استفاده از درمان ضد‐چسبندگی به دنبال هیستروسکوپی جراحی برای درمان قدرت پائین باروری زنان
چکیده
پیشینه
شواهد مشاهدهای، مزیت بالقوهای را در درمانهای ضد‐چسبندگی (مثل گذاشتن وسیله داخل رحمی (IUD) یا بالون، درمان هورمونی، ژلهای سد کننده (barrier gel) یا پیوند غشای آمنیوتیک انسانی) به جهت کاهش چسبندگیهای داخل رحمی (IUA) برای زنان کمبارو که تحت هیستروسکوپی (hysteroscopy) جراحی قرار میگیرند، نشان میدهد.
اهداف
بررسی اثربخشی درمانهای ضد‐چسبندگی در برابر با دارونما (placebo)، عدم درمان یا هر نوع درمان ضد‐چسبندگی دیگر به دنبال هیستروسکوپی جراحی برای درمان قدرت پائین باروری در زنان.
روشهای جستوجو
برای این مرور، بانکهای اطلاعاتی زیر را از آغاز تا تاریخ جون 2017 جستوجو کردیم: پایگاه ثبت تخصصی گروه زنان و باروری در کاکرین، پایگاه ثبت مرکزی مطالعات کاکرین (CRSO)؛ MEDLINE؛ Embase؛ CINAHL و دیگر منابع الکترونیکی کارآزماییها، شامل پایگاههای ثبت کارآزمایی، منابع علمی منتشر نشده و فهرست منابع. همچنین Journal of Minimally Invasive Gynecology, را به صورت دستی جستوجو کردیم و با متخصصان این حیطه تماس برقرار کردیم. همچنین فهرست منابع مطالعات وارد شده را جستوجو کردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) برای مقایسه درمانهای ضد‐چسبندگی در برابر دارونما، عدم درمان یا هر نوع درمان ضد‐چسبندگی به دنبال هیستروسکوپی جراحی در زنان با قدرت پائین باروری. پیامد اولیه تولد زنده بود. پیامدهای ثانویه به صورت بارداری بالینی، سقط جنین و IUAهایی که در هیستروسکوپی second‐look، همراه با شدت یا امتیازهای متوسط چسبندگی آنها بررسی میشد.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم، مطالعات را انتخاب، خطر سوگیری (bias) را بررسی، اطلاعات را استخراج و کیفیت شواهد را با استفاده از سیستم درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) بررسی کردند.
نتایج اصلی
کیفیت کلی شواهد پائین تا بسیار پائین بودند. محدودیتهای اصلی عبارت بودند از خطر سوگیری مرتبط با کورسازی شرکتکنندگان و پرسنل، غیر‐مستقیم بودن و عدم دقت. 16 RCT را پیدا کردیم که به مقایسه یک وسیله تعبیهای در برابر عدم درمان (دو مطالعه؛ 90 زن)، درمان هورمونی در برابر عدم درمان یا دارونما (دو مطالعه؛ 136 زن)، وسیله تعبیهای همراه با درمان هورمونی در برابر عدم درمان (یک مطالعه؛ 20 زن)، ژل محافظ در برابر عدم درمان (پنج مطالعه؛ 464 زن)، وسیله تعبیهای همراه با گرافت در برابر وسیله بدون گرافت (سه مطالعه؛ 190 زن)، یک نوع از وسیله تعبیهای در برابر وسیله دیگر (یک مطالعه؛ 201 زن) ژل همراه با درمان هورمونی و آنتیبیوتیکها در برابر درمان هورمونی و آنتیبیوتیکها (یک مطالعه؛ 52 زن) و وسیله تعبیهای همراه با ژل در برابر وسیله (یک مطالعه؛ 120 زن) پرداخته بودند. تعداد کل زنان شرکتکننده 1273 نفر بود، اما دادههای 1133 زن برای تجزیهوتحلیل موجود بود. فقط دو مطالعه از 16 مورد، 100% زنان نابارور را در برگرفته بودند، در مطالعات دیگر، این نسبت، متغیر یا ناشناخته بود.
هیچ مطالعهای تولد زنده را گزارش نکرد، اما بعضی (پنج مطالعه)، پیامدهایی را گزارش کردند که به عنوان پیامد جایگزین تولد زنده در نظر گرفته شدند (زایمان ترم یا تداوم بارداری).
درمان ضد‐چسبندگی در برابر دارونما یا عدم درمان به دنبال هیسترسکوپی جراحی.
شواهد ناکافی برای تعیین اینکه تفاوتی میان استفاده از یک وسیله یا درمان هورمونال در مقایسه با عدم درمان یا دارونما با در نظر گرفتن نرخ زایمان ترم یا تداوم بارداری (نسبت شانس (OR): 0.94؛ 95% فاصله اطمینان (CI): 0.42 تا 2.12؛ 107 زن؛ 2 مطالعه؛ I² = %0؛ شواهد با کیفیت بسیار پائین) وجود داشته، گزارش شد.
IUAهای کمتری در هیستروسکوپی second‐look با استفاده از یک وسیله با یا بدون درمان هورمونی یا درمان هورمونی یا ژلهای محافظ در مقایسه با عدم درمان یا دارونما دیده شد (OR: 0.35؛ 95% CI؛ 0.21 تا 0.60؛ 560 زن؛ 8 مطالعه؛ I² = %0؛ شواهد با کیفیت پائین). تعداد افراد مورد نیاز جهت درمان تا حصول یک پیامد مثبت بیشتر (number needed to treat for an additional beneficial outcome; NNTB) معادل 9 گزارش شد (95% CI؛ 5 تا 17).
مقایسههای درمانهای مختلف ضد‐چسبندگی به دنبال هیستروسکوپی جراحی
مشخص نبود که تفاوتی میان استفاده از یک وسیله تعبیهای در ترکیب با گرافت در برابر وسیله به تنهایی برای پیامد تداوم بارداری وجود داشته یا خیر (OR: 1.48؛ 95% CI؛ 0.57 تا 3.83؛ 180 زن؛ 3 مطالعه؛ I² = %0؛ شواهد با کیفیت پائین). IUAهای کمتری در هیستروسکوپی second‐look با استفاده از یک وسیله با یا بدون گرافت/ژل یا ژل در ترکیب با درمان هورمونی و آنتیبیوتیکها در مقایسه با استفاده از یک وسیله به تنهایی یا درمان هورمونی در ترکیب با آنتیبیوتیکها دیده شد، اما یافتههای این متاآنالیز تحت تاثیر کیفیت شواهد قرار داشت (OR: 0.55؛ 95% CI؛ 0.36 تا 0.83؛ 451 زن؛ 5 مطالعه؛ I² = %0؛ شواهد با کیفیت پائین).
نتیجهگیریهای نویسندگان
کاربردهای عملی بالینی
کیفیت شواهد به دست آمده، برای تمامی نتایج پائین تا بسیار پائین بود. اثربخشی بالینی درمان ضد‐چسبندگی برای بهبود پیامدهای کلیدی باروری یا برای کاهش IUAها به دنبال هیستروسکوپی جراحی در زنان کمبارور، همچنان نامطمئن باقی مانده است.
کاربردهای تحقیقاتی
برای بررسی مقایسهای ایمنی و (هزینه‐)اثربخشی درمانهای مختلف ضد‐چسبندگی در مقایسه با عدم درمان یا مداخلات دیگر برای بهبود پیامدهای باروری در زنان با قدرت پائین باروری مطالعات بیشتری مورد نیاز است.
PICO
خلاصه به زبان ساده
درمان ضد‐چسبندگی (anti‐adhesion therapy) بعد از هیستروسکوپی (hysterscopy) برای زنانی که مشکل باروری دارند.
سوال مطالعه مروری
بررسی تاثیرات درمانها برای پیشگیری از بافت اسکار (چسبندگیها) (درمان ضد‐چسبندگی) بعد از درمان جراحی برای ضایعات داخل رحمی در زنانی که مشکل بارداری دارند.
پیشینه
چسبندگیهای شکمی ساختارهای شبیه شبکه هستند که بهطور طبیعی سطوح را در شکم از هم جدا میکند و به دلیل آسیب وارده به پوشش شکم، به هم میچسبند. آنها اغلب پس از جراحی شکمی شکل میگیرند. باعث ایجاد وضعیتهای متعددی مانند دردهای مزمن لگنی و ناباروری میشوند. عملی که در حال حاضر در بالین صورت میگیرد، بر اساس مطالعات مرسوم یا مشاهدهای شکل گرفته است.
ویژگیهای مطالعه
مطالعاتی را جستوجو کردیم که هر درمانی را در برابر عدم درمان، دارونما (placebo) (درمان ساختگی)، یا هر مداخله دیگری به صورت تصادفی مقایسه کردند. پیامدها به صورت تولد زنده، بارداری بالینی، سقط جنین و وجود یا شدت بافت اسکار در پروسیجر second‐look تعریف شدند.
نتایج کلیدی
برای این مرور 16 مطالعه پیدا کردیم. درمانها شامل موارد زیر بودند: گذاشتن یک وسیله در مقایسه با عدم درمان (دو مطالعه؛ 90 زن)، درمان هورمونی در برابر عدم درمان یا دارونما (دو مطالعه؛ 136 زن)، وسیله همراه با درمان هورمونی در برابر عدم درمان (یک مطالعه؛ 20 زن)، ژل محافظ در برابر عدم درمان (پنج مطالعه؛ 464 زن)، وسیله با استفاده از غشاهای پس از تولد نوزاد در برابر بدون غشاها (سه مطالعه؛ 190 زن)، یک نوع از وسیله در برابر نوع دیگر (یک مطالعه؛ 201 زن) ژل همراه با درمان هورمونی و آنتیبیوتیکها در برابر درمان هورمونی با آنتیبیوتیکها (یک مطالعه؛ 52 زن) یا وسیله همراه با ژل در مقایسه با وسیله به تنهایی (یک مطالعه؛ 120 زن). از 1273 زنی که تصادفیسازی شدند، دادههای 1133 زن برای تجزیهوتحلیل موجود بودند.
در فقط دو مطالعه، همه زنان مشکل باروری داشتند. اغلب مطالعات (14/16) در معرض خطر سوگیری (bias) بالایی برای حداقل یک دلیل بودند. هیچ یک از مطالعات تولد زنده را گزارش نکردند، به همین دلیل ما دادههای مربوط به زایمان ترم یا تداوم بارداری را به جای تولد زنده در نظر گرفتیم که در پنج مطالعه گزارش شده بودند.
مشخص نبود که تفاوتی میان درمان ضد‐چسبندگی در مقایسه با عدم درمان (دو مطالعه؛ 107 زن) یا دیگر درمانها (سه مطالعه؛ 180 زن) برای افزایش شانس به دنیا آوردن نوزاد زنده، زایمان ترم یا تداوم بارداری وجود دارد یا خیر. استفاده از بعضی درمانهای ضد‐چسبندگی (وسیله با یا بدون درمان هورمونی یا درمان هورمونی یا ژلها) (هشت مطالعه، 560 زن) ممکن است خطر تشکیل بافت اسکار را در مقایسه با عدم درمان کاهش میدهد. ما انتظار داشتیم که از هر 1000 زن درمان شده با جراحی، بین 153 و 365 زن پس از استفاده از ژلها با بافت اسکار روبهرو میشوند، در مقایسه با 545 زنی که درمان نمیشوند. شواهد تا 6 جون 2017 بهروز است.
کیفیت شواهد
کیفیت کلی شواهد مطالعه بسیار پائین تا پائین بود. این مطالعات محدودیتهایی داشتند، مانند خطر جدی سوگیری مرتبط با شرکتکنندگان و ارزیابانی که میدانستند به هر بیمار چه درمانی داده شده است.
پیش از توصیه به درمانهای ضد‐چسبندگی در کار بالینی هر روزه بعد از جراحی رحم در زنانی که مشکل بارداری دارند، پژوهشهای بیشتری مورد نیاز است.
Authors' conclusions
Summary of findings
| Any anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy | ||||||
| Patient or population: women treated by operative hysteroscopy for uterine pathology associated with subfertility or adverse pregnancy outcome Settings: single centre, Hysteroscopy Unit or Department of Obstetrics and Gynaecology of a university or non‐university tertiary care hospital Intervention: any anti‐adhesion therapy Comparison: no treatment or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment or placebo | Anti‐adhesion therapy | |||||
| Live birth a | No treatment or placebo | Device or hormonal treatment | OR 0.94 (0.42 to 2.12) | 107 | ⊕⊝⊝⊝ | ‐ |
| Mean‐risk populationb | ||||||
| 407 per 1000 | 399 per 1000 | |||||
| Presence of intrauterine adhesions at second‐look hysteroscopy (second‐look hysteroscopy at 4‐12 weeks after operative hysteroscopy) | No treatment or placebo | Device ± hormonal treatment or hormonal treatment or barrier gel | OR 0.35 g (0.21 to 0.60) | 560 (8 RCTs) | ⊕⊕⊝⊝ | ‐ |
| Low‐risk populationf | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population f | ||||||
| 545 per 1000 | 234 per 1000 | |||||
| High‐risk population f | ||||||
| 875 per 1000 | 376 per 1000 | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| a The two included studies reported term delivery (Abu Rafea 2013) or ongoing pregnancy (Roy 2014), which we used as a surrogate outcome for live birth. b The assumed risk for the mean‐risk population was the pooled risk of all live births in control groups of the two included studies. c Downgraded one level for serious risk of bias: one study was at high risk of bias in several domains, including allocation concealment. d Downgraded one level for serious imprecision; only 43 events in total. e Downgraded one level for serious indirectness, because only 30% (35/118) of all randomised women in this analysis were subfertile. f The assumed risk for low‐, medium‐ and high‐risk population based on presence of intrauterine adhesions following hysteroscopic removal of endometrial polyps/following removal of submucous fibroids and intrauterine adhesions (mean of both)/removal of uterine septum, respectively, based on findings of a prospective cohort study (Yang 2013). G Two studies reported no events (Lin 2015a; Vercellini 1989). h Downgraded one level for serious risk of bias: all eight studies had several limitations but none was at high risk for selection bias related to random sequence generation or allocation concealment. i Downgraded one level for serious indirectness, because in four of eight studies less than 50% of participants were subfertile and in four of eight studies it was unclear whether subfertile women were included. | ||||||
| Any anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy | ||||||
| Patient or population: women treated by operative hysteroscopy for uterine pathology Settings: multicentric, Hysteroscopy Unit of Department of Obstetrics and Gynaecology of a university, university‐affiliated or non‐university tertiary care hospital Intervention: anti‐adhesion therapy A Comparison: anti‐adhesion therapy B | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Anti‐adhesion therapy B | Anti‐adhesion therapy A | |||||
| Live birth a | Device | Device + graft | OR 1.48 (0.57 to 3.83) | 180 (3 RCTs) | ⊕⊕⊝⊝ Low c,d | ‐ |
| 98 per 1000 b | 138 per 1000 (60 to 315) | |||||
| Presence of intrauterine adhesions at second‐look hysteroscopy (6‐12 weeks) | Device or hormonal treatment with antibiotics | Device ± graft/gel or gel + hormonal treatment + and antibiotics | OR 0.55 (0.36 to 0.83) | 451 (5 RCTs) | ⊕⊕⊝⊝ | ‐ |
| Low‐risk population e | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population e | ||||||
| 545 per 1000 | 403 per 1000 | |||||
| High‐risk population e | ||||||
| 875 per 1000 | 647 per 1000 | |||||
| *The basis for the assumed risk is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| a The three included studies reported term delivery (Wang 2016) or ongoing pregnancy (Amer 2010; Gan 2017; Wang 2016), which we used as a surrogate outcome for live birth. b The assumed risk for the average‐risk population is the pooled risk of all the live births in the control groups of the three included studies. c Downgraded one level for serious risk of bias: despite several limitations none of the studies was at high risk for selection bias related to random sequence generation or allocation concealment. d Downgraded one level for serious imprecision‐ only 21 events in total. e The assumed risk for low/medium/high‐risk population is based on the presence of intrauterine adhesions following hysteroscopic removal of endometrial polyps/following removal of submucous fibroids and IUAs (mean of both)/removal of uterine septum, respectively, based on findings of a prospective cohort study (Yang 2013). f Downgraded one level for serious risk of bias: despite several limitations none of the studies was at high risk for selection bias related to random sequence generation or allocation concealment. g Downgraded one level for serious indirectness because, in two of five studies, less than 50% of participants were subfertile; in one of five studies, it was unclear if subfertile women were included and in two of five studies, the proportion of infertile women was not reported. | ||||||
Background
Description of the condition
Intrauterine adhesions (IUAs) are fibrous strings at opposing walls of the uterus. The spectrum of severity of IUAs ranges from minimal to complete obliteration of the uterine cavity. Any trauma to the endometrium (the inner layer of the uterus) can lead to formation of IUAs; in daily clinical practice, nearly 90% of all IUAs are associated with postpartum or postabortion dilatation and curettage (Nappi 2007). The aetiological role of infection in the formation of IUAs is controversial, with the exception of genital tuberculosis (Deans 2010). IUA formation is the major long‐term complication of hysteroscopic surgery in women of reproductive age.
