Фармакологические вмешательства при рецидивирующих болях в животе в детстве
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Studies (CENTRAL; 2016, Issue 5) in the Cochrane Library
Search dates: 19 April 2013 (990 records); 11 April 2014 (1271 records); 26 March 2015 (49 records); 10 June 2016 (81 records).
#1 Pain*:ti,ab
#2 Ache*:ti,ab
#3 Sore*:ti,ab
#4 Discomfort*:ti,ab
#5 Distress*:ti,ab
#6 Cramp*:ti,ab
#7 Disorder:ti,ab
#8 Disorders:ti,ab
#9 Symptom:ti,ab
#10 Symptoms:ti,ab
#11 Migraine:ti,ab
#12 Migraines:ti,ab
#13 Epilep*:ti,ab
#14 Colic*:ti,ab
#15 Syndrome:ti,ab
#16 Syndromes:ti,ab
#17 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 Stomach*:ti,ab
#19 Abdom*:ti,ab
#20 Intestin*:ti,ab
#21 Viscera*:ti,ab
#22 Tummy:ti,ab
#23 Bowel*:ti,ab
#24 Belly:ti,ab
#25 Gastrointestinal:ti,ab
#26 GI:ti,ab
#27 Gastric:ti,ab
#28 #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27
#29 #17 and #28
#30 Colonic disease*:ti,ab
#31 Irritable bowel:ti,ab
#32 IBS:ti,ab
#33 Functional dyspepsia:ti,ab
#34 MeSH descriptor: [Irritable Bowel Syndrome] explode all trees
#35 MeSH descriptor: [Colonic Diseases, Functional] explode all trees
#36 MeSH descriptor: [Abdominal Pain] explode all trees
#37 MeSH descriptor: [Dyspepsia] explode all trees
#38 MeSH descriptor: [Colic] explode all trees
#39 MeSH descriptor: [Abdomen, Acute] explode all trees
#40 #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39
#41 Recurr*:ti,ab
#42 Chronic*:ti,ab
#43 Intermittent*:ti,ab
#44 Episode*:ti,ab
#45 Bout:ti,ab
#46 Bouts:ti,ab
#47 Spasm*:ti,ab
#48 Transitory:ti,ab
#49 Transient:ti,ab
#50 Functional:ti,ab
#51 Continu*:ti,ab
#52 Paroxysmal:ti,ab
#53 Persistent:ti,ab
#54 Idiopathic:ti,ab
#55 Unspecifi*:ti,ab
#56 Non specifi*:ti,ab
#57 Nonspecific*:ti,ab
#58 Motility:ti,ab
#59 MeSH descriptor: [Recurrence] explode all trees
#60 #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59
#61 #40 and #60
#62 irritable bowel syndrome*:ti,ab
#63 #61 or #62
#64 Child*:ti,ab
#65 Adolescen*:ti,ab
#66 Boy*:ti,ab
#67 Girl*:ti,ab
#68 teen*:ti,ab
#69 Schoolchild*:ti,ab
#70 Young adult*:ti,ab
#71 Youth*:ti,ab
#72 Pediatric*:ti,ab
#73 Paediatric*:ti,ab
#74 Student*:ti,ab
#75 Pupil*:ti,ab
#76 Juvenile*:ti,ab
#77 Young person*:ti,ab
#78 MeSH descriptor: [Child] explode all trees
#79 MeSH descriptor: [Adolescent] explode all trees
#80 MeSH descriptor: [Young Adult] explode all trees
#81 MeSH descriptor: [Students] explode all trees
#82 #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79 or #80 or #81
#83 #63 and #82
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations and Ovid Medline (1946 to present)
Search dates: 11 April 2013 (6238 records); 11 April 2014 (5957 records); 25 March 2015 (223 records); 9 June 2016 (300 records).
1 stomach*.tw.
2 abdom*.tw.
3 intestin*.tw.
4 viscera*.tw.
5 tummy.tw.
6 bowel*.tw.
7 belly.tw.
8 gastrointestinal.tw.
9 gi.tw.
10 gastric.tw.
11 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
12 pain*.tw.
13 Ache*.tw.
14 Sore*.tw.
15 Discomfort*.tw.
16 Distress*.tw.
17 Cramp*.tw.
18 Disorder$1.tw.
19 Symptom$1.tw.
20 Migraine$1.tw.
21 Epilep*.tw.
22 syndrome$1.tw.
23 colic*.tw.
24 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25 irritable bowel$.tw.
26 ibs.tw.
27 functional dyspepsia.tw.
28 25 or 26 or 27
29 ((stomach* or abdom* or intestin* or viscera* or tummy or bowel* or belly or gastrointestinal or gi or gastric) adj3 (pain* or Ache* or Sore* or Discomfort* or Distress* or Cramp* or Disorder$1 or Symptom$1 or Migraine$1 or Epilep* or syndrome$1 or colic*)).tw.
