Bloqueo nervioso periférico para el dolor posoperatorio después de la cirugía mayor de la rodilla
Resumen
Antecedentes
La cirugía mayor de la rodilla es un procedimiento quirúrgico habitual para ayudar a los pacientes con enfermedad terminal de la rodilla o traumatismo a recuperar la movilidad y mejorar la calidad de vida. El control deficiente del dolor inmediatamente después de la cirugía todavía es un aspecto clave en este procedimiento. Los bloqueos nerviosos periféricos son opciones analgésicas localizadas y específicas para el lugar en la cirugía mayor de la rodilla. El aumento del uso de los bloqueos nerviosos periféricos después de la cirugía mayor de la rodilla requiere el resumen de pruebas para evaluar su efectividad y seguridad, en comparación con la analgesia sistémica, con infiltración local, epidural y espinal.
Objetivos
Examinar la eficacia y la seguridad de los bloqueos nerviosos periféricos para el control del dolor posoperatorio después de la cirugía mayor de la rodilla con el uso de métodos que permitan la comparación con la analgesia sistémica, con infiltración local, epidural y espinal.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL) (número 1, 2014), MEDLINE y EMBASE, desde su inicio hasta febrero de 2014. Los estudios en curso se identificaron mediante búsquedas en los registros de ensayos, incluido el metaRegistro de ensayos controlados (mRCT), clinicaltrials.gov y la WHO International Clinical Trials Registry Platform (ICTRP).
Criterios de selección
Se incluyeron los ensayos controlados aleatorios que cegaron a participantes adultos (15 años o más) a los que se les realizó cirugía mayor de la rodilla y que compararon los bloqueos nerviosos periféricos con analgesia sistémica, con infiltración local, epidural y espinal para el alivio del dolor posoperatorio.
Obtención y análisis de los datos
Dos revisores de forma independiente evaluaron la elegibilidad de los estudios y extrajeron los datos. Se registró información sobre los participantes, los métodos, las intervenciones, los resultados (intensidad del dolor, consumo adicional de analgésicos, eventos adversos, amplitud de movimiento de la rodilla, duración de la estancia hospitalaria, costos hospitalarios y satisfacción del participante). Para evaluar la calidad metodológica se utilizó la escala de calidad y validez de Oxford de 5 puntos, así como los criterios esbozados en el Manual Cochrane para revisiones sistemáticas de intervenciones. Se realizó el metanálisis de dos o más estudios con datos suficientes para investigar el mismo resultado. Se utilizó la estadística I² para explorar la heterogeneidad. Si no hubo heterogeneidad significativa (valor de I²: 0% a 40%), se utilizó un modelo de efectos fijos para el metanálisis, de lo contrario se utilizó el modelo de efectos aleatorios. Para los datos dicotómicos los resultados se presentaron como el cociente de riesgos (CR) resumido y el intervalo de confianza del 95% (IC del 95%). Cuando fue posible se calculó el número necesario a tratar para obtener un resultado beneficioso adicional (NNTB) o para obtener un resultado perjudicial adicional (NNTD), junto con los IC del 95%. Para los datos continuos se utilizó la diferencia de medias (DM) y el IC del 95% para medidas de resultado similares. Se describen los hallazgos de los estudios individuales en los que no fue posible el agrupamiento de los datos.