Several intrauterine anomalies have been linked with female subfertility (Bosteels 2015a). Endometrial polyps are benign, endometrial, stalk‐like masses protruding into the uterine cavity. Fibroids are excessive growths originating from the muscular portion of the uterine cavity. A septate uterus is a congenital malformation in which the longitudinal band separating left and right Müllerian ducts, which form the uterus in the human female foetus, has not been entirely resorbed. Hysteroscopy allows direct visualisation of the uterine cavity through a rigid, semi‐rigid or flexible endoscope. The hysteroscope consists of a rigid telescope with a proximal eyepiece and a distal objective lens that may be angled at 0 degrees to allow direct viewing, or offset at various angles to provide a fore‐oblique view. Operative hysteroscopy requires adequate visualisation through continuous fluid circulation using inflow and outflow channels. The sheath system of the operative hysteroscope contains one or two 1.6‐ to 2.0‐mm working channels for insertion of a small grasping or biopsy forceps, scissors, myoma fixation instruments, retraction loops, morcellator (surgical instruments used to divide and remove tissue during endoscopic surgery) and aspiration cannulae or unipolar or bipolar electrodiathermy instruments. Operative hysteroscopic procedures require a complex instrumentation setup, special training of the surgeon, and appropriate knowledge and management of complications. Removal of endometrial polyps by an endoscope is called hysteroscopic polypectomy. Hysteroscopic myomectomy is the procedure by which a fibroid is removed by hysteroscopy. Removal of a uterine septum is termed hysteroscopic septoplasty or septum resection. Removal of IUAs is called hysteroscopic adhesiolysis. A diagnostic or operative hysteroscopy following an operative hysteroscopy is termed a second‐look hysteroscopy.
One randomised controlled trial (RCT) reported the following numbers for the incidence of postsurgical IUAs at second‐look hysteroscopy: 3.6% after polypectomy, 6.7% after resection of uterine septa, 31.3% after removal of a solitary myoma and 45.5% after resection of multiple myomas (Taskin 2000). Mechanisms of tissue repair in the human endometrium are poorly understood (Revaux 2008) despite several hypotheses on the origin of cells for endometrial regeneration (Okulicz 2002). Endometrial stem or progenitor cells, present in women and rodents, may have an important function for endometrial regeneration in normal menstrual cycles and after delivery; this holds promise for new treatments for subfertility associated with IUAs or Asherman's syndrome (Deane 2013). The duration of endometrial wound healing depends on the type of pathology present, according to one prospective cohort study of 163 women undergoing operative hysteroscopy (Yang 2013); these investigators reported that the time needed for complete recovery of the endometrium ranges from one month following hysteroscopic removal of endometrial polyps to three months for the hysteroscopic treatment of submucous fibroids.
IUAs are associated with poor reproductive outcomes. This is due in part to infertility, with a prevalence as high as 43% (922/2151 women) according to one large review of observational studies (Schenker 1982). Poor outcomes also result from the clinical problem of recurrent miscarriage, ranging from 5% to 39% in women with IUAs, according to one review of observational studies (Kodaman 2007), and from major, and at times devastating, obstetrical complications, for example, placenta accreta or increta, as well as higher risks for preterm delivery, uterine rupture and peripartum hysterectomy as the endpoint of a successful hysteroscopic treatment for severe IUAs (Deans 2010).
Description of the intervention
Several observational studies have suggested different anti‐adhesion strategies for preventing IUAs following operative hysteroscopy.
Intrauterine device
An intrauterine device (IUD) may provide a physical barrier between the uterine walls, separating the endometrial layers after lysis of IUAs. At least 13 observational studies have recommended insertion of an IUD as an adjunct therapy for the prevention of IUAs (Deans 2010). Eight observational studies reported the use of a Foley catheter balloon as an alternative for similar purposes (Deans 2010).
Hormonal therapy
In 1964, Wood and Pena suggested use of oestrogen therapy to stimulate regeneration of the endometrium after surgical treatment for IUAs (Wood 1964).
Barrier gels
Hyaluronic acid (HA) or hyaluronan is a water‐soluble polysaccharide that consists of multiple disaccharide units of glucuronic acid and N‐acetylglucosamine bound together by a β1‐3‐type glucoside bond. Solutions of HA have viscoelastic properties that have led to interest in developing applications of HA in surgical procedures, for example, during eye surgery, and for prevention of postsurgical adhesions. However, HA may not be the ideal substance for all procedures because of its limited residence time when applied to a surgical site. It quickly enters the systemic circulation, then is cleared rapidly by catabolic pathways. Attempts to use HA for prevention of postsurgical adhesions have therefore resulted in variable success. Chemically modified derivatives of HA have been developed to circumvent the disadvantages of HA. One such derivative is auto‐cross‐linked polysaccharide (ACP), which is formed by cross‐linking of HA via direct formation of covalent ester bonds between hydroxyl and carboxyl groups of the HA molecule. ACP can be prepared through various degrees of cross‐linking: this allows tailoring of the viscosity properties of ACP gels (Renier 2005). Carboxymethylcellulose (CMC) is a high‐molecular‐weight polysaccharide that has greater viscosity than dextran 70. CMC can be used for adhesion prevention as a membrane barrier, or as a gel attained by mixing chemically derivative sodium hyaluronate and carboxymethylcellulose gel (HA‐CMC) (Leach 1998).
Human amniotic membrane grafting
Since the late 1990s, the surgical community has become more aware of the increasing potential of human amniotic membrane (HAM) as an adjunctive anti‐adhesion intervention. Human whole foetal membranes or amnion alone has been used in surgery to aid the repair of surface epithelial defects in the skin, eye, abdominal wall and peritoneum. HAM grafting has not been very popular in the field of obstetrics and gynaecology; its clinical use is limited as a graft in forming an artificial vagina, as a barrier in preventing postoperative intra‐abdominal adhesion formation and, finally, as a biological dressing following radical vulvectomy or groin dissection (Amer 2006).
How the intervention might work
Hypothetical underlying mechanisms of subfertility associated with IUAs include obstruction of sperm transport into the cervix, impaired embryo migration within the uterine cavity and failure of embryo implantation due to endometrial insufficiency (Deans 2010). Ideal anti‐adhesion adjunctive therapy following operative hysteroscopy would include application of a biologically active mechanical separator that achieves suppression of IUA formation and promotes healing of the endometrium. The bulk of evidence on how different interventions might work has been derived from observational or animal studies, largely in rodents and regrettably not in animal models validated for the study of human reproduction, such as primates (D'Hooghe 2009).
Intrauterine device
Use of an IUD (13 observational studies) or a Foley catheter balloon (eight observational studies) is often recommended following hysteroscopic treatment of IUAs or septoplasty, to act as a physical barrier separating opposing walls of the uterine cavity (Deans 2010). The type of IUD selected may be important; copper‐containing IUDs provoke an inflammatory reaction, probably with detrimental effects, whereas T‐shaped IUDs might provide too small a surface area to be truly effective in providing an efficient physical barrier. The loop IUD (e.g. the Lippes loop) is generally considered the IUD of choice for treatment of IUAs; however, it is no longer available in many countries (Kodaman 2007). One clinical controlled trial (CCT) compared use of a Foley catheter balloon for 10 days (59 women) versus insertion of an IUD for a three‐month period (51 women); fertility rates were poor in both the IUD group (20/59 women, or 34%) and the Foley catheter balloon group (14/51 women, or 28%) (Orhue 2003).
Hormonal therapy
Many studies recommend use of a cyclical oestrogen and progestogen treatment regimen following hysteroscopic treatment of IUAs to promote regeneration of the endometrium (Deans 2010). Various regimens consisting of oestrogen (e.g. conjugated equine oestrogen 2.5 mg twice daily for 30 days) with or without a progestogen (e.g. medroxyprogesterone acetate 10 mg for 10 days) have been proposed (Kodaman 2007). There are no comparative studies that examine dosage, administration or combinations of hormones (Deans 2010). In one RCT, 60 women undergoing dilatation and curettage during the first trimester of pregnancy were allocated to receive oestrogen combined with progestogen or no treatment (Farhi 1993). Women in the intervention group had a significantly thicker endometrium compared with women in the control group (8.4 with intervention vs 6.7 mm with no treatment; P = 0.02). Study authors concluded that postoperative hormonal treatment may be beneficial for IUA prevention following surgical trauma to the uterine cavity. Nevertheless, they provided no data on pregnancy outcomes or IUA recurrence (Farhi 1993). One systematic review of 26 observational studies concluded that hormonal therapy, particularly oestrogen treatment, may be beneficial for women with IUAs, but as adjunctive therapy combined with other anti‐adhesion strategies (Johary 2014).
Barrier gels
Use of biodegradable gel surgical barriers is based on the principle of keeping adjacent wound surfaces mechanically separate (Renier 2005). Several preclinical studies in various animal models demonstrated the effectiveness of ACP (Belluco 2001; Binda 2007; Binda 2009; Binda 2010; De Iaco 1998; Koçak 1999; Shamiyeh 2007; Wallwiener 2006), and HA‐CMC gels (Leach 1998; Schonman 2008), or of HA‐CMC membranes (Kelekci 2004; Rajab 2010), for preventing postsurgical adhesions. Other preclinical studies in animal models suggest that HA gel remains in situ longer than five to six days (Laurent 1992; Nimrod 1992). Similarly, animal studies demonstrated the persistence of HA‐CMC for about seven days after its application (Diamond 1988). The exact mechanisms by which ACP and HA‐CMC are able to reduce adhesion reformation are not well known but may be related to 'hydroflotation' or 'siliconising' effects. One French CCT (54 women) compared application of ACP gel (30 women) versus no gel (24 women) at the end of an operative hysteroscopic procedure performed to treat myomas, polyps, uterine septa or IUAs; investigators reported no statistically significant differences between comparison groups in the rate of adhesion formation, or in mean adhesion scores and severity of adhesions (Ducarme 2006). They provided no data on reproductive outcomes.
Human amniotic membrane grafting
Preclinical data on the effectiveness of HAM grafting in different animal models presented conflicting results. One trial demonstrated a beneficial effect in preventing de novo (new) adhesions (Szabo 2002), whereas two other animal studies reported that HAM grafting failed to prevent IUAs (Arora 1994; Badawy 1989). One observational study provided data on use of a fresh amniotic graft over an inflated Foley catheter balloon to prevent recurrence of IUAs after hysteroscopic lysis in 25 women with moderate‐to‐severe Asherman's syndrome. There was minimal adhesion reformation in 48% of study participants with severe adhesions. Study authors concluded that HAM grafting might be promising as adjunctive therapy following hysteroscopic adhesiolysis; it acts as a biologically active mechanical barrier to suppress adhesion formation while promoting endometrial healing (Amer 2006). A fresh HAM graft preserves its viability for 21 days following application in the pelvic cavity (Trelford Sauder 1977). In addition to serving as an anatomical barrier, HAM may promote the regeneration of epithelium by acting as a basement membrane substrate; HAM may also facilitate migration of epithelial cells, reinforce adhesion of the basal epithelium, promote epithelial cell differentiation (Meller 1999), and prevent cellular apoptosis (Hori 2006). Human amniotic epithelial cells produce factors or create a microenvironment for effective tissue repair and endometrial regeneration, possibly by stimulating endogenous stem cells (Padykula 1991).
Why it is important to do this review
At present, whether anti‐adhesion therapies after operative hysteroscopy might be beneficial for the outcome of pregnancy or live birth is unknown, and there are no relevant clinical guidelines. Providing a summary and critical appraisal of existing evidence on the effectiveness of different anti‐adhesion treatments in subfertile women after operative hysteroscopy is the main objective of this Cochrane Review. Moreover, little is known about the relative contributions of different anti‐adhesion strategies towards increasing reproductive benefit in women wishing to conceive following operative hysteroscopy; performing this head‐to‐head comparison of alternative anti‐adhesion interventions is a secondary objective of the present review.
Adhesions may cause infertility, abdominal pain or bowel obstruction. The healthcare burden associated with these three clinical problems is substantial (DeCherney 1997; diZerega 1994; Renier 2005). The total cost of adhesion‐related morbidity for the US health care system exceeds USD1 billion annually (Baakdah 2005). One trial in the domain of gynaecological oncology evaluated the cost‐effectiveness of an HA‐CMC anti‐adhesion barrier versus routine care, during which no adhesion prevention measures were taken, by applying a decision analysis model in the setting of women undergoing radical hysterectomy and pelvic lymphadenectomy for stage IB cervical cancer (Bristow 2007). Study authors concluded that given a conservative set of clinical and economic assumptions, an adhesion prevention strategy utilising an HA‐CMC barrier in women undergoing radical hysterectomy for stage IB cervical cancer might be cost‐effective from the perspective of society and from the view of a third‐party payer. To the best of our knowledge, no cost‐effectiveness studies have explored adhesion prevention after operative hysteroscopy in an infertile population; evidence retrieved through the present research could serve as the basis for economical studies of different anti‐adhesion treatments. This is another secondary objective of the present review.
Infertility, defined as the inability to conceive after a defined period of unprotected intercourse, is an often neglected aspect of reproductive health worldwide. Official ways of providing assistance for reproductive health care and family planning are few worldwide, despite an increasing absolute number of couples affected by infertility from 42.0 million in 1990 to 48.5 million in 2010 (Mascarenhas 2012). Reproductive health has long been recognised by the World Health Organization (WHO) as a priority global health topic (WHO: Reproductive Health).
Objectives
To assess the effectiveness of anti‐adhesion therapies versus placebo, no treatment or any other anti‐adhesion therapy following operative hysteroscopy for treatment of female subfertility.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished parallel‐group RCTs were eligible for inclusion. We excluded non‐randomised studies (e.g. studies with evidence of inadequate sequence generation such as alternate days, participant numbers), as they are associated with high risk of bias. We planned to include cross‐over trials if individually randomly assigned women were the unit of analysis; we aimed to include data from the first phase only in the meta‐analyses, as the cross‐over trial is not a valid study design in the context of subfertility.
Types of participants
Women of reproductive age undergoing operative hysteroscopy for subfertility associated with suspected or unsuspected intrauterine pathology before spontaneous conception or any subfertility treatment. Studies in which at least a proportion of women were undergoing operative hysteroscopy for subfertility were eligible. Studies excluding women wishing to conceive were not eligible.
Types of interventions
We included the following randomly assigned comparisons.
-
Anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy.
-
Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy.
Types of outcome measures
Primary outcomes
-
Live birth.
