கடுமையான ஆஸ்துமா கொண்ட குழந்தைகளுக்கு அவசர சிகிச்சைப் பிரிவில் சிகிச்சையளிப்பதற்கான சிரைவழி மக்னீசியம் சல்பேட்
Abstract
Background
Acute asthma in children can be life‐threatening and must be treated promptly in the emergency setting. Intravenous magnesium sulfate is recommended by various guidelines for cases of acute asthma that have not responded to first‐line treatment with bronchodilators and steroids. The treatment has recently been shown to reduce the need for hospital admission for adults compared with placebo, but it is unclear whether it is equally effective for children.
Objectives
To assess the safety and efficacy of intravenous magnesium sulfate (IV MgSO4) in children treated for acute asthma in the emergency department (ED).
Search methods
We identified studies by searching the Cochrane Airways Review Group Specialised Register up to 23 February 2016. We also searched ClinicalTrials.gov and reference lists of other reviews, and we contacted study authors to ask for additional information.
Selection criteria
We included randomised controlled trials of children treated in the ED for exacerbations of asthma if they compared any dose of IV MgSO4 with placebo.
Data collection and analysis
Two review authors screened the results of the search and independently extracted data from studies meeting the inclusion criteria. We resolved disagreements through discussion and contacted study authors in cases of missing data and other uncertainties relating to the studies.
We analysed dichotomous data as odds ratios and continuous data as mean differences, both using fixed‐effect models. We assessed each study for risk of bias and rated the quality of evidence for each outcome with GRADE and presented the results in a 'Summary of findings' table. There was insufficient evidence to conduct the planned subgroup analyses.
Main results
Five studies (182 children) met the inclusion criteria, and four contributed data to at least one meta‐analysis. The included studies were overall at low risk of bias, but our confidence in the evidence was generally low, mainly due to the small sample sizes. Treatment with IV MgSO4 reduced the odds of admission to hospital by 68% (odds ratio (OR) 0.32, 95% confidence interval (CI) 0.14 to 0.74; children = 115; studies = 3; I2 = 63%). This result was based on data from just three studies including 115 children. Meta‐analysis for the secondary outcomes was extremely limited by paucity of data. We performed meta‐analysis for the outcome 'return to the emergency department within 48 hours', which showed a very imprecise effect estimate that was not statistically significant (OR 0.40, 95% CI 0.02 to 10.30; children = 85; studies = 2; I2 = 0%). Side effects and adverse events were not consistently reported and meta‐analysis was not possible, however few side effects or adverse events were reported.
Authors' conclusions
IV MgSO4 may reduce the need for hospital admission in children presenting to the ED with moderate to severe exacerbations of asthma, but the evidence is extremely limited by the number and size of studies. Few side effects of the treatment were reported, but the data were extremely limited.
PICO
எளியமொழிச் சுருக்கம்
கடுமையான ஆஸ்துமா கொண்ட குழந்தைகளில், மக்னீசியம் சல்பேட் உட்செலுத்தல்கள் மருத்துவமனை அனுமதித்தல்களை குறைக்கக் கூடுமா?
பின்புலம்
அநேக குழந்தைகள் உயிரை‐அச்சுறுத்தும் ஆஸ்துமா தாக்குதல்களை அனுபவிக்கக் கூடும் மற்றும் மருத்துவமனை அவசர சிகிச்சைப் பிரிவில் சிகிச்சை தேவைப்படக் கூடும். பிற சிகிச்சைகளுக்கு சிறப்பாக இணங்காத ஆஸ்துமா தாக்குதல்களைக் கொண்ட குழந்தைகளில், மக்னீசியம் சல்பேட் (IV MgSO4) என்ற ஒரு மருந்தை உட்செலுத்தலை சில தேசிய மற்றும் சர்வதேச சிகிச்சை வழிகாட்டல்கள் பரிந்துரைக்கின்றன இந்த சிகிச்சை, வயது வந்தவர்களில் மருத்துவமனை அனுமதித்தல்களின் தேவையை குறைப்பதாக காட்டியுள்ளன, ஆனால் குழந்தைகளுக்கு அதே போன்று பாதுகாப்பாக மற்றும் திறன் மிக்கதாக இருக்குமா என்பது தெளிவாக இல்லை
ஆய்வு பண்புகள்
பிற சிகிச்சைகள் (வழக்கமான மூச்சினுள் இழுக்கப்படும் மூச்சுக்குழல் விரிப்பான்கள், ஸ்டீராய்டுகள், மற்றும் சில சமயங்களில் பிராணவாயு ) ஆஸ்துமா தாக்குதலுக்கு நிவாரணம் அளிக்காத நிலையில், குழந்தைகளில் ஒரு போலி உட்செலுத்தலுடன் MgSO4 உட்செலுத்தலை ஒப்பிட்ட ஐந்து ஆய்வுகளை நாங்கள் கண்டோம். இந்த ஐந்து ஆய்வுகள் மொத்தம் 182 குழந்தைகளை உள்ளடக்கின. நாங்கள் பெரிதும் ஆர்வம் கொண்டிருந்த மருத்துவமனையில் அனுமதிப்பதற்கான தேவை என்ற விளைவை மூன்று ஆய்வுகள் மட்டும் அறிக்கையிட்டிருந்தன. இந்த ஆய்வுகள் 1996 முதல் 2000 வரைக்குமிடையே வெளியிடப்பட்டிருந்தன: பெப்ரவரி 2016‐ல் நாங்கள் தேடிய போது, இவற்றையே மிகவும் சமீபத்திய ஆய்வுகளாக எங்களால் காண முடிந்தது.
முக்கிய முடிவுகள் மற்றும் சான்றின் தரம்
போலி சிகிச்சையோடு ஒப்பிடுகையில், MgSO4 உட்செலுத்தல் கொண்ட வெகு சில குழந்தைகளுக்கு மட்டுமே மருத்துவமனையில் அனுமதிப்பதற்கான தேவை ஏற்பட்டது. உண்மையில் உள்ளபடி, MgSO4 கொண்டு சிகிச்சையளிக்கப்பட்ட ஒவ்வொரு ஐந்து குழந்தைகளுக்கும், ஒரு மருத்துவமனை அனுமதி தடுக்கப்பட்டது. எனினும், பிரதான பகுப்பாய்வில் 115 குழந்தைகள் மட்டுமே இருந்த சிறிய ஆய்வுகள் உள்ளடக்கப்பட்டன; மற்றும் முடிவுகள் வேறுப்பட்டிருந்தன, ஆதலால், நன்மைகள் மற்றும் தீங்குகள் பற்றி எங்களால் முழுவதுமாக உறுதியாய் இருக்க முடியவில்லை. வெகு சில ஆய்வுகளே இருந்ததால், மருத்துவமனை அனுமதித்தல்களின் குறைவு வயது, ஆஸ்துமா மோசமாகுதலின் தீவிரம் ஆகியவற்றோடு தொடர்பு கொண்டதாக இருந்ததா அல்லது பிற சிகிச்சைகள் அளிக்கப்பட்ட போது, வித்தியாசத்தை ஏற்படுத்தியதா என்பது பற்றி எங்களால் சொல்ல முடியவில்லை. குழந்தைகள் MgSO4 பெற்ற போது, தீங்கு பற்றிய எந்த அறிக்கைகளும் இல்லை. ஆதலால், குழந்தைகளில் MgSO4‐ன் பயன்பாட்டை இந்த திறனாய்வு ஆதரிக்கிறது, எனினும், அதன் பயன்பாட்டிற்கு மிக வலுவல்லாத ஆதாரமே உள்ளது.
