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.68, 0.68] |
| 5.2 Device + graft vs device | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐4.17, ‐2.03] |
| 5.3 Device + gel vs device | 1 | 111 | Mean Difference (IV, Random, 95% CI) | ‐1.6 [‐2.32, ‐0.88] |
| 6 Mild adhesions at second‐look hysteroscopy Show forest plot | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.53, 2.34] |
| Analysis 2.6  Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 6 Mild adhesions at second‐look hysteroscopy. | ||||
| 6.1 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.53, 2.34] |
| 7 Moderate or severe adhesions at second‐look hysteroscopy Show forest plot | 2 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.10, 0.61] |
| Analysis 2.7  Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 7 Moderate or severe adhesions at second‐look hysteroscopy. | ||||
| 7.1 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.09, 0.63] |
| 7.2 Gel + HT + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.98] |

Study flow diagram: summary of searches since 2015. PICO: population, intervention, comparator, outcome; RCT: randomised controlled trial.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
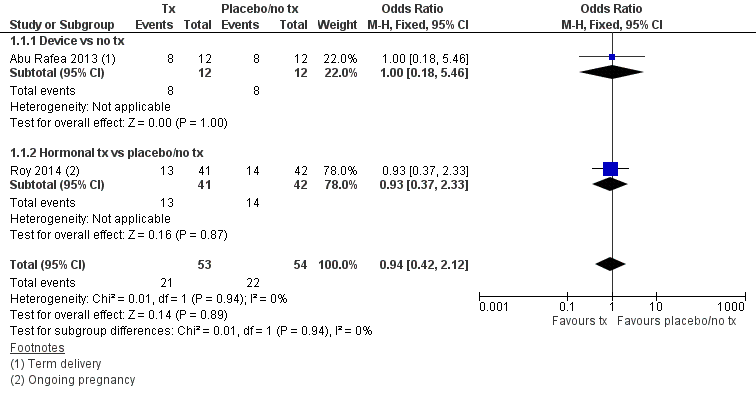
Forest plot of comparison: 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, outcome: 1.1 Live birth.

Forest plot of comparison: 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, outcome: 1.4 Presence of intrauterine adhesions at second‐look hysteroscopy.
Cates' plot of numbers needed to treat for an additional beneficial outcome (NNTB) for Analysis 1.4 assuming medium risk of 545 women per 1000 with intrauterine adhesions at second‐look hysteroscopy in the control group (no treatment or placebo). Randomly compared to control, the use of device with or without hormonal treatment or hormonal treatment or barrier gels (intervention) decreased the number of women with intrauterine adhesions at second‐look hysteroscopy to 234 women per 1000 (95% confidence interval 153 to 365 women per 1000). Figure drawn using www.nntonline.net.

Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 1 Live birth.
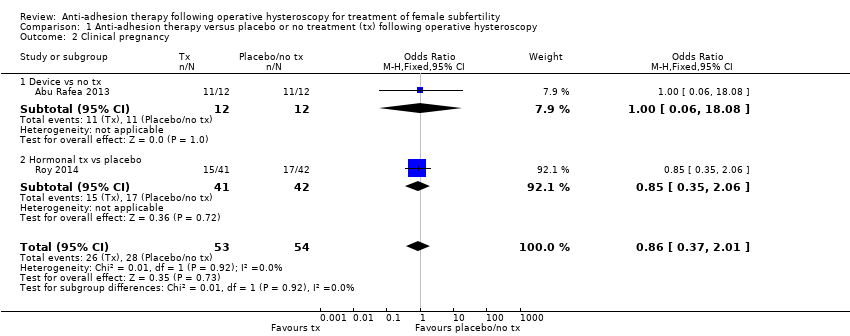
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 2 Clinical pregnancy.

Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 3 Miscarriage.

Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 4 Presence of intrauterine adhesions at second‐look hysteroscopy.
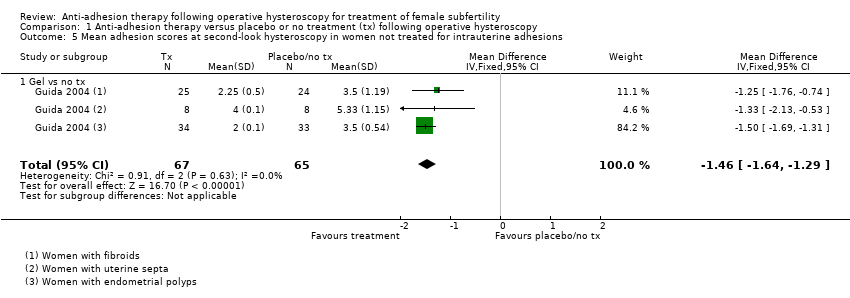
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 5 Mean adhesion scores at second‐look hysteroscopy in women not treated for intrauterine adhesions.

Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 6 Mean adhesion scores at second‐look hysteroscopy in women treated for intrauterine adhesions.
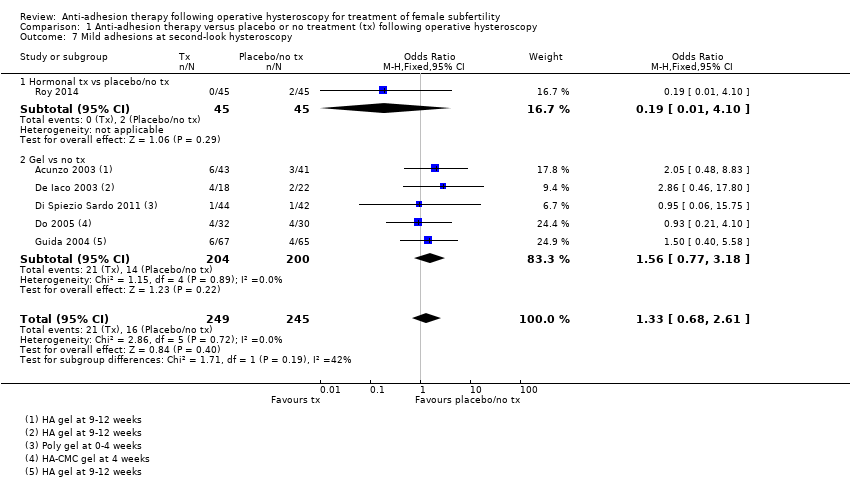
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 7 Mild adhesions at second‐look hysteroscopy.
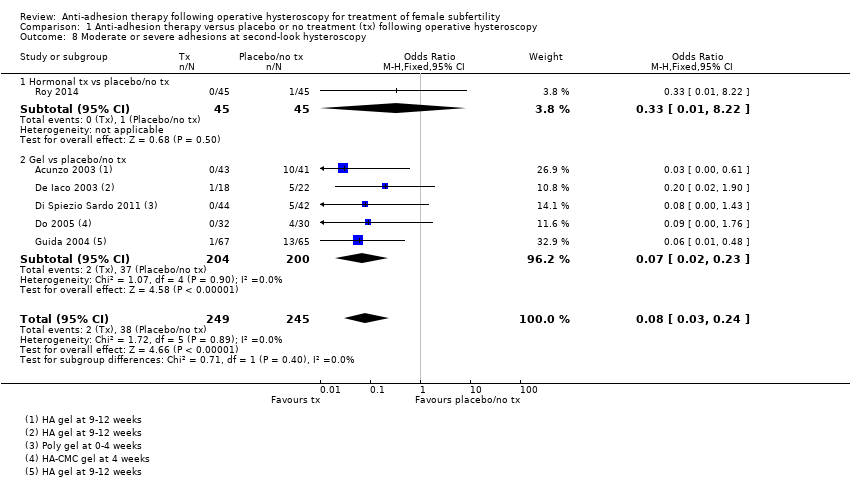
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 8 Moderate or severe adhesions at second‐look hysteroscopy.

Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 1 Live birth.
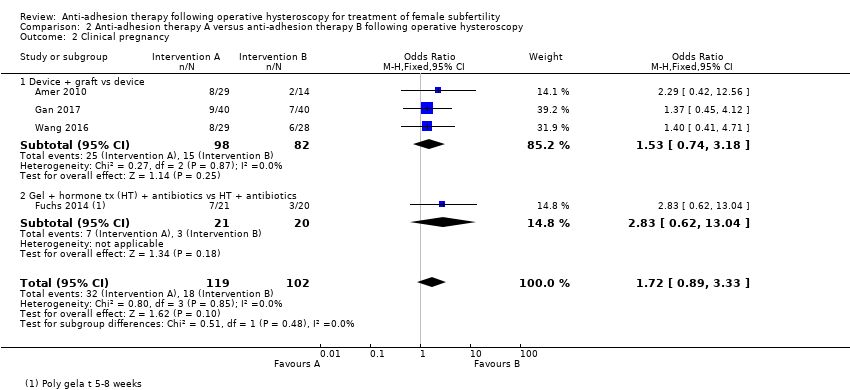
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 2 Clinical pregnancy.

Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 3 Miscarriage.

Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 4 Presence of intrauterine adhesions at second‐look hysteroscopy.

Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 5 Mean adhesion scores in women treated for intrauterine adhesions.
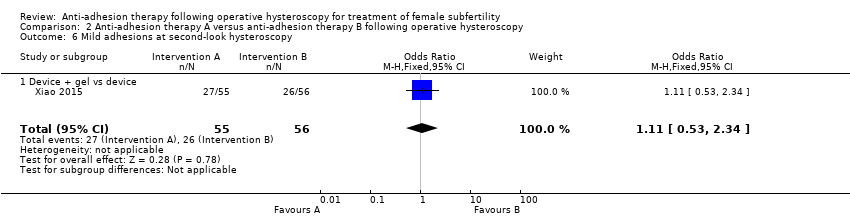
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 6 Mild adhesions at second‐look hysteroscopy.
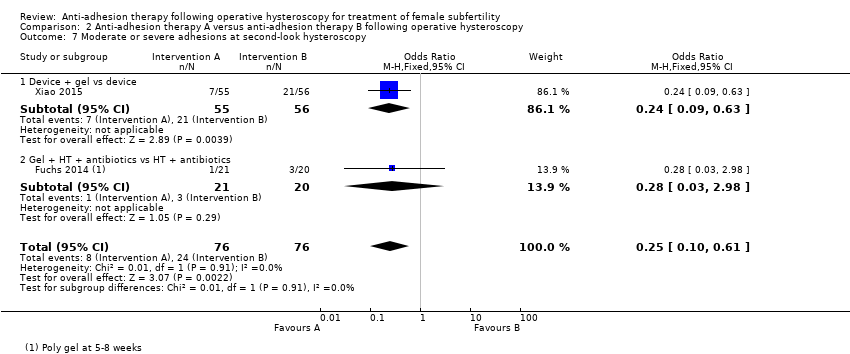
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 7 Moderate or severe adhesions at second‐look hysteroscopy.
| Any anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy | ||||||
| Patient or population: women treated by operative hysteroscopy for uterine pathology associated with subfertility or adverse pregnancy outcome Settings: single centre, Hysteroscopy Unit or Department of Obstetrics and Gynaecology of a university or non‐university tertiary care hospital Intervention: any anti‐adhesion therapy Comparison: no treatment or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment or placebo | Anti‐adhesion therapy | |||||
| Live birth a | No treatment or placebo | Device or hormonal treatment | OR 0.94 (0.42 to 2.12) | 107 | ⊕⊝⊝⊝ | ‐ |
| Mean‐risk populationb | ||||||
| 407 per 1000 | 399 per 1000 | |||||
| Presence of intrauterine adhesions at second‐look hysteroscopy (second‐look hysteroscopy at 4‐12 weeks after operative hysteroscopy) | No treatment or placebo | Device ± hormonal treatment or hormonal treatment or barrier gel | OR 0.35 g (0.21 to 0.60) | 560 (8 RCTs) | ⊕⊕⊝⊝ | ‐ |
| Low‐risk populationf | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population f | ||||||
| 545 per 1000 | 234 per 1000 | |||||
| High‐risk population f | ||||||
| 875 per 1000 | 376 per 1000 | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| a The two included studies reported term delivery (Abu Rafea 2013) or ongoing pregnancy (Roy 2014), which we used as a surrogate outcome for live birth. b The assumed risk for the mean‐risk population was the pooled risk of all live births in control groups of the two included studies. c Downgraded one level for serious risk of bias: one study was at high risk of bias in several domains, including allocation concealment. d Downgraded one level for serious imprecision; only 43 events in total. e Downgraded one level for serious indirectness, because only 30% (35/118) of all randomised women in this analysis were subfertile. f The assumed risk for low‐, medium‐ and high‐risk population based on presence of intrauterine adhesions following hysteroscopic removal of endometrial polyps/following removal of submucous fibroids and intrauterine adhesions (mean of both)/removal of uterine septum, respectively, based on findings of a prospective cohort study (Yang 2013). G Two studies reported no events (Lin 2015a; Vercellini 1989). h Downgraded one level for serious risk of bias: all eight studies had several limitations but none was at high risk for selection bias related to random sequence generation or allocation concealment. i Downgraded one level for serious indirectness, because in four of eight studies less than 50% of participants were subfertile and in four of eight studies it was unclear whether subfertile women were included. | ||||||
| Any anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy | ||||||
| Patient or population: women treated by operative hysteroscopy for uterine pathology Settings: multicentric, Hysteroscopy Unit of Department of Obstetrics and Gynaecology of a university, university‐affiliated or non‐university tertiary care hospital Intervention: anti‐adhesion therapy A Comparison: anti‐adhesion therapy B | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Anti‐adhesion therapy B | Anti‐adhesion therapy A | |||||