30 exp Irritable Bowel Syndrome/
31 exp Colonic Diseases/
32 exp Abdominal Pain/
33 exp Dyspepsia/
34 exp Colic/
35 exp Abdomen, Acute/
36 30 or 31 or 32 or 33 or 34 or 35
37 28 or 29 or 36
38 Recurr*.tw.
39 Chronic*.tw.
40 Intermittent*.tw.
41 Bout$1.tw.
42 spasm*.tw.
43 Transitory.tw.
44 Transient.tw.
45 Functional.tw.
46 Continu*.tw.
47 Paroxysmal.tw.
48 Persistent.tw.
49 Idiopathic.tw.
50 unspecifi*.tw.
51 Non specifi*.tw.
52 nonspecifi*.tw.
53 motility.tw.
54 episod*.tw.
55 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54
56 exp Recurrence/
57 55 or 56
58 37 and 57
59 irritable bowel syndrome*.tw.
60 58 or 59
61 randomized controlled trial.pt.
62 controlled clinical trial.pt.
63 randomi#ed.ab.
64 placebo$.ab.
65 randomly.ab.
66 trial.ab.
67 groups.ab.
68 exp animals/ not humans.sh.
69 or/61‐67
70 69 not 68
71 60 and 70
72 exp Child/
73 exp Adolescent/
74 exp Young Adult/
75 exp Students/
76 Child*.tw.
77 Adolescen*.tw.
78 Young person*.tw.
79 Boy*.tw.
80 Girl*.tw.
81 teen*.tw.
82 Schoolchild*.tw.
83 Young adult*.tw.
84 Youth*.tw.
85 P*ediatric*.tw.
86 Student*.tw.
87 Pupil*.tw.
88 Juvenile*.tw.
89 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88
90 71 and 89
Embase Ovid (1974 to present)
Search dates: 11 April 2013 (2272 records); 11 April 2014 (2523 records); 25 March 2015 (250 records); 9 June 2016 (345 records).
1 recurr*.tw.
2 chronic*.tw.
3 intermittent*.tw.
4 bout$1.tw.
5 spasm*.tw.
6 transitory.tw.
7 transient.tw.
8 functional.tw.
9 continu*.tw.
10 paroxysmal.tw.
11 persistent.tw.
12 idiopathic.tw.
13 unspecifi*.tw.
14 non specifi*.tw.
15 nonspecifi*.tw.
16 motility.tw.
17 episod*.tw.
18 or/1‐17
19 exp recurrent disease/
20 18 or 19
21 stomach*.tw.
22 abdom*.tw.
23 intestin*.tw.
24 viscera*.tw.
25 tummy.tw.
26 bowel*.tw.
27 belly.tw.
28 gastrointestinal.tw.
29 gi.tw.
30 gastric.tw.
31 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30
32 pain*.tw.
33 Ache*.tw.
34 Sore*.tw.
35 Discomfort*.tw.
36 Distress*.tw.
37 Cramp*.tw.
38 Disorder$1.tw.
39 Symptom$1.tw.
40 Migraine$1.tw.
41 Epilep*.tw.
42 syndrome$1.tw.
43 colic*.tw.
44 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43
45 irritable bowel$.tw.
46 ibs.tw.
47 functional dyspepsia.tw.
48 45 or 46 or 47
49 ((stomach* or abdom* or intestin* or viscera* or tummy or bowel* or belly or gastrointestinal or gi or gastric) adj3 (pain* or Ache* or Sore* or Discomfort* or Distress* or Cramp* or Disorder$1 or Symptom$1 or Migraine$1 or Epilep* or syndrome$1 or colic*)).tw.
50 48 or 49
51 exp colic/
52 exp irritable colon/
53 exp abdominal pain/
54 exp dyspepsia/
55 colon disease/
56 50 or 51 or 52 or 53 or 54 or 55
57 20 and 56
58 irritable bowel syndrome*.tw.
59 57 or 58
60 Clinical trial/
61 Randomized controlled trial/
62 Randomization/
63 Single blind procedure/
64 Double blind procedure/
65 Crossover procedure/
66 Placebo/
67 Randomi?ed controlled trial$.tw.
68 Rct.tw.
69 Random allocation.tw.
70 Randomly allocated.tw.
71 Allocated randomly.tw.
72 (allocated adj2 random).tw.
73 Single blind$.tw.
74 Double blind$.tw.
75 ((treble or triple) adj blind$).tw.
76 Placebo$.tw.
77 Prospective study/
78 or/60‐77
79 Case study/
80 Case report.tw.
81 Abstract report/ or letter/
82 or/79‐81
83 78 not 82
84 59 and 83
85 exp Child/
86 exp Adolescent/
87 exp Young Adult/
88 exp Students/
89 Child*.tw.
90 Adolescen*.tw.
91 Young person*.tw.
92 Boy*.tw.
93 Girl*.tw.
94 teen*.tw.
95 Schoolchild*.tw.
96 Young adult*.tw.
97 Youth*.tw.
98 P*ediatric*.tw.
99 Student*.tw.
100 Pupil*.tw.
101 Juvenile*.tw.
102 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101
103 84 and 102
CINAHL Healthcare Databases Advanced Search (Cumulative Index to Nursing and Allied Health Literature; 1981 to present)
Search dates: 18 April 2013 (175 records); 11 April 2014 (195 records); 26 March 2015 (21 records); 9 June 2016 (11 records).