Authors' conclusions
Summary of findings
| MgSO4 compared to placebo for treating children with acute asthma in the emergency department | ||||||
| Patient or population: children with acute asthma in the emergency department | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | MgSO4 | |||||
| Hospital admissions | 767 per 1000 | 513 per 1000 | OR 0.32 | 115 | ⊕⊕⊝⊝ | MgSO4 reduced hospital admissions, but low confidence due to inconsistency and small numbers. Random‐effects sensitivity analysis: OR 0.18, 95% CI 0.02 to 1.59 |
| ED treatment time (minutes) | The mean ED treatment time in the placebo group was 96 minutes | The mean ED treatment time in the intervention group was (24 less to 34 more) | ‐ | 27 | ⊕⊕⊝⊝ low3 | No clear benefit of MgSO4. Based on the subset of children who were discharged home, not those who were admitted |
| Return to ED within 48 hours | 22 per 1000 | 9 per 1000 | OR 0.4 | 85 | ⊕⊕⊝⊝ | No clear benefit of MgSO4 |
| Hospital length of stay (hours) | The mean hospital length of stay (hours) in the placebo group was | The mean hospital length of stay (hours) in the intervention group was | ‐ | 47 | ⊕⊕⊝⊝ | Possible benefit of MgSO4 but based on 1 small study |
| 4 of the planned outcomes were not reported in a way that could be meta‐analysed in any of the included studies (intensive care admissions, vital signs, spirometry, validated paediatric symptom scores, and adverse events) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Test for heterogeneity P = 0.07, I2 = 63% (‐1 inconsistency). | ||||||
Background
Description of the condition
Asthma is a chronic respiratory condition characterised by inflammation of the airways and partially reversible airflow obstruction. Common symptoms include cough, wheezing, difficulty breathing, reduced exercise tolerance, and chest tightness. In asthma airway inflammation and bronchospasm (contraction of the smooth muscle lining the airways) leads to airflow obstruction. The condition follows a varying course in individuals that is driven by genetic and environmental triggers.
Asthma symptoms vary in severity and frequency. It can cause daily chronic symptoms and exacerbations. An exacerbation is defined as an acute worsening of asthma symptoms. Principles of treatment consist of controlling daily symptoms and preventing exacerbations by providing good education and appropriate inhaler usage. National and international guidelines have been published for the treatment of asthma exacerbations (BTS/SIGN 2014; GINA 2015).
Short‐acting bronchodilators are given to relieve bronchospasm, and symptoms of inflammation are treated with corticosteroids; both are usually delivered via inhalers. Depending on the persistence of symptoms, inhalers can be taken regularly (maintenance therapy) or on an as‐needed basis (reliever therapy) (BTS/SIGN 2014; GINA 2015). Beta2‐agonists are recognised as most effective in relieving bronchospasm (Teoh 2012), however anticholinergic inhalers have also proved effective in the treatment of acute asthma (Griffiths 2013).
Children with asthma are most often managed in primary care, however, in severe cases, secondary‐level care by a paediatrician may be necessary. The goal of treatment is to allow a good quality of life while avoiding asthma exacerbations that require a visit to the emergency department (ED) and hospital admission.
In severe exacerbations, which can be life‐threatening, further medications may be required, such as oral or intravenous corticosteroids (BTS/SIGN 2014; GINA 2015; Rowe 2001). Intravenous bronchodilators and magnesium sulfate have also been used to treat children with severe asthma exacerbations.
Description of the intervention
Recent clinical guidelines advise that a single dose of intravenous magnesium sulfate (IV MgSO4) can be considered for children 5 years of age and older with acute severe asthma who have not responded to inhaled bronchodilator therapy and for those with life‐threatening or near‐fatal asthma (BTS/SIGN 2014).
Magnesium sulfate (MgSO4) has been used in a nebulised form in the treatment of acute severe asthma; this is the subject of a separate review, which found no significant reduction in hospital admission (Powell 2012). For the purposes of this review, we have considered only the use of IV MgSO4. Dosage in children is usually based on weight. The British National Formulary for Children advises 40 mg/kg and up to a maximum total dose of 2 g, delivered by intravenous infusion over 20 minutes. However, larger doses of up to 75 mg/kg have been used (Scarfone 2000).
How the intervention might work
The mechanism of action of IV MgSO4 in the context of an exacerbation of asthma is not fully understood. It is believed to play a role in bronchial smooth muscle relaxation via its ability to stop calcium ion movement into smooth muscle cells by blocking the voltage‐dependent calcium channels (Spivey 1990). Some evidence has also been found of its role in reducing the inflammatory response (Cairns 1996). The combination of smooth muscle relaxation and anti‐inflammatory properties provides a theoretical basis for the use of MgSO4 in cases of acute asthma.
Why it is important to do this review
One in 11 children in the UK suffer from asthma. Asthma presentations in EDs are common, peaking at 26,969 admissions in 2006/2007 (Millet 2013). A total of 216 deaths from asthma were reported in the UK in 2014; 16 of these individuals were children 14 years of age or younger (Asthma UK). In fact, between 2005 and 2010, 1% to 4.2% of all admissions to paediatric intensive care units (PICUs) in the UK were due to asthma; this translates to 1640 admissions (in 1410 patients). Furthermore, the number of admissions to PICUs in the UK due to asthma is rising. Asthma‐related admissions increased by 67% (195 to 327 admissions) between 2005 and 2010 (Nyman 2011).
Historically, MgSO4 is a treatment used in the ED. The National Review of Asthma Deaths reviewed 195 deaths from asthma between Febuary 2012 to January 2013 and found that 45% had attended an ED prior to death (NRAD 2014). When these patients present with life‐threatening episodes of asthma, we need effective and safe treatments.
Although current guidelines advocate the use of IV MgSO4 in the treatment of acute asthma (BTS/SIGN 2014; GINA 2015), it is acknowledged that evidence in the literature has provided conflicting results. An earlier version of this review, Rowe 2000, found little evidence to support the use of IV MgSO4 in children based on results from seven studies, five of which studied adult participants.
