Espectroscopia del infrarrojo cercano (NIRS) cerebral para la monitorización perioperatoria de la oxigenación cerebral en niños y adultos
Resumen
Antecedentes
Se han empleado diversas técnicas para la detección temprana de la isquemia y la hipoxia cerebral perioperatorias. La espectroscopia del infrarrojo cercano (NIRS, por sus siglas en inglés) cerebral se usa cada vez más en este escenario clínico para monitorizar la oxigenación cerebral. Sin embargo, no se conoce si la monitorización cerebral perioperatoria con NIRS y las estrategias de tratamiento posteriores son beneficiosas para los pacientes.
Objetivos
Evaluar los efectos de la monitorización cerebral perioperatoria con NIRS y las estrategias de tratamiento correspondientes en adultos y niños, en comparación con la monitorización de la oxigenación cerebral cegada o ninguna monitorización, o la monitorización de la oxigenación cerebral basada en tecnologías diferentes a la NIRS, en la detección de los eventos de desaturación de oxígeno cerebral (EDC), los resultados neurológicos, los resultados no neurológicos y el impacto socioeconómico (incluido el costo de la hospitalización y la duración de la estancia hospitalaria).
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL 2016, número 12), Embase (1974 hasta el 20 diciembre 2016) y en MEDLINE (PubMed) (1975 hasta el 20 diciembre 2016). También se hicieron búsquedas de estudios en curso en la World Health Organization (WHO) International Clinical Trials Registry Platform el 20 diciembre 2016. Se actualizó esta búsqueda en noviembre 2017, pero estos resultados aún no se han incorporado a la revisión. No se impuso ninguna restricción de idioma.
Criterios de selección
Se incluyeron todos los ensayos controlados aleatorios (ECA) relevantes que trataban el uso de NIRS cerebral en el contexto perioperatorio (durante la cirugía y en un plazo de 72 horas después de la cirugía), incluido el quirófano, la unidad de atención de posanestesia y la unidad de cuidados intensivos.
Obtención y análisis de los datos
Dos autores seleccionaron los estudios de forma independiente, evaluaron el riesgo de sesgo y extrajeron los datos. Para los resultados binarios, se calculó el cociente de riesgos (CR) y su intervalo de confianza (IC) del 95%. Para los datos continuos, se calculó la diferencia de medias (DM) entre los grupos y su IC del 95%. Debido a que se esperaba heterogeneidad clínica y metodológica entre los estudios, se empleó un modelo de efectos aleatorios para los análisis y se examinaron los datos de la heterogeneidad (estadística I2). Se creó una tabla de 'Resumen de hallazgos' usando GRADEpro.
Resultados principales
Se incluyeron 15 estudios en la revisión, con un total de 1822 participantes adultos. Hay 12 estudios en espera de clasificación y ocho estudios en curso.
Ninguno de los 15 estudios incluidos consideró a la población pediátrica. Cuatro estudios se realizaron en el contexto de la intervención quirúrgica abdominal y ortopédica (columna lumbar, o reemplazo de rodilla y cadera), un estudio en el contexto de la endarterectomía carótida y los diez estudios restantes en el contexto de la intervención quirúrgica aórtica o cardíaca. Las fuentes principales de sesgo en los estudios incluidos se relacionaron con el conflicto de intereses potencial causado por el patrocinio de la industria, las condiciones poco claras del cegamiento o los datos faltantes de los participantes.
Dos estudios con 312 participantes consideraron la lesión neurológica posoperatoria, sin embargo, no fue posible estimar un cálculo del efecto agrupado debido a la dirección discordante del efecto entre los estudios (evidencia de baja calidad). Un estudio (N = 126) en participantes sometidos a cirugía abdominal mayor informó que 4/66 participantes presentaron lesión neurológica con la monitorización cegada versus 0/56 en el grupo de monitorización activa. Un segundo estudio (N = 195) en participantes sometidos a cirugía de derivación de arterias coronarias informó que 1/96 participantes presentaron lesión neurológica en el grupo de monitorización cegada en comparación con 4/94 participantes en el grupo de monitorización activa.
No existe seguridad en cuanto a si la monitorización cerebral con NIRS activa tiene un efecto importante sobre el riesgo de accidente cerebrovascular posoperatorio debido al número reducido de eventos y al intervalo de confianza amplio (CR 0,25; IC del 95%: 0,03 a 2,20; dos estudios, 240 participantes; evidencia de baja calidad).
No existe seguridad en cuanto a si la monitorización cerebral con NIRS activa tiene un efecto importante sobre el delirio posoperatorio debido al intervalo de confianza amplio (CR 0,63; IC del 95%: 0,27 a 1,45; un estudio, 190 participantes; evidencia de baja calidad).
Dos estudios con 126 participantes revelaron que la monitorización cerebral con NIRS activa puede reducir la incidencia de disfunción cognitiva posoperatoria leve (POCD, por sus siglas en inglés) según lo definieron los estudios originales una semana después de la intervención quirúrgica (CR 0,53; IC del 95%: 0,30 a 0,95; I2 = 49%, evidencia de baja calidad).
Basado en seis estudios con 962 participantes, hubo evidencia de calidad moderada de que la monitorización de la oxigenación cerebral activa probablemente no disminuye la ocurrencia de POCD (disminución en la función cognitiva) una semana después de la intervención quirúrgica (CR 0,62; IC del 95%: 0,37 a 1,04; I2 = 80%). El tipo diferente de equipo de monitorización en un estudio podría ser la causa potencial de la heterogeneidad.
No existe seguridad sobre si la monitorización cerebral con NIRS activa tiene un efecto importante sobre la mortalidad intraoperatoria o la mortalidad posoperatoria debido al número reducido de eventos y el intervalo de confianza amplio (CR 0,63; IC del 95%: 0,08 a 5,03; I2= 0%; tres estudios, 390 participantes; evidencia de baja calidad). No hubo evidencia para determinar si el uso habitual de la monitorización de la oxigenación cerebral basada en NIRS causa efectos adversos.
Conclusiones de los autores
Los efectos de la monitorización cerebral perioperatoria de la oxigenación cerebral con NIRS activa en adultos para reducir la ocurrencia de POCD leve a corto plazo son inciertos debido a la baja calidad de la evidencia. Hay incertidumbre sobre si la monitorización cerebral con NIRS activa tiene un efecto importante sobre el accidente cerebrovascular, el delirio o la muerte luego de la cirugía debido al número reducido de eventos y los intervalos de confianza amplios. Las conclusiones de esta revisión pueden cambiar cuando se publiquen los ocho estudios en curso y se clasifiquen los 12 estudios en espera de evaluación. Se necesitan más ECA realizados en la población pediátrica y en pacientes de alto riesgo sometidos a cirugía no cardíaca (por ejemplo, neurocirugía, endarterectomía carótida y otras cirugías).
PICOs
Resumen en términos sencillos
Uso de espectroscopia del infrarrojo cercano (NIRS) cerebral para la monitorización de la oxigenación cerebral durante o después de la cirugía en adultos y niños
Pregunta de la revisión
Se evaluaron los efectos de la monitorización del cerebro con espectroscopia del infrarrojo cercano cerebral (NIRS), y los tratamientos basados en dicho procedimiento, durante y después de la cirugía en adultos y niños. Se procuró determinar si la NIRS detecta un suministro reducido de oxígeno al cerebro, lo cual permitiría el uso de intervenciones para mejorar el sistema nervioso, el proceso mental (cognición) y otros resultados que pueden tener un impacto en la estancia hospitalaria y los costos para los pacientes.
Antecedentes
El cerebro humano necesita mucho oxígeno (tiene un consumo alto de oxígeno) y es muy sensible al suministro reducido de oxígeno. El tratamiento exitoso para los niveles bajos de oxígeno en el cerebro durante o después de la cirugía depende del diagnóstico temprano de la falta de oxígeno. La NIRS cerebral se usa cada vez más para la detección temprana de la falta de oxígeno al cerebro. Usa la luz del infrarrojo cercano (700 a 1 000 nanómetros) para penetrar a través de las capas de la superficie de la cabeza, incluido el cuero cabelludo y el cráneo, para mostrar el tejido cerebral.
Características de los estudios
La evidencia está actualizada hasta diciembre 2016. Se actualizó la búsqueda en noviembre 2017, pero estos resultados todavía no se han incorporado a la revisión. Se incluyeron 15 ensayos controlados aleatorios completados con 1822 participantes. Hay ocho estudios en curso y 12 en espera de una evaluación adicional.
Ninguno de los estudios finalizados incluyó a neonatos o niños. En cuatro estudios los participantes fueron sometidos a cirugía abdominal u ortopédica, un estudio incluyó a participantes sometidos a un procedimiento para restaurar el flujo sanguíneo adecuado al cerebro y en los diez estudios restantes los participantes fueron sometidos a cirugía cardíaca o de los vasos sanguíneos grandes con o sin derivación cardíaca. Todos los estudios usaron la NIRS cerebral en el quirófano, y sólo dos también utilizaron la NIRS cerebral en la unidad de cuidados intensivos. Los grupos de control fueron monitorizados con métodos como la frecuencia cardíaca y la presión sanguínea arterial media, electroencefalogramas, doppler transcraneal, índice biespectral, saturación de oxígeno en la vena yugular, potenciales evocados o la presión parcial de oxígeno en el tejido cerebral. En términos generales, los diferentes estudios variaron en el enfoque a la pregunta de la revisión.
Resultados clave
No se agruparon (combinaron) los datos del resultado de la lesión neurológica posoperatoria debido a las variaciones entre los estudios. Un estudio con 126 participantes sometidos a cirugía abdominal mayor informó que 4/66 versus 0/56 participantes presentaron lesión neurológica con la monitorización cegada y activa, respectivamente. Un segundo estudio con 195 participantes sometidos a cirugía de derivación de las arterias coronarias informó que 1/96 versus 4/94 participantes sufrieron lesión neurológica en los grupos de monitorización cegada (con ocultación) y activa (con tratamientos activos), respectivamente. No existe seguridad en cuanto a si la monitorización con NIRS activa tiene un efecto importante sobre el riesgo de accidente cerebrovascular y delirio posoperatorios debido a que hubo un número reducido de eventos y el resultado no fue preciso (dos estudios, 240 participantes; un estudio, 190 participantes, respectivamente; evidencia de baja calidad). Basado en dos estudios con 126 participantes, se encontró evidencia de baja calidad de que la monitorización cerebral con NIRS puede reducir el número de participantes con deterioro cognitivo leve una semana después de la intervención quirúrgica. Basado en seis estudios con 962 participantes, se encontró evidencia de calidad moderada de que la monitorización con NIRS cerebral probablemente da lugar a poca o ninguna disminución en el número de participantes con una disminución en la función cognitiva una semana después de la intervención quirúrgica. No existe seguridad sobre si la monitorización de la oxigenación cerebral activa tiene un efecto crucial sobre las muertes intraoperatorias o posoperatorias debido a que hubo un número reducido de eventos y el resultado no fue preciso (tres estudios, 390 participantes; evidencia de baja calidad). No se encontraron efectos perjudiciales del uso habitual de la monitorización de la oxigenación cerebral basada en NIRS.
Calidad de la evidencia
En términos generales, no se conoce si la monitorización con NIRS activa tiene un efecto crucial sobre el accidente cerebrovascular, el delirio o la muerte luego de la cirugía debido a la imprecisión de los resultados (evidencia de baja calidad). Por lo tanto, no se conocen los efectos de la monitorización cerebral con NIRS activa sobre la lesión en el sistema nervioso, el delirio, la disminución en la función cognitiva y la muerte luego de la cirugía. Para algunos resultados, como el accidente cerebrovascular u otra lesión neurológica posterior a la cirugía, la evidencia se basó en pocos estudios con números limitados de participantes. La información de los resultados a menudo fue incompleta para todos los participantes del estudio, al igual que el informe del diseño de estudio, como el cegamiento. Algunos estudios tuvieron conflictos de intereses potenciales debido al patrocinio de la industria.
Authors' conclusions
Summary of findings
| Active cerebral oxygenation monitoring compared to blinded cerebral oxygenation monitoring for perioperative monitoring of brain oxygenation in children and adults | ||||||
| Patient or population: children and adults undergoing surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Blinded cerebral oxygenation monitoring | Active cerebral oxygenation monitoring | |||||
| Postoperative neurological injury (follow‐up: 1 week or hospital discharge) | No pooled effect estimate available due to discordant direction of effect between studies. One study (N = 126) in people undergoing major abdominal surgery reported that 4/66 participants experienced neurological injury with blinded monitoring versus 0/56 in the active monitoring group. A second study (N = 195) in people having coronary artery bypass surgery reported that 1/96 participants experienced neurological injury in the blinded monitoring group compared with 4/94 participants in the active monitoring group. | Not estimable | 312 | ⊕⊕⊝⊝ | Very serious heterogeneity (I2 = 72%) | |
| Postoperative stroke (follow‐up: within 30 days) | 40 per 1000 | 10 per 1000 | RR 0.25 | 240 | ⊕⊕⊝⊝ | — |
| POD: postoperative delirium (follow‐up: 1 week) | 135 per 1000 | 85 per 1000 | RR 0.63 | 190 | ⊕⊕⊝⊝ | — |
| POCD defined by original studies ‐ mild (follow‐up: 1 week) | 641 per 1000 | 340 per 1000 | RR 0.53 | 126 | ⊕⊕⊝⊝ | — |
| POCD: decline in cognitive function (follow‐up: 1 week) | 400 per 1000 | 248 per 1000 | RR 0.62 | 962 | ⊕⊕⊕⊝ | — |
| Intraoperative mortality or postoperative mortality: Death (follow‐up: 30 days) | 10 per 1000 | 6 per 1000 | RR 0.63 | 390 | ⊕⊕⊝⊝ | — |
| Adverse events | See comments | See comments | Not estimable | See comments | See comments | None of the studies reported adverse effects caused by use of NIRS‐based cerebral oxygenation monitoring |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias: downgraded by one level because the body of evidence contained one or more of the following risks of bias: potential conflict of interest from industry sponsorship, unclear blinding status or missing participant data. | ||||||
Background
Description of the condition
The human brain is one of the organs with high oxygen utilization; thus it is extremely susceptible to hypoxic conditions (Gale 2004; Stys 1998). The incidence of intraoperative decrease in cerebral oxygen saturation is about 40% to 70%, depending on the definition of cerebral oxygen desaturation events (CDEs) and the patient population (Fischer 2011; Greenberg 2013; Murkin 2007; Slater 2009; Yao 2004). The prevalence of immediate postoperative CDEs is approximately 50% (Greenberg 2013). Potential contributors to low cerebral regional oxygen saturation (rScO2) include unstable haemodynamics, systemic desaturation, low haematocrit, hypocapnia, cardiac dysfunction, increased cerebral oxygen consumption, malposition of the head/cannula and specific surgical procedures such as deep hypothermic circulatory arrest and rewarming and aortic/carotid cross‐clamping (Closhen 2013; Deschamps 2013; Harilall 2013; Hoffman 2004; Meng 2012; Moerman 2012b; Morimoto 2003; Murkin 2009). Intraoperative CDEs were shown to correlate with increased risk of stroke, postoperative cognitive dysfunction (POCD), major organ morbidity or mortality and prolonged length of hospital stay (Monk 2008; Murkin 2007; Olsson 2006; Schön 2009; Slater 2009; Yao 2004). The hospital mortality of stroke patients after cardiac surgery is much higher than that of patients without stroke (19% versus 4%) (Salazar 2001). Moreover, cerebral oxygen desaturations may be related to postoperative neurological dysfunction and worse neurobehavioural outcome (Aguirre 2014; Colak 2012). In paediatric patients, postoperative neurodevelopmental abnormalities were associated with perioperative CDEs in paediatric biventricular repair operations (Kussman 2010). Intervention for CDEs was shown to result in a decreased incidence of postoperative cognitive decline, less major organ morbidity or mortality and shortened intensive care unit and hospital stay following coronary artery bypass grafting (Murkin 2007; Slater 2009).