1. CINAHL; recurr*.ti,ab;
2. CINAHL; chronic*.ti,ab;
3. CINAHL; intermittent*.ti,ab;
4. CINAHL; (bout OR bouts).ti,ab;
5. CINAHL; spasm*.ti,ab;
6. CINAHL; transitory.ti,ab;
7. CINAHL; transient.ti,ab;
8. CINAHL; functional.ti,ab;
9. CINAHL; continu*.ti,ab;
10. CINAHL; paroxysmal.ti,ab;
11. CINAHL; persistent.ti,ab;
12. CINAHL; idiopathic.ti,ab;
13. CINAHL; unspecifi*.ti,ab;
14. CINAHL; "non specifi*".ti,ab;
15. CINAHL; nonspecifi*.ti,ab;
16. CINAHL; motility.ti,ab;
17. CINAHL; episod*.ti,ab;
18. CINAHL; exp RECURRENCE/;
19. CINAHL; 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18;
20. CINAHL; stomach*.ti,ab;
21. CINAHL; abdom*.ti,ab;
22. CINAHL; intestin*.ti,ab;
23. CINAHL; viscera*.ti,ab;
24. CINAHL; tummy.ti,ab;
25. CINAHL; bowel*.ti,ab;
26. CINAHL; belly.ti,ab;
27. CINAHL; gastrointestinal.ti,ab;
28. CINAHL; gi.ti,ab;
29. CINAHL; gastric.ti,ab;
30. CINAHL; 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29;
31. CINAHL; pain*.ti,ab;
32. CINAHL; Ache*.ti,ab;
33. CINAHL; Sore*.ti,ab;
34. CINAHL; Discomfort*.ti,ab;
35. CINAHL; Distress*.ti,ab;
36. CINAHL; Cramp*.ti,ab;
37. CINAHL; (Disorder OR Disorders).ti,ab;
38. CINAHL; (Symptom OR Symptoms).ti,ab;
39. CINAHL; (Migraine OR Migraines).ti,ab;.
40. CINAHL; Epilep*.ti,ab;
41. CINAHL; (syndrome OR syndromes).ti,ab;
42. CINAHL; colic*.ti,ab;
43. CINAHL; 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42;
44. CINAHL; 30 AND 43;
45. CINAHL; "irritable bowel*".ti,ab;
46. CINAHL; ibs.ti,ab;
47. CINAHL; "functional dyspepsia".ti,ab;
48. CINAHL; exp COLIC/;
49. CINAHL; exp IRRITABLE BOWEL SYNDROME/;
50. CINAHL; exp COLONIC DISEASES, FUNCTIONAL/;
51. CINAHL; exp ABDOMINAL PAIN/;
52. CINAHL; exp DYSPEPSIA/;
53. CINAHL; 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51 OR 52;
54. CINAHL; 44 OR 53;
55. CINAHL; 19 AND 54;
56. CINAHL; (irritable AND bowel AND syndrome*).ti,ab;
57. CINAHL; 55 OR 56;
58. CINAHL; Child*.ti,ab;
59. CINAHL; Adolescen*.ti,ab;
60. CINAHL; "Young person*".ti,ab;
61. CINAHL; Boy*.ti,ab;
62. CINAHL; Girl*.ti,ab;
63. CINAHL; teen*.ti,ab;
64. CINAHL; Schoolchild*.ti,ab;
65. CINAHL; "Young adult*".ti,ab;
66. CINAHL; Youth*.ti,ab;
67. CINAHL; Student*.ti,ab;
68. CINAHL; Pupil*.ti,ab;
69. CINAHL; Juvenile*.ti,ab;
70. CINAHL; exp CHILD/;
71. CINAHL; exp STUDENTS/;
72. CINAHL; 70 OR 71;
73. CINAHL; Pediatric*.ti,ab;
74. CINAHL; Paediatric*.ti,ab;
75. CINAHL; 67 OR 68 OR 69 OR 72 OR 73 OR 74;
76. CINAHL; 63 OR 64 OR 65 OR 66;
77. CINAHL; 58 OR 59 OR 60 OR 61 OR 62;
78. CINAHL; 70 OR 73 OR 74 OR 75;
79. CINAHL; 57 AND 78;
80. CINAHL; exp RANDOMIZED CONTROLLED TRIALS/;
81. CINAHL; random*.ti,ab;
82. CINAHL; "clin* trial*".ti,ab;
83. CINAHL; (singl* OR doubl* OR tripl* OR trebl*).ti,ab;
84. CINAHL; (mask* OR blind*).ti,ab;
85. CINAHL; 83 AND 84;
86. CINAHL; "random* allocate*".ti,ab;
87. CINAHL; "random assign*".ti,ab;
88. CINAHL; exp RANDOM ASSIGNMENT/;
89. CINAHL; exp CLINICAL TRIALS/;
90. CINAHL; exp META ANALYSIS/;
91. CINAHL; 88 OR 89 OR 90;
92. CINAHL; 80 OR 81 OR 82 OR 85 OR 86 OR 87;
93. CINAHL; 91 OR 92;
94. CINAHL; 79 AND 93;
PsycINFO Ovid (1806 to present)
Search dates: 18 April 2013 (238 records); 11 April 2014 (757 records); 25 March 2015 (47 records); 9 June 2016 (87 records).
