Auf der Haut angewendete antimikrobielle Mittel zur Behandlung von Fußgeschwüren bei Menschen mit Diabetes
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011038.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 junio 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Jo Dumville: co‐ordinated the review; extracted data; checked the quality of data extraction; analysed and interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; contributed to writing and editing the review; made an intellectual contribution to the review; approved the final review prior to submission; advised on the review; secured funding; and is a guarantor of the review.
Benjamin Lipsky: conceived, designed, and co‐ordinated the review; checked the quality of data extraction; analysed and interpreted data; checked quality assessment; produced the first draft of the review; contributed to writing and editing the review; made an intellectual contribution to the review; approved the final review prior to submission; advised on the review; performed previous work that was the foundation of the current review; wrote to study author/experts/companies; provided data; and is a guarantor of the review.
Christopher Hoey: extracted data; contributed to writing or editing of the review; made an intellectual contribution to the review; approved the final review prior to submission; advised on the review; performed previous work that was the foundation of the current review; and provided data.
Mario Cruciani: conceived, designed, and co‐ordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; produced the first draft of the review; contributed to writing or editing of the review; made an intellectual contribution to the review; approved the final review prior to submission; and advised on the review.
Marta Fiscon: extracted data; checked the quality of data extraction; contributed to writing or editing of the review; made an intellectual contribution to the review; and approved the final review prior to submission.
Jun Xia: extracted data; checked the quality of data extraction; and approved the final review prior to submission.
Contributions of editorial base
Nicky Cullum (Editor): edited the protocol and the review; advised on methodology, interpretation, and content; approved the final protocol and review prior to submission.
Sally Bell‐Syer and Gill Rizzello (Managing Editors): co‐ordinated the editorial process; advised on interpretation, and content; edited the protocol and review.
Ruth Foxlee and Reetu Child (Information Specialists): designed the search strategy, ran the searches and edited the search methods section.
Ursula Gonthier (Editorial Assistant): checked and edited the Plain Language Summary and reference sections of the review.
Sources of support
Internal sources
-
Division of Nursing, Midwifery and Social Work, School of Health Sciences, University of Manchester, UK.
External sources
-
National of Institute of Health Research, UK.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure and Programme Grant funding (NIHR Cochrane Programme Grant 13/89/08 ‐ High Priority Cochrane Reviews in Wound Prevention and Treatment) to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health
-
National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester Centre, UK.
Jo Dumville was partly funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester. The funder had no role in the design of the studies, data collection and analysis, decision to publish, or preparation of the manuscript. However, the review may be considered to be affiliated to the work of the NIHR CLAHRC Greater Manchester. The views expressed herein are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health.
Declarations of interest
Jo C Dumville: has received research funding from the National Institute for Health Research (NIHR) for the production of systematic reviews focusing on high‐priority Cochrane reviews in the prevention and treatment of wounds. This work was also partly funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC) Greater Manchester.
Benjamin A Lipsky: has been a member of an advisory board or speakers bureau for KCI and Affinium and has served as a consultant to, or received research funding or lecture fees from: Innocoll, Dipexium and Merck. He recused himself from data extraction and from discussions on papers for which he was an author. He asserts that none of his industry‐related activities presented any conflict of interest for this review.
Christopher Hoey: none known
Mario Cruciani: received payments for consultancy work from ViiV Healthcare and Bristol‐Myers Squibb and payment for lectures from Abbott, Gilead Sciences and Merck but states that these were not related to his work with Cochrane or the subject of this review.
Marta Fiscon: none known.
Jun Xia: none known.
Laura Bolton (specialist peer reviewer) states: 'Before I retired in 2006, as a scientist for Johnson & Johnson (13 years), then R&D manager then director of Scientific Affairs for ConvaTec (19 years) I was aware of details of some studies cited, though I never participated directly as investigator or monitor in any of these studies. As a volunteer guideline developer and Cochrane Wounds reviewer I have searched for related study information on prior occasions'.
Acknowledgements
The authors appreciate the contribution of the peer referees ( Jane Burch, Susan O’Meara, Marialena Trivella, Ann‐Marie Glenny, Laura Bolton, Malcolm Brewster and a clinical expert who wishes to remain anonymous) and the copy editors Elizabeth Royle and Lisa Winer. We also thank Carlo Mengoli, who played an important role in helping us develop the protocol.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jun 14 | Topical antimicrobial agents for treating foot ulcers in people with diabetes | Review | Jo C Dumville, Benjamin A Lipsky, Christopher Hoey, Mario Cruciani, Marta Fiscon, Jun Xia | |
| 2014 Mar 22 | Topical antimicrobial agents for preventing and treating foot infections in people with diabetes | Protocol | Benjamin A Lipsky, Christopher Hoey, Mario Cruciani, Carlo Mengoli | |
Differences between protocol and review
We revised the Methods section in keeping with standard Cochrane methods. We also restructured the Types of outcome measures section to improve readability and to add more detailed definitions of assessment. We added 'proportion of ulcers healed' as well as 'time to wound healing', which is standard in wounds review but had been omitted at the protocol stage. We also added details not previously included about how outcome data at different time points would be managed. We renamed the safety outcome 'adverse events' and added a fuller description in line with other Cochrane Wounds reviews.
In the protocol the Types of participants section noted that: "Studies with a mixed population of participants with foot ulcers who do, as well as those who do not, have diabetes will be included if the results for the diabetic patient subset are separately provided." As this approach is essentially a subgroup analysis of an included trial, we revised this approach to consider the use of separately reported data only when stratification by wound type had been used at randomisation, or when the majority of wounds were ulcers of the foot in people with diabetes.
We also clarified the interventions that were not relevant to this review (i.e. those not considered to be topical).
We added GRADE assessment and 'Summary of findings' tables; updated sections with more detailed analytical information; and edited the wording. We made no changes that fundamentally altered the review as planned or in its implementation and do not believe we have biased the review in any way. Changes were made prior to data extraction and the writing of the current draft of the review.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Topical;
- Anti‐Bacterial Agents [*administration & dosage, adverse effects];
- Bacterial Infections [complications, *drug therapy, epidemiology];
- Bandages, Hydrocolloid;
- Diabetic Foot [complications, *drug therapy];
- Foot Ulcer [drug therapy];
- Incidence;
- Intercellular Signaling Peptides and Proteins [administration & dosage];
- Randomized Controlled Trials as Topic [statistics & numerical data];
- Wound Healing [drug effects];
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
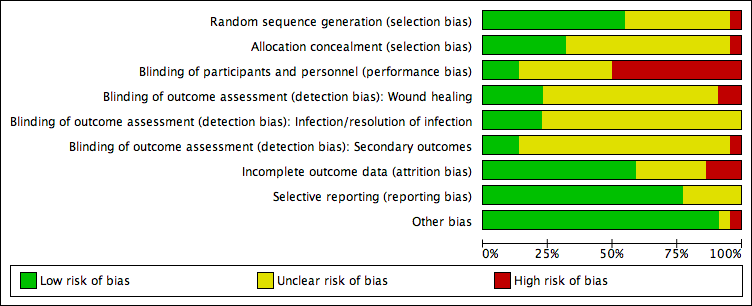
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 1 Proportion of wounds healed.

Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 2 Incidence of infection: medium term follow‐up.

Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 3 Surgical resection: medium term follow‐up.

