Minimalinvasive Chirurgie versus Strahlentherapie / Radiochemotherapie für kleinvolumige primär oropharyngeale Karzinome
Abstract
Background
More than 400,000 cases of oropharyngeal squamous cell carcinoma (OPSCC) are diagnosed each year worldwide and the incidence is rising, partly as a result of human papillomavirus. Human papillomavirus‐associated OPSCC affects younger patients and often presents at a higher stage; however, it is associated with a better prognosis.
Until recently, first‐line management of OPSCC involved chemoradiotherapy, as research had demonstrated comparable survival outcomes when compared with open surgery, with significantly decreased morbidity. However, interventions have now evolved with computerised planning and intensity‐modulated radiotherapy, and the advent of endoscopic head and neck surgery, which provide the potential for decreased treatment‐associated morbidity.
The oropharynx plays an essential role in swallowing, speech and protecting the airway as it is situated at the bifurcation of the respiratory and digestive tracts. Treatment modality recommendations are based on survival outcomes. Given the younger patient demographic, establishing the safety of modalities that potentially have better functional outcome is becoming increasingly important.
Objectives
To assess the efficacy of endoscopic head and neck surgery (transoral robotic surgery or transoral laser microsurgery) for small‐volume, primary (T1‐2, N0‐2) oropharyngeal squamous cell carcinoma (OPSCC) in comparison to radiotherapy/chemoradiotherapy.
Search methods
The Cochrane ENT Information Specialist searched the ENT Trials Register; Central Register of Controlled Trials (CENTRAL 2016, Issue 10); PubMed; EMBASE; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 8 November 2016.
Selection criteria
Randomised controlled trials in patients with carcinoma in the oropharynx subsite (as defined by the World Health Organization classification C09, C10). Cancers included were primary squamous cell carcinomas arising from the oropharyngeal mucosa. The tumours were classified as T1‐T2 with or without nodal disease and with no evidence of distant metastatic spread. The intervention was transoral, minimally invasive surgery with or without adjuvant radiotherapy or adjuvant chemoradiotherapy. The comparator was primary radiotherapy with or without induction or concurrent chemotherapy for the tumour. The treatments received and compared were of curative intent and patients had not undergone prior intervention, other than diagnostic biopsy.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Our primary outcomes were overall survival (disease‐related mortality was to be studied where possible), locoregional control, disease‐free survival and progression‐free survival or time to recurrence. All outcomes were to be measured at two, three and five years after diagnosis. Our secondary outcomes included quality of life, harms associated with treatment, patient satisfaction and xerostomia score.
Main results
No completed studies met the inclusion criteria for the review. Two ongoing trials fulfilled the selection criteria, however neither are complete.
'Early‐stage squamous cell carcinoma of the oropharynx: radiotherapy versus trans‐oral robotic surgery (ORATOR)' is a phase II randomised controlled trial comparing primary radiation therapy with primary transoral robotic surgery for small‐volume primary (T1‐2, N0‐2) OPSCC. It is currently in progress with an estimated completion date of June 2021.
'European Organisation for Research and Treatment of Cancer 1420 (EORTC 1420‐HNCG‐ROG)' is a phase III, randomised study assessing the "best of" radiotherapy compared to transoral robotic surgery/transoral laser microsurgery in patients with T1‐T2, N0 squamous cell carcinoma of the oropharynx and base of tongue. It was due to start accrual mid‐2016.
Authors' conclusions
The role of endoscopic head and neck surgery in the management of OPSCC is clearly expanding as evidenced by its more overt incorporation into the current National Comprehensive Cancer Network guidelines. Data are mounting regarding its outcomes both in terms of survival and lower morbidity. As confidence increases, it is being used in the management of more advanced OPSCC.
Based on this review, there is currently no high‐quality evidence from randomised controlled trials regarding clinical outcomes for patients with oropharyngeal cancer receiving endoscopic head and neck surgery compared with primary chemoradiotherapy.
PICO
Laienverständliche Zusammenfassung
Minimalinvasive (Schlüsselloch) Chirurgie versus Strahlentherapie allein oder Strahlentherapie und Chemotherapie für kehlkopfkrebs in der Frühphase
Reviewfrage
Wir haben die Evidenz begutachtet, um zwei Behandlungen von Kehlkopfkrebs zu vergleichen. Die Behandlungen waren minimalinvasive Chirurgie und Strahlentherapie allein oder in Kombination mit Chemotherapie.
Hintergrund
Mehr als 400.000 Fälle von Krebs im mittleren Teil des Halses (Oropharynx) werden weltweit jedes Jahr diagnostiziert. Diese Zahl steigt, mit dem humanen Papillomviren (HPV) als erheblich beitragender Faktor. Kehlkopfkrebs, verursacht durch diesen Virus, betrifft jüngere Patienten und diese werden oft mit einem fortgeschrittenem Krankheitsbild vorstellig. Es ist jedoch mit einer besseren Prognose verbunden.
Bis vor kurzem war die Erstlinientherapie von dieser Art von Kehlkopfkrebs die Strahlentherapie in Kombination mit Chemotherapie, da die Forschung zeigte, dass diese vergleichbare Überlebensraten wie die operative Behandlung, aber weniger Nebenwirkungen zeigte. Allerdings haben die Behandlungen sich nun durch computergestütze Planung und Verbesserungen in der Strahlentherapie und dem Aufkommen von minimalinvasiver Kopf‐Hals‐Chirurgie weiterentwickelt, was das Potenzial für weniger Nebenwirkungen der Behandlung hat.
Der Oropharynx spielt eine wesentliche Rolle beim Schlucken, Sprechen und beim Schutz der Atemwege, da sich dieser an der Kreuzung der Atemwege und des Verdauungstraktes befindet. Die Wahl der zu verwenden Behandlung ist verbunden mit der besten zu erwartenden Überlebensrate. Da jüngere Patienten betroffen sind, wird das Ermitteln von sicheren Behandlungen mit möglicherweise weniger Nebenwirkungen und weniger Behinderung immer wichtiger.
Recherchedatum
Die Evidenz dieses Reviews ist auf dem Stand von November 2016.