The burden of asthma in children continues to increase and as such it is important to be able to guide treatment based on paediatric evidence. As such, the previous review, Rowe 2000, has been split into adult, Kew 2014, and paediatric reviews, focusing the discussion and conclusions to the respective patient groups. This review has provided the opportunity to review any new evidence that has emerged over the past 16 years and draw conclusions relevant to current paediatric practice.
Objectives
To assess the safety and efficacy of intravenous magnesium sulfate (IV MgSO4) in children treated for acute asthma in the ED.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials of any follow‐up duration. We included studies reported as full text, those published as abstract only, and unpublished data.
Types of participants
We included studies of children (18 months to 18 years of age) treated in the ED for acute asthma (all severities). When studies recruited both adults and children, we only used data if provided for children separately. As wheezy symptoms in children younger than 18 months may represent a different disease process (that is bronchiolitis), we examined participant demographics in trials that included children younger than 18 months to determine percentage of the study population. If they made up more than 10% of the population, we excluded the studies.
Types of interventions
We included studies comparing any dose of IV MgSO4 versus placebo. Because children with acute asthma often require additional treatments, we included studies that allowed other medications provided they were not part of the randomly assigned treatment. We did not include studies of MgSO4 combined with other intravenous bronchodilator agents unless the study set out to test the effect of MgSO4, and all other treatments were the same in both groups. We did not intend to assess IV MgSO4 against nebulised MgSO4 or other active treatments. We have presented the results in a summary characteristics table that includes a list of medications given in each of the included studies (Table 1).
| Study ID | Country (centres) | Total N | Study design | Age range (yrs) | Dose (infusion) | Comedications |
| USA (2) | 30 | R, DB, PC | 6 to 18 | 25 mg/kg 20 minutes | 3 nebulised bronchodilators (albuterol, ipratropium bromide, or both) | |
| USA (1) | 31 | R, DB, PC | 6 to 18 | 40 mg/kg 20 minutes | 3 nebulised beta‐2 adrenergic treatments IV methylprednisolone (2 mg/kg) if not yet given corticosteroids | |
| India (1) | 47 | R, DB, PC | 1 to 12 | 0.2 ml of 50% 35 minutes | Nebulised salbutamol Oxygen, IV aminophylline, corticosteroids | |
| Turkey (1) | 20 | R, DB, PC | 6 to 16 | 40 mg/kg 20 minutes | 3 beta‐2 adrenergic nebuliser treatments | |
| USA (3) | 54 | R, DB, PC | 1 to 18 | 75 mg/kg 20 minutes | Nebulised albuterol Oxygen, methylprednisolone |
R = randomised; DB = double‐blind; PC = placebo‐controlled
Types of outcome measures
Primary outcomes
-
Hospital admissions.
Secondary outcomes
-
ED treatment duration.
-
Intensive care admissions.
-
Hospital length of stay.
-
Vital signs (respiratory rate, oxygen saturations).
-
Spirometry (peak expiratory flow rate (PEFR), forced expiratory volume in one second (FEV1)).
-
Validated paediatric symptom scores.
-
Adverse events.
Reporting in the study one or more of the outcomes listed here was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of multiple bibliographic databases and by handsearching of respiratory journals and meeting abstracts (see Appendix 1 for further details). We searched all records in the CAGR using the search strategy provided in Appendix 2. The most recent search was conducted on 23 Februray 2016.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en/). We searched all databases from their inception to the present, and imposed no restriction on language of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information. We also searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed) on 8 April 2016.
Data collection and analysis
Selection of studies
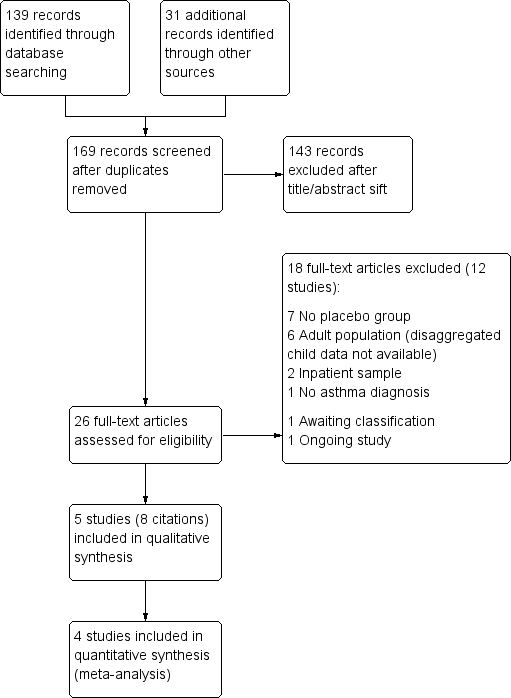
Two review authors (BG and KMK) independently screened titles and abstracts of all citations identified by the search for inclusion and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publications, and both review authors independently screened the full text and identified studies for inclusion. We identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion, or, if required, by consulting a third person. We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and a Characteristics of excluded studies table.
Data extraction and management
To record study characteristics and outcome data, we used a data collection form that had been piloted on at least one study in the review. One review author (KMK) extracted study characteristics from the included studies, and both review authors independently extracted outcome data. We extracted the following study characteristics.
-
Methods: study design, duration of observation and follow‐up, details of any 'run‐in' period, number of study centres and locations, withdrawals, and date of study.
-
Participants: N, mean age, age range, gender, asthma severity, diagnostic criteria, baseline lung function, inclusion criteria, and exclusion criteria.
-
Interventions: intervention, dose, comparison, concomitant and failed treatments, and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for trial and notable conflicts of interest of trial authors.
We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third person. The two review authors transferred data into the Review Manager (version 5.3) file together (RevMan 2014). We double‐checked that data had been entered correctly by comparing data presented in the systematic review with information in the study reports. A second review author (BG) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Both review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), resolving disagreements by discussion. We assessed the risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (for example for unblinded outcome assessment, risk of bias for hospital admissions may be very different than for a participant‐reported scale). When information on risk of bias related to unpublished data or correspondence with a study author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to this published protocol and have reported any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios and continuous data as mean differences or standardised mean differences. If studies reported several validated symptom measures, or if different scales were reported across studies, we analysed the data as standardised mean differences in one analysis to reduce measurement error and enhance precision. We entered the presented data as a scale with a consistent direction of effect. We narratively described skewed data reported as medians and interquartile ranges.
We undertook meta‐analyses only when this was meaningful (that is when treatments, participants, and the underlying clinical question were similar enough for pooling to make sense).
When multiple trial arms were reported in a single trial, we included only the relevant arms. If two relevant comparisons from a single study were combined in the same meta‐analysis, we halved the control group to avoid double‐counting.
Unit of analysis issues
For dichotomous outcomes, we used participants rather than events as the unit of analysis (that is number of children with any adverse events rather than the total number of events).
