Rawatan keletihan dalam penyakit sklerosis lateral amiotrofik/motor neuron
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register and Cochrane Central Register of Controlled Trials (CENTRAL via CRS‐W) search strategy
#1 MeSH DESCRIPTOR Motor Neuron Disease Explode All AND CENTRAL:TARGET
#2 "motor neuron disease*" or "motor neurone disease*" AND CENTRAL:TARGET
#3 "motoneuron disease*" or "motoneurone disease*" AND CENTRAL:TARGET
#4 "motorneuron disease*" or "motorneurone disease*" AND CENTRAL:TARGET
#5 "charcot disease" AND CENTRAL:TARGET
#6 "amyotrophic lateral sclerosis" AND CENTRAL:TARGET
#7 als:ti or als:ab or nmd:ti or mnd:ab AND CENTRAL:TARGET
#8 #1 or #2 or #5 or #6 or #7 AND CENTRAL:TARGET
#9 fatigue or tired* or weariness or weary or exhaust* or lacklustre AND CENTRAL:TARGET
#10 astheni* or lethargic or languidness or languor or lassitude or listlessness AND CENTRAL:TARGET
#11 (lack or loss or lost) near2 (energy or vigour or vigour) AND CENTRAL:TARGET
#12 #9 or #10 or #11 AND CENTRAL:TARGET
#13 #8 and #12 AND CENTRAL:TARGET
#14 MeSH DESCRIPTOR Exercise Therapy Explode All AND CENTRAL:TARGET
#15 MeSH DESCRIPTOR Physical Therapy Modalities Explode All AND CENTRAL:TARGET
#16 rehabilitation or exercise or train or activity or physical or strength or sports AND CENTRAL:TARGET
#17 isometric or isotonic or isokinetic or endurance or kinesiotherap* AND CENTRAL:TARGET
#18 "relaxation therapy" or "behavior therapy" or "behaviour therapy" or "orthotic devices" AND CENTRAL:TARGET
#19 exercise near2 (grading or pacing) AND CENTRAL:TARGET
#20 MeSH DESCRIPTOR Drug Therapy Explode All AND CENTRAL:TARGET
#21 "cognitive therapy" AND CENTRAL:TARGET
#22 #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 AND CENTRAL:TARGET
#23 #13 and #22 AND CENTRAL:TARGET
#24 #13 and #22 AND CENTRAL:TARGET
#25 (#13 and #22) AND (INREGISTER)
#26 181:zsen AND INREGISTER AND ISINCENTRAL
#27 181:zsen AND INSEGMENT AND ISINCENTRAL
#28 #26 OR #27
#29 #24 not #28
#3 "motoneuron disease*" or "motoneurone disease*" AND CENTRAL:TARGET
#30 #25 not #26
#31 #29 OR #30
Appendix 2. MEDLINE Ovid SP search strategy
Database: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Ovid MEDLINE(R) 1946 to August Week 4 2017
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 randomized controlled trial.pt. (476988)
2 controlled clinical trial.pt. (96146)
3 randomized.ab. (417587)
4 placebo.ab. (194961)
5 drug therapy.fs. (2045786)
6 randomly.ab. (289174)
7 trial.ab. (438850)
8 groups.ab. (1782381)
9 or/1‐8 (4222639)
10 exp animals/ not humans.sh. (4531946)
11 9 not 10 (3650505)
12 exp Motor Neuron Disease/ (24501)
13 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$1).mp. (8212)
14 ((Lou Gehrig$1 adj5 syndrome$1) or (Lou Gehrig$1 adj5 disease)).mp. (179)
15 charcot disease.tw. (21)
16 amyotrophic lateral sclerosis.tw. (19305)
17 or/12‐16 (32403)
18 Fatigue/ or fatigue.mp. (92934)
19 (tired$ or weariness or weary or exhaust$ or lacklustre or astheni$ or lethargic or languidness or languor or lassitude or listlessness).mp. (60396)
