Leczenie zmęczenia w stwardnieniu zanikowym bocznym/chorobie neuronu ruchowego
Abstract
Background
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND), is terminal, progressive neurological condition for which there are no curative treatments. Among people with ALS/MND, fatigue is a common and debilitating symptom, which is characterised by reversible motor weakness and whole‐body tiredness that is only partially relieved by rest. The effectiveness of pharmacological or non‐pharmacological treatments for fatigue in ALS/MND is not yet established.
Objectives
To assess the effects of pharmacological and non‐pharmacological interventions for fatigue in ALS/MND.
Search methods
We searched the following databases on 5 September 2017: Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL Plus, and ERIC. We also searched two clinical trials registries.
Selection criteria
We selected randomised and quasi‐randomised controlled trials of any intervention which sought to reduce fatigue for people with ALS/MND. We included studies if reduction in fatigue was a primary or secondary outcome of the trial.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane.
Main results
We included one pharmacological (modafinil) study and three non‐pharmacological studies (resistance exercise, respiratory exercise, and repetitive transcranial magnetic stimulation (rTMS)), involving a total of 86 participants with ALS/MND. None of the included studies were free from risk of bias. Since there was only one trial for each intervention, no meta‐analysis was possible. All studies assessed fatigue using the Fatigue Severity Scale (FSS; scale from 9 to 63, higher scores indicate more fatigue). Information for assessing bias was often lacking in study reports, making the risk of bias unclear across several domains in all trials. Blinding of participants was not possible in exercise trials, but the outcome assessment was blinded.
We found very low‐quality evidence suggesting possible improvements in fatigue for modafinil treatment versus placebo (MD ‐11.00, 95% CI ‐23.08 to 1.08), respiratory exercise versus a sham intervention (MD ‐9.65, 95% CI ‐22.04 to 2.73), and rTMS versus sham rTMS (data not provided), which warrant further investigation to clarify the efficacy of these treatments for fatigue in ALS/MND. We found no clear improvements in fatigue for resistance exercise versus usual care (MD 0.20, 95% CI ‐10.98 to 11.38; very low‐quality evidence).
Three participants in the modafinil group dropped out of the modafinil study, two citing issues with headache and one with chest tightness; other adverse effects were anxiety, nausea, dizziness, and sialorrhoea (probably ALS‐related). The trials reported no adverse effects of exercise or rTMS.
We cannot be certain about the effects of any of the interventions studied because of imprecision (small numbers of participants, wide CI), and possible study limitations.
Authors' conclusions
It is impossible to draw firm conclusions about the effectiveness of interventions to improve fatigue for people with ALS/MND as there are few randomised studies, and the quality of available evidence is very low.
PICO
Streszczenie prostym językiem
Sposoby leczenia skrajnego zmęczenia i braku energii w stwardnieniu zanikowym bocznym/chorobie neuronu ruchowego
Pytanie badawcze
Jakie są efekty leczenia zmęczenia u chorych ze stwardnieniem zanikowym bocznym (ALS) w porównaniu z brakiem leczenia lub placebo?
Wprowadzenie
ALS, które jest również znane jako choroba neuronu ruchowego (MND), to stan, w którym nerwy kontrolujące ruch przestają działać. Chorzy doświadczają problemów z poruszaniem kończynami, utrzymaniem postawy, połykaniem i oddychaniem, które z czasem się pogarszają i skracają życie. Przyczyna nie jest znana i nie ma lekarstwa na tę chorobę. U ludzie żyjących z ALS/MND często występuje zmęczenie, które może powodować stres i obniżać jakość życia. Zmęczenie może być wywołane przez wiele przyczyn, w tym problemy z oddychaniem, leki, złe odżywianie i depresję. W tym przeglądzie skupiono się na sposobach leczenia zmęczenia wynikającego z samego stanu chorobowego. Różne metody terapeutyczne mogą zmniejszyć objawy zmęczenia w ALS/MND. Należą do nich leki, które mogą pomóc chorym czuć się bardziej przebudzonym i inne metody, takie jak ćwiczenia fizyczne. Pozostaje niejasne, czy którykolwiek z tych sposobów leczenia jest skuteczny w zmniejszeniu nasilenia zmęczenia w ALS/MND. Dokonaliśmy przeglądu dostępnych badań dotyczących skuteczności różnych terapii zmęczenia w ALS/MND.
Charakterystyka badań
Przegląd obejmował cztery małe badania, w których uczestniczyło łącznie 86 pacjentów. Każde badanie dotyczyło innej metody leczenia. Były to: leczenie farmakologiczne (modafinil) w porównaniu z placebo, ćwiczenia oddechowe w porównaniu z pozorowanymi (nieaktywnymi) ćwiczeniami oddechowymi, ćwiczenia z ciężarkami w porównaniu ze zwykłą opieką i magnetyczna stymulacja mózgu w porównaniu z pozorowaną powtarzalną przezczaszkową stymulacją magnetyczną.
Główne wyniki i jakość danych
Jesteśmy niepewni co do efektów stosowania modafinilu, ćwiczeń oddechowych, ćwiczeń z ciężarkami czy magnetycznej stymulacji mózgu na zmęczenie u osób z ALS/MND, ponieważ dane były bardzo niskiej jakości. Często nie było jasne, czy badania zostały odpowiednio zaprojektowane i przeprowadzone, ponieważ raporty z badań nie zawierały szczegółowych informacji. Wyniki tych niewielkich badań nie były precyzyjne. Trzech uczestników zaprzestało przyjmowania modafinilu z powodu działań niepożądanych: bólu głowy u dwóch z nich i ucisku w klatce piersiowej u jednego; uczestnicy zgłaszali również niepokój, nudności, zawroty głowy i ślinotok (niezdolność do kontrolowania wydzieliny z ust). Potrzebne są dalsze badania nad skutecznymi metodami terapii zmęczenia w ALS/MND.
Wyszukiwanie badań jest aktualne do września 2017 r.