| Live birth a | Device | Device + graft | OR 1.48 (0.57 to 3.83) | 180 (3 RCTs) | ⊕⊕⊝⊝ Low c,d | ‐ |
| 98 per 1000 b | 138 per 1000 (60 to 315) | |||||
| Presence of intrauterine adhesions at second‐look hysteroscopy (6‐12 weeks) | Device or hormonal treatment with antibiotics | Device ± graft/gel or gel + hormonal treatment + and antibiotics | OR 0.55 (0.36 to 0.83) | 451 (5 RCTs) | ⊕⊕⊝⊝ | ‐ |
| Low‐risk population e | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population e | ||||||
| 545 per 1000 | 403 per 1000 | |||||
| High‐risk population e | ||||||
| 875 per 1000 | 647 per 1000 | |||||
| *The basis for the assumed risk is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| a The three included studies reported term delivery (Wang 2016) or ongoing pregnancy (Amer 2010; Gan 2017; Wang 2016), which we used as a surrogate outcome for live birth. b The assumed risk for the average‐risk population is the pooled risk of all the live births in the control groups of the three included studies. c Downgraded one level for serious risk of bias: despite several limitations none of the studies was at high risk for selection bias related to random sequence generation or allocation concealment. d Downgraded one level for serious imprecision‐ only 21 events in total. e The assumed risk for low/medium/high‐risk population is based on the presence of intrauterine adhesions following hysteroscopic removal of endometrial polyps/following removal of submucous fibroids and IUAs (mean of both)/removal of uterine septum, respectively, based on findings of a prospective cohort study (Yang 2013). f Downgraded one level for serious risk of bias: despite several limitations none of the studies was at high risk for selection bias related to random sequence generation or allocation concealment. g Downgraded one level for serious indirectness because, in two of five studies, less than 50% of participants were subfertile; in one of five studies, it was unclear if subfertile women were included and in two of five studies, the proportion of infertile women was not reported. | ||||||
| Outcome | Balloon group (intervention: n = 82) | IUD group (control: n = 80) | P value |
| AFS score before surgery (median, 95% CI) | 8 (5 to 12) | 8 (5 to 12) | 1.00 |
| Median reduction in AFS score | 7 (2 to 12) | 7 (0 to 12) | 1.00 |
| IUD: intrauterine device; n: number of participants. | |||
| Statistic | Fresh amnion graft (group 2: n = 14) | Dried amnion graft (group 3: n = 15) | No amnion graft (group 1: n = 14) | P value |
| Median | 1.5 | 2 | 2 | ‐ |
| IQR | 1 to 2 | 1 to 2 | 1 to 2 | 0.27 |
| IQR: interquartile range; n: number of participants. | ||||
| Statistic | Amnion graft (intervention: n = 40) | No graft (control: n = 40) | P value |
| Median | 2 | 4 | ‐ |
| IQR | 2 to 5 | 2 to 6 | 0.03 |
| IQR: interquartile range; n: number of participants. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 2 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.42, 2.12] |
| 1.1 Device vs no tx | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.18, 5.46] |
| 1.2 Hormonal tx vs placebo/no tx | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.37, 2.33] |
| 2 Clinical pregnancy Show forest plot | 2 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.37, 2.01] |
| 2.1 Device vs no tx | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 18.08] |
| 2.2 Hormonal tx vs placebo | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.35, 2.06] |
| 3 Miscarriage Show forest plot | 2 | 54 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.18, 2.57] |
| 3.1 Device vs no tx | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 4.00] |
