Intervenções comunitárias e nos sistemas de saúde para melhorar o comparecimento ao pré‐natal e resultados de saúde
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel arm cluster‐RCT conducted at 18 sites in Bangladesh between Feb 2005 and Dec 2008. | |
| Participants | Sample size: 18 clusters (6389 women). Clusters: purposive sampling was performed in 3 different divisions in Bangladesh on the basis of the districts having active Diabetic Association of Bangladesh (BADAS) offices. Within these districts, sub districts (upazilas) and unions (the lowest level administrative units in rural Bangladesh) were also purposefully sampled by use of recommendations from BADAS representatives, the main criteria being perceived limited access to perinatal health care in those unions, and a feasible travelling distance from BADAS district headquarters. Individuals: women were eligible to participate in the study if they were aged 15–49 years, residing in the project area, and had given birth during the study period. | |
| Interventions | Target: health system (re‐organisation of health services intervention) and community (education or IEC intervention). Arm 1 (9 clusters, 17,514 births ITT): in intervention clusters, a facilitator convened 18 groups every month to support participatory action and learning for women, and to develop and implement strategies to address maternal and neonatal health problems. 5 of the 9 clusters became TBA intervention clusters and 4 became controls. 482 TBAs were given basic training in undertaking clean and safe deliveries, providing safe delivery kits, recognising danger signs in mothers and infants, making emergency preparedness plans, accompanying women to facilities, and undertaking mouth‐to‐mouth resuscitation. They also received additional training in neonatal resuscitation with bag valve‐mask. Arm 2 (9 clusters, 18,599 births ITT): health services strengthening intervention and basic training of TBAs. | |
| Outcomes | Trial primary outcome: neonatal mortality rate. Review outcomes reported: Primary: ANC coverage (at least 4 visits), maternal mortality. Secondary: health facility deliveries, tetanus protection, perinatal mortality, neonatal mortality. We have used mortality data from Table 2 (Azad 2010 p. 1197). We used Years 1‐3 combined, excluding the "temporary and tea garden residents" who may not have received the full intervention. We calculated our own cluster adjusted ORs for antenatal care outcomes using the percentages from Table 4, p. 1200 and the denominators from years 1‐3 in Table 2, p. 1197 (all births: intervention n = 15,696 and control n = 15,257). | |
| Notes | Funders: Women and Children First, the UK Big Lottery Fund, Saving Newborn Lives, and the UK Department for International Development. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation sequence was decided upon by the project team before drawing" pg 1194 "and was based on clusters rather than |
| Allocation concealment (selection bias) | Unclear risk | Not clear how allocation was concealed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. |
| Recruitment bias (for cluster RCTs) | Unclear risk | "Additionally, about 10% of mothers in our study area were temporary residents and mainly came into the cluster areas to give birth, since the tradition is for women to go to their mothers’ home just before delivery. These temporary residents were not exposed to the women’s group intervention, and often had returned to their marital homes outside the study area before the postnatal interview." Presumably this would have affected all clusters. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Low risk | Most relevant outcomes reported. |
| Analysis bias | Low risk | Analysis appropriate for clusters; ICC reported; ITT analysis performed. |
| Other bias | Unclear risk | Baseline imbalances not reported. |
| Overall risk assessment | Low risk | No serious risk of bias concerns. |
| Methods | Parallel 3‐arm cluster‐RCT conducted at 24 sites in Bangladesh (Sylhet district) between Jul 2003 and Dec 2005. | |
| Participants | Sample size: 24 clusters (113816 women; 46,444 live births analysed). Clusters: 24 clusters (with a population of about 20,000 each) in Sylhet district, a district with poor access to health care, about 15,000 livebirths per year, and the presence of non‐government organisations with the ability to scale‐up the intervention. The area also has the highest neonatal mortality in Bangladesh. Individuals: ever‐married women of reproductive age (15–49 years old). | |
| Interventions | Target: health system (addition of home visits) and community (IEC). Arm 1 (8 clusters, 36,059 women): (Home care) the CHWs identified pregnancies through routine surveillance during visits to each household once every 2 months; promoted birth and newborn care preparedness through 2 scheduled antenatal and 3 early postnatal home visits; and provided iron and folic acid supplements during birth and newborn‐care preparedness visits. Arm 2 (8 clusters, 40,159 women): (Community care) in the community‐care arm, female volunteers called community resource people were recruited in each village to identify pregnant women, encourage them to attend community meetings held by the community mobilisers, receive routine ANC, and seek care for signs of serious illness in mothers or newborns. Arm 3 (8 clusters, 37,598 women): (Usual care) families received the usual health services provided by the government, non‐government organisations, and private providers. | |
| Outcomes | Trial primary outcome: reduction in neonatal mortality. Review outcomes reported: Primary: not reported. Secondary: tetanus protection, at least 1 ANC visit, neonatal mortality. We have analysed these data by combining the 2 arms with individual interventions (home care or community care) compared to the control arm of standard care. Outcome data are included in our Comparison 1. | |
| Notes | Funders: United States Agency for International Development and saving newborn lives programme by Save the Children (US) with a grant from Bill and Melinda Gates Foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated pseudo random number sequence." |
| Allocation concealment (selection bias) | Low risk | "The computer‐generated randomisation was implemented by a study investigator who had no role in the implementation of the study." |
| Blinding of participants and personnel (performance bias) | Unclear risk | "nature of the intervention meant masking was unachievable." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data described in study flow chart. |
| Selective reporting (reporting bias) | Low risk | Most relevant outcomes reported. |
| Analysis bias | Low risk | Analysis appropriate for clusters; ICC reported; ITT analysis performed. |
| Other bias | Low risk | No baseline imbalances. |
| Overall risk assessment | Low risk | No serious risk of bias concerns. |
| Methods | Parallel‐arm cluster‐RCT conducted at 506 sites in Mexico between 1997 and 2003. | |
| Participants | Sample size: 506 clusters randomised (individuals not reported), 173 clusters analysed. Clusters: "The rural programme established eligibility in two stages: poor communities were first identified, and low‐income households were identified within those communities". Communities and households were randomly selected based on a probability sample proportionate to the number of women of reproductive age women (15–49 years). Individuals: the sample included women who experienced a singleton live birth between 1997 and 2003, were designated as poor and eligible for Oportunidades, and lived in the original treatment and control communities | |
| Interventions | Target: community (financial incentive intervention). Arm 1 (97 clusters, 810 women): Progresa or Opportunidades is a conditional cash transfer program established in 1997 in Mexico, with the dual aim of immediate poverty relief and long‐term impact on the generational transfer of poverty. Every 2 months intervention families received a cash transfer representing approximately a 25% increase in household income (Gertler 2000, p. 3). The cash transfer required specific health behaviours of all members of households. Pregnant women were required to have 5 prenatal visits beginning in the first trimester of pregnancy. Beneficiary births are those births that occurred after the household received their first cash transfer. Households in intervention areas began receiving benefits during the summer of 1998. Arm 2 (61 communities, 215 women): non‐beneficiary births are those that occurred among eligible women prior to receiving the first cash transfer. Households in control clusters began receiving benefits in November 1999. | |
| Outcomes | Trial primary outcome: birthweight. Review outcomes reported: Primary: ANC coverage (at least 4 visits). Secondary: at least 1 ANC visit, health facility deliveries, tetanus protection, low birthweight infants. | |
| Notes | The Mexican social welfare program Oportunidades (now Prospera) has multiple citations. We have incorporated data from a specific analysis conducted on a small sample of women in households involved in this large poverty relief program (Barber 2008). Funders: National Institutes of Health Fogarty International Center TW006084 and National Institute of Child Health and Human Development. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | For the initial cluster‐randomisation, "random assignment was generated at the community level without weighting by use of the randomisation commands in Stata version 2.0" (Fernald 2008). For the survey, areas were randomly assigned "based on a probability sample proportionate to the number of women of reproductive age". p. 20 Barber 2009. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Due to the nature of the intervention participants could not be blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up described but sample sizes vary in the different reports. |
| Selective reporting (reporting bias) | Low risk | Most relevant outcomes reported. |
| Analysis bias | Low risk | Analysis appropriate for clusters; ITT analysis performed. |
| Other bias | Low risk | No baseline imbalances. |
| Overall risk assessment | Low risk | No serious risk of bias concerns. |
| Methods | Parallel‐arm cluster‐RCT conducted at 166 sites in Rwanda between June 2006 and Oct 2006. | |
| Participants | Sample size: 166 clusters (2563 women). Clusters: districts without pre‐existing P4P schemes managed by non‐governmental organisations. Individuals: not described. | |
| Interventions | Target: health system (financial intervention). Arm 1 (80 clusters, 1242 women): P4P scheme to supplement primary health centres’ input‐based budgets. In this P4P scheme, payments are made directly to facilities and are used at each facility’s discretion. Arm 2 (86 clusters, 1321 women): control facilities would continue to receive traditional input‐based financing for an additional 23 months until the rollout of the scheme was complete. | |
| Outcomes | Trial primary outcomes: prenatal care visits and institutional deliveries. Review outcomes reported: Primary: ANC coverage (at least 4 visits). Secondary: ANC coverage (at least 1 visit), health facility deliveries, tetanus protection, use of child preventative care. | |
| Notes | Funders: World Bank’s Bank‐Netherlands Partnership Program, the British Economic and Social Research Council, the Government of Rwanda, and the World Bank’s Spanish Impact Evaluation Fund; Global Development Network and the MacNamara Foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The remaining districts were then grouped into eight blocks based on rainfall, population density, and livelihood data from the 2002 Census.15 Blocks covered between two and 4 districts, depending on district characteristics and size. The blocks were then divided into two sides, and one side of each block was randomly assigned to either the intervention or control group. Randomisation was done by coin toss." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | Women interviewed in households would not have been aware of their local facility's group assignment. Women attending facilities should also not have been aware of the funding scheme in operation at her local health clinic. |
| Blinding of outcome assessment (detection bias) | Low risk | "All surveys were done by trained enumerators hired by external firms specialised in data collection who were masked to whether they were interviewing in an intervention or control area." |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Low risk | 2.1 % of intervention and 1.9% of control households refused to participate. 12% loss to follow‐up between baseline and end of trial surveys. 11.8% attrition in each treatment arm between first and second interviews. Incomplete household surveys were dropped from the sample after each round. |
| Selective reporting (reporting bias) | Low risk | Most relevant outcomes are reported. |
| Analysis bias | Unclear risk | Analysis appropriate for clusters, ICC and ITT not reported. |
| Other bias | High risk | Allocation assignment not respected due to government restructuring. |
| Overall risk assessment | Unclear risk | Due to uncertainties raised above. |
| Methods | Parallel‐arm cluster‐RCT conducted at 16 sites in Pakistan (Hala and Matiari sub districts) between Feb 2006 and Mar 2008. | |
| Participants | Sample size: 16 clusters (51409 individuals). Clusters: catchment areas of primary care facilities with adequate numbers of LHWs. Individuals: not described. Exclusion criteria: areas with low numbers of LHWs and areas with poor access were excluded. | |
| Interventions | Target: health system (health worker education) and community (IEC intervention). Arm 1: the intervention package was delivered by trained LHWs through group sessions consisted of promotion of ANC and maternal health education, use of clean delivery kits, facility births, immediate newborn care, identification of danger signs, and promotion of care seeking. Arm 2: in the control clusters, the LHW programme continued to function as usual and no additional attempt was made to link LHWs with the Dais or communities. | |
| Outcomes | Trial primary outcome: perinatal and all‐cause neonatal mortality. Review outcomes reported: Primary: ANC coverage (at least 4 visits). Secondary: ANC coverage (at least 1 visit), professional ANC, health facility deliveries, perinatal mortality, stillbirth, neonatal mortality. Follow‐up: every 3 months for 2 years. | |
| Notes | Funders: grants from the WHO and the Saving Newborn Lives programme funded by the Bill & Melinda Gates Foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "From this list of balanced allocations, we selected one scheme using a computer generated random number." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) | Low risk | Data collectors and their supervisors were masked to cluster allocation p. 406 Bhutta 2011. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Low risk | Only 414 lost to follow‐up (less than 1%). |
| Selective reporting (reporting bias) | Low risk | Most relevant outcomes reported. |
| Analysis bias | Low risk | Analysis appropriate for clusters; ICC reported; ITT analysis performed. |
| Other bias | Low risk | No baseline imbalances. |
| Overall risk assessment | Low risk | No serious risk of bias concerns. |
| Methods | Parallel arm cluster‐RCT conducted in Mirzapur, Bangladesh, between Dec 2003 and Dec 2006. | |
| Participants | Sample size: 12 clusters (21,140 individuals randomised, 10,700 women with at least 1 pregnancy during 10 preceding months analysed). Clusters: rural unions surrounding an urban central union (excluded from the study) served by a 750 bed private referral‐level hospital. Individuals: all married women of reproductive age (i.e. 15–49 years) in the intervention arm were eligible for enrolment. Women in the survey were eligible if they had had a pregnancy outcome in the last 3 years. | |
| Interventions | Target: health system (addition of home visits). Arm 1: 2 home visits (12‐16 and 32‐34 weeks); they were given a labour card for women to present upon arrival at hospital for delivery and 3 postnatal visits on days 2, 5 and 8. CHWs facilitated free‐of‐charge transfer of ill neonates to hospital.The purpose of the antenatal component of the intervention was to increase uptake of ANC (3 visits taking place at home or at a health centre or satellite clinic ‐ distinct from the 2 antenatal CHW home visits), tetanus toxoid vaccination, general pregnancy and newborn care education, and birth preparedness (including delivery at a health facility). Arm 2: standard ANC. | |
| Outcomes | Trial primary outcomes: antenatal and immediate newborn care behaviours, knowledge of danger signs, care seeking for neonatal complications, and neonatal mortality. Review outcomes reported: Primary: not reported. Secondary: ANC coverage (at least 1 visit), health facility delivery, IPT for malaria, neonatal mortality. | |
| Notes | Funders: The Wellcome Trust: Burroughs Wellcome Fund Infectious Disease Initiative 2000 and the Office of Health, Infectious Diseases and Nutrition, Global Health Bureau, United States Agency for International Development (USAID) through the Global Research Activity Cooperative agreement with the Johns Hopkins Bloomberg School of Public Health (award HRN‐A‐00‐96‐90006‐00). Support for data analysis and manuscript preparation was provided by the Saving Newborn Lives program through a grant by the Bill & Melinda Gates Foundation to Save the Children‐US. The study was registered at clinicaltrials.gov, No. NCT00198627. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of clusters with a computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not blinded. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Low risk | Study flow chart with details of exclusions. Response rate for the endline survey reported as 87.8% (11731/16771). |
| Selective reporting (reporting bias) | Unclear risk | Data for proportion of women who received 2 tetanus toxoid immunisations were not reported. The authors report falling rates during the trial and attribute this to a national shortage of vaccine. |
| Analysis bias | Unclear risk | Method of analysing clusters not clearly described apart from stating ITT analysis performed. |
| Other bias | Low risk | Baseline characteristics similar between arms. |
| Overall risk assessment | Unclear risk | Due to uncertainties raised above. |
| Methods | Parallel‐arm cluster‐RCT conducted at 18 sites/unions in Bangladesh between Jan 2009 and June 2011. | |
| Participants | Sample size: 18 clusters (532,996 population). Clusters: purposeful selection of the 3 districts on the basis of having active Diabetic Association of Bangladesh offices and somewhat representing the social and geographical diversity of Bangladesh..basis of perceived limited access to perinatal health care and feasible accessibility from Diabetic Association of Bangladesh district headquarters. Individuals: women whose childbirths or deaths were recorded in the study areas. | |
| Interventions | Target: community (IEC intervention). Arm 1 (9 clusters, 12,135 women/births): women's participation groups; effect of monthly participatory learning and action cycle focus on maternal and newborn health. Arm 2 (9 clusters, 13,459 women/births): control not described (presumably no women's participation groups). | |
| Outcomes | Trial primary outcome: neonatal mortality rate. Review outcomes reported: Primary: ANC coverage (at least 4 visits). Secondary: health facility deliveries, perinatal and neonatal mortality. 1 of the control areas (with 3 clusters) included "tea‐garden estates". Residents on these estates were described as having more social and economic disadvantage, and separate analyses were carried out including and excluding these areas. For the analyses in this review, we have used the outcome data that excludes these tea garden residents. | |
| Notes | Funders: Big Lottery Fund International Strategic Grant, Wellcome Trust Strategic Award. For the outcome of perinatal mortality we have used stillbirths plus early neonatal deaths. We calculated our own OR using an ICC because the adjusted perinatal deaths OR (without Tea Garden residents) is asymmetrical and would not go into RevMan. See Fottrell 2013 (Table 3, p. 823). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper stated that the sequence "had been decided before drawing the papers" (containing the allocation). |
| Allocation concealment (selection bias) | Low risk | Allocated "by blindly pulling pieces of paper, each representing 1 union from a bottle". |
| Blinding of participants and personnel (performance bias) | Unclear risk | The intervention was not masked, it is not clear how lack of blinding might affect outcomes reported. |
| Blinding of outcome assessment (detection bias) | Low risk | The implementation and in‐country monitoring and evaluation teams were blind to the allocation arms" during interim analysis (June 2011). |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Low risk | Study flow chart included displaying reasons for exclusions. Missing data described as 13% on home delivery practice and 0.8% on other secondary outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Relevant outcomes reported although separate analyses for some control group births meant that results were more difficult to interpret. |
| Analysis bias | Unclear risk | Analysis appropriate for clusters but ICC not reported and ITT analysis only performed for primary outcomes. |
| Other bias | Unclear risk | 1 of the control areas (with 3 clusters) included "tea‐garden estates"; residents on these estates were described as having more social and economic disadvantage and separate analyses were carried out including and excluding these areas. For the analyses in this review, we have used the outcome data that excludes these tea garden residents. |
| Overall risk assessment | Unclear risk | We were uncertain whether some of the above might have significantly biased the results. |
| Methods | Parallel‐arm individual‐randomised RCT conducted at 3 primary care trusts in Birmingham, UK between Jul 2010 and Oct 2011. Trial name: ELSIPS. | |
| Participants | Sample size: 1324 women. Inclusion criteria: nulliparous women < 28 weeks' gestation assessed by a midwife as having specific social risk. (Risk factors included housing problems, lack of social support, smoking, low maternal weight or obesity, teenage, late booking for ANC.) Exclusion criteria: women under 16 years of age, or teenage mothers recruited to another national trial of additional support during pregnancy. | |
| Interventions | Target: health system (re‐organisation of health services: home visits). Arm 1 (662 women): POW provided support, including home visits, in addition to standard ANC and PNC. The POW organised antenatal visits and advised on lifestyle changes. In addition to emotional and health‐related support, the POW helped with financial, legal or benefits problems and with housing. The POW also provided support with care of the newborn, including breastfeeding. Arm 2 (662 women): women in the control group received standard ANC and PNC. | |
| Outcomes | Trial primary outcome: Edinburgh Postnatal Depression Scale1 (EPDS) 8–12 weeks postpartum and antenatal visits attended. Review outcomes reported: Primary: ANC coverage (at least 10 contacts). Secondary: preterm birth (< 34 weeks), low birthweight infants, perinatal mortality. Other: depression scores. We did not include data for preterm birth < 34 weeks because our review's definition of preterm birth < 37 weeks. Outcome data from unpublished paper obtained from author: SL Kenyon, [email protected]. | |
| Notes | Funders: this work was funded by the National Institute for Health Research (NIHR) through the Collaborations for Leadership in Applied Health Research and Care for Birmingham and Black Country (CLAHRC‐BBC) programme. The views expressed in this publication are not necessarily those of the NIHR, the Department of Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation generated by the trial statistician using computer‐generated lists with random block sizes stratified by area. |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation using a registered trial unit ensured allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Inadequate ‐ blinding not possible. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were recorded in maternity care notes by staff providing care. Those who collected and entered data were blind to group assignment. |
| Recruitment bias (for cluster RCTs) | Low risk | Not applicable. Not a cluster‐randomised trial. |
| Incomplete outcome data (attrition bias) | Low risk | Data on antenatal outcomes available for 100% and 99%. Data for the EPDS at 8‐12 weeks postpartum were available for 82% and 85% of the intervention and control arms. respectively. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in protocol are reported in the unpublished paper, with the exception of "engagement with other services, as required (e.g. smoking cessation services)". |
| Analysis bias | Low risk | ITT analysis performed. |
| Other bias | Unclear risk | Baseline characteristics similar between treatment groups. |
| Overall risk assessment | Low risk | No serious risk of bias concerns for primary outcomes. |
| Methods | Parallel‐arm cluster‐RCT conducted in Ghana between Nov 2008 and Dec 2009. | |
| Participants | Sample size: 98 clusters (18,609 individuals). Clusters: residential zones. Individuals: all pregnant women and newborn babies living in the Newhints zones, where pregnancies ended between November 2008 and December 2009. | |
| Interventions | Target: health system (added home visits by community‐based surveillance volunteers) and community (IEC). Arm 1 (49 clusters, 9174 women): training of community‐based surveillance volunteers to identify pregnant women in their community and to undertake 2 home visits during pregnancy and 3 visits after birth on days 1, 3, and 7, to promote essential newborn‐care practices, and to assess and refer sick newborn babies. Arm 2 (49 clusters, 9435 women): control (no intervention). | |
| Outcomes | Trial primary outcome: neonatal mortality rate and coverage of key essential newborn‐care practices. Review outcomes reported: Primary: ANC coverage (at least 4 visits). Secondary: health facility deliveries, neonatal mortality. Other: coverage of key essential newborn care practices. | |
| Notes | Funders: WHO, Save the Children’s Saving Newborn Lives Programme from the Bill & Melinda Gates Foundation, and the UK Department for International Development. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomisation." |
| Allocation concealment (selection bias) | Low risk | An independent epidemiologist conducted the randomisation. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Low risk | 3 groups of pregnancies were not included in the analysis of NMR: 908 (5%) women were lost to follow‐up during pregnancy; 1216 (7%) had pregnancies that ended early and did not result in a livebirth or stillbirth; and 156 (< 1%) women moved, resulting in a change of treatment groups. |
| Selective reporting (reporting bias) | Low risk | Most relevant outcomes reported. |
| Analysis bias | Low risk | Analysis appropriate for clusters; ICC reported; ITT analysis performed. |
| Other bias | Low risk | No baseline imbalances noted. |
| Overall risk assessment | Low risk | No serious risk of bias concerns. |
| Methods | Parallel‐arm individually‐randomised RCT conducted in the USA between Mar 94 and Jun 98. | |
| Participants | Sample size: 656 women. Inclusion criteria: African American, eligible for Medicaid, less than 26 weeks' gestation, at least 16 years old, score of 10 or higher on a risk assessment scale. Exclusion criteria: alcoholism and substance abuse, asthma, cancer, diabetes, epilepsy, high blood pressure, sickle cell disease and HIV/AIDS. | |
| Interventions | Target: health system (additional and longer appointments) and community (IEC). Arm 1: educational intervention informing women about their risk conditions and what behaviours might improve their pregnancy outcome. Augmented care included educationally oriented peer groups, additional appointments, extended time with clinicians, and other supports. Arm 2: control (no intervention). | |
| Outcomes | Trial primary outcomes: pregnancy outcomes, women's knowledge of risks, satisfaction with care. Review outcomes reported: Primary: not reported. Secondary: preterm birth, low birthweight infants. Other: average no. of ANC visits. | |
| Notes | Funders: Federal Agency for Health Care Policy and Research to the University of Alabama at Birmingham, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Low risk | "interviewers blinded" to treatment allocation. Additional outcome data taken from clinic records, data collection forms and a computerised database. |
| Recruitment bias (for cluster RCTs) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Missing data < 10%. 656 women enrolled, but data available for 619; 12 women with fetal deaths excluded from analysis (intervention group: 3 before 20 weeks and 4 after; controls: 3 before 20 weeks and 2 after). |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported. |
| Analysis bias | Unclear risk | ITT not stated. |
| Other bias | Low risk | No baseline imbalances. |
| Overall risk assessment | Low risk | No serious risk of bias concerns. |
| Methods | Parallel 3‐arm cluster‐RCT conducted in India between Jan 2004 and May 2005. | |
| Participants | Sample size: 39 clusters (3891 individuals analysed). Clusters: administrative units. Individuals: all mothers who had delivered during the study period and were available for interview. | |
| Interventions | Target: health system (home visits) and community (IEC). Arm 1 (13 clusters): a preventive package of interventions for essential newborn care (birth preparedness, clean delivery and cord care, thermal care [including skin‐to‐skin care], breastfeeding promotion, and danger sign recognition). The strategy included 2 prenatal (60 days and 30 days before expected date of delivery) and 2 postnatal (day 0 and day 3) home visits, community meetings and folk‐song meetings, maternal and newborn health stakeholder meetings, and meetings for community volunteers. Arm 2 (13 clusters): received same package of essential newborn care plus use of a liquid crystal hypothermia indicator (ThermoSpot; a sticker that indicates hypothermia in the newborn by changing colour). Arm 3 (13 clusters): received the standard care available from government and NGO providers in the area. | |
| Outcomes | Trial primary outcome: newborn care practices and neonatal mortality rate. Review outcomes reported: Primary: not reported. Secondary: maternal mortality (up to 6 weeks postpartum), ANC coverage (at least 1 visit), health facility deliveries, tetanus protection, stillbirths, neonatal mortality. Other: essential newborn care measures, breastfeeding. | |
| Notes | Funders: The United States Agency for International Development, Delhi Mission, and the Saving Newborn Lives program of Save the Children US through a grant from the Bill and Melinda Gates Foundation. Perinatal mortality included neonatal deaths up to 28 days after birth. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified cluster‐randomisation conducted at Johns Hopkins University using a computer program. |
| Allocation concealment (selection bias) | Low risk | Allocation performed remotely. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not possible. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Preliminary analysis (2005) of neonatal mortality rate was said to be masked. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up described in study flow diagram with missing data < 20%. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported. |
| Analysis bias | Low risk | Analysis appropriate for clusters; no ICC reported; ITT analysis performed. |
| Other bias | Low risk | No baseline imbalances. |
| Overall risk assessment | Low risk | No serious risk of bias concerns. |
| Methods | 3‐arm, individually‐randomised RCT conducted in Detroit, USA; recruitment dates not reported. | |
| Participants | Sample size: 205 individuals. Inclusion criteria: low‐income women who entered prenatal care at a local clinic before 32 weeks' gestation and who delivered at a tertiary‐level hospital. Exclusion criteria: not reported. | |
| Interventions | Target: community (financial incentive intervention). Arm 1 (51 women): women received gift certificates for each prenatal appointment. Arm 2 (53 women): women received gift certificates and a chance to win in a $100 raffle. Arm 2 (101 women): no financial incentive. Women in all 3 groups were offered $10 for the postnatal interview. | |
| Outcomes | Triap Primary Outcome: kept appointments for antenatal care and postpartum care Review outcomes reported: Primary: ANC coverage (at least 4 visits). Secondary: maternal mortality, health facility deliveries, perinatal mortality. | |
| Notes | Funders: Michigan Health Care Education and Research Foundation. Authors provided unpublished data for coverage, mortality and health facility deliveries by email on 16/1/15. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random numbers were used" for group assignment. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Clinic staff members were blind to assignment, but women would have been aware of their own assignment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. |
| Recruitment bias (for cluster RCTs) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data < 20% for birth outcomes (low risk) but high loss to follow‐up at the postnatal interview (45%). |
| Selective reporting (reporting bias) | Unclear risk | Relevant outcomes reported, but insufficient data provided. |
| Analysis bias | Unclear risk | Methods not reported in sufficient detail. |
| Other bias | Unclear risk | Insufficient information to make a judgement. |
| Overall risk assessment | Unclear risk | Insufficient information to make a judgement. |
| Methods | Parallel‐arm cluster RCT conducted at 26 sites in South Africa between May 2009 and Sept 2010. | |
| Participants | Sample size: 26 clusters (24 clusters and 1238 individuals analysed). Clusters: neighbourhoods were matched. Eligible neighbourhoods had 450‐600 households, with formal and informal housing, that were within 5 km of health clinics; had 5 to 7 alcohol bars; were noncontiguous or separated by natural barriers; had similar numbers of child care centres, informal shops, and schools; and had households with similar length of residence. Individuals: pregnant women were recruited at an average 26 weeks of pregnancy (range, 3–40 weeks). 94 women in 10 of the 12 standard care neighbourhoods were enrolled post‐birth. Approximately 25% of women in each treatment arm were living with HIV. | |
| Interventions | Target: health system (added home visits) and community (IEC). Arm 1 (12 clusters, 644 women): Philani Intervention Program, home visits by CHWs in addition to standard care. Arm 2 (12 clusters, 594 women): standard care, comprehensive healthcare at clinics. | |
| Outcomes | Trial primary outcomes: composite of maternal and child health and well being measures. Review outcomes reported: Primary: ANC coverage (at least 4 visits). Secondary: HIV screening, complete antiretroviral course, low birthweight infants. Mortality data for women and infants are reported in the primary trial report. However, these data represent all deaths within the particular time frame of data collection (e.g. all deaths to 6 months post birth, p. 1464 of the 2013 trial report). We consulted with trial authors, but we cannot recalculate the mortality data to fit standard definitions for pregnancy‐related deaths or perinatal deaths. We were particularly concerned that maternal deaths may have been unrelated to pregnancy. We were therefore unable to use mortality data in meta‐analysis. | |
| Notes | Funders: NIAAA Grant # 1R01AA017104 and supported by NIH grants MH58107, 5P30AI028697, and UL1TR000124. M.T. is supported by the National Research Foundation (South Africa). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Method described as simple randomisation. |
| Allocation concealment (selection bias) | Low risk | Neighbourhoods were randomised in matched pairs using simple randomisation. Randomisation conducted by an independent research team (UCLA). |
| Blinding of participants and personnel (performance bias) | Unclear risk | "interviewers were blinded but may have known from participants about CHWs." |
| Blinding of outcome assessment (detection bias) | Low risk | A driver transported all participants to a central assessment site, allowing interviewers to be blind to group allocation. |
| Recruitment bias (for cluster RCTs) | High risk | "Initially, however, we identified 22% fewer pregnant women in standard care. By redeploying recruiters, we identified an additional 94 women in 10 of the 12 standard care neighbourhoods who were pregnant during the recruitment period (median of 7 late‐entry participants per neighbourhood; range, 3–24). These women were enrolled post‐birth when their infants were a mean age of 9 months old (range, 1–18 months). The final sample (n = 1238) consisted of a median of 51 pregnant women per neighbourhood (range, 23–72)." Analyses were conducted with and without late‐entry participants and results were similar. |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 matched cluster pair was excluded after 6 months due to poor recruitment. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were reported. |
| Analysis bias | Unclear risk | Analysis appropriate for clusters; no ICC reported; ITT analysis not performed. |
| Other bias | Unclear risk | Not reported. |
| Overall risk assessment | Unclear risk | We were uncertain what impact potential biases mentioned above had on results. |
| Methods | 2 by 2 factorial cluster‐RCT conducted in Malawi between 2005 and 2009. | |
| Participants | Sample size: 42 clusters (18960 pregnancies, 18,744 livebirths analysed). Clusters: the unit of randomisation was a cluster of villages and not an individual village. Cluster design was based on census enumeration areas with population of approximately 3000, surrounded by a buffer zone to reduce contamination. The target population was rural communities; the urban administrative centre of the district was excluded. Individuals: all women aged 10‐49 who were willing to participate were enrolled. Women who had a terminal family planning procedure were excluded from the final sample, but not from participating in the intervention. | |
| Interventions | Target: community (IEC). Arm 1 (12 clusters, 4557 pregnancies): facilitator initiated women's groups to discuss issues of pregnancy, childbirth and newborn and infant health, as well as peer counselling (infant feeding and care counselling via 5 home visits during and after pregnancy (3rd trimester, week after birth, at 1, 3 and 5 months). Arm 2 (12 clusters, 4722 pregnancies): facilitated women's groups. Arm 3 (12 clusters, 4660 pregnancies): peer counselling via home visits. Arm 4 (12 clusters, 5021 pregnancies): no intervention. All clusters benefited from training of staff in health facilities in essential newborn care. | |
| Outcomes | Trial primary outcomes: maternal, perinatal, neonatal and infant mortality rates, and exclusive breastfeeding. Review outcomes reported: Primary: ANC coverage (at least 4 visits), maternal mortality. Secondary: ANC coverage (at least 1 visit), health facility deliveries, IPT for malaria, tetanus protection, HIV screening, perinatal mortality, neonatal mortality. | |
| Notes | Funders: Saving Newborn Lives, UK Department for International Development, Wellcome Trust, Institute of Child Health, and UNICEF Malawi. The primary trial report presents several different analyses, including 1 where Interventions were combined, in order to evaluate the effect of women's groups (arm 1 + 2 combined versus arm 3 + 4 combined) and the effect of peer counselling (arm 1 + 3 combined versus arm 2 + 4 combined) separately. For the analysis in our review's Comparison 1: Lewycka 2013a refers to the women's group intervention only. Lewycka 2013b refers to the peer counselling intervention only. These 2 single‐intervention arms are compared to the arm with no intervention. For the analysis in our review's Comparison 2: Lewycka 2013a refers to the trial arm that received both women's groups and peer counselling. This arm is compared to the arm with no intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done with computer program Stata. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Group assignment was masked for data analysis. Data collection was conducted independently of program implementation and was not fed back to inform the intervention. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Unclear risk | Women with miscarriages were excluded from analysis. Loss to follow‐up about 20%. Miscarriage rates varied across study arms and were more frequent in the combined intervention cluster. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported. |
| Analysis bias | Low risk | Analysis appropriate for clusters; no ICC reported; ITT analysis performed. |
| Other bias | Unclear risk | The authors discuss an interaction between the 2 interventions and baseline imbalances after randomisation across several outcomes. |
| Overall risk assessment | Unclear risk | We were concerned that the exclusion of women with miscarriages might bias maternal death rates. |
| Methods | 2 by 2 factorial cluster RCT conducted in Malawi between 2005 and 2009. Lewycka 2013b describes the same trial as Lewycka 2013a above, and all of the descriptions and risk of bias are identical to that above. We have had to duplicate the 'Risk of bias' judgements below due to RevMan requirements. | |
| Participants | For the analysis in our review's Comparison 1: Lewycka 2013a refers to the women's group intervention only. Lewycka 2013b refers to the peer counselling intervention only. These 2 single‐intervention arms are compared to the arm with no intervention. | |
| Interventions | See Lewycka 2013a. | |
| Outcomes | See Lewycka 2013a. | |
| Notes | See Lewycka 2013a. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done with computer program Stata. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Group assignment was masked for data analysis. Data collection was conducted independently of program implementation and was not fed back to inform the intervention. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Unclear risk | Women with miscarriages were excluded from analysis. Loss to follow‐up about 20%. Miscarriage rates varied across study arms and were more frequent in the combined intervention cluster. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported. |
| Analysis bias | Low risk | Analysis appropriate for clusters; no ICC reported; ITT analysis performed. |
| Other bias | Unclear risk | The authors discuss an interaction between the 2 interventions and baseline imbalances after randomisation across several outcomes. |
| Overall risk assessment | Unclear risk | We were concerned that the exclusion of women with miscarriages might bias maternal death rates. |
| Methods | A parallel arm cluster‐RCT conducted on the island of Unguja, Zanzibar, Tanzania between Mar 2009 and Mar 2010. | |
| Participants | Sample size: 24 clusters (2367 individuals randomised, 2550 analysed). Clusters: government‐run primary healthcare facilities, 4 per district, were selected, based on 2 inclusion criteria: highest number of ANC clients in 2008 and the availability of at least 1 midwife in the facility. All included facilities had mobile phone network coverage. Individuals: women who attended ANC at 1 of the 24 selected healthcare facilities were included on their first ANC visit and followed until 42 days after delivery. Women were eligible for study participation irrespective of their mobile phone and literacy status. Exclusion criteria: PIH, anaemia, multiple pregnancy and malpresentation. | |
| Interventions | Target: health system (policy/practice change). Arm 1 (12 clusters, 1351 women): women received an automated text messaging service for health information and appointment reminders, mobile phone vouchers to enable women to contact health services. The content of messages depended upon gestational age. Women received 2 messages/month < 36 weeks and then 2 per week. Only women with registered phone numbers received text messages; women without received only vouchers with mobile credit. Arm 2 (12 clusters 1286 women): women attending control health facilities received standard ANC, with the goal of at least 4 ANC visits. | |
| Outcomes | Trial primary outcome: skilled delivery attendance. Review outcomes reported: Primary: ANC coverage (at least 4 visits), maternal mortality. Secondary: tetanus protection, perinatal mortality, neonatal mortality. Other: skilled birth attendant (midwife/doctor/nurse) at delivery. Follow‐up: women were offered at least 4 antenatal visits and a postnatal home visit within 48 hours after delivery. Women were interviewed for demographics at trial entry at 6 weeks after delivery. The trial definition of perinatal mortality is non‐standard, stated as stillbirth and death of the infant up to 42 days. | |
| Notes | Funders: Danish International Development Cooperation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation described as 'simple randomisation' but sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Clusters and study participants were not masked due to the nature of the intervention requiring overt participation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Low risk | Exclusions/withdrawals < 20% were due to exclusion criteria (development of complications) or loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported. |
| Analysis bias | Low risk | Analysis appropriate for clusters, ITT analysis was performed. No ICC reported. |
| Other bias | Unclear risk | No baseline imbalances noted. The trial definition of perinatal mortality is non‐standard, stated as stillbirth and death of the infant up to 42 days. |
| Overall risk assessment | Low risk | No serious risk of bias concerns. |
| Methods | Parallel arm cluster‐RCT conducted in a rural setting in Zimbabwe between Jan 1995 and Oct 1997. | |
| Participants | Sample size: 23 clusters (13517 individuals). Clusters: health centres in a rural setting. Gutu district was chosen as the study area because the utilisation of maternity services and reproductive health status of the community had been previously studied.The district had 25 health facilities, comprising a district hospital and 24 rural health centres (RHCs) serving a population of 195 000. Individuals: all mothers booking for ANC since 01/12/94. | |
| Interventions | Target: health system (re‐organisation of ANC). Arm 1: an experimental package of ANC with reduced procedures, clear goals and symphysio‐fundal height measurements. Visits scheduled according to a 5 visit program with reduced routine procedures at these visits. First visit: risk assessment, health education and delivery plan, Hb, rapid plasma reagin, tetanus vaccination and do urinalysis. Visit 2 at 24–28 weeks: exclude multiple pregnancy, check for hypertensive disorders, and do urinalysis. Visit 3 at 32–34 weeks: Exclude anaemia, check fetal growth and review delivery plans, check Hb and do urinalysis. Visit 4 at 36–38 weeks: check fetal growth, exclude abnormal presentation, discuss labour and do urinalysis. Visit 5 at 40–41 weeks: check fetal well being, referral for post‐term induction at 42 weeks and do urinalysis. Arm 2: the control arm followed the standard schedule with a visit every 4 weeks from booking until 28 weeks, every 2 weeks between 28 and 36 weeks and weekly after 36 weeks until delivery. Risk assessment was performed at the booking and subsequent visits, and referral for hospital delivery was made using a list of risk markers recommended by the Zimbabwe Ministry of Health and Child Welfare. Blood pressure, body weight and urinalysis were measured at each visit, while Hb and syphilis test (RPR) were performed at the first visit. Women who tested positive for syphilis had treatment initiated at the booking visit. Oral iron supplementation was provided to all women in both models. | |
| Outcomes | Trial primary outcomes: number of antenatal visits, referrals for antenatal, intrapartum or postpartum problems, place of delivery and low birthweight infant (< 2500 g). Review outcomes reported: Primary: maternal mortality. Secondary: health facility deliveries, preterm birth, perinatal mortality, neonatal mortality. | |
| Notes | Funders: Swedish International Development Cooperation Agency (Sida/SAREC) through the Sida–University of Zimbabwe Reproductive Health Research Programme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. Randomisation described as stratified according to the availability of telecommunication for referrals. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not blinded. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Low risk | 13,517 women randomised. Full records were available for 78% of women with curtailed follow‐up for an additional 20%. Communication with the authors has clarified the numbers used for perinatal deaths, adding back in many women whose records were not retrieved. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported. The authors were contacted to clarify the numbers used to calculate perinatal death. |
| Analysis bias | Low risk | Analysis appropriate for clusters; no ICC reported; ITT analysis performed. |
| Other bias | Low risk | No baseline imbalances. |
| Overall risk assessment | Low risk | No serious risk of bias concerns after contacting authors with data queries. |
| Methods | Parallel arm cluster‐RCT conducted in a rural setting in Nepal between Sept 1999 and Nov 2003. | |
| Participants | Sample size: 24 clusters (28931 individuals). Clusters: Rural Village Development Committees were matched for geography, population and ethnicity; 12 pairs were randomised. Individuals: married women aged 15‐49 residing within the study area who could potentially conceive within the period of the study. Exclusion criteria: unmarried women, permanently separated or widowed women; women under the age of 15 or older than 49. | |
