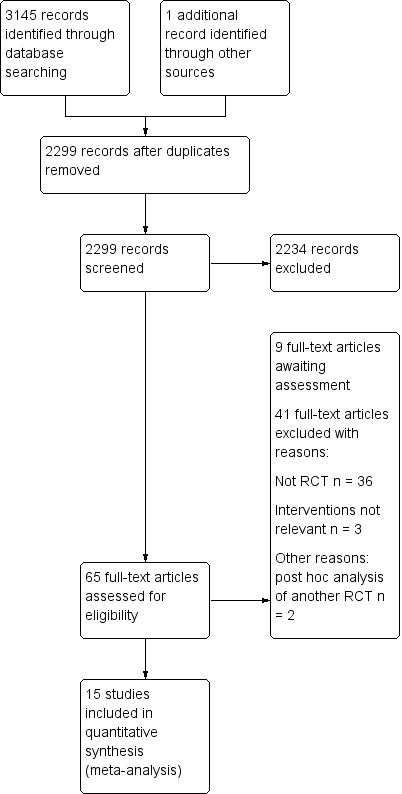
Espectroscopia del infrarrojo cercano (NIRS) cerebral para la monitorización perioperatoria de la oxigenación cerebral en niños y adultos
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: single‐centre, 2‐arm, parallel RCT Follow‐up: 52 weeks Settings: 2 UK regional teaching hospitals | |
| Participants | Total N randomized: N = 72 Surgery type: abdominal 12, orthopaedic 60 Age: mean 75.69 years, SD 7.40 (intervention group), mean 75.16 years, SD 6.51 years (control group) Inclusion criteria: elective major abdominal or orthopaedic surgery under general anaesthesia; over 60 years of age; ASA classification ≤ 3; MMSE score ≥ 23; adequate English Exclusion criteria: unable to complete the outcome measures; Alzheimer's disease or other dementia; undergoing surgical procedures under regional anaesthesia; delirium at 1 week post surgery | |
| Interventions | 2 arms: Intervention group (INVOS in OR): depth of anaesthesia and rSO2 were monitored in all participants using BIS and SICO respectively. Monitoring was performed in the OR throughout the procedure until extubation. An intraoperative management protocol was used to enable optimization of cerebral oxygen saturation (rSO2) during surgery: bring BP to within 10% of baseline value using fluids or inotropes. If there is no change, maintain SpO2 above 95% by increasing the percentage of inspired oxygen to 50%. If there is no change, end tidal carbon dioxide concentration increased to above 5.5%, avoiding excessive hypercarbia as well as hypocarbia. If there is no change, considering transfusion if the Hb level is less than 9 g.dl‐1 where there is ongoing moderate to severe haemorrhage. If all the above fail to correct a decline then increase the ETCO2 to 6% and increase the percentage of inspired oxygen to 100% N = 34 Device type: Somanetics Invos Cerebral Oximeter (SICO, Covidien inc, Co, USA) Control group: rSO2 data were collected, but the anaesthetist was blinded to the monitoring data N = 38 | |
| Outcomes | Postoperative stroke or other neurological injury: cognitive function ‐ MMSE, Vigilance Reaction Time, Trail Making, change data, at 1, 12 and 52 weeks postoperation Postoperative delirium (POD) or POCD: POCD at one, 12 and 52 weeks postoperation (see notes)* The occurrence of abnormal rScO2 during or after surgery: desaturation**, rSO2 below 50% during surgery ‐ Unable to use: Postoperative stroke or other neurological injury: S100; only r value and P values of the relative analysis were reported | |
| Notes | *Authors classified POCD as mild if there was a decline in performance in at least 1of the 7 cognitive domains (i.e. MMSE, simple reaction time, digit vigilance accuracy, digit vigilance reaction time, choice reaction time accuracy, choice reaction time and cognitive reaction time) by greater than 1standard deviation (SD); as moderate if there was an additional decline of at least 1.5 SD in an additional domain; and as severe if there was a decline of greater than 1.96 SD in at least 2 domains **rSO2 drop > 15% baseline. The authors stated that the incidence of rSO2 below 50% was 3.3% vs 17.1% in the intervention group vs the control group (1in the intervention group and 6 in the control group) (page 3; line 10‐13, right). We calculated the total number of participants in each group and deduced that there were 30 and 35 participants in intervention group and control group respectively in this outcome assessment Funding source: funded by the National Institute for Health Research (NIHR) Declarations of interest: Quote: "David Green has received honoraria and expenses for meetings organized by Covidien Inc, manufacturers of the BIS and Invos monitors. Keith Wesnes has a commercial interest in the computerized cognitive assessment battery used in the trial—the Cognitive Drug Research battery. None of the others have any conflicts of interest" Need to contact the author for further information: the result of S100 in each group Author contact details: Prof. Clive Ballard Wolfson Centre for Age‐Related Diseases, King’s College London, London, United Kingdom Trial registration: ISRCTN39503939 We contacted Prof. Clive Ballard by email to request the missing data and detailed information for the study, but we did not receive a reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation lists were generated by the study statistician in the statistical program package R..." (page 7, line 14‐15, left) Comment: the authors gave sufficient information on the generation of randomization |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed envelopes containing the randomization codes were delivered to operating theatres, and an envelope selected randomly by the anaesthetist. The randomization envelope was opened only after the participant's eligibility and willingness to participate were re‐confirmed prior to surgery." (page 7, line 16‐21, left) Comment: sealed envelopes were used to conceal allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The nested RCT trial was double‐blinded; patients and researchers collecting outcome data were blind to treatment allocation. Only the anaesthetist delivering the intervention was aware of the treatment condition." The anaesthesia providers were unlikely to be blind to the treatment condition (page 7, line 10‐13, left) Comment: blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...researchers collecting outcome data were blind to treatment allocation." (page 7, line 11‐12, left) Comment: blinding of outcome assessment ensured |
| Incomplete outcome data (attrition bias) | High risk | Quote: "At 1 week after surgery, 11 and 9 participants declined assessment in intervention group and control group respectively. At 12 week after surgery, 6 and 4 participants declined assessment in intervention group and control group respectively. At 52 weeks after surgery, 6 and 6 participants declined assessment in intervention group and control group respectively." (page 3; Figure 1) Comment: the attrition rates in both groups at 1 week after surgery were 35.3% (intervention group) and 23.7% (control group) respectively, at 12 weeks were 20.6% and 10.5% respectively, at 52 weeks were 17.7% and 15.8% respectively. The proportion of missing outcomes, compared with observed events, is likely to induce important bias in the intervention effect estimate. No reasons for missing data provided. |
| Selective reporting (reporting bias) | Low risk | Comment: the authors did not report the "Trial Making" data at 1 week postoperatively. The authors did not present the S100B data in intervention group and control group. Only the r value and P value for the relative analysis were reported, however, these are unlikely to have had an important influence on the estimate of effects on the prespecified outcomes of this review. |
| Other bias | High risk | Quote: "David Green has received honoraria and expenses for meetings organised by Covidien Inc, manufacturers of the BIS and Invos monitors. Keith Wesnes has a commercial interest in the computerized cognitive assessment battery used in the trial—the Cognitive Drug Research battery." (page 1, line 28, abstract) Comment: 2 authors of this paper may have had conflicts of interest in this study |
| Methods | Design: multicentre, 2‐arm, parallel RCT Follow‐up: not reported Settings: 5 university hospitals; the country is not reported | |
| Participants | Total N randomized: N = 131 Surgery type: scheduled for major abdominal, nonvascular surgery under general anaesthesia (with an expected duration > 2 hours) Age: mean ˜73 years, SD ˜5 years Inclusion criteria: "Patients older than 65 years, scheduled for major abdominal, nonvascular surgery under general anaesthesia (with an expected duration > 2 hours) were considered for the study." (page 741, line 32, left) Exclusion criteria: "Patients with pre‐existing cerebral pathology (such as previous episodes of cerebral ischaemia or stroke) and ASA physical status> = Ⅳ or a baseline Mini Mental State Examination (MMSE) test <=23 and patients whose follow‐up was not probable (not expected to be discharged alive from the hospital or with an expected hospital stay <4 days) were excluded." (page 741, line 40, left) | |
| Interventions | 2 arms: Intervention group (INVOS in the OR): the INVOS monitor in the OR was visible and anaesthesia management was aimed at maintaining rSO2 more than 75% of baseline values. In case of cerebral desaturation in the intervention group, the attending anaesthesiologist activated a 2‐step treatment: The first step included checking the ventilator, head position and tubing system, increasing FiO2, increasing end‐tidal CO2 partial pressure if the ETCO2 was < 35 mmHg, and increasing arterial blood pressure with intravascular fluid administration (250 mL hetastarch) and vasoconstrictors (ethylephrine 2 mg to 5 mg IV) if systolic arterial blood pressure was <= 90 mmHg. If the first step did not restore acceptable rSO2 values within 60 seconds, the second step included the reduction of brain oxygen consumption with an IV bolus of propofol (0.5 mg/kg) N = 63 Device type: Somanetics Invos Cerebral Oximeter (SICO, Covidien inc, Co, USA) Control group: in the control group the screen of the INVOS monitor was blinded and general anaesthesia was managed routinely maintaining arterial blood pressure and heart rate values within 20% of baseline values. N = 68 | |
| Outcomes | POCD: decline of cognitive function at the 7th day postoperation* (see notes) Postoperative stroke or other neurological injury: neurological injury Intraoperative mortality or postoperative mortality: death The occurrence of abnormal rScO2 during or after surgery: desaturation** (see notes), mean rSO2 Any major non‐neurological complications ‐ Unable to use: The occurrence of abnormal rScO2 during or after surgery: AUC rSO2< 50%, AUC rSO2< 75% Length of hospital stay: the authors did not report data for this outcome | |
| Notes | *A decrease in MMSE score of more than 2 points from baseline was also considered as an index of decline in cognitive function **Cerebral desaturation was considered to occur when rSO2 values decreased to < 75% of baseline values for 15 seconds. If baseline rSO2 was less than 50%, the threshold for defining cerebral desaturation was a reduction to less than 80% of baseline values Funding source: the study was entirely supported by funding of the 5 hospitals only Declarations of interest: not reported Need to contact the author for the setting information Author's contact information: Andrea Casati, MD, Department of Anesthesiology, Azienda Ospedaliera di Parma, Via Gramsci 14 – 43100 Parma Email: [email protected] (page 740 footnote) We contacted Dr. Andrea Casati by email to request detailed information for the study, but we did not receive a reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "According to a computer‐generated sequence of numbers and using a sealed envelope technique, patients were randomly allocated to two groups". (page 741, line 3, right) Comment: the authors gave sufficient information on the generation of randomization |
| Allocation concealment (selection bias) | Low risk | Quote: "According to a computer‐generated sequence of numbers and using a sealed envelope technique, patients were randomly allocated to two groups." (page 741, line 3, right) Comment: sealed envelopes were used to conceal allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "... discharge by the surgeons, ward nurses, and research fellows, who were blinded as to intraoperative management and patient grouping..." (page 742, line 26, left) Comment: blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "... discharge by the surgeons, ward nurses, and research fellows, who were blinded as to intraoperative management and patient grouping..." (page 742, line 26, left) Comment: research fellows maybe the outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | Quote: page 743, figure 1 Comment: 131 participants were randomized. 1 participant in the intervention group was excluded because of insertion of epidural catheter, 3 cases were excluded because of cancellation and another 3 cases were excluded because of technical failure In the control group, 1 participant was excluded because of insertion of epidural catheter and a further 1 case was excluded because of technical failure. 1participant in the control group died 20 days after surgery because of surgical complications (rupture of the clonic anastomosis) Comment: reasons for missing outcome data are unlikely to be related to the interventions |
| Selective reporting (reporting bias) | Unclear risk | Comment: we did not have enough information to confidently conclude the status of selective reporting |
| Other bias | Low risk | Quote: "The study was entirely supported by funding of the five hospitals only." (page 740, line 20, right) Comment: none obvious |
| Methods | Design: 2‐arm, parallel RCT Follow‐up: 6 participants in the INVOS group and 4 in the control group did not receive the allocated intervention after randomization (protocol violation). 9 participants (6 in the INVOS group and 3 in the control group) did not perform control cognitive test after surgery (lost to follow‐up) Settings: university hospital centre, Croatia | |
| Participants | Total N randomized: N = 200 Surgery type: on‐pump CABG surgery with the use of cardiopulmonary bypass Age: 61.9 ± 7.1 in the INVOS group; 63.4 ± 8.8 in the no monitoring group Aortic cross‐clamp time, intervention group: mean ± SD ˜63 ± 23 min, control group: 62 ± 26 min Inclusion criteria: "Two hundred participants between 40 and 80 years who underwent on‐pump CABG surgery and signed informed consent were included in the study" Exclusion criteria: "… significant carotid artery stenosis, previous stroke or head injury, seizure, psychiatric illness, decompensated heart failure (NYHA Ⅲ/Ⅳ), left ventricular ejection fraction less than 25%, emergency cardiac surgery, off‐pump CABG, severely impaired renal and liver function, who refuse to participate, reoperations, and dialysis" | |
| Interventions | 2 arms: Intervention group (INVOS in the OR): Quote: "Patients were randomized into the interventional INVOS group, in which rSO2 was maintained above 80% of patient’s baseline value during the operation…During surgery, patients involved in the INVOS group were monitored by cerebral oximetry using the INVOS system...All patients in the INVOS group were monitored with the INVOS system (INVOS 5100C; Somanetics Corp., Troy, MI, USA). The INVOS system uses NIRS for non‐invasive and continuous measurement of changes in cerebral oxygen saturation. The probes for INVOS cerebral oxygen monitoring were attached bilaterally on the patient's forehead overlying the frontal–temporal region to awaken patients who breathed oxygen by nasal catheter, just before induction of anaesthesia. A baseline regional cerebral oxygen saturation (rSO2) value for each side of the brain was determined a short time after probes were attached. The rSO2 values were displayed on a screen and recorded during the entire surgery. If the rSO2 during surgery decreased below 80% of baseline value or below 50% of absolute value, we responded with standardized interventions to maintain rSO2 above those values. These interventions involved measures to eliminate mechanical obstruction to cerebral flow (repositioning of head or bypass cannulae), to increase cerebral oxygen delivery (increasing FiO2, pCO2, mean arterial pressure, cardiac output or pump flow and haematocrit) or to reduce cerebral oxygen consumption (increasing of anaesthetic depth and reduction of temperature)". N = 100 Device type: INVOS system (INVOS 5100C; Somanetics Corp., Troy, MI, USA) Control group: Quote: "Patients in the CONTROL group were not monitored with cerebral oximetry and only standardized monitoring in cardiac surgery was utilized." N = 100 | |
| Outcomes | Postoperative stroke or other neurological injury: neurological deficit (coma, stupor, transient ischaemic attack (TIA), stroke) Postoperative delirium (POD) or POCD: postoperative cognitive impairment Postoperative delirium (POD) or POCD: postoperative delirium Myocardial infarction Atrial fibrillation Prolonged mechanical ventilation Haemodialysis Infection Revision Hospital stay > 7 days (% of patients) ICU length of stay (in days) | |
| Notes | Funding source: the financial support was provided by institutional sources, University of Zagreb Declarations of interest: Quote: "Conflict of interest: none declared" Author's contact information: Corresponding author: Dr. Z. Colak Department of Anesthesiology, Reanimatology and Intensive Care Medicine, University Hospital Center Zagreb, Kispaticeva 12, 10000 Zagreb, Croatia Tel: +385‐91‐5624189 Fax: +385‐1‐2367087 Email: [email protected] We contacted Dr. Zeljko Colak by email to request the missing data and detailed information for the study. He provided the reasons for dropouts and explained the differences between the full text and ClinicalTrials.org. Quote: "In our study, only patients who underwent coronary artery bypass grafting (CABG) with cardiopulmonary bypass were included. Of the 200 randomized patients, 10 of them (4 participants in Control group and 6 participants in INVOS group) were not analysed because they did not receive allocated surgical intervention (CABG), due to changes in intraoperative plan (e.g. additional valvular surgery). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All patients were randomly assigned into the INVOS group or the CONTROL group using a computerized random number generator" (page 488) Comment: using a computerized random number generator |
| Allocation concealment (selection bias) | Low risk | Quote: "After informed consent was obtained, an enclosed assignment in a sequentially numbered, opaque, sealed envelope was allocated to each patient" (page 488) Comment: an enclosed assignment in a sequentially numbered, opaque, sealed envelope was used |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The anaesthesiologists who performed anaesthesia and interventional protocol for maintaining of cerebral oxygenation were involved neither in data collection nor in cognitive test results of patients. Investigators who performed cognitive tests were blinded to patient’s allocation. Patients were also blinded to the allocation group" (page 488) Comment: the anaesthesiologists who performed anaesthesia and the interventional protocol for maintaining cerebral oxygenation were involved neither in data collection nor in the cognitive test results of patients. Patients were also blinded to the allocation of groups. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: the investigators who performed the cognitive tests were blinded to the patient's allocation |
| Incomplete outcome data (attrition bias) | High risk | Comment: of the 200 participants, only 181 (90.5%) had neurocognitive tests at 7 days after surgery. The authors did not report the reasons for dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: none obvious |
| Other bias | Low risk | Comment: none obvious |
| Methods | Design: 2‐arm, parallel RCT Follow‐up: no dropouts Settings: hospital in Victoria, Australia | |
| Participants | Total N randomized: N = 40 Surgery type: total knee or hip replacement or bowel resection surgery Age: mean (95% CI) 78.0 (75.4 to 80.5) years in the intervention group; 77.5 (75.6 to 79.5) in the control group Inclusion criteria: patients over the age of 70 years undergoing total knee or hip replacement or bowel resection surgery Exclusion criteria: emergency or unplanned surgery or the inability to provide informed consent for participation | |
| Interventions | 2 arms: Intervention group (INVOS in the OR): If the participant was randomized to the intervention group (Group I), the anaesthetist was able to monitor the ScO2 throughout the operation. The anaesthetists were instructed to maintain the ScO2 within 25% of the participant's baseline value, which was taken following induction of anaesthesia ... The anaesthetist was provided with a list of suggested methods to improve ScO2, such as avoiding obstruction of neck veins and optimizing mean arterial pressure, oxygen saturation, end‐tidal carbon dioxide and haemoglobin concentration. The use and timing of these interventions was left entirely to the choice of the anaesthetist ... to monitor the ScO2 throughout the operation. The anaesthetists were instructed to maintain the ScO2 within 25% of the participant's baseline value, which was taken following induction of anaesthesia ... At the conclusion of the operation, the cerebral oximetry monitor was turned off prior to leaving the operating theatre. N = 20 Device type: Covidien USA (Mansfield, MA) Control group: If the participant was randomized to the control group (Group C), the monitor was covered throughout the case. N = 20 | |
| Outcomes | Postoperative stroke or other neurological injury: postoperative stroke Postoperative mortality Postoperative acute myocardial infarction Postoperative cardiac arrest Postoperative acute pulmonary oedema Postoperative pulmonary embolism Postoperative acute renal failure Cerebral desaturation rates Length of hospital stay Wound infection Unplanned HDU/ICU admission Management of hypotension without cerebral oximetry reasons Total number of complications | |