1 stomach*.tw.
2 abdom*.tw.
3 intestin*.tw.
4 viscera*.tw.
5 tummy.tw.
6 bowel*.tw.
7 belly.tw.
8 gastrointestinal.tw.
9 gi.tw.
10 gastric.tw.
11 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
12 pain*.tw.
13 Ache*.tw.
14 Sore*.tw.
15 Discomfort*.tw.
16 Distress*.tw.
17 Cramp*.tw.
18 Disorder$1.tw.
19 Symptom$1.tw.
20 Migraine$1.tw.
21 Epilep*.tw.
22 syndrome$1.tw.
23 colic*.tw.
24 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25 irritable bowel$.tw.
26 ibs.tw.
27 functional dyspepsia.tw.
28 25 or 26 or 27
29 ((stomach* or abdom* or intestin* or viscera* or tummy or bowel* or belly or gastrointestinal or gi or gastric) adj3 (pain* or Ache* or Sore* or Discomfort* or Distress* or Cramp* or Disorder$1 or Symptom$1 or Migraine$1 or Epilep* or syndrome$1 or colic*)).tw.
30 exp Irritable Bowel Syndrome/
31 exp Dyspepsia/
32 recurr*.tw.
33 chronic*.tw.
34 intermittent*.tw.
35 bout$1.tw.
36 spasm*.tw.
37 transitory.tw.
38 transient.tw.
39 functional.tw.
40 continu*.tw.
41 paroxysmal.tw.
42 persistent.tw.
43 idiopathic.tw.
44 unspecifi*.tw.
45 non specifi*.tw.
46 nonspecifi*.tw.
47 motility.tw.
48 episod*.tw.
49 or/32‐48
50 irritable bowel syndrome*.tw.
51 exp Students/
52 Child*.tw.
53 Adolescen*.tw.
54 Young person*.tw.
55 Boy*.tw.
56 Girl*.tw.
57 teen*.tw.
58 Schoolchild*.tw.
59 Young adult*.tw.
60 Youth*.tw.
61 P*ediatric*.tw.
62 Student*.tw.
63 Pupil*.tw.
64 Juvenile*.tw.
65 28 or 29 or 30 or 31
66 49 and 65
67 50 or 66
68 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64
69 67 and 68
ERIC ProQuest (Education Resources Information Center; 1966 to present)
Search dates: 19 April 2013 (276 records); 11 April 2014 (294 records); 26 March 2015 (0 records); 9 June 2016 (2 records).
(ab,ti(Pain*) OR ab,ti(Ache*) OR ab,ti(Sore*) OR ab,ti(Discomfort*) OR ab,ti(Distress*) OR ab,ti(Cramp*) OR ab,ti(Disorder) OR ab,ti(Disorders) OR ab,ti(Symptom*) OR ab,ti(Migraine*) OR ab,ti(Epilep*) OR ab,ti(Colic*) OR ab,ti(Syndrome*))
AND
(Ab,ti(Recurr*) OR ab,ti(Chronic*) OR ab,ti(Intermittent*) OR ab,ti(Episode*) OR ab,ti(Bout) OR ab,ti(Bouts) OR ab,ti((Spasm*) OR ab,ti(Transitory) OR ab,ti(Transient) OR ab,ti(Functional) OR ab,ti(Continu*) OR ab,ti(paroxysmal) OR ab,ti(Persistent) OR ab,ti (Idiopathic) OR ab,ti(Unspecifi*) OR ab,ti(Non specifi*) OR ab,ti(motility) OR SU.EXACT.EXPLODE("Recurrence"))
AND
(Ab,ti(Stomach*) OR ab,ti(Abdom*) OR ab,ti(Sore*) OR ab,ti(Intestin*) OR ab,ti(Viscera*) OR ab,ti(Tummy) OR ab,ti(Bowel*) OR ab,ti(Belly) OR ab,ti(Gastrointestinal) OR ab,ti(GI) OR ab,ti(Epilep*) OR ab,ti(Gastric))
OR
(Ab,ti(irritable bowel*) OR ab,ti(ibs) OR ab,ti(colonic disease*) OR ab,ti(functional dyspepsia))
British Education Index ProQuest (1975 to present)
Search dates: 19 April 2013 (46 records); 11 April 2014 (48 records); 26 March 2015 (0 records); 9 June 2016 (5 records).