| Interventions | Target: community (IEC intervention). Arm 1 (12 clusters, 14,884 women): participatory women's groups facilitated by a female facilitator who convened 9 women's group meetings every month. The facilitator supported groups through an action learning cycle in which they identified local perinatal problems and formulated strategies to address them. Arm 2 (12 clusters, 14,047 women): health service strengthening activities were undertaken in both intervention and control areas. These improvements included provision of equipment. | |
| Outcomes | Trial primary outcome: neonatal mortality rate. Review outcomes reported: Primary: not reported. Secondary: ANC coverage (at least 1 visit), health facility deliveries, perinatal mortality, neonatal mortality. The adjusted OR for maternal mortality was taken directly from the systematic review Prost 2013. | |
| Notes | Funders: DFID, with important support from the Division of Child and Adolescent Health, WHO, the United Nations Children's Fund, and the United Nations Fund for Population Activities. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence to randomised pairs was from a random numbers list; to randomise within pairs a coin toss was used. |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was generated centrally (Kathmandu) before enrolment of participants in relevant clusters. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor was blinded to group allocation. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Low risk | All clusters analysed. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported. |
| Analysis bias | Unclear risk | Analysis appropriate for clusters; ITT analysis performed; ICC not reported. |
| Other bias | Low risk | No baseline imbalances noted. |
| Overall risk assessment | Low risk | No serious risk of bias concerns. |
| Methods | Parallel arm, individually‐randomised RCT conducted at 5 sites in the USA between Jan 94 and Dec 95. | |
| Participants | Sample size: 104 women. Inclusion criteria: all pregnant women planning to attend prenatal care at 1 of 5 participating family planning and women's health clinics. All randomised women were eligible for the state of California's Medicaid program (assistance with healthcare costs). Exclusion criteria: women planning prenatal care at another location or women considering abortion. | |
| Interventions | Target: community (financial incentive). Arm 1: 34 women were randomised to receive at taxi voucher and 35 received a coupon for a baby blanket. Arm 2: the control group received no incentive to attend the first antenatal appointment. | |
| Outcomes | Trial primary outcome: compliance with the first prenatal appointment. Review outcomes reported: Primary: not reported. Secondary: not reported. | |
| Notes | Funders: funded in part by a grant from the American Academy of Family Physicians' Foundation. No usable data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence from a table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were prepared remotely from clinics. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Women were not told that the study had to do with appointment compliance. It is not clear if staff were aware of group assignment. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes assessors for the primary outcome were blind to group assignment. |
| Recruitment bias (for cluster RCTs) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) | High risk | 22/69 (32%) in the intervention group lost to follow‐up and excluded; 8/35 in controls. |
| Selective reporting (reporting bias) | Unclear risk | No review outcomes reported. |
| Analysis bias | Low risk | ITT analysis performed. |
| Other bias | Low risk | No baseline imbalances noted. |
| Overall risk assessment | Unclear risk | High loss to follow‐up with no relevant review outcomes. |
| Methods | Parallel arm cluster‐RCT conducted in Balochistan, Pakistan. A baseline survey took place in Aug‐Sept 1998. Intervention package in place by March 2000; follow‐up survey was conducted between March and April 2002. | |
| Participants | Sample size: 32 clusters (2561 individuals analysed). Clusters: Balochistan is an underdeveloped and poor region of Packistan with the highest maternal mortality rate. Each eligible village cluster had between 5‐15 villages. The project area was divided into 3 zones based on distance from the district hospital. Randomisation took place within each zone. Individuals: women who had had a pregnancy in the last 12 months. | |
| Interventions | Target: health system (health worker education and re‐organisation of services ‐ transport) community (IEC intervention). Arm 1: women were provided information on safe motherhood through pictorial booklets and audiocassettes; TBAs were trained in clean delivery and recognition of obstetric and newborn complications; and emergency transportation systems were set up. The intervention was delivered to women only in 1 group and to both women and husbands in another. Arm 2: the project provided training for health professionals at the district hospital, who provided care for women from both intervention and control clusters. Government healthcare providers also trained staff in primary health facilities throughout the study area. | |
| Outcomes | Trial primary outcomes: perinatal or neonatal death; use of iron‐folic acid. Review outcomes reported: Primary: not reported. Secondary: ANC coverage (at least 1 visit), health facility deliveries, tetanus protection, perinatal mortality, neonatal mortality. The perinatal death outcome seems to have been calculated with live births rather than all births. For our analyses, we have used data from the 2002 follow‐up survey only. | |
| Notes | Funders: NICHD, USAID, UNICEF, World Health Organization, British Council, Government of Japan and The Asia Foundation, and implemented by The Asia Foundation’s Islamabad office. Residual impact survey conducted 2 years after project ended, in 2004; on a sample of 900 women randomly selected from immunisation records at the district health office. We have used data from the original cluster‐randomised trial only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by "blindly drawing village cluster names written on folded chits". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Recruitment bias (for cluster RCTs) | Unclear risk | At the 2002 follow‐up survey, intervention clusters were expanded, resulting in 47% increase in size of the control arm. At the 2004 follow‐up survey, refusals or locked households in a selected cluster were replaced by the nearest available household. |
| Incomplete outcome data (attrition bias) | Low risk | The follow‐up survey interviews were completed for 95.2% of visited households. |
| Selective reporting (reporting bias) | Unclear risk | Relevant outcomes were reported. |
| Analysis bias | Unclear risk | Analysis appropriate for clusters; ICC reported; ITT not stated. |
| Other bias | Unclear risk | Information bias. Most of the results are from the 2002 follow‐up survey, but authors state that some data are from the 2004 survey. |
| Overall risk assessment | Unclear risk | We were uncertain whether the risk of bias concerns above might have impacted the results. |
| Methods | A parallel arm cluster‐RCT conducted in India between Oct 2006 and Sept 2009. | |
| Participants | Sample size: 48 clusters (18,197 individuals). Clusters: eligible clusters were communities in urban slums in Mumbai for which a perinatal vital registration was set up as part of the City Initiative for Newborn Health in 2005. The wards were selected purposively for the 2005 Initiative based on accessibility and relative infant mortality rates. Communities with transient populations and areas where resettlement was being negotiated were both excluded. Individuals: women of all ages residing in intervention clusters, whether pregnant or not pregnant, were invited to attend women's groups. | |
| Interventions | Target: community (IEC intervention). Arm 1: women were invited to weekly meetings that emphasized knowledge of local health services, perinatal health care, and negotiating optimal care with family and health providers. Arm 2: no weekly meetings. Surveillance data were collected in both intervention and control areas. 12 interviewers collected these data at 6 weeks postpartum. Unwell mothers or infants in either arm were referred and treatment expedited. | |
| Outcomes | Trial primary outcome: perinatal care, maternal morbidity, and extended perinatal mortality. Review outcomes reported: Primary: maternal mortality. Secondary: professional ANC, health facility delivery, perinatal mortality, neonatal mortality. | |
| Notes | Funders: ICICI Foundation for Inclusive Growth – Centre for Child Health and Nutrition, and the Wellcome Trust. DO was funded by a Wellcome Trust Fellowship (081052/Z/06/Z). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by "drawing of lots". |
| Allocation concealment (selection bias) | Unclear risk | Allocation not concealed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Interviewers may have been aware of the assignment of their particular area, but the authors argue that they "were focused on their task (surveillance) and did not dwell on the comparative nature of the trial." Data analysts were blinded. |
| Recruitment bias (for cluster RCTs) | Unclear risk | 9 clusters were expanded for insufficient births, and 2 clusters reduced for excess births. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was less than 20% in each arm, and authors have provided a study flow diagram with documented reasons for loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were reported. |
| Analysis bias | Low risk | Analysis appropriate for clusters; ITT analysis performed; ICC not reported. |
| Other bias | Unclear risk | Other initiatives during the trial period include outreach services by health volunteers, birth registration and pulse polio campaigns and infectious disease surveillance. Conditions in slums improved over the trial period. |
| Overall risk assessment | Unclear risk | We were uncertain whether the risk of bias concerns above might have impacted the results. |
| Methods | Cluster‐RCT in Bulgan, Mongolia. | |
| Participants | Sample size: 501 women randomised. Clusters: the unit of randomisation was the Soum and bag, small geographic areas in Mongolia. Each Soum has a healthcare facility where women must register their newborn. 18 geographic areas were randomised, after selection for administrative convenience and to avoid contamination. Individuals: pregnant women living in Bulgan, Mongolia. | |
| Interventions | Target: community. Arm 1: distribution of maternal and child health handbooks during pregnancy. The MCH handbook logged maternal health and personal information, pregnancy, delivery and postpartum health and weight, dental health, parenting classes, child developmental milestones from 0‐6 years, immunisation records and height and weight charts for children. Arm 2: women received standard care. | |
| Outcomes | Trial primary outcome: number of antenatal visits; proportion of women attending 6 or more antenatal visits. (The national standard for ANC in Mongolia is 6 visits.) Review outcomes reported: Primary: ANC coverage of at least 4 visits, maternal mortality Secondary: maternal outcomes: morbidity during pregnancy, mode of delivery, breastfeeding initiation, maternal depression and health (EPDS and GHQ). Infant outcomes: birthweight, Apgar score, NICU admission, neonatal mortality at discharge. Maternal healthy behaviours. Follow‐up: data collection at 1 month postpartum. | |
| Notes | Funders: this study was funded by the National Center for Global Health and Medicine, Tokyo, Japan. Significant group differences noted for distances travelled to nearest health centre (greater in the intervention group) and for wealth index (the control group was poorer). The authors report that travel time did not function as an effect modifier; however, women from a higher socioeconomic background attended more ANC visits. Trial authors provided unpublished outcome data upon request. The trial statistician (HN) calculated ORs and 95% confidence intervals using the generalised estimating equations (GEE) method to adjust for cluster design and baseline differences, including wealth. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence according to the shuffling of sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed envelopes at time of randomisation. All areas were randomised at the same time. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Masking was not possible for this intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Masking was not possible for this intervention. |
| Recruitment bias (for cluster RCTs) | Low risk | No problems with recruitment are reported. |
| Incomplete outcome data (attrition bias) | Low risk | 3 randomised areas were excluded; 1 was the subject of a pilot study, and 2 areas were included in another health study. 9 clusters each received the intervention or the control. Missing outcome data for individual women is reported and minimal. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes have been reported. Addtional analyses were obtained from the authors upon request. The trial data file has been published online with the trial report. |
| Analysis bias | Low risk | Analyses were undertaken with methods appropriate for cluster trials; the authors used GEE methods to adjust for the effects of cluster design and baseline variables. A sample size calculation was undertaken and met. |
| Other bias | Unclear risk | The authors reported baseline imbalances between clusters for travel time to health centre and wealth. The authors reported that recall bias may exist due to data collection at 1 month after birth. |
| Overall risk assessment | Low risk | Overall the trial was well planned and conducted. |
| Methods | Parallel arm cluster‐RCT conducted in Honduras between Aug 2000 and Oct 2002. | |
| Participants | Sample size: 70 clusters (˜5600 households). Clusters: municipalities, which were selected because they had the highest prevalence of malnutrition in the country. Individuals: women were eligible who had been pregnant during the previous 12 months but were not pregnant on the day of the interview. | |
| Interventions | Target: health system (financial resources to health team and training) and community (financial incentive and IEC). Arm 1: 1 (20 clusters): a household‐level package consisted of monetary vouchers paid to women in households whose residence in the beneficiary municipalities had been recorded in a special census done in mid‐2000. 3 (20 clusters): financial resources to local health teams combined with a community‐based nutrition intervention involving the training of lay nutrition promoters. 2 (10 clusters): both packages. Arm 2 (20 clusters): neither package. | |
| Outcomes | Trial primary outcome: use of health services. Review outcomes reported: Primary: ANC coverage (at least 4 visits). Secondary: tetanus protection. | |
| Notes | Funders: Government of Honduras. We have calculated a final score by adding the change scores to the baseline scores presented in the trial report, Table 2 Program Effects, p. 2034. For our review's Comparison 1: Morris 2004a is the 2 single intervention trial arms added together and compared with the control group. For our review's Comparison 2: Morris 2004b is the 'both packages' trial arm compared with the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Within each stratum, random allocation was achieved by a child drawing coloured balls from a box, without replacement. Thus, the randomisation was both stratified and blocked." |
| Allocation concealment (selection bias) | Low risk | "The aperture of the box was sufficiently small that once the child had inserted his or her arm, it was impossible for him or her to see the coloured balls. From the day of the randomisation onwards, there was no attempt to conceal the allocation." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Surveys were conducted by an independent data collection company. Not clear if individual interviewers would have been aware of cluster assignment. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Low risk | Study flow chart included. Loss to follow‐up less than 5% in all arms. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported. |
| Analysis bias | Low risk | Analysis appropriate for clusters (no ICC reported); ITT analysis performed. |
| Other bias | High risk | The intervention involving direct transfer of resources to health teams and part of the service‐level package was not successfully implemented in the relevant clusters. Non baseline imbalances noted. |
| Overall risk assessment | Unclear risk | We were uncertain whether the risk of bias concerns relating to poor implementation impacted the results. |
| Methods | This trial is the same as that described in Morris 2004a above. Due to RevMan requirements, we have replicated the 'Risk of bias' assessments below. However, Morris 2004b describes a specific 'both packages' arm of Morris 2004a and not a different study. | |
| Participants | For our review's Comparison 2, Morris 2004b is the 'both packages' trial arm compared with the control group. | |
| Interventions | See Morris 2004a. | |
| Outcomes | See Morris 2004a. | |
| Notes | See Morris 2004a. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Within each stratum, random allocation was achieved by a child drawing coloured balls from a box, without replacement. Thus, the randomisation was both stratified and blocked." |
| Allocation concealment (selection bias) | Low risk | "The aperture of the box was sufficiently small that once the child had inserted his or her arm, it was impossible for him or her to see the coloured balls. From the day of the randomisation onwards, there was no attempt to conceal the allocation." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Surveys were conducted by an independent data collection company. Not clear if individual interviewers would have been aware of cluster assignment. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Low risk | Study flow chart included. Loss to follow‐up less than 5% in all arms. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported. |
| Analysis bias | Low risk | Analysis appropriate for clusters (no ICC reported); ITT analysis performed. |
| Other bias | High risk | The intervention involving direct transfer of resources to health teams and part of the service‐level package was not successfully implemented in the relevant clusters. Non baseline imbalances noted. |
| Overall risk assessment | Unclear risk | We were uncertain whether the risk of bias concerns relating to poor implementation impacted the results. |
| Methods | A parallel, 3‐arm RCT conducted at 1 site in Nepal between Aug 2003 and Jan 2004. | |
| Participants | Sample size: 299 women and 145 couples. Inclusion criteria: currently married women attending their first ANC visit at PGMH (gestational age 16–28 weeks) whose husbands were present at the hospital compound were eligible. Exclusion criteria: women were excluded if they were < 18 years of age or lived > 90 min away from the PGM Hospital. | |
| Interventions | Target: Community (IEC). Arm 1: 2 35 minute health education sessions administered in a private room, for women only, 4‐6 weeks apart, plus a detailed health education flier. Arm 2: 2 35 minute health education sessions administered in a private room, for women and their husbands, 4‐6 weeks apart, plus the detailed health education flier. Arm 3: a brief flier with standardised health messages. Women in the control groups received standard ANC. | |
| Outcomes | Trial primary outcomes: maternal health care utilisation and birth preparedness. Review outcomes reported: Primary: ANC coverage (at least 4 visits). Secondary: health facility deliveries. | |
| Notes | Funders: Hopkins Population Center Dissertation Fieldwork Grant, awarded by the Andrew Mellon Foundation, and a grant awarded by the Bill and Melinda Gates Institute for Population and Reproductive Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using the statistical software program Stata 8.0 (Stata Corp., College Station, TX, USA), a list was generated randomizing the sequence of recruitment of study groups for each day of the recruitment period." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of research assistants administering questionnaires not described. Data entry and coding described as blinded. |
| Recruitment bias (for cluster RCTs) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Data for all women (442) for final ANC visit; data for 386 (87%) for postnatal questionnaire. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported. |
| Analysis bias | Low risk | ITT analysis performed. |
| Other bias | Low risk | No baseline imbalances noted. |
| Overall risk assessment | Low risk | No serious risk of bias concerns noted. |
| Methods | Parallel arm cluster‐RCT conducted at 10 sites in Pakistan (Sindh province) between Jun 2000 and April 2001. | |
| Participants | Sample size: 10 clusters (1070 women interviewed, 969 households visited). Clusters: 10 enumeration areas from 3 districts in the Sindh province of Pakistan were chosen. 8 of the 10 areas were rural. Individuals: women who were pregnant or had delivered in the past 3 years were eligible. | |
| Interventions | Target: health system (change in health worker practice) and community (IEC). Arm 1 (5 clusters, 529 women): a LHW showed an evidence‐based tool and embroidered cloth that depicted 3 important maternal practices, viz. attending antenatal check‐ups, giving colostrum after birth and avoiding heavy work. Arm 2 (5 clusters, 541 women): the LHW delivered standard care. | |
| Outcomes | Trial primary outcome: health practices during pregnancy, including ANC. Review outcomes reported: Primary: not reported. Secondary: ANC coverage (at least 1 visit), professional (LHW) ANC. Other: giving colostrum at birth, stopping heavy work, exclusive breastfeeding for 4 months. | |
| Notes | Funders: Canadian International Development Agency International Development Agency’s (CIDA) Canada Fund for Local Initiatives in Pakistan. Data for ANC visits were not reported by randomisation group and not usable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence from computerised random numbers generator. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not possible due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Fieldworkers collecting data were blinded to the allocation of the community in which they worked but women may have revealed which group they were in if they mentioned the embroideries. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Unclear risk | The total number of women visited by LHW is not stated, only households visited. It is not clear how many households refused the survey or whether there were women approached who refused the interview. There seem to me more women with data for visits than there were households visited or women interviewed. |
| Selective reporting (reporting bias) | Unclear risk | Exclusive breastfeeding for 4 months is mentioned in the abstract, but there are no results for this outcome. |
| Analysis bias | High risk | It is stated that ITT analysis was undertaken but most results were not reported by randomisation group but rather by whether or not women had seen the LHW. There is no evidence of any adjustment made for correlations within or between clusters. The analysis seems to have been done at the individual level. It was stated that baseline imbalance was taken into account in secondary analysis. |
| Other bias | Unclear risk | Baseline imbalances are not clearly stated. |
| Overall risk assessment | High risk | Due to unclear group denominators, attrition and inappropriate analysis methods. |
| Methods | Cluster‐RCT in 6 districts of Southern Tanzania. INSIST (Improving Newborn Survival in Southern Tanzania) community intervention trial. | |
| Participants | Sample size: 512 women delivering 521 babies completed the final survey (9 pairs of twins). Clusters: 65 intervention and 67 control clusters were randomised. 1 control cluster was lost to follow‐up. Individuals: women were eligible for the survey if they had given birth in the previous year and were aged 13‐49. | |
| Interventions | Target: health system Arm 1: the intervention consisted of prenatal (3) and postnatal (2) counselling home visits to support pregnant women and educate women about birth preparation, delivery and recommended newborn care practices. Counselling tools included picture cards and a doll. Arm 2: pregnant women received standard care. | |
| Outcomes | Trial primary outcomes: breastfeeding within an hour of delivery, birth attendants for home deliveries washing hands before childbirth or wearing gloves, and babies fed only breast milk in the first 3 days. Review outcomes reported: Primary: not reported Secondary: other behaviours promoted during counselling to maximise newborn health, e.g. skilled attendance for childbirth, birth preparedness (for home deliveries), immediate drying and wrapping of the baby, clean cord care and delayed bathing of the baby. | |
| Notes | Funders: the study was funded by the Bill & Melinda Gates Foundation through the Saving Newborn Lives program of Save the Children (www.savethechildren.org/ The study was part of INSIST (www.clinicaltrials.gov, NCT01022788), and was approved by the review boards of Ifakara Health Institute, the Medical Research Coordinating Committee of the National Institute for Medical Research, Tanzania Commission for Science and Technology, and the London School of Hygiene and Tropical Medicine, UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence by list of random numbers. Wards with baseline data were randomised using stratification (matched pairs according to baseline neonatal mortality and population). |
| Allocation concealment (selection bias) | Low risk | Allocation performed at same time as sequence generation, by central randomisation team. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not blinded. |
| Recruitment bias (for cluster RCTs) | Low risk | Not noted. |
| Incomplete outcome data (attrition bias) | Low risk | 1 cluster (ward). |
| Selective reporting (reporting bias) | Unclear risk | Outcome data on coverage had to do specifically with the home visits offered by the trial; it is not clear whether any other ANC was available to women. Neonatal mortality data are reported, but perinatal mortality data are not. |
| Analysis bias | Low risk | Methods appropriate for cluster trials. ITT analysis. |
| Other bias | Unclear risk | Baseline variables comparable. Authors state that receipt of counselling visits may have been over‐reported; implementation was difficult with just half of women receiving a postnatal visit (p. 10). There may have been some contamination of control clusters due to women living near intervention clusters receiving visits or women in control areas inadvertently moving into intervention areas to be with family during childbirth (p. 10). |
| Overall risk assessment | Low risk | No serious risk of bias noted. |
| Methods | A parallel arm cluster‐RCT conducted in 90 sites in Vietnam, Quang Ninh Province between Jul 2008 and Jun 2011. | |
| Participants | Sample size: 90 clusters (22,561 births; 1243 mother‐newborn pairs randomly selected for secondary outcomes). Clusters : eligible districts had NMR ≥ 15/1000 in 2005. Individuals: all mother–newborn pairs within the study area with births from July 2008 to June 2011 were eligible. There were 22,561 births registered in the study area during the study period. | |
| Interventions | Target: health system (policy/practice change). Arm 1 (44 clusters, 11,906 births): the intervention consisted of facilitated work with stakeholder groups (primary care staff, local politicians and women's union representatives) on the commune level and included the identification of local perinatal health problems and use of a problem‐solving cycle. The 44 communes in the intervention group had a total of 1508 maternal and newborn health groups (MNHG) meetings, lasting approximately 2 hours each. The problem‐solving process identified 15‐27 unique problems which resulted in 19‐27 unique actions applied 297‐649 times per year. Arm 2 (46 clusters, 10,655 births): standard health care in control communities. A 6% random sample of all registered live births, surviving the neonatal period, was continuously selected each month in order to represent the entire birth cohort for secondary outcome data. Home visits were performed for families of a deceased newborn in another random sample. | |
| Outcomes | Trial primary outcome: neonatal mortality. Review outcomes reported: Primary: maternal mortality. Secondary: ANC coverage (at least 1 visit), health facility deliveries, tetanus protection, perinatal mortality, neonatal mortality. Other: postnatal home visit. | |
| Notes | Funders: Swedish International Development Cooperation Agency (Sida), Swedish Research Council, and Uppsala University. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation for the cluster assignments is described as by random number lists. Randomisation for the sample of women used to assess secondary outcomes is not described. |
| Allocation concealment (selection bias) | Unclear risk | The sequence was "concealed until the intervention assigned". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Data collectors had no contact with the facilitation of maternal and newborn health groups. It is not clear that they were blind to what was happening in their area, though. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Unclear risk | There were attempts to ensure a full data set for the primary outcome (neonatal mortality) but denominators used for the secondary outcomes vary. Loss to follow‐up in this population is not described. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Analysis bias | Low risk | Analysis appropriate for clusters; ICC reported; ITT analysis not stated. |
| Other bias | Unclear risk | In the first year it appeared that women in the control communities were more likely to lack education, be from poor households and be from minority ethnic groups. |
| Overall risk assessment | Unclear risk | We were uncertain how potential risks above impacted on findings. |
| Methods | Cluster‐randomised trial in KwaZulu‐Natal, South Africa. Clinicaltrials.gov identifier: NCT00972699. | |
| Participants | Sample size: intervention clusters (4 clinics; n = 544); control clusters (4 clinics; n = 656). Clusters: unit of randomisation was the clinic (n = 8). 1 clinic in the intervention had 2 sites. Overall sites randomised were the 5 intervention and 4 controls. Individuals: potential participants were pregnant women at least 18 years old living in the neighbourhood from May 2009 to September 2010. Only HIV pregnant women were eligible for the intervention. The control group were determined to be at no‐ or low‐risk for contamination of intervention activities. | |
| Interventions | Target: health system ‐ addition of peer mentors to antenatal and postnatal care. Arm 1: enhanced intervention (EI). The EI consisted of an initial assessment and 4 antenatal and 4 postnatal small group sessions led by Peer Mentors. The intervention targeted 5 domains: HIV prevention, infant health, healthcare and health monitoring, mental health and parenting tasks (p. 707 Richter). Both treatment arms received standard care: dual therapy to prevent HIV transmission, referral and treatment for women with low CDC count (< 400 or WHO stage 4 illness), a recommended single feeding method for the first week of life, and tinned powdered infant milk. Mobile phones were used to collect data in this study. Transport was also provided. Arm 2: standard care as above: dual therapy to prevent HIV transmission, referral and treatment for women with low CDC count (< 400 or WHO stage 4 illness), a recommended single feeding method for the first week of life, and tinned powdered infant milk. | |
| Outcomes | Trial primary outcome: a summary measure of indicators of maternal and infant health, including: child health status, health care and health monitoring, HIV transmission‐related behaviours, mental health and social support. Review outcomes reported: Primary: ANC coverage (at least 4 visits) Secondary: Proportion of women with HIV receiving complete anti‐retroviral course to prevent transmission, low birthweight | |
| Notes | Funders: this work was supported by NIAAA grant R01 AA017104, the Center for HIV Identification, Prevention, and Treatment Services (CHIPTS) NIMH grant P30 We have not used mortality data as reported for this trial because numbers reflect the trial's own definition (all deaths within 12 months, for example) rather than the standard definitions of maternal mortality and perinatal mortality required for use in this review. We contacted authors (Mary Jane Rotheram: [email protected]) for clarification, but it was not possible to recalculate the trial deaths with our definitions. We were also concerned that any maternal deaths reported may have been HIV‐related rather than pregnancy‐related. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clinic were randomised according to matched pairs with a simple randomisation schedule by UCLA. |
| Allocation concealment (selection bias) | Low risk | Not stated, but randomisation and allocation took place remotely (UCLA). |
| Blinding of participants and personnel (performance bias) | Unclear risk | All women were invited into the PeerMentor program while in clinic waiting rooms, and all women gave written consent. It is unclear if women would have been aware of the content of the intervention versus standard care. Research and clinical staff would have been aware of allocation. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Female research assistants were trained to interview women at baseline and post‐birth. Because interviewers were assigned by clinic, they were not blinded to condition. |
| Recruitment bias (for cluster RCTs) | Unclear risk | Women were invited into the PeerMentor program from July 2008, but data collection from March 2009. Data available only for the last 602 women recruited. |
| Incomplete outcome data (attrition bias) | High risk | Follow‐up rates varied: 70% at post‐birth interview; 57% at 6 months; 24% at 12 months. |
| Selective reporting (reporting bias) | Unclear risk | Longer‐term outcomes have been published in multiple reports. Mortality data are not usable due to non‐standard definitions of maternal mortality and perinatal mortality. |
| Analysis bias | Low risk | Adjustments made for cluster‐design. |
| Other bias | Low risk | None noted. |
| Overall risk assessment | Unclear risk | We were uncertain how the above risks, specifically attrition, may have impacted the findings. |
| Methods | A parallel arm cluster‐RCT conducted at 36 sites in India, Jharkhand and Orissa, between Jul 2005 and Jul 2008. | |
| Participants | Sample size: 36 clusters (19030 births). Clusters: not clearly stated. The study area had disproportionately high NMR and an underserved population. Individuals: women aged 15‐49 residing in the project area who gave birth during the study (July 31, 2005‐July 30, 2008). Women who migrated out of the region were excluded from some analyses. 2 women from each arm refused the interview and were excluded. | |
| Interventions | Target: community (IEC). Arm 1 (18 clusters, 9686 births): monthly facilitator‐convened women's groups monthly for a total of 20 meetings. Groups discussed maternal and newborn health problems and practices using pictures, role‐play and storytelling. In addition, health committees were formed to provide village representatives the chance to learn about health services and comment on their design and management. Arm 2 (18 clusters, 9089 births): in control clusters only health committees were formed. | |
| Outcomes | Trial primary outcome: reduction in neonatal mortality rate and maternal depression. Review outcomes reported: Primary: maternal mortality. Secondary: ANC coverage (at least 1 visit), health facility deliveries, tetanus protection, perinatal mortality, neonatal mortality, stillbirth. | |
| Notes | Funders: Health Foundation, UK Department for International Development, Wellcome Trust, and the Big Lottery Fund (UK). Data for years 1‐3 combined excluding migrants were used for our comparison 4 (Table 2, p. 1188). All ORs were taken directly from the published report (Tripathy 2010). The trial authors adjusted data for clustering, stratification, maternal education, assets and any tribal affiliation. Antenatal care outcome data are found in Table 5, p. 1190. It is unclear whether the OR presented for perinatal mortality (excluding migrants) includes infants who died between 0‐6 days or 0‐28 days (Table 3, p. 1188). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing folded papers with numbers corresponding to clusters from a basket. |
| Allocation concealment (selection bias) | Unclear risk | The first 4 numbers drawn were assigned to the intervention; the next 4 to the control group. Participants in the randomisation process would have been aware of the next assignment but as the process was transparent it would not have been possible to manipulate the process. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not blinded. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition and exclusions outlined in study flow diagram with limited missing data. |
| Selective reporting (reporting bias) | Unclear risk | Analysis was presented fully but there were multiple analyses with various adjustments and multiple testing which made results difficult to interpret. |
| Analysis bias | Unclear risk | Both adjusted and unadjusted data were provided. Adjustment for clustering and other factors did not appear to change the main conclusions. ICC of 0.0005 was mentioned but it's unclear if this was actually used for adjustments of data for neonatal death. Analysis was stated as by ITT. |
| Other bias | Unclear risk | There were baseline differences in household assets, maternal education, literacy and tribal membership; the intervention clusters were generally poorer. Some analyses adjusted for baseline differences. |
| Overall risk assessment | Unclear risk | We were uncertain how potential risks above impacted on findings. |
| Methods | Parallel arm RCT conducted in 4 Latin American countries including Argentina (Rosario), Brazil (Pelotas), Cuba (Havana), and Mexico City between Jan 1989 and Mar 1991. | |
| Participants | Sample size: 2235 individuals. Inclusion criteria: 1 or more risk factor for delivering a low birthweight infant, including: a history of low birthweight, premature birth, fetal death, infant death; mothers less than 17 years of age, weighing less than 50 kg, or under 1.5 m tall; low socioeconomic level; less than 3 years of education; smokers; consumption of alcohol; single mothers; started prenatal care between 15‐22 weeks; singleton pregnancy. Exclusion criteria: chronic renal disease, cardiovascular problems, chronic hypertension, cerclage, Rh negative, mental disorders. | |
| Interventions | Target: health system (the addition of home visits to standard ANC package) and community (IEC intervention). Arm 1 (1115 women): 4‐6 home visits from a nurse or social worker during pregnancy, with emphasis on health education, uptake of ANC, improving participants' social networks and individual psychological support. The intervention provided a hotline, a dedicated hospital office, a poster and booklet and a guided tour of the hospital delivery facilities. Arm 2 (1120 women): standard ANC which took place in clinics. | |
| Outcomes | Trial primary outcome: low birthweight (for sample size) and indicators of social support. Review outcomes reported: Primary: not reported. Secondary: preterm birth, low birthweight infants, perinatal mortality, neonatal mortality. Other: mean no. ANC visits (these data were not usable for meta‐analysis). | |
| Notes | Funders: International Development Research Center, Ottawa, Canada. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence from computer‐generated code in blocks of 20, stratified according to centre. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes were used for group assignment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Home visitors and women were aware of group assignment; staff at health clinics were not aware of group assignment unless women themselves disclosed this. |
| Blinding of outcome assessment (detection bias) | Low risk | Langer 1993 reports that the outcome assessors at the Mexico City site were blind to group assignment. |
| Recruitment bias (for cluster RCTs) | Low risk | Not applicable. |
| Incomplete outcome data (attrition bias) | Low risk | Missing data < 20%. Loss to follow‐up not described. 83% of women received the planned number of home visits, with 90% visited at least once. Denominators for delivery outcomes were stated as 1033 for intervention and 1040 for control (< 10%). |
| Selective reporting (reporting bias) | Low risk | None noted. |
| Analysis bias | Unclear risk | ITT not stated. |
| Other bias | Unclear risk | Baseline imbalances not described. |
| Overall risk assessment | Low risk | No serious risk of bias concerns. |
| Methods | Parallel arm cluster‐RCT conducted in 53 clinics in Argentina, Cuba, Saudi Arabia and Thailand between May 1996 and April 1998. | |
| Participants | Sample size: 53 clusters (24526 individuals). Clusters: clinics serving 300 new patients within 24 months. The clinics had to be part of a public or semi‐public health system and not require direct fee‐for‐services payment. Clinics had to have an ANC system in place with adequate staffing and be able to implement and fund tests or activities required by the protocol. Individuals: all women attending prenatal care for the first time at any participating clinic were eligible. Women later found not to be pregnant were excluded. Multiple births were excluded from some outcomes (specifically low birthweight outcomes). Women had to be traceable at delivery, including women transferred to hospitals as high‐risk. | |
| Interventions | Target: health system (reorganisation of services). Arm 1 (27 clusters, 12,568 women): a reduced visits regime of ANC. Women classified as higher risk received standard ANC but were analysed according to ITT. The new model of care included 4 antenatal visits for low‐risk women. The visits were goal‐oriented and focused on scientifically evaluated components of ANC. Arm 2 (26 clusters, 11,958 women): standard ANC. | |
| Outcomes | Trial primary outcomes: low birthweight (< 2500 g), pre‐eclampsia/eclampsia, severe postpartum anaemia (< 90 g/L Hb), treated urinary tract infection. Review outcomes reported: Primary: maternal mortality. Secondary: ANC coverage (< 5 visits), tetanus protection, syphilis treatment, preterm birth, low birthweight infants, perinatal mortality, neonatal mortality, stillbirth. Other: median no. of ANC visits, pre‐eclampsia, antepartum haemorrhage, mode of delivery, and others. | |
| Notes | Funders: UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction of WHO. Additional support from City of Rosario, Argentina, Ministry of Health, Cuba, National Institute of Public Health, Mexico, The Population Council ‐ Regional Office for Latin America and the Caribbean, Ministry of Health, Saudia Arabia, Swedish Agency of Research Cooperation with Developing Countries, Ministry of Public Health and Faculty of Medicine, Khon Kaen University, Thailand, Department for International Development, UK; Mother Care ‐ John Snow, Inc; National Institute for Child Health and Human Development, National Institutes of Health, USA, and The World Bank. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was computer‐generated. Randomisation was stratified according to study site and clinic characteristics. |
| Allocation concealment (selection bias) | Low risk | Allocation kept centrally until each site had completed the basic introductory training of study personnel, which took place in both intervention and control clinics. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding not possible. Staff recording outcome data after the birth were not aware of group allocation but outcomes were recorded by staff providing ANC (not blinded). |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Low risk | Loss of follow‐up varied for delivery outcomes but was reasonably low and balanced across groups (i.e. loss to follow‐up in reduced visits group was 253/12,568 (2.0%) and standard ANC group was 290/11958 (2.4%). For the low birthweight outcome 138/11672 single births were missing for the new model clinics and 81/11121 in the standard care clinics. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were reported. |
| Analysis bias | Low risk | Analysis appropriate for clusters; ICC reported; ITT analysis was performed. |
| Other bias | Low risk | There was some evidence of imbalance at baseline. Women in the new model were less likely to smoke during pregnancy (10.4% versus 12.5%) but it was more likely that women in the new model clinics to have lower levels of education (17.5% education less than primary versus 15.7%). The impact of these differences at baseline are not clear and the differences are taken into account in the adjusted analyses. |
| Overall risk assessment | Low risk | No serious risk of bias concerns. |
| Methods | Cluster‐randomised trial in rural Laos. | |
| Participants | Sample size: 40 clusters randomised. Post‐intervention survey n = 127 intervention and n = 190 controls. Clusters: 2 provinces were selected, with 2 districts in each province. Eligible districts were selected based on having geographically separate populations with different economic standards and having road access. 10 villages were randomly selected in 2 districts (Champasack and Khammouane): in each district 5 randomised villages had health centres and 5 did not. For the Champasack district, the intervention was implemented in the better‐off villages. This was decided by coin. In the district of Khammouane, poorer villages received the intervention with the better‐off villages serving as controls. Individuals: women aged 15‐49 years and currently pregnant with a reported gestational length of 32 weeks or more, or who had recently given birth (during the last year for the pre‐intervention survey, and during the last 6 months for the post‐intervention survey). | |
| Interventions | Tareget: community and health system. Arm 1: 10 community awareness‐raising meetings over 6 months; provision of basic ANC equipment to health centres; a refresher course for healthcare providers. Arm 2: control arm not described and presumed to be standard care. | |
| Outcomes | Trial primary outcomes: difference in overall ANC used as reported by interviewed women and checked with the ANC record book. Review outcomes reported: Primary: ANC coverage at least 4 visits Secondary: ANC from health centres We obtained unpublished outcome data from the author: Rolf Wahlstrom, [email protected]. | |
| Notes | For the review outcome of at least 1 ANC visit, we used the data for the trial outcome 'overall ANC'. Funders: Swedish International Development Cooperation Agency, the Swedish Institute (SI) and the Ministry of Health of Laos. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence described as random. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Women were not blind to the intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Interviewers were aware of the goals of the study. |
| Recruitment bias (for cluster RCTs) | High risk | Problems with recruitment in the intervention arm for the post‐intervention survey. |
| Incomplete outcome data (attrition bias) | Low risk | Pre‐ and post‐intervention surveys were different populations. Authors report problems in recruitment for the post‐intervention survey but no attrition. |
| Selective reporting (reporting bias) | Low risk | Relevant outcome data reported. |
| Analysis bias | Unclear risk | Authors describe adjustments for clustering and intra‐class correlations as "reduced by combining two districts from different provinces as intervention and control areas". |
| Other bias | High risk | Authors report that baseline differences in education favoured the control group and impacted overall results. Women were recruited after 32 weeks of pregnancy, which limited the intervention's capacity to improve rates of the outcome of ANC at least 4 visits. Recall bias: visits were based on women's reports; responses were validated against ANC booklets for a third of respondents. |
| Overall risk assessment | High risk | We were concerned about the impact of baseline group differences and recruitment problems as noted above. |
| Methods | Uganda Newborn Study (UNEST) 2 armed cluster‐randomised trial. | |
| Participants | Sample size: 63 clusters randomised; baseline survey n = 194 intervention and n = 201 controls; endline survey n = 894 intervention and n = 893 control. Clusters: Uganda, Iganga and Mayuge districts in eastern Uganda, predominantly rural with 65 villages and total population of 70,000 at time of the study. Local health services include 1 100 bed hospital and 19 health centres that provide delivery services. 63 villages randomised (31 intervention and 32 control. Individuals: all consenting pregnant women and their newborns residing in the study area between September 2009 and August 2011. women were eligible for the baseline survey if they had a live birth in the last 4 months; women were eligible for the end line survey if they had a live birth in the last 12 months. | |
| Interventions | Target: community and health systems. Arm 1: CHWs made 5 home visits (2 prenatal and 3 postnatal) with extra visits for sick or small newborns. Health facility strengthening in all facilities (both arms) to improve quality of care. Facility strengthening included: "6‐day in‐service training, provision of a once‐off catalytic supply of equipment and medicines, as well as collaboration with the district health team to continuously improve the quality of care provided to mothers and newborns" (p. 4). Arm 2: the control arm received standard care as well as facility strengthening. | |
| Outcomes | Trial primary outcomes: ANC coverage and services. Birth preparedness, skilled attendance at delivery, and postnatal care, as well as increases in healthy practices including breastfeeding, thermal care, and hygiene. Review outcomes reported: Primary: ANC coverage 4 or more visits; Mortality data were collected but not reported in this publication (authors emailed with no reply 5/15). Secondary: deliveries in a health facility, ANC coverage one visit | |