Authors' conclusions
Summary of findings
| Modafinil compared to placebo in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with modafinil | |||||
| Fatigue assessed with: Fatigue Severity Scale (FSS) | The mean FSS score was 43 | MD 11 lower | ‐ | 32 | ⊕⊝⊝⊝ | |
| Adverse events | Three adverse events led to discontinuation of modafinil (2 headache, 1 chest tightness). Anxiety (in 2 people), nausea (in 2), dizziness (in 1), and sialorrhoea (in 1; probably ALS‐related) also occurred with modafinil. Placebo group adverse events were not reported ‐ it is not clear whether there were none. | ‐ | 32 | ⊕⊝⊝⊝ | The trial reported the number of adverse events in the treatment group, but not numbers of events in the placebo group or number of people experiencing adverse events | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the quality of evidence 3 times: once for study limitations and twice for imprecision. The security of blinding in the trial was unclear and the report did not provide enough information to assess attrition and selective reporting. The trial was small and the CI of the effect estimate included appreciable benefit and little or no effect. | ||||||
| Exercise compared to usual care in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with exercise | |||||
| Fatigue Fatigue Severity Scale (FSS) | The mean FSS score was 42.7 | MD 0.2 higher | ‐ | 18 | ⊕⊝⊝⊝ | ‐ |
| Adverse events | No adverse events were reported | ‐ | 18 | ⊕⊝⊝⊝ | None of the people who discontinued did so because they thought the exercise programme was making their condition worse. No participants reported excessive soreness, cramping, or fatigue. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence 3 times to low quality: twice for imprecision as the trial was very small and CI included appreciable benefit and appreciable harm. The third downgrading was for study limitations as the nature of the intervention prevented participant blinding. | ||||||
| Inspiratory muscle training compared to sham intervention in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk or value with sham intervention | Risk or value with inspiratory muscle training | |||||
| Fatigue Fatigue Severity Scale (FSS) follow up: 4 months | An illustrative mean FSS score in the absence of inspiratory muscle training is 42.71 | MD 9.654 lower | ‐ | 24 | ⊕⊝⊝⊝ | |
| Adverse events | The trialists stated that the exercise protocol had no adverse effects. | ‐ | 24 | ⊕⊝⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The mean FSS score in the control group after 4 months was not available from Pinto 2012. The value given here, for illustrative purposes, is the control group mean at 6 months from Dal Bello Haas 2007. 2We downgraded the quality of evidence 3 times to very low: twice for imprecision and once for study limitations. The trial was very small and CI included appreciable benefit and little or no effect. The nature of the intervention meant that the trainer was aware of the intervention group. | ||||||
| Repetitive transcranial magnetic stimulation (rTMS) compared to sham intervention in ALS/MND | |||
| Patient or population: people with ALS/MND | |||
| Outcomes | Impact | Number of participants | Quality of the evidence |
| Fatigue assessed with: Fatigue Severity Scale (FSS) follow‐up: 2 weeks | No FSS scores were given. The investigators assessed fatigue with the FSS using 2‐way analysis of variance (within‐subjects factor time, between‐subjects treatment arm). The trial reported a significant effect for fatigue at the end of the follow‐up period. The effect was non‐significant following post hoc Bonferroni adjustments (data not reported). | 10 | ⊕⊝⊝⊝ |
| Adverse events | No adverse events were reported. | 10 | ⊕⊝⊝⊝ |
| RCT: randomised controlled trial | |||
| GRADE Working Group grades of evidence | |||
| 1We downgraded the quality of evidence 3 times: once for study limitations and twice for imprecision. The risk of bias was unclear as the trial report provided too little detail for assessment. The trial involved 10 people. | |||
Background
Fatigue is a commonly reported symptom in people with amyotrophic lateral sclerosis/motor neuron disease (ALS/MND) (Ramirez 2008), with a large proportion of people reporting 'clinically significant' levels of fatigue (McElhiney 2009). Fatigue in ALS/MND has been defined as "reversible motor weakness and whole‐body tiredness that was predominantly brought on by muscular exertion and was partially relieved by rest" (Gibbons 2013a). In ALS/MND, fatigue is distinguished from sleepiness by feelings of weariness or exhaustion that do not necessarily beget a desire to sleep. This fatigue appears to be experienced predominantly as general (feelings of whole‐body tiredness) and physical (reversible motor weakness). People with other neurological diseases, such as multiple sclerosis, describe an experience of general physical fatigue occurring alongside cognitive fatigue (reversible reduction in concentration or mental performance)(Lou 2003; Mills 2008).
In ALS/MND, fatigue can be experienced as a pervasive feeling of tiredness or lethargy, or as an objective decline in the ability of a muscle to contract to maximum force (Gibbons 2011; Lou 2012). The first type of fatigue is ubiquitous in humans, and may have been experienced by the individual before diagnosis; however, following development of ALS/MND, the severity of this fatigue and precipitating factors substantially change. The second type of fatigue, objective motor fatigue, also known as physical fatigability, is unique to the disease state and is not normally within a person’s previous experience. Both types of fatigue have been shown to be important to people with ALS/MND (Gibbons 2011), and fatigue, more generally, impacts negatively upon quality of life (Lou 2003).
Evidence for the relationship between fatigue and functional ability is conflicting. Although some studies have suggested that fatigue is related to functional capacity (Lo Coco 2012; McElhiney 2009), others have been unable to support this claim (Gibbons 2011; Ramirez 2008). Fatigue appears to be related to common nighttime complaints in ALS/MND, including muscle cramps and nocturia (Lo Coco 2012). Fatigue is related to psychological distress, social withdrawal, and reduced quality of life for people with ALS/MND (Gibbons 2013b; Lou 2003).
Description of the condition
ALS/MND is a progressive, terminal neurodegenerative disease of unknown aetiology that is currently without a cure. The incidence of ALS/MND is around 2.16 per 100,000 person‐years (Logroscino 2010). At any one time, approximately 5000 people in the United Kingdom are affected (Shaw 1999), and 25,000 in North America (McGuire 1996).
Rapid progression of the disease causes weakness and muscular atrophy, impacting upon an individual's ability to carry out activities of daily living, such as dressing, bathing, and eating. Problems with speech and swallowing often occur as the result of weakness in bulbar musculature. As the disease progresses, breathing issues (including nocturnal hypoventilation and reduced sleep quality) become apparent, and may progress until respiratory failure occurs (Bourke 2004). Death has been reported in most people with MND within two to five years following diagnosis, usually from respiratory failure, caused by respiratory muscle weakness (Rowland 2001).
Description of the intervention
Currently, no evidence‐based treatment is available for fatigue in people with ALS/MND. Several drugs have been investigated as treatment for fatigue in this population, including amantadine, pemoline, and bupropion, although evidence regarding their efficacy is lacking (Jackson 2006). Modafinil, a novel wakefulness‐promoting agent that has been approved for the treatment of excessive sleepiness associated with narcolepsy, may also be an effective treatment for fatigue (Carter 2005; McElhiney 2009; Rabkin 2009).