Description of the intervention
Successful intervention for perioperative cerebral ischaemia and hypoxia relies on early diagnosis (Ng 2011). Various modalities, including electroencephalogram (EEG), somatosensory evoked potential (SSEP), motor evoked potential (MEP), transcranial doppler (TCD), bispectral index (BIS), jugular bulb venous blood haemoglobin saturation (SjvO2) and biomarkers, have been adopted for this purpose with varying limitations (Andropoulos 2004; Guo 2011; Inoue 2013; Sanchez‐Pena 2012; Williams 1994). Cerebral near‐infrared spectroscopy (NIRS) is increasingly used in clinical settings to monitor brain oxygenation.
Near‐infrared light (700 to 1000 nanometres) can penetrate through the superficial layers of the head, including the scalp and the skull, and can illuminate the cerebral tissue. The work by Jöbsis pioneered the use of NIRS to monitor rScO2 (Jöbsis 1977). rScO2 is the percentage of oxy‐haemoglobin over the sum of oxy‐ and deoxy‐haemoglobin in pooled arterial, capillary and venous blood in the illuminated brain region. rScO2 is essentially determined by cerebral metabolic rate of oxygen (CMRO2) (demand) and oxygen delivery to the brain (supply). It can be altered by a change in volume percentage of cerebral arterial and/or venous blood (Kurth 1999; Watzman 2000; Yoshitani 2005). CDE treatment aims to augment cerebral blood flow and arterial blood oxygen content, as well as to reduce cerebral oxygen consumption (Subramanian 2016). There is variation among the intervention protocols adopted by previous studies (Ballard 2012; Goldman 2004; Murkin 2007). This may be due to differences in local practice style, types of surgery and patients' individual conditions. Cerebral NIRS has been used in cardiac and major vascular surgeries in both adults and children and in various non‐cardiac surgeries, but mainly in adults (Hirsch 2009).
Current cerebral oximetries used in the clinical setting are based on continuous‐wave technology; newer technologies such as time‐domain and frequency‐domain NIRS are based on the transition from bench to bedside (Ferrari 2012; Watzman 2000). Although algorithm differences exist among continuous‐wave devices used in patients, the fundamental principle is the same as that previously reviewed (Ferrari 2012; McCormick 1991; Murkin 2009). So far, five NIRS‐based cerebral oximetries have been approved for patient use by the US Food and Drug Administration (FDA), including CerOx (Ornim, Inc., Dedham, Massachusetts, USA), EQUANOX 7600 and 8004CA (Nonin Medical, Inc., Plymouth, Minnesota, USA), FORE‐SIGHT (CAS Medical Systems, Branford, Connecticut, USA) and INVOS (Somanetics Corporation, Troy, Michigan, USA). Examples of non‐approved devices include NIMO (Nirox s.r.l., Brescia, Italy), NIRO‐200NX and TRS‐20 (Niro, Hamamatsu, Japan), Oxymon‐II A and PortaLite (Artinis, Elst, Netherlands) and OxiplexTS (ISS, Champaign, Illinois, USA) (Ferrari 2012; Zheng 2013).
How the intervention might work
Clinical studies suggest that NIRS‐based cerebral oximetry can reliably detect perioperative CDEs, especially in cardiac surgery (Bhatia 2007; Casati 2005; Egawa 2009; Greenberg 2013; Kussman 2010; Lovell 1999; Moreno 2013; Murkin 2007; Slater 2009). Various protocols aimed at CDE correction have been proposed (Scheeren 2012; Taillefer 2005). The refined algorithm has improved the accuracy of cerebral NIRS (Fischer 2008). However, bias in NIRS‐measured rScO2 still exists both between individuals and between different devices, likely because of differences in skin colour and gender and in the volume percentage of arterial and venous blood in the monitored brain region (Bickler 2013).
NIRS‐measured rScO2 reflects the balance between cerebral oxygen consumption and supply. Differential diagnosis is needed when the cause of a change in rScO2 is deciphered. Denault et al proposed an intervention algorithm for CDEs that involves increasing cerebral oxygen supply and/or decreasing consumption (Denault 2007). This algorithmic strategy has been tested in a prospective study that successfully used it to reverse decreased rScO2 in high‐risk cardiac surgery patients in both the operating room and the intensive care unit (Deschamps 2013). The effort involved in applying cerebral NIRS in the perioperative setting is encouraged by the fact that NIRS is non‐invasive, continuous and applicable at the patient's bedside. This new, non‐invasive technique may act as a warning sign of cerebral ischaemia and hypoxia (Tsai 2016). However, whether a perioperative monitor can facilitate decision‐making in the implementation of treatment strategies to reverse cerebral desaturation, reduce adverse neurological events and improve overall outcomes in a cost‐effective manner needs to be rigorously tested by randomized controlled trials (RCTs).
Why it is important to do this review
One of the fundamental goals of perioperative management is to avoid tissue ischaemia and hypoxia. This is especially true for vital organs such as the brain. It has been shown that low rScO2 is associated with neurological injury under various cardiopulmonary bypass conditions in piglets (Anttila 2005; Hagino 2005). Several clinical studies in adults have also demonstrated the association between intraoperative CDEs and adverse neurological outcomes, POCD, major organ morbidity or mortality and prolonged length of hospital stay (Monk 2008; Murkin 2007; Slater 2009; Vohra 2009; Yao 2004). In paediatric patients, cerebral NIRS seems to correlate with vital parameters and enhance the prediction of neurodevelopmental outcomes after cardiac surgery (Kussman 2010; Menke 2014; Sood 2013). Cerebral NIRS is also increasingly being applied in the cardiac ICU for perioperative brain oxygenation monitoring (Ghanayem 2016). In addition, real‐time brain oxygenation monitoring with cerebral NIRS may be beneficial for detecting ischaemic events during cerebrovascular procedures and in the neurosurgical ICU (Calderon‐Arnulphi 2007; Grinspan 2014; Mazzeo 2012; Obrig 2014). However, evidence to support the predictive value of cerebral NIRS for adverse neurological outcome is lacking and a very limited number of RCTs have tested the beneficial effect of cerebral NIRS on outcomes in adult cardiac patients (Zheng 2013). Currently, the hypoxic threshold based on cerebral NIRS monitoring for intervention is poorly defined (Denault 2007; Fischer 2011; Murkin 2007; Orihashi 2004). In non‐cardiac surgery, especially routine surgery under general anaesthesia, the role of cerebral NIRS is undefined (Ghosh 2012). Without well‐defined outcomes from the use of NIRS and associated implemented treatment strategies, it is difficult to support the use of NIRS in the clinical setting (Hirsch 2010). In addition, cost‐effectiveness analysis needs to be done to support the benefits of cerebral NIRS. Therefore, an up‐to‐date review of the use of cerebral NIRS in the perioperative setting based on RCTs could be pivotal, not only in defining the areas where clarification or rigorous evidence is needed but also in guiding its application in the clinical setting.
Objectives
To assess the effects of perioperative cerebral NIRS monitoring and corresponding treatment strategies in adults and children, compared with no cerebral oxygenation monitoring or cerebral oxygenation monitoring based on non‐NIRS technologies, on the detection of CDEs, neurological outcomes, non‐neurological outcomes and socioeconomic impact (including cost of hospitalization and length of hospital stay).
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs dealing with the use of cerebral NIRS in the perioperative setting (during the operation and within 72 hours after the operation), including the operating room (OR), the postanaesthesia care unit (PACU) and the intensive care unit (ICU). We included all appropriate studies without regard for publication status or language used. We excluded non‐randomized studies such as cohort studies, which are susceptible to bias.
Types of participants
We included adult participants (aged 18 years or older) and paediatric participants (aged younger than 18 years, excluding neonates) of both genders undergoing any types of surgery under general anaesthesia. We also included operations sometimes undertaken under local anaesthesia, such as carotid artery stenting and carotid endarterectomy. We excluded neonates because there is a review published by the Cochrane Neonatal Group (Hyttel‐Sorensen 2017).
Types of interventions
The intervention group included all surgical participants who received cerebral NIRS monitoring and interventions to correct CDEs in the perioperative setting. So far, five NIRS‐based cerebral oximetries have been approved by the US Food and Drug Administration (FDA). We documented the type of device used in each study and carried out subgroup analyses.
The control group included surgical participants monitored by conventional monitors (e.g. heart rate, mean arterial pressure) or other kinds of monitors such as electroencephalogram (EEG), transcranial doppler (TCD), bispectral index (BIS), jugular bulb oximetry, evoked potentials, cerebral tissue oxygen partial pressure (PbO2), etc. The control group received no monitoring by cerebral NIRS or blinded monitoring where the rScO2 readout was concealed from the anaesthesiologist.
Types of outcome measures
Primary outcomes
-
Postoperative stroke or other neurological injury, including adverse neurodevelopmental outcomes (within 24 hours postoperatively up to discharge or the end of follow‐up). The diagnosis was based on new‐onset neurological deficits, and findings were based on the neurological examination or neuroradiological evidence including computed tomography (CT), magnetic resonance imaging (MRI) or neuroangiography. Neurological deficits included abnormalities of sensory, motor, balance, speech, vision or autonomic nervous system functions.
-
Postoperative delirium (POD) or POCD (within 24 hours postoperatively up to discharge). The diagnosis of POD or POCD was based on the criteria adopted by the authors in each included study.
-
Intraoperative mortality or postoperative mortality (at 24 hours, 30 days and one year after surgery).
Secondary outcomes
-
The occurrence of abnormal rScO2 during or after surgery: the definition was attributed to the varying NIRS devices and differing physical and medical conditions of the participants. We used the criteria adopted by each included study.
-
Any major non‐neurological complications that occurred during the intraoperative or postoperative period.
-
Respiratory insufficiency caused by pneumonia (fever, leukocytosis, chest x‐ray or positive sputum culture), atelectasis (diagnosed based on chest x‐ray), pulmonary emboli (sudden death confirmed by positive radiological findings) or pneumothorax (chest x‐ray).
-
Cardiovascular complications, including myocardial infarction (electrocardiogram (ECG) changes confirmed by abnormal myocardial enzymes), cardiac failure (clinical signs and symptoms or positive radiological findings), malignant arrhythmia (ECG changes) or cardiac arrest (ECG changes).
-
Hepatic or renal insufficiency (clinical manifestations and laboratory evidence).
-
-
ICU length of stay.
-
Hospital length of stay.
-
Cost of hospitalization.
-
Adverse events.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 12), Embase (1974 to 20 December 2016) and MEDLINE (PubMed) (1975 to 20 December 2016), with no language restrictions. YY performed the search on 20 December 2016. We performed a further search in November 2017. We have added the results for the latter search to Studies awaiting classification and we will incorporate them into the review at the next update.
We used search strategies to maximize sensitivity by following Section 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions to search for RCTs in Embase and PubMed (Higgins 2011). We searched CENTRAL, Embase and PubMed using the search terms described in Appendix 1, Appendix 2 and Appendix 3.
Searching other resources
We scanned the reference lists of all eligible articles and reviews to identify further RCTs.
We searched the World Health Organization (WHO) International Clinical Trials Registry Platform on 20 December 2016, including ClinicalTrials.gov, the metaRegister of Controlled Trials and other national trial registries, for ongoing studies. We contacted relevant specialists in this field to identify unpublished research and ongoing trials on 24 March 2016.
We imposed no language or region restrictions.
Data collection and analysis
Selection of studies
Two review authors (YY and KZ) independently screened the results of the searches and recorded separately the reasons for inclusion or exclusion. We excluded duplicate records. We excluded studies in animal models. We resolved disagreements on study selection between review authors via discussion. If needed, we consulted with a third review author (RH or LM) to resolve any disagreements. If further information was required, YY contacted the corresponding author of the study.
Data extraction and management
We used a pre‐designed form to record the data obtained from included studies (Appendix 4). Two review authors (YY and KZ) independently extracted data using a paper data extraction form (Appendix 4). We resolved disagreements via discussion. If we were unable to reach a consensus, we consulted a third review author (RH or LM). If further information was needed, YY contacted the corresponding author of the study.
Assessment of risk of bias in included studies
Two review authors (YY and KZ) independently assessed the risk of bias of all included studies. We tried to resolve disagreements by discussion and, if a consensus could not be reached, a third review author (LZ) was consulted.
We assessed the risk of bias of the included studies using the 'Risk of bias' tool that is described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). A copy of the assessment form that we adopted can be found in Appendix 4.
We evaluated each study according to the following domains: selection bias, performance bias and attrition bias, including randomization and allocation concealment, blinding of participants, blinding of outcome assessment, missing data, selective reporting and any other bias. We assessed blinding separately for subjective (e.g. POCD) and objective (e.g. mortality) outcome measures. Objective outcome measures were less likely to be influenced by the knowledge of research personnel.
We rated a study as having 'low risk of bias' if all of the domains were assessed as adequate. We rated a study as having 'high risk of bias' if one or more of the domains were identified as inadequate or unclear. We performed sensitivity analyses to assess the influence of the exclusion of 'high risk of bias' studies on the results of the meta‐analysis.
We presented a 'Risk of bias' table for each included study using the classification of 'low', 'high' or 'unclear' risk of bias. We also completed a 'Risk of bias' summary figure for each outcome to present a detailed description of all judgements made for all eligible studies in the review.
Measures of treatment effect
We analysed data using the Review Manager software (RevMan 5.3).
Dichotomous data
For dichotomous data, for example, whether cerebral NIRS was associated with postoperative neurological injury during the perioperative period, as well as reduction in mortality at 24 hours, 30 days and one year, we used a random‐effects model for analysis to estimate the overall risk ratio (RR) with the 95% confidence interval (CI).