| Notes | Funders: this study was supported by the Sida/SAREC‐Makerere University‐ Karolinska Institutet Research collaboration as well as by funds provided by Save the Children through a grant from the Bill & Melinda Gates Foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated restricted randomisation was done in a 1‐to‐1 ratio by an independent epidemiologist from the London School of Hygiene and Tropical Medicine. |
| Allocation concealment (selection bias) | Low risk | Allocation done remotely as above. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Women and staff not blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes assessors not blind. |
| Recruitment bias (for cluster RCTs) | Low risk | No problems with recruitment reported. |
| Incomplete outcome data (attrition bias) | Low risk | Different populations for pre and post‐intervention surveys. |
| Selective reporting (reporting bias) | High risk | Mortality data not reported yet in published reports; analysis of a household survey to document mortality outcomes is ongoing; low birthweight not reported according to intervention arm; preterm birth mentioned in discussion but unclear if these data were available. |
| Analysis bias | Low risk | ITT analysis. Authors report adjustments made for cluster design. |
| Other bias | Unclear risk | Demographic variables comparable at baseline (age, parity and household wealth); only women with a live birth were eligible; mortality data are not reported; there were no buffer zones between control and intervention villages. |
| Overall risk assessment | Unclear risk | Conduct of the trial is of low risk of bias, though reporting bias is unclear. |
| Methods | Parallel arm cluster‐RCT conducted in 2 states in Mexico between Jan 2009 and Dec 2010. | |
| Participants | Sample size: 27 clusters (2053 individuals). Clusters: health centres located in the study area had to have > 25 registered births in 2007, have basic equipment and supplies to attend deliveries and be located 1‐2 hours from the referral hospital. Study areas were 2 states, Oaxaca or Guerrero, with high maternal mortality. Individuals: birth outcome data were taken from monthly interviews and chart review instead of direct observation. Staff were asked to recall their 3 most recent deliveries. | |
| Interventions | Target: health system (addition of obstetric nurse or professional midwife to physician‐based health centre). Arm 1 (12 clusters, 1129 births): the addition of an obstetric nurse or professional midwife to the physician‐based team in rural health clinics. Arm 2 (15 clusters, 924 births): women attending control clinics received standard obstetric care. | |
| Outcomes | Trial primary outcome: an index of ANC measures. Primary: ANC coverage (at least 4 visits). Secondary: pregnant women initiating ANC in first trimester The trial author clarified the ANC outcomes presented in the text and in Table 3 of the primary trial report. Prof Walker replied, "Appropriate care in Table 3 signifies first antenatal visit before 12 weeks gestation." This would be our outcome: 1.4 The proportion of women who initiate ANC in the first trimester. We used "In accordance with WHO standards" from Table 3 for our review's primary outcome 1.1 pregnant women attending at least 4 ANC visits. Dilys Walker: [email protected]. | |
| Notes | Funders: The National Center for Gender Equity and Reproductive Health,the National Institute of Women, and the Bill and Melinda Gates Foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was computer‐based. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described, but it appears that all sites were randomised at the same time through a computer program. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not blinded. |
| Recruitment bias (for cluster RCTs) | Low risk | None noted. |
| Incomplete outcome data (attrition bias) | Unclear risk | For delivery outcomes, data are based on provider recall of the most recent 3 deliveries. |
| Selective reporting (reporting bias) | Unclear risk | Few relevant outcomes reported. ANC coverage not clearly reported. Correspondece with author to clarify ANC outcome data for this review. |
| Analysis bias | Unclear risk | Adjusted for clusters but ITT not stated and no ICC reported. |
| Other bias | High risk | Recall bias may have distorted the data for delivery outcomes in both the intervention and control sites. These data have not been reported in usable form. Some outcomes were composites, e.g. care meeting WHO standards, resulting in very low numbers in either group appearing to receive adequate care. Baseline differences were present in the proportion of literate women in each group, with control clusters having significantly less literate women (1021/1259 intervention versus 705/995 control, P < 0.001). It was more likely that information on baseline characteristics was recorded for intervention group women, so it is possible that there were further differences between I and C groups at baseline. |
| Overall risk assessment | High risk | For above reasons. |
| Methods | A parallel arm cluster‐RCT conducted in Anhui province, Eastern China, between Aug 2000 to Jul 2002. | |
| Participants | Sample size: 20 clusters (1264 individuals). Clusters: townships were selected and paired according to: place of birth (hospital, family planning centre or other); per capital income; average number of prenatal care visits; and location. Population, proportion of farmers, infant death rate, number of midwives and number of hospital beds were also taken into account. Townships were required to have an existing health facility and the staff necessary to implement the trial. Individuals: women who had given birth in the past year were eligible for the interview. | |
| Interventions | Target: health system (health worker education and equipment provision) and community (IEC). Arm 1 (10 clusters, 673 women): the intervention had 3 health system components: training of community midwives, a public awareness campaign with posters and leaflets about prenatal care, and provision of equipment to health centres. Arm 2 (10 clusters, 591 women): usual health system. | |
| Outcomes | Trial primary outcomes: prenatal care utilisation and perinatal outcomes. Primary: ANC coverage (at least 4 visits). Secondary: ANC initiation in first trimester, health facility deliveries, stillbirths, perinatal mortality, neonatal mortality. | |
| Notes | Funders: Academy of Finland, Finnish Ministry of Education (DPPH‐program), European Commission INCO Programme "Structural hinders to and promoters of good maternal care in rural China ‐ C HIMACA (015396). Results of a hospital‐based survey are not included in this trial report. We have excluded the perinatal mortality data reported for this trial due to multiple risk of bias concerns, including unclear denominators. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated from a coin toss. |
| Allocation concealment (selection bias) | Unclear risk | 1 township in each matched pair was assigned to intervention or control by a coin toss. Allocation concealment was not described. Matching was checked after randomisation for matching. It was not clear if allocation could be changed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not blinded. |
| Recruitment bias (for cluster RCTs) | Unclear risk | None noted. |
| Incomplete outcome data (attrition bias) | Unclear risk | The denominators for perinatal outcomes were not clear (results expressed at percentages) so it was not possible to assess attrition bias for these outcomes. 1306 women were eligible for the survey, and data were reported for 1264 (missing data ˜ 3%). 42 mothers were described as missing; 2 refused; 27 were out of the village; and 13 cases were missing for "other reasons". |
| Selective reporting (reporting bias) | Unclear risk | Denominators not clearly reported. Mortality stats after the intervention were compared with those before the intervention with major differences reported. |
| Analysis bias | Unclear risk | Adjustments made for clusters, but no information on ICC or what difference adjustment made. ITT not stated. |
| Other bias | High risk | The perinatal data are difficult to interpret due to differences between clusters before the intervention. Data from the community based survey showed group differences for parity, but similarities on other demographic traits. Mortality data were taken from township family planning records. The early neonatal death rate for girls' is much higher than that for boys', causing the authors to doubt the utility of mortality outcomes for the intervention. They wrote, "If the impact of the family planning policy is larger on perinatal mortality than maternal care, then it is hard for any health care intervention to have an effect on perinatal health outcomes". Authors state that the Provincial Health Board implemented a program of ANC in control and intervention townships just 8 months after the trial had begun. 2 intervention districts and 4 control districts also had a prepayment scheme for maternal care implemented during this period. These health initiatives likely contaminated the controls and diluted the effects of the intervention. Furthermore, trialists failed to distribute posters and leaflets because of poor co‐operation between family planning and health sectors, and so this component of the intervention was not completed. |
| Overall risk assessment | High risk | Due to multiple risk of bias concerns above. |
ANC: antenatal care
CHW: community health worker
Hb: haemoglobin
IEC: information, education and communication intervention
ICC: intra‐cluster correlation coefficient
IPT: intermittent prophylactic treatment
ITT: intention‐to‐treat
LHW: lady healthcare worker
NGO: non‐governmental organisations
NICU: neonatal intensive care unit
NMR: neonatal mortality rate
OR: odds ratio
P4P: payment for performance
POW: pregnancy outreach worker
RCT: randomised controlled trial
TBA: traditional birth attendant
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not randomised. This study was a 15‐month longitudinal, observational study of 2 treatment areas. Women in the intervention area received integrated a maternity services, and women in the control area women received standard care. | |
| Intervention does not target ANC. This study compares neonatal assessment by community health workers with assessment carried out by physicians. | |
| This study was a pilot study for the cluster‐randomised trial reported in Bhutta 2011. This study was not randomised. | |
| This study examined the effects of community mobilisation and quality improvement on maternal, neonatal and perinatal mortality. The objective of the trial was not improved ANC coverage. Percentage of women attending women's groups was a secondary outcome. | |
| This trial investigated whether additional care for women who had 1 previous low birthweight baby would effect birthweight and other pregnancy outcomes. An increase of ANC coverage was not an objective of the trial. | |
| The intervention in this trial involved prenatal education and support, with the objective of improved perinatal outcomes among disadvantaged women. An increase in ANC coverage was not an aim of this trial. | |
| Protocol. This trial will investigate whether increased training of healthcare workers of has an impact on perinatal and maternal mortality rates. Availability of resources, resource use, and a process evaluation are also planned. Improved coverage of ANC is not an objective of the trial. | |
| This report describes an observational study to evaluate improved ANC and is not a randomised trial. | |
| This is a trial of an intervention to improve the self‐efficacy of teenage mothers. The intervention does not have the goal of increased ANC coverage. | |
| This trial looked at the possible differences between care delivered by midwives versus lady home visitors. Increased antenatal coverage was not an objective of the trial. | |
| The intention of this trial was to compare community based and standard ANC in order to improve clinical outcomes, especially rate of caesarean birth. Improved ANC coverage was not the objective of the trial. | |
| This trial investigated group versus standard ANC in young pregnant women (aged 18‐25) with a view to improved pregnancy outcomes, psychosocial function, costs and patient satisfaction. Improved coverage of ANC was not an objective of the trial. | |
| This trial investigated group versus standard ANC in young pregnant women (aged 18‐25) with a view to improved pregnancy outcomes, psychosocial function, costs and patient satisfaction. Improved coverage of ANC was not an objective of the trial. | |
| This study examined the effects of a nutrition education program for pregnant women. Improved ANC coverage was not part of the objective of the trial. | |
| The purpose of this intervention was to improve the maternal skills of adolescent mothers. An improved coverage of ANC was not an objective of the trial. | |
| This trial compared an augmented and a standard form of prenatal and follow‐up care for at‐risk pregnant women. Increased coverage of ANC was not an objective of the trial. | |
| This is a quasi‐randomised trial (alternate allocation by odd or even hospital number) with no allocation concealment. The study compares a reduced‐visits regimen of ANC with standard care. An increase in ANC coverage was not an objective of the trial. | |
| The intervention for this trial involved group psychotherapy with the aim of reducing depression and stress in new mothers. Increased ANC coverage was not an objective of the trial. | |
| This study is not a randomised trial but an analysis of patterns of prenatal care utilization for women enrolled in a randomised trial. Data were retrieved from computerised databases and patterns of care were evaluated using the Kotelchuck index. Predictors of visits were explored, and structured interviews with participants were conducted. | |
| This cluster‐randomised trial examined the effects of training for traditional birth attendants in the Dera Ghazi Khan District of Punjab, Pakistan. The objective of the trial was improved birth attendant performance and reduced perinatal mortality. | |
| This trial looked at a program of reduced ANC visits and considered the impact of this change on maternal and infant outcomes. An increase in ANC coverage was not an objective of the trial. | |
| The intervention in this trial of home visitation had the objective of reducing and preventing child abuse and neglect. Outcomes included number of substantiated reports of child abuse from state records and level of domestic violence in the home as measured in the Conflict Tactics Scale. Increased ANC was not an objective of the trial. | |
| This trial investigated the effects of prenatal and postnatal home visits on the maternal life course and on children's longer‐term functioning. Increased ANC coverage was not an objective of this trial. | |
| This trial examined the impact of an intervention to educate women about serious pregnancy complications. Increased ANC coverage was not an objective of the trial. | |
| This trial examined the impact of a community‐based intervention to improve the care of newborn infants. Increased ANC coverage was not an objective of the trial. | |
| This trial compared an augmented and a standard form of prenatal and follow‐up care for at‐risk pregnant women. Increased coverage of ANC was not an objective of the trial. | |
| The intervention in this trial involved 2 prenatal and 5 postnatal visits with the aim of improving neonatal outcomes, including breastfeeding at 12 weeks and decreasing transmission of HIV. Increased coverage of ANC was not an objective of the trial. Data for coverage of antenatal HIV testing were collected. | |
| This trial compared augmented prenatal care with standard care in the Canadian health system, where there is universal coverage. Increased ANC coverage was not an objective of this trial. | |
| This trial compares group with individual prenatal care. Increased coverage of ANC is not an objective of the trial. | |
| Three studies are described in this report. One is a randomised trial of antenatal education sessions for couples, women‐only or control. Increased ANC coverage was not an objective of the trial. |
ANC: antenatal care
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Overcoming barriers to scaling SBA utilization in improving maternal, neonatal and child health in Nepal. Location: Nepal. Trial Registration: ISRCTN78892490. |
| Methods | Cluster‐randomised trial in 3 districts in Nepal, 36 clusters selected for study. Clusters were not eligible if they had already achieved the target coverage of SBAs. |
| Participants | All pregnant women. |
| Interventions | Arm 1: the intervention has 5 components to strengthen the capacity of the existing health system. The intervention is implemented through training community health volunteers, training of health personnel, organisation of community leaders and reorganisation of emergency services. The intervention targets: family support for transport to health facility for childbirth, emergency funding for transport, availability of transport, women‐friendly environment in health facilities, mechanisms to improve security at health facilities. The fifth component of measuring the impact of the intervention will be a survey of process indicators. Arm 2: standard care |
| Outcomes | Target: health system strengthening. Primary outcome: utilisation of SBAs. Secondary outcome: at least 4 ANC visits, postnatal care, availability of transport, functional operation of emergency fund, security of SBA, family support, women‐friendly environment at health facility. |
| Starting date | May 2013‐Aug 2014 intervention; May 2014‐Oct 2014 evaluation. Mid‐term data monitoring in Oct 2013. |
| Contact information | G Bhandari ‐ [email protected] |
| Notes |
| Trial name or title | Implementation of evidence‐based ANC in Mozambique. Location: antenatal clinics in3 regions of Mozambique. Trial Registration: Pan African Clinical Trial Registry database. Identification number: PACTR201306000550192. |
| Methods | Cluster‐randomised trial. |
| Participants | "Clinics were eligible if (1) they were not already implementing the proposed ANC model; (2) they served at least 200 new pregnant women per year; (3) they had midwives or nurses midwives among their personnel; and (4) they were willing to participate. All women attending ANC visits at the participating clinics will be eligible to receive the ANC package." |
| Interventions | Target: health system. "The aim of this study is to determine the effect of an intervention designed to increase the use of evidence‐based practices included in the ANC package by midwives (and other health professionals) in prenatal clinics in Mozambique. Specifically, we will assess the effect of the intervention on practices related to the detection, treatment and prevention of major health‐related conditions (e.g., anaemia, and infectious diseases such as HIV/AIDS, Arm 1: the intervention has 4 components: ANC kits for clinics, storage for ANC kits, tracking system, training for staff. Arm 2: standard care. |
| Outcomes | Primary outcome: administration of a set of practices during first ANC visit Secondary outcome: composite of several practices. • Frequency of women receiving screening for syphilis. • Frequency of women receiving screening for HIV. • Frequency of women receiving screening for anaemia. • Frequency of women receiving screening for hypertension. • Frequency of women receiving tetanus toxoid. • Frequency of women receiving intermittent preventive malaria treatment. • Frequency of women receiving iron supplementation. • Frequency of women receiving anti‐parasitic treatment (de‐worming). • Frequency of syphilis sero‐positive women receiving the administration of syphilis treatment. • Frequency of HIV sero‐positive women receiving administration of antiretroviral treatment. |
| Starting date | July 2013‐March 2015. |
| Contact information | Leonardo Chavane ‐ [email protected] |
| Notes | Funding ‐ World Health Organization, Department of Reproductive Health and Research. |
| Trial name or title | A matched pair cluster‐randomised implementation trial to measure the effectiveness of an intervention package aiming to decrease perinatal mortality and increase institution‐based obstetric care among indigenous women in Guatemala. Location: Guatemala. Trial Registration: ClinicalTrial.gov NCT01653626. |
| Methods | Matched pair cluster‐randomised implementation trial (30 matched pairs randomised evenly). |
| Participants | All women in study areas, aged 10‐49. |
| Interventions | Target: community and health system. Arm 1: intervention involves 3 components: PRONTO a training program for management of emergency obstetric care, social campaign to increase use of health facilities for birth, strengthening lings between trained birth assistants and professional midwives. Arm 2: standard care. |
| Outcomes | Primary outcome: perinatal mortality. Secondary outcome: process indicators, deliveries in a health facility. |
| Starting date | July 2012‐Dec 2013. |
| Contact information | Edgar Kestler ‐ [email protected] |
| Notes | Funding: This study is funded by the Alliance/WHO grant file register: H9‐TSA‐224. We are also grateful for the support of the Guatemalan Reproductive Health Observatory and National Reproductive Health Program, the Guatemalan Ministry of Health and its Department of Nursing and the National Society of Gynecologists and Obstetricians of Guatemala. |
| Trial name or title | Making birth safe for Pakistan women: a cluster‐randomised trial. Location: Jhang, Chiniot and Khanewal districts of Punjab, Pakistan. Trial Registration: Current Controlled Trials ISRCTN86264432. |
| Methods | Cluster‐randomised trial with 3 arms (7 clusters per arm). A costing study and exploratory qualitative study are also proposed. |
| Participants | All pregnant women in the catchment areas. |
| Interventions | Arm 1: structured birth planning and travel facilitation. Arm 2: structured birth planning. Arm 3: control (routine care by Lady Health Workers, as available to all pregnant women). |
| Outcomes | Primary outcome: neonatal mortality and service utilisation. Secondary outcome: maternal mortality. |
| Starting date | February 2011‐May 2013. |
| Contact information | Shirin Mirza, [email protected] |
| Notes | This trial appears to be completed. |
| Trial name or title | Ghana’s Ensure Mothers and Babies Regular Access to Care (EMBRACE) program. Location: Dodowa, Kintampo, and Navrongo, Ghana. Trial registration: Current Controlled Trials ISRCTN90618993. |
| Methods | Cluster‐randomised trial using an effectiveness‐implementation hybrid design. |
| Participants | Women of reproductive age between the ages of 15 and 49 years living in the study areas. |
| Interventions | Arm 1: the package includes: 1) use of a new continuum of care card, 2) continuum of care orientation for health workers, 3) 24‐hour health facility retention of mothers and newborns after delivery, and 4) postnatal care by home visits. Arm 2: standard ANC. |
| Outcomes | Primary outcome: continuum of care completion rate of mothers and infants Secondary outcome: PNC rate within 48 hours, the complication rate requiring mothers’ and newborns' hospitalisations, and the PMR and NMR. The PMR is defined as fetal deaths during any period of pregnancy and newborn deaths within 7 completed days after birth. The NMR includes early neonatal deaths occurring during the first 7 days of life and late neonatal deaths occurring after 7 days but before 28 completed days of life. |
| Starting date | The expected recruitment period will be from August to September 2014 for the baseline survey, from October 2014 to September 2015 for the intervention, and from October to November 2015 for the follow‐up survey. |
| Contact information | Masamine Jimba ‐ [email protected]‐tokyo.ac.jp |
| Notes | The Ghana EMBRACE Implementation Research Project is conducted by the Government of the Republic of Ghana, Japan International Cooperation Agency (JICA) Human Development Department, and JICA Research Institute. |
| Trial name or title | Community mobilisation and health management committee strengthening to increase birth attendance by trained health workers in rural Makwanpur, Nepal. Trial registration: ISRCTN99834806. |
| Methods | Cluster‐randomised trial to test the effect on institutional deliveries and home deliveries by trained health workers of a combination of community mobilisation through women’s groups and strengthening of health management committees. |
| Participants | Women of reproductive age, family members, health service cadres, health management committee members, and communities. |
| Interventions | Intervention arm: community mobilisation through women’s groups, and health management committee strengthening. Control arm: no intervention. We stratified clusters into 4 groups. Group 1 included control clusters from 2002‐2005, and intervention clusters from 2005 to 2008. Group 2 included intervention clusters from 2001‐2008. In group 3 we monitored birth outcomes from 2005‐2008. In group 4, we had not previously conducted any intervention or monitoring activities. There were equal numbers of clusters from each group allocated to intervention and control clusters. |
| Outcomes | Primary outcome measures: |
| Starting date | Overall trial start date: 01/10/2010. Overall trial end date: 30/09/2012. |
| Contact information | Anthony Costello: [email protected] |
| Notes | The Wellcome Trust funded this study. |
| Trial name or title | Building Blocks: Family Nurse Partnership in England trial. 18 study sites around England. Location: England, United Kingdom. Trial registration: ISRCTN23019866. |
| Methods | Individually‐randomised parallel controlled trial with a parallel economic modelling study. |
| Participants | Nulliparous pregnant women aged 19 or under, recruited by 24 weeks' gestation and followed until child is aged 2 are eligible. Women who require a translator or sign interpreter, who plan to move from the trial area, or who plan to have their children adopted are excluded from the trial. |
| Interventions | Arm 1: Family Nurse Partnership and usual care. The Family Nurse Partnership programme is a "structured, intensive programme of home visits delivered by specially trained nurses, provided from early pregnancy until the child is 2 years old. ...Visits cover core content areas of personal and environmental health, life course development, maternal role, family and friends and access to health and social services" (p. 2). Arm 2: usual care. Usual care women receive local maternity services as standard in the UK, including pre and postnatal visits and an assigned Health Visitor after the birth of the baby. |
| Outcomes | Primary outcomes: birthweight, changes in prenatal tobacco use, emergency attendances and hospital admissions for the child within 2 years, proportion of women with a second pregnancy within 2 years. Secondary outcomes: multiple outcomes for mothers and infants, including use of ANC and other services, maternal health and well being, health behaviours, social support, parenting beliefs, behaviours and experience, and for infants/children: Apgar, neonatal intensive care unit admissions, head circumference, feeding and development outcomes, child health and use of services, emergency attendances and admissions. |
| Starting date | 06/04/2009‐19/09/2014. |
| Contact information | Owen‐[email protected] |
| Notes | Michael Robling also an author. The trial appears to be finished. |
| Trial name or title | The Tanzania Connect Project: a cluster‐randomised trial of the child survival impact of adding paid community health workers to an existing facility‐focused health system. Location: 3 rural districts in Tanzania, with a population of roughly 360,000 ( Kilombero, Rufiji, and Ulanga). Trial Registration: ISRCTN96819844. |
| Methods | Cluster‐randomised trial with the following objectives.
|
| Participants | 1. Women who reside within the catchment population of the Ifakara and Rufiji HDSS. 2. The household survey will include roughly 3000 households. Households can be included only if: 2.1. have women of reproductive age (15‐49 years of age), or be the primary care takers of at least 1 under 5‐year old child for women more than 49 years old; 2.2. the population to be enrolled as participants in the household survey will, in most cases, be non‐English speaking, educationally‐ and economically‐disadvantaged. Kiswahili is the national language of Tanzania and residents of rural villages are typically impoverished and with limited means to adequate schooling. No children under the age of 15 will be enrolled. All women of reproductive age will be enrolled as participants; 3. Health workers – any Community Health Agents (CHAs) and government health employees within the study districts. |
| Interventions | Intervention: integrated community health services strengthening (addition of community health agents or CHA, and emergency health services); health service and facility strengthening (health workforce training, communications and information systems improvement, facility and medicines/supplies strengthening, financial and social protection including reducing emergency transport costs, improvements in local planning and referral). Controls: no CHAs. |
| Outcomes | Several maternal, newborn and child health outcomes. Sample size based on child, infant and newborn mortality. |
| Starting date | Overall trial start date: 01/07/2010. Overall trial end date: 31/07/2014. |
| Contact information | Primary contact: Dr James Phillips 60 Haven Ave |
| Notes | Funder: Doris Duke Charitable Foundation DDCF 2009058 (USA). |
| Trial name or title | PROCOMIDA ‐ Strengthening and Evaluating the Preventing Malnutrition in Children under 2 Approach in Guatemala: Report of the Enrollment Survey. Location: Guatemala. Trial Registration: ClinicalTrials.gov Identifier:NCT01072279. |
| Methods | Cluster‐randomised trial ‐ enrolment survey component of the impact evaluation. |
| Participants | "Women can enrol in PROCOMIDA at any stage during pregnancy or lactation if the lactating woman has a child under 6 months of age or can enrol her child between the ages of 6 and 18 months. Children graduate from the program when they are 23 months of age." Participants in the enrolment survey, undertaken to establish a baseline for the trial, were between 3 and 7 months pregnant. |
| Interventions | Target: health systems. The trial "aims to lower the prevalence of child malnutrition by targeting all pregnant women, mothers of children 0–23 months, and children under 2 in food‐insecure areas with a package of health and nutrition interventions. PROCOMIDA is implemented by Mercy Corps in Alta Verapaz, Guatemala." There are 6 study arms: Group A: full family ration (rice, pinto beans, and oil), individual ration (corn‐soy blend [CSB]), BCC, and required health visits. Group B: reduced family ration (rice, pinto beans, and oil), individual ration (CSB), BCC, and required health visits. Group C: no family ration, individual ration (CSB), BCC, and required health visits. Group D: full family ration (rice, pinto beans, and oil), lipid‐based nutrient supplement (LNS) as the individual ration, BCC, and required health visits. Group E: full family ration (rice, pinto beans, and oil), micronutrient powder (MNP) supplement as the individual ration, BCC, and required health visits. Group F: control group: does not receive PROCOMIDA (i.e. does not receive family or individual rations or BCC messages) and is not required to attend health visits; however, families in the control group do have access to standard MOH health services. |
| Outcomes | Primary outcome: child nutritional status. Secondary outcome: multiple outcomes to do with women's and children's health, process indicators. |
| Starting date | April 2010‐Dec 2015. |
| Contact information | Marie Ruel, IFPRI. |
| Notes | Funding ‐ International Food Policy Research Institute, Food and Nutrition Technical Assistance Project 2 of the Academy of Educational Development, United States Agency for International Development (USAID), Catholic Relieve Services‐Burundi, Mercy Corps‐Guatemala, Guatemal Ministry of Health. This 2013 report provides the results of the first round of a longitudinal study, undertaken to establish baseline values and confirm randomisation. |
| Trial name or title | Familia Salama: a cluster‐randomised health systems implementation study. Location: Dar es Salaam, Tanzania. Trial registration: ClinicalTrials.gov: EJF22802. |
| Methods | A 2 by 2 factorial cluster‐randomised trial to test the effectiveness, cost‐effectiveness, feasibility, and acceptability of a community health worker intervention in improving ANC and PMTCT outcomes. |
| Participants | All pregnant women in the study areas. |
| Interventions | 2 by 2 factorial design. The 2 urban districts of Kinondoni and Ilala districts in which the trial takes place have 60 wards. These wards were randomly allocated to either the community health worker intervention (36 wards) or the normal standard of care in Dar es Salaam (24 wards). The 2 arms were then randomised again to receiving either WHO Option A or Option B for prevention of mother‐to‐child transmission of HIV (PMTCT). The number of clusters in the arms varies to reflect the difference in the population sizes in each ward. The community health workers: (1) identify pregnant women through home visits and refer them to ANC; (2) provide education to pregnant women on ANC, PMTCT, birth, and postnatal care; (3) routinely follow up on all pregnant women to ascertain whether they have attended ANC; and (4) follow up on women who have missed ANC or PMTCT appointments. Under the standard of care, facility‐based health workers follow up on patients who have missed scheduled appointments for PMTCT, first through a telephone call and then with a home visit. |
| Outcomes | Primary outcomes: (1) the percentage of pregnant women making at least 4 antenatal clinic visits (as recommended by WHO); (2) the percentage of pregnant women delivering at a healthcare facility; Secondary outcomes: (1) the percentage of pregnant women who were tested for HIV during pregnancy or labour and delivery; (2) the number of weeks of gestation at which pregnant women attend ANC for the first time; (3) the percentage of HIVinfected pregnant women who completed PMTCT; and (4) the percentage of HIV‐exposed infants who received PMTCT. |
| Starting date | The trial is being carried out over a period of 17 months from January 2013 to May 2014. |
| Contact information | Till W Bärnighausen: [email protected] |
| Notes | The study is carried out by Management and Development for Health (MDH, Tanzania). The Harvard School of Public Health provides technical assistance for the trial of the CHW intervention. MDH is a Tanzanian organisation based in Dar es Salaam, which works in partnership with Tanzania’s Ministry of Health and Social Welfare. It provides technical and financial support to 50 HIV treatment sites, 17 tuberculosis clinics, and 180 PMTCT outlets across Dar es Salaam. |
| Trial name or title | Impact Evaluation of the Pilot SMS Mother Reminder System. Location: Uganda. Trial Registration: ClinicalTrials.gov Identifier: NCT02121821. |
| Methods | Randomised trial. |
| Participants | All pregnant women attending ANC. Estimated enrolment: 11,454. |
| Interventions | Target: health systems. Arm 1: village health teams and SMS reminder system. Arm 2: Standard care. |
| Outcomes | Primary outcome: ANC visits: pregnant women attend 4 ANC visits. Secondary outcome: sulfadoxine‐pyrimethamine (SP) doses: pregnant women receive at least 2 doses of intermittent preventive treatment of malaria in pregnancy using SP. Other maternal health outcomes and process outcomes. |
| Starting date | May 2014‐May 2015 (preliminary data). |
| Contact information | Lungi Okoko: [email protected]. PI ‐ Donald Shepard, Brandeis University (Massachussetts, USA). |
| Notes | Funding: African Strategies for Health, United States Agency for International Development (USAID). |
| Trial name or title | MIRA Dhanusha: Community interventions to reduce child mortality in Dhanusha, Nepal. Trial Registration: ISRCTN87820538. |
| Methods | Cluster‐randomised trial. |
| Participants | Pregnant women and infants up to 1 year of age. |
| Interventions | Arm 1: women's groups, female community health volunteers trained to care for vulnerable infants. Arm 2: standard care. |
| Outcomes | Primary outcome: neonatal mortality. Secondary outcome: MIRA Dhanusha community group: stillbirth, infant and under‐2 mortality rates, care practices and healthcare‐seeking behaviour, maternal diet, breastfeeding and complementary feeding practices, maternal and under‐2 anthropometric status. MIRA Dhanusha sepsis management: identification and treatment of neonatal sepsis by community health volunteers, infection‐specific neonatal mortality. |
| Starting date | Jan 2008‐Jan 2010. |
| Contact information | Naomi Saville: [email protected] |
| Notes | Funding: UBS Optimus Foundation (Switzerland). This trial has finished, but we have not located published data. |
| Trial name or title | Effectiveness of score card‐based antenatal risk selection, care pathways, and multidisciplinary consultation in the Healthy Pregnancy 4 All study (HP4ALL). Location: The Netherlands. Trial Registration: Dutch Trial Registry (NTR‐3367). |
| Methods | Cluster‐randomised trial. |
| Participants | All midwives and gynaecologists providing care to women living in these zip codes will be invited to participate in the Healthy Pregnancy 4 All study. All pregnant women living in these selected areas are eligible for this trial. All municipalities deal with an above‐average perinatal mortality rate and many disadvantaged neighbourhoods. Exclusion criteria include an acute obstetric situation during the booking visit (for example, ectopic pregnancy) and women in labour during this initial visit. |
| Interventions | Intervention: use of the R4U scorecard, corresponding care pathways, and multidisciplinary consultation. Control: standard ANC. The follow‐up period consists of 6 weeks. Details of pregnancy, delivery, and maternal follow‐up will be recorded after 6 weeks in a case record form. If necessary, medical records of newborns will be requested (if consent is provided). |
| Outcomes | Primary outcome: small for gestational age (< 10th percentile) and preterm birth (gestational age below 37 weeks). Secondary outcome: |
| Starting date | Randomisation took place January 2011; Recruitment began August 2012. |
| Contact information | Amber A Vos ([email protected]) |
| Notes | This study is funded by the Dutch government, Ministry of Welfare and Sports (VWS), grant 318804. |
ANC: antenatal care
CHA: Community Health Agents
NMR: neonatal mortality rate
PMTCT: prevention of mother‐to‐child transmission
PNC: perinatal mortality rate
SBA: skilled birth attendants
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits Show forest plot | 10 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 One intervention versus no intervention, Outcome 1 ANC coverage: four or more visits. | ||||
| 1.1 Primary analysis (ICC 0.02 for studies Kirkwood and Morris) | 10 | 45022 | Odds Ratio (Random, 95% CI) | 1.11 [1.01, 1.22] |
| 1.2 Sensitivity analysis using ICC 0.08 | 10 | 45022 | Odds Ratio (Random, 95% CI) | 1.11 [1.00, 1.22] |
| 2 Pregnancy‐related deaths Show forest plot | 10 | 114930 | Odds Ratio (Random, 95% CI) | 0.69 [0.45, 1.08] |
| Analysis 1.2 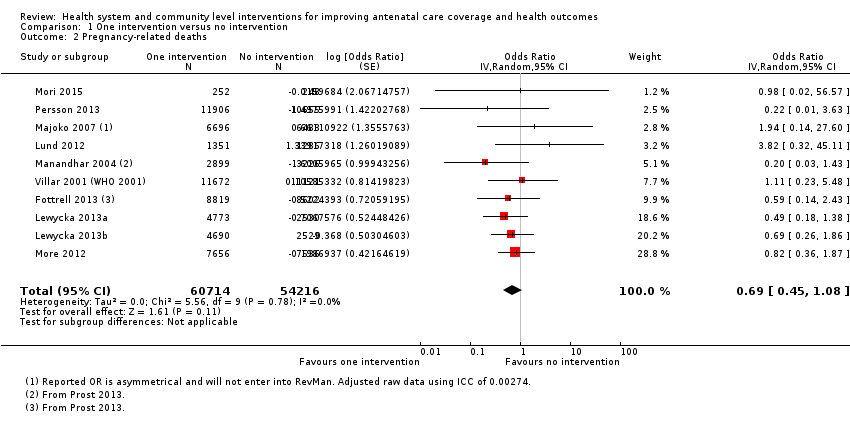 Comparison 1 One intervention versus no intervention, Outcome 2 Pregnancy‐related deaths. | ||||
| 3 ANC coverage: one or more visits Show forest plot | 6 | Odds Ratio (Random, 95% CI) | 1.68 [1.02, 2.79] | |
| Analysis 1.3 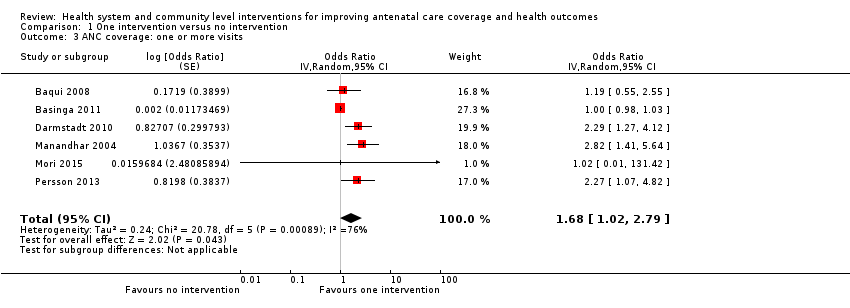 Comparison 1 One intervention versus no intervention, Outcome 3 ANC coverage: one or more visits. | ||||
| 4 Pregnant women initiating ANC in first trimester Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 1.20 [0.99, 1.45] | |
| Analysis 1.4 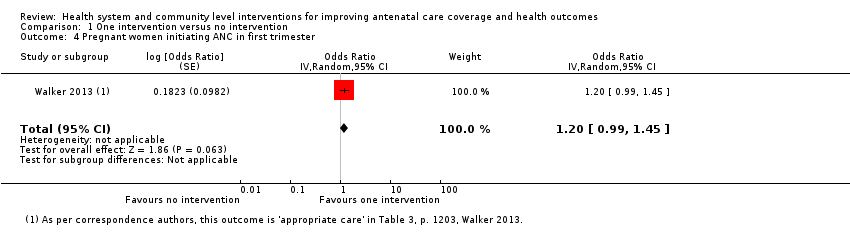 Comparison 1 One intervention versus no intervention, Outcome 4 Pregnant women initiating ANC in first trimester. | ||||
| 5 Pregnant women receiving ANC from health professional Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 1.13 [0.84, 1.52] | |
| Analysis 1.5 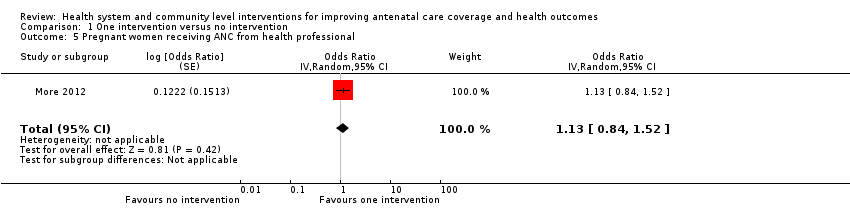 Comparison 1 One intervention versus no intervention, Outcome 5 Pregnant women receiving ANC from health professional. | ||||
| 6 Deliveries in a health facility Show forest plot | 10 | Odds Ratio (Random, 95% CI) | 1.08 [1.02, 1.15] | |
| Analysis 1.6  Comparison 1 One intervention versus no intervention, Outcome 6 Deliveries in a health facility. | ||||
| 7 Intermittent Prophylactic Treatment for malaria | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Proportion of women with tetanus protection at birth Show forest plot | 8 | Odds Ratio (Random, 95% CI) | 1.03 [0.92, 1.15] | |
| Analysis 1.8 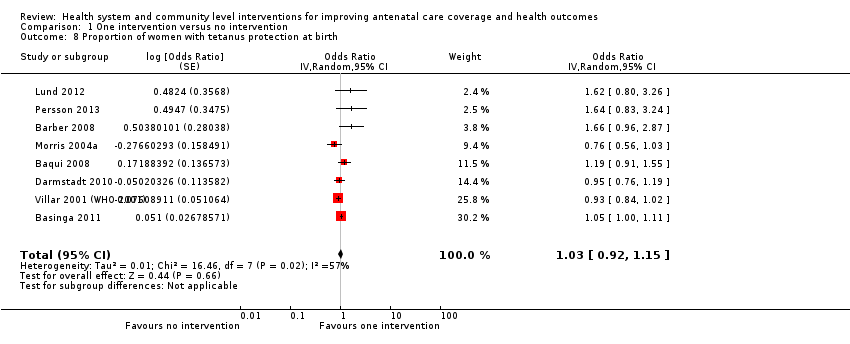 Comparison 1 One intervention versus no intervention, Outcome 8 Proportion of women with tetanus protection at birth. | ||||
| 9 Proportion of women treated for syphilis Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 1.46 [0.94, 2.26] | |
| Analysis 1.9 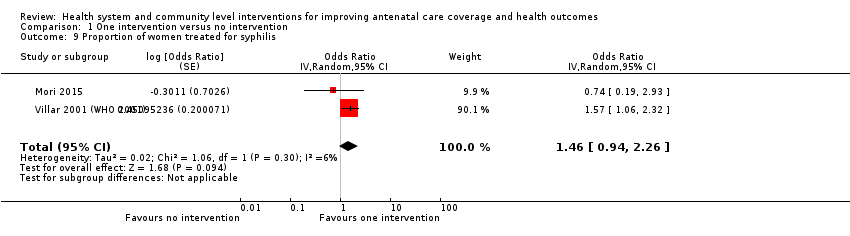 Comparison 1 One intervention versus no intervention, Outcome 9 Proportion of women treated for syphilis. | ||||
| 10 Proportion of women with HIV who receive a complete antiretroviral course for prevention of mother‐to‐child transmission of HIV Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 0.44 [0.26, 0.74] | |
| Analysis 1.10  Comparison 1 One intervention versus no intervention, Outcome 10 Proportion of women with HIV who receive a complete antiretroviral course for prevention of mother‐to‐child transmission of HIV. | ||||
| 11 Preterm labour Show forest plot | 4 | Odds Ratio (Random, 95% CI) | 1.00 [0.93, 1.09] | |
| Analysis 1.11 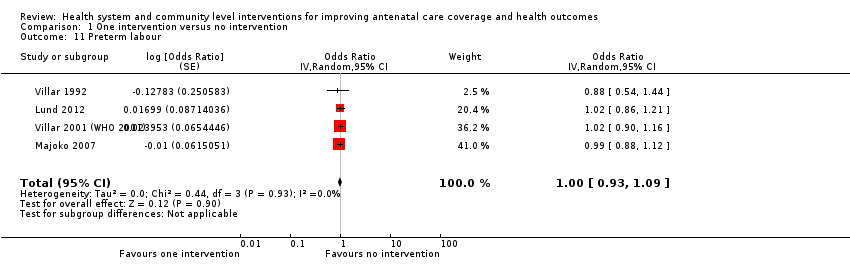 Comparison 1 One intervention versus no intervention, Outcome 11 Preterm labour. | ||||
| 12 Low birthweight Show forest plot | 5 | Odds Ratio (Random, 95% CI) | 0.94 [0.82, 1.06] | |
| Analysis 1.12  Comparison 1 One intervention versus no intervention, Outcome 12 Low birthweight. | ||||
| 13 Perinatal mortality Show forest plot | 15 | Odds Ratio (Random, 95% CI) | 0.96 [0.89, 1.03] | |
| Analysis 1.13 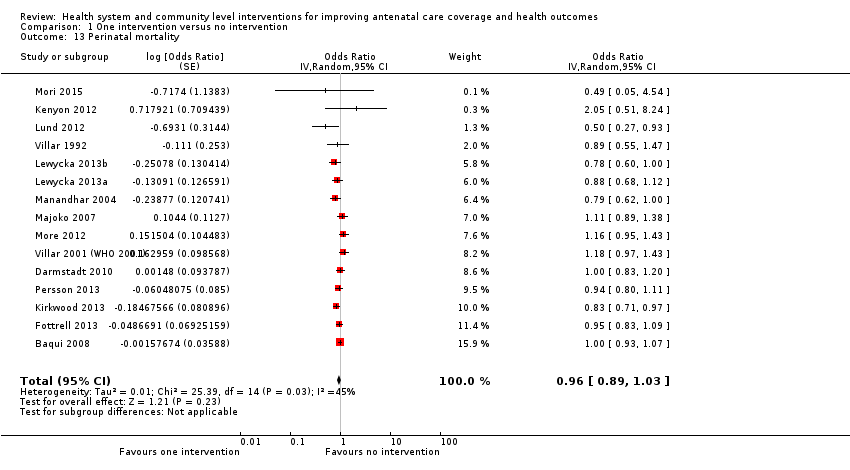 Comparison 1 One intervention versus no intervention, Outcome 13 Perinatal mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits Show forest plot | 6 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 2.1 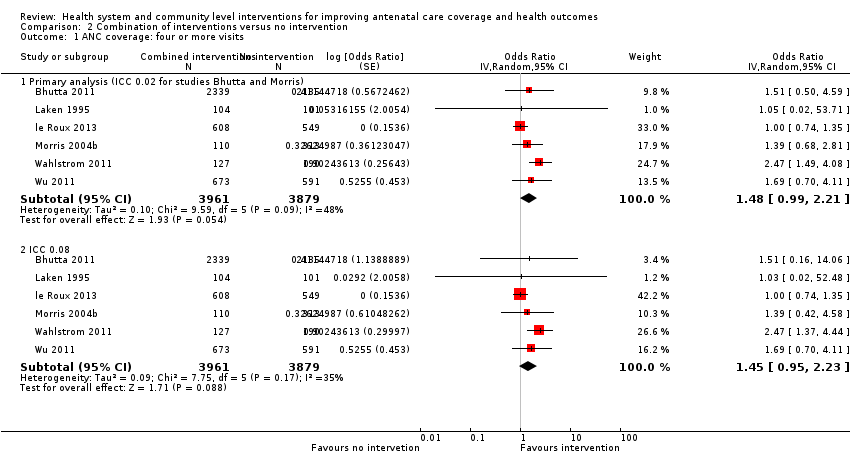 Comparison 2 Combination of interventions versus no intervention, Outcome 1 ANC coverage: four or more visits. | ||||
| 1.1 Primary analysis (ICC 0.02 for studies Bhutta and Morris) | 6 | 7840 | Odds Ratio (Random, 95% CI) | 1.48 [0.99, 2.21] |
| 1.2 ICC 0.08 | 6 | 7840 | Odds Ratio (Random, 95% CI) | 1.45 [0.95, 2.23] |
| 2 Pregnancy‐related deaths Show forest plot | 3 | 13756 | Odds Ratio (Random, 95% CI) | 0.70 [0.39, 1.26] |
| Analysis 2.2  Comparison 2 Combination of interventions versus no intervention, Outcome 2 Pregnancy‐related deaths. | ||||
| 3 ANC coverage: one or more visits Show forest plot | 5 | Odds Ratio (Random, 95% CI) | 1.79 [1.47, 2.17] | |
| Analysis 2.3 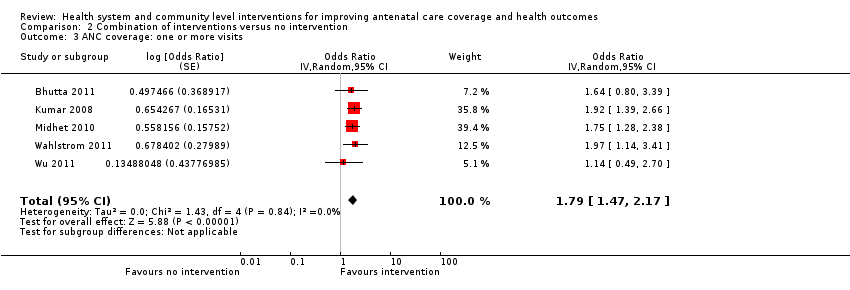 Comparison 2 Combination of interventions versus no intervention, Outcome 3 ANC coverage: one or more visits. | ||||
| 4 Pregnant women initiating ANC in first trimester Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 0.83 [0.47, 1.47] | |
| Analysis 2.4 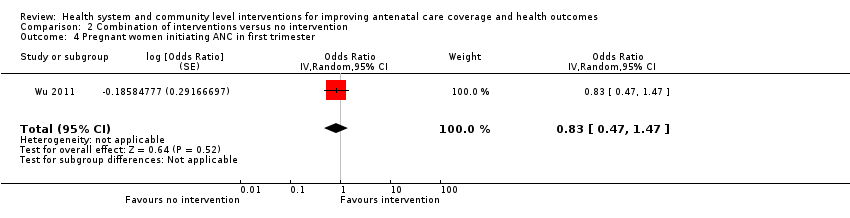 Comparison 2 Combination of interventions versus no intervention, Outcome 4 Pregnant women initiating ANC in first trimester. | ||||
| 5 Pregnant women receiving ANC from health professional Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 2.97 [1.67, 5.30] | |
| Analysis 2.5 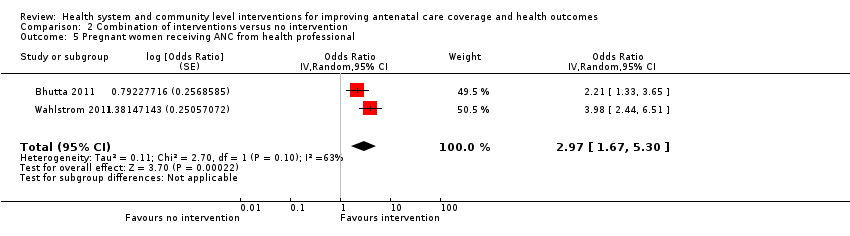 Comparison 2 Combination of interventions versus no intervention, Outcome 5 Pregnant women receiving ANC from health professional. | ||||
| 6 Deliveries in a health facility Show forest plot | 5 | Odds Ratio (Random, 95% CI) | 1.53 [0.96, 2.43] | |
| Analysis 2.6  Comparison 2 Combination of interventions versus no intervention, Outcome 6 Deliveries in a health facility. | ||||
| 7 Intermittent Prophylactic Treatment for malaria | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Proportion of women with tetanus protection at birth Show forest plot | 3 | Odds Ratio (Random, 95% CI) | 1.48 [1.18, 1.87] | |
| Analysis 2.8  Comparison 2 Combination of interventions versus no intervention, Outcome 8 Proportion of women with tetanus protection at birth. | ||||
| 9 Preterm labour Show forest plot | 1 | 607 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.45, 1.20] |
| Analysis 2.9  Comparison 2 Combination of interventions versus no intervention, Outcome 9 Preterm labour. | ||||
| 10 Low birthweight Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 0.61 [0.46, 0.80] | |
| Analysis 2.10  Comparison 2 Combination of interventions versus no intervention, Outcome 10 Low birthweight. | ||||
| 11 Perinatal mortality Show forest plot | 5 | Odds Ratio (Random, 95% CI) | 0.74 [0.57, 0.95] | |
| Analysis 2.11  Comparison 2 Combination of interventions versus no intervention, Outcome 11 Perinatal mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Pregnancy‐related deaths | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 ANC coverage: one or more visits | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Deliveries in a health facility | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Perinatal mortality | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Low birthweight | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Intermittent Prophylactic Treatment for malaria | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 0.99 [0.70, 1.40] | |
| Analysis 4.1 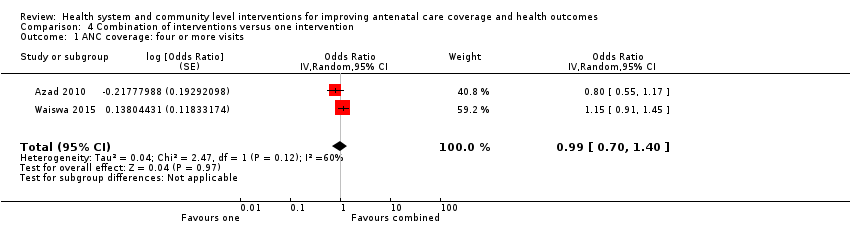 Comparison 4 Combination of interventions versus one intervention, Outcome 1 ANC coverage: four or more visits. | ||||
| 2 Pregnancy‐related deaths Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 1.00 [0.52, 1.96] | |
| Analysis 4.2  Comparison 4 Combination of interventions versus one intervention, Outcome 2 Pregnancy‐related deaths. | ||||
| 3 ANC coverage: one or more visits Show forest plot | 3 | Odds Ratio (Random, 95% CI) | 0.86 [0.61, 1.20] | |
| Analysis 4.3  Comparison 4 Combination of interventions versus one intervention, Outcome 3 ANC coverage: one or more visits. | ||||
| 4 Deliveries in a health facility Show forest plot | 3 | Odds Ratio (Random, 95% CI) | 0.95 [0.69, 1.30] | |
| Analysis 4.4  Comparison 4 Combination of interventions versus one intervention, Outcome 4 Deliveries in a health facility. | ||||
| 5 Perinatal mortality Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 0.88 [0.72, 1.07] | |
| Analysis 4.5 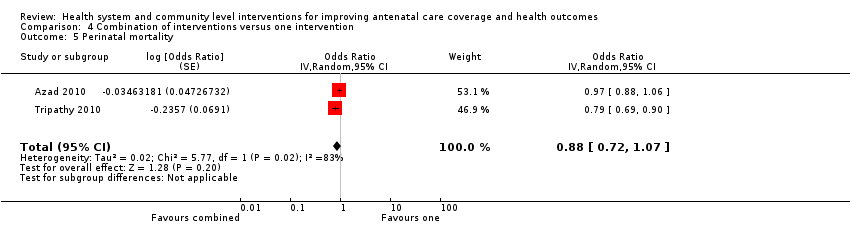 Comparison 4 Combination of interventions versus one intervention, Outcome 5 Perinatal mortality. | ||||
| 6 Proportion of women with tetanus protection at birth Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 1.07 [0.80, 1.43] | |
| Analysis 4.6  Comparison 4 Combination of interventions versus one intervention, Outcome 6 Proportion of women with tetanus protection at birth. | ||||
| 7 Low birthweight | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Intermittent Prophylactic Treatment for malaria | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits Show forest plot | 1 | 383 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.41, 1.43] |
| Analysis 5.1 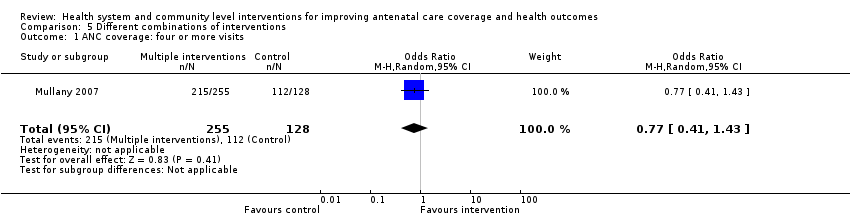 Comparison 5 Different combinations of interventions, Outcome 1 ANC coverage: four or more visits. | ||||
| 2 Pregnancy‐related deaths | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 ANC coverage: one or more visits | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Deliveries in a health facility Show forest plot | 1 | 383 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [0.71, 3.37] |
| Analysis 5.4  Comparison 5 Different combinations of interventions, Outcome 4 Deliveries in a health facility. | ||||
| 5 Perinatal mortality | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Low birthweight | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Intermittent Prophylactic Treatment for malaria | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Health systems vs Population ANC coverage: four or more visits Show forest plot | 9 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 6.1  Comparison 6 Subgroup analysis, Outcome 1 Health systems vs Population ANC coverage: four or more visits. | ||||
| 1.1 Health system interventions | 5 | Odds Ratio (Random, 95% CI) | 1.13 [0.96, 1.34] | |
| 1.2 Population interventions | 4 | Odds Ratio (Random, 95% CI) | 1.04 [0.96, 1.13] | |
| 2 Health systems vs Population Pregnancy‐related deaths Show forest plot | 11 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 6.2  Comparison 6 Subgroup analysis, Outcome 2 Health systems vs Population Pregnancy‐related deaths. | ||||
| 2.1 Health system interventions | 4 | Odds Ratio (Random, 95% CI) | 1.22 [0.41, 3.65] | |
| 2.2 Population intervention | 7 | Odds Ratio (Random, 95% CI) | 0.69 [0.46, 1.03] | |
| 3 Country Income Low vs High ANC at least 4 visits Show forest plot | 18 | Odds Ratio (Random, 95% CI) | 1.14 [1.04, 1.25] | |
| Analysis 6.3  Comparison 6 Subgroup analysis, Outcome 3 Country Income Low vs High ANC at least 4 visits. | ||||
| 3.1 Low or lower middle income countries | 11 | Odds Ratio (Random, 95% CI) | 1.21 [1.04, 1.40] | |
| 3.2 High or higher middle income countries | 7 | Odds Ratio (Random, 95% CI) | 1.12 [0.95, 1.32] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits Show forest plot | 9 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 7.1  Comparison 7 One intervention versus no intervention ‐ Sensitivity analysis by risk of bias, Outcome 1 ANC coverage: four or more visits. | ||||
| 1.1 Primary analysis (ICC 0.02 for studies Kirkwood and Morris) | 9 | Odds Ratio (Random, 95% CI) | 1.07 [0.99, 1.15] | |
| 1.2 Sensitivity analysis using ICC 0.08 | 9 | Odds Ratio (Random, 95% CI) | 1.05 [0.98, 1.14] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits Show forest plot | 4 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 8.1  Comparison 8 Combination of interventions versus no intervention ‐ Sensitivity analysis by risk of bias, Outcome 1 ANC coverage: four or more visits. | ||||
| 1.1 Primary analysis (ICC 0.02 for studies Bhutta and Morris) | 4 | Odds Ratio (Random, 95% CI) | 1.07 [0.82, 1.40] | |
| 1.2 ICC 0.08 | 4 | Odds Ratio (Random, 95% CI) | 1.03 [0.77, 1.37] | |

