کلشیسین برای پیشگیری از وقوع حوادث قلبیعروقی
Referencias
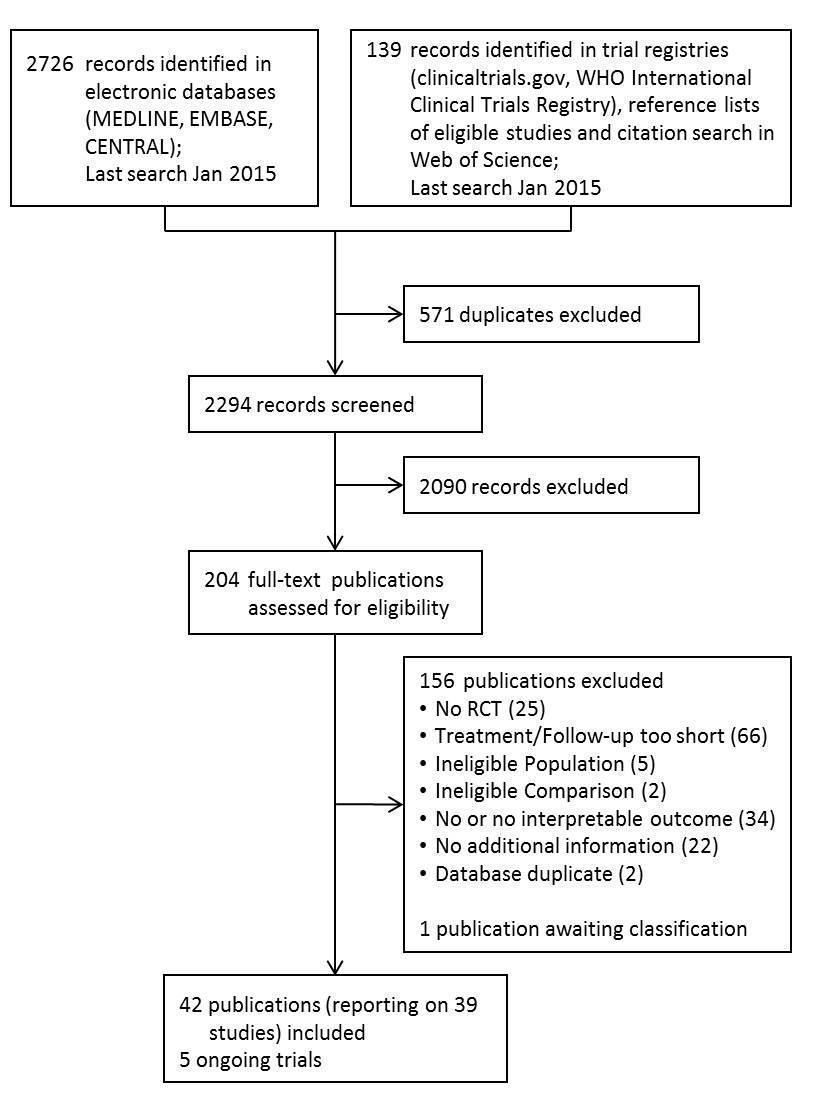
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در انتظار ارزیابی
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | ||
| Methods | Design: Pseudo‐randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 11 years | |
| Participants | Number randomised: Total 52. 29 colchicine. 23 control Condition: Ascitic cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Not reported Age mean (SD) in years: colchicine 54.27 (14.27). control 54.21 (15.04) Sex (women): 13% Inclusion criteria: "The trial included 52 ascitic cirrhotic patients." Exclusion criteria: "According to Kershenobich the patients were excluded if they had evidence of gastrointestinal bleeding or of encephalopathy during a period of two weeks before the trial." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation according to odd/even age |
| Allocation concealment (selection bias) | Low risk | Pharmacy supplied drugs and kept identity of drugs confidential |
| Blinding of participants and personnel (performance bias) | Low risk | "neither staff, nor the patients knew the drug used", double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Analysis unclear; 13% missing data reported for control group ‐ imbalance to colchicine group might cause bias |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, multicentre (6 centres, 2 centres in substudy) Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 3 years | |
| Participants | Number randomised: Total 90. 46 colchicine, 44 control. (Substudy: Total 44. colchicine 22, control 22) Condition: Primary biliary cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Italy Age mean (SD) in years: Colchicine 55.5 (10.9), control 53.3 (10.2). (Substudy: colchicine 55 (11), control 58 (10)) Sex (women): Colchicine 87%, control 93% (Substudy: colchicine 86, control 86) Inclusion criteria: "The criteria for entry into the trial were: an established diagnosis of primary biliary cirrhosis according to Taal et al.; symptomatic disease as defined by presence of pruritus (severe enough to necessitate therapy); and/or serum bilirubin higher than 2 mg/dl; and/or histological or clinical diagnosis of cirrhosis. Patients were included regardless of the duration of symptoms or the stage." Exclusion criteria: "Exclusion criteria were: advanced liver disease (ascites, encephalopathy, portal hypertensive bleeding, bilirubin >10 mg/dl); hepatocellular carcinoma; any concomitant immunosuppressive treatment; evidence of other major diseases unrelated to primary biliary cirrhosis; alcohol abuse; and low compliance. Treatment with cholestyramine, antihistamines, H₂‐blockers or proton‐pump inhibitors, calcium supplementation and liposoluble vitamins was allowed." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Substudy results are reported from 2 of 6 centres (with separate randomisation) with longer follow‐up. We used the main study results for our analyses. Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was performed by a central study unit using the same randomized blocks separately for each centre" |
| Allocation concealment (selection bias) | Low risk | Central randomization |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | ITT; ≤ 10% missing data per group |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, multicentre (8 centres) Blinding: Open‐label Longest mean follow‐up period for a review‐relevant outcome: 25 months | |
| Participants | Number randomised: Total 50. 18 colchicine, 32 control. Condition: Idiopathic pulmonary fibrosis Cardiovascular risk profile: Not reported Setting: outpatient Country: Greece Age median (range) in years: Colchicine 69 (54 ‐ 85), control 66 (42 ‐ 82) Sex (women): 16% Inclusion criteria: "Eligible patients were aged 40–80 yrs, had shown clinical symptoms of IPF for ≥3 months, and had a forced vital capacity (FVC) of ≥55% and ≤90% of the predicted value, a transfer factor of the lung for carbon monoxide (TL,CO) of ≥35% pred and an arterial oxygen tension (Pa,O₂ ) of >7.3 kPa while breathing room air at rest." Exclusion criteria: "Criteria for exclusion were a significant history of exposure to organic or inorganic dust or drugs known to cause pulmonary fibrosis and connective tissue disease or other chronic lung diseases causing pulmonary fibrosis, a ratio of the forced expiratory volume in one second to FVC of <0.6 after bronchodilator use, a residual volume of >120% pred, active infection within 1 week before enrolment, unstable cardiovascular or neurological disease, uncontrolled diabetes, pregnancy, lactation, any active malignancy likely to result in death or any condition other than IPF likely to result in death within 3 yrs." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | "supported by an unrestricted grant from Boehringer Ingelheim Hellas (Athens, Greece) and the Society for Pulmonary and Intensive Care Research in the district of East Macedonia and Thrace (Alexandrou´polis, Greece). The Greek National Health System supported both colchicine and interferon gamma‐1b." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed using a random number table." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Open trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Two readers, blinded to the clinical functional data and type of treatment, examined the HRCT images"; however, we do not measure the outcomes related to the HRCT images; no other blinding reported |
| Incomplete outcome data (attrition bias) | High risk | In total, 17 (11 in the IFN‐c‐1b group and 6 in the colchicine group) of the 50 participants discontinued treatment before 24 months. Of the 11 participants in the IFN‐c‐1b group, 8 stopped because of an adverse event and/or disease progression and 3 for social reasons. Of the colchicine group, 6 participants withdrew; 2 stopped because of disease progression and 4 for social reasons. In addition, median follow‐up was longer (median 5 months) in control group than in colchicine group |
| Selective reporting (reporting bias) | Unclear risk | "The study was originally designed to investigate the molecular perspective after both treatment regimens. Owing to technical difficulties, this aim was only investigated in a subgroup of 10 patients (data not shown). The study did not have prespecified end‐points." |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 26 months | |
| Participants | Number randomised: Total 57. Colchicine 28 control 29 Condition: Primary biliary cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: USA Age mean (SD) in years: Colchicine 53 (not reported), control 51 (not reported) Sex (women): Colchicine 93%, control 90% Inclusion criteria: "History of chronic cholestatic liver disease and liver biopsy results compatible with PBC were entered into the study. Fifty‐one of our patients were followed at Mount Sinai Medical." Exclusion criteria: Not reported | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Endpoint mortality (extracted from Bodenheimer 1985) reported only for 26 months. Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The liver histology was reviewed without knowledge of the clinical status or the drug the patient was receiving." However, we did not look into liver outcomes, so the blinding remains unclear |
| Incomplete outcome data (attrition bias) | High risk | Unbalanced attrition and missing reporting: in the abstract from 1985, Bodenheimer et al report 39% attrition in colchicine group and 24% in the control group at 26 weeks follow‐up. In their publication from 1988, they report 29% attrition in colchicine and 21% in the control group at a mean follow‐up of 33 months. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial Blinding: Not reported Longest mean follow‐up period for a review‐relevant outcome: 36 months | |
| Participants | Number randomised: Total 180. Colchicine 100, placebo 80 Condition: Liver cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Not reported Age: Not reported Sex (women): Not reported Inclusion criteria: Liver cirrhosis Exclusion criteria: Not reported | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | This study is reported only as an abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Analysis unclear, no withdrawal reported |
| Selective reporting (reporting bias) | High risk | Only abstract, objectives unclear |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: mean 45 months | |
| Participants | Number randomised: Total 129. Colchicine 63, control 66 Condition: Alcoholic cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Not reported Age: Not reported Sex (women): Not reported Inclusion criteria: People with alcoholic cirrhosis Exclusion criteria: Not reported | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | This study is only reported as an abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported specifically enough (sequential numbers) |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) | High risk | From initially 129 participants: 41 died, another 41 were withdrawn (26 did not comply, 10 had adverse events, 5 for geographic reasons) |
| Selective reporting (reporting bias) | High risk | Abstract only; no protocol reported |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial Blinding: Unclear Longest mean follow‐up period for a review‐relevant outcome: 2 years | |
| Participants | Number randomised: Total 555. Colchicine 269, control 286 Condition: HCV with advanced liver disease Cardiovascular risk profile: Not reported Setting: Not reported Country: Unclear Age mean (SD) in years: mean 51 (not reported) Sex (women): 30% Inclusion criteria: "IFN failures with Ishak stage 3−6" and "Study patients had no evidence of liver decompensation or HCV (liver cancer); Ishak Fibrosis stage 3 or more; HIV & HbeAg negative." Exclusion criteria: Not reported | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | This study was reported as an abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported specifically enough (sequential numbers) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | ITT; dropouts were censored; unclear how many dropouts |
| Selective reporting (reporting bias) | High risk | Abstract only; results from secondary endpoints not described |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Open‐label Longest mean follow‐up period for a review‐relevant outcome: 20 months | |
| Participants | Number randomised: Total 84. Colchicine 42, control 42 Condition: Recurrent pericarditis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Italy Age mean (SD) in years: Colchicine 56.4 ± 16.9 control 51.2 ± 16.3 Sex (women): Colchicine 62%, control 69% Inclusion criteria: "Inclusion criteria were a diagnosis of recurrent pericarditis (first episode); previous idiopathic, viral, and autoimmune etiologies (including postpericardiotomy syndromes and connective tissue diseases) of the first episode of acute pericarditis; 18 years or older; and informed consent." Exclusion criteria: "Exclusion criteria were tuberculous, neoplastic, or purulent etiologies of the first episode; known severe liver disease or current transaminase levels greater than 1.5 times the upper limit of normal; a current serum creatinine level greater than 2.5 mg/dL (221 µmol/L); known myopathy or a current serum creatine kinase level greater than the upper limit of normal; known blood dyscrasias or gastrointestinal disease; pregnant and lactating women or women of child bearing potential not protected by a contraception method; known hypersensitivity to colchicine; and current treatment with colchicine for any indication." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified ("Randomization was based on permuted blocks, with a block size of 4.") |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Low risk | "The validation of clinical events was ensured by an ad hoc committee of expert cardiologists blinded to patient treatment assignment", "data analyses were performed by an external data analysis committee masked to treatment assignment" |
| Incomplete outcome data (attrition bias) | High risk | ITT, but "clinical follow‐up data were available in all patients for a mean of 20 months (range, 8‐44 months)" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, multicentre (4 centres) Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 2 years | |
| Participants | Number randomised: Total 120. Colchicine 60, control 60 Condition: Recurrent pericarditis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Italy Age mean (SD) in years: Colchicine 47.3 (14.4), control 47.9 (15.4) Sex (women): Colchicine 57%, control 52% Inclusion criteria: "Inclusion criteria were a definite diagnosis of recurrent pericarditis (first recurrence), age 18 years or older, and provision of informed consent. The relevant institutional review boards and ethics committees approved our research protocol, and all participants gave written informed consent" Exclusion criteria: "Patients were excluded if they were having their first episode of acute pericarditis or their second or subsequent recurrence or had pericarditis with tuberculous, purulent, or neoplastic causes; known severe liver disease; current aminotransferase levels greater than 1.5 times the upper limit of normal, current serum creatinine level greater than 221 mol/L(2.5mg/dL); known myopathy; serum creatine kinase level above the upper limit of normal; known blood dyscrasias; gastrointestinal disease; or known hypersensitivity to colchicine. Also excluded were pregnant or lactating women (because of the contraindication to colchicine) and women in their childbearing years who were not using contraception. Finally, we excluded persons who were receiving or had previously received colchicine for any indication." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | "Primary Funding Source: Maria Vittoria Hospital, Torino, Italy." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "central computer– based automated sequence Randomization was based on permuted blocks, with a block size of 4. The random allocation sequence was implemented by using sequentially numbered containers." |
| Allocation concealment (selection bias) | Low risk | "central computer–based automated sequence" |
| Blinding of participants and personnel (performance bias) | Low risk | "participants and trial investigators were blinded to randomized treatment"; double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Low risk | "Data were collected by using case report and clinical events adjudication forms and were managed by investigators who were blinded to treatment assignments. A blinded clinical end point committee adjudicated all events." |
| Incomplete outcome data (attrition bias) | Low risk | ITT, 7% in the control and 8% in the colchicine group discontinued intervention |
| Selective reporting (reporting bias) | Unclear risk | "Study protocol: Available from Dr. Imazio (e‐mail, [email protected]). Statistical code and data set: available from Dr. Imazio (e‐mail, [email protected]) for personal use, with approval from the Steering Committee" |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, multicentre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 20 months | |
| Participants | Number randomised: Total 240. Colchicine 120, control 120 Condition: Recurrent pericarditis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Italy Age mean (SD) in years: Colchicine 48.6 (13.6), placebo 48.9 (15.5) Sex (women): Colchicine 45%, control 55% Inclusion criteria: "Consecutive patients aged 18 years or older with two or more recurrences of pericarditis (idiopathic, viral, post‐cardiac injury, or caused by connective tissue disease) were eligible for enrolment." Exclusion criteria: "Patients with any of the following were ineligible: tuberculous, neoplastic, or purulent pericarditis; severe liver disease or current aminotransferase concentrations more than 1.5 times the upper limit of the normal; serum creatinine concentration more than 221.00 μmol/L; skeletal myopathy or serum creatine kinase concentration more than the upper limit of the normal; blood dyscrasia; inflammatory bowel disease; hypersensitivity to colchicine or other contraindication to colchicine; current treatment with colchicine; and life expectancy of 18 months or less. Pregnant or lactating women or women of childbearing potential not using contraception were also ineligible, as were patients with evidence of myopericarditis as indicated by any increase of serum troponin concentration." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | "supported by the former Azienda Sanitaria 3 of Torino (now ASLTO2) within the Italian National Health service. Acarpia (Madeira, Portugal) provided the study drug and placebo as an unrestricted grant." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned to receive colchicine or placebo (1:1) with a central computer‐based automated sequence." |
| Allocation concealment (selection bias) | Low risk | "The random allocation sequence was implemented with sequentially numbered study drug containers." |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled, "All patients and investigators were masked to treatment allocation." |
| Blinding of outcome assessment (detection bias) | Low risk | "All patients and investigators were masked to treatment allocation." |
| Incomplete outcome data (attrition bias) | Low risk | ITT, 6% in the control and 7% in the colchicine group discontinued intervention |
| Selective reporting (reporting bias) | Low risk | Protocol available. |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 41 months | |
| Participants | Number randomised: Total 62. Colchicine 31, control 31 Condition: Alcoholic cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Not reported Age mean (SD) in years: Colchicine 53.2 (8.5), control 54.4 (9.1) Sex (women): Colchicine 7%, control 15% Inclusion criteria: "Ambulatory subjects aged 18‐65 years, with biopsy proven liver cirrhosis and a well‐documented history of previous daily alcohol intake exceeding 40 g of ethanol in women and 60 g in men for more than 5 years, in whom other causes of liver disease were excluded, were eligible for inclusion." Exclusion criteria: "Exclusion criteria included the presence of other liver diseases, namely haemochromatosis, Wilson's disease, α₁‐antitrypsin deficiency, autoimmune hepatitis, primary biliary cirrhosis, or viral hepatitis, as evaluated by the latter tests. Child‐Pugh class C, serum bilirubin >10 mg/dl, gastrointestinal bleeding in the previous 15 days, refractory ascites, or serious illness, e.g. renal failure (creatinine >2.5 mg/dl), cardiac failure or neoplasia were also exclusion criteria." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Maximum follow‐up 10 years. "supported in part by a grant from the Center of Nutrition and Metabolism (RUN 437)." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization list (blocks of four)" |
| Allocation concealment (selection bias) | Low risk | "either 1 mg colchicine or a placebo identical in appearance, prepared at the hospital pharmacy. The study `drugs' were coded and distributed to the patient, by the hospital pharmacy, according to a computer‐generated randomization list (blocks of four)." |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, "At no time were the treatment codes disclosed for any patient, attending physicians or investigators." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear, although "investigators" are blinded |
| Incomplete outcome data (attrition bias) | High risk | "Two (6%) patients in the colchicine group and five (16%) in the placebo, who did not return for the first follow‐up visit, were not included in the analysis of data." ITT mentioned but not all randomised participants were analysed; "Patient dropouts were as follows: nine patients (16%) before 3 years, 14 patients (25%) before 5 years, and 33 patients (60%) before 10 years." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 6 months | |