20 ((lack or loss or lost) adj2 (energy or vigour or vigour)).mp. (6665)
21 18 or 19 or 20 (154071)
22 17 and 21 (335)
23 Fatigue/dh, dt, th [Diet Therapy, Drug Therapy, Therapy] (2687)
24 exp Exercise Therapy/ (42299)
25 exp Physical Therapy Modalities/ (135665)
26 rehabilitation.mp. or Rehabilitation/ (161213)
27 (exercise or train or activity or physical or exercise or strength or sports or isometric or isotonic or isokinetic or endurance or kinesiotherap$).mp. (3621760)
28 relaxation therapy/ (6260)
29 (exercise adj2 (grading or pacing)).mp. (191)
30 behavior therapy/ (26836)
31 orthotic devices/ (6354)
32 exp drug therapy/ (1261528)
33 cognitive therapy/ (21880)
34 or/23‐33 (4918512)
35 11 and 22 and 34 (63)
36 exp *neoplasms/ (2682771)
37 35 not 36 (62)
38 remove duplicates from 37 (56)
Appendix 3. Embase Ovid SP search strategy
Database: Embase <1980 to 2017 Week 36>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 crossover‐procedure.sh. (53004)
2 double‐blind procedure.sh. (139660)
3 single‐blind procedure.sh. (29379)
4 randomized controlled trial.sh. (467233)
5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1418321)
6 trial.ti. (228280)
7 or/1‐6 (1579561)
8 (animal/ or nonhuman/ or animal experiment/) and human/ (1664741)
9 animal/ or nonanimal/ or animal experiment/ (3783510)
10 9 not 8 (3138210)
11 7 not 10 (1452500)
12 limit 11 to (conference abstracts or embase) (1224945)
13 Motor Neuron Disease/ or Amyotrophic Lateral Sclerosis/ (36673)
14 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease).mp. (11958)
15 ((Lou Gehrig$1 adj5 syndrome$1) or (Lou Gehrig$1 adj5 disease)).mp. (195)
16 charcot disease.tw. (26)
17 amyotrophic lateral sclerosis.tw. (24487)
18 or/13‐17 (40610)
19 Fatigue/ or fatigue.mp. (208432)
20 (tired$ or weariness or weary or exhaust$ or lacklustre or astheni$ or lethargic or languidness or languor or lassitude or listlessness).mp. (103622)
21 ((lack or loss or lost) adj2 (energy or vigour or vigour)).mp. (6165)
22 19 or 20 or 21 (301269)
23 18 and 22 (881)
24 fatigue/dm, dt, th [Disease Management, Drug Therapy, Therapy] (3699)
25 exp Exercise Therapy/ (63627)
26 exp Physical Therapy Modalities/ (75181)
27 rehabilitation.mp. or Rehabilitation/ (307922)
28 (exercise or train or activity or physical or exercise or strength or sports or isometric or isotonic or isokinetic or endurance or kinesiotherap$).mp. (4764154)
29 relaxation therapy/ (9816)
30 (exercise adj2 (grading or pacing)).mp. (217)
31 behavior therapy/ (40378)
32 orthotic devices/ (5247)
33 exp drug therapy/ (2093882)
34 cognitive therapy/ (42135)
35 or/24‐34 (6755799)
36 11 and 23 and 35 (91)
37 exp *neoplasm/ (2854492)
38 36 not 37 (85)
39 remove duplicates from 38 (80)
Appendix 4. PsycINFO Ovid SP search strategy
Database: PsycINFO <1806 to August Week 4 2017>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 (random$ or rct or cct or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. (308023)