Studienmerkmale
Wir fanden keine Studien, die minimalinvasive Chirurgie und Strahlentherapie alleine oder kombiniert verglichen. Zwei laufende Studien haben unsere Auswahlkriterien erfüllt, jedoch sind diese noch nicht abgeschlossen. Eine hat einen geschätzten Fertigstellungstermin im Juni 2021 und die andere plant die Rekrutierung von Patienten Mitte 2016.
Hauptergebnisse
Es gibt noch keine abgeschlossenen Studien im Rahmen der Untersuchung, also gibt es zurzeit keine Ergebnisse.
Qualität der Evidenz und Schlussfolgerungen
Die Bedeutung der minimalinvasiven Chirurgie zur Behandlung von Kehlkopfkrebs wächst, wie durch die Einbindung in die aktuellen nationalen Leitlinien in den USA gezeigt wird. Evidenz für die Endpunkte Überleben und weniger Nebenwirkungen der minimalinvasiven Chirurgie nimmt zu.
Auf der Grundlage dieses Reviews gibt es derzeit keine qualitativ hochwertige Evidenz aus randomisierten kontrollierten Studien, die für Patienten mit Kehlkopfkrebs minimalinvasive Chirurgie mit einer Strahlentherapie und Chemotherapie vergleicht.
Authors' conclusions
Summary of findings
| Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small‐volume primary oropharyngeal carcinoma | ||||||
| Patient or population: patients with small‐volume primary oropharyngeal carcinoma Settings: inpatient Intervention: transoral, minimally invasive surgery (transoral robotic surgery/transoral laser microsurgery) with or without adjuvant radiotherapy or adjuvant chemoradiotherapy Comparison: primary radiotherapy with or without induction or concurrent chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Primary radiotherapy ± induction or concurrent chemotherapy | Transoral, minimally invasive surgery ± adjuvant radiotherapy or adjuvant chemoradiotherapy | |||||
| Overall survival | No data | No data | No data | No data | — | — |
| Locoregional control | No data | No data | No data | No data | — | — |
| Progression‐free survival | No data | No data | No data | No data | — | — |
| Gastrostomy rate (at 1 year) | No data | No data | No data | No data | — | — |
| Tracheostomy rate | No data | No data | No data | No data | — | — |
| Swallowing function (MDADI) | No data | No data | No data | No data | — | — |
| Quality of life (EORTC QLQ‐C30 and H&N35) | No data | No data | No data | No data | — | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
More than 400,000 cases of oropharyngeal squamous cell carcinoma (OPSCC) are diagnosed each year worldwide (Chaturvedi 2013). It is estimated that there will be approximately 48,000 new cases of oral and pharyngeal cancer in the United States in 2016 (Siegel 2016). Worldwide the incidence of OPSCC ranges from 7 to 17 cases per 100,000 persons (Chaturvedi 2013), and in developed countries it is steadily rising, particularly in young males (Chaturvedi 2011).
Human papillomavirus (HPV) is a major carcinogen, with an estimated 4.8% of total worldwide cancers in 2008 linked to the virus (de Martel 2012). HPV now meets the epidemiological criteria for OPSCC causality, especially in non‐smokers (Gillison 2015; Sudhoff 2011). Meta‐analysis of the world literature has demonstrated that the proportion of HPV‐associated oropharyngeal cancer has increased from 40.5% in studies recruiting before the year 2000 to 72.2% in studies reporting after 2005 (Mehanna 2012). However, this is known to vary by individual population (Schache 2016). In contrast to this apparent overall trend, Schache et al have published recent work to show that over the period 2002 to 2011, whilst the overall incidence of OPSCC in the UK doubled, the proportion that were HPV‐positive stayed the same, demonstrating an increase in non‐HPV associated OPSCC (Schache 2016). In the UK population, therefore, the increase cannot be attributed to HPV alone. It is worth noting that this group of HPV‐positive patients has significantly improved rates of both overall and disease‐free survival compared to HPV‐negative tumour groups. HPV‐positive OPSCC displays a 58% reduction in the risk of death compared to HPV‐negative disease (Ang 2010; Fakhry 2008). Indeed the presence or absence of HPV with regard to the tumour may have a greater impact on five‐year survival than T stage or nodal status alone (Haughey 2011).
Risk factors for oral HPV infection include a history of orogenital sexual practice, a large number of sexual partners and first intercourse at an early age. The same factors also reflect changes in modern society and combine to increase the cumulative effect of HPV infection in OPSCC (Chung 2009). HPV‐negative OPSCC tends to affect an older age group and is normally associated with smoking and alcohol. HPV‐positive OPSCC behaves differently, often presenting with a small primary in the oropharynx combined with a metastatic cystic deposit in the neck, thereby entailing a higher stage at presentation for the majority of patients (Ang 2010; Dwivedi 2013; Evans 2010).
Description of the intervention
Over the last 20 years management of oropharyngeal cancer has changed dramatically. In 2002, Parsons et al published a review of 51 studies of patients with OPSCC who were treated with surgery with or without radiation therapy or primary radiation therapy without neck dissection (Parsons 2002). The cumulative five‐year survival was 47% for patients undergoing primary surgical resection with or without neck dissection, and 43% for those undergoing primary radiation therapy with or without neck dissection. However, the severe complication rate was 23% in the primary surgical group and only 6% in the primary radiation group. This led to the conclusion that non‐operative therapy was superior to operative therapy for OPSCC of all stages. More recently a large meta‐analysis comparing primary radiotherapy with chemoradiotherapy in 16,192 head and neck squamous cell carcinoma (HNSCC) patients provided updated results. The study concluded an absolute survival benefit of 8.1% after five years in OPSCC patients treated with concurrent chemoradiotherapy (Blanchard 2011).