| Notes | Funding source: Quote: "This study was supported by an equipment grant (device loan and sensors) from Covidien USA (Mansfield, MA) and also by an Australian and New Zealand College of Anaesthetists pilot trial grant." Declarations of interest: not reported Author's contact information: Corresponding author: Dr. Dean Cowie MB BS, FANZCA, Staff Specialist, Department of Anaesthesia, Austin Health, Heidelberg, Victoria, Australia Email: [email protected] We contacted Dr. Dean Cowie by email to request detailed information for the study, but we did not receive a reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were then randomised to one of two groups using a random number allocation system with permuted blocks" (page 311) Comment: using a random number allocation system with permuted blocks |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "All patients were blinded to group allocation, as were all investigators analysing the data" (page 312) Comment: all patients were blinded to group allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All patients were blinded to group allocation, as were all investigators analysing the data...complications were recorded by blinded investigators via daily visits to the patient on postoperative days 1 to 5, as well as a review of the patient records, pathology tests and radiology results" (page 312) Comment: outcomes were assessed blindly |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no participants left the study early during the period of study |
| Selective reporting (reporting bias) | Low risk | Comment: none obvious |
| Other bias | High risk | Comment: this study was supported by an equipment grant (device loan and sensors) from Covidien USA (Mansfield, MA) and also by an Australian and New Zealand College of Anaesthetists pilot trial grant |
| Methods | Design: 2‐arm, parallel RCT Period: not reported Follow‐up: not reported Settings: tertiary care centre specializing in cardiac surgery, Canada | |
| Participants | Total N randomized: 48 Surgery type: complex cardiac surgery with cardiopulmonary bypass (CPB), except for patients with planned circulatory arrest Age: mean 71.1 years, SD 7.9 years Cardiopulmonary bypass time: mean 119.3 min, SD 39.6 min Inclusion criteria: consecutive patients requiring complex cardiac surgery with CPB regardless of comorbidities, including following 3 situations: 1. High‐risk surgery (defined as redo surgery, adult congenital surgery, thoracic aortic surgery with and without circulatory arrest and combined procedures surgery) 2. Combined surgery, including coronary artery bypass graft and valvular surgery or multiple valvular surgery or valvular and aortic surgery 3. Patients with a perioperative risk estimation score > 15 using the Parsonnet score Exclusion criteria: patients under the age of 18; emergency surgery; first time CABG surgery; single‐valve surgery in patients with a perioperative risk estimation score < 15; patients with planned circulatory arrest (because the anaesthesiologists and surgeons insisted on the use of NIRS in these cases) | |
| Interventions | 2 arms: Intervention group: INVOS in the OR and ICU: rSO2 monitoring in the OR and ICU. The algorithmic approach published previously was followed to reverse significant decreases in rSO2 (Denault 2007). Device type: INVOS 4000 N = 23 Control group: rSO2 was recorded, but "NIRS values were hidden from the anesthesiologists" N = 25 | |
| Outcomes | The occurrence of abnormal rScO2 during or after surgery: desaturation* (OR and ICU) ‐ Unable to use: Duration of mechanical ventilation postoperatively: skewed data (we did not plan to observe this outcome in our protocol) ICU length of stay: skewed data; we present these data as 'other data' in the data analysis section Hospital length of stay: skewed data, we present these data as 'other data' in the data analysis section | |
| Notes | *Significant decreases in rSO2 values were defined as a decrease > 20% from baseline lasting 15 seconds or more Funding source: funded by a grant from the Research Foundation of the Anesthesiology Department of the Université de Montréal and the Foundation of the Montreal Heart Institute Declarations of interest: not reported Corresponding author: Dr. Alain Deschamps PhD, MD, Department of Anesthesiology, Montreal Heart Institute, Université de Montreal, 5000 Belanger Street, Montreal, Quebec H1T 1C8, Canada We contacted Dr. Alain Deschamps by email to request detailed information for the study, but we did not receive a reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomized into 2 groups..." (page 1260, line 13‐21, right) Comment: there was no further description of randomization methods, but we accept the authors' reporting as true and accurate |
| Allocation concealment (selection bias) | Unclear risk | Comment: there was insufficient information about allocation concealment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: there was insufficient information about blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: there was inadequate information about blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | Comment: there were no missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: the authors did not clarify the expected outcomes for the randomized pilot study in the methods section. However, they reported important outcomes such as the incidence of cerebral desaturation, ICU and hospital length of stay, and postoperative complications in the 2 study groups. |
| Other bias | Low risk | Comment: none obvious |
| Methods | Design: 2‐arm, parallel RCT Period: between April 2012 and October 2013 Follow‐up: 30 days postoperatively Settings: 8 Canadian centres; however, detailed information about these hospitals was not presented | |
| Participants | Total N randomized: 201 Surgery type: high‐risk surgery defined as combined surgery (coronary bypass plus valve replacement or repair), or multiple valve replacement and/or redo surgery Age: mean 71 years, SD 11.2 years Cardiopulmonary bypass time: mean 135.9 min, SD 54.2 min Aortic cross clamp time: mean 105.8 min, SD 42.4 min Inclusion criteria: age over 18 years, had a cumulative EuroScore II more than or equal to 10, and/or were undergoing high‐risk surgery defined as combined surgery (coronary bypass plus valve replacement or repair), or multiple valve replacement and/or redo surgery Exclusion criteria: unable to read French or English, undergoing off‐pump coronary artery bypass surgery, emergency surgery (i.e. less than 6 hours after diagnosis) or planned deep hypothermic circulatory arrest, acute endocarditis, or the presence of active delirium or encephalopathy | |
| Interventions | 2 arms: Intervention group: (In the OR and ICU): patients allocated to the intervention group had NIRS values displayed on the monitor. At an intervention threshold of a 10% decrease in rSO2 value relative to baseline for a duration exceeding 15 seconds, anaesthesiologists used an interventional algorithm to reverse desaturations Device type: research sites used 1 of the 3 Health Canada approved rSO2 monitoring devices for the study: FORE‐SIGHT (CAS Medical Systems Inc., USA; 1 site, 9% of participants), EQUANOX Classic 7600 (Nonin Medical Inc., USA; M, 2 sites, 28% of participants) and INVOS 5100C‐PB (Covidien, USA; 5 sites, 62% of participants) N = 102 Control group: patients allocated to the control group had cerebral oximetry probes applied to the forehead but did not have NIRS values displayed on the monitor (i.e. anaesthesiologists were blinded to rSO2 values). Anaesthesiologists relied on standard monitoring for the management of these cases N = 99 | |
| Outcomes | The incidence of decreases in rSO2 more than 20% of baseline in the first 12 hours in the ICU (or until tracheal extubation) Cerebral desaturation below 10% relative to baseline in the operating room ‐ Unable to use: Cerebral desaturation load (CDL) (%.min)* (see notes): skewed data; we present these data as 'other data' in the data analysis section Major organ morbidity and mortality (MOMM) score; a composite endpoint of stroke, renal failure requiring dialysis, prolonged mechanical ventilation for more than 48 hours, deep sternal wound infection, reoperation and death; length of hospital stay: for these outcomes, authors only reported data for participants without cerebral desaturations and for participants with cerebral desaturations in the control group and the intervention group. They did not provide data for all participants in the intervention group and the control group, respectively | |
| Notes | Funding source: funded in part by the Canadian Anesthesia Research Foundation (Toronto, Ontario, Canada); the Montreal Heart Institute Foundation (Montreal, Quebec, Canada); and the Anesthesiology Departments of the University of Manitoba (Winnipeg, Manitoba, Canada), Ottawa Heart Institute (Ottawa, Ontario, Canada), McMaster University (Hamilton, Ontario, Canada), University of Calgary (Calgary, Alberta, Canada), University of Alberta (Edmonton, Alberta, Canada), and the University of British Columbia (Vancouver, British Columbia, Canada) Declarations of interest: "Dr. Deschamps has received speaking honoraria for educational seminars on the use of cerebral saturation monitoring in cardiac surgery patients sponsored by the companies Nonin Medical Inc., Plymouth, Minnesota, and Covidien Inc. (now a part of Medtronic), Boulder, Colorado. Dr. Denault has received speaking honoraria for educational seminars on the use of cerebral saturation monitoring in cardiac surgery patients sponsored by Covidien Inc. (now a part of Medtronic). NONIN Medical Inc. provided equipment and *CDL, defined as the cumulative area under the curve of desaturation over time for decreases in rSO2 values below 20% relative to baseline Corresponding author: Dr. Deschamps Department of Anesthesiology, Montreal Heart Institute, 5000 rue Bélanger, Montreal, Quebec, Canada H1T 1C8 Email: [email protected]; [email protected] Trial registration: https://clinicaltrials.gov/ct2/show/NCT01432184 We contacted Dr. Alain Deschamps by email to request detailed information for the study, but we did not receive a reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed in a 1:1 ratio using a computer‐generated random number table with permuted random blocks stratified by hospital sites" Comment: a computer‐generated random number table was used to generate the random sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: there was insufficient information about allocation concealment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "...the ICU staff (blinded to group assignment)" Comment: the blinding of participants and other staff members was not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: there was inadequate information about blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Follow‐up to 30 days was successful in all 201 patients in the study" Comment: there were no missing data |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes were reported |
| Other bias | High risk | Quote: "Dr. Deschamps has received speaking honoraria for educational seminars on the use of cerebral saturation monitoring in cardiac surgery patients sponsored by the companies Nonin Medical Inc., Plymouth, Minnesota, and Covidien Inc. (now a part of Medtronic), Boulder, Colorado. Dr. Denault has received speaking honoraria for educational seminars on the use of cerebral saturation monitoring in cardiac surgery patients sponsored by Covidien Inc. (now a part of Medtronic). NONIN Medical Inc. provided equipment and sensors for one of the centres implicated in the study." Comment: there might be a conflict of interest, but we do not have enough information to determine |
| Methods | Design: 2‐arm, parallel RCT Period: not reported Follow‐up: not reported Settings: Inkosi Albert Luthuli Central Hospital, South Africa | |
| Participants | Total N randomized: N = 40 Surgery type: undergoing elective on‐pump coronary artery bypass graft surgery Age: mean 55.3 years, SD 9.7 years Cardiopulmonary bypass time: mean 133.3 min, SD 26.86 min Cross clamp time: mean 80.08 min, SD 17.78 min Inclusion criteria: age over 18 years, scheduled for elective on‐pump coronary artery bypass graft surgery and a preoperative haematocrit greater than 36% (haemoglobin > 12 g/dl) Exclusion criteria: ...pregnancy, history of stroke or persistent neurological residue, history of transient ischaemic attack (TIA), unilateral stenosis of carotid artery greater than 70%, bilateral stenosis of carotid artery greater than 50%, combined cardiac procedure, i.e. CABG plus heart valve replacement, left ventricular ejection fraction less than 40%, left main stem stenosis more than 70%, symptomatic chronic pulmonary disease requiring long term medication, renal insufficiency or anuric renal failure or creatinine above 1.5 mg/dl, HIV positive patients, patients in AF (atrial fibrillation), patients presenting with left ventricular thrombosis preoperatively, presence of aortic atheroma detected pre, intra or postoperatively | |
| Interventions | 2 arms: Intervention group: (INVOS in the OR): in the interventional group, rSO2 monitoring in the OR, intraoperative regional cerebral oxygen saturation (rSO2) monitoring was performed with active display and administration of the Murkin treatment interventional protocol (Murkin 2007) Device type: Somanetics INVOS model 5100c cerebral/Somanetics oximeter (Covidien, Midrand South Africa) N = 20 Control group: in the control group, regional cerebral oxygen saturation monitoring was not visible to the cardiovascular perfusionist operating the heart lung machine during cardiopulmonary bypass (blinded) N = 20 | |
| Outcomes | The occurrence of abnormal rScO2 during or after surgery: desaturation* time (see notes) ‐ Unable to use Postoperative stroke or other neurological injury: change in serum S100B: skewed data; we present these data as 'other data' in the data analysis section | |
| Notes | Funding source: not reported Declarations of interest: not reported *Cerebral desaturation was defined as a decrease in oxygen saturation values below 70% of baseline for more than 1 min Corresponding author: Dr. Jamila Kathoon Adam Department of Biomedical and Clinical Technology, Durban University of Technology, South Africa Tel.: +27 31 373 5291 Fax: +27 31 373 5295 We contacted Dr. Jamila Kathoon Adam by email to request detailed information for the study, but we did not receive a reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised into intervention and control groups, and the completeness of the randomisation process was checked statistically by comparison of baseline features between the two groups using t‐tests in the case of quantitative variables and Pearson's chi square tests or Fisher's exact tests for categorical variables" (page 70, line 55‐56, right page 71, line 1‐5, left) Comment: the authors reported that patients were randomly assigned to either group and the randomization produced balanced baseline characteristics |
| Allocation concealment (selection bias) | Low risk | Quote: "... using a sealed envelope system." (page 69, line 32, left) Comment: sealed envelopes were used to conceal allocation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: there was insufficient information about blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: there was insufficient information about blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes were reported |
| Other bias | Low risk | Comment: none obvious |
| Methods | Design: 2‐arm, parallel RCT Period: December 2013 to February 2015 Follow‐up: not reported Settings: in a tertiary healthcare centre, Turkey | |
| Participants | Total N randomized: N = 79 Surgery type: coronary artery bypass grafting (CABG) operation with asymptomatic carotid artery disease for whom no intervention is intended (carotid artery stenosis is between ≥ 50% and < 70% on Doppler ultrasonography (USG)) Age: NIRS group: mean 59.1 years, SD 9.4 years; no NIRS group: mean 61.2 years, SD 10.3 years Cardiopulmonary bypass time: NIRS group: mean 77.7 min, SD 28.3 min; no NIRS group: mean 78.6 min, SD 26.9 min Aortic cross clamp time: NIRS group: mean 48.8 min, SD 23.1 min; no NIRS group: mean 56.3 min, SD 25.8 min Inclusion criteria: participants who had coronary artery bypass grafting (CABG) operation with asymptomatic carotid artery disease for whom no intervention is intended (carotid artery stenosis is between ≥ 50% and < 70% on Doppler ultrasonography (USG)) Exclusion criteria:patients who had an additional procedure other than CABG, who had a ascending aortic atherosclerosis degree of ≥ 2, carotid artery stenosis ≥ 70% lesions on carotid Doppler USG, who had a low level of literacy, who had a clinical history of cerebrovascular attack, fit and who had psychiatric disorders | |
| Interventions | 2 arms: Intervention group (NIRS group) (INVOS in the OR): intraoperative near‐infrared spectroscopy was applied. The algorithm used was the standard that is suggested for the brain oxymetry use when there is a > 20% decrease in the rSO2 values of the NIRS group patients during CPB when compared to their initial values (Akpek 2008) Device type: INVOS 5100C; Somanetics Corp, Troy, MI, USA N = 43 Control group (no NIRS group): intraoperative near‐infrared spectroscopy was not applied N = 36 | |
| Outcomes | Postoperative cognitive function impairment (mild impairment; serious impairment) Intensive care and duration of hospital stay ‐ Unable to use: rSO2 parameters: the authors did not report these data | |
| Notes | Funding source: not reported Declarations of interest: no conflict of interest Corresponding author: Ibrahim Kara, MD Address: Adnan Menderes Cad., Saglık Sok., No: 195, Adapazarı, 54000, Sakarya, Turkey We contacted Dr. Ibrahim Kara by email to request detailed information for the study, but we did not receive a reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization list has been generated by the clinic head nurse and the patients were sent to the surgery in accordance with such list..." Comment: the authors mentioned use of a randomization list but did not provide the detailed methods of randomization; in this case we accepted the authors' reporting as true and accurate |
| Allocation concealment (selection bias) | Low risk | Quote: "… the randomization list was kept hidden until the study is concluded" Comment: the authors mentioned allocation concealment but did not state the methods of allocation concealment in detail; in this case we accepted the authors' reporting as true and accurate |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "... randomized, controlled and with a double blind working design ..." Comment: the authors stated that a double‐blind working design was used. However, the blinding of participants and other staff members was not clearly described. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Preoperative and postoperative cognitive test has been applied to all the patients by two perfusionists under the supervision of the neurologist in accordance with the randomization list of the head nurse and it has been evaluated by two observers by using double blind method" Quote: "... rSO2 changes of the patients during cardiopulmonary bypass (CPB) have been recorded in excel format in computer environment by the perfusionist and were evaluated by two observers with double blind method" Comment: blinding of outcome assessment ensured for the subjective outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Unclear risk | Comment: intraoperative rSO2 parameters were not reported |
| Other bias | Low risk | Comment: none obvious |
| Methods | Design: 2‐arm, parallel RCT Follow‐up: not reported Settings: hospital (detailed information was not given) | |
| Participants | Total N randomized: N = 25 Surgery type: aortic surgery Age: mean ± SD, intervention group: 60.5 ± 9.4 years; control group: 62.3 ± 11.5 years Inclusion criteria: 18 to 80 years; adult male and female patients 18 to 80 years of age scheduled for aortic surgery requiring deep hypothermic circulatory arrest (DHCA) and intention to use antegrade selective cerebral perfusion with or without retrograde cerebral perfusion (RCP) Exclusion criteria: adult male and female patients 18 to 80 years of age undergoing aortic surgery not scheduled for DHCA; patients with ejection fraction < 15%; pregnancy; prisoners; patients mentally impaired (screening criteria i.e. MMSE score ≤ 23); history of stroke | |
| Interventions | 2 arms: Intervention group (INVOS in the OR): INVOS cerebral oximetry monitoring Intervention will be initiated if rSO2 drops > 20% from baseline or rSO2 declines below 50% Sequence of interventions to increase cerebral oxygen saturation 1. Check head and cannula position 2. Increase mean arterial pressure 3. Increase pump flow 4. Increase systemic oxygenation 5. Increase PaCO2 > 45 6. Increase anaesthetic depth by increasing volatile anaesthetic or by administering propofol boluses 7. Consider PRBC transfusion for Hct < 21% N = 12 Device type: INVOS Somanetics Cerebral Oximeter (USA) Control group: blinded cerebral oximetry monitoring with no intervention in surgical procedures and anaesthesia without deviation from standard of care INVOS cerebral oximetry blinded monitoring with no deviation in surgical procedures or standard of care in anaesthesia N = 13 | |
| Outcomes | Adverse events Postoperative stroke or other neurological injury: Mini Mental State Examination (MMSE) | |
| Notes | This is an ongoing trial with results presented in ClinicalTrials.gov (NCT01149148) Funding source: the study was supported by the University of Michigan Declarations of interest: not reported Author's contact information: Corresponding author: Lau WC, MD Wei C. Lau, University of Michigan Health System Phone: 734 9369479 Email: [email protected] We contacted Dr. Wei C. Lau by email to request detailed information for the study, but we did not receive a reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: allocation was stated as randomized, but no further description provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: double‐blind (subject, caregiver, investigator) |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: double‐blind (subject, caregiver, investigator) |
| Incomplete outcome data (attrition bias) | High risk | Comment: 2 of 12 in the intervention group and 3 of 13 in the control group dropped out, but MMSE was analysed with 9 participants in each group |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes were reported |