Dietary supplementation of creatine may increase, or at least preserve, muscular strength, and reduce levels of fatigue (Rosenfeld 2008).
Other non‐pharmacological therapies may be used to ameliorate fatigue. Studies have evaluated the potential benefits of supported treadmill ambulation (Sanjak 2010), muscular exercise (Drory 2001), and repetitive transcranial magnetic stimulation (rTMS) (Guo 2012; Zanette 2008) for this purpose.
How the intervention might work
Pharmacological interventions that stimulate the central nervous system (CNS) may reduce generalised fatigue for people with ALS/MND. Modafinil stimulates the release of norepinephrine and dopamine from synaptic terminals and elevates hypothalamic levels of histamine (Ishizuka 2008). Creatine, which increases maximum availability for energy output in anaerobic activities, may also have a positive effect on muscle strength and fatigue (Ellis 2004; Kley 2013; Rosenfeld 2008). Different treatments may be effective for different forms of fatigue.
Non‐pharmacological interventions may focus on light exercise, including supported or unsupported exercises (e.g. walking), or resistance training using weights (Bello‐Haas 2007).
Why it is important to do this review
Fatigue is prevalent in people with ALS/MND and has a significant impact on quality of life. Presently, clarity regarding the best management of fatigue in this population is lacking. This review is the first systematic review of pharmacological and non‐pharmacological interventions for fatigue in ALS/MND. The purpose of this review is to identify interventions to reduce primary fatigue, which is disease related. We will not consider secondary causes of fatigue, such as malnutrition and chronic respiratory failure, which have been assessed in other Cochrane systematic reviews (Katzberg 2011; Radunovic 2017).
Objectives
To assess the effects of pharmacological and non‐pharmacological interventions for fatigue in ALS/MND.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and quasi‐randomised controlled trials, which assessed the effectiveness of pharmacological and non‐pharmacological treatments for fatigue in people with ALS/MND in this review.
Types of participants
We included studies in which study participants were diagnosed with possible, probable, or definite ALS/MND, according to recognised criteria, preferably the El Escorial criteria (Brooks 2000).
Types of interventions
We included all interventions that aimed to reduce fatigue in people with ALS/MND, and measured it as either a primary or a secondary objective. These pharmacological or non‐pharmacological treatments could be compared to each other, placebo, or standard care. Examples of such treatments included drug treatments (e.g. modafinil) and behavioural interventions.
Types of outcome measures
Primary outcomes
The primary outcome was level of fatigue at the end of the follow‐up period.
Fatigue could be evaluated using any validated patient‐ or clinician‐administered fatigue questionnaire that measures general fatigue, including the Modified Fatigue Impact Scale (MFIS) (Fisk 1994) and the Fatigue Severity Scale (FSS) (Krupp 1989). We also included questionnaires that measured general fatigue and reversible muscle weakness, such as the Neurological Fatigue Index–MND (NFI‐MND) (Gibbons 2011).
Secondary outcomes
Secondary outcomes were assessed at the end of the follow‐up period, and could include the following.
-
Sleepiness of participants measured by a validated scale including the Epworth Sleepiness Scale (ESS) (Johns 1991).
-
Depression measured by a validated scale or by a clinical diagnostic interview, including the Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983), or the Beck Depression Inventory (BDI) (Beck 1988).
-
Quality of life of participants measured by a validated objective or subjective instrument, including the ALS Specific Quality of Life‐Revised (ALSSQOL‐R) (Felgoise 2008), the McGill Quality of Life Questionnaire (Cohen 1995), or the Short Form‐36 Health Survey (SF‐36) (Ware 1992).
-
Functional status of participants measured by a validated scale such as the ALS Functional Rating Scale‐Revised (ALSFRS‐R) (Cedarbaum 1999).
-
Adverse effects.
Search methods for identification of studies
Electronic searches
We searched the following databases on 5 September 2017.
-
Cochrane Neuromuscular Specialised Register and Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web); (searched 5 September 2017) using the search strategy in Appendix 1
-
MEDLINE (1966 to August 2017) using the search strategy in Appendix 2.
-
Embase (1980 to August 2017) using the search strategy in Appendix 3.
-
PsycINFO (1806 to August 2017) using the search strategy in Appendix 4.
-
CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature Plus; 1937 to August 2017) using the search strategy in Appendix 5.
-
ERIC (1966 to August 2017) using the search strategy in Appendix 6
Searching other resources
We scanned available conference abstracts from International ALS/MND Symposia for relevant studies. We checked all references in the identified trials, and contacted trial authors to identify additional published or unpublished data. We searched trial registries (US National Institutes of Health trials registry, ClinicalTrials.gov (clinicaltrials.gov/), and the World Health Organization International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch/)) to identify ongoing trials. We considered trials published in languages other than English to be eligible for inclusion, following translation.
Data collection and analysis
Selection of studies
Three review authors (CAY, CG, and FP) checked titles and abstracts identified during the electronic searches. These review authors obtained the full text of all potentially relevant studies for independent assessment. All review authors independently assessed which trials fit the inclusion criteria. We resolved disagreements about inclusion criteria by discussion and consensus.
We excluded duplicate reports, and collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and a 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors (CG and FP) independently extracted data onto a specially designed form; the other two review authors (CAY and TF) checked the data extraction. One review author entered the data into the software (CG), another (FP) checked the entered data.
Where data allowed, we extracted the following study characteristics.
-
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study settings, withdrawals, and date of study.
-
Participants: number (N), mean age, age range, gender, severity of condition, diagnostic criteria, baseline characteristics, inclusion criteria, and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications, and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for trial, and notable conflicts of interest of trial authors.
Assessment of risk of bias in included studies
Two review authors (CG and FP) independently assessed risk of bias for each study using the Cochrane 'Risk of bias' tool, as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). All review authors discussed disagreements related to risk of bias, and reached a consensus. We assessed risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other sources of bias.
We graded studies as having high, low, or unclear risk of bias in each of these domains, and provided justifications for these judgements in the 'Risk of bias' table in Characteristics of included studies.
Measures of treatment effect
If sufficient studies had been included, we would have evaluated treatment effects for continuous outcomes using mean differences (MDs), or standardised mean differences (SMDs) for results across studies with outcomes that were conceptually the same, but measured in different ways. In the event that studies presented dichotomous data (e.g. responder analyses), we would have used risk ratios (RRs). We would have calculated 95% confidence intervals (CIs) for the measures of treatment effect.