| Participants | Number randomised: Total 222. Colchicine 112, control 110 Condition: PCI Cardiovascular risk profile: Secondary prevention Setting: Outpatient Country: Not reported Age mean (SD) in years: Colchicine 63.7 (6.9), control 63.5 (7.2) Sex (women): Colchicine 37%, control 32% Inclusion Criteria: "Eligible patients were diabetic, 40 to 80 years of age, undergoing PCI in a coronary artery with a diameter of at least 2.5 mm with a BMS. Acceptable reasons for not implanting a drug‐eluting stent were: contraindication to long‐term dual antiplatelet treatment, need for triple antithrombotic therapy, planned or high probability of necessary surgery in the following 12 months, or the patient’s expressed wish in the context of the PCI informed consent procedure. Only 1 lesion per patient was included in the study. (If PCI was performed in >1 coronary site in a patient, the site with the greater artery diameter was included.) Diabetes mellitus had to be previously diagnosed by a specialist, with the patient treated with either oral medication or insulin." Exclusion Criteria: "Exclusion criteria were left main artery disease (>30% in angiography); PCI performed as primary treatment for ST‐segment elevation myocardial infarction, hepatic impairment (Child‐Pugh class B or C); target vessel segment presenting particular technical challenges for intravascular ultrasound (IVUS) (e.g., marked tortuosity, vessel with steep take‐off angle); severe or end‐stage renal failure (estimated glomerular filtration rate ≤20 ml/min/1.73 m² or requiring dialysis); history of intolerance to colchicine, myopathy, and statin hepatotoxicity or myotoxicity; women with child‐bearing potential; and inability or unwillingness to adhere to standard treatment or to provide consent." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary Outcome of the Study:
Outcomes considered in this review:
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation not specified in publication; author reply: “This is not reported in the paper, but randomization was computer‐based, with the random number sequence being produced by a short script in R language.” |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported in the publication; author reply: “Outcome assessment was completely blinded. It is stated in the published paper that 'Captured IVUS data, identified only by a serial number, were analyzed offline.' That means that assessors of IVUS‐defined restenosis were not in knowledge of the images‐patient correspondence (even if they had been they would not know the treatment allocation of each patient). The same was true for the QCA measurements (for angiographic restenosis).” |
| Incomplete outcome data (attrition bias) | Unclear risk | Modified ITT: "All patients who received at least 1 dose of study treatment were included in the analysis"; "Of 222 eligible patients who consented to take part in the study, 26 (12%) were not available for follow‐up catheterization. As a result, 196 (100 in the colchicine and 96 in the placebo group) completed the study procedures and were available for analysis"; according to author reply, all randomised participants were analysed for outcomes pertinent to this review. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 6 months | |
| Participants | Number randomised: Total 279. Colchicine 140, control 139 Condition: Chronic heart failure Cardiovascular risk profile: Secondary prevention Setting: Outpatient Country: Not reported Age mean (SD) in years: Colchicine 66.9 (5.8), control 66.4 (5.7) Sex (women): Colchicine 33%, control 33% Inclusion criteria: "Patients with stable symptomatic heart failure and systolic left ventricular dysfunction (ejection fraction ≤40%) were included." Exclusion criteria: "Recently hospitalized patients (hospital stay for heart failure in the previous 3 months) were excluded. Other exclusion criteria were New York Heart Association (NYHA) class IV, recent (in the previous 6 months) implantation of a cardiac resynchronization treatment device, active inflammatory/infectious disease or malignancy, known autoimmune diseases, corticosteroid or other immunosuppressive or immunomodulatory therapy, moderate or severe hepatic impairment (Child‐Pugh class B or C), severe renal failure (estimated glomerular filtration rate <30 ml/min/1.73 m²), current participation in another research protocol, and inability or unwillingness to adhere to standard treatment or to provide consent." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not reported specifically enough (sequential numbers); from author: "Randomization was computer‐based. Random numbers were produced by a short script written in R language.” |
| Allocation concealment (selection bias) | Unclear risk | Method of randomisation not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Low risk | All personnel blinded |
| Incomplete outcome data (attrition bias) | Low risk | ITT and PP; 3 participants excluded from analysis (1%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Open‐label Longest mean follow‐up period for a review‐relevant outcome: 30 months | |
| Participants | Number randomised: Total 26. Colchicine 14, control 12 Condition: Idiopathic pulmonary fibrosis Cardiovascular risk profile: Not reported Setting: Not reported Country: USA Age mean (SD) in years: Colchicine 65.9 (12.3), control 69.9 (4.0) Sex (women): Colchicine 17%, control 29% Inclusion criteria: "Conforms to clinical plus either high‐resolution computed tomography (HRCT) or histopathologic criteria for the diagnosis of idiopathic UIP; baseline tests performed, including pulmonary function, chest radiograph, serum creatinine, liver function tests, and complete blood count; willing to return for follow‐up examination at 3 month intervals for 1 year; age 18 years or older; written informed consent given." Exclusion criteria: "Exclusion criteria were as follows: history of allergy, intolerance, or unwillingness to take either study drug; pregnancy, lactation, or women capable of becoming pregnant who were without adequate birth control; history of chronic asthma and/or treated for asthma within the previous year; diabetes treated (including dietary therapy) within the previous year; active tuberculosis treated within the previous year; use of either study drug within the previous 2 months." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | "Funding was provided by Mayo Institute funds." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation not specified, but was done by biostatistician: "Randomization was accomplished by the Section of Biostatistics at Mayo Rochester." and "Within each stratum defined by the three stratification factors described previously, the randomization was done in blocks of four, ensuring that after every fourth subject was entered in a given stratum the number of subjects in the stratum assigned to prednisone was the same as the number of subjects in the strata assigned to colchicine." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Open study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not clear; missing data reported for baseline and various outcomes (e.g. baseline symptoms 7.1% vs 16.7% missing data) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Not reported Longest mean follow‐up period for a review‐relevant outcome: 2 years | |
| Participants | Number randomised: Total 22. Colchicine 10, control 12 Condition: Primary biliary cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Not reported Age mean (SE) in years: Colchicine 57 (3), control 64 (3) Sex (women): Colchicine 90%, control 83% Inclusion criteria: "The diagnosis of PBC was made based on the following observations: 1) elevation of alkaline phosphatase (ALP) over the upper limit of normal, 2) presence of antimitochondrial antibody in the serum, 3) compatible histological appearance of liver biopsy specimens, and 4) radiological or ultrasonographic evidence that the bile ducts were patent. Anti‐mitochondrial antibody titers were measured by the immunofluorescence technique. Histological staging of liver biopsy specimens was carried out as previously described." Exclusion criteria: "None of them had a history of blood transfusion, ethanol addiction, or drug abuse, and none had anti‐HCV antibodies (second‐generation RIA assay), HBs antigen, or anti‐HBc antibody in the serum" and "No patients had taken any medicines known to be hepatotoxic nor had been treated with corticosteroids, immunosuppressive agents, colchicine, penicillamine, or UDCA in the previous 6 months." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Previous treatment with 600 mg/d ursodeoxycholic acid during 30 months before randomisation in 2 different groups, and continued for 2 years after randomisation. "supported by a grant from the Intractable Liver Diseases Research Committee, the Ministry of Health and Welfare, Japan." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | No blinding, no placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Analysis unclear; missing data unclear |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 2 years | |
| Participants | Number randomised: Total 60. Colchicine 30, control 30 Condition: Primary biliary cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Not reported Age: 6 participants ≤ 50 years in colchicine group and 6 participants ≤ 50 years in control group (no further information provided in paper) Sex (women): Colchicine 93%, control 97% Inclusion criteria: "The criteria for entry into the trial included a clinical history and biochemical profile consistent with primary biliary cirrhosis, a positive test for antimitochondrial antibody, liver‐biopsy results consistent with or diagnostic of primary biliary cirrhosis, and radiologic or ultrasonographic evidence that the bile ducts were patent." Exclusion criteria: Not reported | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Control group received colchicine after 2 years. "Supported by a Research Grant (AM‐28490), a Training Grant (AM‐07024). and a General Research Center Grant (M01‐RR00054) from the National institutes of Health." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not sufficiently specified ("randomization scheme in which the numbers […] tended to be kept equal") |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind until 24 months; placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | 3 of 60 participants (5%) dropped out and were excluded from the analysis; the rest were included in analyses. 2 of them had early disease and did no tolerate medication, 1 had Stage 4 disease. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 10 years | |
| Participants | Number randomised: 87. Colchicine 43, control 42; (2 never received any drug, allocation not reported) Condition: Primary biliary cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: USA Age mean (SD) in years: Colchicine 51 (1.4), control 51 (1.5) Sex (women): 94% Inclusion criteria: "15 Patients were included provided they had (1) a medical history and blood test results consistent with chronic cholestatic liver disease; (2) a serum alkaline phosphatase that was at least twice that of the upper limit of normal; (3) a serum bilirubin that was not greater than 10 mg/dL; (4) a liver biopsy performed within 12 months of entry that was consistent with primary biliary cirrhosis, and (5) imaging tests that demonstrated that bile ducts were patent." Exclusion criteria: "Patients were excluded if they had end‐stage liver disease, which was defined as: (1) a serum bilirubin level greater than 10 mg/dL or a serum albumin level less than 3.0 g/dL on two examinations two months apart; (2) hepatic encephalopathy; (3) hemorrhage from esophageal varices and/or portal gastropathy; (4) refractory ascites; or (5) signs of hypersplenism (i.e. a hematocrit less than 30, white blood cell count less than 2,500, and platelet count less than 100,000). Other reasons for exclusion were history of alcohol abuse, administration of drugs associated with chronic liver disease, contemplation of pregnancy, or any serious medical illness that might in itself cause liver dysfunction or shorten life expectancy." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Ursodeoxycholic acid was administered to all participants after 2 years. "Supported in part by National Institutes of Health Center for Research Resources, General Clinical Research Center, grant RR 00054; by GRASP (Gastroenterologic Research in Absorptive and Secretory Processes) Digestive Disease Center grant NIH‐NIDDK P30 DK34928; and by a grant from Lederle Laboratories (Pearl River, New York)." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned, using a computer generated list in blocks of four" |
| Allocation concealment (selection bias) | Low risk | Active drug and placebo were kept in the hospital pharmacy and dispensed by a research pharmacist who was the only caregiver with access to the randomisation code. |
| Blinding of participants and personnel (performance bias) | Low risk | "Patients and investigators were blinded" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | ITT; high rate of withdrawals: 14% in both study groups at 2‐year follow‐up, 51% in colchicine group and 67% in control at 10‐year follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 24 months | |
| Participants | Number randomised: Total 28. Colchicine 14, control 14 Condition: Liver fibrosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Not reported Age: Not reported Sex (women): Not reported Inclusion criteria: "Each patient had bilirubin below 1 mg%, prothrombin below 16/12 and pre‐trial histologic evidence of cirrhosis." Exclusion criteria: Not reported | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | This study was reported as an abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing data reported, 2 participants in group B (placebo) died |
| Selective reporting (reporting bias) | High risk | Abstract only |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 14 years | |
| Participants | Number randomised: Total 100.Colchicine 54, control 46 Condition: Liver fibrosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Mexico Age mean (SE) in years: Colchicine 49.7 (1.5), control 50.8 (1.7) Sex (women): Colchicine 55%, control 46% Inclusion criteria: "They had to have a definitive diagnosis of liver cirrhosis, as established by history, physical examination, and biochemical tests, histologic studies of the liver, or both. Second, they had to be at least 18 years of age." Exclusion criteria: "Patients were excluded if they had an episode of gastrointestinal bleeding or encephalopathy within two weeks before entry into the trial, if they had a total serum bilirubin level above 171 µmol per liter (10 mg per deciliter) or a serum albumin level below 220 μmol per liter (1.5 g per deciliter), if they had a severe concomitant disease, or if they were unable to attend the clinic regularly for geographic or other reasons." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not sufficiently specified ("Randomization was carried out by one of us […]") |
| Allocation concealment (selection bias) | Low risk | "At no time […] disclose the treatment code for any patients to the attending physicians"; person who conducted randomisation was in another institution |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | ITT; all randomised participants analysed, 19 participants lost to follow‐up (number of participants balanced between groups, but considerably unbalanced median follow‐up: colchicine 42 months vs control 12 months) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Not reported Longest mean follow‐up period for a review‐relevant outcome: 5 years | |
| Participants | Number randomised: Total 101. Colchicine 52, control 49 Condition: Primary systemic amyloidosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: USA Age median (SD) in years: Colchicine 62 (not reported), control 64 (not reported) Sex (women): Colchicine 43%, control 40% Inclusion criteria: "All patients had clinical or laboratory evidence of systemic amyloidosis." Exclusion criteria: "Excluded from the study were patients with secondary, familial, or localized amyloidosis; patients with overt symptomatic multiple myeloma or diarrhea; patients who had received alkylating agents or colchicine; and patients with brittle diabetes, severe hypertension, or an active peptic ulcer that would prevent the use of prednisone as indicated in the protocol." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | "Forty‐nine patients initially received melphalan/prednisone and eight subsequently had colchicine added to their regimen. Fifty‐two patients lnitially received colchicine and 35 subsequently required melphalan/prednisone because of progressive disease." "supported in part by Research Grant CA‐16835 from the National Institutes of Health, Public Health Service, Bethesda, Maryland, and by the Toor Myeloma Research Fund, West Palm Beach, Florida." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Not specified, but authors report that colchicine dosage was weekly increased until side effects occurred, thus no blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Analysis unclear; it seems all randomised participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial Blinding: Unclear Longest mean follow‐up period for a review‐relevant outcome: 9 years | |
| Participants | Number randomised: Total 148. Colchicine, melphalan and prednisone 71 (MPC), melphalan and prednisone 77 (MP) Condition: Primary amyloidosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: USA Age median (SD) in years: Colchicine 65 (not reported), control 63 (not reported) Sex (women): Not reported Inclusion criteria: "Amyloidosis was confirmed by biopsy in every patient." Exclusion criteria: "Patients with secondary, familial, senile, or localized amyloidosis were not admitted to the study. Patients with overt symptomatic multiple myeloma or diarrhea were ineligible, as were patients who had previously received alkylating drugs or colchicine." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | One‐third treatment group (colchicine without melphalan or prednisone, 72 participants) was not considered for analysis. "Supported in part by grants from the National Institutes of Health (CA62242) and the Quade Amyloidosis Research Fund." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Analysis unclear; missing data unclear (14 participants "were removed from the study" = 6.4%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised trial, single‐centre Blinding: Open‐label Longest mean follow‐up period for a review‐relevant outcome: 4 years | |
| Participants | Number randomised: Total 66. Colchicine 38, control 27. Exclusion of 1 participant after randomisation, assignment unclear Condition: Chronic hepatitis B cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Taiwan Age mean (SD) in years: Cochicine 40 (9), control 40 (13) Sex (women): Colchicine 13%, control 11% Inclusion criteria: "Patients with hepatic decompensation, bridging necrosis or an alpha‐fetoprotein level greater than 100 ng/ml during an exacerbation of hepatitis have a high risk of developing cirrhosis" Exclusion criteria: "Patients under age 25, pregnant, with renal insufficiency (serum creatinine > 2.5mg/ml (normal < 1.0 mg/ml)), a history of idiosyncrasy or who refused the trial after careful explanation were excluded. (...)Those with clinical suspicions of cirrhosis, including spider angioma, palmar erythaema, an albumin <1.5 g m % (normal > 3.5 g m %), and endoscopy documented esophageal varices were also excluded." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | "supported by grants from the National Science Council of the Republic of China: NSC‐79‐0419‐B18209, NSC‐80‐0412‐B‐182A‐32 and NSC‐82‐0412‐B‐182‐029." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random table sequence" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | 12% participants were lost, unclear group allocation |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial Blinding: Not reported Longest mean follow‐up period for a review‐relevant outcome: Colchicine up to 2 years, control up to 7 years | |
| Participants | Number randomised: Total 54. Colchicine 27, control 27 Condition: Renal amyloidosis Cardiovascular risk profile: Not reported Setting: Not reported Country: Not reported Age: Not reported Sex: Not reported Inclusion criteria: People with renal amyloidosis secondary to rheumatic diseases Exclusion criteria: Not reported | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | This study was reported as an abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported specifically enough (sequential numbers) |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Follow‐up in the control group was 5 years longer |
