Imagenología para la exclusión de la embolia pulmonar en el embarazo
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011053.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 26 enero 2017see what's new
- Tipo:
-
- Diagnostic
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vascular
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
The protocol was written by Thijs van Mens and Paulien de Jong, with comments provided by Mathilde Nijkeuter and Saskia Middeldorp. Mariska Leeflang provided support with methodological aspects of the protocol. Thijs van Mens and Luuk Scheres conducted the search and extracted study data. Thijs van Mens wrote the manuscript with input from all review authors and is the contact author and guarantor of this review.
Sources of support
Internal sources
-
Academic Medical Center, Netherlands.
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office
Declarations of interest
Mathilde Nijkeuter and Saskia Middeldorp were investigators in one included study (Nijkeuter 2013). These review authors were not involved in study selection nor in data extraction for this review.
TvM: none known.
LS: none known.
PdJ: none known.
ML: none known.
SM: Dr Middeldorp's institution has received funding for research grants from BMS/Pfizer, Daiichi Sankyo and Sanquin for investigator‐initiated studies in the field of treatment of VTE and assessment of long‐term outcomes of VTE; payment for lectures from GSK, Bayer, Boehringer Ingelheim and Sanofi; and payment for development of educational presentations from Bayer, GSK, Daiichi Sankyo and BMS/Pfizer. These companies had no influence on the content of the educational material.
Acknowledgements
We thank Rene Spijker from the Academic Medical Center, Amsterdam Medical Library, for assisting with the search.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jan 26 | Imaging for the exclusion of pulmonary embolism in pregnancy | Review | Thijs E van Mens, Luuk JJ Scheres, Paulien G de Jong, Mariska MG Leeflang, Mathilde Nijkeuter, Saskia Middeldorp | |
| 2014 Mar 28 | Imaging for the exclusion of pulmonary embolism in pregnancy | Protocol | Paulien G de Jong, Thijs E van Mens, Mariska MG Leeflang, Saskia Middeldorp, Mathilde Nijkeuter | |
Differences between protocol and review
Additions to the protocol
-
We added a minimum sample size of fewer than 25 pregnant women per index test as an exclusion criterion. In this population, a PE is confirmed in 4% of women (Kline 2014). We therefore deemed cohorts of fewer than 25 as uninformative.
-
We regarded cases with an inconclusive index test who were subsequently treated on clinical grounds as having a positive reference standard. After an inconclusive index test, the chance of a PE is relatively high compared with after a negative test. Yet a missed PE is unlikely to be identified as such in the follow‐up if a woman is receiving anticoagulant treatment. This would lead to an underestimation of false negatives. Therefore, the current adjudication was more conservative than the one provided in the original protocol.
-
We determined PE frequency. We considered this informative, but it was not specified in the protocol.
Alterations to the protocol
-
We removed the question in the QUADAS‐2 checklist "If a threshold was used, was it prespecified?" because very few studies described the use of a threshold, and a prespecified threshold is not of the utmost importance when these imaging tests are performed.
Clarifications of the protocol
The original protocol did not provide guidance on all encountered judgement calls in data collection and analysis. We made the following clarifications.
-
CTPA, lung scintigraphy or MRA had to be performed as an index test, not as part of a reference test.
-
When data on both original assessments of the index test result were reported in the clinical setting, and when re‐assessments for study purposes were reported, we preferred the former because this information matches the clinical question more accurately. We deemed re‐assessments as having high risk of bias.
-
We set the maximum duration of the period between confirmed PE and the index test at three months. With later identified cases, we deemed the chance of newly occurring PE as substantial.
-
If patient recruitment was consecutive, but patients received one of multiple index tests on a non‐systematic basis, we still regarded studies as presenting a consecutive patient series.
-
Lung scintigraphy as an index test comprised perfusion scintigraphy ‐ planar or V/Q SPECT and with or without ventilation scanning.