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (for example when we identified a study as an abstract only). When this was not possible and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by performing a sensitivity analysis.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. When we identified substantial heterogeneity, we reported this and explored possible causes by prespecified subgroup analysis.
Assessment of reporting biases
We were not able to pool more than 10 studies, and so could not create and examine a funnel plot to explore possible small‐study and publication biases. We considered the impact of unpublished studies in the GRADE ratings for each outcome.
Data synthesis
We used a fixed‐effect model and performed a sensitivity analysis with random‐effects when we observed significant heterogeneity (I² greater than 30%).
Summary of findings table
We created a 'Summary of findings' table for all five outcomes. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro GDT. We justified all decisions to downgrade or upgrade the quality of studies using footnotes, and made comments to aid readers' understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
Based on observations in previous versions of this review and to focus recommendations about the appropriateness of the intervention within specific patient groups, we planned the following subgroup analyses.
-
Baseline severity of exacerbation (moderate, severe, life‐threatening*).
-
Age (≤ and > 5 years).
We used the formal test for subgroup differences in RevMan 2014
*As no single metric has been accepted for assessing asthma severity in children, we planned to extract baseline data relevant to the following severity criteria as stated in the recent asthma guidelines (BTS/SIGN 2014).
-
Ability to speak and eat.
-
Breaths per minute.
-
Pulse.
-
Pulse oximetry.
-
Peak flow.
-
Arterial (oxygen saturation).
BG labelled study populations as moderate, severe, and life‐threatening based on available data. The judgements were not made by an independent assessor as planned because we were not able to perform the subgroup analysis, so classification was made for descriptive purposes only. If additional studies allow the subgroup analysis to be undertaken in a future update of this review, this will be done by an assessor blinded to the study results.
Sensitivity analysis
We planned the following sensitivity analyses.
-
Studies at high risk of bias for blinding.
-
Studies including children < 18 months of age.
-
Unpublished data.
Results
Description of studies
We have provided full details of the conduct and participant characteristics of each included study in the Characteristics of included studies table, and reasons for excluding full texts in the Characteristics of excluded studies table.
Results of the search
We identified 139 records in the electronic database searches and 31 additional records by searching ClinicalTrials.gov. We removed one duplicate record, screened 169 records, and excluded 143 by looking at the titles and abstracts alone. We retrieved full texts for the remaining 26, of which 18 were excluded for the following reasons: 'no placebo group' (n = 7), 'adult population' (n = 6), 'inpatient sample' (n = 2), no asthma diagnosis (n = 1), awaiting classification because we were unable to locate the publication (Abd El Kader 1997), and ongoing (NCT01522040). The remaining eight citations related to five studies, which we included in the systematic review (Ciarallo 1996; Ciarallo 2000; Devi 1997; Gürkan 1999; Scarfone 2000). We have presented the study flow in Figure 1.

Study flow diagram.
Included studies
Five studies met all the inclusion criteria (Ciarallo 1996; Ciarallo 2000; Devi 1997; Gürkan 1999; Scarfone 2000), randomising a total of 182 children presenting to the ED. Sample sizes were small, ranging from 20 to 54 (median 31). We have provided a summary of study characteristics in Table 1.
All of the included studies were randomised, double‐blind, placebo‐controlled trials, which were conducted at between one and three centres. Three studies were conducted in the USA (Ciarallo 1996; Ciarallo 2000; Scarfone 2000), one in India (Devi 1997), and one in Turkey (Gürkan 1999). The main time of follow‐up measurement was not reported in Devi 1997, but in the remaining four studies it ranged from 90 to 120 minutes after the start of the infusion.
Inclusion and exclusion criteria varied across the five studies with respect to age, severity metrics such as lung function and vital statistics, and allowed and disallowed comedications. The minimum age was 6 years in three studies, and 1 year in two studies (Devi 1997; Scarfone 2000). The upper age for inclusion was 16 or 18 in all studies except Devi 1997, which used a maximum age of 12 years.
We have provided a summary of the characteristics of children included in the studies in Table 2, including mean age, percentage male, and key measures of lung function and vital signs when they entered the ED.
| Study ID | Inclusion | Group | Age (SD) | % Male | % PEF | FEV1 | Other | Classification |
| PEF < 60% predicted (after 3 beta‐2 adrenergic nebuliser treatments) | MgSO4 | 10.8 | 46.7 | 43.8 | 33.1 | RR = 35 BP = 120 SaO2 = 92 | Moderate | |
| Placebo | 11.9 | 43.8 | 43.0 | 45.1 | RR = 30 BP = 123 SaO2 = 94 | |||
| PEF < 70% predicted (after 3 nebulised bronchodilating treatments) | MgSO4 | 10.9 | 68.8 | 29.9 | 28.9 | BP = 120, SaO2 = 92 | Severe | |
| Placebo | 12.0 | 50.0 | 33.1 | 31.3 | BP = 114, SaO2 = 92 | |||
| "Inadequate or poor response to 3 doses of nebulized salbutamol" | MgSO4 | 6.7 | 79.2 | 30.1 | NR | HR = 142 | Severe | |
| Placebo | 6.8 | 73.9 | 27.1 | NR | HR = 138 | |||
| PEF < 60% predicted (after 3 beta‐2 adrenergic nebuliser treatments) | MgSO4 | 10.4 | 60 | 46.8 | NR | HR = 118 BP = 118 SaO2 = 91.8 | Moderate | |
| Placebo | 11.2 | 50 | 46.2 | NR | HR = 120 BP = 116 SaO2 = 91.4 | |||
| "moderate to severe asthma exacerbation" | MgSO4 | 6.8 | 58 | NR | NR | SaO2 = 93.9 | Moderate | |
| Placebo | 4.8 | 47 | NR | NR | SaO2 = 94.1 |
SD = standard deviation; % PEF = percentage predicted peak expiratory flow; FEV1 = forced expiratory volume in one second; HR = heart rate; RR = respiration rate; BP = systolic blood pressure; SaO2 = oxygen saturation; NR: not reported
Across the five studies, 89 children were randomised to receive MgSO4 and 93 to placebo. In four studies, between 25 and 75 mg/kg MgSO4 was administered over 20 minutes, and Devi 1997 gave 0.2 ml of 50% over 35 minutes (100 mg). The placebo was always delivered in a matching saline infusion. The administration of nebulised bronchodilators, usually multiple times, was common across studies. All studies except Gürkan 1999 described the use of corticosteroids, usually methylprednisolone, and two also stated that oxygen had been used (Devi 1997; Scarfone 2000).
Three of the five studies reported the primary outcome (Ciarallo 1996; Ciarallo 2000; Scarfone 2000); the secondary outcomes were generally poorly reported. No data could be analysed for intensive care admissions, vital signs, spirometry, symptom scales, or adverse events.