| Other bias | Low risk | Comment: none obvious |
| Methods | Design: single‐centre, 2‐arm, parallel RCT Follow‐up: not reported Settings: not reported | |
| Participants | Total N randomized: N = 100 Surgery type: undergoing cardiac surgery using CPB Age: mean 38.05 years, SD 15.81 years AoX time: mean 65 min, SD 28.74 Inclusion criteria: patients undergoing cardiac surgery using CPB were selected for the study Exclusion criteria: patients with pre‐existing neuropsychiatric disorders, inability to correctly perform the neurocognitive tests and mini mental state examination (MMSE) scores of less than 23, were excluded from the study | |
| Interventions | 2 arms: Intervention group (Nonin Equanox in the OR): all participants received premedication with oral diazepam (0.1 to 0.2 mg/kg) Upon arrival in the operating room standard monitors were connected, including 5‑lead electrocardiogram, pulse oximeter, capnography and radial artery catheter. Prior to induction of anaesthesia, all the participants in both the groups had Nonin Equanox (model 7600) cerebral oximeter sensor in the OR. The interventions for desaturation included the following: repositioning of the head or perfusion cannulae; increasing arterial CO2 tension; increasing systemic arterial blood pressure; adjusting the pump flow rate; adjusting the anaesthetic depth; reduction of temperature; vasodilatation; increase in haematocrit. "We followed the algorithm proposed... " N = 50 Control group: rSO2 data were collected, but the anaesthetist was blinded to the monitoring data N = 50 | |
| Outcomes | Postoperative neurocognitive impairment*: 1week and 3 months postoperation (see notes) Postoperative neurocognitive impairment: MMSE, ASEM, 1week and 3 months postoperation Length of ICU stay Length of extubation (this outcome was not planned in our protocol) ‐ Unable to use: The occurrence of abnormal rSO2 during or after surgery: desaturation** (see notes): the authors did not report these data The occurrence of abnormal rSO2 during or after surgery: AUC, skewed data; we present these data as 'other data' in the data analysis section | |
| Notes | Funding source: none Declarations of interest: Quote: "Conflict of Interest: None declared" *A decrease in the MMSE and ASEM scores… (page 104, line 38, left; page 105 table 4). "Postoperative MMSE impairment was defined as a decrease in scores by more than 20% of the preoperative values" (page 103, line 21, right) "Postoperative ASEM impairment was defined as a decrease of scores to more than 30% of preoperative values." (page 103, line 41, right) **"Cerebral desaturation was defined as a decrease in saturation values below 80% of the baseline or an absolute value below 50% for one minute or longer." (page 103, line 40, left) Contact the author for further information: 1. Need to contact the author of the original study for more details about randomization, blinding 2. Desaturation rate of rSO2 in each group 3. Statistical tests used for continuous data 4. The person who measured the outcome Author's contact information: Correspondence: Dr. Mohandas BS, Department of Cardiac Anesthesia, Sri Jayadeva Institute of Cardiovascular Sciences and Research, Bannerghatta Road, Bangalore ‑ 560 069, Karnataka, India Email: [email protected] We contacted Dr. Mohandas by email to request detailed information for the study, but we did not receive a reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "100 patients were randomly allocated to...." (page 102, line 3, abstract) Comment: the authors mentioned randomization but did not state the methods; in this case we accepted the authors' reporting as true and accurate |
| Allocation concealment (selection bias) | Unclear risk | Comment: there was insufficient information about allocation concealment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: there was insufficient information about blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The extubation and ICU discharge were decided by the intensivists and they were blinded to the interventions carried out in the operating room for increasing the rSO2" (page 103, line 9, right) Comment: the blinding of other outcome assessment was not described |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | Unclear risk | Quote: "... the number of patients experiencing desaturation is not reported in each group." (page 104, table 1 and table 3) Comment: 1 secondary outcome is not reported |
| Other bias | Low risk | Comment: none obvious |
| Methods | Design: 2‐arm, parallel RCT Follow‐up: not reported Settings: preoperative clinic, Canada | |
| Participants | Total N randomized: N = 200 Surgery type: scheduled for primary elective coronary artery bypass (CAB) surgery with use of cardiopulmonary bypass (CPB) Age: mean 61.8 years, SD 9.3 years Clamp‐time: mean 59.4 min, SD 23.2 min Inclusion criteria: Age > 18 years, scheduled for primary elective CAB surgery with use of CPB Exclusion criteria: Patients were not routinely preoperatively screened for evidence of carotid artery disease | |
| Interventions | 2 study groups: Intervention group (Invos 5100 in the OR): active treatment with cerebral oximetry monitoring using NIRS bilaterally in the OR. A prioritized intraoperative management protocol was used to maintain rSO2 values at or above 75% of the baseline threshold. Device type: Invos 5100; Somanetics Corporation, Troy, MI N = 100 Control group: "...the screen was electronically blinded and continuously recorded after verification of adequate signal strength and baseline values were calculated post hoc by taking the average of data over 1 min, 3 min after beginning recording" N = 100 | |
| Outcomes | Postoperative stroke or other neurological injury: new onset stroke, within the first 30 days after surgery Intraoperative mortality or postoperative mortality: mortality, within the first 30 days after surgery The occurrence of abnormal rSO2 during or after surgery: incidence of prolonged desaturations during surgery*; mean rSO2, rSO2< AUC 75%, rSO2 < AUC 40% Myocardial infarction within the first 30 days after surgery Any major non‐neurological complications: cardiac (myocardial infarction, arrhythmia), renal failure requiring dialysis, mediastinitis, septicaemia, wound infection, major organ morbidity and mortality (MOMM) within the first 30 days after surgery Length of ICU stay ‐ Unable to use Readmission: this outcome was not planned in our protocol Length of hospital stay: skewed data; we present these data as 'other data' in the data analysis section | |
| Notes | *An index of the degree and duration of desaturations was determined by examining the incidence of prolonged desaturations where the AUC of rSO2 values < 70% of baseline was > 150% minutes duration (AUCrSO2 < 70% baseline > 150% min) Funding source: Quote: "Supported in part by Canadian Institutes of Health Research grant MOP37914, and a grant from Somanetics Corporation" Declarations of interest: Quote: "Dr. Murkin has received lecture/travel fees from neuromonitoring companies, including Somanetics, but has no stock equity, consulting agreements, or other financial interests in Somanetics. None of the other authors have any relevant disclosures." Corresponding author: Dr. J. Murkin Rm C3‐112, University Hospital Campus LHSC, 339 Windermere Rd, London, Ontario, Canada N6A 5A5 Email: [email protected] | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was by means of sealed opaque envelopes assigning treatment allocation and placed in computer‐generated random order which were drawn in sequence as each patient was enrolled in the study and were opened in the OR at the time of surgery." (page 53, line 224‐29, right) Comment: the authors gave sufficient information on the generation of randomization |
| Allocation concealment (selection bias) | Low risk | Quote: "... sealed opaque envelopes assigning treatment allocation..." (page 53, line 25‐26, right) Comment: sealed envelopes were used to conceal allocation |
| Blinding of participants and personnel (performance bias) | Low risk | The patients were anaesthetized during the surgery. Quote: "To maintain postoperative blinding, no study group identifiers were included with the patient or in the patients' charts. Postoperatively, all patients were transferred to an autonomous, protocol‐driven, "closed" ICU, under the exclusive care of ICU physicians without direct reference to the attending surgeons or anaesthesiologists" The anaesthesia providers were unlikely to be blind to the treatment condition (page 53, line 41‐47, left) Comment: blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "... data on perioperative complications were compiled and registered concomitantly by an independent blinded observer using the same variables" (page 53, line 59, left) Comment: blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The primary analysis undertaken was "intent‐to‐treat" without exclusion of any patients once randomization had occurred." (page 53, line 56‐57, right) "Technical failure resulted in loss of rSO2 data from the floppy discs of six patients, with resultant NIRS data from 194 patients for cerebral rSO2 analysis." (page 55, line 18‐21, left) Comment: only 3% of the participants were missing. The proportion of missing outcomes, compared with observed events, was not large enough to induce important bias in the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes were reported |
| Other bias | High risk | Quote: "Supported in part by Canadian Institutes of Health Research grant MOP37914, and a grant from Somanetics Corporation." (page 51, line 13, left) "Dr. Murkin has received lecture/travel fees from neuro monitoring companies, including Somanetics, but has no stock equity, consulting agreements, or other financial interests in Somanetics." (page 51, line 18, left) Comment: there might be a conflict of interest, but we do not have enough information to determine |
| Methods | Design: 2‐arm, parallel RCT Follow‐up: of the 240 participants, 202 (84%) had neurocognitive testing at 3 months. The authors did not report the reasons for dropouts. Settings: Morristown Memorial and Gagnon Heart Hospital, New Jersey | |
| Participants | Total N randomized: N = 240 Surgery type: scheduled for primary elective CAB surgery with use of cardiopulmonary bypass (CPB) Age: mean 64.33 years, SD 10.2 years Clamp‐time: mean 39.47 min, SD 13.6 min Inclusion criteria: Patients undergoing primary coronary artery bypass grafting using cardiopulmonary bypass Exclusion criteria: Pre‐existing neuropsychiatric disorders; inability to correctly perform the neurocognitive tests; mini mental state examination score of 23 or less; off‐pump coronary artery bypass grafting; unplanned concomitant intraoperative procedures (i.e. patent foramen ovale repair, mitral valve repair) and one 80‐year‐old male who expired on postoperative day 2 | |
| Interventions | 2 study groups: Intervention group (INVOS in the OR): participants had INVOS cerebral oximeter sensors placed bilaterally on the forehead During surgery, participants in the intervention group had rSO2 values displayed on a screen and recorded continuously during the entire procedure. Interventions to treat decreasing rSO2 included the following: repositioning of the head or perfusion cannulae; increasing arterial carbon dioxide tension, increasing systemic arterial blood pressure, adjusting pump flow rate or anaesthetic depth; reduction of temperature; vasodilation; or blood transfusion. To determine the order of intervention, the value farthest from acceptable range was modified first at the anaesthesiologist's discretion. Interventions were performed only in the operating room. Device type: INVOS 5100BTM; Somanetics Corp, Troy, MI N = 125 Control group: rSO2 was recorded; however, the values were not displayed and no specific treatments were employed to improve cerebral oxygenation N = 115 | |
| Outcomes | Postoperative cognitive decline* (see notes): prior to discharge and at 3 months postoperatively The occurrence of abnormal rScO2 during surgery: prolonged cerebral desaturation during surgery** (see notes) ‐ Unable to use Postoperative delirium: prior to discharge and at 3 months postoperatively. The authors did not report these data Any major postoperative complications the authors did not report these data Hospital stay: prolonged length of hospital stay*** (see notes). The authors did not report these data. | |
| Notes | *Cognitive decline was defined as a decline of 1standard deviation or more in performance on 1or more of the neuropsychologic tests **The rSO2 at 50% represents the average of observed intraoperative cerebral oxygen saturations. Prolonged rSO2 desaturation was defined as rSO2 score greater than 3000%‐second below a 50% saturation threshold ***Length of hospital stay > 6 days Funding source: study funding sources were not reported Declarations of interest: not reported Contact the author for further information: 1. The authors presented the data in percentages; the exact numbers are needed (gender; POCD prior to discharge; prolonged cerebral desaturation) 2. Other information is needed: i. The number of participants who did not complete the neurocognitive test at 3 months in the 2 study groups, respectively ii. The POCD and postoperative delirium data in the intervention group and control group, respectively iii. The incidence of "prolonged length of hospital stay" in the intervention group and control group, respectively iv. The incidence of "major postoperative complications" in the intervention group and control group, respectively v. The length of hospital stay (in days) in the intervention group and control group, respectively Author's contact information: James P. Slater Mid‐Atlantic Surgical Associates, 95 Madison Avenue, Morristown, NJ 07962 Email: [email protected] We contacted Dr. James P. Slater by email to request detailed information for the study, but we did not receive a reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on a table of random numbers. The first number was blindly chosen, subsequent assignments were sequentially dictated by the table. Even numbered patients were assigned to the intervention group and odd numbers to the control group." (page 37, line 12‐16, left) Comment: the authors gave sufficient information on the generation of randomization |
| Allocation concealment (selection bias) | Unclear risk | Comment: there was insufficient information about allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The patient was blinded to their group assignment." (page 37, line 16‐17, left) Comment: blinding of participants was ensured, and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: there was insufficient information about blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Of the 240 patients, 202 (84%) had neurocognitive testing at 3 months." (page 41, line 14‐15, right) Comment: the attrition rate was 16% and the authors did not report the reasons for dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes were reported |
| Other bias | Low risk | Comment: no obvious risk of bias |
| Methods | Design: 2‐arm, parallel RCT Follow‐up: not reported Settings: Clinical Department of Neurosurgery and Oncology of the Central Nervous System, Medical University of Lodz, Poland | |
| Participants | Total N randomized: N = 43 Surgery type: lumbar spine surgery Age: mean (95% CI), intervention group: 50.58 (40.32 to 60.84) years; control group: 49.22 (44.12 to 54.32) years Inclusion criteria: adult patients who qualified for surgical treatment of lumbar spondylosis in the Clinical Department of Neurosurgery and Oncology of the Central Nervous System, Medical University of Lodz, Poland in 2012 Exclusion criteria: patients with a history of neurological and psychiatric disorders which impair cognitive processes were disqualified from the study. These include previously established dementia, stroke, schizophrenia and depression. Individuals undergoing or with a history of treatment with hypnotics, antidepressants, anxiolytics and steroids were also excluded from the study. Also those who reported frequent alcohol consumption (above 50 g per day) and whose preoperative laboratory tests showed elevated GGT (gamma‐glutamyl transpeptidase) and macrocytosis with hyperchromia were disqualified | |
| Interventions | 2 arms: Intervention group (INVOS in the OR): monitored intraoperatively by means of NIRS cerebral oximetry (INVOS 5100, Somanetics Corporation, USA). A downward tendency of ScrO2 oximetry index (cerebral regional oxygen saturation) by more than 20% from the baseline may be a manifestation of a significant tissue hypoxia. The measured values of ScrO2 at the output in INVOS 5100 most frequently oscillated above 70 units. They focused mainly on improving the head positioning whenever ScrO2 declined by 20% from the baseline. When systemic pulse oximetry saturation (SpO2) declines were noted, observers improved the orientation of the pulse oximeter sensor. If this intervention was not effective the increase of FiO2 took place. Device type: Somanetics Invos Cerebral Oximeter (SICO, Covidien inc, Co, USA) N = 13 Control group: without NIRS monitoring N = 30 | |
| Outcomes | Episodes of ScrO2 reduction Duration of episodes of ScrO2 reduction Cognitive test: N‐back Test (NBT), Digit Span Test (DST) Adverse events | |
| Notes | Trial registration: RNN/556/08/KBe approval of the ethics committee at Medical University of Lodz, Poland Funding source: granted by Medical University of Lodz. Project number: 502‐03/7‐128‐03/502‐54‐004. No significant financial support for this work that could have influenced its outcome. Declarations of interest: Quote: "The authors declare that there are no known conflicts of interest associated with this publication" Author's contact information: Corresponding author: K. Nowakowska‐Domagała Email: [email protected] (T. Trafidło), [email protected] (T. Gaszynski), [email protected] (W. Gaszynski), [email protected] (K. Nowakowska‐Domagała) We contacted Dr. K. Nowakowska‐Domagała by email to request detailed information for the study, but we did not receive a reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the procedures the patients were randomized into two subgroups..." (page 24) Comment: there was no further description of randomization methods, but we accepted the authors' reporting as true and accurate |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no information provided about blinding |
| Blinding of outcome assessment (detection bias) | High risk | Comment: observers were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no missing data |
| Selective reporting (reporting bias) | High risk | Comment: all prespecified outcomes were reported, but the numbers of patients in the DST and NBT tests were different from the randomized numbers |
| Other bias | Low risk | Comment: none obvious |
| Methods | Design: 2‐group, parallel RCT Period: over 16 months, but dates were not reported Follow‐up: not reported Settings: in a tertiary care university hospital | |
| Participants | Total N randomized: 150 (sample size calculation was reported) Operation time: 249.9 ± 41.9 min (intervention group), 248.0 ± 59.2 min (control group) Inclusion criteria: patients undergoing elective cardiac surgery under cardiopulmonary bypass, with no age or ASA physical status classification limit Exclusion criteria: patients undergoing emergency or re‐do operations, combined cardiac‐carotid surgery and operations with minimal extracorporeal flow (surgery of the ascending aorta) or circulatory arrest; patients with haematologic disease (including anaemia requiring preoperative blood product transfusion), coagulation abnormality, advanced cirrhosis and renal dysfunction (creatinine > 50% upper limit of normal value) | |
| Interventions | 2 study groups: Intervention group (INVOS group in the OR): "In group A (INVOS), decisions were as follows: If mean INVOS value from both hemispheres was less than 60 regardless of baseline values, (criterion a) or INVOS decreased by 20% or more compared to the mean value during pulmonary artery catheter insertion (criterion b), the patient was candidate for transfusion, but was transfused only if hematocrit from the arterial blood‐gas analysis was indicating the need for transfusion (see below: indications in group B). Patients with low hematocrit values who did not meet the INVOS criteria (a or b, as described above) did not receive blood transfusions" Device type: INVOS 5100 device (Somanetics, USA) N = 75 Control group (without INVOS monitoring): "In group B (control group, no INVOS) transfusion decisions were based on hematocrit‐based rules as follows: During aortic cross‐clamp, allogeneic blood was not given if hematocrit was > 21%. For values ≤17%, one unit of RBC was transfused. When hematocrit was between 17‐21%, anaesthesiologists could decide based on their clinical judgment. After aortic clamp removal and before weaning from CPB (usually near the completion of the last proximal anastomosis or during cardiac reperfusion), RBCs were transfused for hematocrit less than 21%. After weaning from CPB and re‐transfusion of salvaged blood, patients were transfused for hematocrit ≤24%" N = 75 | |
| Outcomes | Postoperative mortality within 30 days of discharge from the hospital Postoperative complications ("defined as events that required some specific acute medical therapy or intervention resulting in prolonged (>9 days) hospital stay or death") Length of ICU stay Length of hospital stay | |
| Notes | 1centre (a tertiary care university hospital) Funding source: Quote: "supported solely by department funds" Declarations of interest: Quote: "All authors declare they have no conflict of interest to report" Corresponding author: Menelaos Karanikolas Address: Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri 63110, USA Email: [email protected] Trial registration: ClinicalTrials.gov NCT00879463 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Group assignment originated from a sequentially numbered sealed envelope containing a randomization code" Comment: randomization was implied |