-
When possible, we included only data on the first index test if the patient had received the same index test more than once. If followed by a different index test, we included this test result.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Angiography [standards, statistics & numerical data];
- Magnetic Resonance Angiography;
- Positron‐Emission Tomography [*standards, statistics & numerical data];
- Pregnancy Complications, Hematologic [*diagnostic imaging];
- Pulmonary Embolism [*diagnostic imaging];
- Radionuclide Imaging [standards, statistics & numerical data];
- Sensitivity and Specificity;
- Tomography, X‐Ray Computed [*standards, statistics & numerical data];
Medical Subject Headings Check Words
Female; Humans; Pregnancy;

Study flow diagram.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.

Primary analysis. Negative predictive values (%) with 95% confidence intervals for CTPA with inconclusives regarded as negative.

Primary analysis. Forest plot of CTPA with inconclusives regarded as negative.

Sensitivity analysis. Negative predictive values (%) with 95% confidence intervals for CTPA with inconclusives regarded as positive.

Sensitivity analysis. Forest plot of CTPA with inconclusives regarded as positive.
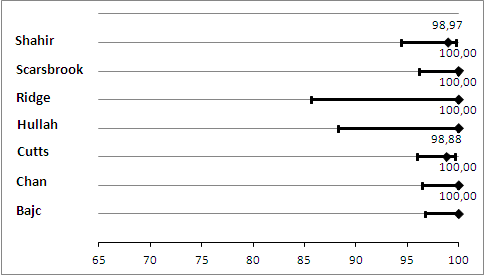
Primary analysis. Negative predictive values (%) with 95% confidence intervals for lung scintigraphy with inconclusives regarded as negative.
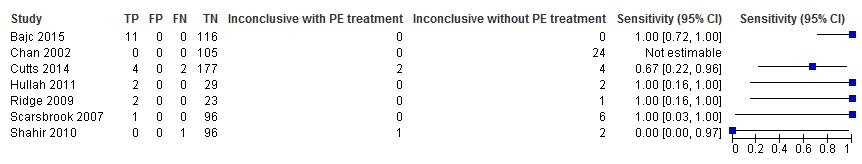
Primary analysis. Forest plot of lung scintigraphy with inconclusives regarded as negative.
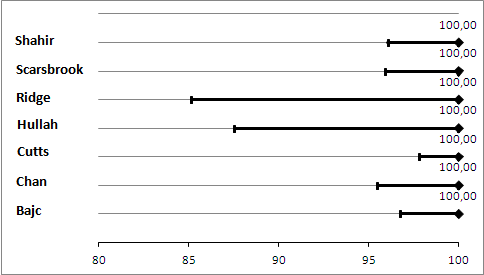
Sensitivity analysis. Negative predictive values (%) with 95% confidence intervals for lung scintigraphy with inconclusives regarded as positive.
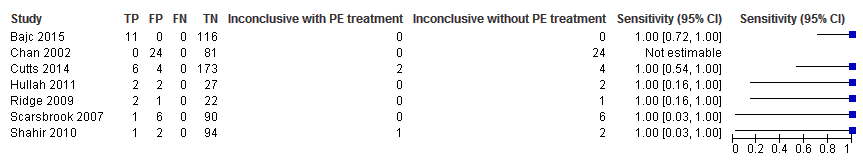
Sensitivity analysis. Forest plot of lung scintigraphy with inconclusives regarded as positive.
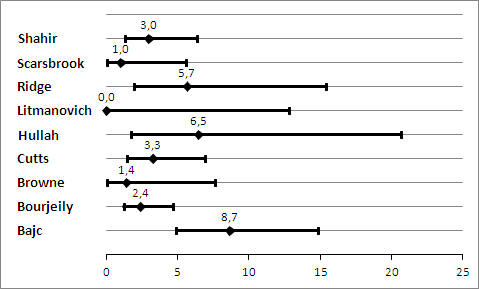
Prevelance of pulmonary embolism (%) with 95% confidence interval.
