Визуализация для исключения эмболии легочной артерии во время беременности
Appendices
Appendix 1. MEDLINE search strategy
| 1 | Thromboembolism/ | 20790 |
| 2 | Venous Thromboembolism/ | 5497 |
| 3 | Thrombosis/ | 57313 |
| 4 | Venous Thrombosis/ | 18922 |
| 5 | Upper Extremity Deep Vein Thrombosis/ | 194 |
| 6 | (thromboemboli$ or microthrombus or thrombus or thrombo$ or thrombilic or thrombotic).ti,ab. | 298311 |
| 7 | (DVT or VTE).ti,ab. | 11960 |
| 8 | Pulmonary Embolism/ | 32316 |
| 9 | (pulmonary adj embol$).ti,ab. | 28329 |
| 10 | (pulmonary adj thrombo$).ti,ab. | 3645 |
| 11 | (lung adj embol$).ti,ab. | 381 |
| 12 | (lung adj thrombo$).ti,ab. | 64 |
| 13 | PE.ti,ab. | 25958 |
| 14 | or/1‐13 | 368515 |
| 15 | exp Pregnancy/ | 732514 |
| 16 | pregnan$.ti,ab. | 390395 |
| 17 | prepartum.ti,ab. | 1747 |
| 18 | antepartum.ti,ab. | 4605 |
| 19 | prenatal.ti,ab. | 71313 |
| 20 | antenatal.ti,ab. | 24738 |
| 21 | exp Pregnancy Trimesters/ | 33767 |
| 22 | trimester?.ti,ab. | 42203 |
| 23 | or/15‐22 | 844362 |
| 24 | diagnostic imaging/ | 32966 |
| 25 | exp Lung/ra, ri, us [Radiography, Radionuclide Imaging, Ultrasonography] | 20697 |
| 26 | Pulmonary Artery/ra, ri, us [Radiography, Radionuclide Imaging, Ultrasonography] | 6616 |
| 27 | Angiography/ | 54573 |
| 28 | (pulmonary adj3 angiogr$).ti,ab. | 3629 |
| 29 | (lung adj3 angiogr$).ti,ab. | 248 |
| 30 | magnetic resonance imaging/ | 303668 |
| 31 | magnetic resonance angiography/ | 18622 |
| 32 | Diffusion Magnetic Resonance Imaging/ | 12340 |
| 33 | ((magnetic resonance or MR or MRI or NMR) adj5 (angiogra$ or arteriogra$)).ti,ab. | 16040 |
| 34 | MRA.ti,ab. | 6223 |
| 35 | tomography/ | 9269 |
| 36 | exp tomography, emission‐computed/ | 83280 |
| 37 | exp tomography, x‐ray/ | 325764 |
| 38 | tomography scanners, x‐ray computed/ | 2035 |
| 39 | ((CT or CAT) adj5 (angiogra$ or arteriogra$ or tomograph$)).ti,ab. | 77957 |
| 40 | (comput$ adj3 tomogra$).ti,ab. | 207656 |
| 41 | tomodensitometry.ti,ab. | 592 |
| 42 | (cat adj4 scan$).ti,ab. | 1285 |
| 43 | (ct adj4 scan$).ti,ab. | 74762 |
| 44 | (CTA or CTPA).ti,ab. | 6817 |
| 45 | three dimensional‐ct.ti,ab. | 1015 |
| 46 | (MDCT or MSCT).ti,ab. | 7493 |
| 47 | multislice.ti,ab. | 5013 |
| 48 | multi‐slice.ti,ab. | 1634 |
| 49 | (multi‐row or multirow).ti,ab. | 175 |
| 50 | (single‐slice or singleslice).ti,ab. | 1145 |
| 51 | 3d‐cta.ti,ab. | 227 |
| 52 | exp ultrasonography, doppler/ | 58611 |
| 53 | ultrasonography/ | 63788 |
| 54 | exp ultrasonography, prenatal/ | 26369 |
| 55 | ultrasonics/ | 20767 |
| 56 | ultrasound.ti,ab. | 166695 |
| 57 | ultrasonogra$.ti,ab. | 82761 |
| 58 | ultrasonic$.ti,ab. | 42986 |
| 59 | echograph$.ti,ab. | 8737 |
| 60 | (USS or DUS or CDUS or CEUS).ti,ab. | 2945 |
| 61 | (doppler or duplex).ti,ab. | 110831 |
| 62 | sonograph$.ti,ab. | 45002 |
| 63 | sonogram$.ti,ab. | 3359 |
| 64 | (contrast adj4 US).ti,ab. | 1506 |
| 65 | Phlebography/ | 10804 |
| 66 | radionuclide imaging/ or perfusion imaging/ | 25611 |
| 67 | Ventilation‐Perfusion Ratio/ | 5543 |
| 68 | (VQ or V?Q or V?P).ti,ab. | 34835 |
| 69 | SPECT.ti,ab. | 21984 |
| 70 | perfusion.ti,ab. | 132776 |