2 amyotrophic lateral sclerosis/ (3288)
3 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$1).mp. (1243)
4 ((Lou Gehrig$1 adj5 syndrome$1) or (Lou Gehrig$1 adj5 disease)).mp. (49)
5 or/2‐4 (4107)
6 (fatigue or tired$ or weariness or weary or exhaust$ or lacklustre or astheni$ or lethargic or languidness or languor or lassitude or listlessness).mp. (38405)
7 ((lack or loss or lost) adj2 (energy or vigour or vigour)).mp. (619)
8 6 or 7 (38838)
9 1 and 5 and 8 (3)
Appendix 5. CINAHL Plus EBSCO host search strategy
Tuesday, September 05, 2017 9:18:08 AM
S30 S28 AND S29 2
S29 EM 20161007‐ 248,271
S28 S27 Limiters ‐ Exclude MEDLINE records
Interface ‐ EBSCOhost Research Databases
Search Screen ‐ Advanced Search
Database ‐ CINAHL Plus 20
S27 S18 AND S23 AND S26 98
S26 S24 OR S25 40,470
S25 (lack or loss or lost) N2 (energy or vigour or vigour) 665
S24 fatigue or tired* or weariness or weary or exhaust* or lacklustre or astheni* or lethargic or languidness or languor or lassitude or listlessness 39,965
S23 S19 or S20 or S21 or S22 7,560
S22 (Lou Gehrig* W5 syndrome*) or (Lou Gehrig* w5 disease*) 42
S21 "amyotrophic lateral sclerosis" 3,397
S20 motor neuron disease or motor neurone disease or motoneuron* disease or motorneuron* disease 1,431
S19 (MH "Motor Neuron Diseases+") 6,785
S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 990,862
S17 ABAB design* 102
S16 TI random* or AB random* 223,975
S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 447,954
S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 171,024
S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) 59,093
S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 34,513
S11 PT ("clinical trial" or "systematic review") 132,151
S10 (MH "Factorial Design") 1,005
S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 315,906
S8 (MH "Meta Analysis") 28,517
S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 92
S6 (MH "Quasi‐Experimental Studies") 8,580
S5 (MH "Placebos") 10,318
S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 36,327
S3 (MH "Clinical Trials+") 220,329
S2 (MH "Crossover Design") 15,021
S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 77,258
Appendix 6. ERIC EBSCO host search strategy
Interface ‐ EBSCOhost Research Databases
Search Screen ‐ Advanced Search
Database ‐ ERIC
Tuesday, September 05, 2017 9:40:20 AM
S8 S4 AND S7 1
S7 S5 OR S6 3,217
S6 (lack or loss or lost) N2 (energy or vigour or vigour) 97
S5 fatigue or tired* or weariness or weary or exhaust* or lacklustre or astheni* or lethargic or languidness or languor or lassitude or listlessness 3,125
S4 S1 OR S2 OR S3 47
S3 (Lou Gehrig* W5 syndrome*) or (Lou Gehrig* w5 disease*) 6
S2 amyotrophic lateral sclerosis 33
S1 motor neuron disease or motor neurone disease or motoneuron* disease or motorneuron* disease
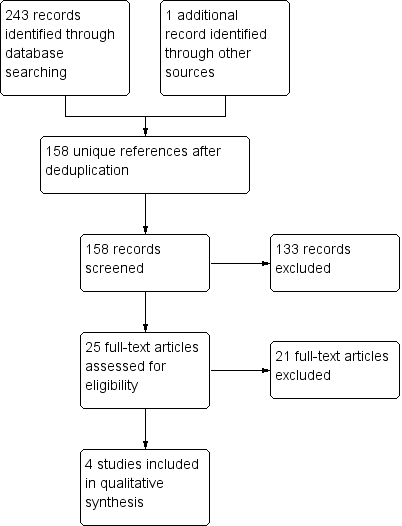
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study.

Comparison 1 Modafinil versus placebo, Outcome 1 Efficacy outcomes (all at 4 weeks).