-
-
Live birth was defined as the delivery of at least one live foetus after 20 weeks of gestation that resulted in at least one live baby; we counted the delivery of singleton, twin or multiple pregnancies as one live birth.
-
In studies that failed to report live birth, we used the following measures as primary effectiveness outcomes:
-
-
Ongoing pregnancy, defined as pregnancy surpassing the first trimester or 12 weeks of pregnancy and was used as a surrogate outcome for live birth.
-
Term delivery, defined as birth at any time between three weeks before and two weeks after the expected date of delivery (37 to 42 weeks of gestation) was also used as a surrogate outcome for live birth.
-
Secondary outcomes
-
Clinical pregnancy, defined as pregnancy diagnosed by ultrasonographic visualisation of one or more gestational sacs or definitive clinical signs of pregnancy; this included ectopic pregnancy. We counted multiple gestational sacs as one clinical pregnancy.
-
Miscarriage, defined as spontaneous loss of a clinical pregnancy that occurred before 20 completed weeks of gestation (18 weeks' postfertilisation) or, if gestational age was unknown, loss of an embryo or foetus of bodyweight less than 400 g.
-
Presence of IUAs at second‐look hysteroscopy.
-
Mean adhesion scores at second‐look hysteroscopy.
-
Severity of adhesions at second‐look hysteroscopy.
We did not exclude studies on the basis of their reported outcome measures. We reviewed all potentially eligible studies that could have measured the outcomes of interest; we aimed to report any lack of data for the key outcomes in the final review.
We adhered as much as possible to terminology of the International Committee for Monitoring Assisted Reproductive Technology (ICMART) (ICMART) for key reproductive outcomes (live birth, pregnancy and miscarriage) (Zegers‐Hochschild 2009); we contacted primary study authors for clarification in cases of unclear definitions. We reported discrepancies or uncertainties in the final review.
At present, seven classification systems are reported for scoring the extent or severity of IUAs. None of these systems has been validated or universally accepted (Deans 2010). Therefore, we avoided pooling data from studies using different scoring systems, and we asked for clarification from primary study authors, when there was any uncertainty on the classification system used in the primary research.
According to a prospective cohort study, the duration of endometrial wound healing may differ according to the type of pathology; study authors concluded that recovery of the endometrium may vary from one month (after hysteroscopic removal of polyps) to three months (following hysteroscopic myomectomy) (Yang 2013). We planned to pool studies when assessment of IUAs by second‐look hysteroscopy was done between four and 12 weeks after operative hysteroscopy.
Search methods for identification of studies
We searched for all published and unpublished RCTs of anti‐adhesion therapies following operative hysteroscopy in subfertile women, with no language restrictions and in consultation with the Information Specialist of the Cochrane Gynaecology and Fertility Group (CGFG).
Electronic searches
We searched the following electronic databases, trial registers and websites using the search strategies provided in the appropriate appendices: the CGFG Specialised Register (6 June 2017) (Appendix 1), the Cochrane Central Register of Studies Online (CENTRAL) (2017, Issue 6) (Appendix 2), MEDLINE using PubMed (1950 to 6 June 2017) (Appendix 3) and Embase using Embase.com (1974 to 6 June 2017) (Appendix 4).
The search strategy combined both index and free‐text terms.
Our MEDLINE search included the Cochrane highly sensitive search strategy for identifying randomised trials as it appears in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Our Embase search included the trial filter developed by the Scottish Intercollegiate Guidelines Network (SIGN).
Electronic sources of trials included the following.
-
CENTRAL.
-
Cochrane Database of Systematic Reviews (CDSR) (2017, Issue 6).
-
Database of Abstracts of Reviews of Effectiveness (DARE) and the Health Technology Assessment Database (HTA Database) through the Centre for Reviews and Dissemination (www.crd.york.ac.uk) (from inception to 6 June 2017).
-
National Guideline Clearinghouse (www.guideline.gov/) for evidence‐based guidelines (from inception to 6 June 2017).
-
Citations, conference abstracts and proceedings in the Institute for Scientific Information (ISI) Web of Science (WOS) core collection, Biosis Previews and Biosis Citation Index through WOS (wcs.webofknowledge.com.kuleuven.ezproxy.kuleuven.be) (from inception to 6 June 2017) (Appendix 5) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (web.b.ebscohost.com.kuleuven.ezproxy.kuleuven.be) (from inception to 6 June 2017) (Appendix 6) through EBSCOhost, available at the Biomedical Library Gasthuisberg of the Catholic University of Leuven.
-
Trial registers for ongoing and registered trials: ISRCTN Registry (www.isrctn.com/) and WHO International Clinical Trials Registry Platform search portal (apps.who.int/trialsearch/) (from inception to 6 June 2017).
-
Latin American Caribbean Health Sciences Literature (LILACS) database, which is a source of trials from the Spanish and Portuguese speaking countries (lilacs.bvsalud.org/en/) (from inception to 6 June 2017).
-
European grey literature through the Open Grey database (www.opengrey.eu/) (from inception to 6 June 2017).
-
General search engines Turning Research Into Practice (TRIP) database (www.tripdatabase.com/), Google Scholar (scholar.google.com/) and Scopus, available at the Biomedical Library Gasthuisberg of the KU Leuven‐ University of Leuven, Leuven, Belgium (www‐scopus‐com.kuleuven.ezproxy.kuleuven.be) (from inception to 6 June 2017).
Searching other resources
Two review authors (JB and SJC) examined reference lists of articles retrieved by the search and contacted experts in the field to request additional data. We contacted the first or corresponding authors of included studies to ascertain whether they were aware of any ongoing or unpublished trials. We handsearched the Journal of Minimally Invasive Gynecology (from inception to 6 June 2017) to look for conference abstracts that were not covered in the CGFG Specialised Register, in liaison with the Information Specialist of the CGFG. We also searched reference lists of appropriate papers. We documented the search process in a PRISMA flow diagram in the final review.
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts retrieved by the search, we retrieved the full texts of all potentially eligible studies. Two review authors (JB and SJC) independently examined these full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We corresponded with study investigators, as required, to clarify study eligibility. We resolved disagreements as to study eligibility by discussion or by consultation with a third review author (BWM). We classified the study as 'awaiting classification' if disagreements between review authors were not resolved, and we reported disagreements in the final review.
Data extraction and management
At least two review authors (JB for all included studies and HT/SW/SJC each for some studies) independently extracted data from all eligible studies using a data extraction form designed and pilot‐tested by the review authors. We resolved disagreements by discussion or by consultation with a third review author (BWM). Extracted data included study characteristics and outcome data (Appendix 7). When studies had multiple publications, we collated multiple reports on the same study, so that each study, rather than each report, was the unit of interest in the review, and we assigned such studies a single study identity with multiple references. We used the main trial report as the reference and derived additional details from secondary papers. We corresponded with study investigators to request further data on methods and results, as required. We included studies irrespective of whether outcomes were reported in a 'usable' way. In multiarm studies, we excluded data from arms that did not meet the eligibility criteria.
Assessment of risk of bias in included studies
At least two review authors (JB for all included studies and HT/SW/SJC each for some studies) independently assessed included studies for risk of bias using the Cochrane 'Risk of bias' tool (Higgins 2011). We assessed the following seven items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting and other potential sources of bias. We resolved disagreements by discussion or by consultation with a third review author (BWM). We fully described all judgements and presented our conclusions in the 'Risk of bias' table, which we incorporated into our interpretation of review findings by conducting sensitivity analyses.
Selective reporting is a type of reporting bias that affects the internal validity of an individual study (see Table 10.1A in the Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011). This term refers to selective reporting of some outcomes (e.g. positive outcomes) and failure to report others (e.g. adverse events). We took care to search for within‐trial selective reporting, such as trials failing to report obvious outcomes, or failing to report them in insufficient detail to allow inclusion. We looked for published protocols and compared outcomes between the protocol and the final published study. When identified studies did not report the primary outcome of live birth but did report interim outcomes such as pregnancy, we planned to undertake informal assessment as to whether the interim values (e.g. pregnancy rates) were similar to those reported in studies that also reported live births.
If any outcomes were defined in the protocol or the study report, and data were insufficient to allow inclusion, we sought to mention this lack of data along with the suggestion that additional clinical trials need to be conducted to clarify these knowledge gaps.
Measures of treatment effect
For dichotomous data (e.g. live births, clinical pregnancy rates), we used the numbers of events in control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (ORs). We treated ordinal data (e.g. adhesion scores) as continuous data. For continuous data, if all studies reported exactly the same outcomes, we calculated mean differences (MDs) between treatment groups. If similar outcomes were reported on different scoring scales, we did not calculate standardised mean differences (SMDs) because the seven different adhesion score classifications had not been validated. We aimed to reverse the direction of effect of individual studies, if required, to ensure consistency across trials. We presented 95% confidence intervals (CIs) for all outcomes and contacted corresponding or first authors of all included trials that reported data in a form that was not suitable for meta‐analysis. We reported data from reports that did not present additional data that could be analysed under 'other data.' When data were not available for calculating ORs or MDs, we planned to utilise the most detailed numerical data provided that might facilitate similar analyses of included studies (e.g. test statistics, P values). We compared the magnitude and direction of effect reported by studies with how they were presented in the review, while taking account of legitimate differences.
Unit of analysis issues
We performed the primary analysis per woman randomly assigned; however, we included per‐pregnancy data for one secondary outcome (miscarriage). If studies had reported only per‐cycle data, we would have contacted primary study authors to request per‐woman data. If these had been available, we would have briefly summarised per‐cycle data in an additional table without performing a meta‐analysis. We would have counted multiple live births (e.g. twins, triplets) as one live birth event only. We would have included only first‐phase data from cross‐over trials if relevant cross‐over trials had been found eligible.
Dealing with missing data
We analysed data on an intention‐to‐treat (ITT) basis; if data had been available, we would have attempted to obtain all missing data from the original researchers. If this had been impossible, we would have undertaken imputation of individual values for the beneficial primary outcome only (live birth); we would have assumed that live births did not occur in women without a reported outcome. For all other outcomes, we would have analysed only available data. We would have subjected any imputation undertaken for missing data for the primary outcome to sensitivity analysis. (See Sensitivity analysis.) If studies had reported sufficient detail to calculate MDs but had not information on associated standard deviations (SDs), we would have assumed that the outcome had an SD equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by measuring the I² statistic. We took an I² statistic greater than 50% to indicate substantial heterogeneity (Higgins 2003).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we minimised their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If we had included 10 or more studies in an analysis, we would have used a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
One review author (JB) entered the data and carried out all statistical analyses of the data in Review Manager 5 (RevMan 2014). When studies were sufficiently similar and substantial statistical heterogeneity could be confidently ruled out, we combined data derived from primary studies in a meta‐analysis using Review Manager 5 (RevMan 2014). We have used summary Mantel‐Haenszel ORs and a fixed‐effect model for the following comparisons.
-
Anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy.
-
Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy.
We considered outcomes of 'live birth' and 'clinical pregnancy' as positive outcomes of effectiveness and, as a consequence, higher numbers of these two outcomes as a benefit. We considered 'miscarriage,' 'presence of IUAs,' 'mean adhesion scores' or 'severity of adhesions' at second‐look hysteroscopy as negative outcomes of safety and interpreted higher numbers as harmful. An increase in the odds of a particular outcome that was beneficial (e.g. live birth) or detrimental (e.g. IUAs) was displayed graphically in the meta‐analyses to the right of the centre line, and a decrease in the odds of an outcome to the left of the centre line.
We defined analyses that were comprehensive and mutually exclusive, so that all eligible study results could be slotted into one stratum for each comparison, and that trials within the same stratum could be sensibly pooled. Stratification was not a requirement, but it allowed consideration of effects within each stratum as well as, or instead of, an overall estimate for the comparison. If we had retrieved no RCTs for some comparisons, we would have indicated their absence in the review to reveal knowledge gaps for which further research is needed. We would have presented a narrative overview if meta‐analysis had not been appropriate.
Subgroup analysis and investigation of heterogeneity
Where data were available, we conducted subgroup analyses to identify separate evidence within the following subgroup:
-
studies with HA gel versus studies with another type of gel for the primary outcome and the presence of IUAs at second‐look hysteroscopy.
We interpreted the findings of subgroup analyses cautiously, even when sufficient data were available; subgroup analysis is by itself observational in nature and the interpretation of formal statistical tests to detect differences between subgroups is problematic.
If we detected substantial heterogeneity, we explored possible explanations in the subgroup analyses (e.g. differing populations) or sensitivity analyses (e.g. differing risk of bias), or both. We took any statistical heterogeneity into account when interpreting the results.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcome to determine whether conclusions were robust to arbitrary decisions made regarding eligibility and analysis of studies. These analyses included consideration of whether review conclusions would have differed if:
-
only studies were included reporting the primary outcome (live birth) versus all studies reporting live birth or a surrogate outcome;
-
eligibility had been restricted to studies without high risk of bias;
-
study used only a random‐effects model;
-
alternative imputation strategies had been implemented;
-
summary effect measure had been risk ratio (RR) rather than OR.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared two 'Summary of findings' tables using GRADEpro GDT and Cochrane methods (Higgins 2011). These 'Summary of findings' tables evaluated the overall quality of the body of evidence for the two most important review outcomes (live birth as the primary outcome of effectiveness and presence of IUAs at second‐look hysteroscopy as the primary outcome of safety) for the two main review comparisons (i.e. anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy; anti‐adhesion therapy following operative hysteroscopy versus another anti‐adhesion therapy). We restricted the content of the 'Summary of findings' tables to these two main review outcomes in the interest of readability of the review. We presented the evidence for all other secondary outcomes in the text of the review. We assessed the quality of the evidence using GRADE criteria, including risk of bias, consistency of effect, imprecision, indirectness and publication bias. Two review authors independently made judgements about evidence quality (high, moderate, low or very low), with disagreements resolved by discussion. Judgements were justified, documented and incorporated into reporting of results for each outcome.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies tables.
Results of the search
Our original search retrieved 11 studies which were included in the original published version of this review in 2015. In the updated search in 2017, we identified 342 records by searching the following databases: CGFG Specialised Register (11 records), CENTRAL (14), MEDLINE (21), Embase (32), WoS (11), CINAHL (1), CRD (26), National Guideline Clearinghouse (3), ISRCTN Register of Controlled Trials (13), WHO ICTRP (22), LILACS (75), Open Grey (107) and Scopus (6). We retrieved 629 additional records through other sources: handsearch of the Journal of Minimally Invasive Gynecology (8) and handsearch of related articles on included studies (621).
After combining 342 records identified through electronic searches with 629 additional records obtained by searching other sources, we screened 971 records for duplicates using specialised software (www.myendnoteweb.com). We removed 899 duplicates. We screened 72 records for titles and abstracts: we excluded 50 records for being obviously irrelevant and six records for being duplicates. We assessed 16 full‐text articles for eligibility: we excluded 11 full‐text articles for various reasons. We identified five potentially eligible studies for the updated search. We included 16 studies in the present Cochrane Review for quantitative synthesis and critical appraisal (Characteristics of included studies table); two trials are ongoing (Characteristics of ongoing studies table) and one trial is awaiting classification (Characteristics of studies awaiting classification table).
See the PRISMA flow chart for a summary of studies retrieved by our search, including both our original search (from inception to 1 March 2015) and an updated search (from 1 March 2015 until 1 June 2017) (Figure 1).

Study flow diagram: summary of searches since 2015. PICO: population, intervention, comparator, outcome; RCT: randomised controlled trial.