Excluded studies
We excluded 17 articles relating to 12 studies after viewing the full texts. Bijani 2001, Bilaceroglu 2001, Boonyavorakul 2000, del Castillo Rueda 1991, and Skobeloff 1989 were all included in the adult review (Kew 2014); although these studies included some participants under 18, they were classified as adult populations and disaggregated data could not be obtained. Singhi 2011 and Torres 2012 used the correct population and intervention and were relatively large compared to the included studies (100 and 143 respectively), but these were open‐label studies that did not use a placebo comparison. Irazuzta 2016 studied children with status asthmaticus and did not use a placebo comparison, and Watanatham 2015 compared nebulised MgSO4 with IV MgSO4 without a placebo group. We excluded Santana 2001 because the study recruited children who had already been admitted to a special paediatric care unit before they were given IV MgSO4. Similarly, Okayama 1987 recruited children from mixed settings, including those already admitted to hospital, which could not be separated from the children who met the inclusion criteria for this review. The remaining study, Liang 1998, did not require that children had a diagnosis of asthma to be included in the study.
Risk of bias in included studies
Overall, the risk of bias across the studies was low, with some uncertainties relating to attrition and methods of allocation, and some issues with selective reporting (Figure 2).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We rated no studies as having a high risk of bias for sequence generation or allocation concealment. We rated only one study as low risk for both selection bias domains (Ciarallo 2000), and rated the other four as unclear in one domain, in Ciarallo 1996 and Scarfone 2000, or both domains, in Devi 1997 and Gürkan 1999.
Blinding
It is unlikely that biases related to insufficient blinding affected the results. All studies used matched placebos and double‐blind procedures, although outcome assessor blinding was unclear in one study that was only reported as a conference abstract (Gürkan 1999).
Incomplete outcome data
There were some uncertainties in this domain, but no study reported drop‐out rates that were high or unbalanced enough that we considered it to be at high risk of bias. Two studies did not report how many children were not accounted for in the analyses (Ciarallo 2000; Gürkan 1999), and we rated the other three as at low risk of bias (Ciarallo 1996; Devi 1997; Scarfone 2000).
Selective reporting
There was evidence of selective reporting in two studies conducted by the same author (Ciarallo 1996; Ciarallo 2000). Both reported some outcomes without variance, with inexact P values, or only in graphs, meaning the data could not contribute to the meta‐analyses. We rated the other three studies as at low risk (Devi 1997; Gürkan 1999; Scarfone 2000).
Other potential sources of bias
Ciarallo 1996 was terminated before the specified sample was reached due to a change in ED practice (intravenous access was used less frequently in the care of status asthmaticus), which slowed the rate at which eligible patients were enrolled. There were also baseline imbalances in lung function between the two groups, which the study authors recognised may have magnified the difference between magnesium and placebo. We noted no additional sources of bias in the other studies (Ciarallo 2000; Devi 1997; Gürkan 1999; Scarfone 2000).
Effects of interventions
A summary of the main results, including absolute effects and an assessment of the quality of the evidence, can be found in summary of findings Table for the main comparison.
Primary outcomes
Hospital admissions
Treatment with IV MgSO4 reduced the odds of admission to hospital by 68% (odds ratio (OR) 0.32, 95% confidence interval (CI) 0.14 to 0.74; children = 115; studies = 3; I2 = 63%; Analysis 1.1). This result was based on data from three studies including 115 children (Ciarallo 1996; Ciarallo 2000; Scarfone 2000). The result was statistically significant (P = 0.008) but had a wide confidence interval that estimated a true population reduction in admission between 86% and 26%. In absolute terms, 767 out of 1000 people given placebo needed a hospital admission, compared with 513 (95% CI 315 to 709) out of 1000 given IV MgSO4. This translates to a number needed to treat for an additional beneficial outcome of 4. There was statistically significant heterogeneity in the analysis (I2 = 63%, P = 0.07) and a sensitivity analysis using a random‐effects model provided a much less precise result that was not statistically significant (OR 0.18, 95% CI 0.02 to 1.59), but the direction and size of the effect was still in favour of IV MgSO4. The studies were of good methodological quality, however the small number of study participants and heterogeneity reduced our confidence in the result to low.
Secondary outcomes
Emergency department treatment duration
In the one study that reported data for duration of treatment in the ED (Scarfone 2000), use of IV MgSO4 caused children to spend an extra five minutes in the ED. However, the effect estimate was very imprecise, and the result was neither statistically nor clinically significant (mean difference (MD) 5.00, 95% CI ‐24.40 to 34.40; children = 27; studies = 1; Analysis 1.2). We downgraded the quality of this evidence twice for imprecision and rated as low because just one small study contributed to the analysis.
Intensive care admissions
No studies reported admission to intensive care in a way that we could include in our analyses.
Return to emergency department within 48 hours
We did not specify this outcome in the protocol for this systematic review, but chose to present the results because it is related to other named outcomes which were not well reported across the studies. Two studies with 85 children reported data for this outcome (Ciarallo 1996; Scarfone 2000), but one did not observe any events and so did not contribute to a pooled effect. In both groups the event rate was low, and the confidence intervals suggest a very imprecise effect estimate that was not statistically significant (OR 0.40, 95% CI 0.02 to 10.30; children = 85; studies = 2; I2 = 0%; Analysis 1.3). The small number of studies and events in this analysis resulted in a very imprecise estimate that warranted downgrading the quality of evidence twice for imprecision and rating as low.
Hospital length of stay
One study with 47 children reported data for this outcome (Devi 1997). Treatment with IV MgSO4 reduced the length of hospital admission by 5.3 hours (MD ‐5.30, 95% CI ‐9.46 to ‐1.14; children = 47; studies = 1; Analysis 1.4). The effect estimate was imprecise but favours the treatment group at each extreme, and the result was statistically significant. Again this analysis was based on one study with very few children; we also downgraded for publication bias because two other studies reported hospital admission but not length of hospital stay, rating the evidence as low quality.
Vital signs (respiratory rate, oxygen saturations)
No studies reported vital signs in a way that we could include in our analyses. Devi 1997 reported oxygen saturations in graphical form, therefore data could not be accurately collected, however they did report a statistically significant difference favouring the treatment group. Examining the graph the effect was seen from 0 to 15 hours postinfusion.
Spirometry
No studies reported peak expiratory flow rate (PEFR) or forced expiratory volume in one second (FEV1) in a way that we could include in our analyses. Ciarallo 1996 reported the outcome for FEV1, forced vital capacity (FVC), and PEFR as a statistical difference between groups after treatment favouring the magnesium group. Devi 1997 reported PEFR in graphical form, therefore data could not be accurately collected, however they again reported a statistically significant difference favouring the treatment group. Examining the graph the effect was seen from 0 to 15 hours postinfusion. Gürkan 1999 reported a significant increase in the mean of percentage of improvement from baseline in PEFR at 30 minutes after initiation of magnesium infusion (P = 0.0002), and they found an even greater improvement at the end of the observation period (P = 0.0001). Ciarallo 2000 found the PEFR and FEV1 improvement from baseline was statistically greater in the group that had received IV MgSO4. They reported that this effect was apparent at all study time points.