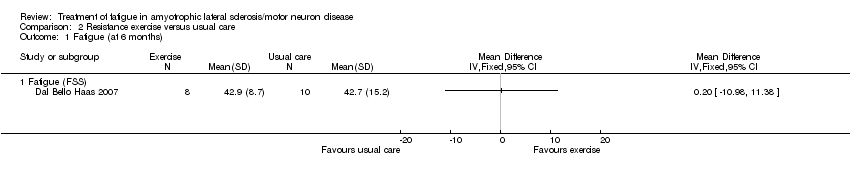
Comparison 2 Resistance exercise versus usual care, Outcome 1 Fatigue (at 6 months).
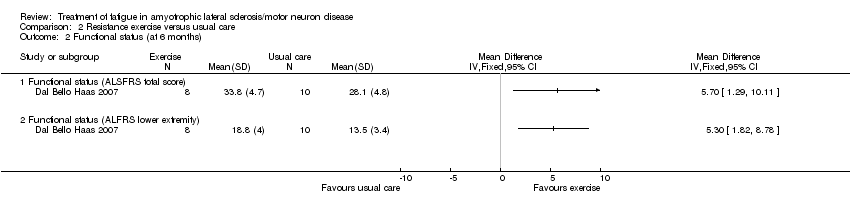
Comparison 2 Resistance exercise versus usual care, Outcome 2 Functional status (at 6 months).

Comparison 2 Resistance exercise versus usual care, Outcome 3 Quality of life (at 6 months).
| Study | Respiratory exercise N | Sham intervention N | MD, 95% CI |
| Fatigue (FSS) | |||
| Pinto 2012 | 12 | 12 | ‐9.654, 95% CI ‐22.037 to 2.729 |
| Sleepiness | |||
| Pinto 2012 | 12 | 12 | 0.308, 95% CI ‐3.48 to 4.096 |
| Depression | |||
| Pinto 2012 | 12 | 12 | 1.769, 95% CI 0.018 to 3.52 |
| Quality of life | |||
| Pinto 2012 | 12 | 12 | 0.769, 95% CI ‐17.093 to 18.631 |
| Functional status | |||
| Pinto 2012 | 12 | 12 | 0.846, 95% CI ‐2.157 to 3.849 |
| Functional status (ALSFRS‐bulbar) | |||
| Pinto 2012 | 12 | 12 | ‐0.385, 95% CI ‐1.378, to 0.609 |
| Functional status (ALSFRS‐respiratory) | |||
| Pinto 2012 | 12 | 12 | 0.077, 95% CI ‐0.254 to 0.407 |
Comparison 3 Respiratory exercise versus sham intervention, Outcome 1 Efficacy outcomes (all at 4 months).
| Study | Number of participants | Analysis of variance (time x treatment arm |
| Fatigue (FSS) | ||
| Zanette 2008 | 10 (5 rTMS, 5 sham intervention) | F[2,16] = 4.0; P = 0.04 |
| Functional status | ||
| Zanette 2008 | 10 (5 rTMS, 5 sham intervention) | F[2,16] = 2.7; P > 0.05 |
Comparison 4 Repetitive transcranial magnetic stimulation (rTMS) versus sham intervention, Outcome 1 Efficacy outcomes (all at 2 weeks).
| Modafinil compared to placebo in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with modafinil | |||||
| Fatigue assessed with: Fatigue Severity Scale (FSS) | The mean FSS score was 43 | MD 11 lower | ‐ | 32 | ⊕⊝⊝⊝ | |
| Adverse events | Three adverse events led to discontinuation of modafinil (2 headache, 1 chest tightness). Anxiety (in 2 people), nausea (in 2), dizziness (in 1), and sialorrhoea (in 1; probably ALS‐related) also occurred with modafinil. Placebo group adverse events were not reported ‐ it is not clear whether there were none. | ‐ | 32 | ⊕⊝⊝⊝ | The trial reported the number of adverse events in the treatment group, but not numbers of events in the placebo group or number of people experiencing adverse events | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the quality of evidence 3 times: once for study limitations and twice for imprecision. The security of blinding in the trial was unclear and the report did not provide enough information to assess attrition and selective reporting. The trial was small and the CI of the effect estimate included appreciable benefit and little or no effect. | ||||||
| Exercise compared to usual care in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with exercise | |||||
| Fatigue Fatigue Severity Scale (FSS) | The mean FSS score was 42.7 | MD 0.2 higher | ‐ | 18 | ⊕⊝⊝⊝ | ‐ |
| Adverse events | No adverse events were reported | ‐ | 18 | ⊕⊝⊝⊝ | None of the people who discontinued did so because they thought the exercise programme was making their condition worse. No participants reported excessive soreness, cramping, or fatigue. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence 3 times to low quality: twice for imprecision as the trial was very small and CI included appreciable benefit and appreciable harm. The third downgrading was for study limitations as the nature of the intervention prevented participant blinding. | ||||||
| Inspiratory muscle training compared to sham intervention in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk or value with sham intervention | Risk or value with inspiratory muscle training | |||||
| Fatigue Fatigue Severity Scale (FSS) follow up: 4 months | An illustrative mean FSS score in the absence of inspiratory muscle training is 42.71 | MD 9.654 lower | ‐ | 24 | ⊕⊝⊝⊝ | |
| Adverse events | The trialists stated that the exercise protocol had no adverse effects. | ‐ | 24 | ⊕⊝⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The mean FSS score in the control group after 4 months was not available from Pinto 2012. The value given here, for illustrative purposes, is the control group mean at 6 months from Dal Bello Haas 2007. 2We downgraded the quality of evidence 3 times to very low: twice for imprecision and once for study limitations. The trial was very small and CI included appreciable benefit and little or no effect. The nature of the intervention meant that the trainer was aware of the intervention group. | ||||||
| Repetitive transcranial magnetic stimulation (rTMS) compared to sham intervention in ALS/MND | |||
| Patient or population: people with ALS/MND | |||
| Outcomes | Impact | Number of participants | Quality of the evidence |
| Fatigue assessed with: Fatigue Severity Scale (FSS) follow‐up: 2 weeks | No FSS scores were given. The investigators assessed fatigue with the FSS using 2‐way analysis of variance (within‐subjects factor time, between‐subjects treatment arm). The trial reported a significant effect for fatigue at the end of the follow‐up period. The effect was non‐significant following post hoc Bonferroni adjustments (data not reported). | 10 | ⊕⊝⊝⊝ |
| Adverse events | No adverse events were reported. | 10 | ⊕⊝⊝⊝ |
| RCT: randomised controlled trial | |||
| GRADE Working Group grades of evidence | |||
| 1We downgraded the quality of evidence 3 times: once for study limitations and twice for imprecision. The risk of bias was unclear as the trial report provided too little detail for assessment. The trial involved 10 people. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Efficacy outcomes (all at 4 weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Fatigue (FSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sleepiness | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Depression | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Fatigue (FSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional status (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Functional status (ALSFRS total score) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Functional status (ALFRS lower extremity) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mental health (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Physical function (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Physical role (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Pain (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 General health (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Vitality (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Social function (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Emotional role (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Efficacy outcomes (all at 4 months) Show forest plot | Other data | No numeric data | ||
| 1.1 Fatigue (FSS) | Other data | No numeric data | ||
| 1.2 Sleepiness | Other data | No numeric data | ||
| 1.3 Depression | Other data | No numeric data | ||
| 1.4 Quality of life | Other data | No numeric data | ||
| 1.5 Functional status | Other data | No numeric data | ||
| 1.6 Functional status (ALSFRS‐bulbar) | Other data | No numeric data | ||
| 1.7 Functional status (ALSFRS‐respiratory) | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Efficacy outcomes (all at 2 weeks) Show forest plot | Other data | No numeric data | ||
| 1.1 Fatigue (FSS) | Other data | No numeric data | ||
| 1.2 Functional status | Other data | No numeric data | ||