Comparison 1 Topical antimicrobial dressing compared with non‐antimicrobial dressing, Outcome 4 Adverse events.

Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 1 Proportion of wounds healed: medium term follow‐up.
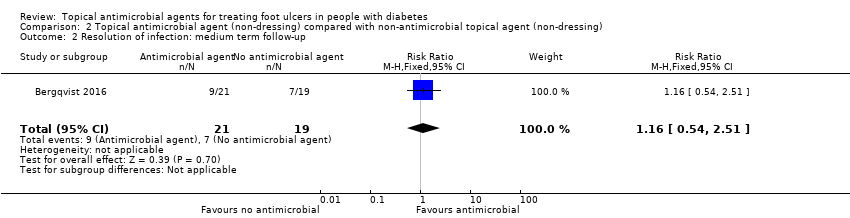
Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 2 Resolution of infection: medium term follow‐up.

Comparison 2 Topical antimicrobial agent (non‐dressing) compared with non‐antimicrobial topical agent (non‐dressing), Outcome 3 Surgical resection: medium term follow‐up.

Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 1 Proportion of wounds healed.

Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 2 Resolution of infection: medium term follow‐up.

Comparison 3 One topical antimicrobial agent compared with an alternative topical antimicrobial agent, Outcome 3 Surgical resection: medium term follow‐up.

Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 1 Resolution of infection.
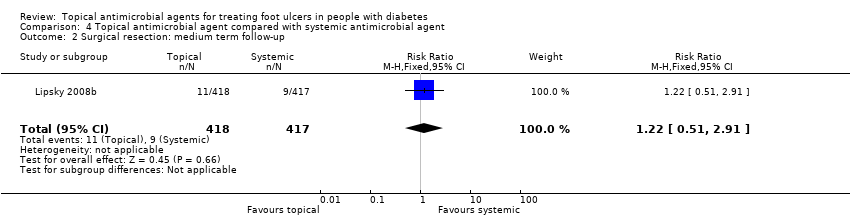
Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 2 Surgical resection: medium term follow‐up.

Comparison 4 Topical antimicrobial agent compared with systemic antimicrobial agent, Outcome 3 Adverse events.
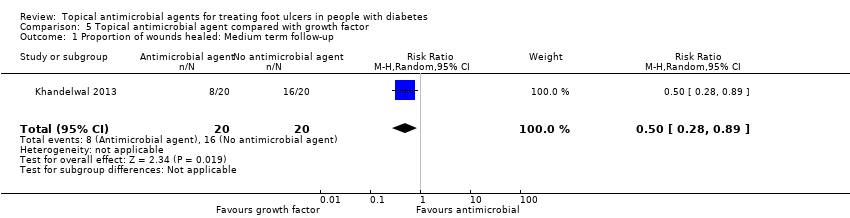
Comparison 5 Topical antimicrobial agent compared with growth factor, Outcome 1 Proportion of wounds healed: Medium term follow‐up.
| Antimicrobial dressings compared with non‐antimicrobial dressings | ||||||
| Patient or population: Foot ulcers in people with diabetes Settings: Mixed Intervention: Antimicrobial dressings Comparison: Standard dressings | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard dressings | Risk with antimicrobial dressings | |||||
| Proportion of wounds healed Up to 24 weeks' follow‐up | 425 per 1000 | 544 per 1000 | RR 1.28 (1.12 to 1.45) | 945 participants (5 studies) | ⊕⊕⊝⊝ | On average, use of an antimicrobial dressing compared with a non‐antimicrobial dressing may increase the number of ulcers healed over a medium‐term follow‐up period. |
| Risk difference: 119 more healed wounds per 1000 (51 more to 191 more) | ||||||
| Incidence of infection Up to 24 weeks' follow‐up | 183 per 1000 | 62 per 100 (7 to 567) | RR 0.34 (0.04 to 3.10) | 173 participants (2 studies) | ⊕⊝⊝⊝ | On average, it is unclear whether or not use of an antimicrobial dressing compared with a non‐antimicrobial dressing reduces the incidence of ulcer infection over a medium‐term follow‐up period. |
| Risk difference: 121 fewer infections per 1000 (176 fewer to 384 more) | ||||||
| Resolution infection | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
| Adverse events Up to 24 weeks' follow‐up | 388 per 1000 | 373 per 1000 (241 to 574) | RR 0.96 (0.62 to 1.48) | 134 participants (1 study) | ⊕⊝⊝⊝ | It is uncertain whether use of an antimicrobial dressing affects the risk of adverse events compared with use of a non‐antimicrobial dressing over a medium‐term follow‐up period. |
| Risk difference: 16 fewer adverse events per 1000 (147 fewer to 186 more) | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for risk of bias due to one study (with the highest weighting in the meta‐analysis) being at unclear risk of selection bias and three studies being at high risk of performance bias (36% weighting in analysis), although the studies were at unclear or low risk of detection bias for this outcome. | ||||||
| Topical antimicrobial agents (non‐dressing) compared with non‐antimicrobial topical agents (non‐dressing) | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with non‐antimicrobial treatment | Risk with topical antimicrobial treatment | |||||
| Proportion of wounds healed Up to 24 weeks' follow‐up | 241 per 1000 | 679 per 1000 (135 to 1000) | RR 2.82 (0.56 to 14.23) | 112 participants (3 studies) | ⊕⊝⊝⊝ | The average effect of antimicrobial agents compared with non‐antimicrobial treatment is uncertain over a medium‐term follow‐up period. |
| Risk difference: 438 more healed wounds per 1000 (106 fewer to 1000 more) | ||||||
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
| Resolution of infection Up to 24 weeks' follow‐up | 368 per 1000 | 427 per 1000 (199 to 925) | RR 1.16 (0.54 to 2.51) | 40 participants (1 study) | ⊕⊕⊝⊝ | It is unclear whether use of an antimicrobial topical agent has an effect on risk of infection over a medium‐term follow‐up period. |
| Risk difference: 59 more resolved infections per 1000 (169 fewer to 556 more) | ||||||
| Adverse events Up to 24 weeks' follow‐up | Not estimable | N/A | 81 participants (2 studies) | ⊕⊝⊝⊝ | 2 studies reported adverse event data. We were unable to extract per‐participant data for 1 study. The second study stated that no adverse events were reported in each arm. We judged this as very low‐certainty evidence. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for risk of bias with two studies at high risk of detection bias, which is of particular concern when healing is being assessed, and one study not accounting for a small number of participants with multiple ulcers in their trial. Downgraded twice for imprecision: small sample size and small number of events. Downgraded once for inconsistency: one small study reported all wounds healed in one arm and few wounds healed in the other. These data are adding heterogeneity to the analysis. | ||||||
| One topical antimicrobial agent compared with another topical antimicrobial agent | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with topical antimicrobial agent | Risk with alternative topical antimicrobial agent | |||||
| Proportion of wounds healed Up to 24 weeks' follow‐up | Data were not pooled due to the 3 studies evaluating different interventions. | N/A | 85 participants (3 studies) | ⊕⊝⊝⊝ | It is generally uncertain whether 1 topical treatment has an increased likelihood of healing compared with the alternative treatment. We judged this as very low‐certainty evidence ‐ downgraded twice for imprecision and once for risk of bias. | |
| Incidence of infection Up to 24 weeks' follow‐up | Not reported for this comparison | N/A | N/A | N/A | This outcome was not reported for this comparison. | |
| Resolution of infection Up to 24 weeks' follow‐up | 625 per 1000 | 906 per 1000 (606 to 1000) | RR 1.45 (0.97 to 2.17) | 37 participants (1 study) | ⊕⊝⊝⊝ | It is uncertain whether 1 specific type of topical antimicrobial agent has a different effect on resolution of infection than another over a medium‐term follow‐up period. |
| Risk difference: 281 more resolved infections per 1000 (19 fewer to 731 more) | ||||||
| Adverse events Up to 24 weeks' follow‐up | Not estimable | N/A | 41 participants (1 study) | ⊕⊝⊝⊝ | The 1 study noted that no events were reported in either group. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for imprecision: small sample size and small number of events. Downgraded for risk of performance and detection bias. | ||||||
| Topical antimicrobial agent compared with systemic antimicrobial agent | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with systemic antibiotic agent | Risk with topical antimicrobial agent | |||||