| 71 | scintigraph$.ti,ab. | 41522 |
| 72 | or/24‐71 | 1366959 |
| 73 | 14 and 23 and 72 | 2303 |
Appendix 2. Embase search strategy
| 1 | thromboembolism/ | 58814 |
| 2 | thrombosis/ | 118468 |
| 3 | vein thrombosis/ | 30660 |
| 4 | THROMBUS/ | 19125 |
| 5 | MICROTHROMBUS/ | 1060 |
| 6 | (thrombus or microthrombus or thrombotic or thrombilic or thromboemboli$ or thrombos$).ti,ab. | 265666 |
| 7 | DEEP VEIN THROMBOSIS/ | 42111 |
| 8 | deep vein$ thrombo$.ti,ab. | 17473 |
| 9 | deep venous thrombo$.ti,ab. | 12470 |
| 10 | (dvt or VTE).ti,ab. | 20840 |
| 11 | exp lung/ | 306946 |
| 12 | pulmonary artery/ | 42030 |
| 13 | lung embolism/ | 71089 |
| 14 | (pulmonary adj embol$).ti,ab. | 43593 |
| 15 | (pulmonary adj thrombo$).ti,ab. | 5203 |
| 16 | (lung adj embol$).ti,ab. | 761 |
| 17 | (lung adj thrombo$).ti,ab. | 112 |
| 18 | PE.ti,ab. | 39080 |
| 19 | or/1‐18 | 759291 |
| 20 | exp pregnancy/ | 669905 |
| 21 | pregnan$.ti,ab. | 536263 |
| 22 | prepartum.ti,ab. | 1840 |
| 23 | trimester?.ti,ab. | 58057 |
| 24 | antepartum.ti,ab. | 6194 |
| 25 | prenatal.ti,ab. | 89611 |
| 26 | antenatal.ti,ab. | 33682 |
| 27 | or/20‐26 | 895638 |
| 28 | exp diagnostic imaging/ | 124191 |
| 29 | exp nuclear magnetic resonance imaging/ | 606198 |
| 30 | angiography/ | 89251 |
| 31 | (pulmonary adj3 angiogr$).ti,ab. | 5532 |
| 32 | (lung adj3 angiogr$).ti,ab. | 367 |
| 33 | ((magnetic resonance or MR or MRI or NMR) adj5 (angiogra$ or arteriogra$)).ti,ab. | 20670 |
| 34 | MRA.ti,ab. | 9552 |
| 35 | Radiodensitometry/ | 3954 |
| 36 | exp tomography/ | 770782 |
| 37 | ((CT or CAT) adj5 (angiogra$ or arteriogra$ or tomograph$)).ti,ab. | 100240 |
| 38 | (comput$ adj3 tomogra$).ti,ab. | 249406 |
| 39 | tomodensitometry.ti,ab. | 697 |
| 40 | (cat adj4 scan$).ti,ab. | 1803 |
| 41 | (ct adj4 scan$).ti,ab. | 114356 |
| 42 | (CTA or CTPA).ti,ab. | 11995 |
| 43 | three dimensional‐ct.ti,ab. | 1274 |
| 44 | (MDCT or MSCT).ti,ab. | 11824 |
| 45 | (multi‐slice or multislice or multi‐row or multirow).ti,ab. | 9184 |
| 46 | (single‐slice or singleslice).ti,ab. | 1372 |
| 47 | 3d‐cta.ti,ab. | 342 |
| 48 | exp echography/ | 568375 |
| 49 | computed tomography scanner/ | 11875 |
| 50 | ultrasound/ | 123540 |
| 51 | ultrasound.ti,ab. | 242300 |
| 52 | ultrasonogra$.ti,ab. | 108294 |
| 53 | ultrasonic$.ti,ab. | 52948 |
| 54 | echograph$.ti,ab. | 11356 |
| 55 | (USS or DUS or CDUS or CEUS).ti,ab. | 5513 |
| 56 | (doppler or duplex).ti,ab. | 146940 |
| 57 | sonograph$.ti,ab. | 58807 |
| 58 | sonogram$.ti,ab. | 4132 |
| 59 | (contrast adj4 US).ti,ab. | 1993 |
| 60 | exp phlebography/ | 24842 |
| 61 | scintiangiography/ or scintiphlebography/ | 2935 |
| 62 | computer assisted scintigraphy/ | 304 |
| 63 | phlebogra$.ti,ab. | 6440 |
| 64 | lung ventilation perfusion ratio/ | 6711 |
| 65 | lung perfusion/ | 6348 |
| 66 | (VQ or V?Q or V?P).ti,ab. | 46317 |
| 67 | imag$.ti,ab. | 1011000 |
| 68 | perfusion.ti,ab. | 180169 |
| 69 | scintigraph$.ti,ab. | 56484 |
| 70 | SPECT.ti,ab. | 33023 |
| 71 | lung angiography/ | 6667 |
| 72 | or/28‐71 | 2638744 |
| 73 | 19 and 27 and 72 | 5841 |

Study flow diagram.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.