Study flow diagram.
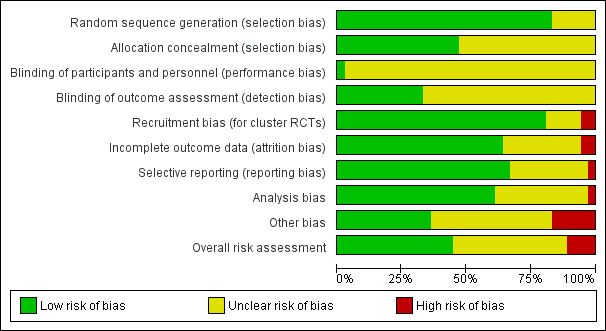
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Funnel plot of comparison: 1 One intervention versus no intervention, outcome: 1.1 ANC coverage: four or more visits.
Funnel plot of comparison: 1 One intervention versus no intervention, outcome: 1.2 Pregnancy‐related deaths.
Funnel plot of comparison: 1 One intervention versus no intervention, outcome: 1.6 Deliveries in a health facility.
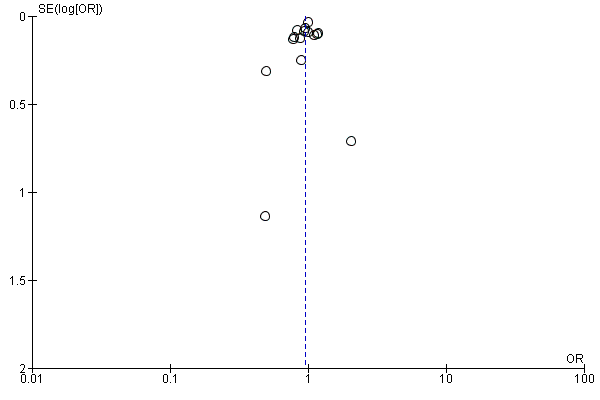
Funnel plot of comparison: 1 One intervention versus no intervention, outcome: 1.13 Perinatal mortality.
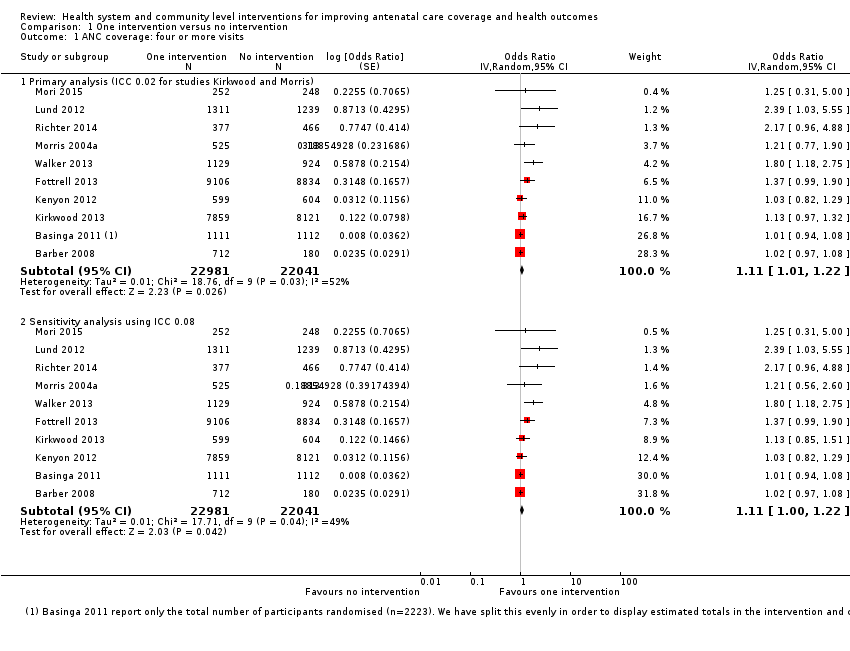
Comparison 1 One intervention versus no intervention, Outcome 1 ANC coverage: four or more visits.