As the biological differences in viral/non‐viral associated OPSCC are further elaborated (Masterson 2015; Pyeon 2007; Slebos 2006), radical change in most therapeutic interventions has taken place. With regard to radiotherapy, intensity modulation and computerised planning have been introduced in addition to external beam therapy. In surgery, the focus has shifted to the use of minimally invasive procedures such as transoral laser microsurgery or transoral robotic surgery, which demonstrate reduced immediate postoperative toxicity, reduced length of hospital stay and faster functional recovery compared with open surgery (Holsinger 2015). Furthermore, these techniques have the potential to improve organ preservation and function, and ameliorate the economic burden of treatment. Additionally, with regard to control of lymphatic spread, neck dissections have become more selective (resulting in the removal of fewer normal structures and therefore lower morbidity) (Adelstein 2012).
Current management, following the US National Comprehensive Cancer Network guidelines for T1‐2, N0‐1 oropharyngeal tumours (NCCN 2015), is either definitive radiotherapy (with or without salvage surgery) or primary surgery (transoral or open, with or without ipsilateral or bilateral neck dissection). Post‐surgery, if there is extracapsular spread or a positive margin not amenable to re‐resection, the patient should have adjuvant chemoradiotherapy. If there are other adverse features (pT3‐4 primary, N2‐3 nodal disease, nodal disease in levels IV or V, perineural invasion or vascular embolism) patients should at least have radiotherapy with consideration of chemoradiotherapy. For T2 lesions the current guidance is for patients to receive induction chemotherapy prior to radiotherapy or to receive chemoradiotherapy, each with the possibility of salvage surgery for residual disease.
The extent of neck dissection depends on the staging. In the case of N0 lesions a selective neck dissection of at least levels II‐IV is recommended. In N1‐N2a‐c cases a selective to comprehensive neck dissection is recommended. The NCCN guidelines state that "In general, patients undergoing selective neck dissection should not have clinical nodal disease; however, selective neck dissection may prevent morbidity in patients with nodal disease and may be appropriate in certain patients with N1‐N2 disease" (NCCN 2015).
Transoral endoscopic head and neck surgery poses several challenges in relation to haemostasis, illumination and tissue manipulation. In particular, transoral laser surgery is limited by a 'line of sight' where the lens of the microscope, used for visualisation, is distant from the patient. The CO2 laser is also not ideally suited to haemostasis, sometimes requiring alternation of the laser with diathermy or surgical clips. The long manipulators can further restrict the surgeon. Piecemeal removal may be required to facilitate the minimally invasive approach, which goes against the traditional tenets of en‐bloc removal to preserve the specimen for the histopathologist and in order to prevent seeding of the tumour. However, data published by several units have provided evidence that challenges this latter point (Christiansen 2006; Haughey 2011).
Robotic surgery has now emerged to overcome some of the limitations of laser surgery and further expand the minimally invasive surgery portfolio. The advantages offered to the surgeon include a three‐dimensional view, 360° robotic arm movement (in restricted spaces with precision of movement), hand tremor filtration and lack of muscle fatigue. Line of sight restrictions can be avoided as optical feedback from the endoscope is near the resection tissue and angled instruments can be used (Genden 2012). Furthermore, combinations of the two techniques have been developed including carbon dioxide and thulium laser attachments for the Da Vinci robot (Solares 2007; Van Abel 2012).
How the intervention might work
In the 1980s, Wolfgang Steiner pioneered the transoral use of lasers to resect tumours of the upper aerodigestive tract (Steiner 1988). Subsequent publications have confirmed that survival using these transoral laser techniques is at least as good as with primary radiotherapy/chemoradiotherapy, whilst long‐term swallowing function is significantly enhanced (Haughey 2011; Rich 2011).
The published literature suggests that survival outcomes are similar for OPSCC treated by either transoral laser microsurgery or lip split mandibulotomy (Jackel 2007). One recent clinical trial comparing both modalities had too few patients in each group to make a valid comparison with respect to survival, but suggested a benefit of transoral laser microsurgery for other parameters such as tracheostomy rate, time to decannulation, mode of feeding, length of hospital stay and cost (Williams 2013).
Most tertiary head and neck ENT departments will already possess or have access to a CO2 laser, given its use in the larynx, and its limitations are well recognised. However, the expertise required for its use in resection of OPSCC is less widespread. Nevertheless, given the possibilities in terms of patient outcomes, its use can only increase (Grant 2009).
In recent years, additional evidence of enhanced functional outcome following transoral tumour resection has been provided by the early proponents of transoral robotic surgery (Lawson 2011; Moore 2009; Weinstein 2012). The da Vinci Surgical Robot (Intuitive Surgical Incorporated, CA, USA) has been utilised for almost all surgical procedures performed in the head and neck region (Weinstein 2012). The system comprises three components: (a) a high‐definition visual display unit, (b) a surgical control platform that is distant from the patient, and (c) a robotic platform adjacent to the patient. The surgeon can remotely operate the robot using EndoWrist technology, which offers several advantages in terms of tremor filtration, three‐dimensional visualisation and wristed instrumentation. The surgical platform allows control of robot manipulators that normally consist of a camera arm (providing true stereoscopic, high‐definition images) and two 8 mm or 5 mm handling arms.
Lack of haptic feedback, extra space and time allocation are common limitations that are likely to improve in the near future with advancing technology. The cost of installation is approximately GBP 1 million (USD 1.2 million) along with a GBP 70,000 to 80,000 (USD 87,000 to 99,000) yearly maintenance fee and GBP 150 to 500 (USD 186 to 620) per case due to disposable instrumentation (Weinstein 2007). Whilst this high price restricts acquisition to larger teaching hospital settings where the capital outlay cost can be shared with urology, general surgery and cardiothoracic departments (who will also have higher case‐loads), the disposable instrument costs are comparable with other endoscopic harmonic or laser instruments (Richmon 2014).
Why it is important to do this review
The oropharynx plays an essential role in swallowing, speech and protecting the airway as it is situated at the bifurcation of the respiratory and digestive tract. Treatment modalities are heavily influenced by the aim of reducing the risk of functional disability where possible.