We would have undertaken meta‐analyses only where meaningful, that is, when treatments, participants, and the underlying clinical questions were similar enough for pooling to make sense.
Assessment of reporting biases
If we had identified a large number of eligible studies, we would have analysed a funnel plot to assess the potential existence of small‐study bias.
Data synthesis
We had planned to use the random‐effects model for the summary effect measure in any meta‐analysis. The weights for the studies would have been be inverse to the variances. The random‐effects model incorporates possible between‐study variation as well as within‐study differences, and so is more conservative. The fixed‐effect model assumes that no between‐study differences exist. Both models yield very similar results, unless significant between‐study differences are noted (heterogeneity).
'Summary of findings' table
We created 'Summary of findings' tables, using fatigue as the outcome. We used the five GRADE considerations (study limitations, inconsistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence (studies that contributed data for the outcome). We also included adverse events. Since we included only RCTs and quasi‐RCTs in this review, we started from an assumption of high‐quality evidence, from which we downgraded the quality to moderate, low, or very low, based on the extent to which the GRADE considerations presented a threat to validity. We used methods and recommendations described in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011) and GRADEpro software (GRADEpro 2008). We justified all decisions to downgrade or upgrade the quality of studies using footnotes, with comments to aid readers' understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
We would have assessed the level of functional impairment, measured using the ALSFRS‐R (Cedarbaum 1999), to identify heterogeneity of participants. If numbers had allowed, we would have used this scale to create subgroups of participants to analyse separately and compare.
Sensitivity analysis
If a study was of doubtful eligibility for the systematic review, appeared to be an outlier, or had missing data that were impossible to retrieve, we had intended to compare the results of analyses with and without the trial. However, there was only one trial for any comparison.
This review has a published protocol (Gibbons 2014).
Results
Description of studies
Our searches identified a total of 243 reports. After we removed duplicates, we had 157, 25 of which were potentially relevant randomised controlled trials (RCT). The number of references returned from each database was: Cochrane Neuromuscular Specialised Register 31, CENTRAL 52, MEDLINE 56, Embase 80, PsycINFO 3, CINAHL Plus 20, and ERIC 1. We identified one additional reference from other sources. See Figure 1 for a PRISMA flow chart illustrating the study selection process.

Study flow diagram
Included studies
Modafinil versus placebo
The double‐blind trial by Rabkin and colleagues aimed to evaluate the effect of modafinil on fatigue in people with probable or definite ALS by modified El Escorial criteria (Rabkin 2009). They used a 3:1 modafinil:placebo design, in doses up to 300 mg/day for four weeks, followed by eight weeks of open maintenance treatment. Measures were collected at baseline, weeks two and four, and biweekly thereafter. Primary outcome analyses were conducted at week four. The primary outcome was fatigue, measured by the FSS. Secondary outcomes included sleepiness and depression.
Resistance exercise versus usual care
Dal Bello Haas and colleagues conducted a RCT of resistance exercise for people with clinically definite, probable, or laboratory‐supported amyotrophic lateral sclerosis/motor neuron disease (ALS/MND) (Dal Bello Haas 2007). Twenty‐seven participants were randomly assigned to either a resistance exercise group (N = 13), or a usual care group, which consisted of stretching exercises (N = 14). Participants in the resistance exercise group were given stretching exercises plus an individualised moderate‐load and moderate‐intensity resistance exercise programme for upper and lower limbs. Participants were assessed at baseline, and once monthly for six months thereafter. The primary outcome of the study was change in global function, measured by the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS). Secondary outcomes were health‐related quality of life (measured using the Short Form 36 (SF‐36) Health Survey), and fatigue (measured using the Fatigue Severity Scale (FSS)).
Respiratory exercise versus sham intervention
This study evaluated the effect of respiratory exercise in people with definite or probable ALS (Pinto 2012). The intervention was inspiratory muscular training, consisting of inhaling and exhaling through a threshold‐inspiratory muscle training (IMT) device, which was a plastic cylinder with a mouthpiece and spring‐loaded valve that could be set to different pressure thresholds. Twenty‐six participants were recruited into the trial; all participants received an IMT device. The participants were randomised into either an active intervention group (IMT resistance set at 30% to 40% of maximal inspiratory pressure (MIP)) or a sham intervention group (IMT resistance set to the lowest possible load, to remove any therapeutic effect). All participants received instructions to blow into the tube for 10 minutes twice daily. After an initial four‐month intervention period, all participants received the active intervention for a further four months. The primary outcome measure was decline in ALSFRS. Other outcomes relevant to this review were the respiratory subscore of the ALSFRS; fatigue assessed using the FSS, depression (Hamilton Rating Scale for Depression (HRSD)), sleepiness (Epworth Daytime Sleepiness Scale (ESS)), the Functional Independent Measure (FIM), which is an 18‐item ordinal scale of independence, and quality of life (measured using the EuroQol‐5D). Trialists evaluated fatigue during respiratory training by the Borg scale.
Repetitive transcranial magnetic stimulation (rTMS) versus sham intervention
Zanette and colleagues conducted a randomised‐controlled pilot study to assess the effect of 5‐Hz repetitive rTMS on motor performance, fatigue, and quality of life (QoL) (Zanette 2008). Ten people with probable or definite ALS attended a two‐week period of either real or sham 5‐Hz rTMS. Participants were examined at baseline, after the first day of treatment, and after two weeks of rTMS treatment. Outcomes relevant to this review included ALSFRSr, FSS, and quality of life, measured using the MOS 36‐Item Short Form Health Survey (SF‐36).
Excluded studies
We excluded 13 studies because (i) they were not RCTs (Carter 2005), (ii) did not assess fatigue as an end point (Drory 2001; Dupuis 2012; Goonetilleke 1995; Gordon 2007), or (iii) did not include a validated, patient‐reported measure for fatigue (Bertorini 2011; Cudkowitz 2003; Desnuelle 2001; Lange 2006; Mazzini 2001; Rosenfeld 2009; Silva 2009; Steele 2004).
Risk of bias in included studies
Figure 2 shows the review authors' 'Risk of bias' assessments using the Cochrane 'Risk of bias' tool.

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study.