| Selective reporting (reporting bias) | High risk | Abstract only |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, multicentre (13 centres) Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 6 years | |
| Participants | Number randomised: Total 549. Colchicine 274, control 275 Condition: Alcoholic cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: USA Age mean (SD) in years: Colchicine 55.2 (8.0), control 55.9 (7.6) Sex (women): Colchicine 2.5%, control 1.4% Inclusion criteria: "Outpatients were eligible for the study if they had a clinical diagnosis of alcoholic cirrhosis (based on a long history of alcohol use and the exclusion of other causes of liver disease and a modified Pugh 27 score of 7 or greater. Liver biopsy demonstrating cirrhosis was required unless contraindications to biopsy were present (eg. ascites, coagulopathy)." Exclusion criteria: "Patients were excluded for the following reasons: gastrointestinal bleeding within the prior 28 days requiring transfusion; explicit drug use in the prior 12 months, human immunodeficiency virus infection; cancer in the prior 10 years; serum creatinine greater than 1.5 mg/dL; total white blood cell (WBC) count less than 3500/mL; age 70 years or greater; serious chronic disease interfering with adherence to the protocol follow‐up schedule; no home telephone; and refusal." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes (time points) considered in this review:
| |
| Notes | "Supported by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation scheme based on permuted bocks of random length separately for each study centre |
| Allocation concealment (selection bias) | Low risk | "Patient enrollment and random assignment to treatment was by telephone call to the data‐coordinating center" and matched placebo |
| Blinding of participants and personnel (performance bias) | Low risk | Neither participants nor study personnel were aware of treatment group assignment |
| Blinding of outcome assessment (detection bias) | Low risk | "physicians assessing the outcomes were aware of the treatment group assignment until all data analysis was complete." |
| Incomplete outcome data (attrition bias) | Low risk | ITT, no losses to follow‐up for survival analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 4 years | |
| Participants | Number randomised: Total 74. Colchicine 37, control 37 Condition: Chronic liver disease Cardiovascular risk profile: Not reported Setting: Outpatient Country: Not reported Age mean (SD) in years: 53 (13) Sex (women): 38% Inclusion criteria: Not reported. Diverse chronic liver diseases Exclusion criteria: "Exclusion criteria were: age < 20 years or a known hypersensitivity to colchicine. Patients were recruited by referral from general practitioner or by self choice and gave informed consent to be assigned to intervention (colchicine) or control by using random allocation." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes (time‐points) considered in this review:
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified ("using random allocation. Randomization was performed by giving 74 consecutive numbers to all patients coming to our clinic") |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Reported as double‐blind in the abstract, not placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Analysis unclear; odd mentioning of ITT ("Inclusion criteria were on an intention‐to‐treat basis."); 12 (colchicine 9 and control 3; 16%) participants withdrew due to personal reasons, another 10 (colchicine 3 and control 7; 14%) died |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Open trial, observer‐blinded endpoint Longest mean follow‐up period for a review‐relevant outcome: 3 years | |
| Participants | Number randomised: Total 532. Colchicine 282, control 250 Condition: Stable coronary disease Cardiovascular risk profile: Secondary prevention Setting: Outpatient Country: Australia Age mean (SD) in years: Colchicine 66 (9.6), control 67 (9.2) Sex (women): Colchicine 11%, control 11% Inclusion criteria: "Patients were eligible for inclusion if they met each of the following criteria: 1) angiographically proven coronary disease; 2) age 35 to 85 years; 3) clinically stable for at least 6 months; 4) no major competing comorbidities or contraindication to colchicine therapy; 5) considered to be compliant with therapy and attending routine cardiology follow‐up appointments; and 6) willing to provide consent and be randomized into the study. Patients with a history of bypass surgery were only eligible if they had undergone bypass surgery more than 10 years before, had angiographic evidence of graft failure, or had undergone stenting since their bypass surgery." Exclusion criteria: Not reported | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | 93% of the participants continued with aspirin and/or clopidogrel and 95% continued with statins; "Patients (N=32) who were intolerant of therapy remained in the study, were followed in the usual manner, and were included in the primary ITT analysis"; Trial "conducted under the auspices of the Heart Research Institute of Western Australia." "There was no external funding source." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was computer‐generated |
| Allocation concealment (selection bias) | Low risk | Concealed from the investigators |
| Blinding of participants and personnel (performance bias) | High risk | Open trial |
| Blinding of outcome assessment (detection bias) | Low risk | Observer‐blinded outcome trial |
| Incomplete outcome data (attrition bias) | Low risk | Modified ITT: "All patients who received at least 1 dose of study treatment were included in the analysis"; ≤ 10% missing data per group that were excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Not reported Longest mean follow‐up period for a review‐relevant outcome: 12 months | |
| Participants | Number randomised: Total 38. Colchicine 21, control 17 Condition: Liver cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Greece Age median (SD) in years: Colchicine 49 (not reported), control 53 (not reported) Sex (women): Colchicine 43%, control 35% Inclusion criteria: Chronic liver disease Exclusion criteria: "Patients were excluded from the study if they had: age <20 and >70 years, evidence of pregnancy, malignancies or renal, cardiopulmonary, hematological, neurological and collagen diseases, diabetes mellitus, hyper/hypothyroidism or Child C liver function." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Liver tissue samples were evaluated by two pathologists (K.P., M.L.) who were blinded to the treatment groups and to response to treatment." However, we did not look into liver outcomes, so the blinding remains unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Analysis unclear, no dropouts reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 6 months | |
| Participants | Number randomised: Total 197. Colchicine 130, control 67 Condition: Coronary angioplasty Cardiovascular risk profile: Secondary prevention Setting: Outpatient Country: USA Age mean (SD) in years: Colchicine 59 (not reported), control 62 (not reported) Sex (women): Colchicine 15%, control 13% Inclusion criteria: "Elegibility criteria for entry into the trial were 1) successful elective coronary angioplasty; 2) single or multivessel angioplasty; 3) bypass graft angioplasty; 4) angioplasty of previously undiluted (new) and restenosed lesions; 5) angioplasty performed for silent ischemia and stable or unstable angina pectoris" Exclusion criteria: "Exclusion criteria were 1) direct angioplasty for acute myocardial infarction; 2) unsuccessful coronary angioplasty; 3) premenopausal women; 4) baseline leukopenia; 5) active peptic ulcer disease; 6) active diarrhea; 7) creatinine ≥2.5 mg/dI at baseline; 8) known colchicine intolerance. Successful angioplasty was defined as the reduction of the dilated lesion to ≤50% lumen diameter stenosis without documented acute reocclusion during the hospital stay." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT analysis reported, incomplete outcome measures (angiographic follow‐up rate 74%) mainly due to refusal of catheterisation, reported dropout rate due to adverse events, Colchicine 6.9% vs control 1.5% |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, multicentre (7 centres) Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 3 years | |
| Participants | Number randomised: Total 84. Colchicine 44, control 40 Condition: Primary sclerosing cholangitis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Sweden Age mean (95% CI) in years: Colchicine 39.5 (36.2 to 42.7), control 43.7 (40.1 to 47.3) Sex (women): Colchicine 39%, control 28% Inclusion criteria: Diagnosis of PSC based on typical cholangiographic appearance Exclusion criteria: Not reported | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | "Supported by G. D. Searle Ltd." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not sufficiently specified ("randomization procedure was performed for each center using the sealed envelope technique") |
| Allocation concealment (selection bias) | Unclear risk | Not sufficiently specified ("sealed envelope technique") |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | ITT; dropout colchicine 18% vs control 5%, overall 12% |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 1 year | |
| Participants | Number randomised: Total 41. Colchicine 21, control 20 Condition: Alcoholic cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Brazil Age mean (SD) in years: Colchicine 49.2 (9.9), control 47.8 (9.8) Sex (women): Colchicine 10%, control 14% Inclusion criteria: Chronic liver disease (confirmed by biopsy and/or ultrasound, endoscopy and clinical findings) Exclusion criteria: Hepatitis B confirmed by laboratory exams; any previous history of post‐transfusion hepatitis; alcoholic hepatitis; heart failure; renal failure; schistosomiasis (bilharzia); gastric bleeding during the last 30 days before the study start; advanced hepatic encephalopathy; refractory ascites; hepatorenal syndrome; type‐II diabetes; use of corticosteroids or sexual hormones | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Study published in Portuguese. The study was funded by Smith‐Kline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information is given. The authors state that the a stratified randomisation was “applied”, but no detail is provided on how the sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) | Low risk | Reported as "double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) | Low risk | There were 3 participants lost during the 12‐month follow‐up (table 2, “perdas deseguimento”/ translation: “loss of follow‐up“): 1 from the colchicine group and 2 from the placebo group. Those participants were included in the final assessment regarding alcohol relapse, all‐cause mortality and hepatic decompensation. Those losses are unlikely to have significant effects on the results. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, multicentre (2 centres) Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 6 months | |
| Participants | Number randomised: Total 52. Colchicine 29, control 23 Condition: Gout Cardiovascular risk profile: Not reported Setting: Outpatient Country: USA Age mean (SD) in years: Colchicine 53 (not reported), control 52 (not reported) Sex (women): 0% Inclusion criteria: "Diagnosis of gout in presence of hyperuricaemia. Patients were selected from the Gout Clinics of the Veterans Administration Hospitals in West Los Angeles and Kansas City, and the UCLA and University of Kansas Medical Center Hospitals." Exclusion criteria: "Patients were excluded if they were known to be uncooperative during treatment or if significant renal disease was present as reflected in a serum creatinine greater than 1.2 mg/100ml." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Mean therapy duration < 6 months: colchicine 5.5 months and control 5.2 months. "Supported by the Veterans Administration, Southern California Arthritis Foundation, Merck Sharpe and Dohme and USPHS Grant GM 15759."; "Merck Sharpe and Dohme Research Laboratories, West Point, Pennsylvania, who kindly provided the colchicine used in this study." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported specifically enough (sequential numbers) |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Dropout: colchicine 31% and control 22%; per protocol analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, multicentre (10 centres) Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 2 years | |
| Participants | Number randomised: Total 74. Colchicine 37, control 37 Condition: Primary biliary cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: France Age mean (SE) in years: Colchicine 55 (2), control 52 (2) Sex (women): Colchicine 89%, control 81% Inclusion criteria: "Criteria for entry to the trial were persistent abnormalities in liver function tests, in particular serum alkaline phosphatase activity of >1.5N (N is the upper limit of normal values), in patients with PBC who have been previously treated with UDCA (13‐15 mg/kg⁻¹/d⁻¹) for at least 8 months. All patients had biopsy‐proven PBC. They were admitted to the trial regardless of the duration of symptoms or the histological stage." Exclusion criteria: "drug therapy (except UDCA) for PBC during the previous 6 months (colchicine, azathioprine, chlorambucil, corticosteroids, D‐penicillamine, and cyclosporine); serum bilirubin concentration of >100 µmol/L; a serum albumin concentration of >25 g/L; past or active bleeding from esophageal varices; ascites; other identified causes of liver or biliary diseases; excessive alcohol consumption (>50 g/d); severe intercurrent disease; and aged older than 75 years." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | "Supported in part by Laboratoires Houde´ (France) and Jouveinal (Canada)." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported, for liver biopsy "assessed by two pathologists unaware of the [...] treatment", no other blindings of assessment specified |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT; missing data not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 24 months | |
| Participants | Number randomised: Total 28. Colchicine 14, control 14 Condition: Primary biliary cirrhosis Cardiovascular risk profile: Not reported Setting: Not reported Country: Germany Age mean (range) in years: Colchicine + control 54 years (37 ‐ 71) Sex (women): 100% Inclusion criteria: Women with primary biliary cirrhosis. "Initially all patients received monotherapy with UDCA in a dose of 10‐12 mg/kg per day for 12 months." Exclusion criteria: Not reported | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Initially all participants received monotherapy with UDCA in a dose of 10 ‐ 12 mg/kg/d for 12 months. After this period the treatment was continued in a randomised fashion with UDCA plus placebo or UCDA plus colchicine. Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported specifically enough (sequential numbers) |
| Allocation concealment (selection bias) | Unclear risk | Method of randomisation not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Reported as double‐blinded; placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specifically reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Analysis unclear; 2 participants were excluded (7%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 3 years | |
| Participants | Number randomised: Total 74. Colchicine 37, control 37 Condition: Liver fibrosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Germany Age: Not reported Sex (women): Not reported Inclusion criteria: Liver fibrosis or cirrhosis. Exclusion criteria: Participants were excluded if they had episodes of gastrointestinal bleedings, hepatic encephalopathy in the last two 2 weeks, bilirubin‐levels > 2 mg %, 34 µmol/l respectively, because of noncompliance, non‐hepatic diseases which change the biochemical parameters of the metabolism of the connective tissue, and the inability to asses the histological process | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | 13 of 74 individuals lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 6 months | |
| Participants | Number randomised: Total 67. Colchicine 33, control 34 Condition: Alcoholic cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Belgium Age mean (SD) in years: Colchicine 52 (8), control 52 (9) Sex (women): Colchicine 36%, control 50% Inclusion criteria: "Alcoholic patients with histologically proven Alcoholic cirrhosis assessed by per‐cutaneous liver biopsy, with or without cirrhosis, were consecutively included in the study." Exclusion criteria: "Patients were not included in case of hepatic encephalopathy, presence of ascites, prothrombin activity below 50 per cent or platelet count below 100.10⁹/l, hepatocellular carcinoma, evident lack of compliance or refusal to participate in the trial." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Houdé Pharmaceutical Laboratories, Paris, France, supplied colchicine and placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified (sealed envelopes, but not opaque) |
| Allocation concealment (selection bias) | Unclear risk | Details of concealment not sufficiently specified ("sealed envelopes") |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Analysis unclear; dropouts 48.5% vs 52.9% at 6 months |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, multicentre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 2 years | |
| Participants | Number randomised: Total 90. Colchicine 29, placebo 31 Condition: Primary biliary cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: Finland Age mean in years: Colchicine 56, placebo 57 Sex: (women): Colchicine 86%, placebo 87% Inclusion criteria: "Criteria for entry into the trial were elevated serum alkaline phosphatase activity, liver biopsy findings diagnostic of or compatible with PBC, and a positive result for serum mitochondrial antibodies. In patients fulfilling the above criteria but negative for antimitochondrial antibodies, other potential causes of liver disease were excluded, and the patency of the bile ducts was evaluated by endoscopic retrograde cholangiography. All patients tested negative for hepatitis B surface antigen and for hepatitis C antibodies." Exclusion criteria: "Patients with endstage PBC and those who used drugs that might affect the course of PBC were excluded from the study. Thus, patients whose serum bilirubin level was >150 btmol/L, serum albumin level was <25 g/L, or TT‐SPA (thrombotest) was <50% in two successive determinations were excluded, as were patients with drug‐resistant ascites and those in whom liver transplantation was indicated. None of the patients had used colchicine, UDCA, D‐penicillamine, or immunosuppressive drugs (corticosteroids, azathioprine, cyclosporin A, methotrexate) for 6 months before the trial. " | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Third study group receiving UDCA (n = 30) not relevant for this review and thus not extracted. "Supported by grants from the Finnish Foundation for Gastroenterological Research and the Mary and Georg C. Ehrnrooth Foundation. Leiras Oy, Finland, supplied the drugs for this study and financial support." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified, "using consecutive case numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not reported specifically enough (sequential numbers) |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT mentioned, data analysis unclear. Dropouts: colchicine 17%, control 26%, UDCA 0% |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: median 26 months | |