| Proportion of wounds healed | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Resolution of infection | 333 per 1000 | 503 per 1000 (303 to 830) | RR 1.51 (0.91 to 2.49) | 102 participants (2 studies) | ⊕⊝⊝⊝ | It is uncertain whether the effects of topical antimicrobial treatment on resolution of infection differ from those of systemic antibiotics. |
| Risk difference: 170 more resolved infections per 1000 (30 fewer to 497 more) | ||||||
| Adverse events | 450 per 1000 | 409 per 1000 (351 to 477) | RR 0.91 (0.78 to 1.06) | 937 participants (4 studies) | ⊕⊕⊕⊝ moderate2 | On average, there is probably little difference in the risk of adverse events between the systemic antibiotics and topical antimicrobial treatments compared here. |
| Risk difference: 40 fewer adverse events per 1000 (99 fewer to 27 more) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded twice for imprecision: small sample size and small number of events. Downgraded once for risk of performance bias. | ||||||
| Topical antimicrobial agent compared with growth factor | ||||||
| Patient or population: Foot ulcers in people with diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with growth factor | Risk with topical antimicrobial | |||||
| Proportion of wounds healed | 800 per 1000 | 400 per 1000 (224 to 712) | RR 0.50 (0.28 to 0.89) | 40 participants (1 study) | ⊕⊝⊝⊝ | It is uncertain whether treatment with growth factor affects the risk of healing when compared with antiseptic dressing. |
| Risk difference: 400 fewer resolved infections 576 fewer to 88 fewer | ||||||
| Incidence of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Resolution of infection | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| Adverse events | Not reported for this comparison | N/A | N/A | N/A | Outcome not reported for this comparison. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once for imprecision: small sample size and small number of events ‐ optimal information size not met and results are fragile. Downgraded twice for risk of performance and attrition bias. | ||||||
| Clinical manifestation of infection | PEDIS grade | IDSA infection |
| No symptoms or signs of infection | 1 | Uninfected |
| Infection present, as defined by the presence of at least 2 of the following items:
| ||
| Local infection involving only the skin and the subcutaneous tissue (without involvement of deeper tissues and without systemic signs as described below). If erythema, must be > 0.5 cm to ≤ 2 cm around the ulcer. | 2 | Mild |
| Local infection (as described above) with erythema > 2 cm, or involving structures deeper than skin and subcutaneous tissues (e.g. abscess, osteomyelitis, septic arthritis, fasciitis), and no systemic inflammatory response signs (as described below) | 3 | Moderate |
| Local infection (as described above) with the signs of SIRS, as manifested by ≥ 2 of the following:
| 4 | Severe* |
| Abbreviations: IDSA, Infectious Diseases Society of America; PaCO2, partial pressure of arterial carbon dioxide; PEDIS, perfusion, extent/size, depth/tissue loss, infection, and sensation; SIRS, systemic inflammatory response syndrome *Ischaemia may increase the severity of any infection, and the presence of critical ischaemia often makes the infection severe. Systemic infection may sometimes manifest with other clinical findings, such as hypotension, confusion, vomiting, or evidence of metabolic disturbances, such as acidosis, severe hyperglycaemia, and new‐onset azotaemia. | ||
| Product and formulations | Formulations | Bacterial spectrum | Advantages | Disadvantages | Costa | Indicationsb and comments |
| Acetic acid | 0.25%, 0.5%, and 1% solutions | Bactericidal against most gram‐positive and gram‐negative organisms, including Pseudomonas aeruginosa | Inexpensive; shown to eliminate P aeruginosa colonisation from burn | Cytotoxic in vitro although maybe not in vivo; limited activity against biofilm | $ | No longer as widely used as in the past |
| Cadexomer iodine | Gel,c ointment, and dressing | Polysaccharide starch lattice; active agent is slowly released free iodine; broad spectrum of activity (same as iodine) | Reduced local toxicity compared to iodine; elemental iodine released on exposure to exudate | Application may cause stinging and erythema, but less tissue damage than other iodine products; effect may not persist, and efficacy may be reduced in body fluids. | $$ | Indicated for use in cleaning wet ulcers and wounds and reducing microbial load in the wound environment |
| Cetrimide | Solution, 40% | Active against bacteria and fungi; not active against P aeruginosa | May be less toxic to wound tissues than other antiseptics | May be corrosive and is potentially harmful if swallowed | $ | Not available in the USA |
| Chlorhexidine gluconate | Solution, 2% and 4%; liquid, 2% and 4%; hand rinse, 0.5%; wipes, 0.5%; sponge/brush, 4%; and foam, 4% | Active against gram‐positive bacteria (e.g. Staphylococcus aureus) and gram‐negative bacteria, including P aeruginosa | Persistent activity up to 6 h after application; few adverse effects | Hypersensitivity, including anaphylaxis, generalised urticaria, bronchospasm, cough, dyspnoea, wheezing, and malaise; may cause serious injury to the eye and middle ear; avoid contact with face or head; some resistance reported | $ | 2% chlorhexidine indicated as surgical hand scrub, hand wash, skin and wound cleanser; polyhexanide is a similar, newer biguanide. |
| Hexachlorophene | Liquid, 3%; foam, 0.23% with 56% alcohol | Biguanide that is bacteriostatic against Staphylococcus species and other gram‐positive bacteria | May retain residual effect on skin for several days | Rapidly absorbed and may result in toxic blood levels; application to burns has resulted in neurotoxicity and death; may cause central nervous system stimulation and convulsions, dermatitis, and photosensitivity reactions | $$$ | Not recommended for routine use on wounds due to potential toxicity |
| Iodine compounds and iodine tincturec | Solution (aqueous) 2% and 2.4%; and tincture (44% to 50% alcohol) 2% and 2.4% | Microbicidal against bacteria, fungi, viruses, spores, protozoa, and yeasts | Broad spectrum | Highly toxic if ingested or significantly absorbed; do not use with occlusive dressings; causes pain and stains skin and clothing; use cautiously in people with thyroid disorders | $ | Iodine compounds are now rarely used for wound management; cadexomer iodine and povidone iodine products are less toxic. |
| Povidone iodinec | Ointment, 1%, 4.7%, 10%; solution, 1% and 10%; also wash, scrub, cleanser, gel, aerosol, gauze pad, swab, and other forms | Broad spectrum includes S aureus and enterococci; active ingredient is liberated free iodine; shares spectrum but is less potent than iodine | Less irritating to skin and allergenic than iodine. Can be covered with dressings. Clinically significant resistance very rare | Antibacterial action requires at least 2 min contact; may cause stinging and erythema; effect may not persist, and efficacy may be reduced in body fluids; prolonged use may cause metabolic acidosis; stains skin and clothing; possible interaction with starches in dressings | $ | Indicated for perioperative skin cleansing and for cleansing and prevention of infection in superficial burns, incisions, and other superficial wounds |