The risk of bias in the included studies was generally unclear, as all study reports tended to omit important information concerning risk of bias. Eighteen of the 28 risk of bias domains were judged to be unclear. However, where this information was reported, the majority (7 out of 10) of the judgements were of a low risk of bias. Two cases in which risk of bias was judged to be high, related to blinding of participants and personnel. In one study the patients received training for the intervention and follow‐up calls to ensure compliance from a physician who was aware of group allocation (Pinto 2012). In the other study, the participants and personnel were unblinded, however the outcome assessor was blinded and participants were asked not to reveal their assignment(Dal Bello Haas 2007). Although the dropout rate in this study was high, reasons for withdrawal were unrelated to the intervention and we considered the risk of attrition bias to be low. We assessed the risk of other bias as high for another study in which the dropout rate was high in the intervention arm (16%) (Rabkin 2009).
Effects of interventions
See: Summary of findings for the main comparison Modafinil compared to placebo in ALS/MND; Summary of findings 2 Exercise compared to usual care in ALS/MND; Summary of findings 3 Inspiratory muscle training compared to sham intervention in ALS/MND; Summary of findings 4 Repetitive transcranial magnetic stimulation (rTMS) compared to sham intervention in ALS/MND
Modafinil versus placebo
Reported in Rabkin 2009. Of the 32 (25 intervention, 7 control) people randomised, 28 completed the four‐week double‐blind phase, with all four dropouts coming from the intervention group.
Primary outcome
Level of fatigue at the end of the follow‐up period
The possible range in Fatigue Severity Scale (FSS, total) is from 9 to 63, with higher scores indicating more fatigue or more fatigue‐related problems.
Level of fatigue was assessed using the FSS in an intention‐to‐treat (ITT) analysis. At the end of the follow‐up period, the mean score in the modafinil group (adjusted mean (SE) 32 (3.6)) was lower than in the placebo group (adjusted mean (SE) 43 (5.0)) (MD ‐11.00, 95% CI ‐23.08 to 1.08; P = 0.066; Analysis 1.1).
We downgraded the quality of evidence to very low (‐3): once for study limitations and twice for imprecision. The security of blinding in the trial was unclear and the report did not provide enough information to assess attrition and selective reporting. The trial was small and the 95% CI of the effect estimate included appreciable benefit and little or no effect. See summary of findings Table for the main comparison.
Secondary outcomes
Sleepiness
The range of possible Epworth Sleepiness Scale (ESS) scores is from 0 to 24, with higher scores indicating more sleepiness.
Sleepiness was assessed using the ESS in an ITT analysis at the end of the follow‐up period. There was little or no difference in adjusted mean scores (SE) for sleepiness between the modafinil group (5 (0.6)) and the placebo group (7 (1.1)) (MD ‐2.00, 95% CI ‐4.46 to 0.46; Analysis 1.1). The result was imprecise; 95% CI included both a potentially clinically relevant effect and no effect.
Depression
Scores in the Beck Depression Inventory‐II (BDI‐II) range from 0 to 63, with higher scores indicating greater severity of depressive symptoms.
The BDI‐II was used to assess depression at the end of the follow‐up period in an ITT analysis. There was no difference in adjusted mean score between the modafinil group (adjusted mean (SE) 9 (1.0)) and the placebo group (adjusted mean (SE) 9 (2.0)) (MD 0.00 95% CI ‐4.38 to 4.38; Analysis 1.1).
Quality of life
Not assessed.
Functional status
Not assessed using the ALS Functional Rating Scale (ALSFRS) or revised ALSFRS (ALSFRS‐R).
Statistically significant improvements were demonstrated in the Clinical Global Impression Score (Chi² = 8.887, degrees of freedom (df) = 1; P = 0.003). Only 1/7 participants (14%) in the placebo group reported that they were much or very much improved, compared to 19/25 participants (75%) in the intervention group. All data were taken from the published report.
Adverse effects
Three participants in the modafinil group dropped out due to side effects (two with headache, one with tightness in chest); a fourth for lack of effect and burden of travel). A participant in the placebo group discontinued modafinil because of 'agitation' during a subsequent open‐label phase of the study. Anxiety (in 2 participants), nausea (in 1), dizziness (in 1), and sialorrhoea (in 1, probably ALS‐related) also occurred with treatment. Numerical analysis was not possible, as the number of participants with adverse events was not reported.
We downgraded the quality of evidence to very low (‐3): once for study limitations and twice for imprecision. Reporting of adverse events was incomplete and security of blinding was unclear. The trial was small and the event rate low. See summary of findings Table for the main comparison.
Resistance exercise versus usual care
Reported in Dal Bello Haas 2007. The study randomly assigned 27 people to receive resistance exercise (N = 13) or usual care (N = 14). Five participants from the intervention group and four participants from the control group dropped out for reasons unrelated to the tolerability of the intervention. Data represent an available case analysis of the remaining eight participants in the intervention group and 10 participants in the usual care group.
Primary outcome
Level of fatigue at the end of the follow‐up period
Dal Bello Haas 2007 measured fatigue using the FSS at six months. The possible range in FSS (total) is from 9 to 63. Higher scores indicate more fatigue or more fatigue‐related problems.
The intervention had little effect on fatigue, as scores on the FSS were 42.9 ± 8.7 in the intervention group (N = 8) and 42.7 ± 15.2 in the usual care group (N = 10) at six‐month follow‐up (MD 0.20, 95% CI ‐10.98 to 11.38; Analysis 2.3).
We downgraded the quality of evidence to very low (‐3): once for study limitations (lack of blinding) and twice for imprecision, as the 95% CI included appreciable benefit and appreciable harm. See summary of findings Table 2.
Secondary outcomes
Sleepiness
Not assessed.
Depression
Mental health was assessed using the Short Form‐36 Health Survey (SF‐36) Mental Health subscale, with a range of possible scores from 0 to 100, with a higher score indicating better health.
SF‐36 Mental Health subscale scores (mean ± SD) showed no clear difference between the resistance exercise and usual care group at 6‐month follow‐up: 22.3 ± 4.0 in the resistance exercise group (N = 8) and 24.0 ± 4.2 in the usual care group (N = 10) (MD ‐1.70, 95% CI ‐5.50 to 2.10; Analysis 2.3).
Quality of life
Quality of life was assessed using the eight subscales of the SF‐36, including mental health, reported above. All of the SF‐36 scales have scores that range from 0 to 100, with higher scores indicating a more favourable state.