Early‐stage tumours arising from the tonsil or base of the tongue region (T1 or T2) can be a technical challenge to excise and may necessitate a trans‐cervical or mandibulotomy approach. Radiotherapy or chemoradiotherapy are alternative primary treatments and will often be given as adjuvant treatment if complete surgical clearance cannot be obtained. Open surgery and radiotherapy/chemoradiotherapy both have drawbacks in terms of cost, overall survival and patient quality of life (Haigentz 2009; Machtay 2008). This latter point has become more important because HPV‐associated OPSCC affects a younger patient cohort who may have to carry the burden of treatment‐related morbidity for a much longer period of time (Quon 2013).
Advanced technology has now made it possible to completely resect a primary oropharyngeal tumour using transoral, minimal access surgery. Lasers can be used to resect tumours under microscopic guidance and three‐dimensional images of the back of the mouth enable robotically guided instruments to dissect tumours free from surrounding tissues. So far, observational studies suggest that these approaches may have an advantage by improving patient quality of life and functional outcome, and reducing the need for adjuvant chemoradiotherapy (Leonhardt 2012; Moore 2013). Furthermore, the use of endoscopic head and neck surgery for the primary site may permit a lower radiation dose to the neck (60 Gy rather than 70 Gy), with a concomitant reduction in radiation‐related morbidity. This has raised the potential option of 'de‐escalation therapy' in the case of HPV‐related OPSCC (Masterson 2014a; Masterson 2014b; Moore 2009). The potential benefits of this are apparent when one considers the well‐established relationship between the radiation dose to the constrictor muscles and long‐term swallowing difficulties: patients in whom more than 78% of their cricopharyngeus inlet receives over 60 Gy have a 50% risk of developing a stricture (Chen 2010).
The potential benefits of a minimally invasive approach were behind the drive to investigate the evidence comparing this with traditional radiotherapy/chemoradiotherapy. We plan to review the evidence for de‐escalation of adjuvant treatment post minimally invasive surgery in a related review of 'Trans‐oral endoscopic head and neck surgery plus adjuvant (chemo)radiotherapy versus trans‐oral endoscopic head and neck surgery plus de‐intensified adjuvant (chemo)radiotherapy for oropharyngeal squamous cell carcinoma' (title registered with Cochrane).
Objectives
To assess the efficacy of endoscopic head and neck surgery (transoral robotic surgery or transoral laser microsurgery) for small‐volume, primary (T1‐2, N0‐2) oropharyngeal squamous cell carcinoma (OPSCC) in comparison to radiotherapy/chemoradiotherapy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials. We excluded quasi‐randomised and cluster‐randomised trials.
Types of participants
We included patients with carcinoma in the oropharynx subsite (as defined by the World Health Organization classification C09, C10). We excluded oral cavity (C01‐C02, C03, C04, C05‐C06), hypopharynx (C13), nasopharynx (C11) and larynx (C32) lesions (WHO 2000).
Cancers included were primary squamous cell carcinomas arising from the oropharyngeal mucosa. The tumours were classified as T1‐T2 with or without nodal disease and with no evidence of distant metastatic spread. A significant proportion of patients are expected to be HPV16‐positiveve (the commonest subtype of HPV responsible for more than 95% of cases of oropharyngeal squamous cell carcinoma (OPSCC)).
Types of interventions
Intervention
Transoral, minimally invasive surgery (transoral robotic surgery/transoral laser microsurgery) with or without adjuvant radiotherapy or adjuvant chemoradiotherapy.
Control
Primary radiotherapy with or without induction or concurrent chemotherapy for the tumour. The treatments received and compared were of curative intent and patients had not undergone prior intervention, other than diagnostic biopsy.
The sole planned comparison was:
-
endoscopic head and neck surgery versus radiotherapy/chemoradiotherapy.
Types of outcome measures
We planned to analyse the following outcomes in the review, but they would not have been used as a basis for including or excluding studies.
Primary outcomes
-
Overall survival (disease‐related mortality was to be studied where possible)
-
Locoregional control
-
Disease‐free survival
-
Progression‐free survival or time to recurrence
All outcomes were to be measured at two, three and five years after diagnosis.
Secondary outcomes
-
Quality of life, dysphagia and patient satisfaction, including the following:
-
MD Anderson Dysphagia Inventory (MDADI)
-
Gastrostomy tube rate (at one year)
-
European Organisation for Research and Treatment of Cancer (EORTC) scales (e.g. QLQ‐C30 and H&N35)
-
Neck Dissection Impairment Index (NDII)
-
Voice Handicap Index‐10 (VHI‐10)
-
-
Harms associated with treatment, including the following:
-
National Cancer Institute Common Toxicity Criteria (NCI‐CTC)
-
Patient Neurotoxicity Questionnaire (PNQ)
-
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 8 November 2016.
Electronic searches
The Information Specialist searched:
-
the Cochrane ENT Trials Register (searched 8 November 2016);
-
the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 10);
-
PubMed (1946 to 8 November 2016);
-
Ovid EMBASE (1974 to 2016 week 45);
-
Ovid CAB Abstracts (1910 to 2016 week 43);
-
EBSCO CINAHL (1982 to 8 November 2016);
-
LILACS, lilacs.bvsalud.org (searched 8 November 2016);
-
KoreaMed (searched via Google Scholar 8 November 2016);
-
IndMed, www.indmed.nic.in (searched 8 November 2016);
-
PakMediNet, www.pakmedinet.com (searched 8 November 2016);
-
Web of Knowledge, Web of Science (1945 to 8 November 2016);
-
CNKI, www.cnki.com.cn (searched via Google Scholar 8 November 2016);
-
ClinicalTrials.gov, (searched via the Cochrane Register of Studies 8 November 2016);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), www.who.int/ictrp (searched 8 November 2016);
-
ISRCTN, www.isrctn.com (searched 8 November 2016);
-
Google Scholar, scholar.google.co.uk (searched 8 November 2016);
-
Google, www.google.com (searched 8 November 2016).
The Information Specialist modelled the subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched PubMed, theCochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
Data collection and analysis
This review is based on a published protocol (Howard 2014).
Selection of studies
Three authors (JH, LM and RD) independently screened the results of the search to identify studies that broadly met the inclusion criteria. We independently assessed studies selected for full‐text review against the predefined inclusion criteria. Any conflict was resolved by referral to a senior author.