Continuous data
For continuous data, such as length of ICU or hospital stay in days, we used mean differences (MDs) as summary statistics in the meta‐analysis.
Time‐to‐event data
There were no time‐to‐event data reported in the included studies, therefore we did not perform survival analysis.
Unit of analysis issues
We included RCTs with a parallel‐group design. However, we did not find any cluster‐randomized controlled trials (cluster‐RCTs).
Dealing with missing data
We (YY) contacted the original investigators to request any missing data required for meta‐analysis. We assumed the data to be missing at random and we analysed only the available data, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We performed sensitivity analyses to assess how sensitive the results were to reasonable changes in our assumptions. In the Discussion section, we addressed the potential impact of missing data on the conclusions of the review.
Assessment of heterogeneity
We appraised the heterogeneity of the included studies based on both clinical diversity (e.g. type of surgery, type of anaesthesia, participants' comorbidities) and methodological diversity (risk of bias assessment). We performed subgroup analysis and sensitivity analysis to address clinical heterogeneity, including visual inspection of the forest plots and determining the I2statistic (Higgins 2011). We considered an I2 statistic exceeding 50% to show high levels of heterogeneity, mandating further analysis.
Assessment of reporting biases
Although we included 15 studies in this review, fewer than 10 were involved in the meta‐analysis for each outcome. As a result, we did not create a funnel plot to qualitatively assess publication or reporting bias.
Data synthesis
If the included studies did not have excessive clinical or statistical heterogeneity, we used the Review Manager software to combine the data on population, interventions and outcomes and performed a meta‐analysis to generate a quantitative summary. As we expected clinical and methodological heterogeneity between studies, we analysed the data with a random‐effects model. If suitable numerical data were insufficient for a meta‐analysis, we carried out a narrative analysis for each study and summarized all of the qualified data.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses as follows, including subgroups of participants and interventions.
Subgroups of participants
Subgroup analysis according to type of surgery
-
Participants undergoing neurosurgery.
-
Participants undergoing cardiac or great vessel surgery
with/without bypass. -
Participants undergoing carotid endarterectomy.
-
Participants undergoing other surgery.
In this review, we did not perform subgroup analysis according to age of participants because none of the included studies considered a paediatric population.
Subgroups of interventions
-
Perioperative cerebral NIRS monitoring versus no cerebral oxygenation monitoring.
-
Perioperative cerebral NIRS monitoring versus other kinds of cerebral oxygenation monitoring.
Subgroup analysis according to documentation of device types
-
INVOS (Somanetics Corporation, Troy, Michigan, USA).
-
CerOx (Ornim, Inc., Dedham, Massachusetts, USA).
-
EQUANOX 7600 (Nonin Medical, Inc., Plymouth, Minnesota, USA).
-
EQUANOX 8004CA (Nonin Medical, Inc., Plymouth, Minnesota, USA).
-
FORE‐SIGHT (CAS Medical Systems, Branford, Connecticut, USA).
Sensitivity analysis
We carried out sensitivity analyses to assess the effects of study risk of bias by excluding each study sequentially and we excluded studies at high risk of bias, which had inadequate allocation of concealment.
'Summary of findings' table and GRADE
We used the principles of the GRADE system to assess the quality of the body of evidence associated with the specific outcomes (rate of postoperative stroke or other neurological injury, POD, POCD or mortality at 24 hours, 30 days and one year, and adverse events) and we constructed a 'Summary of findings' table using the GRADEpro software (Guyatt 2008). We generated a 'Summary of findings' table for 'Active cerebral oxygenation monitoring compared to blinded cerebral oxygenation monitoring'.
The GRADE approach evaluates the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considered risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
Please see Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies for further details.
Results of the search
We identified 3145 potential references from the electronic search and one additional reference through handsearching on 20 December 2016. Two review authors (YY and KZ) independently reviewed the results and were able to exclude 2234 citations through title and abstract screening. We screened 65 full‐text articles for further assessment of which 15 were subsequently included in this review (Ballard 2012; Casati 2005; Colak 2015; Cowie 2014; Deschamps 2013; Deschamps 2016; Harilall 2014; Kara 2015; Lau 2012; Mohandas 2013; Murkin 2007; Slater 2009; Trafidlo 2015; Vretzakis 2013; Zogogiannis 2011). Moreover, there are nine studies awaiting classification, comprising one protocol for a RCT and eight conference abstracts (Aguirre 2016; Baker 2006; Ellis 2015; Gauge 2014; Girgin 2012; Iglesias 2003; Sahan 2014; Trinh 2016; Verborgh 2009). Two study reports (Lei 2017; Rogers 2017) and one conference abstract (Hosang 2017) from an updated search in November 2017 have been added to 'Studies awaiting classification' (Characteristics of studies awaiting classification). There are eight ongoing trials currently without available data from the investigators (Bal 2016; Djaiani 2012; Fischer 2009; Fominskiy 2014; Grocott 2013; Shi 2013; Teurnier 2011;Trinh 2012). The ongoing trials were retrieved from other resources, such as the ClinicalTrials.gov.
Please refer to Figure 1 and Figure 2.
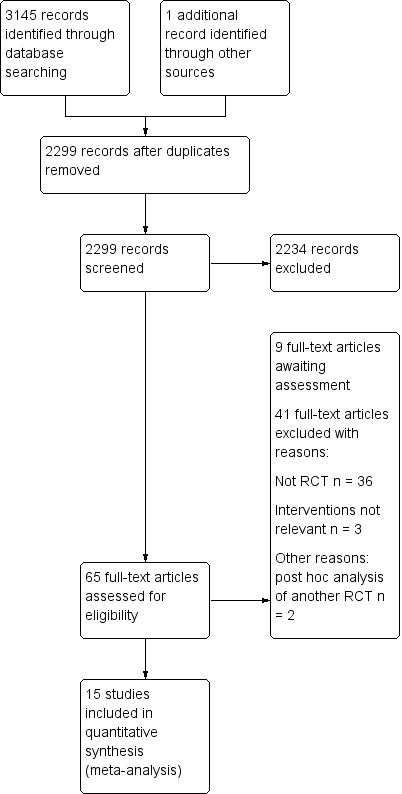
Study flow diagram.
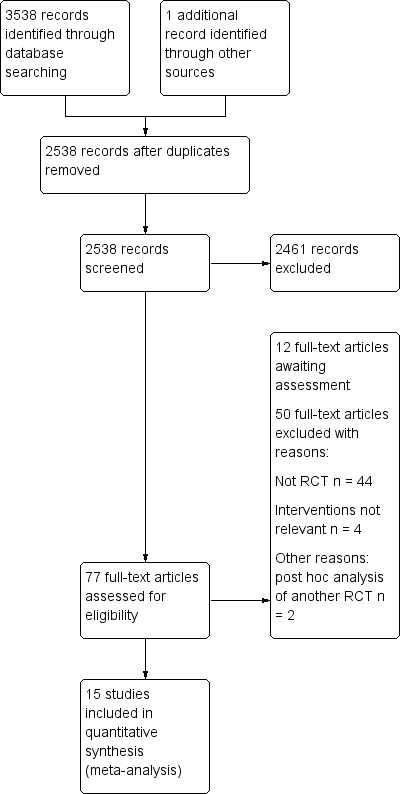
Study flow diagram‐top up search on November 2017.
Included studies
Methods
All the included studies employed a parallel‐group, randomized study design. No studies used a quasi‐ or cluster‐randomized design.
Participants
A total of 1822 adults participated in 15 studies. The total sample size ranged from 25 to 253. None of the 15 included studies considered the paediatric population.
Settings
Four of the 15 included studies were conducted in the abdominal or orthopaedic (lumbar spine, or knee and hip replacement surgery) setting (Ballard 2012; Casati 2005; Cowie 2014; Trafidlo 2015), one study in the carotid endarterectomy setting (Zogogiannis 2011), and the remaining 10 studies in the aortic or cardiac surgery setting (Colak 2015; Deschamps 2013; Deschamps 2016; Harilall 2014; Kara 2015; Lau 2012; Mohandas 2013; Murkin 2007; Slater 2009; Vretzakis 2013). One study was conducted under local anaesthesia (Zogogiannis 2011). Three studies were conducted in Canada (Deschamps 2013; Deschamps 2016; Murkin 2007), two in the United States (Slater 2009; Vretzakis 2013), one in the United Kingdom (Ballard 2012), one in Croatia (Colak 2015), one in Australia (Cowie 2014), one in South Africa (Harilall 2014), one in Turkey (Kara 2015), one in Poland (Trafidlo 2015) and one in Greece (Zogogiannis 2011). The remaining three studies did not provide detailed information about the country in which they were conducted (Casati 2005; Lau 2012; Mohandas 2013).
Study period
Ballard 2012 recruited participants from March 2007 to January 2009, Colak 2015 from June 2009 to September 2011, Cowie 2014 from February 2012 to September 2012, Deschamps 2016 from April 2012 and October 2013, Kara 2015 from December 2013 to February 2015, Lau 2012 from November 2009 to September 2011, Murkin 2007 from September 2002 to April 2004, Slater 2009 from January 2004 to February 2006, and Zogogiannis 2011 from December 2007 to January 2010. The remaining six studies did not report the exact study period (Casati 2005; Deschamps 2013; Harilall 2014; Mohandas 2013; Trafidlo 2015; Vretzakis 2013).
Interventions
All of the included studies used cerebral NIRS in the perioperative setting. In order to correct CDEs, all surgical participants received cerebral NIRS monitoring in the perioperative setting. The types of device documented included EQUANOX 7600 (Mohandas 2013; Deschamps 2016), FORE‐SIGHT (Deschamps 2016) and INVOS (i.e. INVOS Somanetics Cerebral Oximeter (Covidien, USA) or SCIO (i.e. Somanetics Invos Cerebral Oximeter (SICO, Covidien inc, Co, USA)) in the remaining 14 studies (Ballard 2012; Casati 2005; Colak 2015; Cowie 2014; Deschamps 2013; Deschamps 2016; Harilall 2014; Kara 2015; Lau 2012; Murkin 2007; Slater 2009; Trafidlo 2015; Vretzakis 2013; Zogogiannis 2011).
The control group in the following studies either did not receive cerebral oxygenation monitoring (Colak 2015; Kara 2015; Vretzakis 2013; Zogogiannis 2011), or the rScO2 was collected but was concealed from the anaesthesiologist (Ballard 2012; Casati 2005; Cowie 2014; Deschamps 2013; Deschamps 2016; Harilall 2014; Lau 2012; Mohandas 2013; Murkin 2007; Slater 2009). One study described the comparison as INVOS monitoring versus no monitoring, but no further detail about the control group was provided (Trafidlo 2015). In Zogogiannis 2011, cerebral oximetry values were recorded in group B but anaesthesia management was not based on the algorithm mentioned in the full text; therefore, we only used the data from group A as the intervention group.
Outcomes
All 15 included studies reported the pre‐specified primary and secondary outcomes except for the cost of hospitalization. This current review did not analyse additional outcomes measured in five studies (33%), which were not mentioned in our protocol, such as rate of ICU stay, rate of hospitalization and management of hypotension without cerebral oximetry reasons (Cowie 2014; Deschamps 2013; Mohandas 2013; Murkin 2007; Vretzakis 2013).
Two studies (13%) considered loss to follow‐up in the sensitivity analysis (Ballard 2012; Lau 2012). However, no data were obtained through contacting the original investigators (Characteristics of included studies). In terms of funding sources, four studies (27%) may have been potentially influenced by the financial or commercial interests of the investigators (Ballard 2012; Cowie 2014; Deschamps 2016; Murkin 2007).
Excluded studies
We excluded three studies from this review for the reasons listed in the Characteristics of excluded studies table.
Kussman 2009 was a cohort study, which was part of a RCT comparing early postoperative and neurodevelopmental outcomes after haemodilution to a haematocrit of 25% versus 35% during infant heart surgery.
Kussman 2010 was a secondary analysis of data arising from a RCT of haemodilution to a haematocrit of 25% versus 35% during cardiopulmonary bypass in infants. The authors evaluated the correlation between intraoperative cerebral oxygen saturation and postoperative neurological outcomes at the age of one year.
Murkin 2011 was a post hoc analysis of a subset of participants in Murkin 2007 and focused on participants with a preoperative diagnosis of diabetes mellitus. The remaining excluded studies were observational studies and not RCTs.
Studies awaiting classification
Nine studies are awaiting classification (Characteristics of studies awaiting classification). One study was the protocol for a RCT that met our inclusion criteria (Ellis 2015). The remaining eight studies were conference abstracts (Aguirre 2016; Baker 2006; Gauge 2014; Girgin 2012; Iglesias 2003; Sahan 2014; Trinh 2016; Verborgh 2009). We tried to contact the relevant research group through their institutions for further information about these studies (Characteristics of studies awaiting classification). Two RCTs meeting the inclusion criteria (Lei 2017; Rogers 2017) and a conference abstract (Hosang 2017) from an updated search in November 2017 have also been added to 'Studies awaiting classification' (Characteristics of studies awaiting classification).
Ongoing studies
By searching multiple clinical trials registry platforms, we identified eight ongoing studies (Characteristics of ongoing studies). Five of the ongoing studies were conducted in a cardiac surgery setting (Djaiani 2012; Fischer 2009; Fominskiy 2014; Grocott 2013; Trinh 2012), two studies in an oesophagectomy or orthopaedic setting (Bal 2016; Shi 2013), and one study in a carotid endarterectomy setting (Teurnier 2011). Four of these studies were recruiting participants (Fischer 2009; Fominskiy 2014; Grocott 2013; Trinh 2012), one study was completed (Bal 2016), one study was terminated (Teurnier 2011), and the remaining studies were pending (Djaiani 2012; Shi 2013).
Risk of bias in included studies
See also the 'Risk of bias' tables in Characteristics of included studies and Figure 3 and Figure 4.
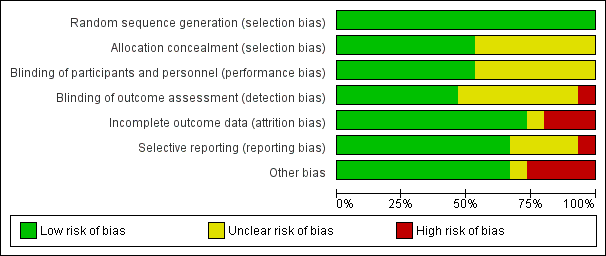
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
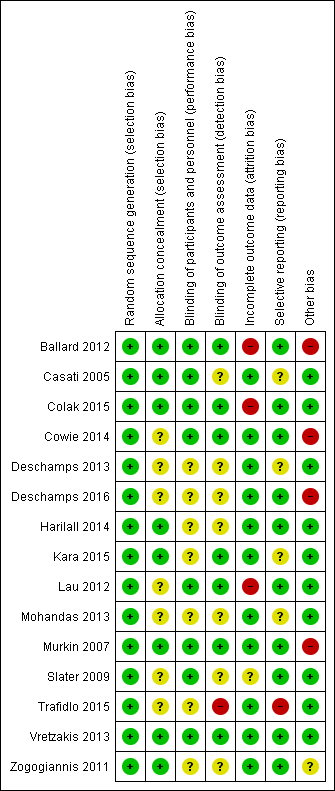
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included studies were RCTs and random sequence generation was employed in all of them. Seven studies, however, did not provide adequate details of their randomization methods and simply stated that they were randomized (Deschamps 2013; Kara 2015; Lau 2012; Mohandas 2013; Trafidlo 2015; Vretzakis 2013; Zogogiannis 2011). As we accepted the trialist's reporting as true and accurate, we rated all 15 studies as being at a low risk of bias. Random sequence generation was adequately described in eight studies (53%) using the statistical program package R, a computer‐generated sequence of numbers or a random numbers table (Ballard 2012; Casati 2005; Colak 2015; Cowie 2014; Deschamps 2016; Harilall 2014; Murkin 2007; Slater 2009). The common method used in seven studies to conceal allocation was sequentially numbered, opaque, sealed envelopes (Ballard 2012; Casati 2005; Colak 2015; Harilall 2014; Murkin 2007; Vretzakis 2013; Zogogiannis 2011). One study described the randomization list being concealed until the study was concluded; in this case we accepted the authors' reporting as true and accurate and rated this study as low risk of bias (Kara 2015). The remaining seven studies did not describe the method of concealment and we rated them as having unclear risk of bias (Cowie 2014; Deschamps 2013; Deschamps 2016; Lau 2012; Mohandas 2013; Slater 2009; Trafidlo 2015).
Blinding
Blinding of participants and key study personnel was ensured in eight studies (53%) and we rated them as at low risk of bias (Ballard 2012; Casati 2005; Colak 2015; Cowie 2014; Lau 2012; Murkin 2007; Slater 2009; Vretzakis 2013). There was insufficient information about blinding of participants and personnel in the remaining seven studies (Deschamps 2013; Deschamps 2016; Harilall 2014; Kara 2015; Mohandas 2013; Trafidlo 2015; Zogogiannis 2011). Hence, we rated them as having unclear risk of bias.
Blinding of outcome assessors was ensured in seven studies (47%) and consequently we rated these as low risk of bias (Ballard 2012; Colak 2015; Cowie 2014; Kara 2015; Lau 2012; Murkin 2007; Vretzakis 2013). In Casati 2005, some research personnel may also have been the outcome assessors, hence we assessed this study as having an unclear risk of bias. However, Trafidlo 2015 stated that the observers were not blinded and thus we rated it as having a high risk of bias. There was insufficient information about blinding of outcome assessment in the remaining six studies (40%) and we thus rated these as having an unclear risk of bias (Deschamps 2013; Deschamps 2016; Harilall 2014; Mohandas 2013; Slater 2009; Zogogiannis 2011).
Incomplete outcome data