The study reported less impairment in physical function in the resistance exercise group (N = 10) than in the usual care group (N = 8) at six‐months follow‐up: intervention group (mean ± SD) 21.1 ± 7.6 versus usual care group 14.0 ± 3.9 (MD 7.10, 95% CI 1.31 to 12.89; Analysis 2.3).
The trial found little or no differences between the resistance exercise (N = 10) and usual care group (N = 8) on any other SF‐36 subscale (Analysis 2.3): Physical Role subscale (mean ± SD): resistance exercise group 5.1 ± 1.0, usual care 4.9 ± 1.7 (MD 0.20, 95% CI ‐1.06 to 1.46}; Pain subscale: resistance exercise 10.2 ± 2.3, usual care 10.3 ± 1.7 (MD ‐0.10, 95% CI ‐2.01 to 1.81); General Health subscale: resistance exercise 16.4 ± 3.4, usual care 16.0 ± 6.6 (MD 0.40, 95% CI ‐4.32 to 5.12); Vitality subscale: resistance exercise 16.4 ± 3.4, usual care 14.8 ± 4.1 (MD 1.60, 95% CI ‐1.87 to 5.07); Social Function subscale: resistance exercise 8.4 ± 1.5, usual care 7.7 ± 2.1 (MD 0.7, 95% CI ‐0.97 to 2.37); and Emotional Role subscale: resistance exercise group 5.3 ± 0.9, usual care 4.7 ± 1.4 (MD 0.60, 95% CI ‐0.47 to 1.67).
Functional status
The study demonstrated less decline in physical function overall in the resistance exercise group: resistance exercise group (mean ± SD) 33.8 ± 4.7, usual care 28.1 ± 4.8 (MD 5.7, 95% CI 1.29 to 10.11) and in the lower extremities: intervention group 18.8 ± 4, usual care 13.5 ± 3.4 (MD 5.50, 95% CI 1.82 to 8.78) (Analysis 2.3).
Adverse effects
None of the participants who discontinued did so because they thought the exercise programme itself was making their condition worse. No participants reported excessive soreness, cramping, or fatigue with either exercise protocol.
We downgraded the quality of evidence to very low (‐3): once for study limitations (lack of blinding) and twice for imprecision, as the 95% CI included appreciable benefit and appreciable harm. See summary of findings Table 2.
Respiratory exercise versus sham intervention
Pinto 2012 randomised 26 participants to receive resistance exercise (N = 13) or sham intervention (N = 13), of whom 12 in each group were evaluable. Due to the delayed‐start design for the control group, we only considered the results of the first four months of the intervention, during which we could compare the active and sham groups.
Primary outcome
Level of fatigue at the end of the follow‐up period
Fatigue was assessed using the FSS, with a possible total range from 9 to 63. Higher scores indicate more fatigue or more fatigue‐related problems.
At the end of the first follow‐up period the intervention group showed less fatigue than the sham intervention group (MD ‐9.65, 95% CI ‐22.04 to 2.73; N = 24; Analysis 3.1).
We downgraded the quality of evidence to very low (‐3): twice for imprecision and once for study limitations. The trial was very small and 95% CI included appreciable benefit and little or no effect. The nature of the intervention meant that the trainer was aware of the intervention group. See summary of findings Table 3.
Secondary outcomes
Sleepiness
The range of possible ESS scores is from 0 to 24. Higher scores indicate more sleepiness.
We found no clear differences between groups in mean scores on the ESS questionnaire at the end of the first follow‐up period (MD 0.31, 95% CI ‐3.48 to 4.10; N = 24; Analysis 3.1).
Depression
The range of possible Hamilton Depression Rating Scale (HDRS, or Ham‐D) scores, which was used to measure depression, is from 0 to 63. Higher scores indicate greater severity of depression.
Depression was lower in the respiratory exercise group than the usual care group at the end of the first follow‐up period (MD 1.77, 95% CI 0.02 to 3.52; N = 24; Analysis 3.1).
Quality of life
Quality of life was assessed using the EuroQol ‐ 5 dimension instrument (EQ‐5D), with a range in scores from 1 to 25. Higher scores indicate lower health‐related quality of life.
There was no significant difference in quality of life at the end of the first follow‐up period between the resistance exercise and the usual care groups (MD 0.77, 95% CI ‐17.10 to 18.63; N = 24; Analysis 3.1).
Functional status
Functional status was assessed with the ALSFRS, administered by a blinded evaluator. There was no difference between groups in mean scores for the overall scale at the end of the first follow‐up (MD 0.85, 95% CI ‐2.16 to 3.85; N = 24), the ALSFRS‐b bulbar subscale (MD ‐0.39, 95% CI ‐1.38 to 0.61; N = 24), or the respiratory subscale (MD 0.08, 95% CI ‐0.25 to 0.41; N = 24; Analysis 3.1).
Adverse effects
There were no adverse effects reported.
We downgraded the quality of evidence for this outcome to very low (‐3): twice for imprecision and once for study limitations. The trial was very small. The nature of the intervention meant that the trainer was aware of the intervention group. See summary of findings Table 3.
Repetitive transcranial magnetic stimulation (rTMS) versus sham intervention
Zanette 2008 randomised 10 participants to receive rTMS (N = 5) or the sham intervention (N = 5).
Primary outcome
Level of fatigue at the end of the follow‐up period
Fatigue was measured using the FSS, and assessed using two‐way repeated measures analysis of variance (ANOVA; the within‐subjects factor was time and the between‐subjects factor was treatment arm). The paper reported a significant effect from rTMS for fatigue at the end of the two‐week follow‐up period (F[2,16] = 4.0; P = 0.04; Analysis 4.1). The effect was non‐significant following post hoc Bonferroni adjustments (data not reported). The paper did not provide mean scores, SDs, or CIs.
We downgraded the quality of evidence to very low (‐3): once for study limitations and twice for imprecision. The trial involved 10 people. The risk of bias was unclear, as the trial report provided too little detail for assessment. See summary of findings Table 4.
Secondary outcomes
Sleepiness
Not assessed.
Depression
Not assessed.
Quality of life
Quality of life was assessed using the global score from the SF‐36, a method for which there is no evidence of psychometric validity (Ware 1992).
Functional status
The ALSFRSr was used to assess functional status at the end of the follow‐up period, using complete data. ANOVA tests demonstrated no significant interaction between time and treatment (F[2,16] = 2.7; P > 0.05; Analysis 4.1). The paper did not report mean scores, SDs, or CIs.
Adverse effects
No adverse events were reported.