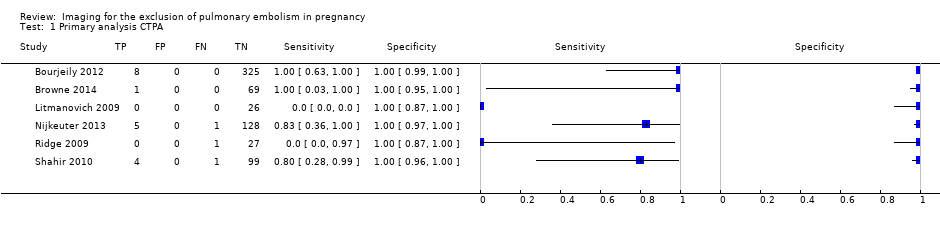
Primary analysis CTPA.
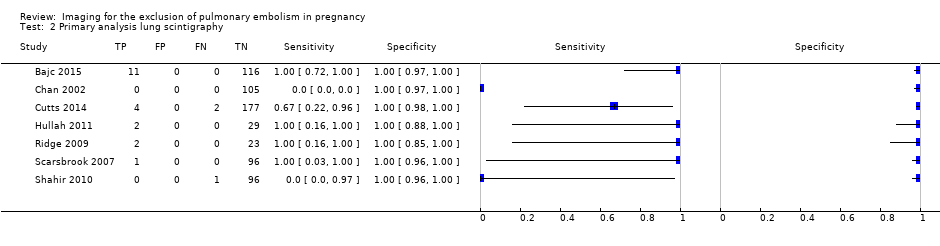
Primary analysis lung scintigraphy.
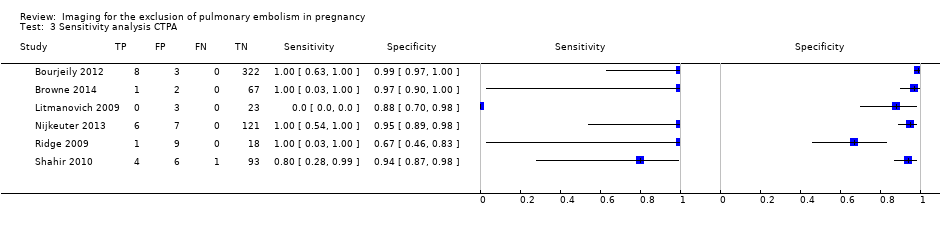
Sensitivity analysis CTPA.

Sensitivity analysis lung scintigraphy.
| What is the diagnostic accuracy of imaging tests for the diagnosis of pulmonary embolism (PE) in pregnancy? | ||||||
| Patients | Pregnant women with clinical suspicion of PE. | |||||
| Prior testing and prevalence | Varied. Most often performed were chest X‐ray and imaging for deep venous thrombosis. The median prevalence of PE was 3.3% (range 0.0% to 8.7%), as assessed by the applied reference standard, which has limitations. | |||||
| Settings | Secondary and tertiary care, both inpatients and outpatients. | |||||
| Index test | Computed tomography pulmonary angiography (CTPA), lung scintigraphy and magnetic resonance angiography (MRA). No studies on MRA were included. Inconclusive test results were regarded as negative in the primary analysis. | |||||
| Importance | Pregnant women are often suspected of PE because of increased risk and physiological signs that mimic symptoms of PE. Pregnant women are often excluded from diagnostic imaging studies. These imaging tests might perform differently during pregnancy, and radiation and other risks are weighed differently. | |||||
| Reference standard | Clinical follow‐up of at least 6 weeks. In almost all studies, follow‐up was performed to identify PE, not to exclude it. Pulmonary angiography was preferred but was applied by none of the studies. | |||||
| Studies | Cross‐sectional cohort studies were included. Case‐control studies were excluded. All studies were retrospective. | |||||
| Test | Number of studies (number of index test results) | Median negative predictive value (range) | Median sensitivity (range) | Median inconclusive test results (range) | Overall risk of bias (QUADAS‐2) | Overall applicability (QUADAS‐2) |
| CTPA | 6 (695) | 100% (96%‐100%) | 83% (0%‐100%) | 5.9% (0.9%‐36%) | High risk | High concern |
| Lung scintigraphy | 7 (665) | 100% (99%‐100%) | 100% (0%‐100%) | 4% (0%‐23%) | High risk | High concern |
| CAUTION: The results in this table should not be interpreted in isolation from results of the individual included studies contributing to each summary test accuracy measure. These are reported in the main body of the text of the review. | ||||||
| CTPA: computed tomography pulmonary angiography. | ||||||
|
| Item plus signalling questions | Criteria for scoring 'yes', 'no' and 'unclear' |
| 1 | PATIENT SELECTION Was a consecutive or random sample of patients enrolled? Was a case‐control design avoided? Did the study avoid inappropriate exclusions? | We will score this item 'yes' when patients were consecutively or randomly selected; 90% or more were evaluated at the hospital; 5% or less of had received anticoagulant therapy within 24 hours before testing; 30% or less were given a diagnosis of comorbidity such as chronic obstructive pulmonary disease or other pulmonary disease, malignancy or pregnancy complications (preeclampsia, syndrome of haemolysis, elevated liver enzymes and low platelets or eclampsia); and 10% or less had undergone prior testing for this episode of suspected PE. We will score 'no' if one of these criteria was not met. |