| Sodium hypochlorite (Dakin’s solution and EUSOL) | Solution, 0.0125%, 0.125%, 0.25%, and 0.5% | Vegetative bacteria, viruses, and some spores and fungi | Inexpensive | No known systemic toxicity. May require prolonged contact for antibacterial action; inactivated by pus; toxic to fibroblasts and keratinocytes, and may cause pain or lyse blood clots | $ | A concentration of 0.025% is both bactericidal and non‐toxic to tissues (Heggers 1991). |
| Hydrogen peroxidec | Solution, 1% and 3%; and cream, 1% | Oxidizing agent active against many gram‐positive and gram‐negative bacteria | Broad‐spectrum, bactericidal, inexpensive; no known 1q11 | May cause some discomfort | $ | Commonly used, but few clinical studies |
| Silver nitrate | Solution 0.5%, 10%, 25%, and 50%; ointment, 10%; and swabs, 25% to 50% | Silver ions are bactericidal against a broad spectrum of gram‐positive and gram‐negative bacteria. | Low cost; easily applied | Painful on application; stains tissues; may delay healing; concentrations 10.5% cause cauterisation; inactivated by wound exudates and chlorine | $ | Previously widely used, but now largely replaced by other compounds, including newer silver dressings |
| Silver dressings | At least 6 approved products with different properties | Slowly released silver ions have broad spectrum, including MRSA and VRE. | Provide sustained levels of active silver ions; microbial resistance is rare; less painful and few adverse effects than silver nitrate; variety of products adaptable to different types of wounds; infrequent application required | Levels of silver ions at wound interface not well defined; may cause silver staining of tissues; may delay epithelialisation; relatively expensive; few published comparative trials | $$ | Should not substitute for non‐medicated dressings for uninfected wounds; may be useful for subclinically infected, highly colonised wounds or for wounds being prepared for skin grafting |
| Abbreviations: EUSOL, Edinburgh University Solution of Lime; MRSA, methicillin‐resistant Staphylococcus aureus; VRE, vancomycin‐resistant enterococci. aCosts are approximate in USD per day for treating 100‐square centimetre wound, as follows: $, < USD 3; $$, USD 3 to 15; and $$$, > USD 15. | ||||||
| Product and | Formulations | Bacterial spectrum | Advantages | Disadvantages | Costa | Indicationsb and comments |
| Bacitracin c | Ointment, 500 units/g; and powder combinations with neomycin, polymyxin B, and zinc | Many gram‐positive organisms, including aerobic staphylococci and streptococci, corynebacteria, anaerobic cocci, and clostridia; inactive against most gram‐negative organisms | Activity not impaired by blood, pus, necrotic tissue, or large bacterial inocula; resistance is rare but increasing among staphylococci; no cross‐resistance with other antibiotics; minimal absorption | May cause allergic reactions, contact dermatitis, and (rarely) anaphylactic reactions; may lead to overgrowth of drug‐resistant organisms, including fungi | $ | Widely used for many years; indicated for prevention of infection in minor skin wounds |
| Fusidic acid | Cream, 2%; ointment, 2%; and gel, 2% | Staphylococcus aureus, streptococci (in topical concentrations), corynebacteria, and clostridia | Penetrates intact and damaged skin as well as crust and cellular debris | Occasional hypersensitive reactions; resistance among staphylococci is emerging; must apply 3 times daily | $$ | Not available in the USA |
| Gentamicin | Cream, 0.1%; and ointment, 0.1% | Streptococci, staphylococci, Pseudomonas aeruginosa, Enterobacter aerogenes, Escherichia coli, Proteus vulgaris, and Klebsiella pneumoniae | Broad spectrum; inexpensive | Must be applied 3 to 4 times daily; may drive resistance to an agent used systemically | $ | Indicated for primary skin infections (pyodermas) and secondary skin infections, including infected excoriations, and for bacterial superinfections |
| Mafenide acetate | Solution, 5%; and cream, 85 mg/g | A sulfonamide that is bacteriostatic against many gram‐negative organisms, including P aeruginosa, and some gram‐positive organisms, but minimal activity against staphylococci and some obligate anaerobes | Remains active in the presence of pus and serum, and its activity is not affected by acidity of environment | Systemic absorption may occur; drug and metabolites may inhibit carbonic anhydrase, potentially causing metabolic acidosis; use cautiously in patients with renal impairment; pain on application; hypersensitive reactions. | $$$ | Indicated as adjunctive therapy in second‐ and third‐degree burns; may be used in rapidly progressing bacterial necrotising fasciitis; limited use in other wounds |
| Metronidazole | Cream, 0.75%; gel, 1%; lotion, 0.75% | Many clinically important anaerobic bacteria | May reduce odour associated with anaerobic infections; application only 1 to 2 times daily | Relatively expensive; systemic formulations available; could drive resistance to these | $–$$ | Indicated for inflammatory papules and pustules of rosacea |
| Mupirocin and mupirocin calcium | Ointment, 2%; for mupirocin calcium, cream, 2.15%; and nasal ointment, | Gram‐positive aerobes, including S aureus (most MRSA), Staphylococcus epidermidis, Staphylococcus saprophyticus, and streptococci (groups A, B, C, and G) but not enterococci, some gram‐negative aerobes (not P aeruginosa), corynebacteria, and obligate anaerobes | Minimal potential for allergic reactions | Rare local burning and irritation; applying ointment to large wounds in azotaemic patients can cause accumulation of polyethylene glycol; long‐term use can lead to resistance among staphylococci, which is increasing | $$ | Indicated for topical treatment of impetigo and eradication of nasal colonisation with S aureus |
| Neomycin sulfatec | Powder; cream, 0.5%; combinations with polymyxin B and pramoxine, and ointment, 0.5%; combinations with bacitracin, polymyxin B, lidocaine, and pramoxine | Good for gram‐negative organisms but not P aeruginosa; active against some gram‐positive | Low cost; applied only 1 to 3 times daily; may | Topical powder in wound irrigating solution | $ | Use of topical powder alone or in solution is not recommended; cream and ointment, in combination with other agents, are indicated for prevention of infection in minor skin injuries. |
| Nitrofurazone | Solution, 0.2%; ointment, 0.2%; and cream, 0.2% | Broad gram‐positive and gram‐negative activity, | Used mainly for burn wounds | Hypersensitive reactions; polyethylene glycols (in some formulations) may be absorbed and can cause problems in azotaemic patients | $$ | Indicated as adjunctive to prevent infections in people with second‐ and third‐degree |
| Polymyxin Bc | Cream, 5000 units/g or | Bactericidal against many gram‐negative organisms, | Inexpensive | Some hypersensitive and neurological or | $ | Only available in combination with other agents, including bacitracin and neomycin; |
| Retapamulin | Ointment, 1% | Active against staphylococci (but uncertain | May be active against some mupirocin‐resistant S aureus strains; broader activity than mupirocin | Not evaluated for use on mucosal surfaces; may cause local irritation | $$$ | Indicated for impetigo due to S aureus (methicillin‐susceptible only) or Streptococcus pyogenes |