| Allocation concealment (selection bias) | Low risk | Quote: "Group assignment originated from a sequentially numbered sealed envelope containing a randomization code" Comment: numbered and sealed envelopes were used to conceal allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "All personnel (including the surgical team, perfusionist, nursing and ICU personnel) involved in the care of these patients were blinded to group assignment." "However, the anaesthesiologist in charge of each case had access to the INVOS data and, obviously, was not blinded" Comment: we feel that an adequate level of blinding was applied |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "…all investigators who collected data were also blinded."... "Data were collected by blinded investigators…" Comment: blinded assessment was used |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "In total, 150 patients were enrolled, and there were no cases of missing data." Comment: no missing data |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcomes were reported |
| Other bias | Low risk | Comment: none obvious |
| Methods | Design: 2‐arm, parallel RCT Follow‐up: not reported Settings: 2 Greek institutions, Greece | |
| Participants | Total N randomized: N = 253 Surgery type: carotid endarterectomy (CEA) Age: mean ˜69.1 years, range: 48 to 82 years Operation time: not reported Inclusion criteria: Patients were American Society of Anesthesiologists physical status II‐III, aged 42 to 82 years who underwent carotid endarterectomy Exclusion criteria: not reported | |
| Interventions | 2 study groups: Group A (INVOS in the OR group): standard monitoring in all participants included electrocardiography (ECG), end tidal carbon dioxide (EtCO2), invasive blood pressure (IBP) and pulse oximetry (SpO2). In addition to the above mentioned monitoring, cerebral oximetry with near‐infrared refracted spectroscopy was used. participants in group A were managed according to the algorithm developed by Denault et al for participants undergoing cardiac surgery. Device type: INVOS 4100, Somanetics Inc., Troy MI N = 83 Group B (INVOS in the OR group): standard monitoring in all participants included ECG, EtCO2, IBP and SpO2. In addition to the above mentioned monitoring, cerebral oximetry with near‐infrared refracted spectroscopy was used. Cerebral oximetry values were recorded but anaesthesia management was not based on the aforementioned algorithm. Device type: INVOS 4100, Somanetics Inc., Troy MI N = 84 Group C (control group, without INVOS monitoring): participants in the third group (Group C) underwent routine CEA without INVOS monitoring and served as the control group. N = 86 | |
| Outcomes | Postoperative stroke or other neurological injury: neurologic deficits* (see notes) Any major non‐neurological complications: cardiac ischaemia ‐ Unable to use The occurrence of abnormal rScO2 during or after surgery: odds of rSO2; the data for the control group were not available | |
| Notes | Funding source: not reported Declarations of interest: Quote: "We have not received financial support (reimbursements, fees, funding, or grants) from an organization that may in any way gain or lose financially from the publication of this manuscript. We do not have any other financial competing interests" *Participants who exhibited new neurological deficits postoperatively that persisted for more than 24 hours underwent a follow‐up brain computed tomography (CT) scan; we used the data from group A to perform aggregation but not group B Need to contact the author: the method of blinding Author's contact information: Vassilios K Dimitriou, Associate Professor of Anesthesia, Head of Department of Anesthesia, "G Gennimatas" General Hospital of Athens, 154 Mesogion Avenue, 11527 Athens, Greece. Fax: +302132032212 Email address: [email protected] We contacted Dr. Vassilios K Dimitriou by email to request detailed information for the study, but we did not receive a reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated by using closed envelopes into three groups" (page 368, line 26, right) Comment: the authors did not describe the method of randomization, however we accepted the authors' reporting as true and accurate |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly allocated by using closed envelopes into three groups" (page 368, line 26, right) Comment: allocation concealment was done via closed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: there was insufficient information about blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: there was insufficient information about blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "2 out of the total 253 patients (0.8%), one in group B and one in group C, expired due to cardiovascular events, postoperatively" (page 371, line 23, left) Comment: the proportion of missing outcomes, compared with observed events, was not large enough to induce important bias in the intervention effect estimate |
| Selective reporting (reporting bias) | Low risk | Comment: all the prespecified outcomes were reported |
| Other bias | Unclear risk | Comment: none obvious |
AF: atrial fibrillation; ASA: American Society of Anesthesiologists; ASEM: antisaccadic eye movement test; AUC: area under curve; BIS: bispectral index; BP: blood pressure; CABG: coronary artery bypass grafting; CI: confidence interval; CO2: carbon dioxide; CPB: cardiopulmonary bypass; CT: computed tomography; DHCA: deep hypothermic circulatory arrest; DST: Digit Span Test; ECG: electrocardiography; epid: epidural; EtCO2: end tidal carbon dioxide; FiO2: fraction of inspired oxygen; g: gram; g.dl‐1: gram per decilitre; GGT: gamma‐glutamyl transpeptidase; Hb: haemoglobin; Hct: haematocrit; HDU: high dependency unit; HIV: human immunodeficiency virus; IBP: invasive blood pressure; ICU: intensive care unit; INVOS: one trademark of cerebral oximetry; IV: intravenous injection; Kg: kilogram; mg: milligram; min: minute; MOMM: major organ morbidity and mortality; mmHg: millimetres of mercury; MMSE: mini‐mental state examination; N: number; NBT: N‐back Test; NIHR: the National Institute for Health Research; NIRS: near‐infrared spectroscopy; NYHA: New York Heart Association; OR: operating room; pCO2: partial pressure of carbon dioxide; POCD: postoperative cognitive dysfunction; POD: postoperative delirium; PRBC: packed red blood cells; RBC: red blood cell; RCP: retrograde cerebral perfusion; RCT: randomized controlled trial; rSO2: regional cerebral oxygen saturation; S100: one biomarker of cerebral damage; ScrO2: local saturation of the cerebral cortex; SD: standard deviation; SICO: Somanetics Invos Cerebral Oximeter; SpO2: pulse oximetry saturation; TIA: transient ischaemic attack; vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This was a cohort study that was part of a RCT comparing early postoperative and neurodevelopmental outcomes after haemodilution to a haematocrit of 25% versus 35% during infant heart surgery. The authors failed to demonstrate a relationship between intraoperative cerebral oxygen saturation and early postoperative outcomes. | |
| This was a secondary analysis of data arising from a RCT of haemodilution to a haematocrit of 25% versus 35% during cardiopulmonary bypass in infants. The authors evaluated the correlation between intraoperative cerebral oxygen saturation and postoperative neurological outcomes at the age of 1 year. | |
| This was a post hoc analysis of a subset of participants in another RCT (Murkin 2007) and focused on patients with a preoperative diagnosis of diabetes mellitus. |
RCT: randomized controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Design: 2‐arm, prospective study Generation of allocation: not reported Allocation concealment: not reported Withdrawals: not reported Follow‐up: not reported Settings: not reported |
| Participants | Total N randomized: N = 80 Surgery type: elective shoulder surgery Age: not reported Inclusion criteria: ASA II‐IV patients scheduled for elective shoulder surgery Exclusion criteria: not reported |
| Interventions | 2 arms: Intervention group: "intravenous general anaesthesia group using a NIRS‐based protocol" Control group: "assessor‐blinded study according to clinical standard into a regional anaesthesia" |
| Outcomes | Postoperative cognitive function Intraoperative cerebral desaturation events |
| Notes | This is an abstract for a study; a full text is required, but there is no contact information for the corresponding author We tried to contact the relevant research group through their institutions and acquired Dr. Aguirre's email address ([email protected]). Dr. Aguirre replied that a preliminary report will be submitted shortly (not all parts were included) |
| Methods | Design: 2‐arm, parallel RCT Generation of allocation: not reported Allocation concealment: not reported Withdrawals: not reported Follow‐up: not reported Settings: not reported |
| Participants | Total N randomized: N = 97 Surgery type: patients undergoing primary coronary artery bypass grafting using cardiopulmonary bypass Age: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 2 arms: Intervention group: not reported N = 45 Device type: Somanetics Invos Cerebral Oximeter (SICO, Covidien inc, Co, USA) Control group: not reported N = 42 |
| Outcomes | ‐ Unable to use* ICU length of stay: median and IQR were reported; we will present these data as 'other data' in the data analysis section Hospital length of stay: median and IQR were reported; we will present these data as 'other data' in the data analysis section |
| Notes | This is an abstract for a study: a full text is required, but there is no contact information for the corresponding author We tried to contact the relevant research group through their institutions and acquired Dr. Baker's email address ([email protected]). We have not yet received a reply. |
| Methods | Design: multicentre, 2‐arm, parallel RCT Generation of allocation: randomized in blocks of varying size in a 1:1 ratio, stratified by centre and planned surgery; allocations are generated by computer in advance of starting recruitment Allocation concealment: concealed using an Internet‐based system; sealed envelopes Withdrawals: not reported Follow‐up: follow‐up on all study participants was completed in April 2014 Settings: Bristol Royal Infirmary in Bristol, Glenfield Hospital in Leicester and Castle Hill Hospital in Hull |
| Participants | Participants: aged over 16 years Total N randomized: N = 200 participants (100 per group) Surgery type: nonemergency valve or combined coronary artery bypass graft (CABG) and valve surgery using cardiopulmonary bypass (CPB) at mild hypothermia (32°C to 35°C) Age: not reported Inclusion criteria: adult (≥ 16 years) cardiac surgical patients undergoing nonemergency valve or combined CABG and valve surgery using CPB at the Bristol Royal Infirmary in Bristol, Glenfield Hospital in Leicester, or Castle Hill Hospital in Hull; given informed consent Exclusion criteria: patients undergoing emergency cardiac surgery; patients who are prevented from having blood and blood products according to a system of beliefs; patients who may have higher perioperative haemoglobin requirements or critical limb ischaemia; patients with congenital or acquired RBC, platelet, or clotting factor disorders; patients with a neurological disorder; patients with a diagnosed psychiatric disorder, drug, or alcohol addiction; patients with an already identified cognitive impairment as defined by psychometric assessment or a preoperative Mini Mental State Examination score of < 24; patients who have previously sustained a stroke, intracerebral haemorrhage, or acquired brain injury; patients with a pre‐existing inflammatory state; patients with end‐stage renal failure or patients who have undergone renal transplantation; patients unable to complete the cognitive assessments required for the trial (e.g., due to language difficulties, visual, or hearing impairment); patients who are unable to give full informed consent for the study; patients already participating in another clinical (interventional) study |
| Interventions | 2 arms: Intervention group: "Patient‐specific algorithm" (including a restrictive transfusion threshold): patient‐specific, goal‐directed algorithm based on the monitoring and optimization of regional cerebral oxygen saturation, combined with a predefined restrictive intraoperative haematocrit transfusion threshold of 18 Target regional oxygen saturation values are specified as 70% or more of pre‐induction values and an absolute value of 50% or more. However, if the regional oxygen saturation remains at or above these target values, but the haematocrit falls to 18, then RBC transfusion is indicated Device type: INVOS 5100 (Somanetics, Troy, MI, USA) Control group: "Generic algorithm" (including a standard transfusion threshold): based on global measures of oxygen utilization and including a predefined intraoperative haematocrit transfusion threshold of 23 |
| Outcomes | Primary outcome: cognitive function measured at 3 months after surgery Secondary outcome: units of RBC and other blood components transfused during the operative period and postoperative hospital stay; cerebral oxygenation during the operative period; oxygen delivery and utilization during CPB; EuroQol EQ‐5D‐3L (a generic health‐related quality of life instrument that measures mobility, self‐care, usual care, pain/discomfort and anxiety/depression) assessed at baseline and at 6 weeks and 3 months after surgery; length of CICU or high dependency unit (HDU) stay; length of postoperative hospital stay; clinical outcomes defined as infectious complications (sepsis and wound infection), stroke (validated by CT scanning), ST elevation myocardial infarction accompanied by troponin >5 ng/mL, postoperative acute kidney injury (defined as AKIN criteria stage 1, 2, or 3), and respiratory complications (i.e. reintubation, ventilation > 48 hours, tracheostomy, or acute respiratory distress syndrome); sepsis; stroke; cumulative resource use, cost and cost‐effectiveness; all‐cause mortality within 30 days of surgery; biochemical markers of organ injury |
| Notes | Funding source: sponsored by NIHR (Grant Reference Number RP‐PG‐0407‐10384) and the University Hospitals Bristol NHS Foundation Trust Declarations of interest: none declared Author contact details: Chris A Rogers, PhD Clinical Trials & Evaluation Unit, University of Bristol, Level 7 Queen's Building, Bristol Royal Infirmary, Bristol, BS2 8HW, United Kingdom Phone: 44 (0)117 342 2507 Fax: 44 (0)117 342 3288 Email: [email protected] Trial registration: http://www.isrctn.com/ISRCTN23557269 This is the protocol for a RCT and the results are to be published (early 2016) We tried to contact the corresponding author, but have not yet received a reply |
| Methods | Design: 2‐arm, parallel RCT Period: no more details in the abstract Generation of allocation: no more details in the abstract Allocation concealment: no more details in the abstract Withdrawals: no more details in the abstract Follow‐up: no more details in the abstract Settings: no more details in the abstract |
| Participants | Participants: aged over 64 years Total N randomized: N = 81 Surgery type: coronary artery bypass graft surgery Age: mean age ˜71.9 years Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 2 study groups: Intervention group: "aimed at a BiSpectral Index (BIS) of 50 ± 10 and standardized interventions were delivered if cerebral oxygenation (rSO2) dropped below 15% of the baseline or below 50%" N = unclear Control group: "blinded to BIS and rSO2" N = unclear |
| Outcomes | Postoperative delirium at 3 ± 1 days after surgery using the Confusion Assessment Method (CAM) |
| Notes | We need the full text for this study, but there is no contact information for the corresponding author We tried to contact the relevant research group through their institutions and acquired Dr. Gudrun Kunst's email address ([email protected]). We have not yet received a reply. |
| Methods | Design: 2‐arm, parallel RCT Period: not reported Generation of allocation: not reported Allocation concealment: not reported Withdrawals: not reported Follow‐up: not reported Settings: not reported |
| Participants | Total N randomized: N = 100 Surgery type: coronary artery bypass grafting (CABG) Age: not reported Inclusion criteria: patients between 18 and 65 years of age undergoing coronary artery bypass grafting (CABG) Exclusion criteria: not reported |
| Interventions | 2 arms: Intervention group: when a 20% decrease from baseline NIRS monitor was detected, a predefined intervention algorithm was used to treat desaturation Control group: "without any evidence of NIRS values, management was maintained in accordance with clinical practice and experience" |
| Outcomes | Postoperative neurocognitive function ICU length of stay Duration of total hospitalization |
| Notes | We need the full text for this study, but there is no contact information for the corresponding author We tried to contact the relevant research group through their institutions and acquired Dr. Murat Aksun's email address ([email protected]). We have not yet received a reply. |
| Methods | Design: 2‐arm, parallel RCT Generation of allocation: not reported Allocation concealment: not reported Withdrawals: not reported Follow‐up: not reported Settings: Otto‐von‐Guericke‐Universität Magdeburg |
| Participants | Total N randomized: N = 10 Surgery type: coronary artery bypass grafting (CABG) Age: median age was 68 (62 to 77) years Inclusion criteria: patients undergoing CABG at high risk of cerebrovascular events Exclusion criteria: patients with symptomatic carotid stenosis and/or previous cerebral infarctions |
| Interventions | 2 arms: Intervention group: "Received intraoperative frontal regional cerebral‐tissue oxygenation (rSO2) monitoring as well as appropriate measures to optimize rSO2 in case of desaturations" N = 5 Control group: no more details in the abstract N = 5 |
| Outcomes | Cognitive function on postoperative days 5 to 7, using the Montreal Cognitive Assessment (MoCA), Trail‐making Test (TMT A/B), Regensburg Word Fluency Test (RWT) and the Boston Naming Test (BNT) The rSO2 area under the curve (desaturations below 50% * time (min), mean value of left and right frontal channel) Length of hospital stay |
| Notes | This is a report of the preliminary results of an ongoing prospective study. We need to find the full text for this study. However, there is no contact information for the author. |
| Methods | Design: 2‐arm, parallel RCT Generation of allocation: not reported Allocation concealment: not reported Withdrawals: not reported Follow‐up: not reported Settings: not reported |
| Participants | Total N randomized: N = 98 Surgery type: coronary artery bypass (CAB) surgery Age: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 2 arms: Intervention group: regional cerebral oxygen saturation (rSO2) "... was visible and efforts to keep the rSO2 on levels ≥ 75% of preinduction value by sequentially increasing perfusion pressure, pump flow, PaCO2 (if < 35 mmHg), FiO2, decrease temperature (if > 37°C), increase PaCO2 > 45 mmHg, increase Hct (if < 20%)" N = 44 Control group: "... the monitor was covered and the patient was managed routinely" N = 54 |
| Outcomes | Neurological complications Length of stay (LOS) |
| Notes | We need to find the full text for this study. However, there is no contact information for the author. We tried to contact the relevant research group through their institutions and acquired Dr. Iglesias's email address ([email protected]). We have not yet received a reply. |
| Methods | Design: 2‐arm, parallel RCT Generation of allocation: computer‐generated randomization code in blocks of 4, aiming at participant allocation in a 1:1 ratio Allocation concealment: not reported Withdrawals: 1 patient from the intervention group died in the operating room Follow‐up: not reported Settings: a single‐centre study in the quaternary referral hospital in Toronto, Canada |
| Participants | Total N randomized: N = 250 (a total of 250 patients were randomly allocated, and 249 analysed. 1 patient from the intervention group died in the operating room) Surgery type: cardiac surgery with cardiopulmonary bypass Age: mean 74.2 years, SD 6.5 years (intervention group), mean 72.9 years, SD 6.3 years (control group) Inclusion criteria: patients ≥ 60 years of age, undergoing combined valve and coronary revascularization procedures, repeat cardiac surgery, multiple valve replacement or repair, or surgery of ascending aorta and aortic arch, with or without circulatory arrest Exclusion criteria: patients with a history of serious mental illness, delirium or who were planned to undergo either emergency or surgery without bypass |
| Interventions | 2 arms: Intervention group: "Bilateral NIRS sensors (INVOSTM 5100C; Covidien) were used to measure rScO2 intra‐operatively and postoperatively, up to 24 h in ICU" "an algorithm was commenced if regional cerebral oxygen saturation decreased below 75% of baseline value for 1 min or longer" N = 124 Control group:"the cerebral oximetry monitor screen was electronically blinded" N = 126 |
| Outcomes | Postoperative delirium for the first 7 postoperative days or until discharge (at 12‐hour intervals), using the confusion assessment method for ICU (CAM‐ICU) or confusion assessment method (CAM) Major adverse outcomes Duration of ICU and hospital length of stay All‐cause in‐hospital mortality |
| Notes | Correspondence author: G. Djaiani |
| Methods | Design: 2‐arm, parallel RCT Generation of allocation: blocked allocations with varying block sizes, generated by computer Allocation concealment: concealed using a secure password‐protected internet‐based randomization system Withdrawals: 3 patients withdrew and 1 patient died before surgery in the intervention group; of the 204 randomized patients, 194 were eligible for follow‐up at 3 months, and 175 completed the 3‐month questionnaire and attended for the neurocognitive assessment Follow‐up: 3 months after randomization Settings: 3 cardiac surgery centres in the UK |