In 11 out of 15 included studies (73%), all randomized participants were analysed for all expected outcomes and we consequently awarded them a low risk of bias (Casati 2005; Cowie 2014; Deschamps 2013; Deschamps 2016; Harilall 2014; Kara 2015; Mohandas 2013; Murkin 2007; Trafidlo 2015; Vretzakis 2013; Zogogiannis 2011). In Casati 2005, after randomization, seven participants were excluded from the treatment group while two participants were excluded from the control group for the following reasons: insertion of an epidural catheter, cancellation, technical failure and death due to surgical complications. The reasons for the missing data were unlikely to influence the outcomes. In Murkin 2007, rSO2 data were lost in six participants because of technical failure (3% of sample size). In Zogogiannis 2011, 2 out of the 253 participants (0.8% of sample size) died because of postoperative cardiovascular events. In two studies, the proportion of missing outcomes compared with observed events was not likely to induce clinically relevant bias in the intervention effect estimate (Murkin 2007; Zogogiannis 2011). However, in Ballard 2012, two participants were excluded from the analysis due to delirium, which was an unacceptable reason for exclusion and the levels of missing data were high (at one week after surgery: 35.3% in the intervention group and 23.7% in the control group, respectively; at 12 weeks: 20.6% and 10.5%, respectively; at 52 weeks: 17.7% and 15.8%, respectively). Hence, we rated it as high risk of bias. In Slater 2009, the authors did not provide information about withdrawal or loss to follow‐up but reported that 16% of participants had no neurocognitive testing at three months. In Colak 2015, out of 200 participants, six in the intervention group and four in the control group were excluded from the analysis because the participants did not receive the allocated intervention. We regarded the reason for excluding participants as unacceptable and thus we rated it as high risk of bias. Lau 2012 was at a high risk of bias due to a significant amount of incomplete outcome data (2 out of 12 in the intervention group and 3 out of 13 in the control group were dropouts).
Selective reporting
All expected outcomes were reported in nine of the included studies (60%), which we rated as low risk of bias (Colak 2015; Cowie 2014; Deschamps 2016; Harilall 2014; Lau 2012; Murkin 2007; Slater 2009; Vretzakis 2013; Zogogiannis 2011). In Ballard 2012, the authors did not present the "Trail Making" data at one week postoperatively or the S100B data in the intervention group and the control group. However, this is unlikely to have had an important influence on the estimate of the effects of the prespecified outcomes of this review, hence we rated it as low risk of bias. Mohandas 2013 had an unclear risk of bias because one secondary outcome (the number of participants experiencing desaturation) was not reported. We rated Kara 2015 as unclear risk of bias because one secondary outcome (intraoperative rSO2 parameters) was not reported. The predefined outcomes were not clarified in the methods section in the other two studies, hence we rated them as unclear risk of bias, as we did not have sufficient information to make a firm judgement (Casati 2005; Deschamps 2013). We also rated Trafidlo 2015 as high risk of bias as although all prespecified outcomes were reported, the numbers of participants in the Digit Span Test (DST) and the N‐back Test (NBT) were different from the randomized number.
Other potential sources of bias
Four of the included studies (27%) appeared to have other potential sources of bias. In Ballard 2012, two of the authors may have had financial or commercial conflicts of interest, hence we rated this domain as high risk of bias. Commercial funding was also apparent in Cowie 2014, Murkin 2007 and Deschamps 2016, therefore we rated them as high risk of bias. Cowie 2014 was supported by an equipment grant (device loan and sensors) from Covidien USA (Mansfield, MA) and also by an Australian and New Zealand College of Anaesthetists pilot trial grant. There was no obvious commercial involvement in the other studies.
Effects of interventions
1. Comparison 1: Active cerebral oxygenation monitoring versus blinded cerebral oxygenation monitoring
Primary outcomes: postoperative stroke or other neurological injury
1.1 Postoperative stroke or other neurological injury
Neurological injury was investigated by two relevant studies (n = 312) (Casati 2005; Colak 2015). Neither study showed a significant difference between interventions. The direction of effect favoured different interventions in these studies, hence when we tried to pool the results we observed a high level of heterogeneity (I2 = 72%). This was probably due to the clinical diversity of participants. Casati 2005 involved participants undergoing major abdominal, nonvascular surgery under general anaesthesia (with an expected duration of more than two hours). In contrast, Colak 2015 included participants undergoing on‐pump coronary artery bypass grafting (CABG) surgery with the use of cardiopulmonary bypass. Consequently, we presented the results of each study separately without meta‐analysis (Casati 2005 n = 126, fixed‐effect model, risk ratio (RR) 0.13, 95% confidence interval (CI) 0.01 to 2.37; Colak 2015 n = 195, fixed‐effect model, RR 4.09, 95% CI 0.47 to 35.88) (Table 1). We downgraded this outcome to low quality due to risk of bias and inconsistency (summary of findings Table for the main comparison).
| 1 Postoperative stroke or other neurological injury | ||||||||
| 1.1 Neurological injury | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Risk ratio | ||||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 0 | 56 | 4 | 66 | 0.13 (0.01 to 2.37) | ||||
| 4 | 94 | 1 | 96 | 4.09 (0.47 to 35.88) | ||||
| 1.2 Stroke | ||||||||
| 0 | 20 | 0 | 20 | Not estimable | ||||
| 1 | 100 | 4 | 100 | 0.25 (0.03 to 2.20) | ||||
| 2 Postoperative stroke or other neurological injury: ASEM (endpoint score) | ||||||||
| 2.1 1 week | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 17.46 | 1.99 | 50 | 15.04 | 4.8 | 50 | 100.0% | 2.42 (0.98 to 3.86) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 2.42 (0.98 to 3.86) | ||||
| Test for overall effect: Z = 3.29 (P = 0.0010) | ||||||||
| 2.2 12 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 17.68 | 1.79 | 50 | 15.69 | 3.99 | 50 | 100.0% | 1.99 (0.78 to 3.20) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 1.99 (0.78 to 3.20) | ||||
| Test for overall effect: Z = 3.22 (P = 0.001) | ||||||||
| 3 Postoperative stroke or other neurological injury: Vigilance Reaction Time (change score) | ||||||||
| 3.1 1 week | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 6.39 | 80.9 | 22 | 27.95 | 54.99 | 29 | 100.0% | ‐21.56 (‐60.85 to 17.73) | |
| Subtotal (95% CI) | 22 | 29 | 100.0% | ‐21.56 (‐60.85 to 17.73) | ||||
| Test for overall effect: Z = 1.08 (P = 0.28) | ||||||||
| 3.2 12 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| ‐11.73 | 33.5 | 27 | 13.61 | 29.69 | 34 | 100.0% | ‐25.34 (‐41.44 to ‐9.24) | |
| Subtotal (95% CI) | 27 | 34 | 100.0% | ‐25.34 (‐41.44 to ‐9.24) | ||||
| Test for overall effect: Z = 3.08 (P = 0.002) | ||||||||
| 3.3 52 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| ‐10.8 | 36.28 | 28 | 15.1 | 40.73 | 32 | 100.0% | ‐25.90 (‐45.39 to ‐6.41) | |
| Subtotal (95% CI) | 28 | 32 | 100.0% | ‐25.90 (‐45.39 to ‐6.41) | ||||
| Test for overall effect: Z = 2.61 (P = 0.009) | ||||||||
| 4 Postoperative stroke or other neurological injury: Trail Making (change score) | ||||||||
| 4.1 12 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| ‐0.23 | 0.73 | 27 | 0.47 | 1.34 | 34 | 100.0% | ‐0.70 (‐1.23 to ‐0.17) | |
| Subtotal (95% CI) | 27 | 34 | 100.0% | ‐0.70 (‐1.23 to ‐0.17) | ||||
| Test for overall effect: Z = 2.60 (P = 0.009) | ||||||||
| 4.2 52 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 0.12 | 0.68 | 28 | ‐0.47 | 0.93 | 32 | 100.0% | 0.59 (0.18 to 1.00) | |
| Subtotal (95% CI) | 28 | 32 | 100.0% | 0.59 (0.18 to 1.00) | ||||
| Test for overall effect: Z = 2.83 (P = 0.005) | ||||||||
| 5 POD: postoperative delirium | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 8 | 94 | 13 | 96 | 100.0% | 0.63 (0.27 to 1.45) | |||
| Total (95% CI) | 94 | 96 | 100.0% | 0.63 (0.27 to 1.45) | ||||
| Total events | 8 | 13 | ||||||
| Test for overall effect: Z = 1.09 (P = 0.27) | ||||||||
| 6 POCD as defined by the original studies ‐ 12 weeks | ||||||||
| 6.1 Mild | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 13 | 24 | 27 | 33 | 100.0% | 0.66 (0.44 to 0.99) | |||
| Subtotal (95% CI) | 24 | 33 | 100.0% | 0.66 (0.44 to 0.99) | ||||
| Total events | 13 | 27 | ||||||
| Test for overall effect: Z = 2.01 (P = 0.04) | ||||||||
| 6.2 Moderate | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 6 | 24 | 9 | 33 | 100.0% | 0.92 (0.38 to 2.23) | |||
| Subtotal (95% CI) | 24 | 33 | 100.0% | 0.92 (0.38 to 2.23) | ||||
| Total events | 6 | 9 | ||||||
| Test for overall effect: Z = 0.19 (P = 0.85) | ||||||||
| 6.3 Severe | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 2 | 24 | 4 | 33 | 100.0% | 0.69 (0.14 to 3.45) | |||
| Subtotal (95% CI) | 24 | 33 | 100.0% | 0.69 (0.14 to 3.45) | ||||
| Total events | 2 | 4 | ||||||
| Test for overall effect: Z = 0.46 (P = 0.65) | ||||||||
| 7 POCD as defined by the original studies ‐ 52 weeks | ||||||||
| 7.1 Mild | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 15 | 27 | 27 | 32 | 100.0% | 0.66 (0.46 to 0.95) | |||
| Subtotal (95% CI) | 27 | 32 | 100.0% | 0.66 (0.46 to 0.95) | ||||
| Total events | 15 | 27 | ||||||
| Test for overall effect: Z = 2.22 (P = 0.03) | ||||||||
| 7.2 Moderate | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 3 | 27 | 12 | 32 | 100.0% | 0.30 (0.09 to 0.94) | |||
| Subtotal (95% CI) | 27 | 32 | 100.0% | 0.30 (0.09 to 0.94) | ||||
| Total events | 3 | 12 | ||||||
| Test for overall effect: Z = 2.06 (P = 0.04) | ||||||||
| 7.3 Severe | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 1 | 27 | 4 | 32 | 100.0% | 0.30 (0.04 to 2.49) | |||
| Subtotal (95% CI) | 27 | 32 | 100.0% | 0.30 (0.04 to 2.49) | ||||
| Total events | 1 | 4 | ||||||
| Test for overall effect: Z = 1.12 (P = 0.26) | ||||||||
ASEM: antisaccadic eye movement test; CI: confidence interval; IQR: interquartile range; N: number; NIRS: near‐infrared spectroscopy; POCD: postoperative cognitive dysfunction; POD: postoperative delirium; S100B: one biomarker of cerebral damage; SD: standard deviation
The estimated effect on stroke based on data from Cowie 2014 and Murkin 2007 was similar between interventions (n = 240, fixed‐effect model, RR 0.25, 95% CI 0.03 to 2.20) (Table 1). We downgraded this outcome to low quality due to risk of bias (commercial funding) and imprecision (small sample size) (summary of findings Table for the main comparison).
1.2 Postoperative stroke or other neurological injury: mini‐mental state examination (MMSE) (endpoint or change score)
Using the MMSE score (maximum score = 30), Ballard 2012, Lau 2012 and Mohandas 2013 measured postoperative stroke or other neurological injury at one, 12 and 52 weeks. Pooled analysis showed that at every measured time point the active cerebral oxygenation monitoring group had a higher mean MMSE score than the blinded monitoring group (2 studies with 151 participants, random‐effects model, mean difference (MD) 2.72, 95% CI 1.42 to 4.03, at one week; 3 studies with 179 participants, random‐effects model, MD 1.11, 95% CI 0.15 to 2.07, at 12 weeks; and one study with 60 participants, MD 1.63 95% CI 0.7 to 2.56 at 52 weeks) (Analysis 1.1).
1.3 Postoperative stroke or other neurological injury: a simplified antisaccadic eye movement test (ASEM) (endpoint score)
Only Mohandas 2013 (n = 100) observed stroke or neurological injury using the ASEM score and reported a significant difference favouring active cerebral oxygenation monitoring at both one and 12 weeks (MD 2.42, 95% CI 0.98 to 3.86; MD 1.99, 95% CI 0.78 to 3.20, respectively) (Table 1).
1.4 Postoperative stroke or other neurological injury: S100B
For this outcome, Harilall 2014 tested the serum 100B level to indicate postoperative neurological injury, which provided the median and interquartile range (IQR) of the endpoint and change data (n = 40). The blinded monitoring group demonstrated a higher level of S100B at endpoint (median 77.7, IQR 71.4 to 87.6 in the active monitoring group; 176.8, 160.8 to 199.5 in the blinded monitoring group) and change value (37.3, 33.1 to 42.7 in the active monitoring group; 139.3, 123.8 to 159.0 in the blinded monitoring group) than the active monitoring group.
1.5 Postoperative stroke or other neurological injury: vigilance reaction time (change score)
Only Ballard 2012 (n = 61) provided data for the vigilance reaction time at one, 12 and 52 weeks. Only the data at 12 and 52 weeks supported the active monitoring group (MD ‐25.34, 95% CI ‐41.44 to ‐9.24 at 12 weeks; MD ‐25.9, 95% CI ‐45.39 to ‐6.41 at 52 weeks) (Table 1), while both groups had a similar change score for vigilance reaction time at one week (MD ‐21.56, 95% CI ‐60.85 to 17.73).
1.6 Postoperative stroke or other neurological injury: trail making test (change score)
Ballard 2012 (n = 61) also provided data for the trail making test at 12 and 52 weeks, which indicated a lower level of trail making at 12 weeks (MD ‐0.7, 95% CI ‐1.23 to ‐0.17) but a higher level at 52 weeks (MD 0.59, 95% CI 0.18 to 1.0) (Table 1) in the active cerebral oxygenation monitoring group compared to the blinded monitoring group.
In Ballard 2012, there were potential risks of bias in terms of incomplete outcome data. In Mohandas 2013, there was insufficient information about allocation concealment and blinding; in addition, the number of participants experiencing desaturation was not reported. In Lau 2012, 2 of 12 in the treatment group and 3 of 13 in the control group were dropouts; the MMSE was analysed with nine participants in each group. Furthermore, the sample size was small and the outcome was indirectly measured via a scale. Therefore, we downgraded these outcomes to very low quality due to risk of bias, imprecision and indirectness (Analysis 1.1; summary of findings Table for the main comparison; Table 1).
Primary outcomes: postoperative delirium or postoperative cognitive dysfunction
1.7 Postoperative delirium (POD)
Only Colak 2015 provided data for this outcome and the result indicated that there was no significant difference between groups (n = 190, RR 0.63, 95% CI 0.27 to 1.45) (Table 1). We downgraded this outcome to low quality because only 181 participants (90.5%) had a neurocognitive test at seven days after surgery and the sample size was small (summary of findings Table for the main comparison).
1.8 Postoperative cognitive dysfunction (POCD) as defined by the original studies
Two studies (n = 126) provided data for this outcome (Ballard 2012; Kara 2015). Kara 2015 reported mild and severe postoperative cognitive function impairment before discharge, but the detailed time point was not described. Since the hospital length of stay was 7.15 ± 1.39 days versus 7.67 ± 1.14 days in the intervention group and control group, respectively, we pooled the data into the analysis of POCD as defined by the original studies ‐ one week. In terms of mild and severe POCD as defined by the original studies up to one week, there was a significant difference between groups (random‐effects model, RR 0.53, 95% CI 0.30 to 0.95; RR 0.18, 95% CI 0.03 to 0.92, respectively) (Analysis 1.2). However, there was no significant difference in the incidence of moderate POCD (RR 0.46, 95% CI 0.2 to 1.04) (Analysis 1.2). See summary of findings Table for the main comparison. Ballard 2012 also reported the incidence of mild POCD by 12 weeks; a significant difference was demonstrated between the two groups (RR 0.66 95% CI 0.44 to 0.99) (Table 1). Ballard 2012 also reported the incidence of POCD by 52 weeks. The active monitoring group saw a significant decrease in the incidence of mild and moderate POCD (RR 0.66, 95% CI 0.46 to 0.95; RR 0.30, 95% CI 0.09 to 0.94, respectively) but the incidence of severe POCD by 52 weeks was similar between the two groups (RR 0.30, 95% CI 0.04 to 2.49) (Table 1). In Ballard 2012, the attrition rates at one week postoperatively were 35.3% (intervention group) and 23.7% (control group), respectively; at 12 weeks they were 20.6% and 10.5%, respectively; and at 52 weeks they were 17.7% and 15.8%, respectively. The proportion of missing outcomes compared with the observed events could possibly induce clinically relevant bias in the intervention effect estimate. As a result, we downgraded this outcome to low quality (summary of findings Table for the main comparison).