Validated paediatric symptom scores
No studies reported validated asthma symptom scores. Gürkan 1999 reported a significant change in mean clinical asthma score of the children in the magnesium group at 90 minutes (P = 0.005). Ciarallo 2000 reported that there were statistically significant differences in the clinical asthma scores between the two groups, which occurred later in the study period at 95 minutes (1.4 IV MgSO4 group versus 2.5 placebo group) and 110 minutes (1.1 IV MgSO4 group versus 2.4 placebo group). Scarfone 2000 reported a pulmonary index score at 7 points and found no statistical difference between groups (P = 0.37).
Adverse events
The included studies did not report enough data to enable meta‐analysis, however reviewing the narrative results revealed a low incidence of adverse events in both groups. Gürkan 1999 reported no significant difference in side effects (but did not report which symptoms this included) and no significant difference in heart rate or blood pressure. Scarfone 2000 reported no episodes of hypotension in either group and no difference between groups in degree of tachycardia. Only one child in the placebo group experienced emesis. Ciarallo 2000 reported no intergroup difference in systolic blood pressure. Ciarallo 1996 also reported no difference in blood pressure. They did state that two children in the treatment group reported a relaxed sensation compared to none in the placebo group. There were no reports of dizziness, fatigue, or any other adverse symptoms.
Devi 1997 reported the following adverse effects in the treatment group: epigastric warmth (12.5%), pain (16.6%), and tingling and numbness (12.5%) at the site of infusion. There were no reported incidents of these symptoms in the control group.
Subgroup and sensitivity analyses
Since there were only three studies in the primary analysis (and fewer in the secondary), we did not consider the planned subgroup analyses on the basis of baseline severity of exacerbation and age to be justified. Similarly, we were unable to conduct the planned sensitivity analyses to test the robustness of the results in relation to detection bias, the inclusion of very young children, and unpublished data.
Discussion
Summary of main results
Children with predominantly moderate to severe asthma presenting to the ED who were treated with IV MgSO4 in addition to standard therapy showed a 68% reduction in the odds of hospital admission (OR 0.32, 95% CI 0.14 to 0.74). This result represents a dramatic reduction in admission rates, however confidence in the result was low due to the small number of children included in the analysis (n = 115) and variability between study results.
The variability in study results was reflected in the estimate of heterogeneity for the primary outcome. Examining the primary analysis, the data from Scarfone 2000 appears to lie outside those reported in the other two studies. It is difficult to explain the heterogeneity, as the studies were very similar in design and study population included. All of the studies were relatively small in size, increasing the imprecision in the reporting of effects. Repeating the analysis using a random‐effects model gave a result that was not statistically significant (OR 0.18, 95% CI 0.02 to 1.59). This again highlights that although the results favour the use of MgSO4, the strength of that conclusion is very limited.
Data for the secondary outcomes was also limited. Two studies provided data for the outcome 'return to the emergency department within 48 hours' (Ciarallo 1996; Scarfone 2000), which favoured IV MgSO4, but the confidence intervals did not exclude no difference (OR 0.40, 95% CI 0.02 to 10.30). Several studies reported improvements in spirometric data with treatment, but did not include the raw data for meta‐analysis. The message from the narrative description of results for symptoms score and spirometry in all but one case favoured treatment with IV MgSO4. However, interpreting these results requires even greater caution.
Traditionally asthma has been referred to in the literature in terms of severity, and as such national guidelines are often framed in this manner. We intended to perform a subgroup analysis by severity subgroup. We had proposed to get a blinded review author to rate the severity of each study based on the inclusion criteria, but the limited amount of available data prevented this subgroup analysis. However, examining the inclusion criteria for the three studies that presented data for the primary outcome (Ciarallo 1996; Ciarallo 2000; Scarfone 2000), we can see that all children had a peak expiratory flow (PEF) less than 70%, which would have traditionally been referred to as moderate to severe exacerbations.
While the evidence is based on a moderate‐to‐severe cohort, it is acknowledged that severity in paediatric practice is often a clinical judgement as spirometry in young children is frequently limited in its accuracy.
Again, data on side effects was inconsistently reported, which limited the meta‐analysis, but on the whole the number of side effects was small. In fact, the only reported side effect was a relaxed sensation in two children (Ciarallo 1996). There was no reported evidence of the haemodynamic instability that is often historically reported with the use of MgSO4. The lack of any reported harm is encouraging, but the ability to extrapolate this result is again limited by the small number of studies included in the review.
If we accept that there is weak evidence of the effectiveness of MgSO4, the question of timing of administration needs to be addressed. The included study protocols administered IV MgSO4 after failure to improve with first‐line nebulised bronchodilator therapy. We would agree that MgSO4 remains a second‐line therapy after patients have failed to respond to more evidence‐based nebulised bronchodilator therapies. In clinical practice IV MgSO4 is often used in conjunction with other IV bronchodilators (salbutamol and aminophylline). As the studies included in this review only examined MgSO4 as a single IV agent, evaluating any possible synergistic interaction was beyond the scope of this review.
Overall completeness and applicability of evidence
Three studies contributed data to the primary outcome assessing the potential effect of IV MgSO4 on preventing hospital admission (Ciarallo 1996; Ciarallo 2000; Scarfone 2000). The studies used between 25 and 75 mg/kg of MgSO4, but all were given in a 20‐minute infusion. We cannot comment on optimal dosing given the limited data in the review; at this time it would be prudent to comply with national guidance which is in line with the range in this review. All three studies were conducted in the USA, which has implications on the applicability of the evidence to other healthcare systems, especially with regard to the comedications administered previous to MgSO4. The US studies gave children nebulised bronchodilators (albuterol, ipratropium bromide, or both) and IV methylprednisolone, which may not be the practice in other countries.
A common criticism of hospital admission as an outcome is that the criteria to make this decision is often not standardised, and it is widely acknowledged that admission rates vary by country and even region. However, where randomisation is appropriate it should provide a reliable between‐group difference in moderate‐to‐severe patients. It may be argued that severe asthma by its nature requires admission to hospital, in which case admission to paediatric intensive care unit (PICU) may be a possible outcome. However, it must also be acknowledged that in some institutions some therapies mandate admission to a PICU (certain IV therapies), in which case the need for intubation and mechanical ventilatory support may represent an alternative. Rightly or wrongly, burden of disease is widely reported as a rising admission rate, therefore it stands to reason we should attempt to use this as an outcome of treatment efficacy.
The children in the studies had a mean age of around 11 in Ciarallo 1996 and Ciarallo 2000 and were younger on average in Scarfone 2000, at around 7 in the active group and 5 in the placebo group. This mean age would support the recommendation in British Thoracic Society guidance, however younger children were also included and treated safely in the studies.