| Participants | Total N randomized: N = 208 Surgery type: open valve or combined CABG and open valve surgery Age: 65.9 (18.5 to 86.6) years (intervention group), 70.0 (29.5 to 88.7) years (control group) Inclusion criteria: adult patients undergoing open valve or combined CABG and open valve surgery scored >= 24 on the Mini Mental State Examination (indicating no cognitive impairment) Exclusion criteria: patients with pre‐existing neurological disease or inflammatory states |
| Interventions | 4 patients withdrew before surgery. The analysis population therefore comprised 204 participants, 106 of whom were allocated to the control group and 98 to the intervention group 2 arms: Intervention group: "This was a patient‐specific, goal‐directed algorithm based on the monitoring and optimization of regional cerebral oxygen saturation measured using the INVOS 5000 NIRS device (Somanetics, IN, USA), combined with a predefined ‘restrictive’ intraoperative haematocrit transfusion threshold of 18%. Optimization of cerebral oxygenation used a modified Murkin protocol (see Supplementary Table S2) that aimed to maintain INVOS values at an absolute value of >50% or at >70% of baseline values obtained in the anaesthetic room before induction whilst breathing room air. If target cerebral oxygenation values were not achieved by modifying aspects of pump flow, gas exchange, or depth of anaesthesia as specified in the algorithm, red cells could be transfused above the 18% haematocrit threshold" N = 102 Control group:"This was a generic algorithm for optimizing tissue oxygenation based on global measures of oxygen utilization and including a predefined intraoperative haematocrit transfusion threshold of 23%" N = 106 |
| Outcomes | Cognitive function on or between 4 and 7 days after surgery and again at 3 months Biomarkers of the inflammatory response (serum interleukin (IL)‐6, IL‐8), brain (serum S100) and myocardial (serum troponin) injury Kidney injury (serum creatinine and calculated creatinine clearance, and urine biomarkers of inflammation (neutrophil gelatinase associated lipocalcin (NGAL), liver‐fatty acid binding protein (L‐FAB)) and tubular epithelial injury (kidney injury molecule‐1 and IL‐18)) Clinical outcomes, resource use and quality of life |
| Notes | Funding: National Institute for Health Research (NIHR) Programme Grants for Applied Research (grant HTA: RP‐PG‐0407‐10384 for The PASPORT Trial); Leicester and Bristol NIHR Cardiovascular Biomedical Research Units (The PASPORT Trial); British Heart Foundation (RG/13/6/29947 and CH/12/1/29419 to G.J.M.) Clinical trial registration. http://www.controlled‐trials.com, ISRCTN 23557269 Corresponding author: GJ Murphy Email: [email protected] |
| Methods | Design: 2‐arm, parallel RCT Generation of allocation: no more details in the abstract Allocation concealment: no more details in the abstract Withdrawals: no more details in the abstract Follow‐up: no more details in the abstract Settings: no more details in the abstract |
| Participants | Participants: aged over 60 years Total N randomized: N = 38 N randomized to intervention group: 17 Surgery type: elective coronary artery bypass graft surgery Age: no more details in the abstract Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 2 study groups: Intervention group: "handled according to NIRS values" N = 17 Control group: "treated under conventional monitoring modalities (blood pressures, SpO2, etc.)" N = 21 |
| Outcomes | Postoperative cognitive dysfunction (POCD) at 1 week and 3 months after surgery |
| Notes | We need the full text for this study, but there is no contact information for the corresponding author We tried to contact the relevant research group through their institutions and acquired Dr. Emre Camci's email address ([email protected]). We have not yet received a reply. |
| Methods | Design: 2‐arm, parallel RCT Period: not reported Generation of allocation: not reported Allocation concealment: not reported Withdrawals: a cognitive function test was performed in 92 and 78 participants postoperatively at 3 and 6 months, respectively Follow‐up: 6 months Settings: not reported |
| Participants | Total N randomized: N = 125 Surgery type: elective cardiac surgery requiring cardiopulmonary bypass (CPB) Age: no more details in the abstract Duration of surgery: 296 (263to 345) min in the intervention group; 308 (258 to 371) min in the control group Inclusion criteria: adult patients (> 18 years) who underwent elective cardiac surgery requiring CPB Exclusion criteria: patients with severe preoperative cognitive impairment or end‐stage organ failure |
| Interventions | 2 study groups: Intervention group: an intervention algorithm was used to improve regional cerebral tissue oxygen saturation (SctO2) if desaturation occurred < 60 for > 1 min at either probe N = 59 Control group: the SctO2 data were hidden from the perioperative team, unless a critical low value, SctO2 < 40 for > 1 min triggered an alarm N = 66 |
| Outcomes | Postoperative cognitive function (at 3 and 6 months) Intraoperative desaturation |
| Notes | This is an abstract for a study. We need the full text of this report. However, there is no contact information provided. We tried to contact the relevant research group through their institutions and acquired Dr. David L Reich's email address ([email protected]). We have not yet received a reply. |
| Methods | Design: 2‐arm, parallel RCT Generation of allocation: randomized; no more details in the abstract Allocation concealment: no more details in the abstract Withdrawals: no more details in the abstract Follow‐up: no more details in the abstract Settings: no more details in the abstract |
| Participants | Total N randomized: N = 44 Surgery type: off‐pump coronary artery bypass grafting (CABG) Age: no more details in the abstract Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | 2 study groups: Intervention group: regional cerebral oxygen saturation (SrO2) was actively monitored and not allowed to decrease more than 20% N = unclear Device type: Invos Control group: the anaesthesiologist and surgeon were blinded to the values of the SrO2 N = unclear |
| Outcomes | ‐ Unable to use Desaturation of regional cerebral oxygen saturation (SrO2): the authors did not report the data Length of ICU stay: mean and standard deviation were reported, but the authors did not report the number of participants in each group Length of hospitalization: mean and standard deviation were reported, but the authors did not report the number of participants in each group |
| Notes | We need the full text of this report. However, there is no contact information provided. We tried to contact the relevant research group through their institutions and acquired the email address of the Anaesthesiology Department ([email protected]). We have not yet received a reply. |
BIS: bispectral index; CAM: confusion assessment method; CABG: coronary artery bypass grafting; Hct: haematocrit; ICU: intensive care unit; IQR: interquartile range; mmHg: millimetres of mercury; N: number; NIRS: near‐infrared spectroscopy; PaCO2: partial pressure of carbon dioxide; POCD: postoperative cognitive dysfunction; RCT: randomized controlled trial; rSO2: regional cerebral oxygen saturation; SrO2: regional cerebral oxygen saturation; vs: versus
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Prospective evaluation of cognitive outcomes after anaesthesia on patients in the beach chair position (BCP) |
| Methods | Randomized, parallel‐group, controlled trial (double‐blind) |
| Participants | Enrollment: 90 Inclusion criteria: 18 to 75 years of age; elective shoulder surgery in the beach chair position |
| Interventions | Intervention group: anaesthesiologist treats based on near‐infrared spectroscopy (NIRS); NIRS monitor allows anaesthesiologist to treat cerebral desaturations according to NIRS results while maintaining mean arterial pressure (MAP) at least 60 mmHg or at least 80% of baseline Control group: anaesthesiologist blinded to NIRS |
| Outcomes | Primary outcomes: number of cerebral desaturation events; cerebral desaturation event while undergoing elective ambulatory surgery in the beach chair position Secondary outcomes: cognitive decline measured by a drop in Mini Mental State Exam (MMSE) > 2 or a score of 23 (up to 2 weeks) |
| Starting date | October 2012 |
| Contact information | George K Bal, MD, West Virginia University, Morgantown, West Virginia, United States, 26506‐9196 |
| Notes | ClinicalTrials.gov identifier: NCT02674334 Recruiting status: completed https://clinicaltrials.gov/ct2/show/study/NCT02674334 We failed to contact Dr. George K Bal because the email address was not available |
| Trial name or title | Role of cerebral oximetry in reducing delirium after complex cardiac surgery |
| Methods | Randomized, parallel‐group, controlled trial (double‐blind) |
| Participants | Inclusion criteria: adults > 60 years; combined valve and CABG; repeat cardiac surgery; multiple valve replacement or repair; surgery of ascending aorta and aortic arch; signed informed consent Exclusion criteria: cardiac surgery without the use of cardiopulmonary bypass; symptomatic cerebrovascular disease; history of delirium; schizophrenia Estimated enrolment: 266 |
| Interventions | Intervention group: cerebral oximetry monitoring (the INVOS® Cerebral/Somatic Oximeter) intraoperatively and during the 24‐hour postoperative period in the ICU; if the threshold of < 75% from baseline rSO2 value is reached for > 1 minute an algorithm geared to restore rSO2 to baseline levels will be implemented Control group: "NIRS monitor screen will be electronically blinded" |
| Outcomes | Primary outcomes: number of participants who suffer from delirium postoperatively assessed postoperatively for 7 days or until discharge |
| Starting date | December 2011 |
| Contact information | George Djaiani, MD, University Health Network, Toronto General Hospital, Toronto, Ontario, Canada, M5G 2C4 Tel: 416‐340‐4800 ext 6205 Email address: [email protected] |
| Notes | ClinicalTrials.gov identifier: NCT01707446 Recruiting status: this study is ongoing, but not recruiting participants http://clinicaltrials.gov/ct2/show/study/NCT01707446 We sent an email to Dr. George Djaiani to check if this ongoing study was included in the Deschamps 2016 multicentre trial. The reply was that this study was not included in Deschamps 2016. We contacted Dr. George Djaiani to request detailed information for the study. The reply was that this study is finished and they are planning to analyse the data soon. |
| Trial name or title | Role of absolute cerebral oximetry to prevent neurocognitive injury in elderly patients undergoing cardiac surgery |
| Methods | Randomized, parallel‐group, controlled trial (double‐blind) |
| Participants | Inclusion criteria: adults > 65 years; elective cardiac or thoracic aortic surgery; capable and willing to consent; literate in English Exclusion criteria: emergency surgery; major neurological disease; gross cognitive dysfunction; patients not expected to be able to complete the 1 week and 3 months postoperative visit Estimated enrolment: 120 |
| Interventions | Intervention group: once the cerebral desaturation (SctO2 < 60% for 5 minutes) is established, "the study personnel will attempt to optimize the level of oxygen within the brain of the study patients." Control group: no intervention even if the SctO2 falls below 60% |
| Outcomes | Primary outcomes: postoperative delirium and postoperative cognitive dysfunction during the first 5 days after surgery Secondary outcomes: postoperative morbidity and mortality at 3 months postoperatively |
| Starting date | September 2009 |
| Contact information | Gregory Fischer, MD, Mount Sinai School of Medicine, New York, United States, 10029 Tel: 212‐241‐7749 Email address: [email protected] Dionne Bobb, M.S, CCRC Email address: [email protected] |
| Notes | ClinicalTrials.gov identifier: NCT00991328 Recruiting status: recruiting http://clinicaltrials.gov/ct2/show/study/NCT00991328 We contacted Dr. Gregory Fischer by email to request detailed information for the study, but we did not receive a reply. |
| Trial name or title | Role of cerebral oximetry in reducing postoperative morbidity following cardiac surgery |
| Methods | Randomized, parallel‐group, controlled trial (single‐blind) |
| Participants | Estimated enrolment:120 Inclusion criteria: high‐risk cardiac surgery patients as determined by at least one of the following: age greater than or equal to 75 years on the day of screening; left ventricle ejection fraction less than 35%; use of a preoperative intraaortic balloon pump; combined valve and coronary artery surgery or multiple valve surgery in patients who have congestive heart failure, or renal insufficiency (creatinine clearance < 60 ml/min) Exclusion criteria: refusal of consent |
| Interventions | Intervention group: Cerebral NIRS monitoring by means of FORE‐SIGHT Universal Cerebral Oximeter MC‐2030C Predefined protocol of interventions for correcting rSO2 desaturation (< 60%) during cardiac surgery and the first 6 hours after it In case of rSO2 decrease less than 60% correct: head position; position of aortic,venous cannulae and central venous catheters; partial pressure of carbon dioxide in arterial blood < 35 mmHg; mean arterial pressure < 60 mmHg; central venous pressure > 10 mmHg; cardiac index < 2.0 l/min/m2; mixed venous oxygen saturation < 60%; haemoglobin < 65 g/L during cardiopulmonary bypass or haemoglobin < 90 g/L after cardiopulmonary bypass; decrease cerebral O2 consumption Control group: standard treatment |
| Outcomes | Primary outcomes: incidence of major organ morbidity and mortality including stroke, acute kidney injury requiring dialysis, mechanical ventilation more than 48 hours, mediastinitis, reoperation and death (up to 30 day after randomization); duration of intensive care unit stay; duration of postoperative hospital stay; death from all causes at 30 days Secondary outcomes: incidence of intraoperative desaturation episodes (desaturation is defined as level of rSO2 less than 60%); severity of intraoperative desaturation episodes (severity is defined as the product of length of time and depth of rSO2 less than 60%) |
| Starting date | June 2014 |
| Contact information | Evgeny V Fominskiy, MD, PhD, Meshalkin Research Institute of Pathology of Circulation Tel: +79139538754 Email: [email protected] |
| Notes | ClinicalTrials.gov identifier: NCT02155868 Recruiting status: recruiting https://clinicaltrials.gov/ct2/show/NCT02155868 We contacted Dr. Evgeny V Fominskiy by email to request detailed information for the study. The reply was that they have just finished enrolling patients and are making the follow‐up. The final results will be ready approximately in March 2017. |
| Trial name or title | Reversing cerebral oxygen desaturations greater that 10% of baseline values using NIRS in the ICU (NIRS ICU) |
| Methods | Randomized controlled trial (single‐blind) |
| Participants | Inclusion criteria: adults > 18 years; patients undergoing cardiac surgery employing CPB Exclusion criteria: patients having DHCA or aorta procedures Estimated enrolment: 50 |
| Interventions | Intervention group: "NIRS derived cerebral oximetry device (EQUANOX) used and the caregiver in the ICU will see the data in order to guide the use of the interventional algorithm to treat the cerebral desaturation" Control group: "NIRS derived cerebral oximetry device (EQUANOX) used but data not visible to ICU caregivers" |
| Outcomes | Primary outcomes: incidence of postoperative cerebral desaturation at 24 hours or unit discharge; endothelial function, delirium and any adverse events by phone interview on postoperation day 30 |
| Starting date | May 2013 |
| Contact information | Hilary P Grocott, MD, Department of Anesthesia and Surgery, University of Manitoba, St. Boniface Hospital Winnipeg, Manitoba, Canada, R2H 2A6 Tel: 204‐258‐1085 Email address: [email protected] John R McVagh, MA Tel: 204‐258‐1380 Email address: [email protected] |
| Notes | ClinicalTrials.gov identifier: NCT01875055 Recruiting status: recruiting http://clinicaltrials.gov/show/NCT01875055 We have sent an email to Dr. Hilary P Grocott to check if this ongoing study was included in the Deschamps 2016 multicentre trial. The reply was that this study was not included in Deschamps 2016. We contacted Dr. Hilary P Grocott by email to request detailed information for the study. The reply was that the study has not yet been submitted for publication. |
| Trial name or title | Cerebral oxygen directed perioperative anaesthesia management and postoperative delirium in elder patients having oesophageal cancer surgery |
| Methods | Randomized, parallel‐group, controlled trial |
| Participants | Inclusion criteria: adults > 70 years; given informed consent; fluent in Chinese without serious hearing or vision impairments; scheduled for oesophagectomy; Geriatric Depression Scale score < 5; no history of neuropsychiatric disorders, alcoholism, substance abuse or intake of psychotropic medications Exclusion criteria: registered in other clinical trials; neurological and psychotic disorders; taking cholinesterase inhibitors, haloperidol or other atypical antipsychotics; diagnosed with stroke, myocardial ischaemia or heart failure in recent 6 months; contraindicated to haloperidol; head skin injury, infections or ulcers which preclude the attachment of electrodes; prolonged corrected QT (QTc) interval of 460 ms or higher for men and 470 ms or higher for women Estimated enrolment: 300 participants (150 in each arm) |
| Interventions | Intervention group: rScO2 and BIS‐guided intraoperative anaesthesia Control group: BIS‐guided intraoperative anaesthesia |
| Outcomes | Incidence of postoperative delirium; time to the onset of postoperative delirium; duration of postoperative delirium; severity of postoperative delirium; MMSE score changes; incidence of ICU mechanical ventilation; duration of ICU mechanical ventilation; length of ICU stay; length of hospital stay; incidence of other postoperative complications; Postoperative Quality Recovery Scale; Postoperative Morbidity Survey Scale; mortality rate |
| Starting date | November 2013 |
| Contact information | Sun Haijing, Dept. of Anesthesiology, Changzheng Hospital, No. 415 Fengyang Road, Shanghai 20003 China Tel: +86 13816364200 Email address: [email protected] Shi Xueyin, Dept. of Anesthesiology, Changzheng Hospital, No. 415 Fengyang Road, Shanghai 20003 China Tel: +86 13601682827 Email address: [email protected] |
| Notes | Chinese Clinical Trial Registry identifier: hiCTR‐TRC‐13003800 Recruiting status: pending http://www.chictr.org/cn/proj/show.aspx?proj=5585 We contacted Dr. Shi Xueyin by email to request detailed information for the study. The reply was that the study has not yet finished. |
| Trial name or title | Medico‐economic evaluation of preoperative cerebral oximetry monitoring during carotid endarterectomy (EMOCAR) |
| Methods | Randomized, parallel‐group, controlled trial (double‐blind) |
| Participants | Inclusion criteria: adults > 18 years; internal carotid stenosis requiring surgery; Mini Mental State Examination > 24 during preoperative examination; informed written consent. Exclusion criteria: severe renal failure or requiring dialysis; liver failure or cirrhosis (Child class ≥ B) or prothrombin activity < 50%; heart failure (NYHA ≥ III), left ventricular ejection fraction < 40%, acute coronary syndrome; associated surgery; pregnancy; contraindication to MRI; history of allergy to modified gelatine or starch; history of allergy to adhesive part of electrode Estimated enrolment: 978 |
| Interventions | Intervention group: Quote: "Continuous perioperative cerebral oximetry monitoring (using INVOS™ cerebral oximeter) associated with haemodynamic optimisation algorithm (excluding norepinephrine) if cerebral oximetry decrease more than 15% under the preoperative baseline." Control group: Quote: "Continuously monitored with cerebral oximeter but this latter is blinded to the medical team, the alarm switch off, and patients are managed with the standard care of the centre" |
| Outcomes | Primary outcomes: incidence of new cerebral ischaemic lesions up to 1 month postoperatively Secondary outcomes: incremental cost‐effectiveness ratio at 4 months postoperatively; hospitalization length of stay and direct medical costs at 4 months postoperatively; neurological and neurocognitive postoperative disorders at 1 month postoperatively; postoperative quality of life (SF36, EQ5D tests) at 4 months postoperatively; cerebral desaturation threshold assessment at 4 months postoperatively |
| Starting date | April 2011 |
| Contact information | Yann Le Teurnier, MD, Nantes University Hospital, Nantes, France, 44000 Tel: +33240165304 Email address: yann.leteurnier@chu‐nantes.fr Bertrand Rozec, MD, Nantes University Hospital, Nantes, France, 44000 Tel: +33240165308 Email address: bertrand.rozec@chu‐nantes.fr |
| Notes | ClinicalTrials.gov identifier: NCT01415648 Recruiting status: this study has been terminated (recruitment time expired) http://clinicaltrials.gov/show/NCT01415648 We contacted Dr. Yann Le Teurnier by email to request detailed information for the study. The reply was that the study has not yet been finished. |
| Trial name or title | Measuring and treating brain oxygen levels in open heart surgery |
| Methods | Randomized, parallel‐group, controlled trial (single‐blind) |