((ab,ti(Stomach*) OR ab,ti(Abdom*) OR ab,ti(Intestin*) OR ab,ti(Viscera*) OR ab,ti(Tummy) OR ab,ti(Bowel*) OR ab,ti(Belly) OR ab,ti(Gastrointestinal) OR ab,ti(GI) OR ab,ti(Gastric))
AND
((ab,ti(Pain*) OR ab,ti(Ache*) OR ab,ti(Sore*) OR ab,ti(Discomfort*) OR ab,ti(Distress*) OR ab,ti(Cramp*) OR ab,ti(Disorder) OR ab,ti(Disorders) OR ab,ti(Symptom) OR OR ab,ti(Symptoms) OR ab,ti(Migraine) OR ab,ti(Migraines) OR ab,ti(Epilep*) OR ab,ti(Colic*) OR ab,ti(Syndrome) OR ab,ti(Syndromes))
OR
(Ab,ti(irritable bowel*) OR ab,ti(ibs) OR ab,ti(Functional dyspepsia))
Applied Social Sciences Index and Abstracts ProQuest (ASSIA; 1987 to present)
Search dates: 19 April 2013 (179 records); 11 April 2014 (545 records); 26 March 2015 (27 records); 9 June 2016 (48 records).
((ab,ti(Stomach*) OR ab,ti(Abdom*) OR ab,ti(Intestin*) OR ab,ti(Viscera*) OR ab,ti(Tummy) OR ab,ti(Bowel*) OR ab,ti(Belly) OR ab,ti(Gastrointestinal) OR ab,ti(GI) OR ab,ti(gastric)
AND
(ab,ti(Pain*) OR ab,ti(Ache*) OR ab,ti(Sore*) OR ab,ti(Discomfort*) OR ab,ti(Distress*) OR ab,ti(Cramp*) OR ab,ti(Disorder) OR ab,ti(Disorders) OR ab,ti(Symptom*) OR ab,ti(Symptoms) OR ab,ti(Migraine*) OR ab,ti(Epilep*) OR ab,ti(Syndrome) OR ab,ti(Syndromes) OR ab,ti(colic*)
AND
(ab,ti(Recurr*) OR ab,ti(Chronic*) OR ab,ti(Intermittent*) OR ab,ti(Episode*) OR ab,ti(Bout) OR ab,ti(bouts) OR ab,ti(Spasm*) OR ab,ti(Transitory) OR ab,ti(Transient) OR ab,ti(Functional) OR ab,ti(Continu*) OR ab,ti(Paroxysmal) OR ab,ti(Persistent) OR ab,ti(Idiopathic) OR ab,ti(Unspecifi*) OR ab,ti(Non specifi*) OR ab,ti(motility))
OR
(ab,ti(irritable bowel) OR ab,ti(ibs) OR ab,ti(functional dyspepsia))
Allied and Complementary Medicine Healthcare Databases Advanced Search (AMED; 1985 to present)
Search dates: 18 April 2013 (63 records); 11 April 2014 (74 records); 25 March 2015 (1 record); 9 June 2016 (1 record).