Comparison 1 One intervention versus no intervention, Outcome 2 Pregnancy‐related deaths.
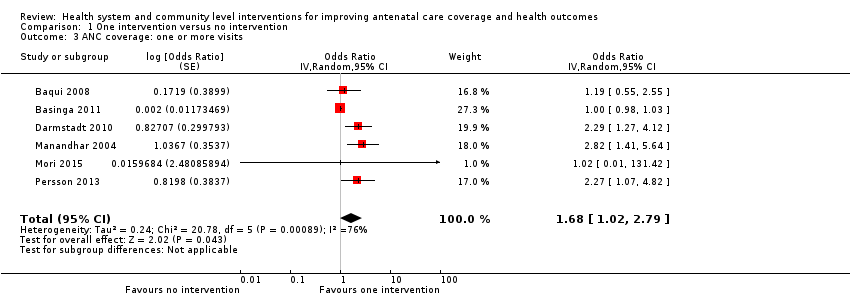
Comparison 1 One intervention versus no intervention, Outcome 3 ANC coverage: one or more visits.
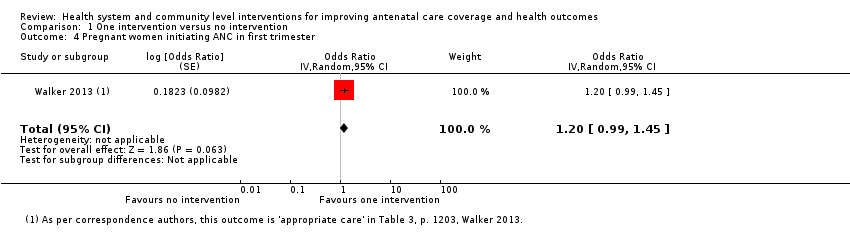
Comparison 1 One intervention versus no intervention, Outcome 4 Pregnant women initiating ANC in first trimester.
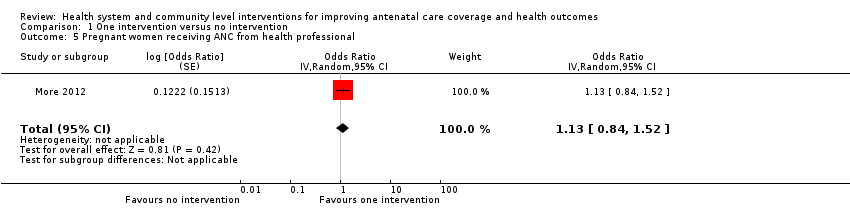
Comparison 1 One intervention versus no intervention, Outcome 5 Pregnant women receiving ANC from health professional.