| Silver sulphadiazine | Cream, 1% | A sulfonamide; the released silver ions are the primary active ingredient; active against many gram‐positive and gram‐negative organisms, including P aeruginosa | Applied only once or twice daily; soothing | Potential cross‐reaction with other sulphonamides; may rarely cause skin staining | $ | Indicated as adjunctive treatment to prevent |
| Sulfacetamide Na+ | Lotion, 10% | Bacteriostatic against many gram‐positive and gram‐negative pathogens | Broad spectrum; can be combined with sulphur | Systemic absorption and rarely severe side | $$$ | Indicated for secondary bacterial skin infections |
| There are no published studies supporting the use of topical erythromycin, clindamycin, aminoglycosides other than neomycin, gramicidin, or tetracyclines for treating chronically infected wounds. Abbreviations: FDA, US Food and Drug Administration; MRSA, methicillin‐resistant Staphylococcus aureus. aCosts are approximate in USD per day for treating 100‐square centimetre wound, as follows: $, < USD 3; $$, USD 3 to 15; and $$$, > USD 15. | ||||||
| Title (comparator) | Current status | Relevant outcomes listed | Database | Results (# enrolled) | Listed contact | Company and any further information received |
| Phase IIa Randomised, Placebo Controlled Trial to Investigate Antimicrobial Photodynamic Therapy in Chronic Leg Ulcers and Diabetic Foot Ulcers (placebo = “cream”) | Prematurely ended (date unclear) | Photodynamic therapy using the combined effect of 3,7‐bis(N,N‐dibutylamino) phenothiazin‐5‐ium bromide (PPA904) and light; measure reduction of bacterial content of diabetic foot ulcers | ClincialTrialsRegister.eu EudraCT number: 2005‐001363‐58 | None (not listed) | None listed. | Photopharmacia |
| Pexiganan Versus Placebo Control for the Treatment of Mild Infections of Diabetic Foot Ulcers (OneStep‐1 and 2) | Completed (August 2016) | 1°: clinical response (resolution of infection); 2°: microbiological response; safety | ClinicalTrials.gov; NCT01594762 | No results (200 for each of the 2 trials) reported on website. | Robert Deluccia, Dipexium | Dipexium Pharmaceuticals, Inc. Multicentre study; all sites outpatient centre in USA |
| Comparison of Resin Salve and Octenidine in Patients with Neuropathic Diabetic Foot Ulcers (comparator: octenidine dihydrochloride‐impregnated gauze) | Completed (May 2015) | Investigate healing rate and healing time of neuropathic diabetic foot ulcer in people suffering from infected fore‐ or mid‐foot ulceration. 2°: eradication of bacteria; wound healing and infection | ClinicalTrials.gov; NCT02169167 | No results on website (n = 35) (see addendum in “comments”) | Janne J Jokinen | Salve prepared from Norway spruce (Repolar Ltd.) |
| Clinical Outcomes for Diabetic Foot Ulcers Treated With Clostridial Collagenase (SANTYL®) Ointment or With a Comparator Product Containing Silver (investigator choice of silver) | Running until January 2017 (last updated November 2016) | Randomly assigned to apply SANTYL or a topical treatment containing silver to their to foot ulcer. 1°: mean change in ulcer area at end of treatment; 2°: target ulcer infection rate | ClinicalTrials.gov; NCT02581488 | No results (102) | Jaime E Dickerson, PhD (Smith & Nephew) | (Smith & Nephew) Information from the sponsor received end of December 2016 stated that the trial is not yet complete but last participant out will be achieved in the next week. The trial enrolled its target number of participants, with the last participant completed December 2016. The evaluability will be carried out prior to the scheduled database lock in January 2017. As intention‐to‐treat is the analysis set for primary inference, it is anticipated that all participants will be included. Final study report is timed for April 2017 (15 December 2016). Waiting for further information to assess eligibility for review |
| Randomized, Controlled Study to Investigate the Efficacy and Safety of a Topical Gentamicin‐Collagen Sponge in Combination with Systemic Antibiotic Therapy in Diabetic Patients With a Moderate or Severe Foot Ulcer Infection | Recruiting (as of September 2013) | 1°: "clinical cure" at the test of cure; 2°: clinical response; time to clinical cure; eradication of baseline pathogen | ClinicalTrials.gov; NCT01951768 | No results (estimate 144) | Ilker Uckay, MD; Hospital of the University of Geneva | Innocoll, Inc. |
| Comparison of the Efficacy of Standard Treatment Associated with Phage Therapy Versus Standard Treatment Plus Placebo for Diabetic Foot Ulcers Monoinfected by Staphylococcus aureus: a Randomized, Multi‐centre, Controlled, 2‐parallel‐group, Double‐blind, Superiority Trial | Starting January 2017 | 1°: reduction in wound surface area; 2°: safety; changes in resistance and virulence of S aureus isolates; production of anti‐phage antibodies | ClinicalTrials.gov; NCT026647401 | No results (estimate 60) | Albert Sotto, MD, PhD +33.(0)6.09.56.66.55 | Centre Hospitalier Universitaire de Nīmes; Pherecydes Pharma. Per correspondence from Prof Sotto on 8 January 2017, National Agency for the Safety of Medicines and Health Products requested “pre‐clinical phase complements”, causing a postponement of the start of the clinical trial. |
| A Phase I/IIa, Randomized Double Blind, Placebo‐Controlled, Dose Escalating Study to Evaluate the Safety and Tolerability of Topically Applied Bisphosphocin Nu‐3 on Infected Diabetic Ulcers of Subjects With Type I or II Diabetes Mellitus (placebo) | Enrolling by invitation only (last verified April 2016) | Diabetic foot ulcers; infection localised to area of ulcer and mild. 1° outcome: treatment‐related adverse events, safety 2°: microbiological activity evaluated by wound assessments, presence of pathogenic bacteria | ClinicalTrials.gov; NCT02737722 | No results (estimate 30) | Paul DiTullio, MSc | Lakewood‐Amedex, Inc. |
| A Phase II, Randomized, Parallel, Double‐blind, Placebo‐controlled Study to Assess Prevention of Infection Using a Topical Gentamicin‐Collagen Sponge in Diabetic Patients With Uninfected Lower Extremity Skin Ulcers (placebo sponge) | Terminated (last verified March 2012) | 1° outcome: uninfected diabetic foot ulcers that remain free of signs/symptoms of infection to end of study 2°: days to wound closure; time to any signs/symptoms of infection; decrease in wound area; pathogen burden in infected wounds | ClinicalTrials.gov; NCT00658957 | No results (49) | David Prior, PhD; Chesapeake Foot and Ankle Center, Pasadena (MD), USA | Innocoll Pharmaceuticals |
| A Phase 3 Randomized, Placebo‐Controlled, Blinded Study to Investigate the Safety and Efficacy of a Topical Gentamicin‐Collagen Sponge in Combination With Systemic Antibiotic Therapy in Diabetic Patients With an Infected Foot Ulcer (COACT 1 and 2) (placebo is no sponge) | Last updated June 2016 | Sponge is adjunctive treatment to systemic antibiotic therapy. 1° outcome: per cent of participants with a clinical outcome of clinical cure (resolution of all clinical signs and symptoms of infection) ˜10 days after end of treatment; 2° outcomes: baseline pathogen eradication; re‐infection; time to clinical cure; amputation; ulcer closure | ClinicalTrials.gov: NCT02447172 | No results posted. | Nigel Jones, VP, Global Clinical Operations, Innocoll Pharmaceuticals | Innocoll Pharmaceuticals |