1. AMED; Recurr*.ti,ab;
2. AMED; Chronic*.ti,ab;
3. AMED; Intermittent*.ti,ab;
4. AMED; Episod*.ti,ab;
5. AMED; (Bout OR Bouts).ti,ab;
6. AMED; Spasm*.ti,ab;
7. AMED; Transitory.ti,ab;
8. AMED; Transient.ti,ab;
9. AMED; Functional.ti,ab;
10. AMED; Continu*.ti,ab;
11. AMED; Paroxysmal.ti,ab;
12. AMED; Persistent.ti,ab;
13. AMED; Idiopathic.ti,ab;
14. AMED; Unspecifi*.ti,ab;
15. AMED; "Non specifi*".ti,ab;
16. AMED; Nonspecific*.ti,ab;
17. AMED; Motility.ti,ab;
18. AMED; exp RECURRENCE/;
19. AMED; 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18;.
20. AMED; Pain*.ti,ab;
21. AMED; Ache*.ti,ab;
22. AMED; Sore*.ti,ab;
23. AMED; Discomfort*.ti,ab;
24. AMED; Distress*.ti,ab;
25. AMED; Cramp*.ti,ab;
26. AMED; (Disorder OR Disorders).ti,ab;
27. AMED; (Symptom OR Symptoms).ti,ab;
28. AMED; (Migraine OR Migraines).ti,ab;
29. AMED; Epilep*.ti,ab;
30. AMED; Colic*.ti,ab;
31. AMED; (Syndrome OR Syndromes).ti,ab;
32. AMED; 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31;
33. AMED; Stomach*.ti,ab;
34. AMED; Abdom*.ti,ab;
35. AMED; Intestin*.ti,ab;
36. AMED; Viscera*.ti,ab;
37. AMED; Tummy.ti,ab;
38. AMED; Bowel*.ti,ab;
39. AMED; Belly.ti,ab;
40. AMED; Gastrointestinal.ti,ab;
41. AMED; GI.ti,ab;
42. AMED; Gastric.ti,ab;
43. AMED; 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42;
44. AMED; 32 AND 43;
45. AMED; "Colonic disease*".ti,ab;
46. AMED; "Irritable bowel".ti,ab;
47. AMED; IBS.ti,ab; 86
48. AMED; "Functional dyspepsia".ti,ab;
49. AMED; exp IRRITABLE BOWEL SYNDROME/;
50. AMED; exp COLONIC DISEASE/;
51. AMED; exp ABDOMINAL PAIN/;
52. AMED; exp DYSPEPSIA/;
53. AMED; 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51 OR 52;
54. AMED; 44 OR 53;
55. AMED; 19 AND 54;
56. AMED; (irritable AND bowel AND syndrome*).ti,ab;
57. AMED; Child*.ti,ab;
58. AMED; Adolescen*.ti,ab;
59. AMED; Boy*.ti,ab;
60. AMED; Girl*.ti,ab;
61. AMED; teen*.ti,ab;
62. AMED; Schoolchild*.ti,ab;
63. AMED; "Young adult*".ti,ab;
64. AMED; Youth*.ti,ab; 767 results.
65. AMED; (Pediatric* OR Paediatric*).ti,ab;
66. AMED; Student*.ti,ab;
67. AMED; Pupil*.ti,ab;
68. AMED; Juvenile*.ti,ab;
69. AMED; "Young person*".ti,ab;
70. AMED; exp CHILD/;
71. AMED; exp ADOLESCENT/;
72. AMED; exp STUDENTS/;
73. AMED; 57 OR 58 OR 59 OR 60 OR 61 OR 62 OR 63 OR 64 OR 65 OR 66 OR 67 OR 68 OR 69 OR 70 OR 71 OR 72;
74 AMED; 55 OR 56;
75. AMED; 74 AND 73;
LILACS (Latin American and Caribbean Health Science Information database; lilacs.bvsalud.org/en; all available years)
Search dates: 19 April 2013 (11 records); 11 April 2014 (13 records); 26 March 2015 (0 records); 9 June 2016 (0 records).
((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) (trial$ OR ensa$ OR estud$ OR experim$ OR investiga$ OR singl$ OR simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) [Palavras]
and ((recurr$ or chronic$ or intermittent$ or bout or bouts or spasm$ or transitory or transient or functional or continu$ or Paroxysmal or Persistent or Idiopathic or unspecifi$ or Non specifi$ or nonspecific$ or motility or episode$) [Palavras] and (pain$ or ache$ or sore$ or discomfort$ or distress$ cramp$ or colic$ or disorder or disorders or symptom or symptoms or Migraine$ or Epilep* or syndrome$) and (stomach$ or abdom$ or intestin$ or viscera$ or tummy$ or bowel$ or belly or gastrointestinal or gi or gastric)) [Palavras]
OpenGrey (www.opengrey.eu; 1980 to present)
Search dates : 19 April 2013 (1 record); 11 April 2014 (1 record); 26 March 2015 (0 records); 9 June 2016 (0 records).
Irritable bowel syndrom*
Ibs
functional dyspepsia
Chronic* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
Recurr* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
Intermittent* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
Bout* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
spasm* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
Transitory AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
Transient AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
Functional AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
Continu* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
Paroxysmal AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
Persistent AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
Idiopathic AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
unspecifi* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
Non specifi* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
nonspecifi* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
motility AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
episod* AND (abdom* OR stomach* OR intestin* OR viscera* OR tummy OR bowel* OR belly or gastrointestinal OR gi OR gastric))
ClinicalTrials.gov (clinicaltrials.gov; 2007 to present)
Search dates: 11 April 2014 (69 records); 26 March 2015 (35 records); 9 June 2016 (62 records).
“irritable bowel” OR “abdominal pain” in the condition field. Limited to children.
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch; 1999 to present)
Search dates: 11 April 2014 (106 records); 26 March 2015 (4 records); 9 June 2016 (32 records).
“irritable bowel” OR “abdominal pain” in the condition field. Limited to children and interventional studies.
Appendix 2. Additional methods
We detailed methods for each of the topics below in our protocol (Martin 2014a), but did not use them in the review because we had insufficient data to perform a meta‐analysis.
-
Types of outcome measures.
-
Unit of analysis issues.
-
Dealing with missing data.
-
Assessment of heterogeneity.
-
Assessment of reporting bias.
-
Data synthesis.
-
Subgroup analysis and investigation of heterogeneity.
-
Sensitivity analysis.
We describe these methods, which have been archived for use in future updates of this review, in the table below.
| Types of outcome measures |
| We expect studies to vary in their duration of postintervention follow‐up. We will therefore group studies according to duration of follow‐up: immediate outcome measurement, short term (less than three months), medium term (three to 12 months), and long term (greater than 12 months). |
| Unit of analysis issues |
| If we find the following three types of trials, we will consider their results as per guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c, section 9.3). Cross‐over trials For cross‐over trials with random allocation to period and an appropriate washout period, we will include the relevant effect estimate within the meta‐analysis, using the generic inverse variance method in Review Manager 5 (Review Manager 2014). An appropriate washout period may vary with the interventions (including drug pharmacokinetics) and outcome measurements. Considering that RAP can be a stable and chronic condition, a washout period of several weeks may be sufficient. Cluster‐RCTs Cluster‐randomised trials randomise groups of people rather than individuals. For each cluster‐randomised trial, we will first determine whether or not the data incorporate sufficient controls for clustering (such as robust standard errors or hierarchical linear models). If data do not have proper controls, then we will attempt to obtain an appropriate estimate of the data's intracluster correlation coefficient. If we cannot find an estimate in the trial report, then we will request an estimate from the trial report authors. If the authors do not provide an estimate, if possible, we will obtain one from a similar study and conduct a sensitivity analysis to determine if the results are robust when different values are imputed. We will do this according to procedures described in Higgins 2011c (section 9.3). This will prevent the meta‐analysis from being based on clustered data that has not been properly controlled. Trials with multiple intervention groups This is a common scenario. To avoid any unit of analysis errors in the meta‐analysis, we will use the following approach for a study that could contribute multiple comparisons.