| Participants | Inclusion criteria: adults > 18 years; patients scheduled to undergo elective cardiac or thoracic aortic surgery requiring cardiopulmonary bypass Exclusion criteria: severe preoperative cognitive impairment (i.e. dementia or developmental intellectual disability); sensory or motor impairment that would preclude reliable operation of a computer and keyboard; lack of access to use computer‐based cognitive evaluation; non‐English speaking patients; renal failure requiring dialysis; respiratory failure requiring home oxygen use; Child's B or C hepatic failure |
| Interventions | Intervention group: Quote: "Cerebral oxygenation levels for people in this group will be monitored and maintained above 60%. If levels decrease to below 60%, a protocol is followed to guide possible interventions to increase cerebral oxygenation levels above 60%" Control group: Quote: "Cerebral oxygenation levels for people in this group will be masked and thus doctors and care staff will not use the cerebral oxygenation levels to make any interventions. If the cerebral oxygenation levels drop to below 40%, the cerebral oxygenation levels will be unmasked so that doctors can follow the protocol to increase levels to above 60%" |
| Outcomes | Primary outcomes: postoperative neurocognitive decline before surgery; postoperative neurocognitive decline at 3 months and 6 months after surgery Secondary outcomes: neurological dysfunction during the hospitalization for postoperative recovery; multiple organ dysfunction during the hospitalization for postoperative recovery |
| Starting date | November 2011 |
| Contact information | Muoi Trinh, MD, Icahn School of Medicine at Mount Sinai, New York, United States, 10029 Tel: 212‐241‐4203 Email address: [email protected] Suzan Uysal, PhD, Icahn School of Medicine at Mount Sinai, New York, United States, 10029 Tel: 212‐241‐1836 Email address: [email protected] |
| Notes | ClinicalTrials.gov identifier: NCT01539382 Recruiting status: recruiting http://clinicaltrials.gov/show/NCT01539382 We contacted Dr. Muoi Trinh by email to request detailed information for the study, but we did not receive a reply. |
BCP: beach chair position; CAB: coronary artery bypass; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; DHCA: deep hypothermic circulatory arrest; EQ5D: EuroQol 5‐domain instrument; EQUANOX: one of the NIRS‐based cerebral oximetries; FORE‐SIGHT: one of the NIRS‐based cerebral oximetries; ICU: intensive care unit; INVOS: one of the NIRS‐based cerebral oximetries; MRI: magnetic resonance imaging; ms: millisecond; NIRS: near‐infrared spectroscopy; NYHA: New York Heart Association; rSO2: regional oxygen saturation; SctO2: cerebral tissue oxygen saturation; SF36: Short Form 36‐Item
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score). | ||||
| 1.1 1 week | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| 1.2 12 weeks | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| 1.3 52 weeks | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.63 [0.70, 2.56] |
| 2 POCD defined by original studies ‐ 1 week Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 2 POCD defined by original studies ‐ 1 week. | ||||
| 2.1 Mild | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.95] |
| 2.2 Moderate | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.20, 1.04] |
| 2.3 Severe | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 0.92] |
| 3 POCD: decline in cognitive function ‐ 1 week Show forest plot | 6 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.04] |
| Analysis 1.3  Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 3 POCD: decline in cognitive function ‐ 1 week. | ||||
| 4 Intraoperative mortality or postoperative mortality: Death Show forest plot | 3 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.08, 5.03] |
| Analysis 1.4  Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 4 Intraoperative mortality or postoperative mortality: Death. | ||||
| 5 The occurrence of abnormal rScO2 during or after surgery: Desaturation Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 5 The occurrence of abnormal rScO2 during or after surgery: Desaturation. | ||||
| 5.1 In OR | 7 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 5.2 In ICU | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.34] |
| 6 Any major non‐neurological complications as defined by individual study Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 6 Any major non‐neurological complications as defined by individual study. | ||||
| 6.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 6.2 Non‐specific respiratory complications | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.56] |
| 6.3 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 6.4 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 6.5 Pneumothorax | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.05] |
| 6.6 Postoperative acute pulmonary edema | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 3.92] |
| 6.7 Postoperative pulmonary embolism | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 Prolonged mechanical ventilation | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.54] |
| 6.9 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 6.10 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 6.11 Cardiac ischaemia | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.27] |
| 6.12 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 6.13 Reoperation for bleeding | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.77] |
| 6.14 MOMM | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.08, 0.95] |
| 6.15 Rupture of the colonic anastomosis | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.36, 2.83] |
| 6.16 Surgical reintervention | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.09] |
| 6.17 Patients experiencing more than 1 complication | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.56] |
| 6.18 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 6.19 Revision | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.25] |
| 6.20 Hospital stay > 7 days (% of patients) | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.48] |
| 6.21 Mediastinitis | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.09] |
| 6.22 Septicaemia | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.09] |
| 6.23 Unplanned HDU/ICU admission | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.08] |
| 7 Length of ICU stay (days) Show forest plot | 3 | 379 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.48, ‐0.09] |
| Analysis 1.7  Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 7 Length of ICU stay (days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: Neurological injury Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 1 Postoperative stroke or other neurological injury: Neurological injury. | ||||
| 1.1 Cardiac or great vessel surgery | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 4.09 [0.47, 35.88] |
| 1.2 Other surgery | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 2.37] |
| 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 1 week Show forest plot | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| Analysis 2.2  Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 1 week. | ||||
| 2.1 Cardiac or great vessel surgery | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 3.16 [0.98, 5.34] |
| 2.2 Other surgery | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 2.48 [0.85, 4.11] |
| 3 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks Show forest plot | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| Analysis 2.3  Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 3 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks. | ||||
| 3.1 Cardiac or great vessel surgery | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 1.58 [‐0.10, 3.25] |
| 3.2 Other surgery | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 0.75 [‐0.21, 1.71] |
| 4 POCD defined by original studies ‐ 1 week ‐ mild Show forest plot | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.95] |
| Analysis 2.4  Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 4 POCD defined by original studies ‐ 1 week ‐ mild. | ||||
| 4.1 Cardiac or great vessel surgery | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.17, 0.79] |
| 4.2 Other surgery | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 0.97] |
| 5 POCD defined by original studies ‐ 1 week ‐ severe Show forest plot | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 0.92] |
| Analysis 2.5  Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 5 POCD defined by original studies ‐ 1 week ‐ severe. | ||||
| 5.1 Cardiac or great vessel surgery | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 2.25] |
| 5.2 Other surgery | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.03, 1.58] |
| 6 POCD: decline in cognitive function ‐ 1 week Show forest plot | 6 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.04] |
| Analysis 2.6  Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 6 POCD: decline in cognitive function ‐ 1 week. | ||||
| 6.1 Cardiac or great vessel surgery | 4 | 671 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.19, 1.11] |
| 6.2 Carotid endarterectomy | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.42, 2.10] |
| 6.3 Other surgery | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 1 week Show forest plot | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| Analysis 3.1  Comparison 3 Subgroup of interventions: cerebral oxygenation monitoring (EQUANOX) or INVOS vs blinded monitoring, Outcome 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 1 week. | ||||
| 1.1 INVOS | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 2.48 [0.85, 4.11] |
| 1.2 EQUANOX | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 3.16 [0.98, 5.34] |
| 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks Show forest plot | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| Analysis 3.2 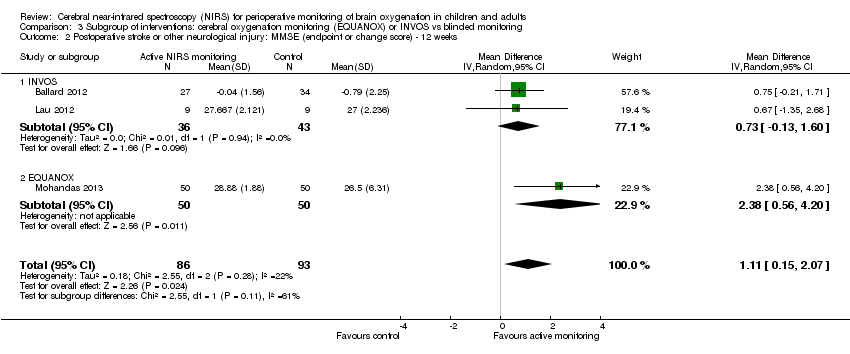 Comparison 3 Subgroup of interventions: cerebral oxygenation monitoring (EQUANOX) or INVOS vs blinded monitoring, Outcome 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks. | ||||
| 2.1 INVOS | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 0.73 [‐0.13, 1.60] |
| 2.2 EQUANOX | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 2.38 [0.56, 4.20] |
| 3 POCD: decline in cognitive function ‐ 1 week Show forest plot | 6 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.04] |
| Analysis 3.3  Comparison 3 Subgroup of interventions: cerebral oxygenation monitoring (EQUANOX) or INVOS vs blinded monitoring, Outcome 3 POCD: decline in cognitive function ‐ 1 week. | ||||
| 3.1 INVOS | 5 | 862 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.04] |
| 3.2 EQUANOX | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any major non‐neurological complications as defined by individual study: including studies with detection bias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Sensitivity analysis: detection bias, Outcome 1 Any major non‐neurological complications as defined by individual study: including studies with detection bias. | ||||
| 1.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 1.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 1.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 1.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 1.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 1.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 1.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 2 Any major non‐neurological complications as defined by individual study: including studies without detection bias Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Sensitivity analysis: detection bias, Outcome 2 Any major non‐neurological complications as defined by individual study: including studies without detection bias. | ||||
| 2.1 Non‐specific any reported complications | 5 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 0.99] |
| 2.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 2.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 2.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 2.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 2.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 2.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with missing data Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Sensitivity analysis: missing data, Outcome 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with missing data. | ||||
| 1.1 1 week | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| 1.2 12 weeks | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| 2 POCD: decline in cognitive function ‐ 1 week: including studies with missing data Show forest plot | 6 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.04] |
| Analysis 5.2  Comparison 5 Sensitivity analysis: missing data, Outcome 2 POCD: decline in cognitive function ‐ 1 week: including studies with missing data. | ||||
| 3 Occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies with missing data Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 Sensitivity analysis: missing data, Outcome 3 Occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies with missing data. | ||||
| 3.1 In OR | 7 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 3.2 In ICU | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.34] |
| 4 Any major non‐neurological complications as defined by individual study: including studies with missing data Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 Sensitivity analysis: missing data, Outcome 4 Any major non‐neurological complications as defined by individual study: including studies with missing data. | ||||
| 4.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 4.2 Non‐specific respiratory complications | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.56] |
| 4.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 4.4 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 4.5 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 4.6 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 4.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 5 POCD: decline in cognitive function ‐ 1 week: without missing data Show forest plot | 5 | 781 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.31, 1.21] |
| Analysis 5.5  Comparison 5 Sensitivity analysis: missing data, Outcome 5 POCD: decline in cognitive function ‐ 1 week: without missing data. | ||||
| 6 Occurrence of abnormal rScO2 during or after surgery: Desaturation: without missing data Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.6  Comparison 5 Sensitivity analysis: missing data, Outcome 6 Occurrence of abnormal rScO2 during or after surgery: Desaturation: without missing data. | ||||
| 6.1 In OR | 6 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 6.2 In ICU | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.34] |
| 7 Any major non‐neurological complications as defined by individual study: without missing data Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.7 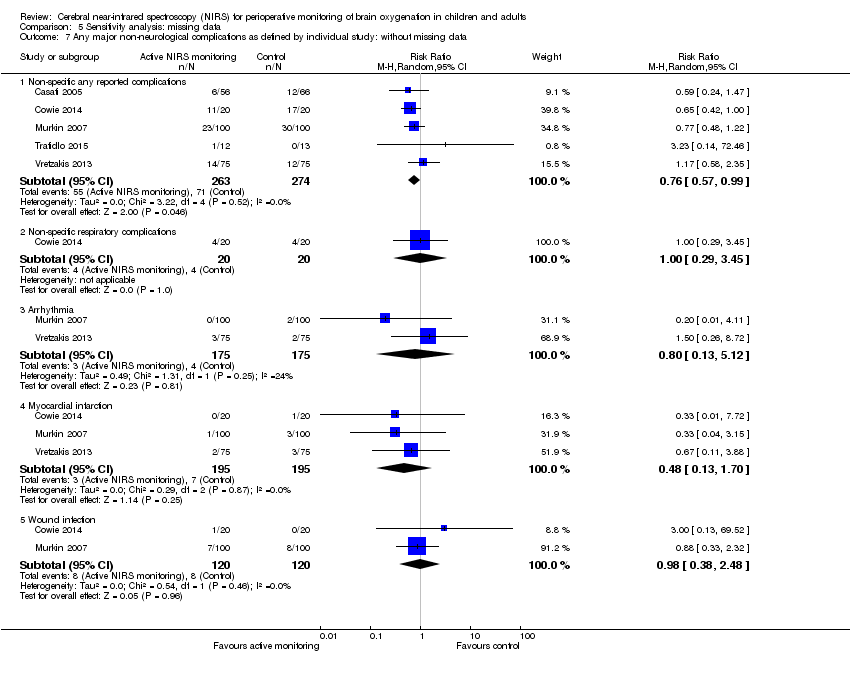 Comparison 5 Sensitivity analysis: missing data, Outcome 7 Any major non‐neurological complications as defined by individual study: without missing data. | ||||
| 7.1 Non‐specific any reported complications | 5 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 0.99] |
| 7.2 Non‐specific respiratory complications | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.29, 3.45] |
| 7.3 Arrhythmia | 2 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.13, 5.12] |
| 7.4 Myocardial infarction | 3 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.13, 1.70] |
| 7.5 Wound infection | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.38, 2.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any major non‐neurological complications as defined by individual study: including studies with reporting bias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.1  Comparison 6 Sensitivity analysis: reporting bias, Outcome 1 Any major non‐neurological complications as defined by individual study: including studies with reporting bias. | ||||
| 1.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 1.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 1.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 1.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 1.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 1.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 1.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 2 Any major non‐neurological complications as defined by individual study: including studies without reporting bias Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.2  Comparison 6 Sensitivity analysis: reporting bias, Outcome 2 Any major non‐neurological complications as defined by individual study: including studies without reporting bias. | ||||
| 2.1 Non‐specific any reported complications | 5 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 0.99] |
| 2.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 2.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 2.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 2.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 2.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 2.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with other bias Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 7.1  Comparison 7 Sensitivity analysis: other bias, Outcome 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with other bias. | ||||
| 1.1 1 week | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| 1.2 12 weeks | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| 2 POCD defined by original studies ‐ 1 week: including studies with other bias Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.2  Comparison 7 Sensitivity analysis: other bias, Outcome 2 POCD defined by original studies ‐ 1 week: including studies with other bias. | ||||
| 2.1 Mild | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.95] |
| 2.2 Severe | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 0.92] |
| 3 Intraoperative mortality or postoperative mortality: Death: including studies with other bias Show forest plot | 3 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.08, 5.03] |
| Analysis 7.3  Comparison 7 Sensitivity analysis: other bias, Outcome 3 Intraoperative mortality or postoperative mortality: Death: including studies with other bias. | ||||
| 4 The occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies with other bias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.4  Comparison 7 Sensitivity analysis: other bias, Outcome 4 The occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies with other bias. | ||||
| 4.1 In OR | 7 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 4.2 In ICU | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.34] |
| 5 Any major non‐neurological complications as defined by individual study: including studies with other bias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.5  Comparison 7 Sensitivity analysis: other bias, Outcome 5 Any major non‐neurological complications as defined by individual study: including studies with other bias. | ||||
| 5.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 5.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 5.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 5.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 5.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 5.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 5.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 6 Length of ICU stay (days): including studies with other bias Show forest plot | 3 | 379 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.48, ‐0.09] |
| Analysis 7.6  Comparison 7 Sensitivity analysis: other bias, Outcome 6 Length of ICU stay (days): including studies with other bias. | ||||
| 7 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies without other bias Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 7.7  Comparison 7 Sensitivity analysis: other bias, Outcome 7 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies without other bias. | ||||
| 7.1 12 weeks | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 1.58 [‐0.10, 3.25] |
| 8 The occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies without other bias Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.8  Comparison 7 Sensitivity analysis: other bias, Outcome 8 The occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies without other bias. | ||||
| 8.1 In OR | 3 | 410 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.69, 1.13] |
| 9 Any major non‐neurological complications as defined by individual study: including studies without other bias Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.9  Comparison 7 Sensitivity analysis: other bias, Outcome 9 Any major non‐neurological complications as defined by individual study: including studies without other bias. | ||||
| 9.1 Non‐specific any reported complications | 4 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.57, 1.68] |
| 9.2 Non‐specific cardiac complications | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.42, 3.44] |
| 9.3 Non‐specific renal complications | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.25] |
| 9.4 Arrhythmia | 2 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.53, 1.39] |
| 9.5 Myocardial infarction | 2 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.38, 2.20] |
| 9.6 Cardiac arrest | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 9.7 Wound infection | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.53, 1.76] |
| 10 Length of ICU stay (days): including studies without other bias Show forest plot | 2 | 179 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.39, ‐0.07] |
| Analysis 7.10  Comparison 7 Sensitivity analysis: other bias, Outcome 10 Length of ICU stay (days): including studies without other bias. | ||||
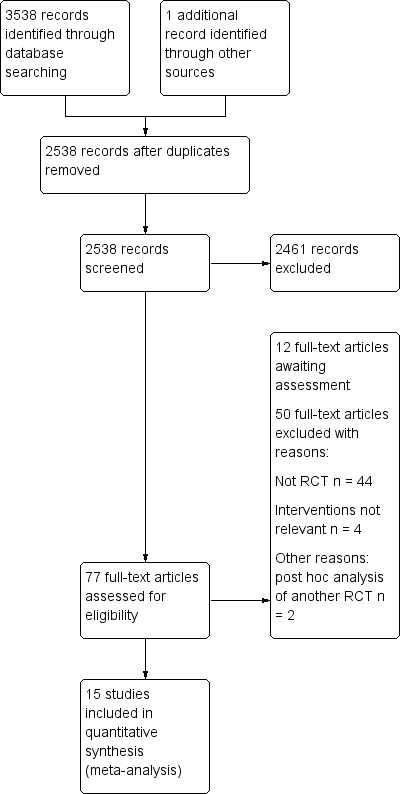
Study flow diagram‐top up search on November 2017.
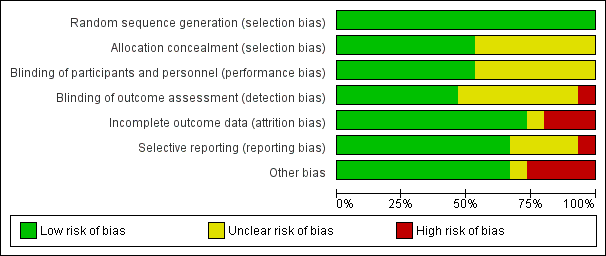
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
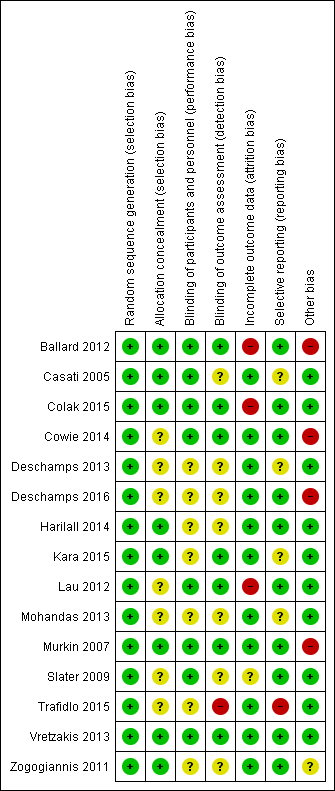
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score).