The age imbalance within Scarfone 2000 may have affected the results and suggests that the sample sizes of the included studies, of which Scarfone 2000 is the largest at 54, are unlikely to have been sufficiently large enough to assume that important baseline variables were evenly distributed by randomisation. These differences and limitations in the design and conduct of the studies contributing data means that the implications for practice must be carefully considered.
No more than two studies contributed to any of the secondary outcomes, and we were unable to conduct a meta‐analysis for five of the eight secondary outcomes. As such, even the three secondary outcomes for which we could include data (duration in ED, return to ED within 48 hours, and duration of hospital stay) included data for no more than 88 children, which severely limits the applicability of any conclusions that can be drawn. Outcomes that could not be supported by analyses included admission to intensive care unit, spirometry, and adverse events, which we fully expected to be better reported in studies conducted in an emergency setting. For these outcomes, we tried to describe narratively information in the studies that was not fully reported in order to give the fullest picture possible, but they represent an important gap in the evidence base.
Previous reviews have examined subgroups based on exacerbation severity and concluded that IV MgSO4 has a role primarily in the treatment of severe exacerbations. We have already alluded to the difficulties of assessing asthma severity in children, but our results are most applicable to children with moderate or severe asthma attacks. This group of patients is more likely to present to the ED, therefore we would agree that currently the role of IV MgSO4 would be in moderate to severe patients who have failed to respond to inhaled therapies, and not to children presenting in primary care. Clinically MgSO4 is also used prior to or concurrently with nebuliser therapy in severe life‐threatening episodes where severe obstruction to air flow limits the effectiveness of inhaled therapies. Studies in this review did not test IV MgSO4 in these situations, but we excluded a study recruiting children in status asthmaticus that did not include a placebo group (Irazuzta 2016). Prioritisation of therapies in extremis needs to be based on sound clinical judgement and experience. It is important to recognise that asthma attacks run a continuum, often lapsing and relapsing over several days, and what role magnesium has in altering the course of an exacerbation is unknown.
Quality of the evidence
While there were some uncertainties in the study procedures, particularly with selection and attrition bias, we considered the included studies to be of good methodological quality overall, and so none of the outcomes were downgraded for risk of bias. Additionally, the five studies closely matched the inclusion criteria set out in the protocol, therefore we did not consider the evidence to be compromised by indirectness.
However, our confidence in the findings was reduced by serious imprecision in the estimates, largely due to very small numbers of studies and participants. In the case of the primary outcome, serious differences in what the three studies found resulted in the effect becoming imprecise when we performed a random‐effects sensitivity analysis, and this reduced our confidence in the main finding.
Incomplete outcome reporting in two studies affected some of the secondary outcomes that we were not able to meta‐analyse (spirometry, intensive care admissions, vital signs). As mentioned above, the small number of included studies overall and the very small amount of data suitable for meta‐analysis may suggest publication bias, and limits the conclusions that can be drawn.
Potential biases in the review process
This review examined included studies for bias against predetermined criteria as specified by current Cochrane methodology. We identified internal reporting biases in two of the included studies (Ciarallo 1996; Ciarallo 2000), which may have impacted on the completeness of our results, but this was considered in the relevant GRADE ratings. Ciarallo 1996 reported in their methodology a wide range of parameters that were collected on participants but only reported some of these outcomes, which all had significant results. The authors stated that the trial recruitment period was cut short because of a change in ED practice. Their original power calculation stipulated 40 participants to detect a 25% difference in PEFR; only 31 participants were enrolled, and as such the estimate of effect can be exaggerated. However, we did not include PEFR in our meta‐analysis, and so our results were not affected. The authors also note there was a difference in the baseline characteristic (FEV1) between the groups, which could again have led to an overestimate of the effect in the treatment group.
We implemented the planned methods as far as possible, but in some cases the small number of studies meant this was not possible or valid. We have listed these instances in Differences between protocol and review.
Agreements and disagreements with other studies or reviews
Having urged caution in the interpretation of the primary analysis, it can be seen that the result favouring admission reduction in the IV MgSO4 group is in keeping with the recently published adult review. A recent review of IV MgSO4 for acute asthma in the ED in adult patients reported a reduction in the odds of hospital admission of 25% (OR 0.75, 95% CI 0.60 to 0.92) based on 11 studies including 1769 participants (Kew 2014). This systematic review of the adult evidence contains new data from the largest adult study to date (Goodacre 2013, n = 752). They also found an improvement in spirometric parameters in participants treated with IV MgSO4. An older Cochrane review that assessed adults and children together only found a treatment effect in a severe patient subgroup (Rowe 2000).
Caution is required in extrapolating results from adult asthma studies, as childhood asthma is reported to respond differently to some established therapies.
Cheuk 2005 published a meta‐analysis of paediatric patients, which reported hospital admission as its primary outcome. It included four studies in this analysis, three of the studies presented here and also admission data for Devi 1997 (admission data not included in published article and was not made available despite attempts to contact the study author). The meta‐analysis concluded that magnesium was likely to reduce hospital admission and improve bronchospasm.
Most recently in the paediatric literature Ohn 2014 published a review stating magnesium should be given to all children presenting to hospital with acute severe asthma. This conclusion was based on two meta‐analyses that included adult and paediatric patients, Rowe 2001 and Shan 2013, and one recent randomised controlled trial, Torres 2012, which was excluded from this review as it compared IV MgSO4 versus standard care and not placebo. Torres 2012 reported a statistically significant reduction in the need for mechanical ventilation in the IV MgSO4 group.
MgSO4 can also be administered as an aerosol by nebuliser devices. There has been increasing interest in its use in adults and children with acute asthma, and a Cochrane review in 2012 found no improvement in lung function and no decrease in hospital admission with its use (Powell 2012). A large paediatric randomised controlled trial examining its use in children in the ED (N = 508) was recently published (Powell 2013), and while the new study is yet to be incorporated in to their Cochrane review (Powell 2012), the study did not find a clinical difference in asthma severity score and did not report hospital admission data. Admission to PICU/high dependency unit was reported and was required by 35 out of 508 children (9% in the treatment group versus 6% in the placebo group).
IV MgSO4 is commonly used in paediatric practice. In a survey of 183 ED consultants in the UK and Ireland, 94.5% report using it in their management of acute wheeze, and nearly one‐third (28.4%) use it as their first‐line intravenous agent (Lyttle 2015). The overwhelming narrative from the paediatric and adult literature supports the use of IV MgSO4. This conclusion, while in keeping with this review, has at times been based on a very limited evidence base. Despite the largest adult trial to date showing no statistical difference, its inclusion in the most recent Cochrane analysis has provided a more robust base for the use of IV MgSO4 in adults. In paediatric practice the literature has often drawn from adult data and paediatric data not obtained from randomised controlled trials. This review does draw a similar conclusion to the previous paediatric meta‐analysis, but highlights the very severe limitations of the evidence.