We will not perform a multiple‐treatment meta‐analysis, as the clinical heterogeneity would mean the results have little clinical meaning. |
| Dealing with missing data |
| We may carry out a sensitivity analysis to establish if the inclusion of studies with high levels of missing data significantly alters the findings of the review. |
| Assessment of reporting bias |
| Publication bias If we identify sufficient trials (at least 10), we will use the outcome data to produce a funnel plot to investigate the likelihood of overt publication bias (Sutton 2000). Any asymmetry of the funnel plot may indicate possible publication bias. We will explore other reasons for asymmetry such as poor methodological quality or heterogeneity. We will look for publication bias by comparing the results of the published and unpublished data. |
| Assessment of heterogeneity |
| We will describe statistical heterogeneity (observed variability in study results that is greater than that expected to occur by chance) by calculating I² (Higgins 2003). I² describes approximately the proportion of variation in point estimates due to heterogeneity rather than sampling error. An I² more than 50% may indicate significant heterogeneity. We will use a Chi² test to further assess the role of heterogeneity on the strength of the evidence. We will regard any result with a P value lower than 0.10 as indicative of heterogeneity. We will interpret this cautiously and use it to help quantify the impact of heterogeneity on the results of the meta‐analysis (Higgins 2003). |
| Data synthesis |
| We will use Review Manager 5 for statistical analysis (Review Manager 2014). Two review authors (RAA, AB, JTC, TVND, or AEM) will independently enter data into Review Manager 5. We will report summary statistics for continuous data as mean differences or standardised mean differences using a random‐effects model. For dichotomous data, we will report odds ratios using a random‐effects model. We intend to use a a random‐effects model as we anticipate significant statistical and clinical heterogeneity. |
| Subgroup analysis and investigation for heterogeneity |
| If sufficient trials are available, we will examine the following subgroups to explore clinical heterogeneity:
Subgroup analysis can be misleading because the studies may not be designed and powered to show difference within subgroups. We will therefore undertake subgroup analyses with caution. |
| Sensitivity analysis |
| We will conduct primary analyses based on available data on the outcomes of interest. Following this, we will use a sensitivity analysis to assess the robustness of conclusions in relation to two aspects of study design:
We will also conduct a sensitivity analysis to establish the effect of missing data on the estimate of treatment effect, by performing the analysis with and without the studies with significant missing data to determine if this alters the conclusions. |
Footnotes
RAP: recurrent abdominal pain
RCT: randomised controlled trial
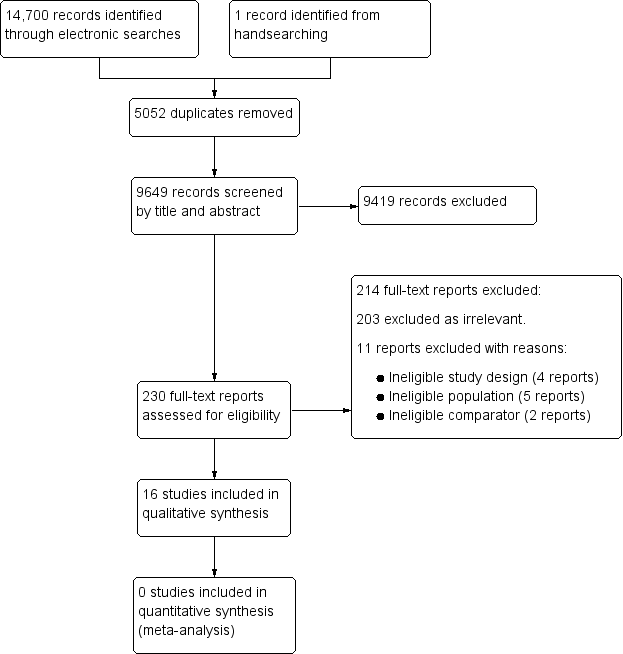
Study flow diagram.