Comparison 1 One intervention versus no intervention, Outcome 6 Deliveries in a health facility.

Comparison 1 One intervention versus no intervention, Outcome 8 Proportion of women with tetanus protection at birth.
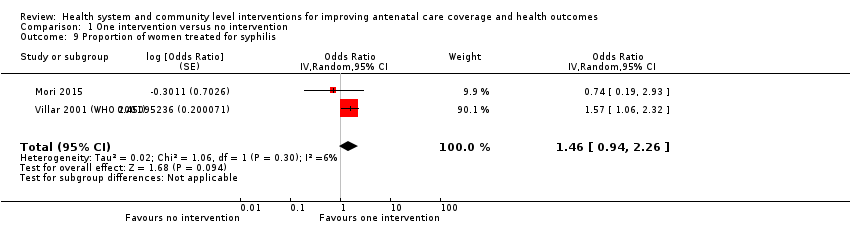
Comparison 1 One intervention versus no intervention, Outcome 9 Proportion of women treated for syphilis.
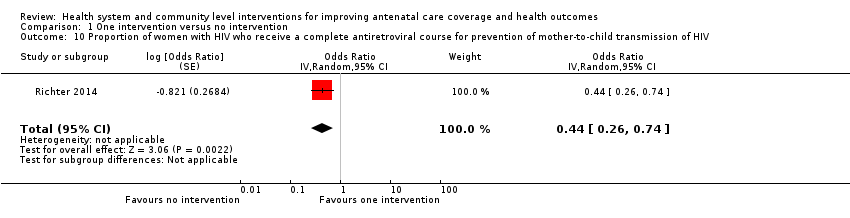
Comparison 1 One intervention versus no intervention, Outcome 10 Proportion of women with HIV who receive a complete antiretroviral course for prevention of mother‐to‐child transmission of HIV.