Data extraction and management
Three review authors (JH, LM and RD) independently extracted data using a specially designed data extraction form. We piloted the data extraction forms on several papers and modified them as required before use. We discussed any disagreements in full and consulted a senior review author where necessary. Where required, we contacted the authors for clarification or missing information.
For each trial we recorded the following data.
-
Year of publication, country of origin and source of study funding.
-
Details of the participants, including demographic characteristics and criteria for inclusion and exclusion.
-
Details of the type of intervention, timing and duration.
-
Details of quality of life after treatment (EORTC QLQ‐HN35 or equivalent questionnaire) (Singer 2013).
-
Details of treatment‐related morbidity, categorised as acute (less than 90 days after treatment) or late (more than 90 days) and classified according to the Common Terminology Criteria for Adverse Events (CTCAE 2010).
-
Details of all other outcomes reported, including method of assessment and time intervals.
-
HPV status for each patient established by displaying p16 activity (a surrogate marker of viral activity) utilising immunohistochemistry and, if positive, confirmed by polymerase chain reaction or DNA/RNA in situ hybridisation (Masterson 2016; Schache 2011; Westra 2009).
Assessment of risk of bias in included studies
Had suitable trials been retrieved JH, LM and RD would have assessed the risk of bias of the included trials independently, with the following taken into consideration, as guided by theCochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
-
sequence generation;
-
allocation concealment;
-
blinding;
-
incomplete outcome data;
-
selective outcome reporting; and
-
other sources of bias.
We planned to use the Cochrane 'Risk of bias' tool in RevMan 5.3 (RevMan 2014), which involves describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias.
Measures of treatment effect
We planned to express continuous outcomes as a mean difference with standard deviation. For continuous outcomes measured using different (but compatible) scales we planned to express treatment effects as a standardised mean difference.
For the key outcomes in the 'Summary of findings' table we also planned to present the results as absolute percentages.
Unit of analysis issues
We planned to use data only from individually randomised controlled trials to avoid unit of analysis issues. We planned to exclude cluster‐randomised trials.
Dealing with missing data
We planned to contact study authors:
-
where a study protocol suggested an outcome of interest had been measured but was not reported;
-
if not all data required for meta‐analysis were reported;
-
if standard deviation data were not available or estimable from other reported data (using the methods detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011)).
Assessment of heterogeneity
We planned to assess heterogeneity by inspecting the overlap of confidence intervals for the results of individual studies and to utilise horizontal lines to depict these graphically. We planned to conduct formal assessment of heterogeneity using the Chi² test (with a significance level of α = 0.1 in view of the low power of this test) and the I² statistic (with 75% or more indicating a considerable level of inconsistency), both available in RevMan 5.3 (RevMan 2014).
Assessment of reporting biases
We planned to assess reporting bias as within‐study (outcome reporting) bias and between‐study (publication) bias.
Outcome reporting bias
Bias can occur if outcomes are not adequately reported to allow further analysis. We planned to assess outcome reporting bias by comparing the reported outcomes against the outcomes listed in the trial protocol or methods section. Where there was insufficient detail we planned to contact study authors. If no further information could be found this would be judged a 'high' risk of bias. If insufficient information was found to allow adequate judgement we would judge this an 'unclear' risk of bias.
Publication bias
If sufficient trials were available we planned to assess funnel plots for each outcome. If asymmetry was found we planned to investigate this further according to the methodology in the Cochrane Handbook of Systematic Reviews of Interventions (Handbook 2011).
Data synthesis
We planned to extract data from the included studies and enter the data into RevMan 5.3 for statistical analysis. In the event of incomplete data, we intended to contact the study authors to obtain further information and to seek statistical advice where necessary. We planned to approach survival and disease recurrence in one of two ways depending on the data available and aimed to include analysis of the proportion surviving at two, three and five years as dichotomous outcomes or hazard ratios for comparison in meta‐analysis. Secondary outcomes were to be restricted to assessment of validated questionnaires (where appropriate). If the data provided were in the form of means and standard deviations, we intended to display the effects on outcomes as standardised mean differences (SMD) with 95% confidence intervals (CIs) and using an intention‐to‐treat analysis. If hazard ratios were not quoted in studies, we planned to calculate them from available summary statistics such as observed events, expected events, variance, confidence intervals, P values or survival curves (Parmar 1998).
Subgroup analysis and investigation of heterogeneity
We intended to assess clinical heterogeneity by examining the types of participants, interventions and outcomes in each study. We would have attempted a meta‐analysis if studies were available with similar comparisons and reported the same outcome measures. If appropriate, we intended to calculate pooled estimates using a random‐effects model (Handbook 2011), as there is likely to be significant statistical or clinical heterogeneity (an I² value > 50%, as specified in the Cochrane Handbook for Systematic Reviews of Interventions).
If the data provided were in the form of means and standard deviations, we intended to display the effects on outcomes as standardised mean differences (SMD) with 95% CIs and using an intention‐to‐treat analysis.
Furthermore, if the subgroups showed a clinically relevant difference we planned to report them separately using a fixed‐effect model (Handbook 2011).
Sensitivity analysis
We aimed to consider a sensitivity analysis to compare fixed‐effect and random‐effects estimates. We also planned to undertake a sensitivity analysis to examine the effects of allocation concealment, randomisation, quality of follow‐up and blind outcome assessment (if appropriate).
GRADE and 'Summary of findings' table
We planned to use the GRADE approach to rate the overall quality of evidence. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we planned to apply this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high quality of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
-
study limitations (risk of bias);
-
inconsistency;
-
indirectness of evidence;
-
imprecision; and
-
publication bias.
We planned to include a 'Summary of findings' table, constructed according to the recommendations described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), which would include the following outcomes: 1) overall survival; 2) locoregional control; 3) progression‐free survival; 4) gastrostomy rate (at one year); 5) tracheostomy rate; 6) swallowing function (MDADI); 7) quality of life (EORTC QLQ‐C30 and H&N35).