| Participants | Number randomised: Total 100. Colchicine 50, control 50 Condition: Chronic hepatitis B Cardiovascular risk profile: Not reported Setting: Outpatient Country: Taiwan Age mean (SD) in years: Colchicine 60 (not reported), control 59 (not reported) Sex (women): Colchicine 6%, control 6% Inclusion criteria: People with HBsAg‐positive cirrhosis Exclusion criteria: "Patients were excluded if they had end‐stage liver cirrhosis (serum albumin level below 25 g/I or total bilirubin level above 171 µmol/l ), episodes of variceal bleeding, or hepatic encephalopathy within 2 weeks before recruitment into this trial, a concomitant debilitating illness, or if they were unable to attend the clinic regularly." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | "supported by grant NSC No. 82‐0412‐B075‐027 from the National Science Council, Republic of China." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The random numbers were computer generated and arranged in numerical order and divided in two." |
| Allocation concealment (selection bias) | Low risk | "a nurse [...] prepared coded supplies of colchicine or placebo according to the random numbers for the staff physicians and each patient at entry and every follow‐up" |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled. "Neither the patients nor the physicians knew which treatment was given." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Analysis unclear; missing data 9%, but unclear how allocated to the groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 18 months | |
| Participants | Number randomised: Total 64. Colchicine 34, placebo 30 Condition: Primary biliary cirrhosis Cardiovascular risk profile: Not reported Setting: Outpatient Country: UK Age: Not reported Sex (women): Not reported Inclusion criteria: "All patients gave their informed consent and the study was approved by the hospital ethical committee. At entry, 48% of patients had 'classical PBC' with pruritus followed by jaundice, whilst the other 52% had few, if any, symptoms directly referrable to the disease. All patients in the study had a raised serum alkaline phosphatase, a positive anti‐mitochondrial antibody test, and liver histology compatible with, or diagnostic of, PBC." Exclusion criteria: Not reported | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | Survival data is reported for 12 and 18 months. Dropouts reported until 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The first patient in any pair was allocated by the staff pharmacist to the treatment or placebo group by reference to random tables." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | ITT for survival data. 16% participants dropped out within 12 months (colchicine 24%, control 7%), but were available for survival analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Study characteristics | ||
| Methods | Design: Randomised controlled trial, single‐centre Blinding: Double‐blind Longest mean follow‐up period for a review‐relevant outcome: 2 years | |
| Participants | Number randomised: Total 116. Colchicine 58. Control 58. Condition: Behçet's syndrome Cardiovascular risk profile: Not reported Setting: Outpatient Country: Turkey Age mean (SD) in years: Women:cColchicine 26.7 (4.8), control 27.2 (5.5). Men: colchicine 27.0 (5.5), control 27.3 (5.3) Sex (women): Colchicine 47%, control 47% Inclusion criteria: "All patients were required to meet the inclusion criteria, which meant that they had to 1) be consecutive patients (male or female), 2) be 18–35 years of age, 3) have active disease, 4) have a disease duration of ≤2 years, and 5) live at a reasonable traveling distance from our center. Active disease was defined as the minimum presence of oral or genital ulceration or erythema nodosum occurring at least 3 times within the preceding 6 months. The disease duration was defined as the time that had elapsed since the diagnostic criteria had been fulfilled." Exclusion criteria: "We excluded patients who 1) had received immunosuppressive agents, steroids, or colchicine within the preceding 6 months, 2) had organ involvement requiring immunosuppression, or 3) had eye disease, especially with retinal involvement, during the recruitment period. However, patients who had only a few cells in vitreous body were included if their visual acuity was >9/10 (assessed on a 10‐line scale, with a best vision of 10/10). Patients were to be withdrawn from the study in the event of a major systemic or life‐threatening manifestation such as severe eye, major vein, or central nervous system involvement." | |
| Interventions | Colchicine:
Control:
| |
| Outcomes | Primary outcome of the study:
Outcomes considered in this review:
| |
| Notes | "Supported by TUBITAK (Turkish Scientific and Technical Research Council; TAG 0754) and, in part, by the Research Fund of the University of Istanbul." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was done separately for each sex. In each sex group, equal numbers of cards that were assigned to either the active drug or the placebo arm were mixed, drawn, and placed sequentially on a list by a secretary not involved in running the trial." |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were not stated as opaque in the publication; from author request: "The sealed envelop was really opaque but was forgotten to mention in the paper." |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Low risk | Not reported in publication; from author request: "Here all patients and physicians were blinded and as well as the outcome assessment." |
| Incomplete outcome data (attrition bias) | High risk | ITT; 27% did not complete month 24, but "There were no differences in the number of dropouts or reasons for withdrawal (Figure 1) between the 2 treatment arms." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
HCC: hepatocellular carcinoma
HCV: hepatitis C virus
ITT: intention‐to‐treat
PCI: Percutaneous coronary intervention
PP: per protocol
PSC: Primary Sclerosing Cholangitis
UDCA: ursodeoxycholic acid
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Other publications of this study available; this publication provides no additional information | |
| Other publications of this study available; this publication provides no additional information | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| No pertinent outcome reported at all | |
| No pertinent outcome reported at all | |
| No pertinent outcome reported at all | |
| Duration of follow‐up or treatment was too short | |
| There are outcomes but reported in an uninterpretable way | |
| Not an RCT | |
| Other publications of this study available; this publication provides no additional information | |
| No pertinent outcome reported at all | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Other publications of this study available; this publication provides no additional information | |
| Other publications of this study available; this publication provides no additional information | |
| Other publications of this study available; this publication provides no additional information | |
| Duration of follow‐up or treatment was too short | |
| No pertinent outcome reported at all | |
| Study was not an RCT | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Study was not conducted in adults | |
| No pertinent outcome reported at all | |
| Other publications of this study available; this publication provides no additional information | |
| Other publications of this study available; this publication provides no additional information | |
| Other publications of this study available; this publication provides no additional information | |
| Duration of follow‐up or treatment was too short | |
| There are outcomes but reported in an uninterpretable way | |
| There are outcomes but reported in an uninterpretable way | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Study was not an RCT | |
| No pertinent outcome reported at all | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| No pertinent outcome reported at all | |
| There are outcomes but reported in an uninterpretable way | |
| Duration of follow‐up or treatment was too short | |
| No pertinent outcome reported at all | |
| No pertinent outcome reported at all | |
| Study was not an RCT | |
| Study was not an RCT | |
| No pertinent outcome reported at all | |
| Study was not an RCT | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| No pertinent outcome reported at all | |
| Study was not conducted in adults | |
| Study was not an RCT | |
| Study was not an RCT | |
| No pertinent outcome reported at all | |
| No pertinent outcome reported at all | |
| Study was not conducted in adults | |
| No pertinent outcome reported at all | |
| No pertinent outcome reported at all | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Other publications of this study available; this publication provides no additional information | |
| Duration of follow‐up or treatment was too short | |
| Database duplicate | |
| Duration of follow‐up or treatment was too short | |
| Other publications of this study available; this publication provides no additional information | |
| Study was not an RCT | |
| Duration of follow‐up or treatment was too short | |
| Other publications of this study available; this publication provides no additional information | |
| Other publications of this study available; this publication provides no additional information | |
| Other publications of this study available; this publication provides no additional information | |
| Colchicine not part of the treatment | |
| Study was not an RCT | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| No pertinent outcome reported at all | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Study was not an RCT | |
| Duration of follow‐up or treatment was too short | |
| Study was not conducted in adults | |
| Study was not an RCT | |
| Study was not an RCT | |
| Duration of follow‐up or treatment was too short | |
| Study was not an RCT | |
| Duration of follow‐up or treatment was too short | |
| No pertinent outcome reported at all | |
| Duration of follow‐up or treatment was too short | |
| Other publications of this study available; this publication provides no additional information | |
| Study was not an RCT | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| No pertinent outcome reported at all | |
| No pertinent outcome reported at all | |
| Study was not an RCT | |
| Study was not an RCT | |
| Duration of follow‐up or treatment was too short | |
| No pertinent outcome reported at all | |
| There are outcomes but reported in an uninterpretable way | |
| Study was not an RCT | |
| Other publications of this study available; this publication provides no additional information | |
| Other publications of this study available; this publication provides no additional information | |
| No pertinent outcome reported at all | |
| No pertinent outcome reported at all | |
| No pertinent outcome reported at all | |
| No pertinent outcome reported at all | |
| Study was not an RCT | |
| Duration of follow‐up or treatment was too short | |
| Study was not an RCT | |
| Study was not an RCT | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| No pertinent outcome reported at all | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Study was not conducted in adults | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| No pertinent outcome reported at all | |
| Duration of follow‐up or treatment was too short | |
| No pertinent outcome reported at all | |
| Duration of follow‐up or treatment was too short | |
| Other publications of this study available; this publication provides no additional information | |
| Study was not an RCT | |
| Other publications of this study available; this publication provides no additional information | |
| Study was not an RCT | |
| Other publications of this study available; this publication provides no additional information | |
| Duration of follow‐up or treatment was too short | |
| Other publications of this study available; this publication provides no additional information | |
| Other publications of this study available; this publication provides no additional information | |
| Duration of follow‐up or treatment was too short | |
| Duration of follow‐up or treatment was too short | |
| Study was not an RCT | |
| No pertinent outcome at all | |
| Duration of follow‐up or treatment was too short | |
| Study was not an RCT | |
| Duration of follow‐up or treatment was too short | |
| All participants receive colchicine |
Characteristics of studies awaiting classification [ordered by study ID]
| Methods | N/A |
| Participants | N/A |
| Interventions | N/A |
| Outcomes | N/A |
| Notes | There is neither abstract nor full‐text available for this reference. |
None
Characteristics of ongoing studies [ordered by study ID]
| Study name | LoDoCo2 |
| Methods | Double‐blind, randomised trial |
| Participants | Coronary heart disease |
| Interventions | Colchicine (0.5 mg/d) vs placebo Treatment duration (colchicine): 3 ‐ 4 years |
| Outcomes | Primary outcome: Time to first occurrence of either non‐fatal myocardial infarction, unstable angina, non‐cardio‐embolic ischaemic stroke or fatal or non‐fatal out‐of‐hospital cardiac arrest; minimum of 3 years follow‐up for each individual, estimated median follow‐up of 4 years and a maximum follow‐up of 4 to 5 years (information through personal communication with the principal investigator who is also author of this review (MN)). Secondary outcome: Time to first occurrence of either non‐fatal myocardial infarction or episode of unstable angina, unrelated to stent disease; other cardiovascular endpoints including new onset atrial fibrillation, deep vein thrombosis, pulmonary embolism as evidenced from the participant record; safety measures including rate of intolerance or serious adverse events including rhabdomyolysis as evidenced by the participant records. Rhabdomyolysis determined by acute onset of severe myonecrosis evident by marked elevation in serum creatinine kinase |
| Starting date | February 2014 |
| Contact information | Mark Nidorf, MD, +61 413145410, [email protected] |
| Notes | ACTRN12614000093684 |
| Study name | IRCT138807112539N1 |
| Methods | Double‐blind, randomised trial |
| Participants | Chronic hepatitis B |
| Interventions | Lamivudine + colchicine (0.5 mg/d) vs lamivudine + placebo Treatment duration (colchicine): 6 months |
| Outcomes | Treatment of chronic hepatitis B. time point: 1 month before intervention and 1 month after intervention. Method of measurement: laboratory measurement of serum ALT‐ Albumin‐bilirubin and PT |
| Starting date | July 2009 |
| Contact information | Amir Hassanpour, MD, +988614173608, +989166134349, +988614173630, [email protected] |
| Notes | IRCT138807112539N1 |
| Study name | COACS |
| Methods | Multicentre, double‐blind, randomised trial |
| Participants | Acute coronary syndrome |
| Interventions | Colchicine (0.5 mg/d) vs placebo Treatment duration (colchicine): 24 months |
| Outcomes | Primary outcome: Combined endpoint (all‐cause mortality, new acute coronary syndrome, and ischaemic stroke); at 24 months Secondary outcomes: Each of the combined outcome separately at 24 months |
| Starting date | June 2013 |
| Contact information | Massimo Imazio, MD +39011439 ext 3391 [email protected] |
| Notes | NCT01906749 |
| Study name | CQMU‐2013‐QLi |
| Methods | Double‐blind, randomised trial |
| Participants | Type 2 diabetes mellitus and microalbuminuria |
| Interventions | Colchicine (0.5 mg/d) vs placebo Treatment duration (colchicine): unclear |
| Outcomes | Primary outcome: changes in UACR from baseline to the 6th month; changes in CIMT from baseline to the 18th month; incidence of overt nephropathy;composite cardiovascular events Secondary outcome: Changes in 24 h urinary albumin; proportion of participants achieving at least a 15% reduction in UACR; changes in estimated glomerular filtration rate (eGFR); new or worsening diabetic neuropathy; new or worsening diabetic retinopathy; death from any cause; each component of primary outcomes of phase 4; overt nephropathy; new or worsening diabetic neuropathy; new or worsening diabetic retinopathy |
| Starting date | December 2013 |
| Contact information | Qifu Li, First Affiliated Hospital of Chongqing Medical University |
| Notes | NCT02035891 |
| Study name | COLPET |
| Methods | Double‐blind, randomised trial |
| Participants | Atherosclerotic vascular disease |
| Interventions | Colchicine (0.6 mg/d) vs placebo Treatment duration (colchicine): 6 months |
| Outcomes | Primary outcome: Change in the average of maximum target‐to‐background (TBR) values (mean MAX TBR) of the ascending aorta; participants will be followed over a period of 6 months Secondary outcome: Change in the mean maximum target‐to‐background (Mean MAX TBR) of carotid arteries; change in the average of the mean TBR values; change in the most diseased segment TBR values (MDS TBR) in the carotid arteries and ascending aorta; change in soluble biomarkers of inflammation |
| Starting date | May 2014 |
| Contact information | Jean‐Claude Tardif, MD Montreal Heart Institute |
| Notes | NCT02162303 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 1.1  Comparison 1: Colchicine vs control, Outcome 1: Mortality (all‐cause) | ||||
| 1.1.1 Participants with high cardiovascular risk | 4 | 1230 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.26, 1.14] |
| 1.1.2 Other | 26 | 2944 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.84, 1.12] |
| 1.2 Myocardial infarction (total) Show forest plot | 2 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.57] |
| Analysis 1.2  Comparison 1: Colchicine vs control, Outcome 2: Myocardial infarction (total) | ||||
| 1.2.1 Participants with high cardiovascular risk | 1 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.57] |
| 1.2.2 Other | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.3 Myocardial infarction (non‐fatal) Show forest plot | 2 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.07, 0.61] |
| Analysis 1.3  Comparison 1: Colchicine vs control, Outcome 3: Myocardial infarction (non‐fatal) | ||||
| 1.3.1 Participants with high cardiovascular risk | 1 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.07, 0.61] |
| 1.3.2 Other | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.4 Myocardial Infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| Analysis 1.4  Comparison 1: Colchicine vs control, Outcome 4: Myocardial Infarction (fatal) | ||||
| 1.4.1 Participants with high cardiovascular risk | 1 | 532 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.00, 6.04] |
| 1.4.2 Other | 5 | 378 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.05, 2.47] |
| 1.5 Adverse event (serious) Show forest plot | 4 | 472 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| Analysis 1.5  Comparison 1: Colchicine vs control, Outcome 5: Adverse event (serious) | ||||
| 1.5.1 Participants with high cardiovascular risk | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.5.2 Other | 4 | 472 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.6 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| Analysis 1.6  Comparison 1: Colchicine vs control, Outcome 6: Adverse event (total) | ||||
| 1.6.1 Participants with high cardiovascular risk | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.6.2 Other | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| 1.7 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| Analysis 1.7  Comparison 1: Colchicine vs control, Outcome 7: Adverse event (gastrointestinal) | ||||
| 1.7.1 Participants with high cardiovascular risk | 2 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.43, 4.06] |
| 1.7.2 Other | 9 | 757 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.82, 3.02] |
| 1.8 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| Analysis 1.8  Comparison 1: Colchicine vs control, Outcome 8: Mortality (cardiovascular) | ||||
| 1.8.1 Participants with high cardiovascular risk | 2 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.02, 2.66] |
| 1.8.2 Other | 5 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.09, 3.32] |
| 1.9 Stroke (total) Show forest plot | 3 | 874 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.09, 1.70] |
| Analysis 1.9  Comparison 1: Colchicine vs control, Outcome 9: Stroke (total) | ||||
| 1.9.1 Participants with high cardiovascular risk | 2 | 754 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.09, 1.70] |
| 1.9.2 Other | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.10 Stroke (fatal) Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1: Colchicine vs control, Outcome 10: Stroke (fatal) | ||||
| 1.10.1 Participants with high cardiovascular risk | 2 | 754 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.26 [0.14, 365.85] |