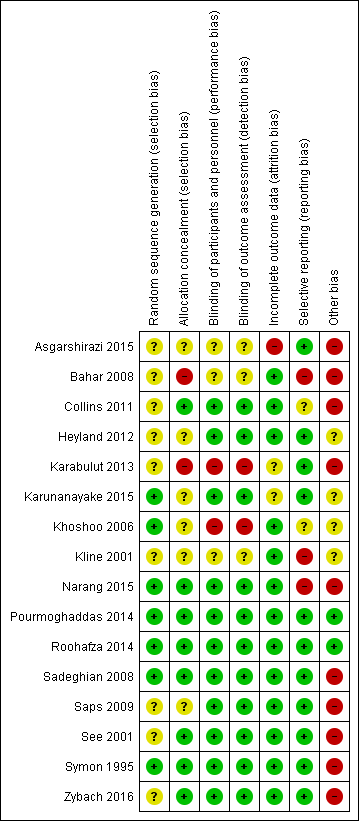
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
| Antispasmodics compared to placebo for recurrent abdominal pain | ||||||
| Patient or population: school‐aged children (5 to 18 years of age) with recurrent abdominal pain Settings: hospital paediatric outpatient clinics Intervention: antispasmodic drugs Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (SD) | Relative effect (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Intervention | |||||
| Pain duration (mean pain duration, assessed at 4 weeks) | The mean duration of pain in the control group was 6.17 (± 11.61). | The mean duration of pain in the intervention group was51.6 (± 23.74). | MD ‐25.4 (‐35.5 to ‐15.3) | 120 (1) | ⊕⊝⊝⊝ | No evidence of efficacy |
| Pain improvement (clinician judged, assessed at 2 weeks) | 9 of 21 children in the control group had an improvement in pain. | 15 of 21 children in the intervention group had an improvement in pain. | OR 3.33 (0.93 to 12.01) | 42 (1) | ⊕⊝⊝⊝ | No evidence of efficacy |
| Pain frequency (episodes of pain in 4 weeks, assessed after 4 weeks) | The mean number of episodes of pain in the control group was 21.6 (32.4). | The mean number of episodes of pain in the intervention group was10.3 (14). | MD 11.3 (2.4 to 20.1) | 132 (1) | ⊕⊝⊝⊝ | No evidence of efficacy |
| Pain improvement (self reported response to treatment, assessed at 4 weeks) | The response to treatment in the control group was 30.3%. | The response to treatment in the intervention group was 40.6%. | OR 1.6 (0.7 to 3.4) | 115 (1) | ⊕⊕⊝⊝ | No evidence of efficacy |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded for low methodological quality due to single, small study; risk of bias from incomplete outcome data and differential loss of participants between groups. The placebo differed in preparation and dose timing compared to the intervention drug. | ||||||
| Domain | 'Risk of bias' judgement | ||
| Low | High | Unclear | |
| Selection bias | |||
| Random sequence generation | If the study details any of the following methods: (1) simple randomisation (such as coin‐tossing, throwing dice, or dealing previously shuffled cards, a list of random numbers, or computer‐generated random numbers); or (2) restricted randomisation such as blocked, ideally with varying block sizes or stratified groups, provided that within‐group randomisation is not affected | If the study details no randomisation or an inadequate method such as alternation, assignment based on date of birth, case record number, and date of presentation. These latter methods may be referred to as ‘quasi‐random’. | If there is insufficient detail to judge the risk of bias
|
| Allocation concealment | If the study details concealed allocation sequence in sufficient detail to determine that allocations could not have been foreseen in advance of, or during, enrolment | If the study details a method where the allocation is known prior to assignment | If there is insufficient detail to judge the risk of bias |
| Performance bias | |||
| Blinding of participants and personnel | If the study details a method of blinding participants and personnel. Detail would need to be sufficient to show that participants and personnel were unable to identify the therapeutic intervention from the control intervention. | If the methods detail that the participants or study personnel were not blinded to the study medication or placebo | If there is insufficient detail to judge the risk of bias |
| Detection bias | |||
| Blinding of outcome assessment | If the study details a blinded outcome assessment. This may only be possible for outcomes that are externally assessed. | If the outcome assessment is not blinded. We expect this may be unavoidable for self rated outcomes of unblinded interventions. | If there is insufficient detail to judge the risk of bias
|
| Attrition bias | |||
| Incomplete outcome data | If the study reports attrition and exclusions, including the numbers in each intervention group (compared with total randomised participants), reasons for attrition or exclusions, and any re‐inclusions; the impact of missing data is not believed to have altered the conclusions; and reasons for the missing data are acceptable | We may judge the risk of attrition bias to be high due to the amount, nature, or handling (such as per‐protocol analysis) of incomplete outcome data. | If there is insufficient detail to judge the risk of bias, e.g. if the number of children randomised to each treatment is not reported |
| Reporting bias | |||
| Selective reporting | If there is complete reporting of all outcome data. This will be determined based on comparison of the protocol and published study, if available. | If the reporting is selective so that some outcome data are not reported | If there is insufficient detail to judge the risk of bias, e.g. protocols are unavailable |
| Other sources of bias | |||
| Other bias | If the study is judged to be at low of risk of other potential sources of bias, such as no differential loss to follow‐up or an adequate washout period in cross‐over trials | If there are other sources of bias, such as differential loss to follow‐up or an inadequate washout period in cross‐over trials | If there is insufficient detail to judge the risk of bias |