Resultados principales
De acuerdo con los criterios de elegibilidad, se incluyen 23 estudios con 1571 participantes, con alta calidad metodológica general. Los estudios compararon los bloqueos nerviosos periféricos junto con analgesia sistémica con analgesia sistémica sola (19 estudios), los bloqueos nerviosos periféricos con infiltración local (tres estudios) y los bloqueos nerviosos periféricos con analgesia epidural (un estudio). Ningún estudio comparó los bloqueos nerviosos periféricos con analgesia espinal. En comparación con la analgesia sistémica sola los bloqueos nerviosos periféricos junto con analgesia sistémica dieron lugar a una puntuación de intensidad del dolor significativamente inferior en reposo al utilizar una escala analógica visual de 100 mm en todos los períodos en el transcurso de 72 horas posoperatoriamente, que incluyó el intervalo de cero a 23 de horas (DM ‐11,85; IC del 95%: ‐20,45 a ‐3,25; siete estudios, 390 participantes), el intervalo de 24 a 47 horas (DM ‐12,92; IC del 95%: ‐19,82 a ‐6,02; seis estudios, 320 participantes) y el intervalo de 48 a 72 horas (DM ‐9,72; IC del 95%: ‐16,75 a ‐2,70; cuatro estudios, 210 participantes). Los análisis de subgrupos indicaron que los altos niveles de variación estadística en los análisis se podrían explicar por efectos más grandes en los pacientes que recibieron artroplastia total de rodilla en comparación con otros tipos de cirugía. La intensidad del dolor al movimiento también se redujo significativamente en el intervalo de 48 a 72 horas posoperatoriamente (DM ‐6,19; IC del 95%: ‐11,76 a ‐0,62; dos estudios, 112 participantes). En la intensidad del dolor al movimiento no hubo diferencias significativas entre estos dos grupos en el período de cero a 23 horas (DM ‐6,95; IC del 95%: ‐15,92 a 2,01; cinco estudios, 304 participantes) y de 24 a 47 horas (DM ‐8,87; IC del 95%: ‐27,77 a 10,03; tres estudios, 182 participantes). Los estudios incluidos informaron diferentes tipos de eventos adversos y no se realizó un metanálisis sobre tipos específicos de eventos adversos. La cantidad de estudios y participantes también fue muy escasa para establecer conclusiones sobre los otros resultados preespecificados: consumo adicional de analgésicos; mediana del tiempo hasta la remedicación; amplitud de movimiento de la rodilla; mediana del tiempo hasta la ambulación; duración de la estancia hospitalaria; costos hospitalarios; y satisfacción de los participantes. No hubo datos suficientes para comparar los bloqueos nerviosos periféricos y la infiltración local o entre los bloqueos nerviosos periféricos y la analgesia epidural.
Conclusiones de los autores
Todos los estudios incluidos informaron el resultado principal intensidad del dolor pero no abarcaron todos los resultados secundarios de interés. La revisión actual proporciona pruebas de que el uso de bloqueos nerviosos periféricos como técnicas coadyuvantes a la analgesia sistémica redujo la intensidad del dolor en comparación con la analgesia sistémica sola después de la cirugía mayor de la rodilla. Hubo muy pocos datos para establecer conclusiones sobre otros resultados de interés. Se necesitan más ensayos para demostrar una diferencia significativa en comparación con la infiltración local, la analgesia epidural y la analgesia espinal.
Resumen en términos sencillos
Bloqueo nervioso periférico para el dolor posoperatorio después de la cirugía mayor de la rodilla
Hay diferentes tipos de cirugía de la rodilla que tienen como objetivo tratar la rigidez articular o reconstruir o reparar la articulación de la rodilla. El control deficiente del dolor inmediatamente después de la cirugía todavía es un aspecto clave.
Las medicinas que alivian el dolor (analgésicos) y que se inyectan en el nervio se llaman bloqueos nerviosos periféricos. Con frecuencia los bloqueos nerviosos se utilizan después de la cirugía mayor de la rodilla, con o sin otra medicina que alivie el dolor.
La revisión actual incluyó 23 estudios de alta calidad con 1571 participantes adultos (15 años o más) a los que se les realizó cirugía mayor de la rodilla. La última fecha de búsqueda fue febrero de 2014.
Esta revisión demostró que los bloqueos nerviosos periféricos más los analgésicos (tomados por vía oral o administrados mediante inyección) aliviaron el dolor en comparación con la analgesia sin bloqueo nervioso en el transcurso de las primeras 72 horas. Sin embargo, los efectos beneficiosos pueden estar limitados a los pacientes que reciben artroplastia total de la rodilla. El dolor al movimiento también se alivió significativamente a las 48 a 72 horas después de la cirugía, pero no hubo diferencias en el dolor al movimiento antes de ese momento. La revisión no encontró pruebas suficientes para establecer conclusiones sobre la ingesta de morfina, la amplitud de movimiento de la rodilla, la duración de la estancia hospitalaria, los costos hospitalarios ni la satisfacción de los pacientes.
La revisión no encontró pruebas suficientes para extraer conclusiones sobre los eventos adversos.
Se necesitan más ensayos para demostrar una diferencia significativa cuando los bloqueos nerviosos se comparan con otras formas de aliviar el dolor.