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 2 POCD defined by original studies ‐ 1 week.

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 3 POCD: decline in cognitive function ‐ 1 week.

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 4 Intraoperative mortality or postoperative mortality: Death.

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 5 The occurrence of abnormal rScO2 during or after surgery: Desaturation.

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 6 Any major non‐neurological complications as defined by individual study.

Comparison 1 Active cerebral oxygenation monitoring vs blinded cerebral oxygenation monitoring, Outcome 7 Length of ICU stay (days).

Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 1 Postoperative stroke or other neurological injury: Neurological injury.

Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 1 week.

Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 3 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks.

Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 4 POCD defined by original studies ‐ 1 week ‐ mild.

Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 5 POCD defined by original studies ‐ 1 week ‐ severe.

Comparison 2 Subgroup of participants: participants with carotid endarterectomy, cardiac or great vessel surgery, or other surgery, Outcome 6 POCD: decline in cognitive function ‐ 1 week.

Comparison 3 Subgroup of interventions: cerebral oxygenation monitoring (EQUANOX) or INVOS vs blinded monitoring, Outcome 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 1 week.

Comparison 3 Subgroup of interventions: cerebral oxygenation monitoring (EQUANOX) or INVOS vs blinded monitoring, Outcome 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks.

Comparison 3 Subgroup of interventions: cerebral oxygenation monitoring (EQUANOX) or INVOS vs blinded monitoring, Outcome 3 POCD: decline in cognitive function ‐ 1 week.

Comparison 4 Sensitivity analysis: detection bias, Outcome 1 Any major non‐neurological complications as defined by individual study: including studies with detection bias.

Comparison 4 Sensitivity analysis: detection bias, Outcome 2 Any major non‐neurological complications as defined by individual study: including studies without detection bias.

Comparison 5 Sensitivity analysis: missing data, Outcome 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with missing data.

Comparison 5 Sensitivity analysis: missing data, Outcome 2 POCD: decline in cognitive function ‐ 1 week: including studies with missing data.

Comparison 5 Sensitivity analysis: missing data, Outcome 3 Occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies with missing data.

Comparison 5 Sensitivity analysis: missing data, Outcome 4 Any major non‐neurological complications as defined by individual study: including studies with missing data.

Comparison 5 Sensitivity analysis: missing data, Outcome 5 POCD: decline in cognitive function ‐ 1 week: without missing data.

Comparison 5 Sensitivity analysis: missing data, Outcome 6 Occurrence of abnormal rScO2 during or after surgery: Desaturation: without missing data.

Comparison 5 Sensitivity analysis: missing data, Outcome 7 Any major non‐neurological complications as defined by individual study: without missing data.

Comparison 6 Sensitivity analysis: reporting bias, Outcome 1 Any major non‐neurological complications as defined by individual study: including studies with reporting bias.

Comparison 6 Sensitivity analysis: reporting bias, Outcome 2 Any major non‐neurological complications as defined by individual study: including studies without reporting bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 2 POCD defined by original studies ‐ 1 week: including studies with other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 3 Intraoperative mortality or postoperative mortality: Death: including studies with other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 4 The occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies with other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 5 Any major non‐neurological complications as defined by individual study: including studies with other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 6 Length of ICU stay (days): including studies with other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 7 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies without other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 8 The occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies without other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 9 Any major non‐neurological complications as defined by individual study: including studies without other bias.