Included studies
Study design and setting
We included 16 parallel‐design RCTs: 15 studies used two comparison groups (Abu Rafea 2013; Acunzo 2003; Dabir‐Ashrafi 1996; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Fuchs 2014; Gan 2017; Guida 2004; Lin 2015a; Lin 2015b; Roy 2014; Vercellini 1989; Wang 2016; Xiao 2015), and one study used three comparison groups (Amer 2010). All but one (Xiao 2015) were single‐centre studies: six from Italy (Acunzo 2003; De Iaco 2003; Guida 2004; Di Spiezio Sardo 2011; Guida 2004; Vercellini 1989), four from China (Gan 2017; Lin 2015b; Wang 2016; Xiao 2015), one from Egypt (Amer 2010), one from Saudi Arabia (Abu Rafea 2013), one from Iran (Dabir‐Ashrafi 1996), one from India (Roy 2014), one from Taiwan (Lin 2015a), and one from South Korea (Do 2005).
Funding sources
See Characteristics of included studies table.
In six of 16 studies, primary authors stated that they had obtained no external funding (Amer 2010; De Iaco 2003; Di Spiezio Sardo 2011; Fuchs 2014; Guida 2004; Roy 2014). In seven of 16 studies, reporting of external funding was unclear; we failed to obtain clarification from corresponding authors of the primary study report despite several queries (Abu Rafea 2013; Acunzo 2003; Dabir‐Ashrafi 1996; Do 2005; Lin 2015a; Vercellini 1989; Xiao 2015). Three studies reported external funding by the Chinese Government (Gan 2017; Lin 2015b; Wang 2016).
Potential conflicts of interest
In nine of 16 studies, primary authors declared no potential conflicts of interest (Amer 2010; De Iaco 2003; Di Spiezio Sardo 2011; Fuchs 2014; Gan 2017; Guida 2004; Lin 2015b; Roy 2014; Wang 2016). In seven of 16 studies, reporting of potential conflicts of interest was unclear despite several queries to the corresponding authors (Abu Rafea 2013; Acunzo 2003; Dabir‐Ashrafi 1996; Do 2005; Lin 2015a; Vercellini 1989; Xiao 2015).
Participants
See Characteristics of included studies table for a detailed description of the main participant characteristics.
Abu Rafea 2013 randomly assigned 28 women diagnosed with an intrauterine septum with from infertility or adverse pregnancy outcomes, or both.
Acunzo 2003 included 92 women with irregular menses and IUAs treated by hysteroscopy.
Amer 2010 included 45 women with severe IUAs, all with subfertility, bound to undergo operative hysteroscopy.
Dabir‐Ashrafi 1996 randomly assigned 46 participants with subfertility and recurrent miscarriage with a fundal defect on hysterosalpingography (HSG).
De Iaco 2003 included 60 women bound to undergo endometrial ablation or hysteroscopic removal of submucosal fibroids, endometrial polyps, septate uterus or intrauterine synechiae.
Di Spiezio Sardo 2011 included 110 women diagnosed at clinic diagnostic hysteroscopy with single or multiple lesions suitable for surgical treatment or with resistant dysfunctional uterine bleeding requiring endometrial ablation.
Do 2005 included 64 women who underwent intrauterine surgery.
Fuchs 2014 included 52 women of confirmed fertility who underwent hysteroscopic surgery because of suspected retained products of conception.
Gan 2017 included 88 women with infertility or at least one spontaneous miscarriage and severe IUAs following hysteroscopic adhesiolysis.
Guida 2004 included 138 women with surgically treatable single lesions (fibroids, polyps and uterine septa, subgroups I to III) at diagnostic hysteroscopy.
Lin 2015a included 62 women undergoing hysteroscopy.
Lin 2015b included 201 women with moderate‐to‐severe IUAs (no prioritisation of the outcomes reported. or greater) after hysteroscopic adhesiolysis.
Roy 2014 included 90 women with septate uterus with a history of miscarriage or subfertility.
Vercellini 1989 included 20 women with two or more unexplained spontaneous miscarriages with a uterine septum.
Wang 2016 included 57 women following hysteroscopic adhesiolysis for severe IUAs.
Xiao 2015 included 120 women that underwent hysteroscopic adhesiolysis for moderate‐to‐severe IUAs.
The proportion of subfertile women was as follows:
-
0% (two studies; 72 women; Fuchs 2014; Vercellini 1989);
-
less than 50% (six studies; 567 women; Abu Rafea 2013; Acunzo 2003; Dabir‐Ashrafi 1996; Di Spiezio Sardo 2011; Lin 2015b; Roy 2014);
-
unknown (six studies; 532 women; De Iaco 2003; Do 2005; Gan 2017; Guida 2004; Lin 2015a; Xiao 2015).
Interventions and comparators
See Characteristics of included studies table.
1. Any intervention versus no treatment or placebo
-
Device versus no treatment (Abu Rafea 2013; Lin 2015a).
-
Hormonal treatment versus no treatment or placebo (Dabir‐Ashrafi 1996; Roy 2014).
-
Device combined with hormonal treatment versus no treatment (Vercellini 1989).
-
Barrier gel versus no treatment (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004).
2. Any intervention versus any other intervention
-
Device with graft versus device without graft (Amer 2010; Gan 2017; Wang 2016).
-
One type of device versus another type of device (Lin 2015b).
-
Gel combined with hormonal treatment and antibiotics versus hormonal treatment combined with antibiotics (Fuchs 2014).
-
Device combined with gel versus device (Xiao 2015).
In the previous version of this review Amer 2010 and Fuchs 2014 were erroneously classified under the comparison "Any therapy versus no treatment or placebo".
Outcomes
See Characteristics of included studies table.
-
Primary outcome
-
Secondary outcomes.
-
-
Clinical pregnancy (Abu Rafea 2013; Amer 2010; Fuchs 2014). Three studies reported pregnancy, not further defined which we used as a surrogate outcome for clinical pregnancy (Gan 2017; Roy 2014; Wang 2016).
-
Miscarriage (Abu Rafea 2013; Amer 2010; Gan 2017; Roy 2014; Wang 2016).
-
Presence of IUAs at second‐look hysteroscopy (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Fuchs 2014; Gan 2017; Guida 2004; Lin 2015a; Lin 2015b; Roy 2014; Vercellini 1989; Wang 2016; Xiao 2015).
-
Adhesion scores of IUAs at second‐look hysteroscopy (Acunzo 2003; Amer 2010; Gan 2017; Guida 2004; Lin 2015b; Wang 2016; Xiao 2015).
-
Severity of IUAs at second‐look hysteroscopy (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Fuchs 2014; Guida 2004; Roy 2014; Xiao 2015).
-
While several studies measured outcomes other than the key outcomes prespecified in our Cochrane Review's protocol (Amer 2010; Di Spiezio Sardo 2011; Do 2005; Gan 2017; Lin 2015b; Roy 2014; Vercellini 1989; Wang 2016; Xiao 2015), two studies reported none of the outcomes relevant for the quantitative synthesis and critical appraisal (Dabir‐Ashrafi 1996; Lin 2015a).
Excluded studies
See Characteristics of excluded studies table.
We excluded 15 potentially eligible studies for the following reasons.
-
Five were observational studies (Chen 2017; Hu 2014a; Hu 2014b; Liu 2016; NCT02328742).
-
Two were quasi‐randomised studies (Pabuccu 2008; Tonguc 2010).
-
Seven did not answer the PICO (population, intervention, comparator, outcome) research questions of this Cochrane Review (Bednarek 2011; Cheong 2016; Johns 2001; Kurtz 2002; NTR3120; Tsapanos 2002; Yaşar 2004).
-
One study explicitly excluded subfertile women from participation in the trial (Kim 2012).
Risk of bias in included studies
See the 'Risk of bias' summary for the review authors' judgements about each risk of bias item in the included study (Figure 2).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
See the 'Risk of bias' graph for the review authors' judgements about each risk of bias item presented as percentages across the 16 included studies (Figure 3).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged 14 of 16 studies at low risk of selection bias in relation to random sequence generation because all used computer‐generated randomisation lists (Abu Rafea 2013; Acunzo 2003; Amer 2010; De Iaco 2003; Di Spiezio Sardo 2011; Fuchs 2014; Gan 2017; Guida 2004; Lin 2015a; Lin 2015b; Roy 2014; Vercellini 1989; Wang 2016; Xiao 2015). We judged two studies at unclear risk of selection bias in relation to random sequence generation: the study reports claim that both trials were RCTs but did not describe the method of randomisation (Dabir‐Ashrafi 1996; Do 2005). We obtained no clarification from the authors of the primary studies despite several mailings. None of the included studies were at high risk of selection bias in relation to random sequence generation.
We judged seven of 16 studies at low risk of selection bias in relation to allocation concealment because investigators used sequentially numbered opaque sealed envelopes containing the allocated treatment (Amer 2010; Di Spiezio Sardo 2011; Fuchs 2014; Guida 2004; Lin 2015a; Vercellini 1989), or a code referring to the allocated treatment (Roy 2014). We judged eight of 16 studies at unclear risk of selection bias in relation to allocation concealment because study authors did not describe the method of allocation concealment and did not provide clarification as requested (Acunzo 2003; Dabir‐Ashrafi 1996; Do 2005; Lin 2015b; Wang 2016; Xiao 2015), or provided insufficient information (De Iaco 2003; Gan 2017). We judged one study at high risk of selection bias in relation to allocation concealment: randomisation was based on a computer‐generated list of numbers, but study authors reported that the allocation was unconcealed (Abu Rafea 2013).
Blinding
Performance bias
Five of 16 studies reported live births (or ongoing pregnancy or term delivery as surrogate outcomes for live birth) (Abu Rafea 2013; Amer 2010; Gan 2017; Roy 2014; Wang 2016), and six of 16 studies reported clinical pregnancy (or pregnancy not further specified a surrogate outcome) (Abu Rafea 2013; Amer 2010; Fuchs 2014; Gan 2017; Roy 2014; Wang 2016). We judged all six studies at low risk of performance bias in relation to blinding of participants and personnel because live birth and clinical pregnancy are unequivocal outcomes (Abu Rafea 2013; Amer 2010; Fuchs 2014; Gan 2017; Roy 2014; Wang 2016). We judged the remaining 10 studies at low risk as none reported live birth or clinical pregnancy (or a surrogate for these predefined outcomes). See Figure 3.
We judged only one of 16 studies at low risk of performance bias in relation to blinding of participants and personnel for the key outcome of adhesions as placebo pills containing folic acid were used for blinding participants and personnel (Roy 2014). We judged three studies at unclear risk of performance bias in relation to blinding of participants and personnel for the outcome of adhesions because the method of blinding of participants and personnel was not described (Dabir‐Ashrafi 1996; Gan 2017), or was not sufficiently clarified after contact with the study authors (De Iaco 2003). We judged 12 of 16 studies at high risk of performance bias in relation to blinding of participants and personnel for the outcome of presence of IUAs, as personnel (Amer 2010; Di Spiezio Sardo 2011; Do 2005; Fuchs 2014; Guida 2004; Lin 2015b; Wang 2016; Xiao 2015), or both participants and personnel (Abu Rafea 2013; Acunzo 2003; Lin 2015a; Vercellini 1989), were not blinded.
Detection bias
Five of 16 studies reported live births (or ongoing pregnancy or term delivery as surrogate outcomes for live birth) (Abu Rafea 2013; Amer 2010; Gan 2017; Roy 2014; Wang 2016), and six of 16 studies reported clinical pregnancy (or pregnancy not further specified a surrogate outcome) (Abu Rafea 2013; Amer 2010; Fuchs 2014; Gan 2017; Roy 2014; Wang 2016). We judged all six studies at low risk of detection bias in relation to blinding of outcome assessors because live birth and clinical pregnancy are unequivocal outcomes (Abu Rafea 2013; Amer 2010; Fuchs 2014; Gan 2017; Roy 2014; Wang 2016). We judged the remaining 10 studies at low risk as none reported live birth or clinical pregnancy (or a surrogate for these predefined outcomes). See Figure 3.
We judged nine of 16 studies at low risk of detection bias for the key outcome of adhesions because outcome assessors were independent observers blinded to treatment allocation (Amer 2010; De Iaco 2003; Di Spiezio Sardo 2011; Fuchs 2014; Gan 2017; Guida 2004; Lin 2015b; Roy 2014; Xiao 2015). We judged five of 16 studies to be at unclear risk of detection bias in relation to blinding of outcome assessors for the key outcome of adhesion formation because the method of blinding was not reported and clarification could not be obtained from the authors of the primary study (Abu Rafea 2013; Acunzo 2003; Do 2005; Wang 2016). We judged one study at unclear risk of performance and detection bias in relation to blinding of participants, personnel and outcome assessors for a subjective outcome not prespecified in this Cochrane Review: the method was unclear, and we obtained no clarification from the authors (Dabir‐Ashrafi 1996). Two studies were at high risk of detection bias in relation to blinding of outcome assessors for the outcome of adhesion formation: the outcome assessors in these two trials were not blinded (Lin 2015a; Vercellini 1989).
Incomplete outcome data
We judged 12 of 16 studies at low risk of attrition bias because all participants with relevant outcome data were included in the final data analysis (Abu Rafea 2013; Di Spiezio Sardo 2011; Vercellini 1989; Wang 2016), or loss to follow‐up was small (less than 10%) without imbalance across comparison groups for numbers or reasons for loss to follow‐up (Acunzo 2003; Amer 2010; Do 2005; Gan 2017; Guida 2004; Lin 2015a; Roy 2014; Xiao 2015). We judged one study at unclear risk of attrition bias because four of 50 (8%) participants were excluded and distribution among comparison groups was not reported: we obtained no clarification from the study authors (Dabir‐Ashrafi 1996). We judged three of 16 studies at high risk of attrition bias (De Iaco 2003; Fuchs 2014; Lin 2015b). In one study, loss to follow‐up after randomisation involved 20/60 included participants (De Iaco 2003). The second study excluded five of 26 participants in the intervention group and six of 26 participants in the control group after randomisation from the analysis (11/52 or 21%): reasons for discontinuation of the trial were not clarified (Fuchs 2014). Loss to follow‐up in the third trial was 19% (Lin 2015b).
Selective reporting
We judged 12 of 16 studies at low risk of reporting bias in relation to selective outcome reporting (Abu Rafea 2013; Acunzo 2003; Amer 2010; Dabir‐Ashrafi 1996; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004; Roy 2014; Vercellini 1989; Wang 2016; Xiao 2015). We judged one study at unclear risk of selective outcome reporting because we noted discrepancies between outcomes prespecified in the registered study protocol NCT01167296 and results reported in the abstract and in the results section (Lin 2015a). We judged three of 16 studies at high risk of reporting bias in relation to selective outcome reporting (Fuchs 2014; Gan 2017; Lin 2015b). One study failed to report data for the primary outcome of live birth despite a study duration of 27 months (Fuchs 2014). In the study protocol of Gan 2017, registered as NCT02496052, all secondary outcomes mentioned in the final study report were not predefined. A third study failed to report data for pregnancy rates in the published report of the study, although pregnancy was prespecified as a main outcome in the study protocol ISRCTN69690272 (Lin 2015b).