Results
Description of studies
Results of the search
The search performed on 8 November 2016 identified 2180 results that dropped to 1314 after removal of duplicates. We added a further two trials from handsearching. Review of title and abstracts identified six articles/reports covering four studies for full‐text review and one trial report. Figure 1 depicts a PRISMA flow chart of the search history.

Process for sifting search results and selecting studies for inclusion.
No completed studies met the inclusion criteria for the review. We excluded three studies, ADEPT, ECOG‐E3311 and PATHOS, and identified two ongoing studies that will meet our inclusion criteria when they are complete (EORTC 1420; ORATOR). There are no studies awaiting assessment.
Included studies
There are no included studies.
Excluded studies
We excluded three studies after full‐text review (see the table of Characteristics of excluded studies for individual study details).
ADEPT is a phase III, prospective trial of de‐escalated adjuvant treatment post endoscopic head and neck surgery (transoral robotic surgery or transoral laser microsurgery) for human papillomavirus (HPV)‐positive oropharyngeal squamous cell carcinoma (OPSCC) (T1‐4a) patients noted to have extracapsular spread detected in the nodal disease. The study is currently in progress with an estimated completion date of October 2021. Patients are allocated to two arms post endoscopic head and neck surgery through randomisation or patient choice, to receive either postoperative radiotherapy (60 Gy) alone or with concurrent systemic cisplatin therapy.
The primary outcomes are disease‐free survival and locoregional control at two years. Secondary outcomes include overall survival and distant metastasis rate up to five years, quality of life and functional outcomes to two years, and toxicities up to 4.5 months.
We excluded this study because it includes advanced tumours (T1‐4a) and all patients have nodal neck disease with extracapsular spread. Again all patients receive the surgical intervention so there is no radiotherapy/chemoradiotherapy‐alone cohort.
ECOG‐E3311 is a phase II, prospective randomised trial of reduced adjuvant treatment post transoral robotic surgery for HPV‐positive locally advanced OPSCC (stages III‐IVa + IVb without distant metastases). The study is in progress with an estimated completion date of October 2016. The primary foci for investigation are the feasibility of risk‐adjusted adjuvant therapy and oncologic outcomes for intermediate‐risk patients post transoral robotic surgery and standard or de‐escalated treatment.
Patients will be stratified into three groups depending on their surgical histology. The low‐risk group will have no adjuvant therapy as per standard treatment. The medium‐risk group will be randomised to receive either standard (60 Gy) or de‐escalated (50 Gy) postoperative radiotherapy. The high‐risk group will receive postoperative radiotherapy (60 Gy) with concurrent cisplatin.
The primary outcomes include progression‐free survival at two years, risk distribution and grade 3‐4 bleeding events during surgery. Secondary outcomes include overall survival, swallow and voice function up to two years post‐treatment, and change in patient‐reported quality of life up to six months post‐radiation treatment.
We excluded this study because it includes advanced tumours (T3‐4) only and again because all patients receive a surgical intervention so there is no radiotherapy/chemoradiotherapy‐alone cohort.
PATHOS is a phase II/III, prospective randomised controlled trial of reduced‐intensity adjuvant treatment for HPV‐positive OPSCC (T1‐3, N0‐2b) patients treated with transoral robotic surgery. It is not yet open for recruitment and the estimated completion is December 2019.
Patients will be stratified into three groups depending on their surgical histology. The low‐risk group will have no adjuvant therapy as per standard treatment. The medium‐risk group will be randomised to receive either standard (60 Gy) or de‐escalated (50 Gy) postoperative radiotherapy. The high‐risk group will be randomised to receive postoperative radiotherapy (60 Gy) with or without concurrent cisplatin.
The primary outcome measure is swallowing function (MD Anderson Dysphagia Inventory) at one year. Secondary outcomes include disease‐free and overall survival at six months, and swallowing function, quality of life and acute or late toxicities measured at up to 24 months.
We excluded this study because it includes patients with T1‐3, N0‐2b disease and all patients receive endoscopic head and neck surgery. There is therefore no radiotherapy/chemoradiotherapy‐alone cohort. Randomisation is also performed only after stratification by stage/features of the excised tumour.
All three of these studies are potentially of interest for our related review on de‐escalation of adjuvant therapy (title registered with Cochrane).
Ongoing studies
Two studies met the inclusion criteria for this review, however neither are completed. One is in preparation and the other in progress with initial results expected in 2021.
'Early‐stage squamous cell carcinoma of the oropharynx: Radiotherapy versus Trans‐oral Robotic Surgery' (ORATOR) is a phase II, randomised controlled trial comparing primary radiotherapy with primary transoral robotic surgery for early‐stage (T1‐2, N0‐2) OPSCC. It is currently in progress with an estimated completion date of June 2021. Patients will be randomised to receive either primary radiotherapy or primary transoral robotic surgery with or without neck dissection. The primary outcome is quality of life (at one year) and the secondary outcomes include overall and progression‐free survival (at three and five years), toxicity and swallowing function (at five years).
'European Organisation for Research and Treatment of Cancer 1420 (EORTC 1420‐HNCG‐ROG)' is a phase III, randomised study assessing the "best of" radiotherapy compared to the "best of" endoscopic head and neck surgery in patients with T1‐T2, N0 squamous cell carcinoma (EORTC 1420). This study will include stage I and II tonsil, glosso‐tonsillar sulcus, lateralised base of tongue as well as lateral pharyngeal wall squamous cell carcinoma. The study is in preparation and was expected to start accruing patients around mid‐2016. Treatment of the neck will be with surgery or radiotherapy depending on the randomisation. The primary outcome will be the assessment of swallowing function within the first year after the two treatment strategies. Secondary outcomes include locoregional tumour control rate at one and two years, overall survival at one, two and five years, functional assessment at two years, complication rate at two years, quality of life up to two years and cost‐effectiveness. One hundred and seventy patients will be randomised to demonstrate a difference between the two treatment arms of at least eight points on the MD Anderson Dysphagia Inventory swallowing scale.
Risk of bias in included studies
We found no completed studies that met the inclusion criteria.
Effects of interventions
No studies could be included in this current review.