| 3.2 Hormonal tx vs placebo | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.10, 5.01] |
| 4 Presence of intrauterine adhesions at second‐look hysteroscopy Show forest plot | 8 | 560 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.21, 0.60] |
| 4.1 Device vs no tx | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Device + hormonal tx vs placebo/no tx | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Hormonal tx vs placebo | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.72] |
| 4.4 Gel vs no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.21, 0.64] |
| 5 Mean adhesion scores at second‐look hysteroscopy in women not treated for intrauterine adhesions Show forest plot | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐1.64, ‐1.29] |
| 5.1 Gel vs no tx | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐1.64, ‐1.29] |
| 6 Mean adhesion scores at second‐look hysteroscopy in women treated for intrauterine adhesions Show forest plot | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐3.3 [‐3.37, ‐3.23] |
| 6.1 Gel vs no tx | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐3.3 [‐3.37, ‐3.23] |
| 7 Mild adhesions at second‐look hysteroscopy Show forest plot | 6 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.68, 2.61] |
| 7.1 Hormonal tx vs placebo/no tx | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.10] |
| 7.2 Gel vs no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.77, 3.18] |
| 8 Moderate or severe adhesions at second‐look hysteroscopy Show forest plot | 6 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.03, 0.24] |
| 8.1 Hormonal tx vs placebo/no tx | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.22] |
| 8.2 Gel vs placebo/no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.57, 3.83] |
| 1.1 Device + graft vs device | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.57, 3.83] |
| 2 Clinical pregnancy Show forest plot | 4 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.89, 3.33] |
| 2.1 Device + graft vs device | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.74, 3.18] |
| 2.2 Gel + hormone tx (HT) + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.62, 13.04] |
| 3 Miscarriage Show forest plot | 3 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.20, 3.19] |
| 3.1 Device + graft vs device | 3 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.20, 3.19] |
| 4 Presence of intrauterine adhesions at second‐look hysteroscopy Show forest plot | 5 | 451 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.36, 0.83] |
| 4.1 Device vs device | 1 | 162 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.42, 1.57] |
| 4.2 Device + graft vs device | 2 | 137 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.10] |
| 4.3 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.13, 0.76] |
| 4.4 Gel + HT + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.98] |
| 5 Mean adhesion scores in women treated for intrauterine adhesions Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Device vs device | 1 | 162 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.68, 0.68] |
| 5.2 Device + graft vs device | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐4.17, ‐2.03] |
| 5.3 Device + gel vs device | 1 | 111 | Mean Difference (IV, Random, 95% CI) | ‐1.6 [‐2.32, ‐0.88] |
| 6 Mild adhesions at second‐look hysteroscopy Show forest plot | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.53, 2.34] |
| 6.1 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.53, 2.34] |
| 7 Moderate or severe adhesions at second‐look hysteroscopy Show forest plot | 2 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.10, 0.61] |
| 7.1 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.09, 0.63] |
| 7.2 Gel + HT + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.98] |