Authors' conclusions
Background
Description of the condition
Major knee surgery includes operations such as total knee arthroplasty, arthrolysis (Capdevila 1999) and anterior cruciate ligament reconstruction (Fowler 2008). People with end‐stage knee disease or trauma can regain mobility and have improved quality of life after undergoing these procedures. Major knee surgery is becoming increasingly common; the annual number of total knee arthroplasties performed in the United States has doubled in the last decade (Weinstein 2013). Despite advances in surgical techniques, poorly controlled pain immediately after surgery is still a key issue associated with this procedure (Chan 2013). The incidence of moderate‐to‐severe pain after total knee arthroplasty is reported to be about 50%, greater than that reported for all surgeries in a general population or for total hip arthroplasty (Grosu 2014). Thus, an effective analgesic strategy is required. Patients are advised to undergo physical therapy after surgery to restore their ability to carry out daily activities. Physical therapy can include passive and active knee flexion and extension exercises. Postoperative muscle spasm is an obstacle to these exercises, as well as a major source of pain. Efficacious muscle relaxation should therefore be considered so as to optimize pain relief (Sakai 2013).
Description of the intervention
There are several analgesic options for major knee surgery, such as systemic analgesia, local infiltration, and epidural and spinal analgesia. Systemic analgesia with opioids may cause various adverse events, including nausea, vomiting, pruritus, sedation and drowsiness (Chan 2013). Local infiltration analgesia has been proven to be most effective when the person is at rest, and may be less effective when they are walking or engaging in continuous passive movement (Yadeau 2013). There are many adverse events associated with epidural and spinal analgesia, such as perioperative hypotension, urinary retention, nausea, vomiting, and sensory and motor blockade of the non‐operated leg. To some extent, these adverse events may cause harm to patients and interfere with early ambulation (Mugabure Bujedo 2012; Teng 2012). Moreover, the co‐administration of anticoagulant drugs, used to prevent thromboembolic events, increases the potential risk of spinal epidural hematoma (Choi 2003).
Peripheral nerve blocks are localized and site‐specific. Their history dates back to 1930, when the technique was first reported to relieve the pain in obliterative vascular disease of the lower leg (Smithwick 1930). In 1980, Rosenblatt 1980 reported the first use of peripheral nerve blocks as the sole means of postoperative analgesia after a knee operation.
For decades, the accurate performance of peripheral nerve blocks has been supported by peripheral nerve stimulation techniques. In 1989, ultrasonography was first utilized to confirm the location of the needle and observe the spread of local anesthetic while performing peripheral nerve blocks (Ting 1989). Subsequently, ultrasound‐guided blocks have become increasingly popular with clinicians, owing to the precise action and faster onset of the block (Sala‐Blanch 2012).
Bupivacaine and ropivacaine are the most commonly used local anesthetics for peripheral nerve blocks. In addition, drugs such as opioids, epinephrine and clonidine are used as adjuvants to increase the duration of the analgesic effect, but there are no reports of their safety or efficacy (Aguirre 2012).
There are several types of peripheral nerve block that aim to relieve lower extremity pain, including femoral nerve, sciatic nerve and lumbar plexus blocks. Among them, the femoral nerve block is considered one of the primary options following major knee surgery. The femoral nerve is the largest branch of the lumbar plexus and provides sensation to the anterior aspect of the knee, whereas the sciatic nerve innervates the posterior aspect. Therefore, one method of ensuring complete reduction of pain transmission in the knee is to perform a sciatic nerve block in combination with a femoral nerve block (Tantry 2012). Another combination of peripheral nerve blocks used to ensure optimum pain relief is the three‐in‐one technique, which blocks three branches of the lumbar plexus: the femoral nerve (L2 ‐ L4), the obturator nerve (L2 ‐ L4), and the lateral femoral cutaneous nerve (L2 ‐ L3) (Hogan 2009).
Peripheral nerve blocks can be administered as a single shot or continuously via a catheter and a pump. Continuous peripheral nerve blocks (CPNBs) were introduced by Ansbro in 1946, later than single‐shot peripheral nerve blocks (SSPNBs), to increase the duration of the effect of the brachial plexus block (Ansbro 1946). Since then, continuous peripheral nerve blocks have evolved into reliable analgesic techniques that are widely used in the postoperative period. Continuous peripheral nerve blocks take approximately one‐and‐a‐half to four times longer to perform than single shots, but the duration of the analgesic effect is increased (Chan 2013). Furthermore, the well‐designed instrumentation used for a continuous peripheral nerve block provides patients with adequate and continuous analgesia after discharge (Dervin 2012).