Comparison 1 One intervention versus no intervention, Outcome 11 Preterm labour.

Comparison 1 One intervention versus no intervention, Outcome 12 Low birthweight.
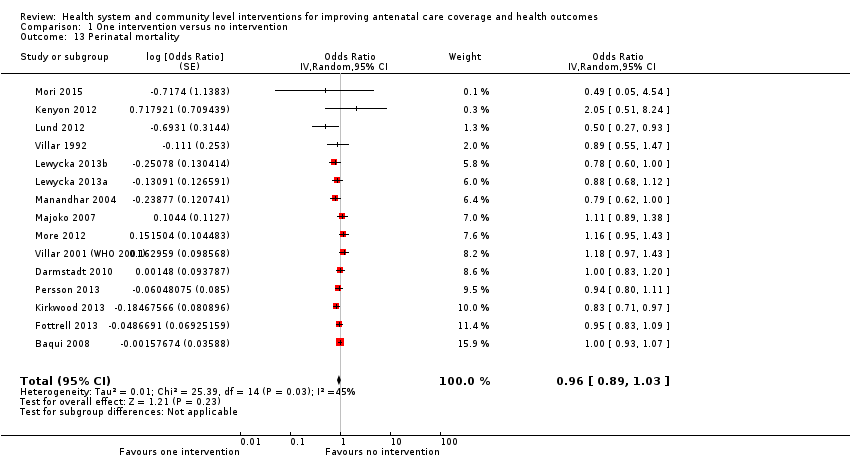
Comparison 1 One intervention versus no intervention, Outcome 13 Perinatal mortality.

Comparison 2 Combination of interventions versus no intervention, Outcome 1 ANC coverage: four or more visits.
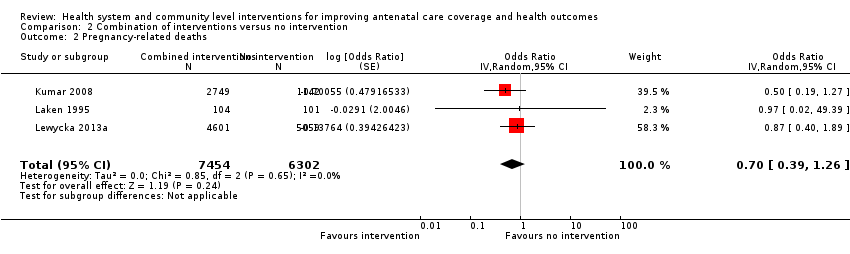
Comparison 2 Combination of interventions versus no intervention, Outcome 2 Pregnancy‐related deaths.
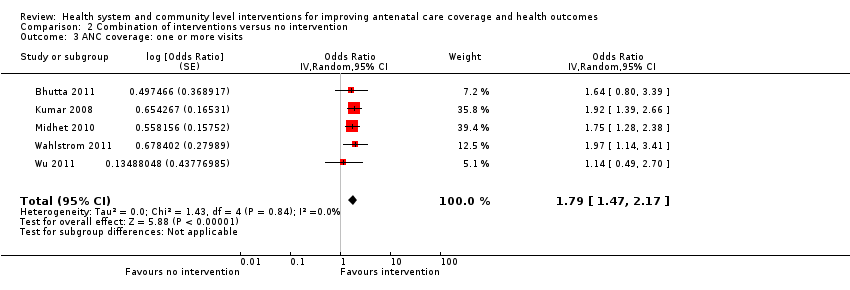
Comparison 2 Combination of interventions versus no intervention, Outcome 3 ANC coverage: one or more visits.

Comparison 2 Combination of interventions versus no intervention, Outcome 4 Pregnant women initiating ANC in first trimester.
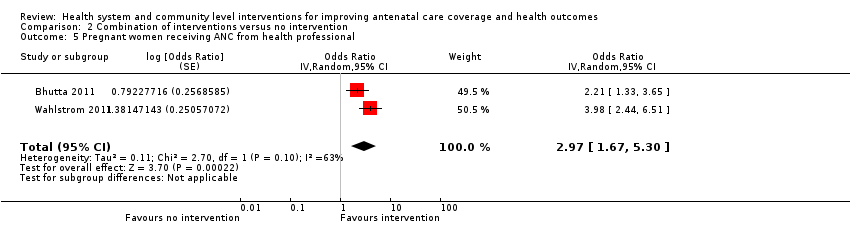
Comparison 2 Combination of interventions versus no intervention, Outcome 5 Pregnant women receiving ANC from health professional.
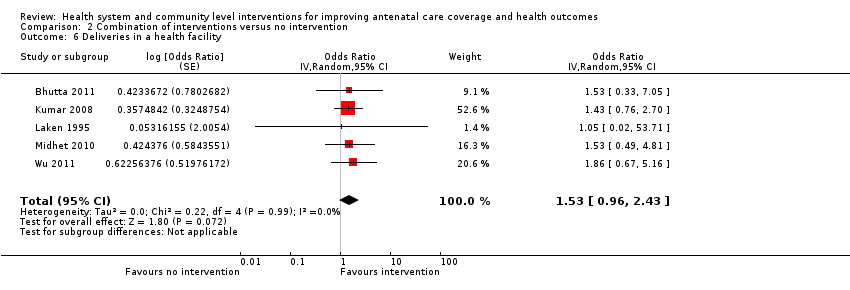
Comparison 2 Combination of interventions versus no intervention, Outcome 6 Deliveries in a health facility.
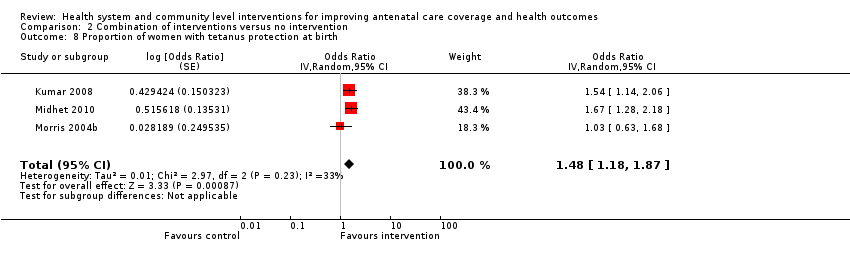
Comparison 2 Combination of interventions versus no intervention, Outcome 8 Proportion of women with tetanus protection at birth.
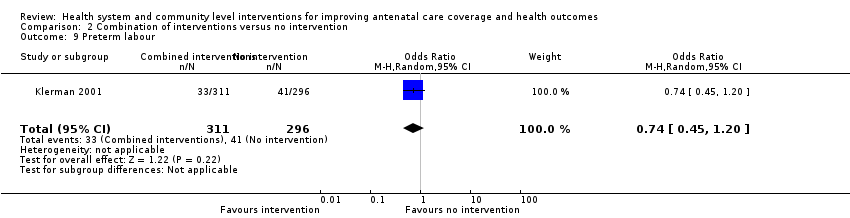
Comparison 2 Combination of interventions versus no intervention, Outcome 9 Preterm labour.

Comparison 2 Combination of interventions versus no intervention, Outcome 10 Low birthweight.
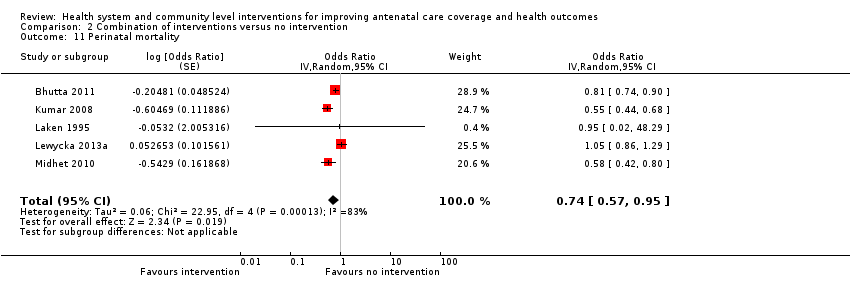
Comparison 2 Combination of interventions versus no intervention, Outcome 11 Perinatal mortality.

Comparison 4 Combination of interventions versus one intervention, Outcome 1 ANC coverage: four or more visits.

Comparison 4 Combination of interventions versus one intervention, Outcome 2 Pregnancy‐related deaths.

Comparison 4 Combination of interventions versus one intervention, Outcome 3 ANC coverage: one or more visits.
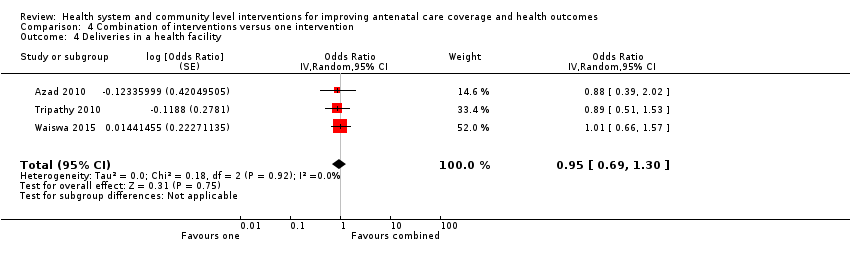
Comparison 4 Combination of interventions versus one intervention, Outcome 4 Deliveries in a health facility.

Comparison 4 Combination of interventions versus one intervention, Outcome 5 Perinatal mortality.
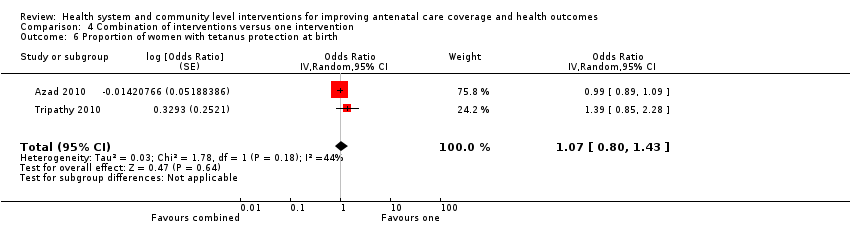
Comparison 4 Combination of interventions versus one intervention, Outcome 6 Proportion of women with tetanus protection at birth.
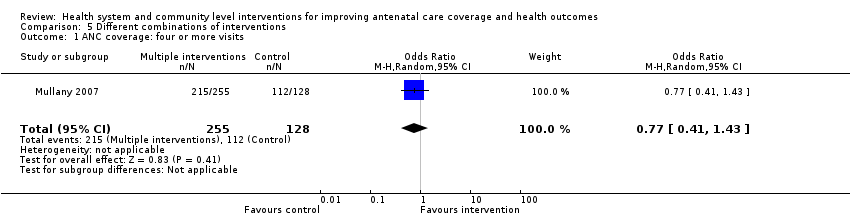
Comparison 5 Different combinations of interventions, Outcome 1 ANC coverage: four or more visits.

Comparison 5 Different combinations of interventions, Outcome 4 Deliveries in a health facility.
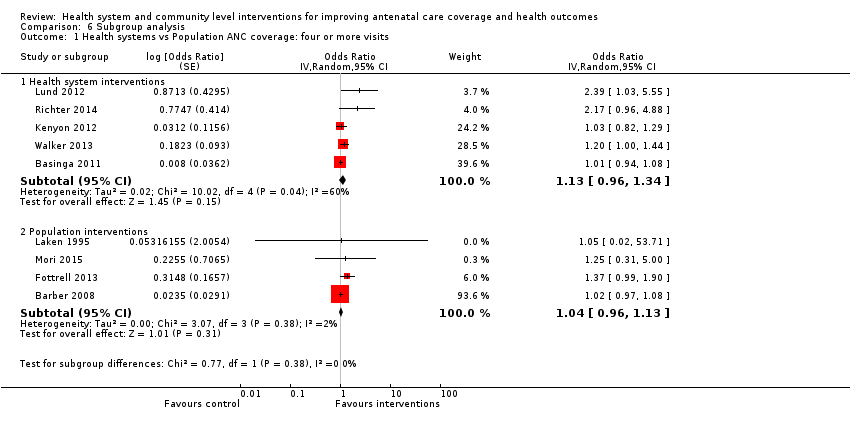
Comparison 6 Subgroup analysis, Outcome 1 Health systems vs Population ANC coverage: four or more visits.
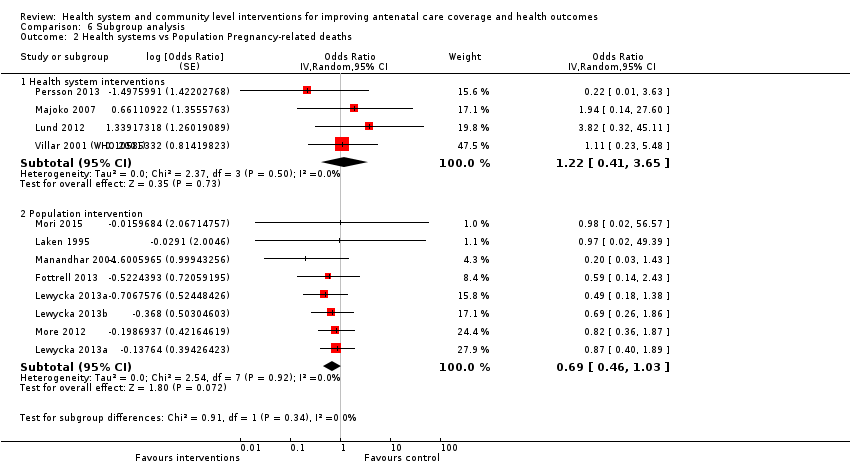
Comparison 6 Subgroup analysis, Outcome 2 Health systems vs Population Pregnancy‐related deaths.

Comparison 6 Subgroup analysis, Outcome 3 Country Income Low vs High ANC at least 4 visits.