| 1.10.2 Other | 2 | 161 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.11 Stroke (non‐fatal) Show forest plot | 3 | 874 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.17] |
| Analysis 1.11  Comparison 1: Colchicine vs control, Outcome 11: Stroke (non‐fatal) | ||||
| 1.11.1 Participants with high cardiovascular risk | 2 | 754 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.17] |
| 1.11.2 Other | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.12 Heart failure (total) Show forest plot | 3 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.10, 3.88] |
| Analysis 1.12  Comparison 1: Colchicine vs control, Outcome 12: Heart failure (total) | ||||
| 1.12.1 Participants with high cardiovascular risk | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.69] |
| 1.12.2 Other | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.46, 2.51] |
| 1.13 Heart failure (fatal) Show forest plot | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1: Colchicine vs control, Outcome 13: Heart failure (fatal) | ||||
| 1.13.1 Participants with high cardiovascular risk | 1 | 222 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.70] |
| 1.13.2 Other | 2 | 161 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.14 Heart failure (non‐fatal) Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1: Colchicine vs control, Outcome 14: Heart failure (non‐fatal) | ||||
| 1.14.1 Participants with high cardiovascular risk | 1 | 222 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.12] |
| 1.14.2 Other | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.15 Non‐scheduled hospitalisation (total) Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.77, 0.99] |
| Analysis 1.15  Comparison 1: Colchicine vs control, Outcome 15: Non‐scheduled hospitalisation (total) | ||||
| 1.15.1 Participants with high cardiovascular risk | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.15.2 Other | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.77, 0.99] |
| 1.16 Non‐scheduled cardiovascular interventions Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1: Colchicine vs control, Outcome 16: Non‐scheduled cardiovascular interventions | ||||
| 1.16.1 Participants with high cardiovascular risk | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.22, 2.85] |
| 1.16.2 Other | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 2.1  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 1: Mortality (all‐cause) | ||||
| 2.1.1 ≤ 1 mg/d | 21 | 2420 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.67, 0.99] |
| 2.1.2 > 1 mg/d | 9 | 1754 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.25] |
| 2.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| Analysis 2.2  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 2: Mortality (cardiovascular) | ||||
| 2.2.1 ≤ 1 mg/d | 5 | 955 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.06, 0.82] |
| 2.2.2 > 1 mg/d | 2 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [0.13, 73.12] |
| 2.3 Myocardial infarction (total) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 3: Myocardial infarction (total) | ||||
| 2.3.1 ≤ 1 mg/d | 1 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.57] |
| 2.3.2 > 1 mg/d | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.4 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| Analysis 2.4  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 4: Myocardial infarction (fatal) | ||||
| 2.4.1 ≤ 1 mg/d | 4 | 733 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.02, 0.87] |
| 2.4.2 > 1 mg/d | 2 | 177 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.66 [0.15, 386.16] |
| 2.5 Myocardial infarction (non‐fatal) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 5: Myocardial infarction (non‐fatal) | ||||
| 2.5.1 ≤ 1 mg/d | 1 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.07, 0.61] |
| 2.5.2 > 1 mg/d | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.6 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| Analysis 2.6  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 6: Adverse event (total) | ||||
| 2.6.1 ≤ 1 mg/d | 8 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.74, 4.14] |
| 2.6.2 > 1 mg/d | 3 | 626 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.72, 2.97] |
| 2.7 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| Analysis 2.7  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 7: Adverse event (gastrointestinal) | ||||
| 2.7.1 ≤ 1 mg/d | 9 | 1104 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.26, 3.66] |
| 2.7.2 > 1 mg/d | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.61, 2.19] |
| 2.8 Adverse event (serious) Show forest plot | 4 | 472 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| Analysis 2.8  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 8: Adverse event (serious) | ||||
| 2.8.1 ≤ 1 mg/d | 4 | 472 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.8.2 > 1 mg/d | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.9 Stroke (total) Show forest plot | 3 | 874 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.09, 1.70] |
| Analysis 2.9  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 9: Stroke (total) | ||||
| 2.9.1 ≤ 1 mg/d | 2 | 754 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.09, 1.70] |
| 2.9.2 > 1 mg/d | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.10 Stroke (fatal) Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.10  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 10: Stroke (fatal) | ||||
| 2.10.1 ≤ 1 mg/d | 3 | 795 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.26 [0.14, 365.85] |
| 2.10.2 > 1 mg/d | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.11 Stroke (non‐fatal) Show forest plot | 3 | 874 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.17] |
| Analysis 2.11  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 11: Stroke (non‐fatal) | ||||
| 2.11.1 ≤ 1 mg/d | 2 | 754 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.17] |
| 2.11.2 > 1 mg/d | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.12 Heart failure (total) Show forest plot | 3 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.10, 3.88] |
| Analysis 2.12  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 12: Heart failure (total) | ||||
| 2.12.1 ≤ 1 mg/d | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.69] |
| 2.12.2 > 1 mg/d | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.46, 2.51] |
| 2.13 Heart failure (fatal) Show forest plot | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.13  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 13: Heart failure (fatal) | ||||
| 2.13.1 ≤ 1 mg/d | 2 | 263 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.70] |
| 2.13.2 > 1 mg/d | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.14 Heart failure (non‐fatal) Show forest plot | 2 | 342 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.12] |
| Analysis 2.14  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 14: Heart failure (non‐fatal) | ||||
| 2.14.1 ≤ 1 mg/d | 1 | 222 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.12] |
| 2.14.2 > 1 mg/d | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.15 Non‐scheduled hospitalisation (total) Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.77, 0.99] |
| Analysis 2.15  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 15: Non‐scheduled hospitalisation (total) | ||||
| 2.15.1 ≤ 1 mg/d | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.31, 2.55] |
| 2.15.2 > 1 mg/d | 1 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.77, 0.99] |
| 2.16 Non‐scheduled cardiovascular interventions Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.16  Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 16: Non‐scheduled cardiovascular interventions | ||||
| 2.16.1 ≤ 1 mg/d | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.22, 2.85] |
| 2.16.2 > 1 mg/d | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 3.1  Comparison 3: Colchicine vs control: sensitivity analysis ‐ placebo or other inactive control, Outcome 1: Mortality (all‐cause) | ||||
| 3.1.1 Placebo, inactive control | 26 | 3479 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.84, 1.11] |
| 3.1.2 Active control | 4 | 695 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.95] |
| 3.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| Analysis 3.2  Comparison 3: Colchicine vs control: sensitivity analysis ‐ placebo or other inactive control, Outcome 2: Mortality (cardiovascular) | ||||
| 3.2.1 Placebo, inactive control | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| 3.2.2 Active control | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.3 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| Analysis 3.3  Comparison 3: Colchicine vs control: sensitivity analysis ‐ placebo or other inactive control, Outcome 3: Myocardial infarction (fatal) | ||||
| 3.3.1 Placebo, inactive control | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| 3.3.2 Active control | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 3.4 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| Analysis 3.4  Comparison 3: Colchicine vs control: sensitivity analysis ‐ placebo or other inactive control, Outcome 4: Adverse event (total) | ||||
| 3.4.1 Placebo, inactive control | 9 | 725 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.90, 2.84] |
| 3.4.2 Active control | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.41, 4.74] |
| 3.5 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| Analysis 3.5  Comparison 3: Colchicine vs control: sensitivity analysis ‐ placebo or other inactive control, Outcome 5: Adverse event (gastrointestinal) | ||||
| 3.5.1 Placebo, inactive control | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| 3.5.2 Active control | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 4.1  Comparison 4: Colchicine vs control: sensitivity analysis ‐ selection bias (random sequence generation and allocation concealment), Outcome 1: Mortality (all‐cause) | ||||
| 4.1.1 Low risk | 6 | 1411 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.87, 1.22] |
| 4.1.2 High or unclear risk | 24 | 2763 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.11] |
| 4.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| Analysis 4.2  Comparison 4: Colchicine vs control: sensitivity analysis ‐ selection bias (random sequence generation and allocation concealment), Outcome 2: Mortality (cardiovascular) | ||||
| 4.2.1 Low risk | 2 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.69] |
| 4.2.2 High or unclear risk | 5 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.15, 2.95] |
| 4.3 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| Analysis 4.3  Comparison 4: Colchicine vs control: sensitivity analysis ‐ selection bias (random sequence generation and allocation concealment), Outcome 3: Myocardial infarction (fatal) | ||||
| 4.3.1 Low risk | 2 | 652 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.00, 6.04] |
| 4.3.2 High or unclear risk | 4 | 258 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.05, 2.47] |
| 4.4 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| Analysis 4.4  Comparison 4: Colchicine vs control: sensitivity analysis ‐ selection bias (random sequence generation and allocation concealment), Outcome 4: Adverse event (total) | ||||
| 4.4.1 Low risk | 3 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.61, 4.04] |
| 4.4.2 High or unclear risk | 8 | 898 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.81, 2.91] |
| 4.5 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| Analysis 4.5  Comparison 4: Colchicine vs control: sensitivity analysis ‐ selection bias (random sequence generation and allocation concealment), Outcome 5: Adverse event (gastrointestinal) | ||||
| 4.5.1 Low risk | 4 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.59, 2.04] |
| 4.5.2 High or unclear risk | 7 | 727 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [1.49, 3.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 5.1  Comparison 5: Colchicine vs control: sensitivity analysis ‐ performance bias (double‐blinded studies), Outcome 1: Mortality (all‐cause) | ||||
| 5.1.1 Double‐blind | 22 | 2538 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.07] |
| 5.1.2 Not clearly double‐blind | 8 | 1636 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.82, 1.32] |
| 5.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| Analysis 5.2  Comparison 5: Colchicine vs control: sensitivity analysis ‐ performance bias (double‐blinded studies), Outcome 2: Mortality (cardiovascular) | ||||
| 5.2.1 Double‐blind | 6 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.15, 2.95] |
| 5.2.2 Not clearly double‐blind | 1 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.69] |
| 5.3 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| Analysis 5.3  Comparison 5: Colchicine vs control: sensitivity analysis ‐ performance bias (double‐blinded studies), Outcome 3: Myocardial infarction (fatal) | ||||
| 5.3.1 Double‐blind | 5 | 378 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.05, 2.47] |
| 5.3.2 Not clearly double‐blind | 1 | 532 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.00, 6.04] |
| 5.4 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| Analysis 5.4  Comparison 5: Colchicine vs control: sensitivity analysis ‐ performance bias (double‐blinded studies), Outcome 4: Adverse event (total) | ||||
| 5.4.1 Double‐blind | 6 | 581 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.01, 3.12] |
| 5.4.2 Not clearly double‐blind | 5 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.49, 3.11] |
| 5.5 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| Analysis 5.5  Comparison 5: Colchicine vs control: sensitivity analysis ‐ performance bias (double‐blinded studies), Outcome 5: Adverse event (gastrointestinal) | ||||
| 5.5.1 Double‐blind | 9 | 1198 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.98, 3.14] |
| 5.5.2 Not clearly double‐blind | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 5.91 [0.32, 110.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 6.1  Comparison 6: Colchicine vs control: sensitivity analysis ‐ detection bias (blinding of outcome assessment), Outcome 1: Mortality (all‐cause) | ||||
| 6.1.1 Outcome assessment blinded | 4 | 1582 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.88, 1.25] |
| 6.1.2 Not clearly blinded | 26 | 2592 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.09] |
| 6.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| Analysis 6.2  Comparison 6: Colchicine vs control: sensitivity analysis ‐ detection bias (blinding of outcome assessment), Outcome 2: Mortality (cardiovascular) | ||||
| 6.2.1 Outcome assessment blinded | 3 | 874 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.02, 2.66] |
| 6.2.2 Not clearly blinded | 4 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.09, 3.32] |
| 6.3 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| Analysis 6.3  Comparison 6: Colchicine vs control: sensitivity analysis ‐ detection bias (blinding of outcome assessment), Outcome 3: Myocardial infarction (fatal) | ||||
| 6.3.1 Outcome assessment blinded | 2 | 652 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.00, 6.04] |
| 6.3.2 Not clearly blinded | 4 | 258 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.05, 2.47] |
| 6.4 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| Analysis 6.4  Comparison 6: Colchicine vs control: sensitivity analysis ‐ detection bias (blinding of outcome assessment), Outcome 4: Adverse event (total) | ||||
| 6.4.1 Outcome assessment blinded | 3 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.58, 1.93] |
| 6.4.2 Not clearly blinded | 8 | 869 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [1.03, 4.16] |
| 6.5 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| Analysis 6.5  Comparison 6: Colchicine vs control: sensitivity analysis ‐ detection bias (blinding of outcome assessment), Outcome 5: Adverse event (gastrointestinal) | ||||
| 6.5.1 Outcome assessment blinded | 5 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.77, 2.68] |
| 6.5.2 Not clearly blinded | 6 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 5.06 [0.92, 27.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 7.1  Comparison 7: Colchicine vs control: sensitivity analysis ‐ attrition bias (incomplete outcome data), Outcome 1: Mortality (all‐cause) | ||||
| 7.1.1 Low risk | 6 | 1548 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.25] |
| 7.1.2 High or unclear risk | 24 | 2626 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.73, 1.09] |
| 7.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| Analysis 7.2  Comparison 7: Colchicine vs control: sensitivity analysis ‐ attrition bias (incomplete outcome data), Outcome 2: Mortality (cardiovascular) | ||||
| 7.2.1 Low risk | 3 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.01, 13.68] |
| 7.2.2 High or unclear risk | 4 | 502 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.07, 2.30] |
| 7.3 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| Analysis 7.3  Comparison 7: Colchicine vs control: sensitivity analysis ‐ attrition bias (incomplete outcome data), Outcome 3: Myocardial infarction (fatal) | ||||
| 7.3.1 Low risk | 3 | 630 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.06, 15.36] |
| 7.3.2 High or unclear risk | 3 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.01, 1.18] |
| 7.4 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| Analysis 7.4  Comparison 7: Colchicine vs control: sensitivity analysis ‐ attrition bias (incomplete outcome data), Outcome 4: Adverse event (total) | ||||
| 7.4.1 Low risk | 2 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.66, 2.51] |
| 7.4.2 High or unclear risk | 9 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.89, 3.33] |
| 7.5 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| Analysis 7.5  Comparison 7: Colchicine vs control: sensitivity analysis ‐ attrition bias (incomplete outcome data), Outcome 5: Adverse event (gastrointestinal) | ||||
| 7.5.1 Low risk | 3 | 639 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.87, 3.16] |
| 7.5.2 High or unclear risk | 8 | 619 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.94, 5.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| Analysis 8.1  Comparison 8: Colchicine vs control: sensitivity analysis ‐ reporting bias (selective reporting, i.e. abstract publication only), Outcome 1: Mortality (all‐cause) | ||||
| 8.1.1 Full journal publication | 26 | 3303 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.09] |
| 8.1.2 Abstract only | 4 | 871 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.58, 1.57] |
| 8.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| Analysis 8.2  Comparison 8: Colchicine vs control: sensitivity analysis ‐ reporting bias (selective reporting, i.e. abstract publication only), Outcome 2: Mortality (cardiovascular) | ||||
| 8.2.1 Full journal publication | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| 8.2.2 Abstract only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.3 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| Analysis 8.3  Comparison 8: Colchicine vs control: sensitivity analysis ‐ reporting bias (selective reporting, i.e. abstract publication only), Outcome 3: Myocardial infarction (fatal) | ||||
| 8.3.1 Full journal publication | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| 8.3.2 Abstract only | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 8.4 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| Analysis 8.4  Comparison 8: Colchicine vs control: sensitivity analysis ‐ reporting bias (selective reporting, i.e. abstract publication only), Outcome 4: Adverse event (total) | ||||
| 8.4.1 Full journal publication | 9 | 725 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.90, 2.84] |
| 8.4.2 Abstract only | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.41, 4.74] |
| 8.5 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| Analysis 8.5  Comparison 8: Colchicine vs control: sensitivity analysis ‐ reporting bias (selective reporting, i.e. abstract publication only), Outcome 5: Adverse event (gastrointestinal) | ||||
| 8.5.1 Full journal publication | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| 8.5.2 Abstract only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot: Mortality (all‐cause)