Discussion
Summary of main results
Unfortunately, although we deemed two randomised controlled trials suitable for inclusion, neither are complete (EORTC 1420; ORATOR). The earliest results are expected in 2021.
Quality of the evidence
Most studies were either retrospective or prospective in design without evidence of randomisation. The two randomised trials with adequate inclusion criteria will report from 2021. The overall risk of bias is therefore unclear at present.
Potential biases in the review process
The search for this topic is comprehensive and up to date to November 2016, allowing for identification of all appropriate studies. We made some minor post hoc changes to our protocol (Howard 2014), which are described in Differences between protocol and review.
Agreements and disagreements with other studies or reviews
This review is in agreement with other reviews in that there are no prospective data available on oncologic or quality of life outcomes for patients with oropharyngeal cancer receiving endoscopic head and neck surgery alone compared with radiotherapy/chemoradiotherapy.
De Almeida et al reviewed the literature and found similar oncologic outcomes at two years when comparing eight intensity‐modulated radiotherapy (IMRT) and 12 transoral robotic surgery studies (de Almeida 2014). The main difference they found between the two groups was in the adverse outcomes. For IMRT these were: gastrostomy tube (43%), oesophageal stenosis (4.8%) and osteoradionecrosis (2.6%). For transoral robotic surgery these were: fistula (2.5%), haemorrhage (2.4%) or gastrostomy tube at the time of surgery (1.4%) or during adjuvant therapy (30%).
Morisod et al examined the literature for survival post radiotherapy or endoscopic head and neck surgery (transoral robotic surgery and transoral laser microsurgery) for early oropharyngeal squamous cell carcinoma (OPSCC) (T1‐2, N0, M0) (Morisod 2016). When analysing studies published from 2004 onwards they found no difference in five‐year overall and disease‐specific survival. Locoregional failure rates were similar (21.3% radiotherapy versus 15.2% endoscopic head and neck surgery) but salvage success rates appeared better after endoscopic head and neck surgery (50.1% radiotherapy versus 93.6% endoscopic head and neck surgery), although these data were not present in all studies. This review is limited as it did not stratify by IMRT or conformal radiotherapy. Also, the endoscopic head and neck surgery trials had a high degree of heterogeneity.
Yeh et al reviewed the literature and found 14 studies following IMRT and six following transoral robotic surgery (Yeh 2015). They found similar disease‐free survival between the two modalities. The follow‐up was generally longer in the IMRT studies (˜2.5 years) than in the transoral robotic surgery studies (˜1.5 years). No meta‐analysis was performed, possibly due to incompatibility/heterogeneity of the data.
Tracheostomy, gastrostomy and quality of life
De Almeida et al found that there were no recorded tracheostomies in the IMRT studies (de Almeida 2014). Tracheostomy was required for 12% of transoral robotic surgery patients at the time of surgery with the majority decannulated prior to discharge.
For transoral robotic surgery, with the exception of one study where 100% of patients had a gastrostomy tube (a protocol that has now changed), the percutaneous endoscopic gastrostomy rate was low, with three studies giving a cumulative rate of 1.4% (2/139) at the time of surgery, and two studies quoting 0% and 2% rates at one year. For patients requiring adjuvant treatment this rose to 30% (32/107) in the three studies reporting this. The rate was 43% for the three IMRT studies reporting this outcome.
Validity of the comparison can be criticised in that there were only 772 transoral robotic surgery patients (502 T1‐2) compared to 1337 IMRT patients (1010 T1‐2). There was significant heterogeneity between studies, which may have been exacerbated by the inclusion of studies with over 75% of participants with low T stage and any N stage. Patients with advanced disease may therefore have been included with resultant increases in the rates of concurrent chemotherapy and neck dissection. This may explain the high frequency of adjuvant treatment found in both the IMRT and transoral robotic surgery trials although early experience (patient selection and technical expertise) is likely to have played a role too.
Yeh et al found three IMRT and nine transoral robotic surgery papers quoting tracheostomy dependency at one year (Yeh 2015). This ranged from 0.1% to 4.5% for the IMRT studies and 0% to 3.5% for the transoral robotic surgery studies. Seven of the transoral robotic surgery studies had no tracheostomies.
Feeding tube dependence at one year was available in 14 IMRT and 13 transoral robotic surgery studies. For IMRT studies the average was 6.1% (range 0% to 18%) and was more likely in older, higher N stage, greater pack‐year smoking history or concurrent chemotherapy patients. For transoral robotic surgery studies the average was 3.8% (range 0% to 20.7%). The study with a 20.7% rate, Al‐Khudari 2013, included salvage and advanced cases, and no patients who underwent transoral robotic surgery alone were feeding tube dependent. Five of the 13 transoral robotic surgery studies had no cases dependent.
The authors also looked at quality of life and swallowing outcomes, however there was very little consensus on the scoring or scales used, which precluded more formal comparison. Two studies compared swallowing quality of life between IMRT and transoral robotic surgery. More et al found that at 6 and 12 months patients treated with transoral robotic surgery and adjuvant treatment had significantly better (MD Anderson Dysphagia Inventor scores; More 2013). Chen et al found no statistical difference, however the swallowing scores were better (91.5 versus 72.1) (Chen 2015).
Rathod et al reviewed the literature on quality of life outcomes in surgery versus radiotherapy/chemoradiotherapy for OPSCC (Rathod 2015). Unfortunately, no study met their inclusion criteria (endoscopic head and neck surgery or IMRT), preventing a meta‐analysis of the results.
Margins and adjuvant treatment
Cracchiolo et al searched the National Cancer Data Base for T1‐2 OPSCC patients from 2004 and found that 25% of patients treated with primary surgery (both open and endoscopic) had positive margins, and 25% had extracapsular spread (Cracchiolo 2016). The rate of positive margins was highest in 2007 (34%) and lowest in 2013 (18%) and decreased significantly with time (P < 0.0001). The authors suggested a possible correlation between the peak value and the early work with transoral robotic surgery. Higher‐volume centres had fewer positive margins (P < 0.0001) and were more likely to use surgical management than lower‐volume centres. Surgery was also more likely to be used for patients with a lower nodal status (P < 0.0001) and who were younger. Of the patients treated with surgery with full pathological staging available 47% of candidates originally suitable for monotherapy (T1‐2, N0‐1) had at least one adverse factor (T3‐4, N2‐3, positive margins or extracapsular spread).