Comparison 7 Sensitivity analysis: other bias, Outcome 10 Length of ICU stay (days): including studies without other bias.
| Active cerebral oxygenation monitoring compared to blinded cerebral oxygenation monitoring for perioperative monitoring of brain oxygenation in children and adults | ||||||
| Patient or population: children and adults undergoing surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Blinded cerebral oxygenation monitoring | Active cerebral oxygenation monitoring | |||||
| Postoperative neurological injury (follow‐up: 1 week or hospital discharge) | No pooled effect estimate available due to discordant direction of effect between studies. One study (N = 126) in people undergoing major abdominal surgery reported that 4/66 participants experienced neurological injury with blinded monitoring versus 0/56 in the active monitoring group. A second study (N = 195) in people having coronary artery bypass surgery reported that 1/96 participants experienced neurological injury in the blinded monitoring group compared with 4/94 participants in the active monitoring group. | Not estimable | 312 | ⊕⊕⊝⊝ | Very serious heterogeneity (I2 = 72%) | |
| Postoperative stroke (follow‐up: within 30 days) | 40 per 1000 | 10 per 1000 | RR 0.25 | 240 | ⊕⊕⊝⊝ | — |
| POD: postoperative delirium (follow‐up: 1 week) | 135 per 1000 | 85 per 1000 | RR 0.63 | 190 | ⊕⊕⊝⊝ | — |
| POCD defined by original studies ‐ mild (follow‐up: 1 week) | 641 per 1000 | 340 per 1000 | RR 0.53 | 126 | ⊕⊕⊝⊝ | — |
| POCD: decline in cognitive function (follow‐up: 1 week) | 400 per 1000 | 248 per 1000 | RR 0.62 | 962 | ⊕⊕⊕⊝ | — |
| Intraoperative mortality or postoperative mortality: Death (follow‐up: 30 days) | 10 per 1000 | 6 per 1000 | RR 0.63 | 390 | ⊕⊕⊝⊝ | — |
| Adverse events | See comments | See comments | Not estimable | See comments | See comments | None of the studies reported adverse effects caused by use of NIRS‐based cerebral oxygenation monitoring |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias: downgraded by one level because the body of evidence contained one or more of the following risks of bias: potential conflict of interest from industry sponsorship, unclear blinding status or missing participant data. | ||||||
| 1 Postoperative stroke or other neurological injury | ||||||||
| 1.1 Neurological injury | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Risk ratio | ||||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 0 | 56 | 4 | 66 | 0.13 (0.01 to 2.37) | ||||
| 4 | 94 | 1 | 96 | 4.09 (0.47 to 35.88) | ||||
| 1.2 Stroke | ||||||||
| 0 | 20 | 0 | 20 | Not estimable | ||||
| 1 | 100 | 4 | 100 | 0.25 (0.03 to 2.20) | ||||
| 2 Postoperative stroke or other neurological injury: ASEM (endpoint score) | ||||||||
| 2.1 1 week | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 17.46 | 1.99 | 50 | 15.04 | 4.8 | 50 | 100.0% | 2.42 (0.98 to 3.86) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 2.42 (0.98 to 3.86) | ||||
| Test for overall effect: Z = 3.29 (P = 0.0010) | ||||||||
| 2.2 12 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 17.68 | 1.79 | 50 | 15.69 | 3.99 | 50 | 100.0% | 1.99 (0.78 to 3.20) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 1.99 (0.78 to 3.20) | ||||
| Test for overall effect: Z = 3.22 (P = 0.001) | ||||||||
| 3 Postoperative stroke or other neurological injury: Vigilance Reaction Time (change score) | ||||||||
| 3.1 1 week | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 6.39 | 80.9 | 22 | 27.95 | 54.99 | 29 | 100.0% | ‐21.56 (‐60.85 to 17.73) | |
| Subtotal (95% CI) | 22 | 29 | 100.0% | ‐21.56 (‐60.85 to 17.73) | ||||
| Test for overall effect: Z = 1.08 (P = 0.28) | ||||||||
| 3.2 12 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| ‐11.73 | 33.5 | 27 | 13.61 | 29.69 | 34 | 100.0% | ‐25.34 (‐41.44 to ‐9.24) | |
| Subtotal (95% CI) | 27 | 34 | 100.0% | ‐25.34 (‐41.44 to ‐9.24) | ||||
| Test for overall effect: Z = 3.08 (P = 0.002) | ||||||||
| 3.3 52 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| ‐10.8 | 36.28 | 28 | 15.1 | 40.73 | 32 | 100.0% | ‐25.90 (‐45.39 to ‐6.41) | |
| Subtotal (95% CI) | 28 | 32 | 100.0% | ‐25.90 (‐45.39 to ‐6.41) | ||||
| Test for overall effect: Z = 2.61 (P = 0.009) | ||||||||
| 4 Postoperative stroke or other neurological injury: Trail Making (change score) | ||||||||
| 4.1 12 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| ‐0.23 | 0.73 | 27 | 0.47 | 1.34 | 34 | 100.0% | ‐0.70 (‐1.23 to ‐0.17) | |
| Subtotal (95% CI) | 27 | 34 | 100.0% | ‐0.70 (‐1.23 to ‐0.17) | ||||
| Test for overall effect: Z = 2.60 (P = 0.009) | ||||||||
| 4.2 52 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 0.12 | 0.68 | 28 | ‐0.47 | 0.93 | 32 | 100.0% | 0.59 (0.18 to 1.00) | |
| Subtotal (95% CI) | 28 | 32 | 100.0% | 0.59 (0.18 to 1.00) | ||||
| Test for overall effect: Z = 2.83 (P = 0.005) | ||||||||
| 5 POD: postoperative delirium | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 8 | 94 | 13 | 96 | 100.0% | 0.63 (0.27 to 1.45) | |||
| Total (95% CI) | 94 | 96 | 100.0% | 0.63 (0.27 to 1.45) | ||||
| Total events | 8 | 13 | ||||||
| Test for overall effect: Z = 1.09 (P = 0.27) | ||||||||
| 6 POCD as defined by the original studies ‐ 12 weeks | ||||||||
| 6.1 Mild | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 13 | 24 | 27 | 33 | 100.0% | 0.66 (0.44 to 0.99) | |||
| Subtotal (95% CI) | 24 | 33 | 100.0% | 0.66 (0.44 to 0.99) | ||||
| Total events | 13 | 27 | ||||||
| Test for overall effect: Z = 2.01 (P = 0.04) | ||||||||
| 6.2 Moderate | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 6 | 24 | 9 | 33 | 100.0% | 0.92 (0.38 to 2.23) | |||
| Subtotal (95% CI) | 24 | 33 | 100.0% | 0.92 (0.38 to 2.23) | ||||
| Total events | 6 | 9 | ||||||
| Test for overall effect: Z = 0.19 (P = 0.85) | ||||||||
| 6.3 Severe | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 2 | 24 | 4 | 33 | 100.0% | 0.69 (0.14 to 3.45) | |||
| Subtotal (95% CI) | 24 | 33 | 100.0% | 0.69 (0.14 to 3.45) | ||||
| Total events | 2 | 4 | ||||||
| Test for overall effect: Z = 0.46 (P = 0.65) | ||||||||
| 7 POCD as defined by the original studies ‐ 52 weeks | ||||||||
| 7.1 Mild | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 15 | 27 | 27 | 32 | 100.0% | 0.66 (0.46 to 0.95) | |||
| Subtotal (95% CI) | 27 | 32 | 100.0% | 0.66 (0.46 to 0.95) | ||||
| Total events | 15 | 27 | ||||||
| Test for overall effect: Z = 2.22 (P = 0.03) | ||||||||
| 7.2 Moderate | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 3 | 27 | 12 | 32 | 100.0% | 0.30 (0.09 to 0.94) | |||
| Subtotal (95% CI) | 27 | 32 | 100.0% | 0.30 (0.09 to 0.94) | ||||
| Total events | 3 | 12 | ||||||
| Test for overall effect: Z = 2.06 (P = 0.04) | ||||||||
| 7.3 Severe | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 1 | 27 | 4 | 32 | 100.0% | 0.30 (0.04 to 2.49) | |||
| Subtotal (95% CI) | 27 | 32 | 100.0% | 0.30 (0.04 to 2.49) | ||||
| Total events | 1 | 4 | ||||||
| Test for overall effect: Z = 1.12 (P = 0.26) | ||||||||
| ASEM: antisaccadic eye movement test; CI: confidence interval; IQR: interquartile range; N: number; NIRS: near‐infrared spectroscopy; POCD: postoperative cognitive dysfunction; POD: postoperative delirium; S100B: one biomarker of cerebral damage; SD: standard deviation | ||||||||
| 1 The occurrence of abnormal rScO2 during or after surgery: desaturation time | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 24.7 | 11.819 | 20 | 63.85 | 23.424 | 20 | 100.0% | ‐39.15 (‐50.65 to ‐27.65) | |
| Total (95% CI) | 20 | 20 | 100.0% | ‐39.15 (‐50.65 to ‐27.65) | ||||
| Test for overall effect: Z = 6.67 ( P < 0.00001) | ||||||||
| 2 The occurrence of abnormal rScO2 during or after surgery: rScO2 below 50% | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 1 | 30 | 6 | 35 | 100.0% | 0.19 (0.02 to 1.53) | |||
| Total (95% CI) | 30 | 35 | 100.0% | 0.19 (0.02 to 1.53) | ||||
| Total events | 1 | 6 | ||||||
| Test for overall effect: Z = 1.56 (P = 0.12) | ||||||||
| 3 Length of hospital stay (days) | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 7.15 | 1.39 | 43 | 7.67 | 1.14 | 36 | 100.0% | ‐0.52 (‐1.08 to 0.04) | |
| Total (95% CI) | 43 | 36 | 100.0% | ‐0.52 (‐1.08 to 0.04 | ||||
| Test for overall effect: Z = 1.83 (P = 0.07) | ||||||||
| 4 Length of hospital stay (days) | ||||||||
| Study ID | Group | Mean | SD/95% CI | N | ||||
| Intervention group | 7.9 | 4.8 to 10.9 | 20 | |||||
| Control group | 10.6 | 5.5 to 15.8 | 20 | |||||
| Intervention group | 7.6 | 5.4 | 23 | |||||
| Control group | 7.9 | 3.2 | 25 | |||||
| Intervention group | 6.1 | 4.4 | 100 | |||||
| Control group | 6.9 | 5.5 | 100 | |||||
| Intervention group | 10.9 | 3.6 | 75 | |||||
| Control group | 10.2 | 10.7 | 75 | |||||
| CI: confidence interval; N: number; NIRS: near‐infrared spectroscopy; rScO2: regional cerebral oxygen saturation; SD: standard deviation | ||||||||
| 1 Postoperative stroke or other neurological injury: including studies with missing data | ||||||||
| 1.1 Neurological injury | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Risk ratio | ||||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 0 | 56 | 4 | 66 | 0.13 (0.01 to 2.37) | ||||
| 4 | 94 | 1 | 96 | 4.09 (0.47 to 35.88) | ||||
| 1.2 Stroke | ||||||||
| 0 | 20 | 0 | 20 | Not estimable | ||||
| 1 | 100 | 4 | 100 | 0.25 (0.03 to 2.20) | ||||
| 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with missing data | ||||||||
| 2.1 52 weeks | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 0.69 | 1.47 | 28 | ‐0.94 | 2.18 | 32 | 100.0% | 1.63 (0.70 to 2.56) | |
| Subtotal (95% CI) | 28 | 32 | 100.0% | 1.63 (0.70 to 2.56) | ||||
| Test for overall effect: Z = 3.43 (P = 0.0006) | ||||||||
| 3 Postoperative stroke or other neurological injury: without missing data | ||||||||
| 3.1 Neurological injury | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Risk ratio | ||||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 0 | 56 | 4 | 66 | 0.13 (0.01 to 2.37) | ||||
| 3.2 Stroke | ||||||||
| 0 | 20 | 0 | 20 | Not estimable | ||||
| 1 | 100 | 4 | 100 | 0.25 (0.03 to 2.20) | ||||
| 4 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): without missing data | ||||||||
| 4.1 1 week | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 28.58 | 2.29 | 50 | 25.42 | 7.54 | 50 | 100.0% | 3.16 (0.98 to 5.34) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 3.16 (0.98 to 5.34) | ||||
| Test for overall effect: Z = 2.84 (P = 0.005) | ||||||||
| 65.2 12 weeks | ||||||||
| 28.88 | 1.88 | 50 | 26.5 | 6.31 | 50 | 100.0% | 2.38 (0.56 to 4.20) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 2.38 (0.56 to 4.20) | ||||
| Test for overall effect: Z = 2.56 (P = 0.01) | ||||||||
| CI: confidence interval; MMSE: mini‐mental state examination; NIRS: near‐infrared spectroscopy; SD: standard deviation | ||||||||
| 1 POCD defined by original studies ‐ 1 week: including studies without other bias | ||||||||
| 1.1 Mild | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 7 | 43 | 16 | 36 | 100.0% | 0.37 (0.17 to 0.79) | |||
| Subtotal (95% CI) | 43 | 36 | 100.0% | 0.37 (0.17 to 0.79) | ||||
| Total events | 7 | 16 | ||||||
| Heterogeneity: not applicable | ||||||||
| Test for overall effect: Z = 2.56 (P = 0.01) | ||||||||
| 1.2 Severe | ||||||||
| 0 | 43 | 3 | 36 | 100.0% | 0.12 (0.01 to 2.25) | |||
| Subtotal (95% CI) | 43 | 36 | 100.0% | 0.12 (0.01 to 2.25) | ||||
| Total events | 0 | 3 | ||||||
| Heterogeneity: not applicable | ||||||||
| Test for overall effect: Z = 1.42 (P = 0.16) | ||||||||
| 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies without other bias | ||||||||
| 2.1 1 week | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Mean difference | |||||
| Mean | SD | Total | Mean | SD | Total | Inverse variance, fixed‐effect model, 95% CI | ||
| 28.58 | 2.29 | 50 | 25.42 | 7.54 | 50 | 100.0% | 3.16 (0.98 to 5.34) | |
| Subtotal (95% CI) | 50 | 50 | 100.0% | 3.16 (0.98 to 5.34) | ||||
| Test for overall effect: Z = 2.84 (P = 0.005) | ||||||||
| 3 Intraoperative mortality or postoperative mortality: death: including studies without other bias | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 1 | 75 | 1 | 75 | 100.0% | 1.00 (0.06 to 15.69) | |||
| Total (95% CI) | 75 | 75 | 100.0% | 1.00 (0.06 to 15.69) | ||||
| Total events | 1 | 1 | ||||||
| Heterogeneity: not applicable | ||||||||
| Test for overall effect: Z = 0.00 (P = 1.00) | ||||||||
| 4 The occurrence of abnormal rScO2 during or after surgery: desaturation: including studies without other bias | ||||||||
| 4.1 In ICU | ||||||||
| Active NIRS monitoring | Blinded NIRS monitoring | Weight | Risk ratio | |||||
| Events | Total | Events | Total | Mantel‐Haenszel, fixed‐effect model, 95% CI | ||||
| 6 | 23 | 14 | 25 | 100.0% | 0.47 (0.22 to 1.01) | |||
| Subtotal (95% CI) | 23 | 25 | 100.0% | 0.47 (0.22 to 1.01) | ||||
| Total events | 6 | 14 | ||||||
| Heterogeneity: not applicable | ||||||||
| Test for overall effect: Z = 1.94 (P = 0.05) | ||||||||
| CI: confidence interval; ICU: intensive care unit; MMSE: mini‐mental state examination; NIRS: near‐infrared spectroscopy; POCD: postoperative cognitive dysfunction; SD: standard deviation; rScO2: regional cerebral oxygen saturation | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 week | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| 1.2 12 weeks | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| 1.3 52 weeks | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.63 [0.70, 2.56] |
| 2 POCD defined by original studies ‐ 1 week Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Mild | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.95] |
| 2.2 Moderate | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.20, 1.04] |
| 2.3 Severe | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 0.92] |
| 3 POCD: decline in cognitive function ‐ 1 week Show forest plot | 6 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.04] |
| 4 Intraoperative mortality or postoperative mortality: Death Show forest plot | 3 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.08, 5.03] |
| 5 The occurrence of abnormal rScO2 during or after surgery: Desaturation Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 In OR | 7 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 5.2 In ICU | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.34] |
| 6 Any major non‐neurological complications as defined by individual study Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 6.2 Non‐specific respiratory complications | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.56] |
| 6.3 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 6.4 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 6.5 Pneumothorax | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.05] |
| 6.6 Postoperative acute pulmonary edema | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 3.92] |
| 6.7 Postoperative pulmonary embolism | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 Prolonged mechanical ventilation | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.54] |
| 6.9 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 6.10 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 6.11 Cardiac ischaemia | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.27] |
| 6.12 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 6.13 Reoperation for bleeding | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.77] |
| 6.14 MOMM | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.08, 0.95] |
| 6.15 Rupture of the colonic anastomosis | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.36, 2.83] |
| 6.16 Surgical reintervention | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.09] |
| 6.17 Patients experiencing more than 1 complication | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.56] |
| 6.18 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 6.19 Revision | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.25] |
| 6.20 Hospital stay > 7 days (% of patients) | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.48] |
| 6.21 Mediastinitis | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.09] |
| 6.22 Septicaemia | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.09] |
| 6.23 Unplanned HDU/ICU admission | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.08] |
| 7 Length of ICU stay (days) Show forest plot | 3 | 379 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.48, ‐0.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: Neurological injury Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Cardiac or great vessel surgery | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 4.09 [0.47, 35.88] |
| 1.2 Other surgery | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 2.37] |
| 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 1 week Show forest plot | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| 2.1 Cardiac or great vessel surgery | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 3.16 [0.98, 5.34] |
| 2.2 Other surgery | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 2.48 [0.85, 4.11] |
| 3 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks Show forest plot | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| 3.1 Cardiac or great vessel surgery | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 1.58 [‐0.10, 3.25] |
| 3.2 Other surgery | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 0.75 [‐0.21, 1.71] |
| 4 POCD defined by original studies ‐ 1 week ‐ mild Show forest plot | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.95] |
| 4.1 Cardiac or great vessel surgery | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.17, 0.79] |
| 4.2 Other surgery | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 0.97] |
| 5 POCD defined by original studies ‐ 1 week ‐ severe Show forest plot | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 0.92] |
| 5.1 Cardiac or great vessel surgery | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 2.25] |
| 5.2 Other surgery | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.03, 1.58] |
| 6 POCD: decline in cognitive function ‐ 1 week Show forest plot | 6 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.04] |
| 6.1 Cardiac or great vessel surgery | 4 | 671 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.19, 1.11] |
| 6.2 Carotid endarterectomy | 1 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.42, 2.10] |
| 6.3 Other surgery | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 1 week Show forest plot | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| 1.1 INVOS | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 2.48 [0.85, 4.11] |
| 1.2 EQUANOX | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 3.16 [0.98, 5.34] |
| 2 Postoperative stroke or other neurological injury: MMSE (endpoint or change score) ‐ 12 weeks Show forest plot | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| 2.1 INVOS | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 0.73 [‐0.13, 1.60] |
| 2.2 EQUANOX | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 2.38 [0.56, 4.20] |
| 3 POCD: decline in cognitive function ‐ 1 week Show forest plot | 6 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.04] |
| 3.1 INVOS | 5 | 862 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.04] |
| 3.2 EQUANOX | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any major non‐neurological complications as defined by individual study: including studies with detection bias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 1.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 1.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 1.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 1.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 1.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 1.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 2 Any major non‐neurological complications as defined by individual study: including studies without detection bias Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Non‐specific any reported complications | 5 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 0.99] |
| 2.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 2.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 2.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 2.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 2.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 2.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with missing data Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 week | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| 1.2 12 weeks | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| 2 POCD: decline in cognitive function ‐ 1 week: including studies with missing data Show forest plot | 6 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.37, 1.04] |
| 3 Occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies with missing data Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 In OR | 7 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 3.2 In ICU | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.34] |
| 4 Any major non‐neurological complications as defined by individual study: including studies with missing data Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 4.2 Non‐specific respiratory complications | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.03, 2.56] |
| 4.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 4.4 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 4.5 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 4.6 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 4.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 5 POCD: decline in cognitive function ‐ 1 week: without missing data Show forest plot | 5 | 781 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.31, 1.21] |
| 6 Occurrence of abnormal rScO2 during or after surgery: Desaturation: without missing data Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 In OR | 6 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 6.2 In ICU | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.34] |
| 7 Any major non‐neurological complications as defined by individual study: without missing data Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Non‐specific any reported complications | 5 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 0.99] |
| 7.2 Non‐specific respiratory complications | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.29, 3.45] |
| 7.3 Arrhythmia | 2 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.13, 5.12] |
| 7.4 Myocardial infarction | 3 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.13, 1.70] |
| 7.5 Wound infection | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.38, 2.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any major non‐neurological complications as defined by individual study: including studies with reporting bias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 1.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 1.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 1.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 1.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 1.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 1.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 2 Any major non‐neurological complications as defined by individual study: including studies without reporting bias Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Non‐specific any reported complications | 5 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 0.99] |
| 2.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 2.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 2.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 2.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 2.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 2.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies with other bias Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 week | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.42, 4.03] |
| 1.2 12 weeks | 3 | 179 | Mean Difference (IV, Random, 95% CI) | 1.11 [0.15, 2.07] |
| 2 POCD defined by original studies ‐ 1 week: including studies with other bias Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Mild | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.95] |
| 2.2 Severe | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 0.92] |
| 3 Intraoperative mortality or postoperative mortality: Death: including studies with other bias Show forest plot | 3 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.08, 5.03] |
| 4 The occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies with other bias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 In OR | 7 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.99] |
| 4.2 In ICU | 2 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.34] |
| 5 Any major non‐neurological complications as defined by individual study: including studies with other bias Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Non‐specific any reported complications | 6 | 562 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 5.2 Non‐specific cardiac complications | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.28, 2.31] |
| 5.3 Non‐specific renal complications | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.27, 2.76] |
| 5.4 Arrhythmia | 3 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 5.5 Myocardial infarction | 4 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.35, 1.67] |
| 5.6 Cardiac arrest | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 5.7 Wound infection | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.60] |
| 6 Length of ICU stay (days): including studies with other bias Show forest plot | 3 | 379 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.48, ‐0.09] |
| 7 Postoperative stroke or other neurological injury: MMSE (endpoint or change score): including studies without other bias Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 12 weeks | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 1.58 [‐0.10, 3.25] |
| 8 The occurrence of abnormal rScO2 during or after surgery: Desaturation: including studies without other bias Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 In OR | 3 | 410 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.69, 1.13] |
| 9 Any major non‐neurological complications as defined by individual study: including studies without other bias Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Non‐specific any reported complications | 4 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.57, 1.68] |
| 9.2 Non‐specific cardiac complications | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.42, 3.44] |
| 9.3 Non‐specific renal complications | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.25] |
| 9.4 Arrhythmia | 2 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.53, 1.39] |
| 9.5 Myocardial infarction | 2 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.38, 2.20] |
| 9.6 Cardiac arrest | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.49] |
| 9.7 Wound infection | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.53, 1.76] |
| 10 Length of ICU stay (days): including studies without other bias Show forest plot | 2 | 179 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.39, ‐0.07] |