1.9 POCD: decline in cognitive function ‐ one week
Six studies reported decline in cognitive function at one week (Casati 2005; Colak 2015;Mohandas 2013;Slater 2009;Vretzakis 2013; Zogogiannis 2011). We found that there was no significant difference between groups (n = 962, random‐effects model, RR 0.62, 95% CI 0.37 to 1.04) (Analysis 1.3). This analysis indicated a high level of heterogeneity (Chi2 = 24.66; P = 0.0002; I2 = 80%). Mohandas 2013 used a different type of monitoring equipment (Nonin Equanox) to the other studies (INVOS), which could potentially be the cause of the heterogeneity. After excluding Mohandas 2013, the analysis of the other five studies still showed no significant difference between groups (n = 862, random‐effects model, RR 0.79, 95% CI 0.61 to 1.04). We downgraded this outcome from high quality to moderate due to the risk of bias with respect to incomplete outcome data (Colak 2015) (summary of findings Table for the main comparison).
1.10 POCD: decline in cognitive function ‐ 12 weeks
Two studies investigated this outcome at 12 weeks (Mohandas 2013; Slater 2009), but the result was heterogeneous when we attempted meta‐analysis. We suspect that the heterogeneity was caused by the use of different monitoring equipment (Nonin Equanox in Mohandas 2013 versus INVOS in Slater 2009). We therefore presented these results separately without pooling. Mohandas 2013 favoured the active oxygenation group (odds ratio (OR) 0.01, 95% CI 0.00 to 0.21), whereas Slater 2009 showed no difference between groups (OR 0.96, 95% CI 0.51 to 1.81). We downgraded this outcome to low quality due to imprecision (small sample size) and inconsistency (summary of findings Table for the main comparison).
1.11 POCD: cognitive tests ‐ one week
Trafidlo 2015 (n = 43) used the Digit Span Test and reported a U statistic of 77.0 for overall numbers of forward and backward repetition (P = 0.003) and 82.5 for the Digit Span Test ‐ forward (P = 0.005) at one week, favouring the use of active monitoring.
1.12 POCD: cognitive tests ‐ four weeks
Trafidlo 2015 also reported a U statistic of 69.0 (P = 0.004) for the N‐back Test (NBT 1 back) at four weeks, favouring the use of active monitoring.
Primary outcomes: intraoperative mortality or postoperative mortality
1.13 Intraoperative mortality or postoperative mortality
Regarding rate of death, we were able to pool data from three studies in a meta‐analysis (Cowie 2014; Murkin 2007; Vretzakis 2013). We did not find a difference between active cerebral oxygenation monitoring and blinded cerebral oxygenation monitoring (n = 390, random‐effects model, RR 0.63, 95% CI 0.08 to 5.03) (Analysis 1.4). We downgraded this outcome to low quality due to risk of bias (commercial funding) and imprecision (small sample size) (summary of findings Table for the main comparison).
Secondary outcomes: the occurrence of abnormal rScO2 during or after surgery
1.14 The occurrence of abnormal rScO2 during or after surgery: desaturation
Seven studies provided data on desaturation in the operating room (Ballard 2012; Casati 2005;Cowie 2014; Deschamps 2013;Deschamps 2016; Murkin 2007; Slater 2009) and two studies in the intensive care unit (ICU) (Deschamps 2013; Deschamps 2016). We found that active cerebral near‐infrared spectroscopy (NIRS) monitoring decreased the incidence of cerebral desaturation in the operating room (n = 916, random‐effects model, RR 0.81, 95% CI 0.67 to 0.99) (Analysis 1.5). However, there was no significant difference in the occurrence of desaturation between the active cerebral oxygenation monitoring group and the blinded cerebral oxygenation monitoring group in the ICU (n = 249, random‐effects model, RR 0.71, 95% CI 0.37 to 1.34) (Analysis 1.5).
1.15 The occurrence of abnormal rScO2 during or after surgery: desaturation time
Harilall 2014 (n = 40) reported the desaturation time (minutes) during surgery. The result indicated a shorter duration of desaturation in the active cerebral oxygenation monitoring group than the blinded cerebral oxygenation monitoring group (MD ‐39.15, 95% CI ‐50.65 to ‐27.65) (Table 2).
| 1 The occurrence of abnormal rScO2 during or after surgery: desaturation time | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 24.7 | 11.819 | 20 | 63.85 | 23.424 | 20 | 100.0% | ‐39.15 (‐50.65 to ‐27.65) | |
| Total (95% CI) | 20 | 20 | 100.0% | ‐39.15 (‐50.65 to ‐27.65) | ||||
| Test for overall effect: Z = 6.67 ( P < 0.00001) | ||||||||
| 2 The occurrence of abnormal rScO2 during or after surgery: rScO2 below 50% | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 1 | 30 | 6 | 35 | 100.0% | 0.19 (0.02 to 1.53) | |||
| Total (95% CI) | 30 | 35 | 100.0% | 0.19 (0.02 to 1.53) | ||||
| Total events | 1 | 6 | ||||||
| Test for overall effect: Z = 1.56 (P = 0.12) | ||||||||
| 3 Length of hospital stay (days) | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 7.15 | 1.39 | 43 | 7.67 | 1.14 | 36 | 100.0% | ‐0.52 (‐1.08 to 0.04) | |
| Total (95% CI) | 43 | 36 | 100.0% | ‐0.52 (‐1.08 to 0.04 | ||||
| Test for overall effect: Z = 1.83 (P = 0.07) | ||||||||
| 4 Length of hospital stay (days) | ||||||||
| Study ID | Group | Mean | SD/95% CI | N | ||||
| Intervention group | 7.9 | 4.8 to 10.9 | 20 | |||||
| Control group | 10.6 | 5.5 to 15.8 | 20 | |||||
| Intervention group | 7.6 | 5.4 | 23 | |||||
| Control group | 7.9 | 3.2 | 25 | |||||
| Intervention group | 6.1 | 4.4 | 100 | |||||
| Control group | 6.9 | 5.5 | 100 | |||||
| Intervention group | 10.9 | 3.6 | 75 | |||||
| Control group | 10.2 | 10.7 | 75 | |||||
CI: confidence interval; N: number; NIRS: near‐infrared spectroscopy; rScO2: regional cerebral oxygen saturation; SD: standard deviation
1.16 The occurrence of abnormal rScO2 during or after surgery: rScO2 below 50%
In terms of the occurrence of rSO2 below 50% during surgery, analysis of Ballard 2012 demonstrated that there was no significant difference between the active cerebral oxygenation monitoring group and the blinded cerebral oxygenation monitoring group (n = 65, RR 0.19, 95% CI 0.02 to 1.53) (Table 2).
1.17 The occurrence of abnormal rScO2 during or after surgery: cerebral desaturation load (CDL) (%.min) or AUC rScO2 (min, %)
Deschamps 2016 provided skewed data for the cerebral desaturation load (CDL) (%.min). The results demonstrated that active cerebral NIRS monitoring decreased the CDL either in the operating room (n = 102, 104 ± 217 versus n = 99, 398 ± 870) or in the ICU (n = 102, 454 ± 870 versus n = 99, 1070 ± 961).
The AUC rScO2 (min, %) during surgery was reported by Mohandas 2013. The results indicated that the blinded monitoring group had significantly higher values (n = 100; right side (Rt) measurement, mean 2.993 ± standard deviation (SD) 8.87 in the active monitoring group versus 92.48 ± 58.31 in the blinded monitoring group; left side (Lt) measurement, 3.056 ± 8.96 versus 92.74 ± 58.61). The data were skewed, therefore we did not combine together the means and SDs of both left and right side measurements.
1.18 The occurrence of abnormal rScO2 during or after surgery: episodes of rScO2 decrease (counts)
Trafidlo 2015 reported 2.46 ± 3.2 episodes of rScO2 decrease in the active monitoring group (n = 13), but no data for the control group were provided.
1.19 The occurrence of abnormal rScO2 during or after surgery: durations of rScO2 decrease (minutes)
Trafidlo 2015 reported 7.46 ± 9.19 minutes' duration of rScO2 decrease in the active monitoring group (n = 13), but no data for the control group were provided.
Secondary outcomes: any major non‐neurological complications
1.20 Any major non‐neurological complications as defined by the individual study
In terms of complications, we categorized eight studies into various outcomes as defined by the individual study (Casati 2005; Colak 2015; Cowie 2014; Lau 2012; Murkin 2007; Trafidlo 2015; Vretzakis 2013;Zogogiannis 2011) (Analysis 1.6).
For non‐specific 'any reported complications' (i.e. only considering the total number of participants with complications regardless of the types of complications), meta‐analysis of six studies demonstrated a similar rate of adverse events between the active cerebral oxygenation monitoring group and the blinded cerebral oxygenation monitoring group (n = 562, random‐effects model, RR 0.76, 95% CI 0.58 to 1.00) (Analysis 1.6) (Casati 2005; Cowie 2014; Lau 2012; Murkin 2007; Trafidlo 2015; Vretzakis 2013).
In terms of non‐specific respiratory complications, only Casati 2005 (n = 122) was included, which did not report a significant difference between groups (RR 0.29, 95% CI 0.03 to 2.56) (Analysis 1.6).
Three studies were in the analysis of non‐specific cardiac complications (Casati 2005; Murkin 2007; Vretzakis 2013). Again, we did not find a difference between groups (n = 472, random‐effects model, RR 0.80, 95% CI 0.28 to 2.31) (Analysis 1.6).
Two studies were included into analysis of non‐specific renal complications (Colak 2015; Cowie 2014). The result did not indicate a difference between groups (n = 230, random‐effects model, RR 0.87, 95% CI 0.27 to 2.76) (Analysis 1.6).
Among the other adverse events, we found a difference between groups in terms of the rate of major organ morbidity and mortality (MOMM) (n = 200, RR 0.33, 95% CI 0.08 to 0.95) (Analysis 1.6), but not for the others. This included surgical complications: rupture of the colonic anastomosis, mediastinitis, septicaemia, wound infection, reoperation for bleeding, surgical intervention, arrhythmia, myocardial infarction, cardiac arrest, pneumothorax and cardiac ischaemia (Analysis 1.6).
Secondary outcomes: ICU length of stay
1.21 Length of ICU stay (days)
Three studies were included in the analysis of length of ICU stay (Kara 2015; Mohandas 2013; Murkin 2007). We found that the use of active monitoring resulted in a shorter length of ICU stay than blinded cerebral oxygenation monitoring (n = 379, random‐effects model, MD ‐0.29, 95% CI ‐0.48 to ‐0.09) (Analysis 1.7).
1.22 Length of ICU stay (hours/days)
Three studies provided the length of ICU stay as skewed data (Colak 2015; Deschamps 2013; Vretzakis 2013). Deschamps 2013 reported 71.9 ± 54.4 hours of ICU stay in the active monitoring group and 9.4 ± 49.3 hours in the control group. Colak 2015 and Vretzakis 2013 demonstrated no difference between groups in the length of ICU stay (in days) (2.7 ± 6.2 versus 1.9 ± 0.9 and 2.7 ± 3.8 versus 2.7 ± 3.6, respectively).
Secondary outcomes: hospital length of stay
1.23 Length of hospitalization (days)
One study was included in the analysis of length of hospitalization (Kara 2015). The result showed that the active cerebral NIRS monitoring group had a similar length of hospital stay to the conventional monitoring group (n = 79, MD ‐0.52, 95% CI ‐1.08 to 0.04) (Table 2).
1.24 Length of hospitalization (hours/days)
Four studies reported the length of hospitalization as skewed data or as the mean and range (Cowie 2014; Deschamps 2013; Murkin 2007; Vretzakis 2013). The results indicate that the active monitoring group had a similar length of hospital stay to the blinded monitoring group (Table 2).
Secondary outcomes: cost of hospitalization
No study reported this outcome.
Secondary outcomes: adverse events
No study considered this outcome.
Subgroup analysis: only primary outcomes
We conducted subgroup analyses by participants and interventions to explore the differences between them. However, none of the included studies included children (< 18 years) or compared perioperative cerebral NIRS monitoring versus other kinds of cerebral oxygenation monitoring. Therefore, we only investigated subgroups of the various types of surgery (i.e. carotid endarterectomy, cardiac or great vessel surgery with/without bypass, or other surgery) and the various active interventions (i.e. EQUANOX 7600 versus INVOS). We were unable to carry out subgroup analysis for neurosurgery because no study enrolled participants undergoing this type of surgery. We carried out the subgroup analyses for the pre‐specified primary outcomes only.
Comparison 2: Subgroup of participants: participants undergoing cardiac or great vessel surgery, carotid endarterectomy or other surgery
We carried out the following subgroup analyses for subsets of studies with participants undergoing various types of surgery. Only Zogogiannis 2011 fell into the carotid endarterectomy subgroup; Colak 2015, Deschamps 2013, Deschamps 2016, Harilall 2014, Kara 2015, Lau 2012, Mohandas 2013, Murkin 2007, Slater 2009 and Vretzakis 2013 enrolled participants undergoing cardiac or great vessel surgery with/without bypass. Ballard 2012, Casati 2005, Cowie 2014 and Trafidlo 2015 considered other surgeries.
2.1 Postoperative stroke or other neurological injury: neurological injury
For this outcome, Colak 2015 considered cardiac or great vessel surgery and Casati 2005 fell into the other surgery group. Although the direction of effect favoured different interventions in these two studies, there was no statistical difference between interventions. Subgroup analysis of the results demonstrated no significant subgroup differences in postoperative neurological injury (Analysis 2.1). No data were available for the analysis of the carotid endarterectomy subgroup.
2.2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ one week
For this outcome, Mohandas 2013 considered cardiac or great vessel surgery and Ballard 2012 fell into the other surgery group. Subgroup analysis showed that there were no significant subgroup differences in MMSE score at one week after surgery (Analysis 2.2).
2.3 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks
Regarding this outcome, two studies enrolled participants undergoing cardiac or great vessel surgery (Lau 2012; Mohandas 2013) and one study considered other surgery (Ballard 2012). In either subgroup, active monitoring had a similar value at 12 weeks compared with blinded monitoring (Analysis 2.3), indicating no significant subgroup differences.
2.4 POCD as defined by the original studies ‐ one week
For this outcome, Kara 2015 fell into the cardiac or great vessel surgery subgroup and Ballard 2012 the other surgery subgroup. In either subgroup, there was a significant difference between groups in mild POCD as defined by the original studies and but no difference in severe POCD at one week after surgery (Analysis 2.4; Analysis 2.5). We found no significant subgroup differences.
2.5 POCD: decline in cognitive function ‐ one week
Regarding this outcome, four studies included participants undergoing the cardiac or great vessel surgery (Colak 2015; Mohandas 2013; Slater 2009; Vretzakis 2013), one study was in the carotid endarterectomy subgroup (Zogogiannis 2011) and one study fell into the other surgery subgroup (Casati 2005). Subgroup analysis of the data indicated no significant subgroup differences in terms of decline in cognitive function at one week after surgery. We did not find any significant difference between interventions in any of the subgroups (Analysis 2.6).
Comparison 3: Subgroup of interventions: cerebral oxygenation monitoring (EQUANOX) or INVOS versus blinded monitoring
Only Mohandas 2013 fell into the EQUANOX 7600 subgroup, and 13 studies were included in the comparison of INVOS versus blinded monitoring (Ballard 2012; Casati 2005; Colak 2015; Cowie 2014; Deschamps 2013; Harilall 2014; Kara 2015; Lau 2012; Murkin 2007; Slater 2009; Trafidlo 2015; Vretzakis 2013; Zogogiannis 2011). The multicentre study Deschamps 2016 used three types of rSO2 monitoring devices including FORE‐SIGHT, EQUANOX 7600 and INVOS in different centres, therefore it was not included in the subgroup analyses of interventions.
1. Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ one week
For this outcome, one study used the INVOS equipment (Ballard 2012) and one study used the EQUANOX equipment in the intervention group (Mohandas 2013). Compared with blinded monitoring, both INVOS and EQUANOX had higher MMSE scores at one week after surgery (Analysis 3.1). We found no significant subgroup differences.
2. Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks
In terms of MMSE score at 12 weeks after surgery, two studies considered the INVOS group (Ballard 2012; Lau 2012) and one study fell into the EQUANOX group (Mohandas 2013). The results indicated that compared with blinded monitoring, EQUANOX had higher MMSE scores at 12 weeks (n = 100, random‐effects model, MD 3.16, 95% CI 0.98 to 5.34) but INVOS has similar scores (n = 79, random‐effects model, MD 0.73, 95% CI ‐0.13 to 1.60) (Analysis 3.2).
3. POCD: decline in cognitive function ‐ one week
Regarding this outcome, five studies employed the INVOS equipment (Casati 2005; Colak 2015; Slater 2009; Vretzakis 2013; Zogogiannis 2011) and one study employed the EQUANOX equipment (Mohandas 2013). The INVOS subgroup had a similar incidence of decline in cognitive function compared with the control group at one week (n = 862, random‐effects model, RR 0.79, 95% CI 0.61 to 1.04) (Analysis 3.3). In the EQUANOX subgroup, compared with the control group, we found a lower incidence of participants with decline of cognitive function at one week (n = 100, RR 0.06, 95% CI 0.01 to 0.23) (Analysis 3.3).
Sensitivity analysis
1. Excluding studies at high risk of bias for allocation concealment
We did not conduct any sensitivity analysis because no study met the criteria for exclusion on the basis of inadequate allocation concealment.
2. Excluding studies at high risk of detection bias (blinding of outcome assessment)
One study was at high risk of detection bias (blinding of outcome assessment) and we excluded it from the analyses affected (Trafidlo 2015). After excluding Trafidlo 2015, the analysis with the other five studies showed a statistically significant and homogeneous effect estimate favouring the active oxygenation group (n = 537, random‐effects model, RR 0.76, 95% CI 0.57 to 0.99) (Analysis 4.1; Analysis 4.2).
3. Excluding studies at high risk of attrition bias (missing data)
Three studies were at high risk of attrition bias and we excluded them from the affected analyses (Ballard 2012; Colak 2015; Lau 2012). The results indicated that there was no change as a result of their exclusion (Analysis 5.1; Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6; Analysis 5.7; Table 3).
| 1 Postoperative stroke or other neurological injury: including studies with missing data | ||||||||
| 1.1 Neurological injury | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Risk ratio | ||||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 0 | 56 | 4 | 66 | 0.13 (0.01 to 2.37) | ||||
| 4 | 94 | 1 | 96 | 4.09 (0.47 to 35.88) | ||||
| 1.2 Stroke | ||||||||
| 0 | 20 | 0 | 20 | Not estimable | ||||
| 1 | 100 | 4 | 100 | 0.25 (0.03 to 2.20) | ||||
| 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with missing data | ||||||||
| 2.1 52 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 0.69 | 1.47 | 28 | ‐0.94 | 2.18 | 32 | 100.0% | 1.63 (0.70 to 2.56) | |
| Subtotal (95% CI) | 28 | 32 | 100.0% | 1.63 (0.70 to 2.56) | ||||
| Test for overall effect: Z = 3.43 (P = 0.0006) | ||||||||
| 3 Postoperative stroke or other neurological injury: without missing data | ||||||||
| 3.1 Neurological injury | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Risk ratio | ||||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 0 | 56 | 4 | 66 | 0.13 (0.01 to 2.37) | ||||
| 3.2 Stroke | ||||||||
| 0 | 20 | 0 | 20 | Not estimable | ||||
| 1 | 100 | 4 | 100 | 0.25 (0.03 to 2.20) | ||||
| 4 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): without missing data | ||||||||
| 4.1 1 week | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 28.58 | 2.29 | 50 | 25.42 | 7.54 | 50 | 100.0% | 3.16 (0.98 to 5.34) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 3.16 (0.98 to 5.34) | ||||
| Test for overall effect: Z = 2.84 (P = 0.005) | ||||||||
| 65.2 12 weeks | ||||||||
| 28.88 | 1.88 | 50 | 26.5 | 6.31 | 50 | 100.0% | 2.38 (0.56 to 4.20) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 2.38 (0.56 to 4.20) | ||||
| Test for overall effect: Z = 2.56 (P = 0.01) | ||||||||
CI: confidence interval; MMSE: mini‐mental state examination; NIRS: near‐infrared spectroscopy; SD: standard deviation
4. Excluding studies at high risk of reporting bias
One study was at high risk of reporting bias and we excluded it from the affected analyses (Trafidlo 2015). The results indicated that there was a statistically significant but marginal difference between the two groups after excluding Trafidlo 2015 (n = 537, random‐effects model, RR 0.76, 95% CI 0.57 to 0.99) (Analysis 6.1; Analysis 6.2).
5. Excluding studies at high risk of other bias
Four studies were at high risk of other bias due to conflicts of interest, equipment grants and speaking honoraria (Ballard 2012; Cowie 2014; Deschamps 2016; Murkin 2007). We excluded these studies from the affected analyses. The results indicated changes in several outcomes after their exclusion (Analysis 7.7; Analysis 7.8; Table 4). The two groups had a similar mean MMSE score at 12 weeks after surgery (n = 118, random‐effects model, MD 1.58, 95% CI ‐0.10 to 3.25) (Analysis 7.7). In terms of severe POCD as defined by the original studies up to one week, there was no significant difference between groups (n = 79, RR 0.12, 95% CI 0.01 to 2.25) (Table 4). We found no significant difference in the occurrence of desaturation between the active cerebral oxygenation monitoring group and the blinded cerebral oxygenation monitoring group in the operating room (n = 410, random‐effects model, RR 0.88, 95% CI 0.69 to 1.13) (Analysis 7.8). There was no change in the remaining outcomes (Analysis 7.9; Analysis 7.10; Table 4).
| 1 POCD defined by original studies ‐ 1 week: including studies without other bias | ||||||||
| 1.1 Mild | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 7 | 43 | 16 | 36 | 100.0% | 0.37 (0.17 to 0.79) | |||
| Subtotal (95% CI) | 43 | 36 | 100.0% | 0.37 (0.17 to 0.79) | ||||
| Total events | 7 | 16 | ||||||
| Heterogeneity: not applicable | ||||||||
| Test for overall effect: Z = 2.56 (P = 0.01) | ||||||||
| 1.2 Severe | ||||||||
| 0 | 43 | 3 | 36 | 100.0% | 0.12 (0.01 to 2.25) | |||
| Subtotal (95% CI) | 43 | 36 | 100.0% | 0.12 (0.01 to 2.25) | ||||
| Total events | 0 | 3 | ||||||
| Heterogeneity: not applicable | ||||||||
| Test for overall effect: Z = 1.42 (P = 0.16) | ||||||||
| 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies without other bias | ||||||||
| 2.1 1 week | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 28.58 | 2.29 | 50 | 25.42 | 7.54 | 50 | 100.0% | 3.16 (0.98 to 5.34) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 3.16 (0.98 to 5.34) | ||||
| Test for overall effect: Z = 2.84 (P = 0.005) | ||||||||
| 3 Intraoperative mortality or postoperative mortality: death: including studies without other bias | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 1 | 75 | 1 | 75 | 100.0% | 1.00 (0.06 to 15.69) | |||
| Total (95% CI) | 75 | 75 | 100.0% | 1.00 (0.06 to 15.69) | ||||
| Total events | 1 | 1 | ||||||
| Heterogeneity: not applicable | ||||||||
| Test for overall effect: Z = 0.00 (P = 1.00) | ||||||||
| 4 The occurrence of abnormal rScO2 during or after surgery: desaturation: including studies without other bias | ||||||||
| 4.1 In ICU | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 6 | 23 | 14 | 25 | 100.0% | 0.47 (0.22 to 1.01) | |||
| Subtotal (95% CI) | 23 | 25 | 100.0% | 0.47 (0.22 to 1.01) | ||||
| Total events | 6 | 14 | ||||||
| Heterogeneity: not applicable | ||||||||
| Test for overall effect: Z = 1.94 (P = 0.05) | ||||||||
CI: confidence interval; ICU: intensive care unit; MMSE: mini‐mental state examination; NIRS: near‐infrared spectroscopy; POCD: postoperative cognitive dysfunction; SD: standard deviation; rScO2: regional cerebral oxygen saturation
Publication bias
Funnel plots of the primary outcome were not applicable due to the small number of studies involved in each meta‐analysis.
Summary of findings
Using the principles of the GRADE system, we found a low‐quality body of evidence associated with most of the primary outcomes, such as rate of postoperative stroke or other neurological injury, POCD and intraoperative or postoperative mortality (summary of findings Table for the main comparison). We rated the quality of the evidence as moderate only for the decline in cognitive function (as defined by the individual study) at one week after surgery.
Discussion
Summary of main results
We identified 15 studies comparing active cerebral oxygenation monitoring with blinded or no cerebral oxygenation monitoring during the perioperative period. These 15 studies comprised a total of 1822 adult participants.
For the primary outcomes, two studies with 312 participants reported postoperative neurological injury, however we did not pool the data because of clinical heterogeneity. Evidence from two studies in 240 participants demonstrated that active cerebral near‐infrared spectroscopy (NIRS) monitoring showed little or no effect on the risk of postoperative stroke. Two studies with 126 participants showed that active cerebral NIRS monitoring may reduce the incidence of short‐term mild postoperative cognitive dysfunction (POCD; as defined by the original studies) by 47% (risk ratio (RR) 0.53, 95% confidence interval (CI) 0.30 to 0.95) and this difference was clinically important. Six studies with 962 participants provided moderate‐quality evidence that active cerebral oxygenation monitoring probably does not decrease the occurrence of POCD (decline in cognitive function) at one week after surgery. There is uncertainty over postoperative delirium, intraoperative mortality and postoperative mortality.
In terms of secondary outcomes, seven studies comprising 916 participants demonstrated that active cerebral NIRS monitoring decreased the incidence of cerebral desaturation in the operating room by 19% (RR 0.81, 95% CI 0.67 to 0.99), but this difference was not clinically important. Three studies with 379 participants indicated that active cerebral NIRS monitoring shortened intensive care unit (ICU) length of stay by 0.29 days (mean difference (MD) ‐0.29, 95% CI ‐0.48 to ‐0.09), but this difference was not clinically important. However, the cost of hospitalization was not reported in any of the included studies. There is no evidence to determine whether routine use of NIRS‐based cerebral oxygenation monitoring causes any adverse effects.
Overall completeness and applicability of evidence
None of the included studies in the review included a paediatric population or people undergoing neurosurgery. In addition, only two of the 15 included studies used cerebral NIRS monitoring in both the operating room and the ICU, whereas the others used it in the operating room. INVOS (Somanetics Corporation, USA) was used for the NIRS‐based cerebral oximetry in most of the included studies. Some other kinds of cerebral NIRS monitor were not used in any of these studies.
With the exception of the cost of hospitalization and adverse events, all our other pre‐specified primary outcomes and secondary outcomes were reported by the included studies. However, for some outcomes, such as postoperative stroke or other neurological injury and ICU length of stay, the evidence synthesis was based on a few studies with limited numbers of participants. As a result, further information will be required for the results to have internal and external validity.
The pooled data analyses in this review were limited mainly to cardiac or great vessel surgery with/without bypass. Consequently, extrapolating the results of the current meta‐analyses to patients undergoing other types of surgery should be performed with caution and further high‐quality clinical research is required.
Quality of the evidence
See also Risk of bias in included studies and summary of findings Table for the main comparison.
We rated the quality of the evidence as low or moderate based on GRADE for most of the outcomes included in the review. We judged the evidence to be of low quality for postoperative mini‐mental state examination (MMSE) or antisaccadic eye movement (ASEM) test scores due to risk of bias and the use of indirect measurement via a scale. We also rated the outcome of POCD as defined by the original studies and mortality as low‐quality evidence due to several unclear risks of bias such as missing participant data and small sample sizes. For the outcome of decline in cognitive function at one week after surgery, we downgraded the level of the evidence to moderate quality due to the high risk of bias related to incomplete outcome data and selective reporting in Colak 2015.
Only one of the included studies was of good methodological quality (Vretzakis 2013). In the other studies, the most questionable bias was related to incomplete outcome data, selective reporting, unclear blinding status and other biases such as potential conflict of interest from industry sponsorship. In most of the included studies, the sample size was smaller than the optimal information size, which prompted us to downgrade the evidence for imprecision. However, sensitivity analysis showed that missing data did not have an impact on the conclusions of this review. In this review, we did not create a funnel plot to qualitatively assess publication or reporting bias because fewer than 10 studies were included for each outcome.
Potential biases in the review process
We intended the search for studies to be as extensive as possible and we made every attempt to include international studies and not just those published in the English language. There remains the possibility that there may be other unpublished trials of this intervention (e.g. in the grey literature) that we do not currently have access to. This means that we may unwittingly have perpetuated a publication bias. Furthermore, one included study did not provide any email addresses for the study investigators (Harilall 2014). We attempted to conduct a comprehensive search for studies, but the fact that the studies meeting the inclusion criteria in an updated search have not yet been incorporated may be a source of potential bias. Despite this, our extensive search for complete and ongoing studies a strength of this review.
The definitions of cerebral desaturation were not identical in all the included studies, varying from below 70% of baseline values to below 90% of baseline values, which may potentially result in clinical heterogeneity. In addition, in Zogogiannis 2011, cerebral oximetry values were recorded in group B but anaesthesia management was not based on the algorithm mentioned in the full text; therefore, we only used the data from group A as the intervention group and group C as the control group, which may have a potential impact on the findings of the review.
Agreements and disagreements with other studies or reviews
A recently published systematic review discussed whether NIRS‐based cerebral oxygenation monitoring could improve neurological outcomes in adults undergoing cardiac surgery (Zheng 2013). Only two randomized controlled trials (RCTs) and 40 other studies such as case reports and observational studies were included, but meta‐analysis was not performed. Zheng 2013 identified two RCTs involving 440 participants. However, these two studies did not meet the eligibility criteria for our review, so we did not include them. In Zheng 2013, the authors concluded that there was insufficient evidence to demonstrate that interventions to improve intraoperative desaturation prevent stroke or POCD.