Discussion
Summary of main results
We included four studies (86 participants) that met the eligibility criteria in this review. They evaluated pharmacological interventions (modafinil) (Rabkin 2009) and non‐pharmacological interventions, including resistance exercise (Dal Bello Haas 2007), respiratory exercise (Pinto 2012), and repetitive transcranial magnetic stimulation (rTMS) (Zanette 2008) We are very uncertain about the effects of these interventions on fatigue in ALS, as the quality of evidence was too low to rule out any negative effect or establish clinical benefit.
Rabkin 2009 (32 participants) conducted an ITT analysis that showed improved fatigue at the end of a brief double‐blind phase for the modafinil group, although the 95% CI were wide, and allowed for the possibility of no effect.
Resistance exercise had little effect on fatigue in Dal Bello Haas 2007 (27 participants).
In Pinto 2012 (26 participants), there was reduced fatigue in the respiratory exercise group over the sham intervention group, but the results were imprecise and 95% CI were consistent with both a large effect and none. No clear differences between groups were reported for sleepiness, quality of life, or functional status, but there was small reduction of depressive symptoms in the respiratory exercise group.
Zanette 2008 (10 participants) reported that improvements in fatigue in the rTMS group showed a "trend towards" statistical significance before post hoc tests corrected for multiple comparisons. These effects were short‐lived, and after two weeks with no treatment, the trialists reported that there was no clear difference between the two groups on any of the reported outcomes.
In the modafinil study, three participants dropped out from the intervention arm due to side effects including headache and headache with chest tightness. The trial authors noted that similar adverse events were not reported in another RCT they had run with over 100 people with HIV/AIDS (Rabkin 2009). Similarly, an open‐label trial of modafinil for ALS/MND reported that the drug was well tolerated in both the 200 mg and 400 mg groups (Carter 2005). Rabkin and colleagues also ran a completer's analysis, in which the positive effect of modafinil in alleviating fatigue was more pronounced, with effect sizes significant at the 5% level for fatigue, sleepiness, energy, and stamina (Rabkin 2009).
No adverse events were reported in any of the non‐pharmacological trials.
Overall completeness and applicability of evidence
We identified four studies. Each evaluated different interventions which, at best, reported weak or transient beneficial effects. The heterogeneity in the assessed interventions precluded meta‐analysis of the disparate outcomes. Therefore, no reliable information was available to assess whether different treatments for fatigue may be beneficial or harmful for people with ALS/MND.
This review highlights a clear need to commit high‐quality evidence to the corpus of literature relating to treatment for fatigue in people with ALS/MND.
Quality of the evidence
We assessed the overall risk of bias from low to unclear for two of the four studies. We found issues relating to blinding of participants or personnel in two of the trials. In Pinto 2012, the trainer was aware of the group allocation and in Dal Bello Haas 2007, the participants were unblinded due to the nature of the intervention. Though they were asked not to reveal their allocation to the assessors, no formal evaluation took place to confirm that assessors remained unblinded throughout the trial period. High dropout introduced a high risk of bias for the study by Rabkin and colleagues of modafinil versus placebo (Rabkin 2009). It is notable that more than half of the risk of bias domains could not be ascertained from the published reports. All results lacked precision and were based on findings from single small studies. There is an additional risk that we were unable to identify all relevant controlled research studies, due to publication bias.
Potential biases in the review process
We followed the Cochrane Neuromuscular search strategies, which include a search of the Cochrane Neuromuscular Specialised Register, which is updated weekly to monthly from a range of databases. Therefore, it is unlikely that studies have been missed, although it is possible that studies that have not been published could be missing. Should any further studies be identified, we will include them in future updates of the review. We followed the recommended Cochrane review process to reduce potential biases, which included having at least two review authors independently assess identified studies, extract data, and evaluate risk of bias. The small size of included studies means that estimates of adverse event rate frequency are unlikely to be accurate, particularly for rare events.
Agreements and disagreements with other studies or reviews
To our knowledge, there are no other systematic reviews on this topic for people with ALS/MND.
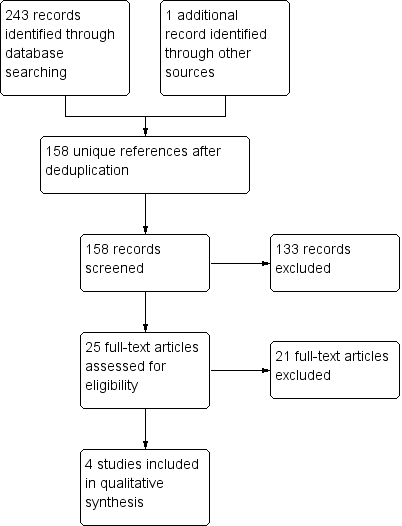
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study.

Comparison 1 Modafinil versus placebo, Outcome 1 Efficacy outcomes (all at 4 weeks).
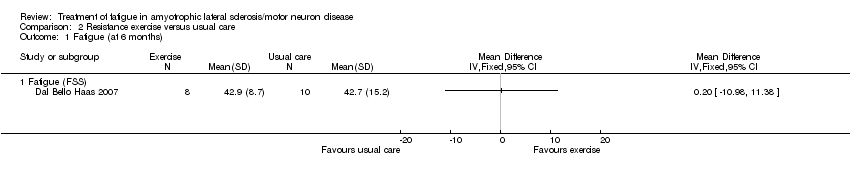
Comparison 2 Resistance exercise versus usual care, Outcome 1 Fatigue (at 6 months).
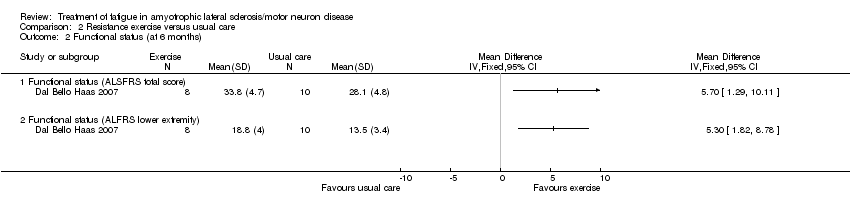
Comparison 2 Resistance exercise versus usual care, Outcome 2 Functional status (at 6 months).