Concerns have been raised regarding the proportion of endoscopic head and neck surgery patients requiring adjuvant treatment and thereby nullifying some of the potential gains regarding treatment de‐escalation (Huang 2015), and placing them at risk of complications, for example soft tissue necrosis (Hee 2016; Luckens 2014). Huang et al found that only 11% to 36% of primary surgical cases were treated with transoral robotic surgery alone, with 15% to 54% rates of postoperative radiotherapy and 11% to 63% rates of postoperative chemoradiotherapy, raising concerns regarding appropriate patient selection (Huang 2015). Notably the adjuvant treatment rate for transoral robotic surgery appears to be decreasing with time. Recent data from the safety monitoring committee for ECOG E3311 reveal that a majority of patients (69%) have avoided concurrent chemoradiation (Holsinger 2015).
The presence of extracapsular spread is an important factor influencing the rate of adjuvant treatment. In surgically treated patients, according to the SEER database, the incidence of extracapsular spread in OPSCC is 23% (Byrd 2016). The relevance of extracapsular spread as a high‐risk feature in HPV‐positive OPSCC has been questioned (Sinha 2012). The ADEPT trial (currently in progress) is specifically investigating this and may result in a change in the guidelines in the future.
It is worth remembering that whilst current efforts are focused on de‐escalation and the avoidance of triple therapy, a proportion of primary radiotherapy/chemoradiotherapy patients will also require salvage surgery and to a certain extent this is unavoidable at present (de Almeida 2014). Furthermore, surgical treatment also provides valuable pathological staging information, which may be especially helpful in HPV‐negative patients (Byrd 2016).
Transoral robotic surgery has also been employed for salvage surgery. White et al found that TNM stage‐matched patients who underwent salvage transoral robotic surgery rather than open surgery had a significantly lower incidence of tracheostomy (P < 0.001) and feeding tube (P < 0.001), and a shorter hospital stay (White 2013). Whilst the healing time is prolonged for these patients (in the context of prior radiation) the two‐year disease‐free survival rate was also significantly higher (74% versus 43%; P = 0.01) for the transoral robotic surgery group.
Cost
In terms of cost, transoral robotic surgery has been shown to be cheaper than open surgery for the treatment of OPSCC (Yeh 2015). De Almeida et al found that there was a cost saving associated with transoral robotic surgery over IMRT, however this was lost in low‐volume centres and with the addition of adjuvant treatment (de Almeida 2014).
In summary, the current literature reports mainly heterogeneous and retrospective data. The cumulative evidence would suggest an equivalent survival rate for both IMRT and endoscopic head and neck surgery. Tracheostomy rates are similar with possibly better quality of life, feeding tube and swallowing outcomes for endoscopic head and neck surgery. Rates of positive margins are decreasing with time and these (along with costs) appear to be better at high‐volume centres.
Whilst concerns have been raised regarding high rates of adjuvant treatment (reported in retrospective trials), this appears to improving as evidenced by early data from current randomised controlled trials. Furthermore, the results of trials currently in progress may change future guidelines, allowing more patients to receive de‐escalated treatment.
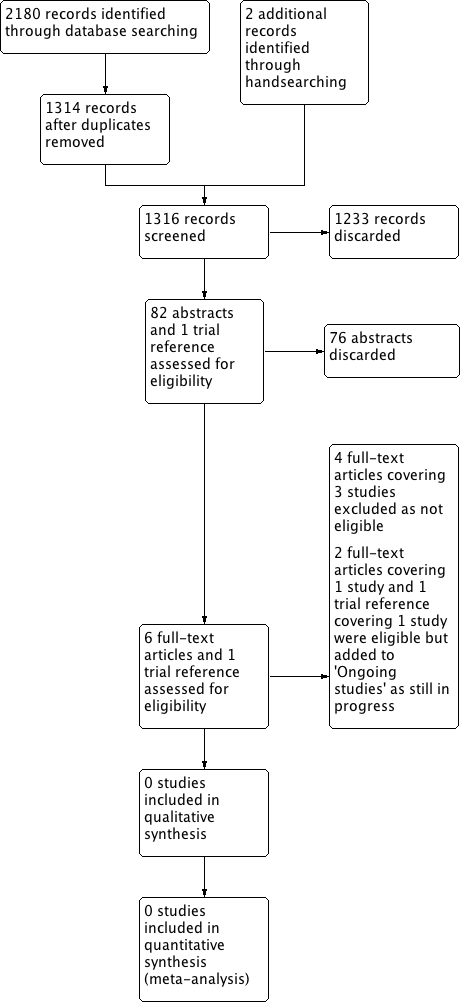
Process for sifting search results and selecting studies for inclusion.
| Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small‐volume primary oropharyngeal carcinoma | ||||||
| Patient or population: patients with small‐volume primary oropharyngeal carcinoma Settings: inpatient Intervention: transoral, minimally invasive surgery (transoral robotic surgery/transoral laser microsurgery) with or without adjuvant radiotherapy or adjuvant chemoradiotherapy Comparison: primary radiotherapy with or without induction or concurrent chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Primary radiotherapy ± induction or concurrent chemotherapy | Transoral, minimally invasive surgery ± adjuvant radiotherapy or adjuvant chemoradiotherapy | |||||
| Overall survival | No data | No data | No data | No data | — | — |
| Locoregional control | No data | No data | No data | No data | — | — |
| Progression‐free survival | No data | No data | No data | No data | — | — |
| Gastrostomy rate (at 1 year) | No data | No data | No data | No data | — | — |
| Tracheostomy rate | No data | No data | No data | No data | — | — |
| Swallowing function (MDADI) | No data | No data | No data | No data | — | — |
| Quality of life (EORTC QLQ‐C30 and H&N35) | No data | No data | No data | No data | — | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||