Study flow diagram‐top up search on November 2017.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score).

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 2 POCD defined by original studies ‐ 1 week.

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 3 POCD: decline in cognitive function ‐ 1 week.

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 4 Intraoperative mortality or postoperative mortality: Death.

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 5 The occurrence of abnormal rScO2 during or after surgery: Desaturation.

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 6 Any major non‐neurological complications as defined by individual study.

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 7 Length of ICU stay (days).

Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 1 Postoperative stroke or other neurological injury: Neurological injury.

Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 1 week.

Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 3 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks.

Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 4 POCD defined by original studies ‐ 1 week ‐ mild.

Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 5 POCD defined by original studies ‐ 1 week ‐ severe.

Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 6 POCD: decline in cognitive function ‐ 1 week.

Comparison 3 Subgroup of interventions: cerebral oxygenation monitoring (EQUANOX) or INVOS vs blinded monitoring, Outcome 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 1 week.
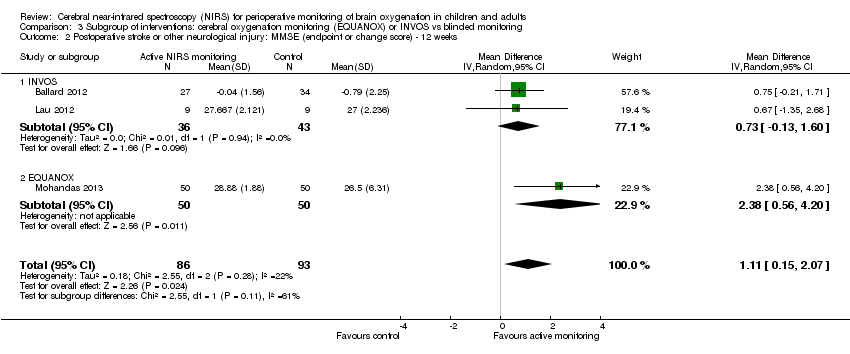
Comparison 3 Subgroup of interventions: cerebral oxygenation monitoring (EQUANOX) or INVOS vs blinded monitoring, Outcome 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks.

Comparison 3 Subgroup of interventions: cerebral oxygenation monitoring (EQUANOX) or INVOS vs blinded monitoring, Outcome 3 POCD: decline in cognitive function ‐ 1 week.

Comparison 4 Sensitivity analysis: detection bias, Outcome 1 Any major non‐neurological complications as defined by individual study: including studies with detection bias.

Comparison 4 Sensitivity analysis: detection bias, Outcome 2 Any major non‐neurological complications as defined by individual study: including studies without detection bias.

Comparison 5 Sensitivity analysis: missing data, Outcome 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with missing data.

Comparison 5 Sensitivity analysis: missing data, Outcome 2 POCD: decline in cognitive function ‐ 1 week: including studies with missing data.

Comparison 5 Sensitivity analysis: missing data, Outcome 3 Occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies with missing data.

Comparison 5 Sensitivity analysis: missing data, Outcome 4 Any major non‐neurological complications as defined by individual study: including studies with missing data.

Comparison 5 Sensitivity analysis: missing data, Outcome 5 POCD: decline in cognitive function ‐ 1 week: without missing data.

Comparison 5 Sensitivity analysis: missing data, Outcome 6 Occurrence of abnormal rScO2 during or after surgery: Desaturation: without missing data.
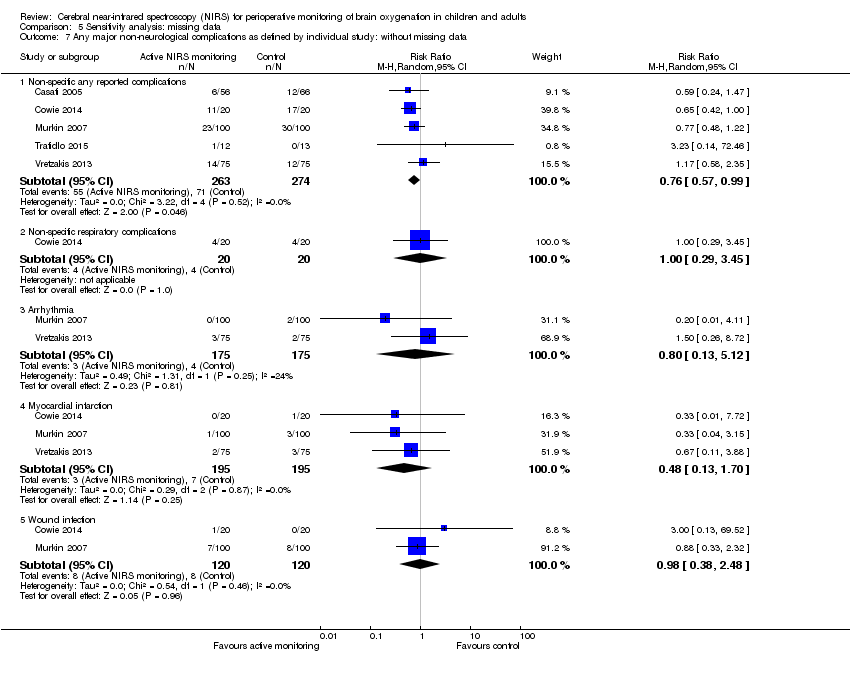
Comparison 5 Sensitivity analysis: missing data, Outcome 7 Any major non‐neurological complications as defined by individual study: without missing data.

Comparison 6 Sensitivity analysis: reporting bias, Outcome 1 Any major non‐neurological complications as defined by individual study: including studies with reporting bias.