Primary analysis. Negative predictive values (%) with 95% confidence intervals for CTPA with inconclusives regarded as negative.

Primary analysis. Forest plot of CTPA with inconclusives regarded as negative.

Sensitivity analysis. Negative predictive values (%) with 95% confidence intervals for CTPA with inconclusives regarded as positive.

Sensitivity analysis. Forest plot of CTPA with inconclusives regarded as positive.
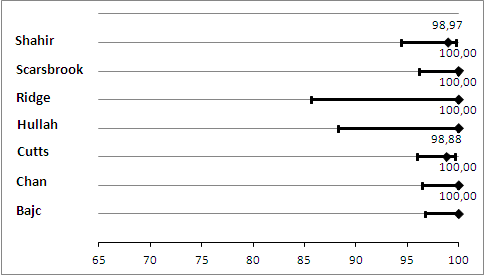
Primary analysis. Negative predictive values (%) with 95% confidence intervals for lung scintigraphy with inconclusives regarded as negative.
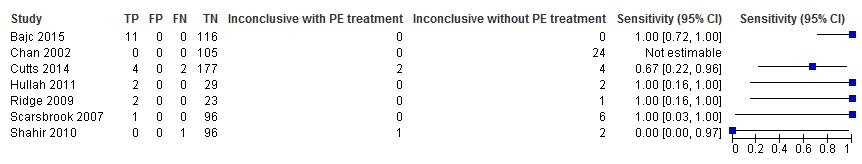
Primary analysis. Forest plot of lung scintigraphy with inconclusives regarded as negative.
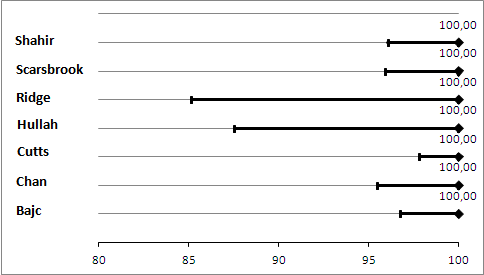
Sensitivity analysis. Negative predictive values (%) with 95% confidence intervals for lung scintigraphy with inconclusives regarded as positive.
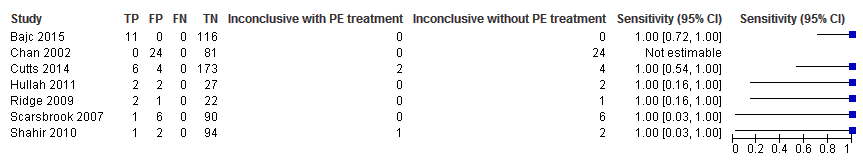
Sensitivity analysis. Forest plot of lung scintigraphy with inconclusives regarded as positive.
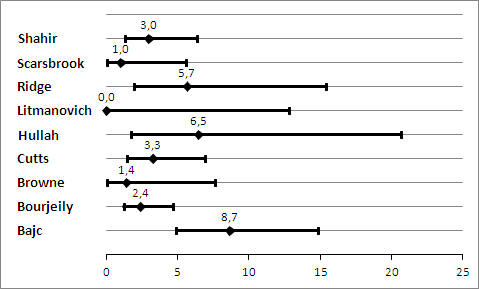
Prevelance of pulmonary embolism (%) with 95% confidence interval.