| Study of the Efficacy of Topical Application of Royal Jelly and Panthenol (PedyPhar® Ointment) on the Diabetic Foot Ulcers, an Open Label, Randomized, Non‐placebo‐controlled Study (active comparator panthenol ointment) | Terminated; (last updated February 2015) | Diabetic foot ulcers at any stage after proper surgical treatment (if needed) 1° outcome: healing of ulcer; 2°: reduction of infection in ulcer site; local reaction possibly related to study drug | ClinicalTrials.gov; NCT01531517 | No results (estimate 120; 47 enrolled) | (?) | European Egyptian Pharmaceutical Industries |
| Platelet Rich Fibrin in Combination With Topical Antibiotics or Antiseptics in the Treatment of Chronic Wounds ‐ a Prospective, Randomized, Active Controlled, Double Blind Pilot Trial With an Observer‐blinded Control Group (3 platelet rich fibrin arms & 1 active comparator (Acticoat)) | Recruiting (last verified January 2016) | People with infected chronic wounds (unclear if diabetic foot) 1° outcome: reduction of wound area; 2°: number requiring systemic antimicrobial therapy; C‐reactive protein level; wound volume; occurrence of drug‐resistant bacteria | ClinicalTrials.gov; NCT02652169 | No results (estimate 120) | Florian Thalhammer, Medical University of Vienna; 0043140400 ext 44400; [email protected] | Medical University of Vienna |
| Double Blind, Randomized, Placebo Controlled Clinical Trial for the Treatment of Diabetic Foot Ulcers, Using a Nitric Oxide Releasing Patch: PATHON | Completed (last verified November 2012) | 1° outcome: per cent reduction in ulcer size; 2°: complete cure of any infection; development of infection during treatment; adverse events | ClinicalTrials.gov; NCT00428727 | No results (?) | Fundación Cardiovascular de Colombia | (?) |
| A Phase I/II, Open Label, Controlled Study to Evaluate the Safety and Efficacy of AppliGel‐G (Gentamicin Sulfate Topical Gel) for Treatment of Mild to Moderately Infected Diabetic Foot Ulcers in Patients With Type 1 and Type 2 Diabetes (comparator oral ciprofloxacin and doxycycline alone) | Terminated (last verified May 2015) | For mild to moderately infected diabetic foot ulcers 1°: complete wound clearing of infection 2°: incidence infection cleared; wound volume and area change | ClinicalTrials.gov; NCT02036528 | No results | Royer Biomedical, Inc. | Royer Biomedical, Inc. |
| A Randomised, Double‐blind, Dose‐response, Placebo‐controlled, Multicenter, Phase IIA Clinical Study to Evaluate the Efficacy and Safety of Topical Application of G.68.y/EtOH in Patients with Type 1 or Type 2 Diabetes With Infected Foot Ulcers (placebo topical gel) | Completed | Enrolling patients with infected “grade 2 PEDIS” diabetic foot ulcers 1°: reduction of bacterial load 2°: maintenance of efficacy; tolerability and safety | EudraCT number: 2010‐019598‐13 | No results (plan for 60) | Molteni | |
| Trial to Assess Safety and Efficacy of Topical MBN‐101 (BisEDT ) in Patients With Moderate/ Severe Diabetic Foot Infections (placebo – vehicle‐controlled) | Not yet open for participant recruitment (last update March 2016) | Part I, participants will be enrolled into 1 of 3 escalating dose cohorts at a ratio of 3:1 (active to placebo). In Part II, participants will be randomised in a 1:1 ratio (active to placebo) based on the optimal dose demonstrated in Part I. People with infected foot ulcer | ClinicalTrials.gov; NCT02723539 | No results (plan for 88) | Department of Vascular Surgery, Rigshospitalet Copenhagen, Denmark, 2100 | Microbion Corporation |
| Abbreviations: PEDIS, perfusion, extent/size, depth/tissue loss, infection, and sensation | ||||||
| Intervention 1 | Intervention 2 | Foot ulcer grade | Infection status at baseline | Follow‐up | Review‐relevant outcomes with reportable data | |
| Group 1: (n = 30) Pyodine bath and saline and vaseline gauze dressing | Group 2: (n = 30) Phenytoin powder | Grade I or II | Not reported | 8 weeks | None reported | |
| Group 1: (n = 19) Gentamicin solution | Group 2: (n = 22) Cadexomer iodine ointment | Grade I or II | Not reported | 12 weeks |
| |
| Group 1: (n = 19) Standard care | Group 2: (n = 21) Chloramine plus standard care | Not reported | Infected | 24 weeks |
| |
| Group 1: (n = 10) Saline solution | Group 2: (n = 10) Super‐oxidised aqueous solution | Grade I or II | Not infected | 4 weeks |
| |
| Group 1: (n = 15) Foam dressing | Group 2: (n = 24) Silver collagen/oxidised regenerated cellulose dressing | Grade II or III | Not infected | 14 weeks |
| |
| Group 1: (n = 40) Routine debridement plus standard care (including blood glucose control, nutritional support, improve microcirculation | Group 2: (n = 40) Silver ion dressing plus standard care | Not reported | Not reported | 4 weeks |
| |
| Group 1: (n = not reported) Iodine gauze | Group 2: (n = not reported) Hydrofiber dressing with silver | Ulcers with bone and tendon exposure | Not reported | Not reported | Not reported | |
| Group 1: (n = 180) Saline dressing | Group 2: (n = 195) Honey dressing | Grade I or II | Not reported | 17 weeks |
| |
| Group 1: (n = 20) Silver sulphadiazine cream | Group 2: (n = 20) Formulation of benzoic acid, 6%; salicylic acid, 3%; and extract of oak bark (Quercus rubra), 3% (Bensal HP with QRB7), with silver sulphadiazine cream | Grade I or II | Not reported | 6 weeks |
| |
| Group 1: (n = 108) Non‐adherent dressing, viscose filament gauze Group 2: (n = 103) Hydrocolloid (Hydrofiber) dressing | Group 3: (n = 106) Iodine‐containing dressing | Not reported | Not reported | 24 weeks |
| |
| Group 1: (n = 67) Calcium‐alginate dressing | Group 2: (n = 67) Fibrous‐hydrocolloid (Hydrofiber) dressing with 1.2% ionic silver | Grade I or II | Mixed infected and not infected | 8 weeks |
| |
| Group 1: (n = 20) Hyperbaric oxygen therapy (not considered further) Group 2: (n = 20) Recombinant human platelet‐derived growth factor | Group 3: (n = 20) Antiseptic treatments (EUSOL, hydrogen peroxide, and povidone iodine) | Grade III or IV | Not reported | More than 8 weeks |
| |
| Group 1: (n = 21) Topical saline solution plus 750 mg levofloxacin once per day Group 2: (n = 21) Super‐oxidised aqueous solution (topical Microcyn) alone (not considered) | Group 3: (n = 21) super‐oxidised aqueous solution (topical Microcyn) therapy plus 750 mg levofloxacin once per day | Eligible foot ulcers involved skin and deeper soft tissue | Infected | 4 weeks |
| |
| Group 1: (n = 246) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream | Group 2: (n = 247) Topical pexiganan cream (1% or 2%) and placebo oral tablets | Not reported | Infected | Up to 42 days |
| |
| Group 1: (n = 171) Ofloxacin (200 mg) oral tablets and a topical placebo (vehicle) cream | Group 2: (n = 171) Topical pexiganan cream (1%) and placebo oral tablets | Full‐thickness wounds | Infected | Up to 42 days |
| |
| Group 1: (n = 38) Systemic antibiotic therapy alone | Group 2: (n = 18) Daily topical application of the gentamicin‐collagen sponge combined with systemic antibiotic therapy | Not reported | Infected | Up to 42 days |
| |
| Group 1: (n = 16) Povidone iodine and saline | Group 2: (n = 21) Neutral pH super‐oxidised aqueous solution | Not reported | Infected | 20 weeks |
| |
| Group 1: (n = 25) Conventional treatment (no further details translated) | Group 2: (n = 25) Zinc hyaluronate | Not reported | Unclear | 20 weeks |
| |
| (30 participants randomised, but number in each group not specified) | Group 1: Standard‐dressing group (povidone iodine solution 10%) (n not reported) | Group 2: Honey dressing group (n not reported) | Grade II | Not reported | Not reported | No useable data |
| Group 1: Normal saline solution, 11 ulcers (in 10 participants) | Group 2: Tretinoin group, 13 ulcers (in 12 participants) | Not reported | Not reported | 16 weeks |
| |
| Group 1: (n = 2) Povidone iodine and metronidazole 1% gel dressing | Group 2: (n = 2) Honey and metronidazole 1% gel dressing | Grade I and II | Not reported | Not reported |
| |
| Group 1: (n = 19) Polyherbal formulation | Group 2: (n = 19) silver sulphadiazine cream | Grade I, II, and III | Unclear | 20 weeks | No useable data | |
| Abbreviations: EUSOL, Edinburgh University Solution of Lime | ||||||
| Resolution of infection | Incidence of wound infection | Time to healing | Proportion of wounds healed | Microbial counts | Health‐related quality of life | Need for surgical resection, including partial or complete lower limb amputation | Safety (adverse events) | |
| Group 1: (n = 30) Povidone iodine bath and saline Vaseline gauze dressing Group 2: (n = 30) Phenytoin powder plus povidone iodine bath and saline Vaseline gauze dressing Not infected at baseline | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 19) Gentamicin solution Group 2: (n = 22) Cadexomer iodine ointment Baseline infection status not reported. | Not reported | Not reported | Not reported | Group 1: 2/18 Group 2: 5/17 | Not reported | Not reported | Surgical resection was reported: Group 1: 5/19 Group 2: 3/22 | Study reports that no adverse reactions related to the topical treatment were documented. |
| Group 1: (n = 19) Standard care alone Group 2: (n = 21) Chloramine plus standard care Infected at baseline | Group 1: 7/15 Group 2: 9/13 | Not reported | Time‐to‐event data presented with no reported hazard ratio. Given the small number of participants and events, no further attempts were made to calculate time‐to‐event values. | Healed at 24 weeks Group 1: 9/17 Group 2: 10/17 | Not reported | Not reported | Vascular procedure or amputation Group 1: 3/17 Group 2: 5/17 | Adverse event data reported but unable to get a per‐participant value, as it is noted that some participants had more than 1 event. |
| Group 1: (n = 10) Saline solution Group 2: (n = 10) Super‐oxidised aqueous solution Not infected at baseline | Not reported | Not reported | Not reported | Study notes that 15% of the study ulcers were healed, but this information not reported by group. | The bacterial load in the wound bed was defined as scattered (0/+), light (+), medium (++), or heavy (+++). At week 4 there was a reduction of 33% in the bacterial load versus baseline. Figure presented but difficult to interpret data by group. | Not reported | Not reported | No safety concerns were reported in either the super‐oxidised aqueous solution group or the saline group; no adverse reactions were recorded. |
| Group 1: (n = 15) Foam dressing Group 2: (n = 24) Silver collagen/oxidised regenerated cellulose dressing Not infected at baseline | Not reported | Wound infection Group 1: 4/13 Group 2: 0/23 | Not reported | Healed by week 14 Group 1: 4/13 Group 2: 12/23 | Not reported | Not reported | Not reported | Limited details of adverse events (in addition to infection data already recorded). There were no reported adverse events related to the use of collagen/oxidised regenerated cellulose/silver dressing, and 5 cases of adverse events (no further details) related to foam dressing. |
| Group 1: (n = 40) Routine debridement plus standard care Group 2: (n = 40) Silver ion dressing plus standard care Baseline infection status not reported. | Not reported | Not reported | Mean wound healing time in days: Group 1: 47.4 ± 11.5 Group 2: 31.3 ± 8.2 Mean granulation tissue occurrence time in days: Group 1: 10.8 ± 1.9 Group 2: 6.4 ± 0.72 | Group 1: 15/40 Group 2: 24/40 | Not reported | Not reported | Not reported | Not reported |
| Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | |
| Group 1: (n = 180) Treated with normal saline dressing Group 2: (n = 195) Treated with honey dressing | Not reported | Not reported | Median healing time in honey group is 18 days (IQR is 6 to 120), and in the saline group is 29 days (IQR 7 to 120). Data do not seem to have been calculated using correct time‐to‐event approaches and were not considered further. | Group 1: 97/169 Group 2: 136/179 | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 20) Silver sulphadiazine Group 2: (n = 20) Formulation of benzoic acid, 6%; salicylic acid, 3%; and extract of oak bark (Quercus rubra), 3% (Bensal HP with QRB7), with silver sulphadiazine cream Baseline infection status not reported. | Not reported | Not reported | Not reported | Healed by week 6 Group 1: 6/20 Group 2: 8/20 | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 108) Non‐adherent dressing, viscose filament gauze (Johnson & Johnson) Group 2: (n = 103) Hydrocolloid (Hydrofiber) dressing (Aquacel, ConvaTec) Group 3: (n = 106) Iodine‐containing dressing (Inadine, Systagenix) Baseline infection status not reported. | Not reported | Number of infected ulcers at 24 weeks: not reported by group Study reports the number of episodes of infection listed as serious adverse events, but it is unclear if foot infections, and not clear how many people had how many infection events. | Mean time to healing in days (SD) (fixed at max of 168 days) Group 2: 125.8 (55.9) Group 3: 127.8 (54.2) Not all ulcers healed, so mean is inappropriate measure of time to healing. | Number of ulcers healed at 24 weeks: Group 2: 46/103 Group 3: 48/106 | Not reported | Mean Cardiff Wound Impact Schedule score at 24 weeks (SD) Group 1: Physical functioning: 68.9 (19.1). Social functioning: 69.8 (23.5). Well‐being: 50.2 (21.1) Group 2: Physical functioning: 71.4 (19.5). Social functioning: 70.3 (25.4). Well‐being: 53.1 (19.9) Other | Minor amputations (below ankle): Group 2: 1/103 n not clear; assumed to be all participants | Non‐serious adverse events Group 2: 227/103 Group 3: 239/106 Serious adverse events Group 2: 28/103 Group 3: 37/106 Not clear how many participants had how many events, but seems to be more than 1 per person; data not analysed further |
| Group 1: (n = 67) Calcium‐alginate dressing Group 2: (n = 67) Fibrous‐hydrocolloid (Hydrofiber) dressing with 1.2% ionic silver Mixed wound infection status at baseline | Not reported | Group 1: 11/67 Group 2: 8/67 | Time to 100% healing also reported, but this is only for a subset of those that healed, so not a useful pan‐study measure. Not reported Mean time to healing in days Group 1: 52.6 ± 1.8 Group 2: 57.7 ± 1.7 | Number of ulcers healed in 8 weeks | Not reported | Not reported | Not reported | Group 1: 26/67 participants experienced adverse event. Death = 1; Infection = 8. 13 participants discontinued treatment due to adverse event. Group 2: 25/67 participants experienced 1 or more events. Death = 1; Infection = 14. 8 participants discontinued treatment due to adverse event. |
| Group 1: (n = 20) Hyperbaric oxygen therapy (not considered in review) Group 2: (n = 20) Recombinant human platelet‐derived Group 3: (n = 20) Antiseptic dressings | Not reported | Not reported | Mean time to healing in weeks (standard error) Group 1: 6.83 (2.5) Group 2: 7.6 (2.5) Group 3: 6.75 (2.7) Not all ulcers healed, so mean is inappropriate measure of time to healing. | Number of ulcers healed Group 1: 12/20 Group 2: 16/20 Group 3: 8/20 Review authors calculated figures from graph. | Not reported | Not reported | Not reported | Not recorded |
| Group 1: (n = 21) Levofloxacin plus saline Group 2: (n = 21) Super‐oxidised aqueous solution alone (not considered) Group 3: (n = 25) Levofloxacin plus super‐oxidised aqueous solution Ulcers infected at baseline. | Group 1: 6/21 Group 2: 11/21 Group 3: 11/25 | Not reported | Not reported | Mentioned, but data not presented. | Not reported | Not reported | Not reported | Adverse events (number of participants with 1 or more event) Group 1: 7/21 Group 2: 7/21 Group 3: 9/25 |
| Group 1: (n = 246) Ofloxacin Group 2: (n = 247) Pexiganan Ulcers infected at baseline. | Not reported Resolution ("cure") and improvement data presented together, so unclear how many participants had resolution. | Not reported | Not reported | Not reported | Not reported | Not reported | See below ‐ results presented by study authors cumulatively for these 2 studies only. | Adverse events (number of participants with > 1 adverse event) Group 1: 109/246 Group 2: 98/247 |
| Group 1: (n = 171) Ofloxacin Group 2: (n = 171) Pexiganan Ulcers infected at baseline. | Not reported Resolution ("cure") and improvement data presented together, so unclear how many participants had resolution. | Not reported | Not reported | Not reported | Not reported | Not reported | Group 1: 9/417 Group 2: 11/418 (cumulative of two RCTs reported in single paper) | Adverse events (number of participants with > 1 adverse event) Group 1: 84/171 Group 2: 76/171 |
| Group 1: (n = 18) Systemic antibiotic therapy alone Group 2: (n = 38) Topical application of the gentamicin‐collagen sponge + systemic antibiotic therapy Ulcers infected at baseline. | Resolution of infection by 7 days Group 1: 7/18 Group 2: 22/38 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Adverse events (number of participants with 1 or more events) Group 1: 5/18 Group 2: 11/38 |
| Group 1: (n = 16) Standard management with chemical antiseptics such as soap or povidone iodine Group 2: (n = 21) Super‐oxidised aqueous solution | Advances from infection to granulating tissue: Group 1: 10/16 Group 2: 19/21 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 25) Conventional treatment (no further details translated) Group 2: (n = 25) Zinc hyaluronate | Not reported/translated | Not reported/translated | Mean time to healing in weeks (not clear if standard deviation or standard error presented) Group 1: Only 2 ulcers healed; no time‐to‐event data reported Group 2: 7.80 (3.49) with all ulcers healing | Group 1: 2/25 Group 2: 25/25 | Not reported/translated | Not reported/translated | Not reported/translated | Not reported/translated |
| Group 1: Standard‐dressing group (povidone iodine solution 10%) Group 2: Honey dressing group 30 participants randomised, but number in each group not specified. | Not reported | Not reported | Time to healing in days Group 1: 15.4 days (range 9 to 36 days) Group 2: 14.4 days (range 7 to 26 days) Comment: mean and range, but no measure of variation provided. Unclear how many participants in each group and how many ulcers healed, thus if this measure is a valid time‐to‐healing measure | Not reported | Not reported | Not reported | Not reported | Not reported |
| Group 1: Normal saline solution, 11 ulcers (in 10 participants) Group 2: Tretinoin group, 13 ulcers (in 12 participants) | Not reported | Not reported | Data presented as time‐to‐event figure with no further data. Given the small number of participants and events, we have not tried to analyse further. | 16 weeks Group 1: 2/10 Group 2: 6/12 Unclear if ulcers were healed in the same or different participants; for the analysis we have assumed in different participants | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 2) Povidone iodine and metronidazole 1% gel dressing Group 2: (n = 2) Honey and metronidazole 1% gel dressing | Not reported | Not reported | Not reported | Group 1: 0/2 Group 2: 2/2 | Not reported | Not reported | Not reported | Not reported |
| Group 1: (n = 19) Polyherbal formulation Group 2: (n = 19) Silver sulphadiazine cream | Not reported | Not reported | "Number of days taken for healing of the wound: Group 1: 43.1 ± 26.8 Group 2: 43.6 ± 30.7" Not clear what sort of analysis was conducted | Healing was defined as complete epithelialisation either by secondary intention or by split skin graft. However, figures are not reported. | "the microbiological investigations were not done" | Not reported | Not reported | "There were no adverse events reported in both the groups." |
| Abbreviations: IQR, interquartile range; SD, standard deviation | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 5 | 945 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.12, 1.45] |
| 1.1 Short term follow up | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.6 [1.00, 2.57] |
| 1.2 Medium term follow‐up | 4 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.10, 1.44] |
| 2 Incidence of infection: medium term follow‐up Show forest plot | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.10] |
| 3 Surgical resection: medium term follow‐up Show forest plot | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.72] |
| 4 Adverse events Show forest plot | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed: medium term follow‐up Show forest plot | 3 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.56, 14.23] |
| 2 Resolution of infection: medium term follow‐up Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.54, 2.51] |
| 3 Surgical resection: medium term follow‐up Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.47, 5.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Medium term follow‐up | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Unknown follow‐up period | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Resolution of infection: medium term follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Surgical resection: medium term follow‐up Show forest plot | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.53, 7.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of infection Show forest plot | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.91, 2.49] |
| 1.1 Short‐term follow‐up | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.69, 3.45] |
| 1.2 Medium term follow‐up | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.79, 2.82] |
| 2 Surgical resection: medium term follow‐up Show forest plot | 1 | 835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.51, 2.91] |
| 3 Adverse events Show forest plot | 4 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.05] |
| 3.1 Short‐term follow‐up | 3 | 891 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.05] |
| 3.2 Medium term follow‐up | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.49, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed: Medium term follow‐up Show forest plot | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.28, 0.89] |