Comparison 6 Sensitivity analysis: reporting bias, Outcome 2 Any major non‐neurological complications as defined by individual study: including studies without reporting bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 2 POCD defined by original studies ‐ 1 week: including studies with other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 3 Intraoperative mortality or postoperative mortality: Death: including studies with other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 4 The occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies with other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 5 Any major non‐neurological complications as defined by individual study: including studies with other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 6 Length of ICU stay (days): including studies with other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 7 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies without other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 8 The occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies without other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 9 Any major non‐neurological complications as defined by individual study: including studies without other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 10 Length of ICU stay (days): including studies without other bias.
| Active cerebral oxygenation monitoring compared to blinded cerebral oxygenation monitoring for perioperative monitoring of brain oxygenation in children and adults | ||||||
| Patient or population: children and adults undergoing surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Blinded cerebral oxygenation monitoring | Active cerebral oxygenation monitoring | |||||
| Postoperative neurological injury (follow‐up: 1 week or hospital discharge) | No pooled effect estimate available due to discordant direction of effect between studies. One study (N = 126) in people undergoing major abdominal surgery reported that 4/66 participants experienced neurological injury with blinded monitoring versus 0/56 in the active monitoring group. A second study (N = 195) in people having coronary artery bypass surgery reported that 1/96 participants experienced neurological injury in the blinded monitoring group compared with 4/94 participants in the active monitoring group. | Not estimable | 312 | ⊕⊕⊝⊝ | Very serious heterogeneity (I2 = 72%) | |
| Postoperative stroke (follow‐up: within 30 days) | 40 per 1000 | 10 per 1000 | RR 0.25 | 240 | ⊕⊕⊝⊝ | — |
| POD: postoperative delirium (follow‐up: 1 week) | 135 per 1000 | 85 per 1000 | RR 0.63 | 190 | ⊕⊕⊝⊝ | — |
| POCD defined by original studies ‐ mild (follow‐up: 1 week) | 641 per 1000 | 340 per 1000 | RR 0.53 | 126 | ⊕⊕⊝⊝ | — |
| POCD: decline in cognitive function (follow‐up: 1 week) | 400 per 1000 | 248 per 1000 | RR 0.62 | 962 | ⊕⊕⊕⊝ | — |
| Intraoperative mortality or postoperative mortality: Death (follow‐up: 30 days) | 10 per 1000 | 6 per 1000 | RR 0.63 | 390 | ⊕⊕⊝⊝ | — |
| Adverse events | See comments | See comments | Not estimable | See comments | See comments | None of the studies reported adverse effects caused by use of NIRS‐based cerebral oxygenation monitoring |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias: downgraded by one level because the body of evidence contained one or more of the following risks of bias: potential conflict of interest from industry sponsorship, unclear blinding status or missing participant data. | ||||||
| 1 Postoperative stroke or other neurological injury | ||||||||
| 1.1 Neurological injury | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Risk ratio | ||||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 0 | 56 | 4 | 66 | 0.13 (0.01 to 2.37) | ||||
| 4 | 94 | 1 | 96 | 4.09 (0.47 to 35.88) | ||||
| 1.2 Stroke | ||||||||
| 0 | 20 | 0 | 20 | Not estimable | ||||
| 1 | 100 | 4 | 100 | 0.25 (0.03 to 2.20) | ||||
| 2 Postoperative stroke or other neurological injury: ASEM (endpoint score) | ||||||||
| 2.1 1 week | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 17.46 | 1.99 | 50 | 15.04 | 4.8 | 50 | 100.0% | 2.42 (0.98 to 3.86) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 2.42 (0.98 to 3.86) | ||||
| Test for overall effect: Z = 3.29 (P = 0.0010) | ||||||||
| 2.2 12 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 17.68 | 1.79 | 50 | 15.69 | 3.99 | 50 | 100.0% | 1.99 (0.78 to 3.20) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 1.99 (0.78 to 3.20) | ||||
| Test for overall effect: Z = 3.22 (P = 0.001) | ||||||||
| 3 Postoperative stroke or other neurological injury: Vigilance Reaction Time (change score) | ||||||||
| 3.1 1 week | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 6.39 | 80.9 | 22 | 27.95 | 54.99 | 29 | 100.0% | ‐21.56 (‐60.85 to 17.73) | |
| Subtotal (95% CI) | 22 | 29 | 100.0% | ‐21.56 (‐60.85 to 17.73) | ||||
| Test for overall effect: Z = 1.08 (P = 0.28) | ||||||||
| 3.2 12 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| ‐11.73 | 33.5 | 27 | 13.61 | 29.69 | 34 | 100.0% | ‐25.34 (‐41.44 to ‐9.24) | |
| Subtotal (95% CI) | 27 | 34 | 100.0% | ‐25.34 (‐41.44 to ‐9.24) | ||||
| Test for overall effect: Z = 3.08 (P = 0.002) | ||||||||
| 3.3 52 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| ‐10.8 | 36.28 | 28 | 15.1 | 40.73 | 32 | 100.0% | ‐25.90 (‐45.39 to ‐6.41) | |
| Subtotal (95% CI) | 28 | 32 | 100.0% | ‐25.90 (‐45.39 to ‐6.41) | ||||
| Test for overall effect: Z = 2.61 (P = 0.009) | ||||||||
| 4 Postoperative stroke or other neurological injury: Trail Making (change score) | ||||||||
| 4.1 12 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| ‐0.23 | 0.73 | 27 | 0.47 | 1.34 | 34 | 100.0% | ‐0.70 (‐1.23 to ‐0.17) | |
| Subtotal (95% CI) | 27 | 34 | 100.0% | ‐0.70 (‐1.23 to ‐0.17) | ||||
| Test for overall effect: Z = 2.60 (P = 0.009) | ||||||||
| 4.2 52 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 0.12 | 0.68 | 28 | ‐0.47 | 0.93 | 32 | 100.0% | 0.59 (0.18 to 1.00) | |
| Subtotal (95% CI) | 28 | 32 | 100.0% | 0.59 (0.18 to 1.00) | ||||
| Test for overall effect: Z = 2.83 (P = 0.005) | ||||||||
| 5 POD: postoperative delirium | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 8 | 94 | 13 | 96 | 100.0% | 0.63 (0.27 to 1.45) | |||
| Total (95% CI) | 94 | 96 | 100.0% | 0.63 (0.27 to 1.45) | ||||
| Total events | 8 | 13 | ||||||
| Test for overall effect: Z = 1.09 (P = 0.27) | ||||||||
| 6 POCD as defined by the original studies ‐ 12 weeks | ||||||||
| 6.1 Mild | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 13 | 24 | 27 | 33 | 100.0% | 0.66 (0.44 to 0.99) | |||
| Subtotal (95% CI) | 24 | 33 | 100.0% | 0.66 (0.44 to 0.99) | ||||
| Total events | 13 | 27 | ||||||
| Test for overall effect: Z = 2.01 (P = 0.04) | ||||||||
| 6.2 Moderate | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 6 | 24 | 9 | 33 | 100.0% | 0.92 (0.38 to 2.23) | |||
| Subtotal (95% CI) | 24 | 33 | 100.0% | 0.92 (0.38 to 2.23) | ||||
| Total events | 6 | 9 | ||||||
| Test for overall effect: Z = 0.19 (P = 0.85) | ||||||||
| 6.3 Severe | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 2 | 24 | 4 | 33 | 100.0% | 0.69 (0.14 to 3.45) | |||
| Subtotal (95% CI) | 24 | 33 | 100.0% | 0.69 (0.14 to 3.45) | ||||
| Total events | 2 | 4 | ||||||
| Test for overall effect: Z = 0.46 (P = 0.65) | ||||||||
| 7 POCD as defined by the original studies ‐ 52 weeks | ||||||||
| 7.1 Mild | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 15 | 27 | 27 | 32 | 100.0% | 0.66 (0.46 to 0.95) | |||
| Subtotal (95% CI) | 27 | 32 | 100.0% | 0.66 (0.46 to 0.95) | ||||
| Total events | 15 | 27 | ||||||
| Test for overall effect: Z = 2.22 (P = 0.03) | ||||||||
| 7.2 Moderate | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 3 | 27 | 12 | 32 | 100.0% | 0.30 (0.09 to 0.94) | |||
| Subtotal (95% CI) | 27 | 32 | 100.0% | 0.30 (0.09 to 0.94) | ||||
| Total events | 3 | 12 | ||||||
| Test for overall effect: Z = 2.06 (P = 0.04) | ||||||||
| 7.3 Severe | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 1 | 27 | 4 | 32 | 100.0% | 0.30 (0.04 to 2.49) | |||
| Subtotal (95% CI) | 27 | 32 | 100.0% | 0.30 (0.04 to 2.49) | ||||
| Total events | 1 | 4 | ||||||
| Test for overall effect: Z = 1.12 (P = 0.26) | ||||||||
| ASEM: antisaccadic eye movement test; CI: confidence interval; IQR: interquartile range; N: number; NIRS: near‐infrared spectroscopy; POCD: postoperative cognitive dysfunction; POD: postoperative delirium; S100B: one biomarker of cerebral damage; SD: standard deviation | ||||||||
| 1 The occurrence of abnormal rScO2 during or after surgery: desaturation time | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 24.7 | 11.819 | 20 | 63.85 | 23.424 | 20 | 100.0% | ‐39.15 (‐50.65 to ‐27.65) | |
| Total (95% CI) | 20 | 20 | 100.0% | ‐39.15 (‐50.65 to ‐27.65) | ||||
| Test for overall effect: Z = 6.67 ( P < 0.00001) | ||||||||
| 2 The occurrence of abnormal rScO2 during or after surgery: rScO2 below 50% | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 1 | 30 | 6 | 35 | 100.0% | 0.19 (0.02 to 1.53) | |||
| Total (95% CI) | 30 | 35 | 100.0% | 0.19 (0.02 to 1.53) | ||||
| Total events | 1 | 6 | ||||||
| Test for overall effect: Z = 1.56 (P = 0.12) | ||||||||
| 3 Length of hospital stay (days) | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 7.15 | 1.39 | 43 | 7.67 | 1.14 | 36 | 100.0% | ‐0.52 (‐1.08 to 0.04) | |
| Total (95% CI) | 43 | 36 | 100.0% | ‐0.52 (‐1.08 to 0.04 | ||||
| Test for overall effect: Z = 1.83 (P = 0.07) | ||||||||
| 4 Length of hospital stay (days) | ||||||||
| Study ID | Group | Mean | SD/95% CI | N | ||||
| Intervention group | 7.9 | 4.8 to 10.9 | 20 | |||||
| Control group | 10.6 | 5.5 to 15.8 | 20 | |||||
| Intervention group | 7.6 | 5.4 | 23 | |||||
| Control group | 7.9 | 3.2 | 25 | |||||
| Intervention group | 6.1 | 4.4 | 100 | |||||
| Control group | 6.9 | 5.5 | 100 | |||||
| Intervention group | 10.9 | 3.6 | 75 | |||||
| Control group | 10.2 | 10.7 | 75 | |||||
| CI: confidence interval; N: number; NIRS: near‐infrared spectroscopy; rScO2: regional cerebral oxygen saturation; SD: standard deviation | ||||||||
| 1 Postoperative stroke or other neurological injury: including studies with missing data | ||||||||
| 1.1 Neurological injury | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Risk ratio | ||||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 0 | 56 | 4 | 66 | 0.13 (0.01 to 2.37) | ||||
| 4 | 94 | 1 | 96 | 4.09 (0.47 to 35.88) | ||||
| 1.2 Stroke | ||||||||
| 0 | 20 | 0 | 20 | Not estimable | ||||
| 1 | 100 | 4 | 100 | 0.25 (0.03 to 2.20) | ||||
| 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with missing data | ||||||||
| 2.1 52 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 0.69 | 1.47 | 28 | ‐0.94 | 2.18 | 32 | 100.0% | 1.63 (0.70 to 2.56) | |
| Subtotal (95% CI) | 28 | 32 | 100.0% | 1.63 (0.70 to 2.56) | ||||
| Test for overall effect: Z = 3.43 (P = 0.0006) | ||||||||
| 3 Postoperative stroke or other neurological injury: without missing data | ||||||||
| 3.1 Neurological injury | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Risk ratio | ||||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 0 | 56 | 4 | 66 | 0.13 (0.01 to 2.37) | ||||
| 3.2 Stroke | ||||||||
| 0 | 20 | 0 | 20 | Not estimable | ||||
| 1 | 100 | 4 | 100 | 0.25 (0.03 to 2.20) | ||||
| 4 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): without missing data | ||||||||
| 4.1 1 week | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 28.58 | 2.29 | 50 | 25.42 | 7.54 | 50 | 100.0% | 3.16 (0.98 to 5.34) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 3.16 (0.98 to 5.34) | ||||
| Test for overall effect: Z = 2.84 (P = 0.005) | ||||||||
| 65.2 12 weeks | ||||||||
| 28.88 | 1.88 | 50 | 26.5 | 6.31 | 50 | 100.0% | 2.38 (0.56 to 4.20) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 2.38 (0.56 to 4.20) | ||||
| Test for overall effect: Z = 2.56 (P = 0.01) | ||||||||
| CI: confidence interval; MMSE: mini‐mental state examination; NIRS: near‐infrared spectroscopy; SD: standard deviation | ||||||||
| 1 POCD defined by original studies ‐ 1 week: including studies without other bias | ||||||||
| 1.1 Mild | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 7 | 43 | 16 | 36 | 100.0% | 0.37 (0.17 to 0.79) | |||
| Subtotal (95% CI) | 43 | 36 | 100.0% | 0.37 (0.17 to 0.79) | ||||
| Total events | 7 | 16 | ||||||
| Heterogeneity: not applicable | ||||||||
| Test for overall effect: Z = 2.56 (P = 0.01) | ||||||||
| 1.2 Severe | ||||||||
| 0 | 43 | 3 | 36 | 100.0% | 0.12 (0.01 to 2.25) | |||
| Subtotal (95% CI) | 43 | 36 | 100.0% | 0.12 (0.01 to 2.25) | ||||
| Total events | 0 | 3 | ||||||
| Heterogeneity: not applicable | ||||||||
| Test for overall effect: Z = 1.42 (P = 0.16) | ||||||||
| 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies without other bias | ||||||||
| 2.1 1 week | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 28.58 | 2.29 | 50 | 25.42 | 7.54 | 50 | 100.0% | 3.16 (0.98 to 5.34) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 3.16 (0.98 to 5.34) | ||||
| Test for overall effect: Z = 2.84 (P = 0.005) | ||||||||
| 3 Intraoperative mortality or postoperative mortality: death: including studies without other bias | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 1 | 75 | 1 | 75 | 100.0% | 1.00 (0.06 to 15.69) | |||
| Total (95% CI) | 75 | 75 | 100.0% | 1.00 (0.06 to 15.69) | ||||
| Total events | 1 | 1 | ||||||
| Heterogeneity: not applicable | ||||||||
| Test for overall effect: Z = 0.00 (P = 1.00) | ||||||||
| 4 The occurrence of abnormal rScO2 during or after surgery: desaturation: including studies without other bias | ||||||||
| 4.1 In ICU | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 6 | 23 | 14 | 25 | 100.0% | 0.47 (0.22 to 1.01) | |||
| Subtotal (95% CI) | 23 | 25 | 100.0% | 0.47 (0.22 to 1.01) | ||||
| Total events | 6 | 14 | ||||||
| Heterogeneity: not applicable | ||||||||
| Test for overall effect: Z = 1.94 (P = 0.05) | ||||||||
| CI: confidence interval; ICU: intensive care unit; MMSE: mini‐mental state examination; NIRS: near‐infrared spectroscopy; POCD: postoperative cognitive dysfunction; SD: standard deviation; rScO2: regional cerebral oxygen saturation | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 week | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| 1.2 12 weeks | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| 1.3 52 weeks | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.63 [0.70, 2.56] |
| 2 POCD defined by original studies ‐ 1 week Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Mild | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.95] |
| 2.2 Moderate | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.20, 1.04] |
| 2.3 Severe | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 0.92] |
| 3 POCD: decline in cognitive function ‐ 1 week Show forest plot | 6 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.04] |
| 4 Intraoperative mortality or postoperative mortality: Death Show forest plot | 3 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.08, 5.03] |
| 5 The occurrence of abnormal rScO2 during or after surgery: Desaturation Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 In OR | 7 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 5.2 In ICU | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.34] |
| 6 Any major non‐neurological complications as defined by individual study Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 6.2 Non‐specific respiratory complications | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.56] |
| 6.3 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 6.4 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 6.5 Pneumothorax | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.05] |
| 6.6 Postoperative acute pulmonary edema | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 3.92] |
| 6.7 Postoperative pulmonary embolism | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 Prolonged mechanical ventilation | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.54] |
| 6.9 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 6.10 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 6.11 Cardiac ischaemia | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.27] |
| 6.12 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 6.13 Reoperation for bleeding | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.77] |
| 6.14 MOMM | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.08, 0.95] |
| 6.15 Rupture of the colonic anastomosis | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.36, 2.83] |
| 6.16 Surgical reintervention | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.09] |
| 6.17 Patients experiencing more than 1 complication | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.56] |
| 6.18 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 6.19 Revision | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.25] |
| 6.20 Hospital stay > 7 days (% of patients) | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.48] |
| 6.21 Mediastinitis | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.09] |
| 6.22 Septicaemia | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.09] |
| 6.23 Unplanned HDU/ICU admission | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.08] |
| 7 Length of ICU stay (days) Show forest plot | 3 | 379 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.48, ‐0.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: Neurological injury Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Cardiac or great vessel surgery | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 4.09 [0.47, 35.88] |
| 1.2 Other surgery | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 2.37] |
| 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 1 week Show forest plot | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| 2.1 Cardiac or great vessel surgery | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 3.16 [0.98, 5.34] |
| 2.2 Other surgery | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 2.48 [0.85, 4.11] |
| 3 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks Show forest plot | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| 3.1 Cardiac or great vessel surgery | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 1.58 [‐0.10, 3.25] |
| 3.2 Other surgery | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 0.75 [‐0.21, 1.71] |
| 4 POCD defined by original studies ‐ 1 week ‐ mild Show forest plot | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.95] |
| 4.1 Cardiac or great vessel surgery | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.17, 0.79] |
| 4.2 Other surgery | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 0.97] |
| 5 POCD defined by original studies ‐ 1 week ‐ severe Show forest plot | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 0.92] |
| 5.1 Cardiac or great vessel surgery | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 2.25] |
| 5.2 Other surgery | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.03, 1.58] |
| 6 POCD: decline in cognitive function ‐ 1 week Show forest plot | 6 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.04] |
| 6.1 Cardiac or great vessel surgery | 4 | 671 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.19, 1.11] |
| 6.2 Carotid endarterectomy | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.42, 2.10] |
| 6.3 Other surgery | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 1 week Show forest plot | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| 1.1 INVOS | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 2.48 [0.85, 4.11] |
| 1.2 EQUANOX | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 3.16 [0.98, 5.34] |
| 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks Show forest plot | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| 2.1 INVOS | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 0.73 [‐0.13, 1.60] |
| 2.2 EQUANOX | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 2.38 [0.56, 4.20] |
| 3 POCD: decline in cognitive function ‐ 1 week Show forest plot | 6 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.04] |
| 3.1 INVOS | 5 | 862 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.04] |
| 3.2 EQUANOX | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any major non‐neurological complications as defined by individual study: including studies with detection bias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 1.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 1.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 1.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 1.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 1.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 1.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 2 Any major non‐neurological complications as defined by individual study: including studies without detection bias Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Non‐specific any reported complications | 5 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 0.99] |
| 2.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 2.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 2.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 2.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 2.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 2.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with missing data Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 week | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| 1.2 12 weeks | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| 2 POCD: decline in cognitive function ‐ 1 week: including studies with missing data Show forest plot | 6 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.04] |
| 3 Occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies with missing data Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 In OR | 7 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 3.2 In ICU | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.34] |
| 4 Any major non‐neurological complications as defined by individual study: including studies with missing data Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 4.2 Non‐specific respiratory complications | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.56] |
| 4.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 4.4 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 4.5 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 4.6 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 4.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 5 POCD: decline in cognitive function ‐ 1 week: without missing data Show forest plot | 5 | 781 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.31, 1.21] |
| 6 Occurrence of abnormal rScO2 during or after surgery: Desaturation: without missing data Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 In OR | 6 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 6.2 In ICU | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.34] |
| 7 Any major non‐neurological complications as defined by individual study: without missing data Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Non‐specific any reported complications | 5 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 0.99] |
| 7.2 Non‐specific respiratory complications | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.29, 3.45] |
| 7.3 Arrhythmia | 2 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.13, 5.12] |
| 7.4 Myocardial infarction | 3 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.13, 1.70] |
| 7.5 Wound infection | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.38, 2.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any major non‐neurological complications as defined by individual study: including studies with reporting bias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 1.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 1.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 1.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 1.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 1.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 1.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 2 Any major non‐neurological complications as defined by individual study: including studies without reporting bias Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Non‐specific any reported complications | 5 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 0.99] |
| 2.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 2.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 2.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 2.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 2.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 2.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with other bias Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 week | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| 1.2 12 weeks | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| 2 POCD defined by original studies ‐ 1 week: including studies with other bias Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Mild | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.95] |
| 2.2 Severe | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 0.92] |
| 3 Intraoperative mortality or postoperative mortality: Death: including studies with other bias Show forest plot | 3 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.08, 5.03] |
| 4 The occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies with other bias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 In OR | 7 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 4.2 In ICU | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.34] |
| 5 Any major non‐neurological complications as defined by individual study: including studies with other bias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 5.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 5.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 5.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 5.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 5.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 5.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 6 Length of ICU stay (days): including studies with other bias Show forest plot | 3 | 379 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.48, ‐0.09] |
| 7 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies without other bias Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 12 weeks | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 1.58 [‐0.10, 3.25] |
| 8 The occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies without other bias Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 In OR | 3 | 410 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.69, 1.13] |
| 9 Any major non‐neurological complications as defined by individual study: including studies without other bias Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Non‐specific any reported complications | 4 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.57, 1.68] |
| 9.2 Non‐specific cardiac complications | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.42, 3.44] |
| 9.3 Non‐specific renal complications | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.25] |
| 9.4 Arrhythmia | 2 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.53, 1.39] |
| 9.5 Myocardial infarction | 2 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.38, 2.20] |
| 9.6 Cardiac arrest | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 9.7 Wound infection | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.53, 1.76] |
| 10 Length of ICU stay (days): including studies without other bias Show forest plot | 2 | 179 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.39, ‐0.07] |