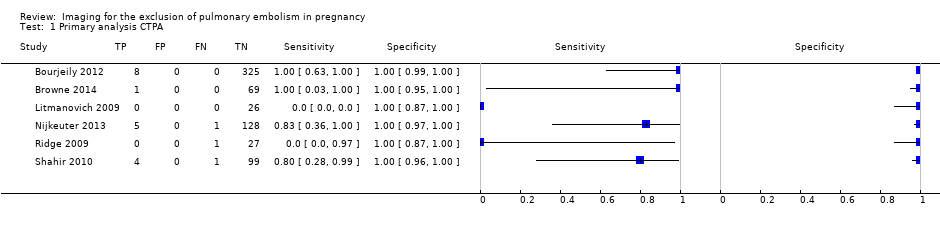
Primary analysis CTPA.
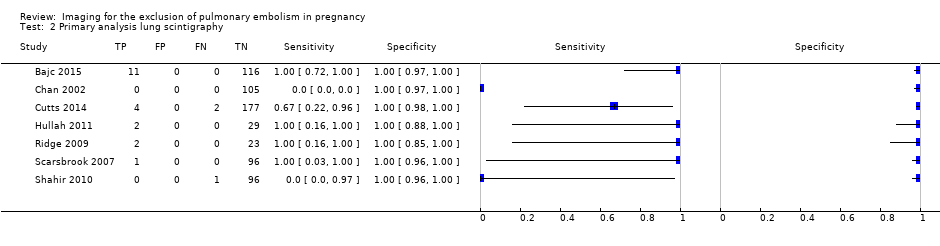
Primary analysis lung scintigraphy.
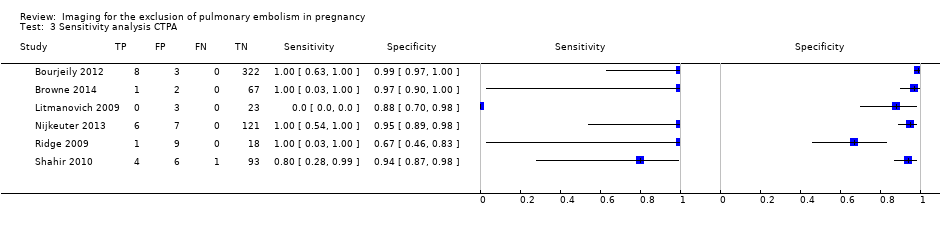
Sensitivity analysis CTPA.

Sensitivity analysis lung scintigraphy.
| What is the diagnostic accuracy of imaging tests for the diagnosis of pulmonary embolism (PE) in pregnancy? | ||||||
| Patients | Pregnant women with clinical suspicion of PE. | |||||
| Prior testing and prevalence | Varied. Most often performed were chest X‐ray and imaging for deep venous thrombosis. The median prevalence of PE was 3.3% (range 0.0% to 8.7%), as assessed by the applied reference standard, which has limitations. | |||||
| Settings | Secondary and tertiary care, both inpatients and outpatients. | |||||
| Index test | Computed tomography pulmonary angiography (CTPA), lung scintigraphy and magnetic resonance angiography (MRA). No studies on MRA were included. Inconclusive test results were regarded as negative in the primary analysis. | |||||
| Importance | Pregnant women are often suspected of PE because of increased risk and physiological signs that mimic symptoms of PE. Pregnant women are often excluded from diagnostic imaging studies. These imaging tests might perform differently during pregnancy, and radiation and other risks are weighed differently. | |||||
| Reference standard | Clinical follow‐up of at least 6 weeks. In almost all studies, follow‐up was performed to identify PE, not to exclude it. Pulmonary angiography was preferred but was applied by none of the studies. | |||||
| Studies | Cross‐sectional cohort studies were included. Case‐control studies were excluded. All studies were retrospective. | |||||
| Test | Number of studies (number of index test results) | Median negative predictive value (range) | Median sensitivity (range) | Median inconclusive test results (range) | Overall risk of bias (QUADAS‐2) | Overall applicability (QUADAS‐2) |
| CTPA | 6 (695) | 100% (96%‐100%) | 83% (0%‐100%) | 5.9% (0.9%‐36%) | High risk | High concern |
| Lung scintigraphy | 7 (665) | 100% (99%‐100%) | 100% (0%‐100%) | 4% (0%‐23%) | High risk | High concern |
| CAUTION: The results in this table should not be interpreted in isolation from results of the individual included studies contributing to each summary test accuracy measure. These are reported in the main body of the text of the review. | ||||||
| CTPA: computed tomography pulmonary angiography. | ||||||
|
| Item plus signalling questions | Criteria for scoring 'yes', 'no' and 'unclear' |
| 1 | PATIENT SELECTION Was a consecutive or random sample of patients enrolled? Was a case‐control design avoided? Did the study avoid inappropriate exclusions? | We will score this item 'yes' when patients were consecutively or randomly selected; 90% or more were evaluated at the hospital; 5% or less of had received anticoagulant therapy within 24 hours before testing; 30% or less were given a diagnosis of comorbidity such as chronic obstructive pulmonary disease or other pulmonary disease, malignancy or pregnancy complications (preeclampsia, syndrome of haemolysis, elevated liver enzymes and low platelets or eclampsia); and 10% or less had undergone prior testing for this episode of suspected PE. We will score 'no' if one of these criteria was not met. |