| 2 | INDEX TEST Were index test results interpreted without knowledge of results of the reference standard? | We will score this item 'yes' in the following cases: if study authors state that the index test interpreter was unaware of the result of the reference test; or if the order of testing was index test before reference test for every patient. Even if clinical follow‐up was the reference test, the order of testing has to be stated for the item to be scored 'yes'. We will score the item ‘no’ for studies in which it is stated that the interpreter of the index test was aware of the result of the reference test. In other cases, we will score this as 'unclear'. In cases of studies directly comparing the diagnostic accuracy of 2 index tests against the reference standard, these test results had to be interpreted without knowledge of the results of the comparator index test, and we will score this item similarly to the approach described above. |
| 3 | REFERENCE STANDARD Is the reference standard likely to correctly classify the target condition? Were reference standard results interpreted without knowledge of results of the index test? | We considered both PA and clinical follow‐up of at least 6 weeks as useful for correct classification of the target condition, the latter only if objective diagnostic tests are used in cases of suspected venous thromboembolism. We will score this item 'yes' if study authors state that reference tests were interpreted without knowledge of results of the index test. Furthermore, in cases of clinical follow‐up as a reference standard, any clinical suspicion of venous thrombosis during follow‐up needs to be followed by objective diagnostic testing (i.e. CUS or venography for suspicion of DVT, and scintigraphy, CTPA or pulmonary angiography for clinical suspicion of PE). If a patient died during follow‐up, we classified death as caused by PE in cases of confirmation by autopsy, in cases of an objective test positive for PE before death or if PE could not be confidently excluded as the cause of death. |
| 4 | FLOW AND TIMING Was an appropriate interval between index test(s) and reference standard provided? Did all patients receive a reference standard? Did all patients receive the same reference standard? Were all patients included in the analysis? | With PA, we will consider a time period of less than 24 hours between index and reference tests as short enough to ensure that the target condition did not change between tests, either because of natural progression of the disease or because of therapeutic intervention. For studies using pulmonary angiography as the reference test, we will score this item 'yes' if the time between index and reference tests was less than 24 hours. Similarly, for studies directly comparing diagnostic accuracy of index tests, we will consider a time period of less than 24 hours between index tests and the reference test as short enough. During clinical follow‐up, the disease may diminish through natural progression or through intervention. Or the condition may arise during follow‐up if it was not present at the time of the index test. Therefore, we will score studies using clinical follow‐up 'no' for this item. We will score this item 'no' if less than 90% or a non‐random selection of patients underwent the reference test. We will score this item 'no' if less than 90% of patients who had an index test result underwent pulmonary angiography or had clinical follow‐up as the reference test. |
| CTPA: computed tomography pulmonary angiography. | ||
| Test | No. of studies | No. of participants |
| 1 Primary analysis CTPA Show forest plot | 6 | 695 |
| 2 Primary analysis lung scintigraphy Show forest plot | 7 | 665 |
| 3 Sensitivity analysis CTPA Show forest plot | 6 | 695 |
| 4 Sensitivity analysis lung scintigraphy Show forest plot | 7 | 665 |