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
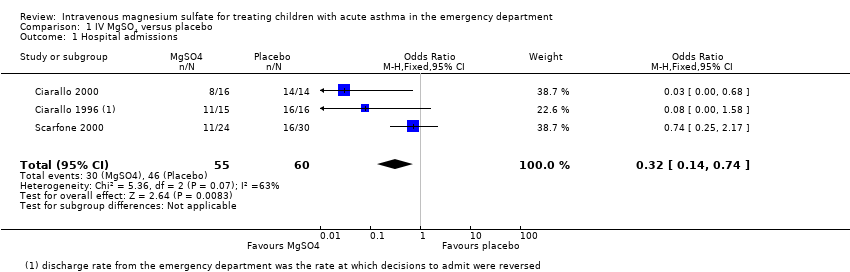
Comparison 1 IV MgSO4 versus placebo, Outcome 1 Hospital admissions.
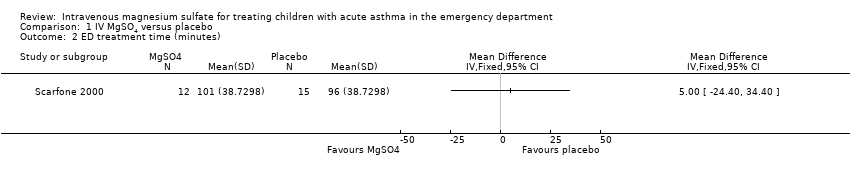
Comparison 1 IV MgSO4 versus placebo, Outcome 2 ED treatment time (minutes).

Comparison 1 IV MgSO4 versus placebo, Outcome 3 Return to ED within 48 hours.

Comparison 1 IV MgSO4 versus placebo, Outcome 4 Hospital length of stay (hours).
| MgSO4 compared to placebo for treating children with acute asthma in the emergency department | ||||||
| Patient or population: children with acute asthma in the emergency department | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | MgSO4 | |||||
| Hospital admissions | 767 per 1000 | 513 per 1000 | OR 0.32 | 115 | ⊕⊕⊝⊝ | MgSO4 reduced hospital admissions, but low confidence due to inconsistency and small numbers. Random‐effects sensitivity analysis: OR 0.18, 95% CI 0.02 to 1.59 |
| ED treatment time (minutes) | The mean ED treatment time in the placebo group was 96 minutes | The mean ED treatment time in the intervention group was (24 less to 34 more) | ‐ | 27 | ⊕⊕⊝⊝ low3 | No clear benefit of MgSO4. Based on the subset of children who were discharged home, not those who were admitted |
| Return to ED within 48 hours | 22 per 1000 | 9 per 1000 | OR 0.4 | 85 | ⊕⊕⊝⊝ | No clear benefit of MgSO4 |
| Hospital length of stay (hours) | The mean hospital length of stay (hours) in the placebo group was | The mean hospital length of stay (hours) in the intervention group was | ‐ | 47 | ⊕⊕⊝⊝ | Possible benefit of MgSO4 but based on 1 small study |
| 4 of the planned outcomes were not reported in a way that could be meta‐analysed in any of the included studies (intensive care admissions, vital signs, spirometry, validated paediatric symptom scores, and adverse events) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Test for heterogeneity P = 0.07, I2 = 63% (‐1 inconsistency). | ||||||
| Study ID | Country (centres) | Total N | Study design | Age range (yrs) | Dose (infusion) | Comedications |
| USA (2) | 30 | R, DB, PC | 6 to 18 | 25 mg/kg 20 minutes | 3 nebulised bronchodilators (albuterol, ipratropium bromide, or both) | |
| USA (1) | 31 | R, DB, PC | 6 to 18 | 40 mg/kg 20 minutes | 3 nebulised beta‐2 adrenergic treatments IV methylprednisolone (2 mg/kg) if not yet given corticosteroids | |
| India (1) | 47 | R, DB, PC | 1 to 12 | 0.2 ml of 50% 35 minutes | Nebulised salbutamol Oxygen, IV aminophylline, corticosteroids | |
| Turkey (1) | 20 | R, DB, PC | 6 to 16 | 40 mg/kg 20 minutes | 3 beta‐2 adrenergic nebuliser treatments | |
| USA (3) | 54 | R, DB, PC | 1 to 18 | 75 mg/kg 20 minutes | Nebulised albuterol Oxygen, methylprednisolone | |
| R = randomised; DB = double‐blind; PC = placebo‐controlled | ||||||
| Study ID | Inclusion | Group | Age (SD) | % Male | % PEF | FEV1 | Other | Classification |
| PEF < 60% predicted (after 3 beta‐2 adrenergic nebuliser treatments) | MgSO4 | 10.8 | 46.7 | 43.8 | 33.1 | RR = 35 BP = 120 SaO2 = 92 | Moderate | |
| Placebo | 11.9 | 43.8 | 43.0 | 45.1 | RR = 30 BP = 123 SaO2 = 94 | |||
| PEF < 70% predicted (after 3 nebulised bronchodilating treatments) | MgSO4 | 10.9 | 68.8 | 29.9 | 28.9 | BP = 120, SaO2 = 92 | Severe | |
| Placebo | 12.0 | 50.0 | 33.1 | 31.3 | BP = 114, SaO2 = 92 | |||
| "Inadequate or poor response to 3 doses of nebulized salbutamol" | MgSO4 | 6.7 | 79.2 | 30.1 | NR | HR = 142 | Severe | |
| Placebo | 6.8 | 73.9 | 27.1 | NR | HR = 138 | |||
| PEF < 60% predicted (after 3 beta‐2 adrenergic nebuliser treatments) | MgSO4 | 10.4 | 60 | 46.8 | NR | HR = 118 BP = 118 SaO2 = 91.8 | Moderate | |
| Placebo | 11.2 | 50 | 46.2 | NR | HR = 120 BP = 116 SaO2 = 91.4 | |||
| "moderate to severe asthma exacerbation" | MgSO4 | 6.8 | 58 | NR | NR | SaO2 = 93.9 | Moderate | |
| Placebo | 4.8 | 47 | NR | NR | SaO2 = 94.1 | |||
| SD = standard deviation; % PEF = percentage predicted peak expiratory flow; FEV1 = forced expiratory volume in one second; HR = heart rate; RR = respiration rate; BP = systolic blood pressure; SaO2 = oxygen saturation; NR: not reported | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hospital admissions Show forest plot | 3 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.14, 0.74] |
| 2 ED treatment time (minutes) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Return to ED within 48 hours Show forest plot | 2 | 85 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 10.30] |
| 4 Hospital length of stay (hours) Show forest plot | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐9.46, ‐1.14] |