Funnel plot: Adverse event (total)

Funnel plot: Adverse event (gastrointestinal)

Overview of results of meta‐analyses for colchicine treatment vs. control
ES: Effect Estimate; OR: Peto Odds Ratio; RR: risk ratio. * including one study without events ** including two studies without events.
Effects for some outcomes were estimated using Peto Odds Ratios because this is a more appropriate method when event rates are very low.

Comparison 1: Colchicine vs control, Outcome 1: Mortality (all‐cause)

Comparison 1: Colchicine vs control, Outcome 2: Myocardial infarction (total)

Comparison 1: Colchicine vs control, Outcome 3: Myocardial infarction (non‐fatal)

Comparison 1: Colchicine vs control, Outcome 4: Myocardial Infarction (fatal)

Comparison 1: Colchicine vs control, Outcome 5: Adverse event (serious)

Comparison 1: Colchicine vs control, Outcome 6: Adverse event (total)

Comparison 1: Colchicine vs control, Outcome 7: Adverse event (gastrointestinal)

Comparison 1: Colchicine vs control, Outcome 8: Mortality (cardiovascular)

Comparison 1: Colchicine vs control, Outcome 9: Stroke (total)

Comparison 1: Colchicine vs control, Outcome 10: Stroke (fatal)

Comparison 1: Colchicine vs control, Outcome 11: Stroke (non‐fatal)

Comparison 1: Colchicine vs control, Outcome 12: Heart failure (total)

Comparison 1: Colchicine vs control, Outcome 13: Heart failure (fatal)

Comparison 1: Colchicine vs control, Outcome 14: Heart failure (non‐fatal)

Comparison 1: Colchicine vs control, Outcome 15: Non‐scheduled hospitalisation (total)

Comparison 1: Colchicine vs control, Outcome 16: Non‐scheduled cardiovascular interventions

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 1: Mortality (all‐cause)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 2: Mortality (cardiovascular)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 3: Myocardial infarction (total)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 4: Myocardial infarction (fatal)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 5: Myocardial infarction (non‐fatal)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 6: Adverse event (total)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 7: Adverse event (gastrointestinal)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 8: Adverse event (serious)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 9: Stroke (total)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 10: Stroke (fatal)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 11: Stroke (non‐fatal)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 12: Heart failure (total)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 13: Heart failure (fatal)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 14: Heart failure (non‐fatal)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 15: Non‐scheduled hospitalisation (total)