Comparison 2 Resistance exercise versus usual care, Outcome 3 Quality of life (at 6 months).
| Study | Respiratory exercise N | Sham intervention N | MD, 95% CI |
| Fatigue (FSS) | |||
| Pinto 2012 | 12 | 12 | ‐9.654, 95% CI ‐22.037 to 2.729 |
| Sleepiness | |||
| Pinto 2012 | 12 | 12 | 0.308, 95% CI ‐3.48 to 4.096 |
| Depression | |||
| Pinto 2012 | 12 | 12 | 1.769, 95% CI 0.018 to 3.52 |
| Quality of life | |||
| Pinto 2012 | 12 | 12 | 0.769, 95% CI ‐17.093 to 18.631 |
| Functional status | |||
| Pinto 2012 | 12 | 12 | 0.846, 95% CI ‐2.157 to 3.849 |
| Functional status (ALSFRS‐bulbar) | |||
| Pinto 2012 | 12 | 12 | ‐0.385, 95% CI ‐1.378, to 0.609 |
| Functional status (ALSFRS‐respiratory) | |||
| Pinto 2012 | 12 | 12 | 0.077, 95% CI ‐0.254 to 0.407 |
Comparison 3 Respiratory exercise versus sham intervention, Outcome 1 Efficacy outcomes (all at 4 months).
| Study | Number of participants | Analysis of variance (time x treatment arm |
| Fatigue (FSS) | ||
| Zanette 2008 | 10 (5 rTMS, 5 sham intervention) | F[2,16] = 4.0; P = 0.04 |
| Functional status | ||
| Zanette 2008 | 10 (5 rTMS, 5 sham intervention) | F[2,16] = 2.7; P > 0.05 |
Comparison 4 Repetitive transcranial magnetic stimulation (rTMS) versus sham intervention, Outcome 1 Efficacy outcomes (all at 2 weeks).
| Modafinil compared to placebo in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with modafinil | |||||
| Fatigue assessed with: Fatigue Severity Scale (FSS) | The mean FSS score was 43 | MD 11 lower | ‐ | 32 | ⊕⊝⊝⊝ | |
| Adverse events | Three adverse events led to discontinuation of modafinil (2 headache, 1 chest tightness). Anxiety (in 2 people), nausea (in 2), dizziness (in 1), and sialorrhoea (in 1; probably ALS‐related) also occurred with modafinil. Placebo group adverse events were not reported ‐ it is not clear whether there were none. | ‐ | 32 | ⊕⊝⊝⊝ | The trial reported the number of adverse events in the treatment group, but not numbers of events in the placebo group or number of people experiencing adverse events | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the quality of evidence 3 times: once for study limitations and twice for imprecision. The security of blinding in the trial was unclear and the report did not provide enough information to assess attrition and selective reporting. The trial was small and the CI of the effect estimate included appreciable benefit and little or no effect. | ||||||
| Exercise compared to usual care in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with exercise | |||||
| Fatigue Fatigue Severity Scale (FSS) | The mean FSS score was 42.7 | MD 0.2 higher | ‐ | 18 | ⊕⊝⊝⊝ | ‐ |
| Adverse events | No adverse events were reported | ‐ | 18 | ⊕⊝⊝⊝ | None of the people who discontinued did so because they thought the exercise programme was making their condition worse. No participants reported excessive soreness, cramping, or fatigue. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded the evidence 3 times to low quality: twice for imprecision as the trial was very small and CI included appreciable benefit and appreciable harm. The third downgrading was for study limitations as the nature of the intervention prevented participant blinding. | ||||||
| Inspiratory muscle training compared to sham intervention in ALS/MND | ||||||
| Patient or population: people with ALS/MND | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk or value with sham intervention | Risk or value with inspiratory muscle training | |||||
| Fatigue Fatigue Severity Scale (FSS) follow up: 4 months | An illustrative mean FSS score in the absence of inspiratory muscle training is 42.71 | MD 9.654 lower | ‐ | 24 | ⊕⊝⊝⊝ | |
| Adverse events | The trialists stated that the exercise protocol had no adverse effects. | ‐ | 24 | ⊕⊝⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The mean FSS score in the control group after 4 months was not available from Pinto 2012. The value given here, for illustrative purposes, is the control group mean at 6 months from Dal Bello Haas 2007. 2We downgraded the quality of evidence 3 times to very low: twice for imprecision and once for study limitations. The trial was very small and CI included appreciable benefit and little or no effect. The nature of the intervention meant that the trainer was aware of the intervention group. | ||||||
| Repetitive transcranial magnetic stimulation (rTMS) compared to sham intervention in ALS/MND | |||
| Patient or population: people with ALS/MND | |||
| Outcomes | Impact | Number of participants | Quality of the evidence |
| Fatigue assessed with: Fatigue Severity Scale (FSS) follow‐up: 2 weeks | No FSS scores were given. The investigators assessed fatigue with the FSS using 2‐way analysis of variance (within‐subjects factor time, between‐subjects treatment arm). The trial reported a significant effect for fatigue at the end of the follow‐up period. The effect was non‐significant following post hoc Bonferroni adjustments (data not reported). | 10 | ⊕⊝⊝⊝ |
| Adverse events | No adverse events were reported. | 10 | ⊕⊝⊝⊝ |
| RCT: randomised controlled trial | |||
| GRADE Working Group grades of evidence | |||
| 1We downgraded the quality of evidence 3 times: once for study limitations and twice for imprecision. The risk of bias was unclear as the trial report provided too little detail for assessment. The trial involved 10 people. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Efficacy outcomes (all at 4 weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Fatigue (FSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sleepiness | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Depression | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Fatigue (FSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Functional status (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Functional status (ALSFRS total score) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Functional status (ALFRS lower extremity) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Quality of life (at 6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mental health (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Physical function (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Physical role (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Pain (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 General health (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Vitality (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Social function (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Emotional role (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Efficacy outcomes (all at 4 months) Show forest plot | Other data | No numeric data | ||
| 1.1 Fatigue (FSS) | Other data | No numeric data | ||
| 1.2 Sleepiness | Other data | No numeric data | ||
| 1.3 Depression | Other data | No numeric data | ||
| 1.4 Quality of life | Other data | No numeric data | ||
| 1.5 Functional status | Other data | No numeric data | ||
| 1.6 Functional status (ALSFRS‐bulbar) | Other data | No numeric data | ||
| 1.7 Functional status (ALSFRS‐respiratory) | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Efficacy outcomes (all at 2 weeks) Show forest plot | Other data | No numeric data | ||
| 1.1 Fatigue (FSS) | Other data | No numeric data | ||
| 1.2 Functional status | Other data | No numeric data | ||