Other potential sources of bias
We judged seven of 16 studies at low risk of other potential sources of bias (Acunzo 2003; Dabir‐Ashrafi 1996; Di Spiezio Sardo 2011; Guida 2004; Lin 2015b; Roy 2014; Xiao 2015). We judged two of 16 studies to be at unclear risk of other potential sources of bias (Vercellini 1989; Wang 2016). Vercellini 1989 did not report the baseline characteristics of both comparison groups. In two women in the intervention group, the IUD was removed early and in one woman of the control group had a Foley balloon catheter inserted for persistent heavy bleeding. These three women should have been excluded from the analysis because these interventions could have affected the outcomes. We did not do sensitivity analyses comparing all data versus data excluding these three participants: the study was completed almost 30 years ago and it was no longer possible to retrieve data for individual participant data analysis (IPD). Wang 2016 offered cotreatment with artificial fertility treatment but it was unclear if comparable proportions of women received similar treatments in both comparison groups. We judged seven of 16 studies at high risk of other potential sources of bias (Abu Rafea 2013; Amer 2010; De Iaco 2003; Do 2005; Fuchs 2014; Gan 2017; Lin 2015a). One study excluded four of 28 participants (14%) from the final analysis after randomisation because they were not trying to conceive (Abu Rafea 2013). The reason for this postrandomisation exclusion was a lack of explicit inclusion and exclusion criteria. Analysis of study results showed that poor inclusion and exclusion criteria may lead to increased risk of bias. Moreover, researchers measured outcomes in this study over 12 to 18 months: this could have affected final pregnancy results if imbalance occurred across comparison groups for the time points at which this key outcome was measured. Finally, although there were no evident statistically significant differences in mean age of participants in both comparison groups, the MD was three years, and more women of a younger age were included in the intervention group. This baseline imbalance between comparison groups is clinically relevant, irrespective of P values. Amer 2010 provided evidence of baseline imbalance among participant characteristics in relation to differences in the prevalence of prior caesarean section as a cause of IUAs. Moreover, investigators provided cotreatment with laparoscopy and in vitro fertilisation (IVF) for some women but failed to reported data on the distribution in numbers among comparison groups. De Iaco 2003 recalculated data for the outcomes of presence of IUAs at second look and severity of IUAs and reported no statistically significant differences between comparison groups, although study authors concluded that the use of anti‐adhesion barrier gel improved outcomes of hysteroscopic surgery. This conclusion was not based on the available evidence. Investigators did not report baseline characteristics of both comparison groups. Do 2005 is at high risk of selection bias because there were clinically relevant differences in baseline characteristics between both comparison groups for age, parity and the number of miscarriages. Moreover, it was unclear if micro‐hysteroscopy or transvaginal ultrasound was used for outcome assessment of IUAs. Therefore, it is unclear if this study was at risk for information bias. Fuchs 2014 at follow‐up hysteroscopy offered cotreatment with hysteroscopic adhesiolysis to women with AFS grade II or III IUAs. They offered cotreatment to three of 20 (14%) women in the control group and to one of 21 (4%) women in the intervention group. This may have affected the magnitude and direction of the treatment effect. For Gan 2017, we had some concerns for performance bias related to cotreatments with IVF and laparoscopy whose proportions in both treatment arms were not reported. There was no fixed endpoint for measuring the secondary outcomes: the total duration of follow‐up via direct contact or telephone every three months lasted between six and 12 months. The longer the follow‐up period, the higher the cumulative pregnancy rate. Therefore, we judged this study at high risk for detection bias. We have some concern for imbalance in baseline characteristics between the two comparison groups of Lin 2015a: the number of participants with IUAs in the intervention group (17/31 women) was nearly doubled compared to the control group (10/31 women).
Effects of interventions
See: Summary of findings for the main comparison Any anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy; Summary of findings 2 Any anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy
1. Anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy
We identified 10 studies any intervention versus no treatment or placebo (Abu Rafea 2013; Acunzo 2003; Dabir‐Ashrafi 1996; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004; Lin 2015a; Roy 2014; Vercellini 1989).
1.1. Live birth
No study reported live birth, but two studies reported outcomes that were used as surrogate outcomes for live birth (term delivery or ongoing pregnancy) (Abu Rafea 2013; Roy 2014). Based on a pooling of these two small studies, there was insufficient evidence to determine whether there was a difference in surrogate outcomes for live birth rate between the use of any intervention compared to no treatment or placebo (OR 0.94, 95% CI 0.42 to 2.12; 107 women; 2 studies; I² = 0%; very‐low‐quality evidence; Analysis 1.1). We stratified data according to device versus no treatment or placebo and hormonal treatment versus no treatment or placebo.
1.1.1. Device versus no treatment and hormonal treatment versus placebo or no treatment
One study reported data for the outcome of term delivery at 12 to 18 months (Abu Rafea 2013). There was insufficient evidence to determine whether there was a difference in term delivery rate at 12 to 18 months between the use of an intrauterine Foley catheter balloon and no treatment following hysteroscopic septum division (OR 1.00, 95% CI 0.18 to 5.46; 24 women; 1 study; Analysis 1.1).
1.1.2. Hormonal treatment versus placebo or no treatment
Roy 2014 reported data on ongoing pregnancy. We used these data as a surrogate for live birth. It was unclear whether there was a difference between treatment with oestradiol valerate 2 mg daily versus folic acid 5 mg as a placebo for 30 days following hysteroscopic septum division (OR 0.93, 95% CI 0.37 to 2.33; 83 women; 1 study; Analysis 1.1).
Sensitivity analysis
We conducted a sensitivity analysis for Analysis 1.1. The choice to include two studies regardless of study quality (Abu Rafea 2013; Roy 2014), or to include only one study at low risk for selection bias related to random sequence generation and allocation concealment (Roy 2014), did not affect the direction/magnitude of the summary effect estimate or the statistical significance tests.
Sensitivity analyses on the choice of the summary effect measure (OR versus RR) or the analysis model (fixed‐effect versus random‐effects model) demonstrated no differences of the direction of the treatment effect or the statistical significance tests.
In Abu Rafea 2013, some women (4/28 (14%)) were not trying to conceive after treatment, although they had been randomly assigned (1/13 women in the intervention group and 3/15 women in the control group). As prespecified in the protocol under 'Dealing with missing data,' we conducted a sensitivity analysis on the choice to use an available data analysis rather than an ITT analysis with the imputation that no live births would have occurred in women without a reported outcome. There was no impact on the direction/magnitude of the effect size or on the statistical significance tests.
1.2. Clinical pregnancy
According to a meta‐analysis of Abu Rafea 2013 and Roy 2014, there was insufficient evidence to determine whether there was a difference in clinical pregnancy rates between the use of any intervention compared to no treatment or placebo (OR 0.86, 95% CI 0.37 to 2.01; 107 women; 2 studies; I² = 0%; Analysis 1.2). We stratified data according to device versus no treatment or placebo and hormonal treatment versus no treatment or placebo.
1.2.1. Device versus placebo or no treatment
Abu Rafea 2013 did not define the outcome of pregnancy, and we obtain no clarification from study authors. Moreover, some women could have had more than one pregnancy during the follow‐up period of 12 to 18 months ‐ a point that could not be clarified. It was unclear whether there was a difference between the use of an intrauterine Foley catheter balloon versus no treatment following hysteroscopic septum division (OR 1.00, 95% CI 0.06 to 18.08; 24 women; 1 study; Analysis 1.2).
1.2.2. Hormonal treatment versus placebo or no treatment
According to Roy 2014, there was insufficient evidence to determine whether there was a difference between treatment with oestradiol valerate 2 mg daily versus folic acid 5 mg as a placebo for 30 days following hysteroscopic septum division (OR 0.85, 95% CI 0.35 to 2.06; 83 women; 1 study; Analysis 1.2).
1.3. Miscarriage
According to a meta‐analysis of Abu Rafea 2013 and Roy 2014, there was insufficient evidence to determine whether there was a difference in miscarriage rates between the use of any intervention compared to no treatment or placebo (OR 0.68, 95% CI 0.18 to 2.57; 54 women; 2 studies; I² = 0%; Analysis 1.3). We stratified data according to device versus no treatment or placebo and hormonal treatment versus no treatment or placebo.
1.3.1. Device versus placebo or no treatment
According to Abu Rafea 2013, there was insufficient evidence to determine whether there was a difference between the use of an intrauterine Foley catheter balloon versus no treatment following hysteroscopic septum division (OR 0.66, 95% CI 0.11 to 4.00; 24 women; 22 clinical pregnancies; 1 study; Analysis 1.3).
1.3.2. Hormonal treatment versus placebo or no treatment
According to Roy 2014, there was insufficient evidence to determine whether there was a difference between treatment with oestradiol valerate 2 mg daily versus folic acid 5 mg as a placebo for 30 days following hysteroscopic septum division (OR 0.72, 95% CI 0.10 to 5.01; 83 women; 32 clinical pregnancies; 1 study; Analysis 1.3).
1.4. Presence of intrauterine adhesions at second‐look hysteroscopy
According to a meta‐analysis of eight studies, anti‐adhesion treatment decreases the occurrence of IUAs at second‐look hysteroscopy compared to no treatment or placebo (OR 0.35, 95% CI 0.21 to 0.60; 560 women; 8 studies; I² = 0%; low‐quality evidence; Analysis 1.4) (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004; Lin 2015a; Roy 2014; Vercellini 1989).
The number needed to treat for an additional beneficial outcome (NNTB) was 9 (95% CI 5 to 17). We stratified data according to device versus no treatment or placebo, device plus hormonal treatment versus no treatment or placebo, hormonal treatment versus no treatment or placebo and gel versus no treatment or placebo.
1.4.1. Device versus placebo or no treatment
There was insufficient evidence from Lin 2015a to determine whether there was a difference between inserting an intrauterine balloon stent compared with no treatment following operative hysteroscopy for decreasing the occurrence of IUAs: there were no events in both treatment arms (OR not estimable; 60 women; 1 study; Analysis 1.4).
1.4.2. Device plus hormonal treatment versus placebo or no treatment
There was insufficient evidence from Vercellini 1989 to determine whether there was a difference between the insertion of an IUD followed by combined oestrogen‐progestin treatment for 30 days (intervention) versus no treatment (control) following hysteroscopic metroplasty for septate uterus in 20 women with two or more unexplained spontaneous miscarriages. A follow‐up HSG was done to detect uterine cavity abnormalities (residual fundal notch 1 cm or greater) and hysteroscopy was done in women with a residual notch (five women in intervention group and six women in control group). There were no IUAs detected in these 11 women: the effect size was, therefore, not determined (OR not estimable; 20 women; 1 study; Analysis 1.4).
1.4.3. Hormonal treatment versus placebo or no treatment
Based on the findings of Roy 2014, there is insufficient evidence to determine whether there is a difference between treatment with oestradiol valerate 2 mg daily versus folic acid 5 mg as a placebo for 30 days following hysteroscopic septum division (OR 0.14, 95% CI 0.01 to 2.72; 85 women; 1 study; Analysis 1.4).
1.4.4. Gel versus placebo or no treatment
Based on the pooled data of five studies, the use of gel decreases the occurrence of IUAs at second‐look hysteroscopy compared to no treatment or placebo (OR 0.37, 95% CI 0.21 to 0.64; 404; 5 studies; I² = 0%; Analysis 1.4) (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004).
The NNTB was 7 (95% CI 4 to 14).
Subgroup analyses
A subgroup analysis according to the type of gel used demonstrated a consistent decrease of the occurrence of IUAs at second‐look hysteroscopy in favour of the use of ACP gel, CMC gel or HA‐CMC gel compared to no gel. There was no evidence for subgroup differences (Chi² = 0.88, degrees of freedom (df) = 2; P = 0.65; I² = 0%). Data from this subgroup analysis should be treated with caution as subgroup analysis by itself is observational in nature, and statistical interpretation of results is not without problems.
1.5. Mean adhesion scores at second‐look hysteroscopy in women not treated for intrauterine adhesions
We aimed to pool the findings of Acunzo 2003 and Guida 2004 to estimate a summary effect size for the outcome of mean adhesion scores at second‐look hysteroscopy at 12 weeks in women treated by operative hysteroscopy for any intrauterine pathology after use of HA gel compared with no treatment. Statistical heterogeneity beyond chance was very high (I² = 99%) suggesting highly inconsistent findings across studies. The reason for this statistical heterogeneity was obvious: the prevalence of the outcome of interest (IUAs) at baseline in Guida 2004 was 0% as opposed to a 100% prevalence at baseline in Acunzo 2003. The populations were very different with respect to the risk of the adverse outcomes and the potential benefit on the adhesion scores. Therefore, we decided to report data for the mean adhesion scores at second‐look hysteroscopy in women not treated for IUAs and women treated for IUAs separately.
1.5.1. Gel versus placebo or no treatment
Guida 2004 demonstrated lower mean adhesion scores at second‐look hysteroscopy after the use of gel compared to no treatment in women treated for fibroids, endometrial polyps or uterine septa (MD in adhesion score ‐1.46, 95% CI ‐1.64 to ‐1.29; 132 women; 3 studies; I² = 0%; Analysis 1.5).
1.6. Mean adhesion scores at second‐look hysteroscopy in women treated for intrauterine adhesions
1.6.1. Gel versus placebo or no treatment
Acunzo 2003 reported lower mean adhesion scores at second‐look hysteroscopy after the use of HA gel compared to no gel in women treated for IUAs (MD in adhesion score ‐3.30, 95% CI ‐3.37 to ‐3.23, 84 women; 1 study; Analysis 1.6).
1.7. Severity of adhesions at second‐look hysteroscopy: mild
Based on a pooling of six studies, there was no clear evidence of a difference between any anti‐adhesion treatment compared to no treatment or placebo for the occurrence of mild adhesions at second‐look hysteroscopy (OR 1.33, 95% CI 0.68 to 2.61; 494 women; 6 studies; I² = 0%; Analysis 1.7). We stratified the data for Analysis 1.7 according to hormonal treatment versus no treatment or placebo and gel versus no treatment or placebo.
1.7.1. Hormonal treatment versus placebo or no treatment
Based on the results of Roy 2014, there was insufficient evidence to determine whether there was a difference between treatment with oestradiol valerate 2 mg daily compared to the intake of folic acid 5 mg as a placebo for 30 days following hysteroscopic septum division for the occurrence of mild adhesions at second‐look hysteroscopy (OR 0.19, 95% CI 0.01 to 4.10; 90 women; 1 study; Analysis 1.7).
1.7.2. Gel versus placebo or no treatment
Based on the pooled findings of five RCTs, there was no clear evidence of a difference between the use of any gel versus no gel for the occurrence of mild adhesions at any second‐look hysteroscopy (OR 1.56, 95% CI 0.77 to 3.18; 404 women; 5 studies; I² = 0%; Analysis 1.7) (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004).
Subgroup analyses
According to a subgroup analysis for Analysis 1.7, there was no clear evidence of a difference in the type of gel used in the occurrence of mild IUAs at second‐look hysteroscopy between the use of ACP gel, CMC gel or HA‐CMC gel compared to no gel. There was no evidence for subgroup differences (Chi² = 0.83, df = 2; P = 0.66; I² = 0%). Data from this subgroup analysis should be treated with caution as subgroup analysis by itself is observational in nature, and statistical interpretation of results is not without problems.
1.8. Severity of adhesions at second‐look hysteroscopy: moderate or severe
Based on the statistical pooling of the findings of six studies, the use of anti‐adhesion treatment decreases the occurrence of moderate or severe adhesions compared to no treatment or placebo (OR 0.08, 95% CI 0.03 to 0.24; 494 women; 6 studies; I² = 0%; Analysis 1.8) (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004; Roy 2014).
The NNTB was 6 (95% CI 5 to 10). We stratified data according to hormonal treatment versus no treatment or placebo and gel versus no treatment or placebo.
1.8.1. Hormonal treatment versus placebo or no treatment
Based on the results of Roy 2014, there was insufficient evidence to determine whether there was a difference between treatment with oestradiol valerate 2 mg daily compared to the intake of folic acid 5 mg as a placebo for 30 days following hysteroscopic septum division for the occurrence of moderate or severe adhesions at second‐look hysteroscopy (OR 0.33, 95% CI 0.01 to 8.22; 90 women; 1 study; Analysis 1.8).
1.8.2. Gel versus placebo or no treatment
Based on the pooled findings of five RCTs, the use of any anti‐adhesion barrier gel decreased the occurrence of moderate or severe adhesions at second‐look hysteroscopy (OR 0.07, 95% CI 0.02 to 0.23; 404 women; 5 studies; I² = 0%; Analysis 1.8) (Acunzo 2003; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Guida 2004).