Comparison 2: Colchicine vs control: subgroup analysis ‐ colchicine dose fixed ≤ 1 mg/d, Outcome 16: Non‐scheduled cardiovascular interventions

Comparison 3: Colchicine vs control: sensitivity analysis ‐ placebo or other inactive control, Outcome 1: Mortality (all‐cause)

Comparison 3: Colchicine vs control: sensitivity analysis ‐ placebo or other inactive control, Outcome 2: Mortality (cardiovascular)

Comparison 3: Colchicine vs control: sensitivity analysis ‐ placebo or other inactive control, Outcome 3: Myocardial infarction (fatal)

Comparison 3: Colchicine vs control: sensitivity analysis ‐ placebo or other inactive control, Outcome 4: Adverse event (total)

Comparison 3: Colchicine vs control: sensitivity analysis ‐ placebo or other inactive control, Outcome 5: Adverse event (gastrointestinal)

Comparison 4: Colchicine vs control: sensitivity analysis ‐ selection bias (random sequence generation and allocation concealment), Outcome 1: Mortality (all‐cause)

Comparison 4: Colchicine vs control: sensitivity analysis ‐ selection bias (random sequence generation and allocation concealment), Outcome 2: Mortality (cardiovascular)

Comparison 4: Colchicine vs control: sensitivity analysis ‐ selection bias (random sequence generation and allocation concealment), Outcome 3: Myocardial infarction (fatal)

Comparison 4: Colchicine vs control: sensitivity analysis ‐ selection bias (random sequence generation and allocation concealment), Outcome 4: Adverse event (total)

Comparison 4: Colchicine vs control: sensitivity analysis ‐ selection bias (random sequence generation and allocation concealment), Outcome 5: Adverse event (gastrointestinal)

Comparison 5: Colchicine vs control: sensitivity analysis ‐ performance bias (double‐blinded studies), Outcome 1: Mortality (all‐cause)

Comparison 5: Colchicine vs control: sensitivity analysis ‐ performance bias (double‐blinded studies), Outcome 2: Mortality (cardiovascular)

Comparison 5: Colchicine vs control: sensitivity analysis ‐ performance bias (double‐blinded studies), Outcome 3: Myocardial infarction (fatal)

Comparison 5: Colchicine vs control: sensitivity analysis ‐ performance bias (double‐blinded studies), Outcome 4: Adverse event (total)

Comparison 5: Colchicine vs control: sensitivity analysis ‐ performance bias (double‐blinded studies), Outcome 5: Adverse event (gastrointestinal)

Comparison 6: Colchicine vs control: sensitivity analysis ‐ detection bias (blinding of outcome assessment), Outcome 1: Mortality (all‐cause)

Comparison 6: Colchicine vs control: sensitivity analysis ‐ detection bias (blinding of outcome assessment), Outcome 2: Mortality (cardiovascular)

Comparison 6: Colchicine vs control: sensitivity analysis ‐ detection bias (blinding of outcome assessment), Outcome 3: Myocardial infarction (fatal)

Comparison 6: Colchicine vs control: sensitivity analysis ‐ detection bias (blinding of outcome assessment), Outcome 4: Adverse event (total)

Comparison 6: Colchicine vs control: sensitivity analysis ‐ detection bias (blinding of outcome assessment), Outcome 5: Adverse event (gastrointestinal)

Comparison 7: Colchicine vs control: sensitivity analysis ‐ attrition bias (incomplete outcome data), Outcome 1: Mortality (all‐cause)

Comparison 7: Colchicine vs control: sensitivity analysis ‐ attrition bias (incomplete outcome data), Outcome 2: Mortality (cardiovascular)

Comparison 7: Colchicine vs control: sensitivity analysis ‐ attrition bias (incomplete outcome data), Outcome 3: Myocardial infarction (fatal)

Comparison 7: Colchicine vs control: sensitivity analysis ‐ attrition bias (incomplete outcome data), Outcome 4: Adverse event (total)

Comparison 7: Colchicine vs control: sensitivity analysis ‐ attrition bias (incomplete outcome data), Outcome 5: Adverse event (gastrointestinal)

Comparison 8: Colchicine vs control: sensitivity analysis ‐ reporting bias (selective reporting, i.e. abstract publication only), Outcome 1: Mortality (all‐cause)

Comparison 8: Colchicine vs control: sensitivity analysis ‐ reporting bias (selective reporting, i.e. abstract publication only), Outcome 2: Mortality (cardiovascular)

Comparison 8: Colchicine vs control: sensitivity analysis ‐ reporting bias (selective reporting, i.e. abstract publication only), Outcome 3: Myocardial infarction (fatal)

Comparison 8: Colchicine vs control: sensitivity analysis ‐ reporting bias (selective reporting, i.e. abstract publication only), Outcome 4: Adverse event (total)