| 2 | INDEX TEST Were index test results interpreted without knowledge of results of the reference standard? | We will score this item 'yes' in the following cases: if study authors state that the index test interpreter was unaware of the result of the reference test; or if the order of testing was index test before reference test for every patient. Even if clinical follow‐up was the reference test, the order of testing has to be stated for the item to be scored 'yes'. We will score the item ‘no’ for studies in which it is stated that the interpreter of the index test was aware of the result of the reference test. In other cases, we will score this as 'unclear'. In cases of studies directly comparing the diagnostic accuracy of 2 index tests against the reference standard, these test results had to be interpreted without knowledge of the results of the comparator index test, and we will score this item similarly to the approach described above. |
| 3 | REFERENCE STANDARD Is the reference standard likely to correctly classify the target condition? Were reference standard results interpreted without knowledge of results of the index test? | We considered both PA and clinical follow‐up of at least 6 weeks as useful for correct classification of the target condition, the latter only if objective diagnostic tests are used in cases of suspected venous thromboembolism. We will score this item 'yes' if study authors state that reference tests were interpreted without knowledge of results of the index test. Furthermore, in cases of clinical follow‐up as a reference standard, any clinical suspicion of venous thrombosis during follow‐up needs to be followed by objective diagnostic testing (i.e. CUS or venography for suspicion of DVT, and scintigraphy, CTPA or pulmonary angiography for clinical suspicion of PE). If a patient died during follow‐up, we classified death as caused by PE in cases of confirmation by autopsy, in cases of an objective test positive for PE before death or if PE could not be confidently excluded as the cause of death. |
| 4 | FLOW AND TIMING Was an appropriate interval between index test(s) and reference standard provided? Did all patients receive a reference standard? Did all patients receive the same reference standard? Were all patients included in the analysis? | With PA, we will consider a time period of less than 24 hours between index and reference tests as short enough to ensure that the target condition did not change between tests, either because of natural progression of the disease or because of therapeutic intervention. For studies using pulmonary angiography as the reference test, we will score this item 'yes' if the time between index and reference tests was less than 24 hours. Similarly, for studies directly comparing diagnostic accuracy of index tests, we will consider a time period of less than 24 hours between index tests and the reference test as short enough. During clinical follow‐up, the disease may diminish through natural progression or through intervention. Or the condition may arise during follow‐up if it was not present at the time of the index test. Therefore, we will score studies using clinical follow‐up 'no' for this item. We will score this item 'no' if less than 90% or a non‐random selection of patients underwent the reference test. We will score this item 'no' if less than 90% of patients who had an index test result underwent pulmonary angiography or had clinical follow‐up as the reference test. |
| CTPA: computed tomography pulmonary angiography. | ||
| Test | No. of studies | No. of participants |
| 1 Primary analysis CTPA Show forest plot | 6 | 695 |
| 2 Primary analysis lung scintigraphy Show forest plot | 7 | 665 |
| 3 Sensitivity analysis CTPA Show forest plot | 6 | 695 |
| 4 Sensitivity analysis lung scintigraphy Show forest plot | 7 | 665 |