The NNTB was 6 (95% CI 4 to 9).
Subgroup analyses
According to a subgroup analysis for Analysis 1.8, there was a consistent effect in favour of the use of ACP gel, CMC gel or HA‐CMC gel compared to no gel for decreasing the occurrence of moderate or severe IUAS at second‐look hysteroscopy. The subgroup interaction test did not identify any between‐group differences.
2. Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy
We identified six studies comparing any intervention versus any other intervention (Amer 2010; Fuchs 2014; Gan 2017; Lin 2015b; Wang 2016; Xiao 2015).
2.1. Live birth
2.1.1. Device plus graft versus device
No study reported live birth, but three studies reported outcomes that we used as surrogate outcomes for live birth (Amer 2010; Gan 2017; Wang 2016). The three studies compared the insertion of a Foley catheter balloon wrapped with fresh or freeze‐dried amniotic graft versus a Foley catheter balloon without graft for one to two weeks following hysteroscopic adhesiolysis in women with severe IUAs. Amer 2010 reported data on ongoing pregnancies or delivered at term. Gan 2017 reported data on ongoing pregnancies beyond 12 weeks of gestational age. Wang 2016 reported data on term delivery and ongoing pregnancy (but not yet delivered at the time of the survey) separately. For reasons of consistency throughout the review, we extracted data for term delivery and ongoing pregnancy and used these data as a surrogate for live birth. There was no clear evidence of a difference between inserting a Foley catheter balloon with fresh or freeze‐dried HAM graft compared to inserting a Foley catheter balloon only (OR 1.48, 95% CI 0.57 to 3.83; 180 women; 3 studies; I² = 0%; low‐quality evidence; Analysis 2.1).
Sensitivity analysis
We conducted a sensitivity analysis for Analysis 2.1. The choice to include all studies regardless of study quality or to include only one study at low risk for selection bias related to random sequence generation and allocation concealment did not affect the statistical significance tests. Sensitivity analyses on the choice of the summary effect measure (OR versus RR) or the analysis model (fixed‐effect versus random‐effects model) did not demonstrate differences of the direction of the treatment effect or the statistical significance tests.
2.2. Clinical pregnancy
There was no clear evidence of a difference between treatment A versus treatment B for improving clinical pregnancy rates (OR 1.72, 95% CI 0.89 to 3.33; 221 women; 4 studies; I² = 0%; Analysis 2.2). We stratified data according to device plus graft versus device and gel plus hormonal treatment plus antibiotics versus hormonal treatment plus antibiotics.
2.2.1. Device plus graft versus device
Three studies compared the insertion of a Foley catheter balloon wrapped with fresh or freeze‐dried amniotic graft versus a Foley catheter balloon without graft following hysteroscopic adhesiolysis in women with severe IUAs (Amer 2010; Gan 2017; Wang 2016). There was no clear evidence of a difference between inserting a Foley catheter balloon wrapped with fresh or freeze‐fried HAM graft compared to inserting a Foley catheter for increasing the chance for a clinical pregnancy (OR 1.53, 95% CI 0.74 to 3.18; 180 women; 3 studies; I² = 0%; Analysis 2.2).
2.2.2. Gel plus hormonal treatment plus antibiotics versus hormonal treatment plus antibiotics
One study compared the application of Oxiplex gel with sequential hormonal treatment for three weeks and antibiotic therapy for one week to sequential hormonal treatment and antibiotic therapy only in women with confirmed fertility undergoing operative hysteroscopy for retained products of conception (Fuchs 2014). This study reported data on pregnancy without further specification. We used these data as a surrogate outcome for clinical pregnancy. There was insufficient evidence from Fuchs 2014 to determine whether there was a difference between the application of Oxiplex gel combined with sequential hormonal treatment and antibiotics compared to sequential hormonal treatment combined with antibiotics (OR 2.83, 95% CI 0.62 to 13.04; 41 women; 1 study; I² = 0%; Analysis 2.2).
2.3. Miscarriage
2.3.1. Device plus graft versus device
According to a pooled analysis of data from three studies, there was no clear evidence of a difference between the insertion of a Foley catheter balloon wrapped with fresh or freeze‐dried amniotic graft versus a Foley catheter balloon without graft following hysteroscopic adhesiolysis in women with severe IUAs for the outcome miscarriage (OR 0.80, 95% CI 0.20 to 3.19; 180 women; 40 clinical pregnancies; 3 studies; I² = 0%; Analysis 2.3) (Amer 2010; Gan 2017; Wang 2016).
2.4. Presence of intrauterine adhesions at second‐look hysteroscopy
A pooled analysis of five RCTs demonstrated a decrease in the occurrence of IUAS with anti‐adhesion treatment consisting of barrier gel or intrauterine balloon with or without gel or graft compared to IUD plus balloon only or hormonal treatment plus antibiotics (OR 0.55, 95% CI 0.36 to 0.83; 451 women; 5 studies; I² = 0%; low‐quality evidence; Analysis 2.4) (Fuchs 2014; Gan 2017; Lin 2015b; Wang 2016; Xiao 2015).
The NNTB was 8 (95% CI 5 to 25). We stratified data according to device versus device, device plus graft versus device, device plus gel versus device and gel plus hormonal treatment plus antibiotics versus hormonal treatment plus antibiotics.
2.4.1. Device versus device
There was insufficient evidence from Lin 2015b to determine whether there was a difference between inserting a specially designed intrauterine balloon compared to the Yantai Contraceptive Instrument, a heart‐shaped copper IUD with thread knitted tail for decreasing the occurrence of IUAs (OR 0.81, 95% CI 0.42 to 1.57; 162 women; 1 study; I² = 0%; Analysis 2.4).
2.4.2. Device plus graft versus device
Gan 2017 studied the rate of IUA reformation in women undergoing hysteroscopic adhesiolysis for severe IUAs: a clear definition of adhesion reformation was not given and further clarification could not be obtained from the study authors. Wang 2016 presented data on the recurrence of IUAs grade 5 or greater according to the 1988 AFS classification as evidence of adhesion reformation in women treated with hysteroscopic adhesiolysis for moderate or severe IUAs. Based on a pooling of the findings of two studies, it was unclear whether there was a difference between inserting a Foley catheter balloon wrapped with HAM versus a Foley catheter balloon without graft following hysteroscopic adhesiolysis in women with severe IUAs (OR 0.53, 95% CI 0.25 to 1.10; 137 women; 2 studies; I² = 0%; Analysis 2.4) (Gan 2017; Wang 2016).
2.4.3. Device plus gel versus device
Based on the findings of Xiao 2015 the injection of 2 mL of medical self‐cross‐linking sodium hyaluronate gel from the lumen of a Foley balloon catheter left in situ for 72 hours decreased the occurrence of IUAs compared to a Foley balloon catheter only following operative hysteroscopy in women with severe IUAs (OR 0.31, 95% CI 0.13 to 0.76; 111 people; 1 study; I² = 0%; Analysis 2.4).
The NNTB is 5 (95% CI 2 to 17).
2.4.4. Gel plus hormonal treatment plus antibiotics versus hormonal treatment plus antibiotics
Fuchs 2014 compared the application of Oxiplex gel with sequential hormonal treatment for three weeks and antibiotic therapy for one week to sequential hormonal treatment and antibiotic therapy only in women with confirmed fertility undergoing operative hysteroscopy for retained products of conception. There was insufficient evidence to determine whether there was a difference between groups for decreasing the occurrence of IUAs (OR 0.28, 95% CI 0.03 to 2.98; 41 women; 1 study; I² = 0%; Analysis 2.4).
2.5. Mean adhesion scores at second‐look hysteroscopy
We aimed to pool three studies randomly comparing two anti‐adhesion treatments head‐to‐head measuring mean adhesion scores at second‐look hysteroscopy (Lin 2015b; Wang 2016; Xiao 2015). Statistical heterogeneity beyond chance was very high (I² = 92.1%) suggesting highly inconsistent findings across studies. The reason for this statistical heterogeneity was obvious: the interventions were clinically too diverse to allow statistical pooling. We stratified data according to device versus device, device plus graft versus device and device plus gel versus device.
2.5.1. Device versus device
Lin 2015b reported the median adhesion scores in both comparison groups before the operation and the median reduction of AFS scores in both groups. According to this study, it was unclear whether there was a difference in favour of the insertion of a specially designed intrauterine balloon compared to the Yantai Contraceptive Instrument for the median adhesion scores at second‐look hysteroscopy (Table 1). We considered converting the medians to means and the 95% CI to SD but the method for conversion is not robust.
| Outcome | Balloon group (intervention: n = 82) | IUD group (control: n = 80) | P value |
| AFS score before surgery (median, 95% CI) | 8 (5 to 12) | 8 (5 to 12) | 1.00 |
| Median reduction in AFS score | 7 (2 to 12) | 7 (0 to 12) | 1.00 |
IUD: intrauterine device; n: number of participants.
2.5.2. Device plus graft versus device
Two studies reported data on the median adhesion scores and their interquartile ranges (IQR) (Amer 2010; Gan 2017).
We considered converting the medians to means and the 95% CI to SD but the method for conversion is not robust.
Amer 2010 reported similar median adhesion scores and IQRs at second‐look hysteroscopy across the three intervention arms (Table 2). In contrast, Gan 2017 demonstrated lower median adhesion scores at second‐look hysteroscopy with the use of amniotic membrane graft compared to inserting a balloon catheter only without amnion graft (Table 3).
| Statistic | Fresh amnion graft (group 2: n = 14) | Dried amnion graft (group 3: n = 15) | No amnion graft (group 1: n = 14) | P value |
| Median | 1.5 | 2 | 2 | ‐ |
| IQR | 1 to 2 | 1 to 2 | 1 to 2 | 0.27 |
IQR: interquartile range; n: number of participants.
| Statistic | Amnion graft (intervention: n = 40) | No graft (control: n = 40) | P value |
| Median | 2 | 4 | ‐ |
| IQR | 2 to 5 | 2 to 6 | 0.03 |
IQR: interquartile range; n: number of participants.
According to Wang 2016, the mean adhesion scores after inserting a balloon catheter with amniotic graft were significantly lower compared to inserting a balloon catheter alone without amniotic graft following hysteroscopic adhesiolysis in women with moderate or severe IUAs (MD in adhesion score ‐3.10, 95% CI ‐4.17 to ‐2.03; 57 women; 1 study; Analysis 2.5).
2.5.3. Device plus gel versus device
According to Xiao 2015, the injection of 2 mL of medical self‐cross‐linking sodium hyaluronate gel from the lumen of a Foley balloon catheter left in situ for 72 hours was associated with lower mean adhesion scores at second‐look hysteroscopy compared to a Foley balloon catheter only following operative hysteroscopy in women with severe IUAs (MD in adhesion score ‐1.60, 95% CI ‐2.32 to ‐0.88; 111 women; 1 study; Analysis 2.5).
2.6. Severity of adhesions at second‐look hysteroscopy: mild
2.6.1. Device plus gel versus device
There was insufficient evidence from Xiao 2015 to determine whether there was a difference between the injection of 2 mL of medical self‐cross‐linking sodium hyaluronate gel from the lumen of a Foley balloon catheter left in situ for 72 hours compared to a Foley balloon catheter only following operative hysteroscopy in women with severe IUAs for the occurrence of mild adhesions at second‐look hysteroscopy (OR 1.11, 95% CI 0.53 to 2.34; 111 women; 1 study; Analysis 2.6).
2.7. Severity of adhesions at second‐look hysteroscopy: moderate or severe
According to a pooling of the findings of two studies, the application of a combined anti‐adhesion treatment consisting of barrier gel decreased the occurrence of moderate or severe IUAs following operative hysteroscopy compared to anti‐adhesion treatment not consisting of barrier gel (OR 0.25, 95% CI 0.10 to 0.61; 152 women; 2 studies; I² = 0%; Analysis 2.7) (Fuchs 2014; Xiao 2015).
The NNTB was 5 (95% CI 3 to 12). We stratified data according to device plus gel versus device and gel plus hormonal treatment plus antibiotics versus hormonal treatment plus antibiotics.
2.7.1. Device plus gel versus device
Based on the findings of Xiao 2015, the injection of 2 mL of medical self‐cross‐linking sodium hyaluronate gel from the lumen of a Foley balloon catheter left in situ for 72 hours decreased the occurrence of moderate or severe IUAs compared to a Foley balloon catheter only following operative hysteroscopy in women with severe IUAs (OR 0.24, 95% CI 0.09 to 0.63; 111 women; 1 study; I² = 0%; Analysis 2.7).
The NNTB was 4 (95% CI 2 to 12).
2.7.2. Gel plus hormonal treatment plus antibiotics versus hormonal treatment plus antibiotics
There was insufficient evidence from Fuchs 2014 to determine whether there was a difference between the application of Oxiplex gel with sequential hormonal treatment for three weeks and antibiotic therapy for one week to sequential hormonal treatment and antibiotic therapy only in women with confirmed fertility undergoing operative hysteroscopy for retained products of conception (OR 0.28, 95% CI 0.03 to 2.98; 41 women; 1 study; Analysis 2.7).
Discussion
Summary of main results
This systematic review aimed to investigate whether the use of anti‐adhesion therapy following operative hysteroscopy made a difference in the main outcomes of live birth or ongoing pregnancy, clinical pregnancy and miscarriage, or in the prevalence, extent or severity of IUAs in women with subfertility.
We searched for studies randomly comparing any anti‐adhesion therapy versus no treatment or placebo or any other anti‐adhesion treatment in subfertile women following operative hysteroscopy.
We retrieved 16 studies involving 1273 women randomly comparing the use of a device versus no treatment (two studies; 90 women), hormonal treatment versus no treatment or placebo (two studies; 136 women), device combined with hormonal treatment versus no treatment (one study; 20 women), barrier gel versus no treatment (five studies; 464 women), device with graft versus device without graft (three studies; 190 women), one type of device versus another device (one study; 201 women), gel combined with hormonal treatment and antibiotics versus hormonal treatment with antibiotics (one study; 52 women) or device combined with gel versus device (one study; 120 women). Only two of 16 studies included 100% infertile women; in all other studies, the proportion of infertile women was variable or unknown. Most studies (14/16) had at least one item at high risk of bias, and nine of 16 studies had two or more items at high risk of bias. Seven studies were at low risk for selection bias related to random sequence generation and allocation concealment (Amer 2010; Di Spiezio Sardo 2011; Fuchs 2014; Guida 2004; Lin 2015a; Roy 2014; Vercellini 1989). Only one study had all items at low risk of bias (Roy 2014).
Anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy
Based on a pooled analysis of the results from two studies there was insufficient evidence to determine whether there was a difference between inserting a device in the uterine cavity or starting hormonal treatment compared to no treatment or placebo for increasing the chance for term delivery or ongoing pregnancy (Figure 4).

Forest plot of comparison: 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, outcome: 1.1 Live birth.
The pooled findings from eight studies demonstrated a summary effect in favour of the insertion of a device with or without hormonal treatment or hormonal treatment or anti‐adhesion barrier gels compared to no treatment or placebo for decreasing the occurrence of IUAs at any second‐look hysteroscopy (Figure 5). For the use of anti‐adhesion treatment in a medium‐risk population, we would expect that out of 1000 women treated by operative hysteroscopy, between 153 and 365 women would develop IUAs, compared with 545 women when no anti‐adhesion treatment was used (Figure 6).
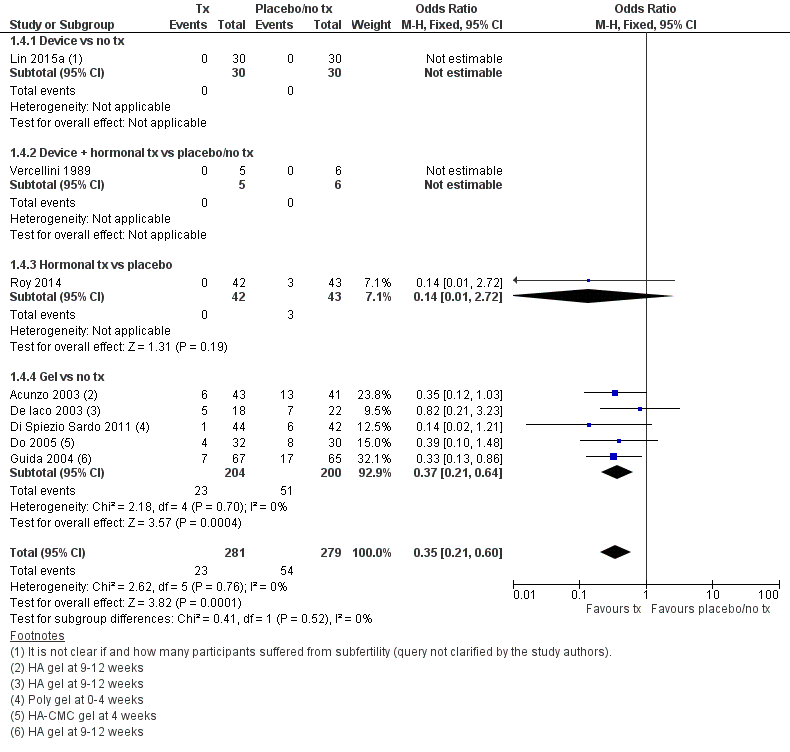
Forest plot of comparison: 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, outcome: 1.4 Presence of intrauterine adhesions at second‐look hysteroscopy.

Cates' plot of numbers needed to treat for an additional beneficial outcome (NNTB) for Analysis 1.4 assuming medium risk of 545 women per 1000 with intrauterine adhesions at second‐look hysteroscopy in the control group (no treatment or placebo). Randomly compared to control, the use of device with or without hormonal treatment or hormonal treatment or barrier gels (intervention) decreased the number of women with intrauterine adhesions at second‐look hysteroscopy to 234 women per 1000 (95% confidence interval 153 to 365 women per 1000). Figure drawn using www.nntonline.net.
Anti‐adhesion therapy versus any other therapy following operative hysteroscopy
According to the pooled findings of three studies there was no clear evidence of a difference between the insertion of a Foley catheter balloon wrapped in amniotic membrane versus the insertion of a Foley catheter balloon without amniotic membrane for improving the ongoing pregnancy rates.
A meta‐analysis of the findings of five trials demonstrated differences head‐to‐head between the use of a device with or without graft/gel or gel plus hormonal treatment plus antibiotics randomly compared to the use of a device only or hormonal treatment plus antibiotics for decreasing the occurrence of IUAs at second‐look hysteroscopy. The findings of this meta‐analysis were not robust and highly affected by evidence quality.
Overall completeness and applicability of evidence
We retrieved only one small study that randomly compared the insertion of an IUD versus no treatment (Vercellini 1989). In everyday clinical practice, worldwide an IUD is very often inserted following the hysteroscopic treatment of IUAs or the resection of an intrauterine septum.
Only five of 16 studies reported data on the primary outcome of live birth but all five used surrogate outcomes. Only five of 16 studies reported data on an adverse reproductive outcome (miscarriage). Thirteen of 16 trials reported the secondary outcomes of prevalence, mean adhesion scores and severity of IUAs at second‐look hysteroscopy.
Only eight of 16 studies reported data on the proportion of women with subfertility. Out of 682 participants from these eight studies, only 247 women had subfertility (36%). Therefore, the evidence retrieved in this Cochrane Review is indirect for the target population of subfertile women undergoing operative hysteroscopy.
There were differences in the HA anti‐adhesion gel used by Acunzo 2003 and Guida 2004 compared to Xiao 2015. Xiao 2015 suggested that the use of their gel (a newly developed highly viscous and elastic self‐cross‐linking sodium hyaluronate gel, which uses fermentation technology on natural sodium hyaluronate gel) may be more advantageous: animal‐derived HA may stimulate immunological rejection and provoke inflammation.
We did not find any cost‐effectiveness studies on the use of anti‐adhesion treatment following operative hysteroscopy in a subfertile population.
In conclusion, we judged that the body of evidence retrieved was insufficient to address all research questions that were predefined for this Cochrane Review.
Quality of the evidence
Several limitations at study and outcome levels were related to performance bias, other potential sources of bias, attrition bias, and reporting and selection bias in decreasing order of frequency. Reasons for risk of bias at the study level and across studies are discussed in detail in the Risk of bias in included studies section and are graphically presented in Figure 2 and Figure 3.
Anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy
See summary of findings Table for the main comparison.
We graded the overall quality of the evidence as very low for the outcome of live birth. The main limitations were serious risk for selection bias related to allocation concealment, serious imprecision and serious indirectness.
For the outcome of IUAs at second‐look hysteroscopy, we graded the overall quality of the evidence as low. The main limitations were high risk of performance bias related to blinding of participants/personnel and serious indirectness.
Anti‐adhesion therapy versus any other therapy following operative hysteroscopy
See summary of findings Table 2.
We graded the overall quality of the evidence as low for the outcome of live birth. The main limitations were high risk of bias for selective outcome reporting and serious imprecision.
For the outcome of IUAs at second‐look hysteroscopy, we graded the overall quality of the evidence as very low. The main limitations were high risk of bias for selective outcome reporting and serious indirectness.
Potential biases in the review process
Limitations at the review level include the following.
-
We conducted no formal study of reporting bias because we retrieved a limited number of studies (fewer than 10 studies) for each randomised comparison. Nevertheless, we aimed to minimise the potential impact of reporting and publication bias by conducting a comprehensive search for all potentially eligible studies, and by staying alert for duplication of data as predefined in the protocol of this Cochrane Review (Bosteels 2013a). We consistently searched for related articles in published and secondary reports of included studies. We contacted all authors of included studies to ask if they were aware of any published or ongoing trials; we also contacted experts in the field.
-
We rigorously subjected to sensitivity analyses all choices to include only studies at low risk of bias versus all studies, to use available data analyses rather than ITT analyses or to exclude participants who were treated by an intervention not indicated for treating subfertility; we considered any observed substantial changes when interpreting results.
-
We used surrogate outcomes for the primary outcome of live birth: term delivery at 12 to 18 months for Abu Rafea 2013, ongoing pregnancies or delivered at term for Amer 2010, ongoing pregnancies beyond 12 weeks of gestational age for Gan 2017, and ongoing pregnancy for Roy 2014 and Wang 2016.
-
We used surrogate outcomes for the secondary outcome of clinical pregnancy: pregnancy without clear definition for Amer 2010, Fuchs 2014, Gan 2017, and Wang 2016.
-
At least two review authors independently and simultaneously extracted all data for the previous version of this Cochrane Review: JB extracted data from all studies, and TD/FB/JK/SW divided all studies between them, and each extracted data from only a portion of all the finally included studies. For the present update (five additional studies retrieved), JB extracted data from all five additional studies while independently and simultaneously HT (three), SW (three) and SJC (two) extracted data from some studies divided between them. In case of disagreement, BWM acted as a third review author for arbitration. This implies that JB may have had a larger influence than any one of all the authors involved in data extraction on the final decisions concerning this part of the review.
-
One of the Cochrane authors (SJC) translated two Chinese articles into English to allow data extraction and assessment of the risk of bias (Wang 2016; Xiao 2015). Queries in English and Chinese were sent to Lin 2015b, Wang 2016, and Xiao 2015. Despite several queries, we were able to obtain answers from the authors of primary study reports in six of 15 included studies (40%). For nine of 15 (60%) included studies, several queries remained unanswered. As predefined in the study protocol, we classified these items as 'unclear evidence' or 'unclear risk of bias.'
Agreements and disagreements with other studies or reviews
We found six systematic reviews that have summarised and critically appraised the available evidence on the effectiveness of anti‐adhesion therapy.
-
One Cochrane Review included 18 RCTs in 1262 women undergoing gynaecological pelvic surgery by laparoscopy (eight RCTs) or laparotomy (10 RCTs) (Ahmad 2015). The authors found no evidence on the effects of barrier agents used during pelvic surgery on either pain or fertility outcomes in women of reproductive age. The quality of the evidence ranged from very low to moderate. The most common limitations were imprecision and poor reporting of study methods. Most studies were commercially funded, and publication bias could not be ruled out.
-
Mais 2012 was a systematic review with a meta‐analysis performed to study the effectiveness of ACP gel for adhesion prevention in laparoscopic and hysteroscopic surgery. Data from three RCTs included in the Cochrane Review were pooled (Acunzo 2003; De Iaco 2003; Guida 2004): the proportion of women with adhesions at second look was significantly lower in women who received ACP gel than in the control group (RR 0.50, 95 % CI 0.31 to 0.85; P = 0.009; 3 studies; 256 women). Mais 2012 used the Jadad scale (an older and less valid tool for assessing the validity of intervention studies) and not the Cochrane 'Risk of bias' tool leading to 'high quality' for the three studies in Mais 2012 as opposed to the grading of the available evidence in the present Cochrane Review for the outcome of IUAs at second‐look hysteroscopy as 'low quality.'
-
Healy 2015 was a systematic review partially sponsored by a grant from the Intramural research program of the Program in Reproductive and Adult Endocrinology, National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH). The review included 13 studies. Seven studies that compared similar treatment methods were statistically pooled. The authors concluded that the use of HA gel or Intercoat gel after operative hysteroscopy may decrease IUA formation. According to their meta‐analysis, the data does not support the use of oestrogen therapy. Additional quality RCTs are needed to further establish better preventive measures of IUA formation.
-
Healy 2016 was a systematic review including 12 studies. Nine studies included by Healy 2016 were also included in this Cochrane Review (Acunzo 2003; Amer 2010; Dabir‐Ashrafi 1996; De Iaco 2003; Di Spiezio Sardo 2011; Fuchs 2014; Guida 2004; Roy 2014; Vercellini 1989); three studies included by Healy 2016 were excluded from this Cochrane review for being non‐randomised (Pabuccu 2008; Tonguc 2010) or excluding subfertile women (Kim 2012). Three studies demonstrating a benefit with the gels in preventing adhesion formation were all conducted by the same research group (Acunzo 2003; Di Spiezio Sardo 2011; Guida 2004); according to Healy 2016 these beneficial results have not been confirmed by other research groups. The final conclusion of Healy 2016 stated that there was a lack of definitive evidence to conclude that any treatment was effective in preventing IUAs following operative hysteroscopy. The available literature "has significant heterogeneity and a high risk of bias, making any definitive conclusions difficult."
-
Di Spiezio Sardo 2016 was a systematic review including 29 studies. Eight studies included by Di Spiezio Sardo 2016 were included in this Cochrane Review (Acunzo 2003; Amer 2010; De Iaco 2003; Di Spiezio Sardo 2011; Do 2005; Fuchs 2014; Guida 2004; Roy 2014). Three studies included by Di Spiezio Sardo 2016 were excluded from this Cochrane Review for being non‐randomised (Pabuccu 2008; Tonguc 2010) or excluding subfertile women (Kim 2012). Eighteen studies were observational studies that were not eligible for this Cochrane Review. Di Spiezio Sardo 2016 concluded that "robust and high quality randomized trials to assess the effectiveness of different anti‐adhesion therapies are still needed before one or more of these strategies may be strongly recommended for improving clinical outcomes in women treated by operative hysteroscopy."
-
One systematic review by Salma 2014 including 28 observational studies of 1806 women with meta‐analysis of five studies and qualitative assessment of 23 studies reported a clinical benefit with the insertion of an IUD for all women with IUAs regardless of their severity. In the opinion of the authors of this review, use of IUDs should be combined with other anti‐adhesion therapies "to obtain maximal outcomes, in particular in patients with moderate to severe IUAs." This review had several methodological limitations, including the lack of a formal assessment of risk of bias, lack of appreciation of the role of confounding variables, lack of adjustment for confounders in data calculation for pooled analyses, evidence of substantial statistical heterogeneity for pooled analyses of the five included studies and lack of formal assessment of reporting bias.

Study flow diagram: summary of searches since 2015. PICO: population, intervention, comparator, outcome; RCT: randomised controlled trial.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
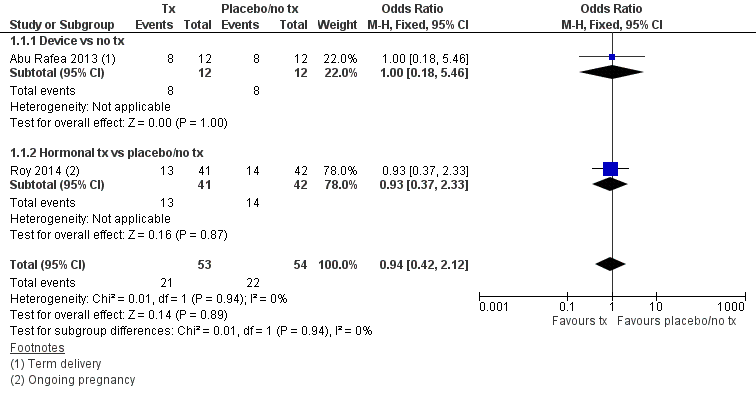
Forest plot of comparison: 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, outcome: 1.1 Live birth.

Forest plot of comparison: 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, outcome: 1.4 Presence of intrauterine adhesions at second‐look hysteroscopy.
Cates' plot of numbers needed to treat for an additional beneficial outcome (NNTB) for Analysis 1.4 assuming medium risk of 545 women per 1000 with intrauterine adhesions at second‐look hysteroscopy in the control group (no treatment or placebo). Randomly compared to control, the use of device with or without hormonal treatment or hormonal treatment or barrier gels (intervention) decreased the number of women with intrauterine adhesions at second‐look hysteroscopy to 234 women per 1000 (95% confidence interval 153 to 365 women per 1000). Figure drawn using www.nntonline.net.

Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 1 Live birth.
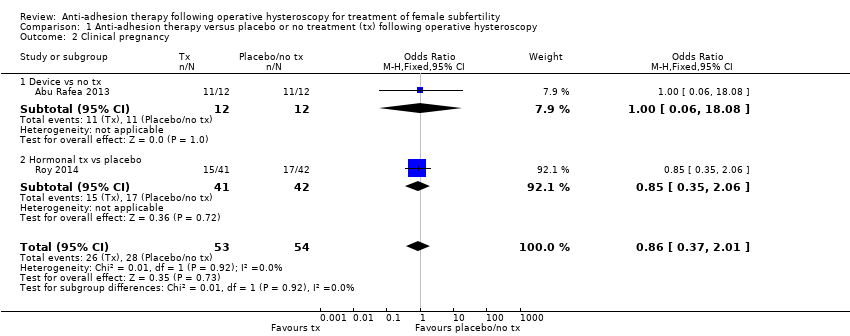
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 2 Clinical pregnancy.

Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 3 Miscarriage.

Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 4 Presence of intrauterine adhesions at second‐look hysteroscopy.
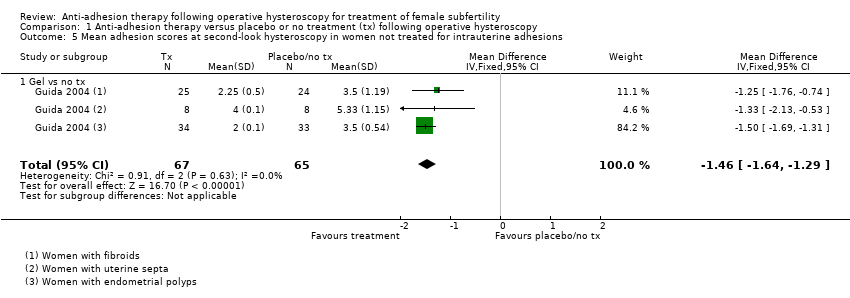
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 5 Mean adhesion scores at second‐look hysteroscopy in women not treated for intrauterine adhesions.

Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 6 Mean adhesion scores at second‐look hysteroscopy in women treated for intrauterine adhesions.
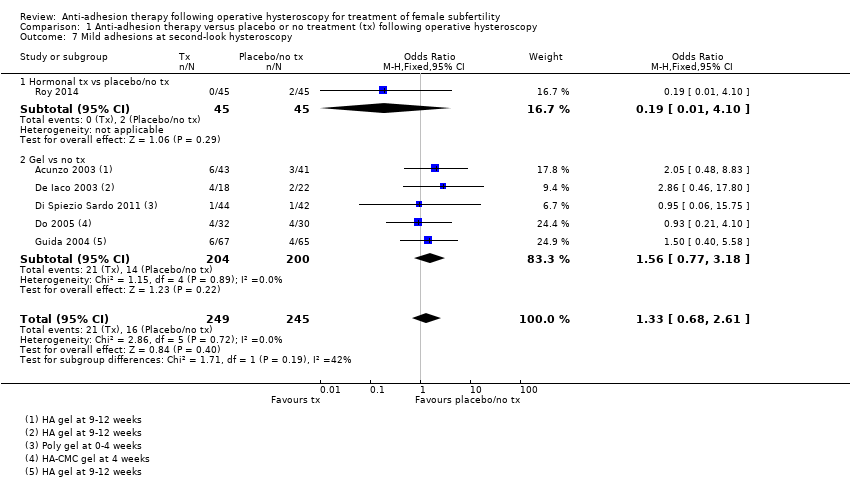
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 7 Mild adhesions at second‐look hysteroscopy.
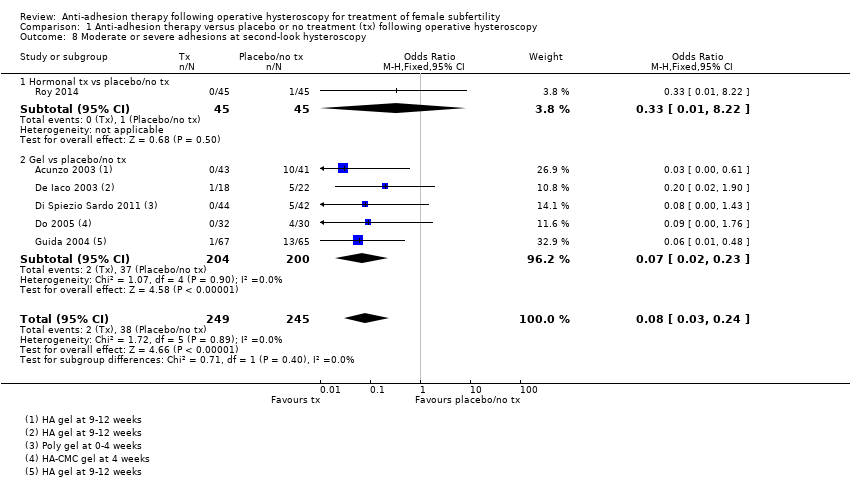
Comparison 1 Anti‐adhesion therapy versus placebo or no treatment (tx) following operative hysteroscopy, Outcome 8 Moderate or severe adhesions at second‐look hysteroscopy.

Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 1 Live birth.
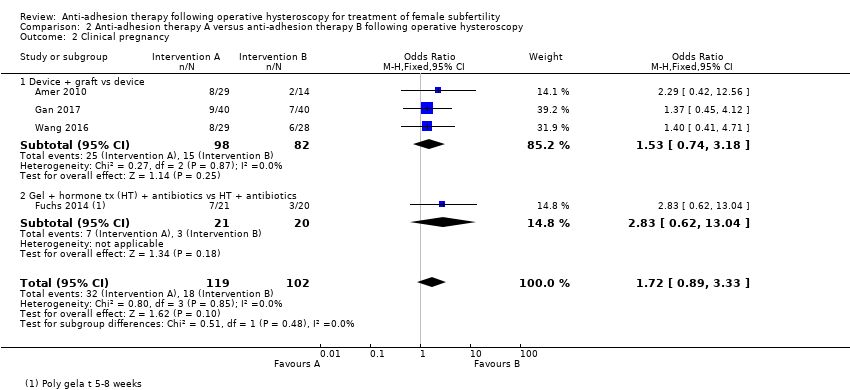
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 2 Clinical pregnancy.

Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 3 Miscarriage.

Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 4 Presence of intrauterine adhesions at second‐look hysteroscopy.

Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 5 Mean adhesion scores in women treated for intrauterine adhesions.
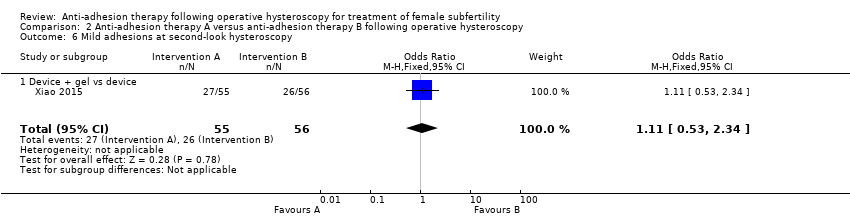
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 6 Mild adhesions at second‐look hysteroscopy.
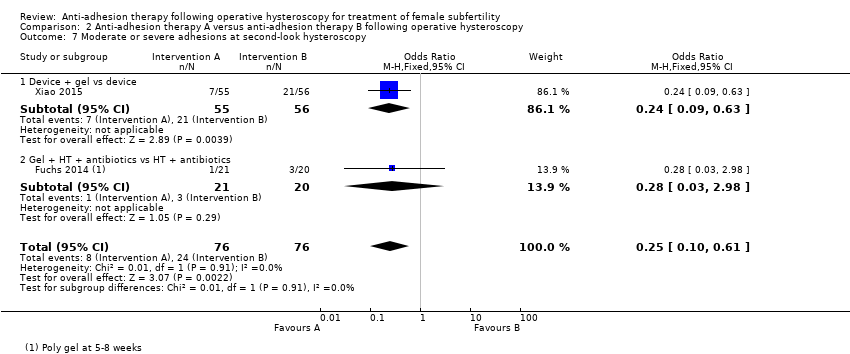
Comparison 2 Anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy, Outcome 7 Moderate or severe adhesions at second‐look hysteroscopy.
| Any anti‐adhesion therapy versus placebo or no treatment following operative hysteroscopy | ||||||
| Patient or population: women treated by operative hysteroscopy for uterine pathology associated with subfertility or adverse pregnancy outcome Settings: single centre, Hysteroscopy Unit or Department of Obstetrics and Gynaecology of a university or non‐university tertiary care hospital Intervention: any anti‐adhesion therapy Comparison: no treatment or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment or placebo | Anti‐adhesion therapy | |||||
| Live birth a | No treatment or placebo | Device or hormonal treatment | OR 0.94 (0.42 to 2.12) | 107 | ⊕⊝⊝⊝ | ‐ |
| Mean‐risk populationb | ||||||
| 407 per 1000 | 399 per 1000 | |||||
| Presence of intrauterine adhesions at second‐look hysteroscopy (second‐look hysteroscopy at 4‐12 weeks after operative hysteroscopy) | No treatment or placebo | Device ± hormonal treatment or hormonal treatment or barrier gel | OR 0.35 g (0.21 to 0.60) | 560 (8 RCTs) | ⊕⊕⊝⊝ | ‐ |
| Low‐risk populationf | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population f | ||||||
| 545 per 1000 | 234 per 1000 | |||||
| High‐risk population f | ||||||
| 875 per 1000 | 376 per 1000 | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| a The two included studies reported term delivery (Abu Rafea 2013) or ongoing pregnancy (Roy 2014), which we used as a surrogate outcome for live birth. b The assumed risk for the mean‐risk population was the pooled risk of all live births in control groups of the two included studies. c Downgraded one level for serious risk of bias: one study was at high risk of bias in several domains, including allocation concealment. d Downgraded one level for serious imprecision; only 43 events in total. e Downgraded one level for serious indirectness, because only 30% (35/118) of all randomised women in this analysis were subfertile. f The assumed risk for low‐, medium‐ and high‐risk population based on presence of intrauterine adhesions following hysteroscopic removal of endometrial polyps/following removal of submucous fibroids and intrauterine adhesions (mean of both)/removal of uterine septum, respectively, based on findings of a prospective cohort study (Yang 2013). G Two studies reported no events (Lin 2015a; Vercellini 1989). h Downgraded one level for serious risk of bias: all eight studies had several limitations but none was at high risk for selection bias related to random sequence generation or allocation concealment. i Downgraded one level for serious indirectness, because in four of eight studies less than 50% of participants were subfertile and in four of eight studies it was unclear whether subfertile women were included. | ||||||
| Any anti‐adhesion therapy A versus anti‐adhesion therapy B following operative hysteroscopy | ||||||
| Patient or population: women treated by operative hysteroscopy for uterine pathology Settings: multicentric, Hysteroscopy Unit of Department of Obstetrics and Gynaecology of a university, university‐affiliated or non‐university tertiary care hospital Intervention: anti‐adhesion therapy A Comparison: anti‐adhesion therapy B | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Anti‐adhesion therapy B | Anti‐adhesion therapy A | |||||
| Live birth a | Device | Device + graft | OR 1.48 (0.57 to 3.83) | 180 (3 RCTs) | ⊕⊕⊝⊝ Low c,d | ‐ |
| 98 per 1000 b | 138 per 1000 (60 to 315) | |||||
| Presence of intrauterine adhesions at second‐look hysteroscopy (6‐12 weeks) | Device or hormonal treatment with antibiotics | Device ± graft/gel or gel + hormonal treatment + and antibiotics | OR 0.55 (0.36 to 0.83) | 451 (5 RCTs) | ⊕⊕⊝⊝ | ‐ |
| Low‐risk population e | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Medium‐risk population e | ||||||
| 545 per 1000 | 403 per 1000 | |||||
| High‐risk population e | ||||||
| 875 per 1000 | 647 per 1000 | |||||
| *The basis for the assumed risk is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| a The three included studies reported term delivery (Wang 2016) or ongoing pregnancy (Amer 2010; Gan 2017; Wang 2016), which we used as a surrogate outcome for live birth. b The assumed risk for the average‐risk population is the pooled risk of all the live births in the control groups of the three included studies. c Downgraded one level for serious risk of bias: despite several limitations none of the studies was at high risk for selection bias related to random sequence generation or allocation concealment. d Downgraded one level for serious imprecision‐ only 21 events in total. e The assumed risk for low/medium/high‐risk population is based on the presence of intrauterine adhesions following hysteroscopic removal of endometrial polyps/following removal of submucous fibroids and IUAs (mean of both)/removal of uterine septum, respectively, based on findings of a prospective cohort study (Yang 2013). f Downgraded one level for serious risk of bias: despite several limitations none of the studies was at high risk for selection bias related to random sequence generation or allocation concealment. g Downgraded one level for serious indirectness because, in two of five studies, less than 50% of participants were subfertile; in one of five studies, it was unclear if subfertile women were included and in two of five studies, the proportion of infertile women was not reported. | ||||||
| Outcome | Balloon group (intervention: n = 82) | IUD group (control: n = 80) | P value |
| AFS score before surgery (median, 95% CI) | 8 (5 to 12) | 8 (5 to 12) | 1.00 |
| Median reduction in AFS score | 7 (2 to 12) | 7 (0 to 12) | 1.00 |
| IUD: intrauterine device; n: number of participants. | |||
| Statistic | Fresh amnion graft (group 2: n = 14) | Dried amnion graft (group 3: n = 15) | No amnion graft (group 1: n = 14) | P value |
| Median | 1.5 | 2 | 2 | ‐ |
| IQR | 1 to 2 | 1 to 2 | 1 to 2 | 0.27 |
| IQR: interquartile range; n: number of participants. | ||||
| Statistic | Amnion graft (intervention: n = 40) | No graft (control: n = 40) | P value |
| Median | 2 | 4 | ‐ |
| IQR | 2 to 5 | 2 to 6 | 0.03 |
| IQR: interquartile range; n: number of participants. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 2 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.42, 2.12] |
| 1.1 Device vs no tx | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.18, 5.46] |
| 1.2 Hormonal tx vs placebo/no tx | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.37, 2.33] |
| 2 Clinical pregnancy Show forest plot | 2 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.37, 2.01] |
| 2.1 Device vs no tx | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 18.08] |
| 2.2 Hormonal tx vs placebo | 1 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.35, 2.06] |
| 3 Miscarriage Show forest plot | 2 | 54 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.18, 2.57] |
| 3.1 Device vs no tx | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 4.00] |
| 3.2 Hormonal tx vs placebo | 1 | 32 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.10, 5.01] |
| 4 Presence of intrauterine adhesions at second‐look hysteroscopy Show forest plot | 8 | 560 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.21, 0.60] |
| 4.1 Device vs no tx | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Device + hormonal tx vs placebo/no tx | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Hormonal tx vs placebo | 1 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.72] |
| 4.4 Gel vs no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.21, 0.64] |
| 5 Mean adhesion scores at second‐look hysteroscopy in women not treated for intrauterine adhesions Show forest plot | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐1.64, ‐1.29] |
| 5.1 Gel vs no tx | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐1.46 [‐1.64, ‐1.29] |
| 6 Mean adhesion scores at second‐look hysteroscopy in women treated for intrauterine adhesions Show forest plot | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐3.3 [‐3.37, ‐3.23] |
| 6.1 Gel vs no tx | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐3.3 [‐3.37, ‐3.23] |
| 7 Mild adhesions at second‐look hysteroscopy Show forest plot | 6 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.68, 2.61] |
| 7.1 Hormonal tx vs placebo/no tx | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.10] |
| 7.2 Gel vs no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.77, 3.18] |
| 8 Moderate or severe adhesions at second‐look hysteroscopy Show forest plot | 6 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.03, 0.24] |
| 8.1 Hormonal tx vs placebo/no tx | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.22] |
| 8.2 Gel vs placebo/no tx | 5 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth Show forest plot | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.57, 3.83] |
| 1.1 Device + graft vs device | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.57, 3.83] |
| 2 Clinical pregnancy Show forest plot | 4 | 221 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.89, 3.33] |
| 2.1 Device + graft vs device | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.74, 3.18] |
| 2.2 Gel + hormone tx (HT) + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.62, 13.04] |
| 3 Miscarriage Show forest plot | 3 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.20, 3.19] |
| 3.1 Device + graft vs device | 3 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.20, 3.19] |
| 4 Presence of intrauterine adhesions at second‐look hysteroscopy Show forest plot | 5 | 451 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.36, 0.83] |
| 4.1 Device vs device | 1 | 162 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.42, 1.57] |
| 4.2 Device + graft vs device | 2 | 137 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.10] |
| 4.3 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.13, 0.76] |
| 4.4 Gel + HT + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.98] |
| 5 Mean adhesion scores in women treated for intrauterine adhesions Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Device vs device | 1 | 162 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.68, 0.68] |
| 5.2 Device + graft vs device | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐4.17, ‐2.03] |
| 5.3 Device + gel vs device | 1 | 111 | Mean Difference (IV, Random, 95% CI) | ‐1.6 [‐2.32, ‐0.88] |
| 6 Mild adhesions at second‐look hysteroscopy Show forest plot | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.53, 2.34] |
| 6.1 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.53, 2.34] |
| 7 Moderate or severe adhesions at second‐look hysteroscopy Show forest plot | 2 | 152 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.10, 0.61] |
| 7.1 Device + gel vs device | 1 | 111 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.09, 0.63] |
| 7.2 Gel + HT + antibiotics vs HT + antibiotics | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.98] |