Comparison 8: Colchicine vs control: sensitivity analysis ‐ reporting bias (selective reporting, i.e. abstract publication only), Outcome 5: Adverse event (gastrointestinal)
| Colchicine compared to any control treatment for prevention of cardiovascular events | ||||||
| Patient or population: any patient population and people with high cardiovascular risk | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Any control treatment | Colchicine | |||||
| Mortality (all‐cause) | 193 per 1000 | 182 per 1000 | RR 0.94 | 4174 | ⊕⊕⊕⊝ | |
| Patients with high cardiovascular risk | ||||||
| 32 per 1000 | 17 per 1000 | RR 0.54 | 1230 | ⊕⊕⊕⊝ | Follow‐up: 0.5 ‐ 3 years | |
| Mortality (cardiovascular) | 27 per 1000 | 9 per 1000 | RR 0.34 | 1132 | ⊕⊕⊕⊝ | |
| Patients with high cardiovascular risk | ||||||
| 31 per 1000 | 8 per 1000 | RR 0.25 | 754 | ⊕⊕⊝⊝ | Follow‐up: 0.5 ‐ 3 years | |
| Myocardial Infarction (total) | 58 per 1000 | 12 per 1000 | RR 0.20 | 652 | ⊕⊕⊕⊝ | Most evidence provided by a single study |
| Patients with high cardiovascular risk | ||||||
| Study population | RR 0.20 | 532 | ⊕⊕⊕⊝ | Evidence provided by a single study. Follow‐up: mean 3 years Please see footnote6 | ||
| 72 per 1000 | 18 per 1000 | |||||
| Assumed 1‐year risk | ||||||
| 25 per 1000 | 6 per 1000 | |||||
| Adverse event (total) | Study population | RR 1.52 | 1313 | ⊕⊝⊝⊝ | No study in participants with high cardiovascular risk reported on total adverse events.9 | |
| 89 per 1000 | 135 per 1000 | |||||
| Assumed 1‐year risk | ||||||
| 89 per 1000 | 135 per 1000 | |||||
| Adverse event (gastrointestinal) | Study population | RR 1.83 | 1258 | ⊕⊕⊝⊝ | Please see footnote9 | |
| 132 per 1000 | 242 per 1000 | |||||
| Assumed 1‐year risk | ||||||
| 132 per 1000 | 242 per 1000 | |||||
| Adverse event (serious) | See comment | See comment | Not estimable | 472 | ⊕⊕⊝⊝ | No illustration of comparative risks due to very uncertain assumed risks10,11 |
| Heart failure (total) | See comment | See comment | RR 0.62 | 426 | ⊕⊕⊝⊝ | No illustration of comparative risks due to very uncertain assumed risks |
| Stroke (total) | See comment | See comment | OR 0.38 | 874 | ⊕⊕⊝⊝ | No illustration of comparative risks due to very uncertain assumed risks |
| *The basis for the assumed risk is the mean control group risk across studies if not otherwise stated in comments/footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Confidence interval is compatible with participant‐relevant benefit and harm. | ||||||
| All Studies | Studies in participants with | |
|---|---|---|
| Total number of studies | 39 (100%) | 4 (100%) |
| No. of participants in all studies | 4992 | 1230 |
| Publication year, median (range) | 1996 (1974 ‐ 2014) | 2013 (1992 ‐ 2014) |
| Publication year < 2000 | 24 (62%) | 1 (25%) |
| Multicentre studies | 9 (23%) | 0 (0%) |
| Study size, median (IQR) | 84 (54 ‐ 129) | 251 (210 ‐ 406) |
| Participant age, median (IQR) | 54 (51 ‐ 61) | 66 (63 ‐ 67) |
| Men, median (IQR) | 62 (25 ‐ 87) | 77 (66 ‐ 88) |
| Follow‐up¹ | ||
| 0.5 to 1 year | 6 (15%) | 3 (75%) |
| 1 to 3 years | 17 (44%) | 0 (0%) |
| > 3 years | 15 (38%) | 1 (25%) |
| Colchicine treatment | ||
| ≤ 1 mg/d | 27 (69%) | 3 (75%) |
| > 1 mg/d | 12 (31%) | 1 (25%) |
| Control treatment, n (%) | ||
| Active treatment | 8 (21%) | 0 (0%) |
| Inactive, placebo | 31 (79%) | 4 (100%) |
| Clinical setting | ||
| CVD, arteriosclerotic | 3 (8%) | 3 (75%) |
| CVD, other | 1 (3%) | 1 (25%) |
| Hepatobiliary disease | 25 (64%) | 0 (0%) |
| Other | 10 (26%) | 0 (0%) |
| Cardiovascular risk profile | ||
| Primary prevention | 0 (0%) | 0 (0%) |
| Secondary prevention | 4 (10%) | 4 (100%) |
| Not specified | 34 (87%) | 0 (0%) |
| Number of studies (% of column total) if not stated otherwise. | ||
| Study | Participants | Centres | Clinical | Age* | Men (%) | Colchicine dose (mg/d) | Control | Follow‐up |
|---|---|---|---|---|---|---|---|---|
| Studies in patients with high cardiovascular risk | ||||||||
| 222 | Single | PCI/CVD | 64 | 65 | 2 x 0.5 | Placebo | 0.5 | |
| 279 | Single | Heart failure | 67 | 67 | 1 ‐ 2 x 0.5² | Placebo | 0.5 | |
| 532 | Single | PCI/CVD | 67 | 89 | 0.5 | Usual care³ | 3 | |
| 197 | Single | PCI/CVD | 61 | 86 | 2 x 0.6 | Placebo | 0.5 | |
| Other studies | ||||||||
| 52 | Single | Liver disease | 54 | 87 | 1⁴ | Placebo | 11 | |
| 90 | Multi | PBC | 55 | 10 | 1 | Placebo⁵ | 3 | |
| 50 | Multi | Other | 68 | 84 | 1 | IFN‐gamma⁶ | 2.1 | |
| 57 | N/R | PBC | 52 | 9 | 2 x 0.6 | Placebo | 2.2 | |
| 180 | N/R. | Liver disease | N/R | N/R | 1 | “Conventional therapy” | 3 | |
| 129 | N/R | Liver disease | N/R | N/R | 1 | Placebo | 3.8 | |
| 555 | N/R | Liver disease | 51 | 70 | 2 x 0.6 | Peg‐IFN‐alpha | 2 | |
| 84 | Single | Other | 54 | 35 | 1 ‐ 2 x 0.5² | Usual care³,⁷ | 1.7 | |
| 120 | Multi | Other | 48 | 46 | 0.5 ‐ 1² | Placebo⁸ | 2 | |
| 240 | Multi | Other | 49 | 50 | 1 ‐ 2 x 0.5² | Placebo | 1.7 | |
| 62 | Single | Liver disease | 54 | 89 | 1⁴ | Placebo | 3.4 | |
| 26 | Single | Other | 68 | 77 | 0.6 ‐ 1.2⁹ | Prednisone | 2.5 | |
| 22 | Single | PBC | 61 | 14 | 1 | Usual care ³,⁵ | 2 | |
| 60 | Single | PBC | N/Rₑ⁰ | 5 | 2 x 0.6 | Placebo | 2 | |
| 87 | Single | PBC | 51 | 6 | 2 x 0.6 | Methotrexateₑₑ | 10 | |
| 28 | N/R | Liver disease | N/R | N/R | 1⁴ | Placebo | 2 | |
| 100 | Single | Liver disease | 51 | 50 | 1⁴ | Placebo | 14 | |
| 101 | Single | Other | 63 | 58 | 2 x 0.6ₑ² | Melphalan/prednisone | 5 | |
| 148 | N/R | Other | 64 | N/R | 2 x 0.6 | Usual care ³,ₑ³ | 9 | |
| 66 | Single | Liver disease | 40 | 88 | 1⁴ | Usual care³ | 4 | |
| 54 | N/R | Other | N/R | N/R | 1 ‐ 2 | Dimethyl sulfoxide | 2 or 7ₑ⁴ | |
| 549 | Multi | Liver disease | 56 | 98 | 2 x 0.6 | Placebo | 6 | |
| 74 | Single | Liver disease | 53 | 62 | 1 | "usual treatment for cirrhosis" | 4 | |
| 38 | Single | Liver disease | 51 | 61 | 1d | Usual care³ | 1 | |
| 84 | Multi | PSC | 42 | 67 | 1 | Placebo | 3 | |
| 41 | Single | Liver disease | 49 | 88 | 1 | Placebo | 1 | |
| 52 | Multi | Other | 53 | 100 | 3 x 0.5 | Placeboₑ⁵ | 0.5 | |
| 74 | Multi | PBC | 54 | 15 | 1⁴ | Placebo⁵ | 2 | |
| 28 | Single | PBC | 54 | 0 | 1 | Placebo⁵ | 2 | |
| 74 | Single | Liver disease | N/R | N/R | 4 x 0.25d | Placebo | 3 | |
| 67 | Single | Liver disease | 52 | 57 | 1 | Placebo | 0.5 | |
| 90 | Multi | Liver disease | 57 | 14 | 2x 0.5 | Placebo | 2 | |
| 100 | Single | Liver disease | 60 | 94 | 1 | Placebo | 2.2 | |
| 64 | Single | PBC | N/R | N/R | 2x 0.5 | Placebo | 1.5 | |
| 116 | Single | Other | 27 | 53 | 2 ‐ 4 x 0.5² | Placebo | 2 | |
| *Age is reported as mean in all studies but three: Antoniou 2006; Kyle 1985; Nikolaidis 2006 reported median age. | ||||||||
| Outcome | Studies (n) | Events (n) | Participants (n) | Summary effect | Heterogeneity (I²), % | Subgroup effect (P value) |
|---|---|---|---|---|---|---|
| Participants with high cardiovascular risk | ||||||
| All‐cause mortality | 4 | 29 | 1230 | RR 0.54 (0.26 to 1.14) | 0% | 0.13 |
| Cardiovascular mortality | 2 | 13 | 754 | RR 0.25 (0.02 to 2.66) | 49% | N/C |
| Myocardial infarction | ||||||
|
| 1 | 22 | 532 | RR 0.20 (0.07 to 0.57) | ‐ | N/C |
|
| 1 | 1 | 532 | RR 0.30 (0.01 to 7.22) | ‐ | N/C |
|
| 1 | 21 | 532 | RR 0.21 (0.07 to 0.61) | ‐ | N/C |
| Stroke | ||||||
|
| 2 | 7 | 754 | OR 0.38 (0.09 to 1.70) | 0% | N/C |
|
| 2 | 1 | 754 | OR 7.26 (0.14 to 365.85) | ‐ | N/C |
|
| 2 | 6 | 754 | OR 0.23 (0.05 to 1.17) | 0% | N/C |
| Heart failure | ||||||
|
| 1 | 3 | 222 | RR 0.14 (0.01 to 2.69) | ‐ | N/C |
|
| 1 | 1 | 222 | RR 0.33 (0.01 to 7.95) | ‐ | N/C |
|
| 1 | 2 | 222 | RR 0.20 (0.01 to 4.05) | ‐ | N/C |
| Hospitalisation | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Cardiovascular intervention | 1 | 9 | 222 | RR 0.79 (0.22 to 2.85) | ‐ | N/C |
| Adverse event, any | 0 | ‐ | ‐ | ‐ | ‐ | N/C |
| Adverse event, gastrointestinal | 2 | 62 | 501 | RR 2.41 (1.43 to 4.06) | 0% | N/C |
| Colchicine dose | ||||||
| All‐cause mortality | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| ≤ 1mg/d | 21 | 268 | 2420 | RR 0.82 (0.67 to 0.99) | 0% | 0.03 |
| > 1mg/d | 9 | 505 | 1754 | RR 1.08 (0.93 to 1.25) | 31% | ‐ |
| Adverse event, any | ||||||
| ≤ 1mg/d | 7 | 60 | 687 | RR 1.75 (0.74 to 4.14) | 40% | 0.75 |
| > 1mg/d | 3 | 87 | 626 | RR 1.47 (0.72 to 2.97) | 73% | ‐ |
| Peto odds ratio for outcomes with event rates between 1% and 5% | ||||||
| All‐cause mortality in participants with high cardiovascular risk | 4 | 29 | 1230 | OR 0.53 (0.25 to 1.11) | 0% | ‐ |
| Cardiovascular mortality | 7 | 17 | 1132 | OR 0.24 (0.09 to 0.64) | 15% | ‐ |
| Mantel‐Haenszel risk ratio without zero correction for outcomes with event rates between 1% and 5% | ||||||
| All‐cause mortality in participants with high cardiovascular risk | 4 | 29 | 1230 | RR 0.54 (0.26 to 1.12) | 0% | ‐ |
| Cardiovascular mortality | 7 | 17 | 1132 | RR 0.20 (0.06 to 0.68) | 0% | ‐ |
| N/C: not calculated | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| 1.1.1 Participants with high cardiovascular risk | 4 | 1230 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.26, 1.14] |
| 1.1.2 Other | 26 | 2944 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.84, 1.12] |
| 1.2 Myocardial infarction (total) Show forest plot | 2 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.57] |
| 1.2.1 Participants with high cardiovascular risk | 1 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.57] |
| 1.2.2 Other | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.3 Myocardial infarction (non‐fatal) Show forest plot | 2 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.07, 0.61] |
| 1.3.1 Participants with high cardiovascular risk | 1 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.07, 0.61] |
| 1.3.2 Other | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.4 Myocardial Infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| 1.4.1 Participants with high cardiovascular risk | 1 | 532 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.00, 6.04] |
| 1.4.2 Other | 5 | 378 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.05, 2.47] |
| 1.5 Adverse event (serious) Show forest plot | 4 | 472 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.5.1 Participants with high cardiovascular risk | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.5.2 Other | 4 | 472 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.6 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| 1.6.1 Participants with high cardiovascular risk | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.6.2 Other | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| 1.7 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| 1.7.1 Participants with high cardiovascular risk | 2 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.43, 4.06] |
| 1.7.2 Other | 9 | 757 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.82, 3.02] |
| 1.8 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| 1.8.1 Participants with high cardiovascular risk | 2 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.02, 2.66] |
| 1.8.2 Other | 5 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.09, 3.32] |
| 1.9 Stroke (total) Show forest plot | 3 | 874 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.09, 1.70] |
| 1.9.1 Participants with high cardiovascular risk | 2 | 754 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.09, 1.70] |
| 1.9.2 Other | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.10 Stroke (fatal) Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.10.1 Participants with high cardiovascular risk | 2 | 754 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.26 [0.14, 365.85] |
| 1.10.2 Other | 2 | 161 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.11 Stroke (non‐fatal) Show forest plot | 3 | 874 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.17] |
| 1.11.1 Participants with high cardiovascular risk | 2 | 754 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.17] |
| 1.11.2 Other | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.12 Heart failure (total) Show forest plot | 3 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.10, 3.88] |
| 1.12.1 Participants with high cardiovascular risk | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.69] |
| 1.12.2 Other | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.46, 2.51] |
| 1.13 Heart failure (fatal) Show forest plot | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.13.1 Participants with high cardiovascular risk | 1 | 222 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.70] |
| 1.13.2 Other | 2 | 161 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.14 Heart failure (non‐fatal) Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.14.1 Participants with high cardiovascular risk | 1 | 222 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.12] |
| 1.14.2 Other | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 1.15 Non‐scheduled hospitalisation (total) Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.77, 0.99] |
| 1.15.1 Participants with high cardiovascular risk | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.15.2 Other | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.77, 0.99] |
| 1.16 Non‐scheduled cardiovascular interventions Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.16.1 Participants with high cardiovascular risk | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.22, 2.85] |
| 1.16.2 Other | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| 2.1.1 ≤ 1 mg/d | 21 | 2420 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.67, 0.99] |
| 2.1.2 > 1 mg/d | 9 | 1754 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.25] |
| 2.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| 2.2.1 ≤ 1 mg/d | 5 | 955 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.06, 0.82] |
| 2.2.2 > 1 mg/d | 2 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [0.13, 73.12] |
| 2.3 Myocardial infarction (total) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3.1 ≤ 1 mg/d | 1 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.57] |
| 2.3.2 > 1 mg/d | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.4 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| 2.4.1 ≤ 1 mg/d | 4 | 733 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.02, 0.87] |
| 2.4.2 > 1 mg/d | 2 | 177 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.66 [0.15, 386.16] |
| 2.5 Myocardial infarction (non‐fatal) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.5.1 ≤ 1 mg/d | 1 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.07, 0.61] |
| 2.5.2 > 1 mg/d | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.6 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| 2.6.1 ≤ 1 mg/d | 8 | 687 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.74, 4.14] |
| 2.6.2 > 1 mg/d | 3 | 626 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.72, 2.97] |
| 2.7 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| 2.7.1 ≤ 1 mg/d | 9 | 1104 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.26, 3.66] |
| 2.7.2 > 1 mg/d | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.61, 2.19] |
| 2.8 Adverse event (serious) Show forest plot | 4 | 472 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.8.1 ≤ 1 mg/d | 4 | 472 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.8.2 > 1 mg/d | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.9 Stroke (total) Show forest plot | 3 | 874 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.09, 1.70] |
| 2.9.1 ≤ 1 mg/d | 2 | 754 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.09, 1.70] |
| 2.9.2 > 1 mg/d | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.10 Stroke (fatal) Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.10.1 ≤ 1 mg/d | 3 | 795 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.26 [0.14, 365.85] |
| 2.10.2 > 1 mg/d | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.11 Stroke (non‐fatal) Show forest plot | 3 | 874 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.17] |
| 2.11.1 ≤ 1 mg/d | 2 | 754 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.17] |
| 2.11.2 > 1 mg/d | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.12 Heart failure (total) Show forest plot | 3 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.10, 3.88] |
| 2.12.1 ≤ 1 mg/d | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.69] |
| 2.12.2 > 1 mg/d | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.46, 2.51] |
| 2.13 Heart failure (fatal) Show forest plot | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.13.1 ≤ 1 mg/d | 2 | 263 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.70] |
| 2.13.2 > 1 mg/d | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.14 Heart failure (non‐fatal) Show forest plot | 2 | 342 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.12] |
| 2.14.1 ≤ 1 mg/d | 1 | 222 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.12] |
| 2.14.2 > 1 mg/d | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.15 Non‐scheduled hospitalisation (total) Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.77, 0.99] |
| 2.15.1 ≤ 1 mg/d | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.31, 2.55] |
| 2.15.2 > 1 mg/d | 1 | 549 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.77, 0.99] |
| 2.16 Non‐scheduled cardiovascular interventions Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.16.1 ≤ 1 mg/d | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.22, 2.85] |
| 2.16.2 > 1 mg/d | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| 3.1.1 Placebo, inactive control | 26 | 3479 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.84, 1.11] |
| 3.1.2 Active control | 4 | 695 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.95] |
| 3.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| 3.2.1 Placebo, inactive control | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| 3.2.2 Active control | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.3 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| 3.3.1 Placebo, inactive control | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| 3.3.2 Active control | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 3.4 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| 3.4.1 Placebo, inactive control | 9 | 725 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.90, 2.84] |
| 3.4.2 Active control | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.41, 4.74] |
| 3.5 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| 3.5.1 Placebo, inactive control | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| 3.5.2 Active control | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| 4.1.1 Low risk | 6 | 1411 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.87, 1.22] |
| 4.1.2 High or unclear risk | 24 | 2763 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.11] |
| 4.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| 4.2.1 Low risk | 2 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.69] |
| 4.2.2 High or unclear risk | 5 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.15, 2.95] |
| 4.3 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| 4.3.1 Low risk | 2 | 652 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.00, 6.04] |
| 4.3.2 High or unclear risk | 4 | 258 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.05, 2.47] |
| 4.4 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| 4.4.1 Low risk | 3 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.61, 4.04] |
| 4.4.2 High or unclear risk | 8 | 898 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.81, 2.91] |
| 4.5 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| 4.5.1 Low risk | 4 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.59, 2.04] |
| 4.5.2 High or unclear risk | 7 | 727 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [1.49, 3.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| 5.1.1 Double‐blind | 22 | 2538 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.07] |
| 5.1.2 Not clearly double‐blind | 8 | 1636 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.82, 1.32] |
| 5.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| 5.2.1 Double‐blind | 6 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.15, 2.95] |
| 5.2.2 Not clearly double‐blind | 1 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.69] |
| 5.3 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| 5.3.1 Double‐blind | 5 | 378 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.05, 2.47] |
| 5.3.2 Not clearly double‐blind | 1 | 532 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.00, 6.04] |
| 5.4 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| 5.4.1 Double‐blind | 6 | 581 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.01, 3.12] |
| 5.4.2 Not clearly double‐blind | 5 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.49, 3.11] |
| 5.5 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| 5.5.1 Double‐blind | 9 | 1198 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.98, 3.14] |
| 5.5.2 Not clearly double‐blind | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 5.91 [0.32, 110.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| 6.1.1 Outcome assessment blinded | 4 | 1582 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.88, 1.25] |
| 6.1.2 Not clearly blinded | 26 | 2592 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.09] |
| 6.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| 6.2.1 Outcome assessment blinded | 3 | 874 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.02, 2.66] |
| 6.2.2 Not clearly blinded | 4 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.09, 3.32] |
| 6.3 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| 6.3.1 Outcome assessment blinded | 2 | 652 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.00, 6.04] |
| 6.3.2 Not clearly blinded | 4 | 258 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.05, 2.47] |
| 6.4 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| 6.4.1 Outcome assessment blinded | 3 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.58, 1.93] |
| 6.4.2 Not clearly blinded | 8 | 869 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [1.03, 4.16] |
| 6.5 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| 6.5.1 Outcome assessment blinded | 5 | 977 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.77, 2.68] |
| 6.5.2 Not clearly blinded | 6 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 5.06 [0.92, 27.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| 7.1.1 Low risk | 6 | 1548 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.25] |
| 7.1.2 High or unclear risk | 24 | 2626 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.73, 1.09] |
| 7.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| 7.2.1 Low risk | 3 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.01, 13.68] |
| 7.2.2 High or unclear risk | 4 | 502 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.07, 2.30] |
| 7.3 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| 7.3.1 Low risk | 3 | 630 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.06, 15.36] |
| 7.3.2 High or unclear risk | 3 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.01, 1.18] |
| 7.4 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| 7.4.1 Low risk | 2 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.66, 2.51] |
| 7.4.2 High or unclear risk | 9 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.89, 3.33] |
| 7.5 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| 7.5.1 Low risk | 3 | 639 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.87, 3.16] |
| 7.5.2 High or unclear risk | 8 | 619 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.94, 5.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Mortality (all‐cause) Show forest plot | 30 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.09] |
| 8.1.1 Full journal publication | 26 | 3303 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.09] |
| 8.1.2 Abstract only | 4 | 871 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.58, 1.57] |
| 8.2 Mortality (cardiovascular) Show forest plot | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| 8.2.1 Full journal publication | 7 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.21] |
| 8.2.2 Abstract only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.3 Myocardial infarction (fatal) Show forest plot | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| 8.3.1 Full journal publication | 6 | 910 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.05, 1.62] |
| 8.3.2 Abstract only | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 8.4 Adverse event (total) Show forest plot | 11 | 1313 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.93, 2.46] |
| 8.4.1 Full journal publication | 9 | 725 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.90, 2.84] |
| 8.4.2 Abstract only | 2 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.41, 4.74] |
| 8.5 Adverse event (gastrointestinal) Show forest plot | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| 8.5.1 Full journal publication | 11 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.03, 3.26] |
| 8.5.2 Abstract only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |