Tratamiento de la fatiga en la esclerosis lateral amiotrófica / enfermedad de la motoneurona
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011005.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 02 enero 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neuromuscular
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Professor Carolyn Young conceived of the review.
All authors assisted in designing the review and the search strategies detailed in this review.
All authors assisted in drafting and providing critical appraisal of this review.
Sources of support
Internal sources
-
National Institute for Health Care and Research Greater Manchester Collaboration for Leadership in Applied Health Research and Care (NIHR CLAHRC‐GM), UK.
Chris Gibbons is supported by the NIHR‐CLAHRC‐GM.
External sources
-
No sources of support supplied
Declarations of interest
CG: no conflicts of interest.
FP: no conflicts of interest.
TF has received personal fees for consultancies (including data monitoring committees) from AstraZeneca, Bayer, Boehringer Ingelheim, CTCT, DaiichiSankyo, Feldmann Patent Attourneys, Galapagos, Grünenthal, Janssen, Mediconomics, Novartis, Parexel, Penumbra, Pharmalog, Roche, SGS, and UCB, but not for the indication concerned (fatigue in ALS/MND).
CY has published on fatigue in various neurological conditions, including MND, and advised a pharmaceutical company about a potential trial for fatigue in multiple sclerosis.
Acknowledgements
The Methods section includes sections of standard text provided by Cochrane Neuromuscular. Editorial assistance was provided by Ruth Brassington.
The search strategy was developed by the Cochrane Neuromuscular Information Specialist, Angela Gunn, in collaboration with the review authors.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health. Cochrane Neuromuscular is also supported by the Motor Neurone Disease Assocation and the MRC Centre for Neuromuscular Diseases.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Jan 02 | Treatment of fatigue in amyotrophic lateral sclerosis/motor neuron disease | Review | Chris Gibbons, Francesco Pagnini, Tim Friede, Carolyn A Young | |
| 2014 Mar 02 | Treatment for fatigue in amyotrophic lateral sclerosis/motor neuron disease (ALS/MND) | Protocol | Carolyn A Young, Chris Gibbons, Francesco Pagnini, Tim Friede | |
Differences between protocol and review
We included an explanation of the process of downgrading the evidence in 'Summary of findings' tables.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
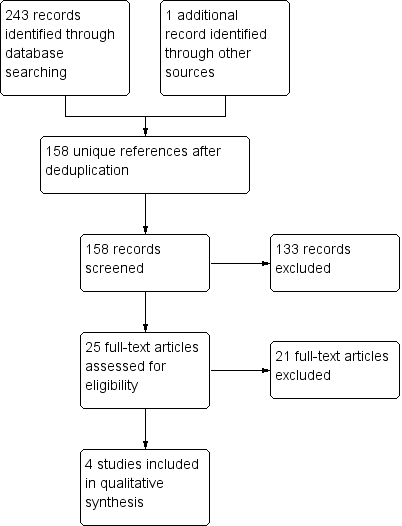
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study.

Comparison 1 Modafinil versus placebo, Outcome 1 Efficacy outcomes (all at 4 weeks).
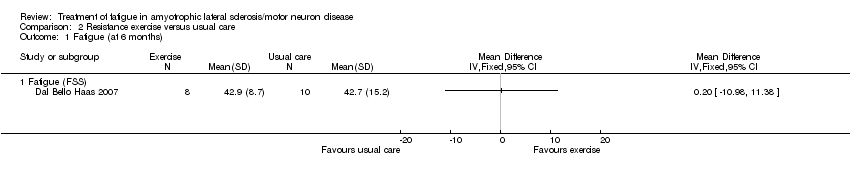
Comparison 2 Resistance exercise versus usual care, Outcome 1 Fatigue (at 6 months).
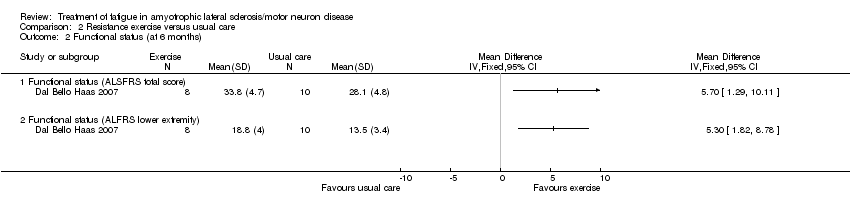
Comparison 2 Resistance exercise versus usual care, Outcome 2 Functional status (at 6 months).

Comparison 2 Resistance exercise versus usual care, Outcome 3 Quality of life (at 6 months).
| Study | Respiratory exercise N | Sham intervention N | MD, 95% CI |
| Fatigue (FSS) | |||
| Pinto 2012 | 12 | 12 | ‐9.654, 95% CI ‐22.037 to 2.729 |
| Sleepiness | |||
| Pinto 2012 | 12 | 12 | 0.308, 95% CI ‐3.48 to 4.096 |
| Depression | |||
| Pinto 2012 | 12 | 12 | 1.769, 95% CI 0.018 to 3.52 |
| Quality of life | |||
| Pinto 2012 | 12 | 12 | 0.769, 95% CI ‐17.093 to 18.631 |
| Functional status | |||
| Pinto 2012 | 12 | 12 | 0.846, 95% CI ‐2.157 to 3.849 |
| Functional status (ALSFRS‐bulbar) | |||
| Pinto 2012 | 12 | 12 | ‐0.385, 95% CI ‐1.378, to 0.609 |
| Functional status (ALSFRS‐respiratory) | |||
| Pinto 2012 | 12 | 12 | 0.077, 95% CI ‐0.254 to 0.407 |
Comparison 3 Respiratory exercise versus sham intervention, Outcome 1 Efficacy outcomes (all at 4 months).
| Study | Number of participants | Analysis of variance (time x treatment arm |
| Fatigue (FSS) | ||
| Zanette 2008 | 10 (5 rTMS, 5 sham intervention) | F[2,16] = 4.0; P = 0.04 |
| Functional status | ||
| Zanette 2008 | 10 (5 rTMS, 5 sham intervention) | F[2,16] = 2.7; P > 0.05 |
Comparison 4 Repetitive transcranial magnetic stimulation (rTMS) versus sham intervention, Outcome 1 Efficacy outcomes (all at 2 weeks).
| Modafinil compared to placebo in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with modafinil | |||||
| Fatigue assessed with: Fatigue Severity Scale (FSS) | The mean FSS score was 43 | MD 11 lower | ‐ | 32 | ⊕⊝⊝⊝ | |
| Adverse events | Three adverse events led to discontinuation of modafinil (2 headache, 1 chest tightness). Anxiety (in 2 people), nausea (in 2), dizziness (in 1), and sialorrhoea (in 1; probably ALS‐related) also occurred with modafinil. Placebo group adverse events were not reported ‐ it is not clear whether there were none. | ‐ | 32 | ⊕⊝⊝⊝ | The trial reported the number of adverse events in the treatment group, but not numbers of events in the placebo group or number of people experiencing adverse events | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the quality of evidence 3 times: once for study limitations and twice for imprecision. The security of blinding in the trial was unclear and the report did not provide enough information to assess attrition and selective reporting. The trial was small and the CI of the effect estimate included appreciable benefit and little or no effect. | ||||||
| Exercise compared to usual care in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with exercise | |||||
| Fatigue Fatigue Severity Scale (FSS) | The mean FSS score was 42.7 | MD 0.2 higher | ‐ | 18 | ⊕⊝⊝⊝ | ‐ |
| Adverse events | No adverse events were reported | ‐ | 18 | ⊕⊝⊝⊝ | None of the people who discontinued did so because they thought the exercise programme was making their condition worse. No participants reported excessive soreness, cramping, or fatigue. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence 3 times to low quality: twice for imprecision as the trial was very small and CI included appreciable benefit and appreciable harm. The third downgrading was for study limitations as the nature of the intervention prevented participant blinding. | ||||||
| Inspiratory muscle training compared to sham intervention in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk or value with sham intervention | Risk or value with inspiratory muscle training | |||||
| Fatigue Fatigue Severity Scale (FSS) follow up: 4 months | An illustrative mean FSS score in the absence of inspiratory muscle training is 42.71 | MD 9.654 lower | ‐ | 24 | ⊕⊝⊝⊝ | |
| Adverse events | The trialists stated that the exercise protocol had no adverse effects. | ‐ | 24 | ⊕⊝⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The mean FSS score in the control group after 4 months was not available from Pinto 2012. The value given here, for illustrative purposes, is the control group mean at 6 months from Dal Bello Haas 2007. 2We downgraded the quality of evidence 3 times to very low: twice for imprecision and once for study limitations. The trial was very small and CI included appreciable benefit and little or no effect. The nature of the intervention meant that the trainer was aware of the intervention group. | ||||||
| Repetitive transcranial magnetic stimulation (rTMS) compared to sham intervention in ALS/MND | |||
| Patient or population: people with ALS/MND | |||
| Outcomes | Impact | Number of participants | Quality of the evidence |
| Fatigue assessed with: Fatigue Severity Scale (FSS) follow‐up: 2 weeks | No FSS scores were given. The investigators assessed fatigue with the FSS using 2‐way analysis of variance (within‐subjects factor time, between‐subjects treatment arm). The trial reported a significant effect for fatigue at the end of the follow‐up period. The effect was non‐significant following post hoc Bonferroni adjustments (data not reported). | 10 | ⊕⊝⊝⊝ |
| Adverse events | No adverse events were reported. | 10 | ⊕⊝⊝⊝ |
| RCT: randomised controlled trial | |||
| GRADE Working Group grades of evidence | |||
| 1We downgraded the quality of evidence 3 times: once for study limitations and twice for imprecision. The risk of bias was unclear as the trial report provided too little detail for assessment. The trial involved 10 people. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Efficacy outcomes (all at 4 weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Fatigue (FSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sleepiness | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Depression | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Fatigue (FSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional status (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Functional status (ALSFRS total score) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Functional status (ALFRS lower extremity) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mental health (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Physical function (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Physical role (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Pain (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 General health (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Vitality (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Social function (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Emotional role (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Efficacy outcomes (all at 4 months) Show forest plot | Other data | No numeric data | ||
| 1.1 Fatigue (FSS) | Other data | No numeric data | ||
| 1.2 Sleepiness | Other data | No numeric data | ||
| 1.3 Depression | Other data | No numeric data | ||
| 1.4 Quality of life | Other data | No numeric data | ||
| 1.5 Functional status | Other data | No numeric data | ||
| 1.6 Functional status (ALSFRS‐bulbar) | Other data | No numeric data | ||
| 1.7 Functional status (ALSFRS‐respiratory) | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Efficacy outcomes (all at 2 weeks) Show forest plot | Other data | No numeric data | ||
| 1.1 Fatigue (FSS) | Other data | No numeric data | ||
| 1.2 Functional status | Other data | No numeric data | ||