Comparison 7 One intervention versus no intervention ‐ Sensitivity analysis by risk of bias, Outcome 1 ANC coverage: four or more visits.
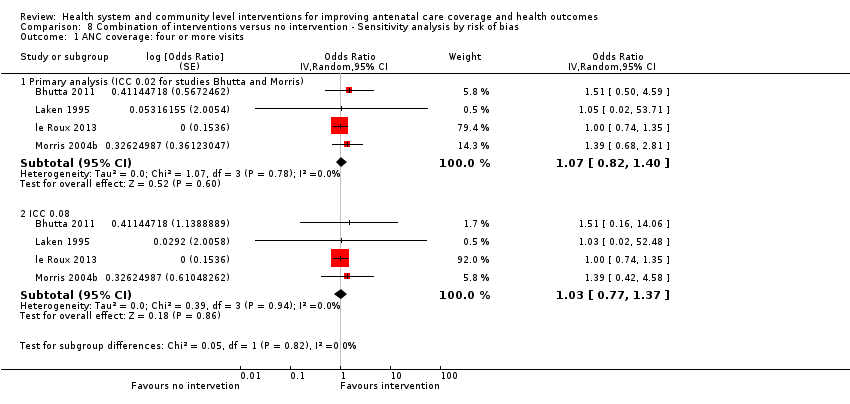
Comparison 8 Combination of interventions versus no intervention ‐ Sensitivity analysis by risk of bias, Outcome 1 ANC coverage: four or more visits.
| Comparison 1: One intervention versus no intervention | ||||||
| Patient or population: improving antenatal care coverage and health outcomes among pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no intervention | Risk with One intervention | |||||
| ANC coverage: four or more visits | Moderate | Average OR 1.11 | 45022 | ⊕⊕⊕⊕ | This is the primary analysis, ICC 0.02. | |
| 529 per 1000 | 555 per 1000 | |||||
| Pregnancy‐related deaths | Moderate | Average OR 0.69 | 114930 | ⊕⊕⊝⊝ | ||
| 700 per 1000000 | 483 per 1000000 | |||||
| ANC coverage: one or more visits | Moderate | Average OR 1.68 | 19281 | ⊕⊕⊕⊝ | ||
| 490 per 1000 | 617 per 1000 | |||||
| Deliveries in a health facility | Moderate | Average OR 1.08 | 74299 | ⊕⊕⊕⊕ | ||
| 645 per 1000 | 662 per 1000 | |||||
| Perinatal mortality | Moderate | Average OR 0.96 | 189164 | ⊕⊕⊕⊝ | ||
| 40 per 1000 | 38 per 1000 | |||||
| Low birthweight | Moderate | Average OR 0.94 | 27154 | ⊕⊕⊕⊕ | ||
| 125 per 1000 | 118 per 1000 | |||||
| Intermittent Prophylactic Treatment for malaria | Study population | not pooled | 00 | No trial included in this review reported this outcome. | ||
| not pooled | not pooled | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio. Denominators for the calculation of the absolute comparative effects have been taken from individual trial reports or from Prost 2013. Where different denominators are stated in different reports, we have taken the larger. The median control group risk has been calculated from event and participant raw data, where this was available. If we found no raw event and participant data in published reports, these trials were not included in the calculation of the median control group risk. Both the participant totals and the median control group risk are for illustrative purposes only. In the majority of the trials in this review, the final odds ratio presented will not correspond with raw event and participant data due to adjustments made for the effects of cluster design. We have designated the control risk as moderate because it is based on the median of a wide range of baseline rates in control groups. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Statistical heterogeneity, I2 = 52%; we did not downgrade for heterogeneity unless the I2 > 60%. 2 Downgraded one level due to serious risk of bias. Most weight from trials with design limitations (‐1). 3 Downgraded one level due to serious imprecision. Wide confidence interval crossing the line of no effect (‐1). 4 Downgraded one level due to serious inconsistency. Statistical heterogeneity, I2= 76% (‐1). 5 Statistical heterogeneity, I2= 58%; we did not downgrade for heterogeneity unless the I2 > 60%. | ||||||
| Comparison 2: Combination of interventions versus no intervention | ||||||
| Patient or population: improving antenatal care coverage and health outcomes among pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no intervention | Risk with Combination of interventions | |||||
| ANC coverage: four or more visits | Moderate | Average OR 1.48 | 7840 | ⊕⊕⊝⊝ | This is the primary analysis, ICC 0.02. | |
| 430 per 1000 | 528 per 1000 | |||||
| Pregnancy‐related deaths | Moderate | Average OR 0.70 | 13756 | ⊕⊕⊕⊝ | ||
| 600 per 100000 | 421 per 100000 | |||||
| ANC coverage: one or more visits | Moderate | Average OR 1.79 | 12426 | ⊕⊕⊕⊝ | ||
| 580 per 1000 | 712 per 1000 | |||||
| Deliveries in a health facility | Moderate | Average 1.53 | 12314 | ⊕⊕⊕⊝ | ||
| 165 per 1000 | 252 per 1000 | |||||
| Perinatal mortality | Moderate | Average 0.74 | 39130 | ⊕⊕⊕⊝ | ||
| 90 per 1000 | 67 per 1000 | |||||
| Low birthweight | Moderate | Average 0.61 | 2084 | ⊕⊕⊕⊝ | ||
| 165 per 1000 | 101 per 1000 | |||||
| Intermittent Prophylactic Treatment for malaria | Study population | not pooled | 00 | No trial eligible for this comparison reported this outcome | ||
| not pooled | not pooled | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).CI: Confidence interval; RR: Risk ratio; OR: Odds ratio. Denominators for the calculation of the absolute comparative effects have been taken from individual trial reports or from Prost 2013. Where different denominators are stated in different reports, we have taken the larger. The median control group risk has been calculated from event and participant raw data, where this was available. If we found no raw event and participant data in published reports, these trials were not included in the calculation of the median control group risk. Both the participant totals and the median control group risk are for illustrative purposes only. In the majority of the trials in this review, the final odds ratio presented will not correspond with raw event and participant data due to adjustments made for the effects of cluster design. We have designated the control risk as moderate because it is based on the median of a wide range of baseline rates in control groups. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most weight from trials with design limitations (‐1). 2 Statistical heterogeneity, I2 = 48% ; we did not downgrade for heterogeneity unless the I2 > 60%. 3 Wide confidence interval crossing the line of no effect (‐1). 4 Statistical heterogeneity, I2 = 83% (‐1). | ||||||
| Study | Measure of effect | Statistical approach1 | Intervention | Control |
| Barber 2008 | Beta coefficient: 0.0235 (Cash transfer, instrumental variable model; p. 1411, Barber 2008) | Calculate exp(beta) to get OR and CI | 712 | 180 |
| Basinga 2011 | Beta (95% CI): 0.008 (‐0.063 to 0.079) | Calculate exp(beta) to get OR and CI | Reported only the total n, stated as = 2223 | |
| Fottrell 2013 | Adjusted Odds Ratio (95%CI) 1.37 (0.99 to 1.88) | Cluster adjusted, straight into RevMan | 9106 | 8834 |
| Kenyon 2012 | RR = 1.01 (95% CI 0.91 to 1.13) | Non‐cluster trial, calculated OR | 322/599 | 320/604 |
| Kirkwood 2013 | RR 1·02 (0·96 to 1·09) | Adjusted using ICC 0.02 | 5975/7859 | 5988/8121 |
| Lund 2012 | Adjusted OR 2.39 (1.03 to 5.55) adjusted for cluster effect and significant variables | Cluster adjusted, straight into RevMan | 574/1311 | 385/1239 |
| Mori 2015 | Adjusted OR 1.25 (0.31 to 5.00) trial statistician (H Noma) calculated unpublished OR for this systematic review. OR adjusted for cluster effect and significant variables. | Cluster adjusted, straight into RevMan | 243/252 | 237/248 |
| Morris 2004 | Calculated from change scores, Table 2 Program effects p. 2034 of main report. | Adjusted using ICC 0.02 | 166/293 + 112/232 | 151/313 |
| Walker 2013 | Adjusted OR 95% CI 1.8(1.2 to 2.8). OR adjusted various characteristics and cluster ('in accordance with WHO standards' from Table 3, p. 1203 Walker 2013) | Cluster adjusted, straight into RevMan | 78/1129 | 39/924 |
| 1Main analysis with ICC of 0.02. We decided to use the same ICC for this outcome as for Proportion of women with one ANC visit. ICC of 0.02 provided in Manandhar 2004 and Wu 2011. We performed sensitivity analyses with the ICC of 0.08, a midrange of the ICC values in Pagel 2011. | ||||
| Study | Measure of effect | Statistical approach1 | Intervention | Control |
| Lewycka 20132 women's groups only | Adjusted | 14/4773 | 15/2530 | |
| Lewycka 2013a peer counselling only | Adjusted | 18/4690 | 14/2529 | |
| Lund 2014 | Adjusted | 4/1351 | 1/1286 | |
| Majoko 2007 | OR 1.94 (0.31‐15.2) | Adjusted raw data because the reported OR is asymmetrical. No reply from trial authors regarding our query. | 4/6696 | 2/6483 |
| Manandhar 2004 | Adjusted | 2/2899 | 11/3226 | |
| More 2012 | Adjusted | 20/7656 | 24/7536 | |
| Mori 2015 | Continuity correction for no events in either treatment arm. Unpublished data provided by trial author | 0/252 | 0/248 | |
| Persson 2013 | Adjusted | 1/11,906 | 4/10655 | |
| Fottrell 2013 | Adjusted | 14/8819 | 23/8602 | |
| Villar 2001 | Adjusted | 7/11672 | 6/11121 | |
| 1All data adjusted using an ICC of 0.00247 as suggested by Professor J P Souza by email. 2Mortality data from Trial Profile (Lewycka 2013, p. 1724). Control group (n = 5059) split for this analysis. | ||||
| Study | Measure of effect | Statistical approach1 | Intervention | Control |
| Basinga 2011 | beta (95% CI): 0.002 (‐0.021 to 0.025); | Calculated ln (OR) and se (ln OR) | Reported only the total n = 2309 | |
| Baqui 2008 | Adjusted | 2297/3421 | 828/1689 | |
| Darmstadt 2010 | Adjusted | 1192/1732 | 864/1759 | |
| Manandhar 2004 | Adjusted OR 2.82 (1.41 to 5.62) | Straight into RevMan. Worked out what ICC they used to adjust for clustering | 1747/3190 | 1051/3524 |
| Mori 2015 | Adjusted OR 1.02 (0.01 to 131.42) trial statistician provided unpublished OR adjusted for clustering and significant variables | Straight into RevMan | 252/252 | 248/248 |
| Persson 2013 | Adjusted OR 2.27 95% CI 1.07 to 4.80. For years 1‐3. | Straight into RevMan | 596/656 | 482/587 |
| 1ICC of 0.02 provided in Manandhar 2004 and Wu 2011. | ||||
| Study | Measure of effect | Statistical approach1 | Intervention | Control |
| Barber 2008 | Adjusted | 480/776 | 130/203 | |
| Basinga 2011 | beta (95% CI): 0.081 (0.015 to 0.146) | Calculated ln (OR) and se (ln OR) | Reported only total n = 2108 | |
| Darmstadt 2010 | Adjusted | 350/1732 | 290/1759 | |
| Fottrell 2013 | Adjusted Odds Ratio (95%CI) 1.05 (0.88 to 1.25) | Straight into RevMan | ||
| Kirkwood 2013 | Adjusted | 5373/7859 | 5539/8121 | |
| Mojoko 2007 | Adjusted OR for delivery at health centre 1.7 (0.88 to 3.0) ICC used 0.103 | Decided to use delivery at health centre outcome rather than hospital outcome. The OR is asymmetrical so will not go in RevMan ‐ have adjusted the data using the reported ICC 0.103 | Health centre 2660/5261 | 1986/5137 |
| Manandhar 2004 | Adjusted | 201/2945 | 66/3270 | |
| More 2012 | Adjusted OR 0.92 (0.58 to 1.47) | Straight into RevMan | 6602 / 7656 | 6573/ 7536 |
| Penfold 2014 | Adjusted | 187/256 | 166/254 | |
| Persson 2013 | Adjusted OR 1.88 95% CI 0.60 to 5.87. For years 1‐3. | Straight into RevMan | 594/656 | 510/587 |
| 1Data adjusted using ICC 0.103 from Majoko 2007. | ||||
| Trial | Measure of effect | Statistical approach1 | Intervention | Control |
| Kenyon 2012 | RR 0.92 (0.74, 1.14) P = 0.43 | Not cluster trial ‐ calculated OR | 127/605 | 141/613 |
| Mori 2015 | Adjusted OR 0.65 (0.26 to 1.59) trial statistician (H Noma) calculated unpublished OR adjusted for cluster effects and significant variables | Straight into RevMan | 8/251 | 12/247 |
| Richter 2014 | Adjusted | 67/377 | 52/414 | |
| Villar 1992 | OR 0.88 (95% CI 0.67 to 1.16) | Straight into RevMan | 115/1033 | 130/1040 |
| Villar 2001 | Adjusted | 910/11534 | 852/11040 | |
| 1ICC from Piaggio 2001 paper of 0.0004 for ANC trial with average cluster size 426. | ||||
| Trial | Effect Measure | Statistical approach1 | Intervention | Control |
| Baqui 2008 | Adjusted | 2552/32279 | 1260/15914 | |
| Darmstadt 2010 | Adjusted | 224/4800 | 255/5472 | |
| Fottrell 2013 | Risk Ratio (95% CI) adjusted without Tea‐garden residents 0.87 (0.62 to 1.22) | Calculated OR | 474/9106 | 503/8834 |
| Kenyon 2012 | RR 2.04 (0.51 to 8.12) P = 0.30 | Not a cluster trial. Calculated adjusted OR | 6/604 | 3/616 |
| Kirkwood 2013 | Adjusted | 288/8035 | 355/8294 | |
| Lewycka 2013a peer counselling only | Adjusted | 150/4690 | 103/2529 | |
| Lewycka 2013 women's groups only | Adjusted | 173/4773 | 104/2530 | |
| Lund 2012 | Adjusted OR 0.50 (0.27 to 0.93) adjusted for clustering and covariates associated with perinatal mortality | Straight into RevMman | 25/1300 | 44/1236 |
| Majoko 2007 | OR 1.11 (0.89 to 1.39) | 185/6614 | 161/6384 | |
| Manandhar 2004 | Adjusted as below | 123/2972 | 147/3303 | |
| More 2012 | Adjusted | 205/8017 | 173/7844 | |
| Mori 2015 | Unpublished OR 0.49 (0.05 to 4.54) provided by trial statistician (H Noma) adjusted for cluster effect and significant variables | Straight into RevMan | 1/253 | 2/248 |
| Persson 2013 | Adjusted | 283/11906 | 290/10655 | |
| Villar 1992 | OR 0.89 (95% CI 0.55 to 1.47) | Straight into RevMan | 3.6% of 1033 | 4% of 1040 |
| Villar 2001 (WHO 2001) | From Vogel 2013 Adjusted RR 1.18 (1.01 to 1.37). Adjusted for clustering and for risk strata, smoking, education less than primary, hospital admission in last pregnancy, previous surgery on reproductive tract, late booking, vaginal bleeding in the first trimester, age, previous low birth weight, parity. | Calculated adjusted OR | 234/11672 | 190/11121 |
| 1Piaggio 2001 reports ICC for perinatal mortality of 0. | ||||
| Study | Measure of effect | Statistical approach1 | Intervention | Control |
| Bhutta 2011 | Adjusted | 302/2339 | 191/2135 | |
| Laken 1995 | Not cluster trial – two intervention arms added together. calculated ln (OR) and se (Ln OR) | 104/194 | 101/101 | |
| Le Roux 2013 | 1.00 (0.74, 1.34) OR Random effects logistic regression, adjusted for neighbourhood clustering. Models for outcomes among HIV‐positive mothers control for baseline employment. | Straight into RevMan | 474/608 | 439/549 |
| Morris 2004 | Calculated from change scores, Table 2 Program effects p. 2034 of main report. Intervention 38.1% + 18.4% = 56.5% of the final sample size in this group which is 110; 48.9% ‐ 0.7% = 48.2 % in the control group (n = 313). | 62/110 | 151/313 | |
| Manthip 2015 | Adjusted | 62/127 | 53/190 | |
| Wu 2011 | Calculated adjusted SE from P value 0.246 | 376/673 | 253/591 | |
| 1Primary analysis using ICC of 0.02. | ||||
| Study | Measure of effect | Statistical approach | Intervention | Control |
| Kumar 2008 | Adjusted | 12/2749 | 10/1142 | |
| Laken 1995 | 0.9713 (0.0191 to 49.4211) | Straight into RevMan | 0/104 | 0/101 |
| Lewycka 2013 | Adjusted | 23/4601 | 29/5059 | |
| 1All data adjusted using an ICC of 0.00247 as suggested by Professor J P Souza by email. | ||||
| Study | Measure of effect | Statistical approach1 | Intervention | Control |
| Bhutta 2011 | Adjusted | 1616/2339 | 1230/2135 | |
| Kumar 2008 | 24.5% of 2681 intervention and 14.4% of 1129 controls: RR (95%CI); 1.67 (1.47 to 1.91) | Calculated adjusted OR | 657/2681 | 163/1129 |
| Wahlstrom 2011 | Adjusted | 99/127 | 122/190 | |
| Midhet 2010 | Adjusted | Womens group 254/836 | 191/1022 | |
| Wu 2011 | Calculated the ICC that they used to adjust the results using the reported P = 0.758 | 85/88 | 74/77 | |
| 1As for first comparison, ICC of 0.02 used for this outcome. Both Manandhar 2004 and Wu 2011 use ICCS of approximately 0.02 | ||||
| Study | Measure of effect | Statistical approach1 | Intervention | Control |
| Bhutta 2011 | Adjusted as below | 1272/2339 | 936/2135 | |
| Kumar 2008 | RR (95%CI); 1.35 (0.88 to 2.07) | Calculated adjusted OR | 507/2681 | 158/1129 |
| Laken 1995 | NOT A CLUSTER TRIAL ‐ calculated OR and confidence interval | 104/104 | 101/101 | |
| Midhet 2010 | Adjusted as below | 46/ 836 in women's group | 39/1022 | |
| Wu 2011 | Calculated what the ICC would have been using the reported adjusted p‐value (P = 0.231) | 625/673 | 517/591 | |
| 1ICC from Wu (0.099). | ||||
| Trial | Statistical approach1 | Intervention | Control |
| Klerman 2001 | Straight into RevMan ‐ not cluster trial | 39/311 | 33/296 |
| Le roux 2013 | Calculated adjusted OR | 88/608 | 123/549 |
| ICC from Piaggio paper of 0.0004 for ANC trial with average cluster size 426 | |||
| Trial | Statistical approach1 | Intervention | Control |
| Bhutta 2011 | Calculated OR | 880/12028 | 972/11005 |
| Laken 1995 | Calculated OR | 0/96 | 0/91 |
| Lewycka 2013 | Calculated OR | 198/4601 | 207/5059 |
| Midhet 2010 | Calculated OR | Women's group 36/836 plus couple's group 42/703 | Control 86/1022 |
| Kumar 2008 | Calculated OR | 219/ 2609 | 155/ 1079 |
| 1Piaggio 2001 reports and ICC of 0 for perinatal deaths. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits Show forest plot | 10 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Primary analysis (ICC 0.02 for studies Kirkwood and Morris) | 10 | 45022 | Odds Ratio (Random, 95% CI) | 1.11 [1.01, 1.22] |
| 1.2 Sensitivity analysis using ICC 0.08 | 10 | 45022 | Odds Ratio (Random, 95% CI) | 1.11 [1.00, 1.22] |
| 2 Pregnancy‐related deaths Show forest plot | 10 | 114930 | Odds Ratio (Random, 95% CI) | 0.69 [0.45, 1.08] |
| 3 ANC coverage: one or more visits Show forest plot | 6 | Odds Ratio (Random, 95% CI) | 1.68 [1.02, 2.79] | |
| 4 Pregnant women initiating ANC in first trimester Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 1.20 [0.99, 1.45] | |
| 5 Pregnant women receiving ANC from health professional Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 1.13 [0.84, 1.52] | |
| 6 Deliveries in a health facility Show forest plot | 10 | Odds Ratio (Random, 95% CI) | 1.08 [1.02, 1.15] | |
| 7 Intermittent Prophylactic Treatment for malaria | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Proportion of women with tetanus protection at birth Show forest plot | 8 | Odds Ratio (Random, 95% CI) | 1.03 [0.92, 1.15] | |
| 9 Proportion of women treated for syphilis Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 1.46 [0.94, 2.26] | |
| 10 Proportion of women with HIV who receive a complete antiretroviral course for prevention of mother‐to‐child transmission of HIV Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 0.44 [0.26, 0.74] | |
| 11 Preterm labour Show forest plot | 4 | Odds Ratio (Random, 95% CI) | 1.00 [0.93, 1.09] | |
| 12 Low birthweight Show forest plot | 5 | Odds Ratio (Random, 95% CI) | 0.94 [0.82, 1.06] | |
| 13 Perinatal mortality Show forest plot | 15 | Odds Ratio (Random, 95% CI) | 0.96 [0.89, 1.03] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits Show forest plot | 6 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Primary analysis (ICC 0.02 for studies Bhutta and Morris) | 6 | 7840 | Odds Ratio (Random, 95% CI) | 1.48 [0.99, 2.21] |
| 1.2 ICC 0.08 | 6 | 7840 | Odds Ratio (Random, 95% CI) | 1.45 [0.95, 2.23] |
| 2 Pregnancy‐related deaths Show forest plot | 3 | 13756 | Odds Ratio (Random, 95% CI) | 0.70 [0.39, 1.26] |
| 3 ANC coverage: one or more visits Show forest plot | 5 | Odds Ratio (Random, 95% CI) | 1.79 [1.47, 2.17] | |
| 4 Pregnant women initiating ANC in first trimester Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 0.83 [0.47, 1.47] | |
| 5 Pregnant women receiving ANC from health professional Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 2.97 [1.67, 5.30] | |
| 6 Deliveries in a health facility Show forest plot | 5 | Odds Ratio (Random, 95% CI) | 1.53 [0.96, 2.43] | |
| 7 Intermittent Prophylactic Treatment for malaria | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Proportion of women with tetanus protection at birth Show forest plot | 3 | Odds Ratio (Random, 95% CI) | 1.48 [1.18, 1.87] | |
| 9 Preterm labour Show forest plot | 1 | 607 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.45, 1.20] |
| 10 Low birthweight Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 0.61 [0.46, 0.80] | |
| 11 Perinatal mortality Show forest plot | 5 | Odds Ratio (Random, 95% CI) | 0.74 [0.57, 0.95] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Pregnancy‐related deaths | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 ANC coverage: one or more visits | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Deliveries in a health facility | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Perinatal mortality | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Low birthweight | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Intermittent Prophylactic Treatment for malaria | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 0.99 [0.70, 1.40] | |
| 2 Pregnancy‐related deaths Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 1.00 [0.52, 1.96] | |
| 3 ANC coverage: one or more visits Show forest plot | 3 | Odds Ratio (Random, 95% CI) | 0.86 [0.61, 1.20] | |
| 4 Deliveries in a health facility Show forest plot | 3 | Odds Ratio (Random, 95% CI) | 0.95 [0.69, 1.30] | |
| 5 Perinatal mortality Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 0.88 [0.72, 1.07] | |
| 6 Proportion of women with tetanus protection at birth Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 1.07 [0.80, 1.43] | |
| 7 Low birthweight | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Intermittent Prophylactic Treatment for malaria | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits Show forest plot | 1 | 383 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.41, 1.43] |
| 2 Pregnancy‐related deaths | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 ANC coverage: one or more visits | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Deliveries in a health facility Show forest plot | 1 | 383 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [0.71, 3.37] |
| 5 Perinatal mortality | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Low birthweight | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Intermittent Prophylactic Treatment for malaria | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Health systems vs Population ANC coverage: four or more visits Show forest plot | 9 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Health system interventions | 5 | Odds Ratio (Random, 95% CI) | 1.13 [0.96, 1.34] | |
| 1.2 Population interventions | 4 | Odds Ratio (Random, 95% CI) | 1.04 [0.96, 1.13] | |
| 2 Health systems vs Population Pregnancy‐related deaths Show forest plot | 11 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Health system interventions | 4 | Odds Ratio (Random, 95% CI) | 1.22 [0.41, 3.65] | |
| 2.2 Population intervention | 7 | Odds Ratio (Random, 95% CI) | 0.69 [0.46, 1.03] | |
| 3 Country Income Low vs High ANC at least 4 visits Show forest plot | 18 | Odds Ratio (Random, 95% CI) | 1.14 [1.04, 1.25] | |
| 3.1 Low or lower middle income countries | 11 | Odds Ratio (Random, 95% CI) | 1.21 [1.04, 1.40] | |
| 3.2 High or higher middle income countries | 7 | Odds Ratio (Random, 95% CI) | 1.12 [0.95, 1.32] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits Show forest plot | 9 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Primary analysis (ICC 0.02 for studies Kirkwood and Morris) | 9 | Odds Ratio (Random, 95% CI) | 1.07 [0.99, 1.15] | |
| 1.2 Sensitivity analysis using ICC 0.08 | 9 | Odds Ratio (Random, 95% CI) | 1.05 [0.98, 1.14] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ANC coverage: four or more visits Show forest plot | 4 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Primary analysis (ICC 0.02 for studies Bhutta and Morris) | 4 | Odds Ratio (Random, 95% CI) | 1.07 [0.82, 1.40] | |
| 1.2 ICC 0.08 | 4 | Odds Ratio (Random, 95% CI) | 1.03 [0.77, 1.37] | |