How the intervention might work
Peripheral nerve blocks following major knee surgery reduce local pain transmission by blocking one or more major nerves supplying the lower limb. Clinical trials have also shown that they may reduce the postoperative inflammatory response (Bagry 2008; Martin 2008).
Peripheral nerve blocks offer a number of advantages for postoperative analgesia following major knee surgery. It has been reported that they result in better analgesic control, fewer opioid‐related side effects, earlier improvements in knee flexion, and less pain during rehabilitation (Chan 2013; Sakai 2013). Additionally, they avoid motor blockade to the non‐operated leg, thereby encouraging early ambulation and relieving psychological stress to some degree.
It is worth noting that performing a peripheral nerve block is limited by certain factors such as the operative position of the patient. In addition, safety factors, such as the potential infection risk and the risk of exceeding the safe dose of local anesthetic, may even potentially become life‐threatening (Tantry 2012).
Why it is important to do this review
Peripheral nerve blocks may be effective for postoperative pain after major knee surgery. Most randomized controlled trials (RCTs) conclude that they relieve postoperative pain and decrease consumption of opioids, although studies have included a relatively small number of participants.
A number of meta‐analyses have been conducted on related topics in recent years. However, most of them focus on specific types of peripheral nerve block, such as the femoral nerve (Chan 2014; Paul 2010), sciatic nerve (Abdallah 2011) and continuous peripheral nerve block (Richman 2006), and the surgical procedures studied have mainly been limited to total knee arthroplasty. It is important to evaluate the effect of a range of peripheral nerve blocks after major knee surgery.
Objectives
To examine the efficacy and safety of peripheral nerve blocks for postoperative pain control following major knee surgery using methods that permit comparison with systemic, local infiltration, epidural and spinal analgesia.
Methods
Criteria for considering studies for this review
Types of studies
We include participant‐blind, prospective, randomized controlled clinical trials. We exclude concurrent cohort studies and observational studies, as well as case series and case reports without a control group.
In order to maintain participant blinding, placebo catheters normally need to be inserted, which may cause unnecessary discomfort and raise the risk of infection (Chan 2013). Therefore, this insertion is not obligatory but studies should ensure that participants have the same expectations of treatment throughout the entire treatment process. For example, all procedures could be performed behind a drape to block the participant's view, dressings could be placed on injection sites and blocks could be performed by anesthetists who are not involved with data collection (Allen 1998).
Types of participants
We include studies of adult participants (15 years or older) who underwent major knee surgery. The procedure could be either total knee arthroplasty, arthrolysis, anterior cruciate ligament reconstruction or any other major surgeries performed on the knee.
Types of interventions
We include studies that compare the analgesic effect of peripheral nerve block versus systemic, local infiltration, epidural or spinal analgesia following major knee surgery. We include all subtypes of peripheral nerve block. Co‐interventions are permitted in the review, but are required to be the same in both the intervention and comparator groups.
Types of outcome measures
We consider pain intensity measured as greater than 30 mm on a 100 mm visual analogue scale (VAS) to equate to 'at least moderate pain'. To clarify, we consider an intensity of less than or equal to 30 mm to be 'no worse than mild pain' (Collins 1997; Moore 2013).
Primary outcomes
-
Pain intensity assessed on a 100 mm VAS on the day of surgery and within the 72 hours following surgery (divided into three time intervals: zero to 23 hours, 24 to 47 hours, 48 to 72 hours) at rest or on movement. We standardized pain intensity data described by other means than a 100 mm VAS to such a scale.
Secondary outcomes
-
Proportion of participants with 'no worse than mild pain' (pain intensity of less than or equal to 30 mm of 100 mm VAS).
-
Additional analgesic consumption within 72 hours after surgery (divided into three time intervals: zero to 23 hours, 24 to 47 hours, 48 to 72 hours) and median time to remedication.
-
Adverse events within 72 hours after surgery (divided into three time intervals: zero to 23 hours, 24 to 47 hours, 48 to 72 hours).
-
Knee range of motion within 72 hours after surgery (divided into three time intervals: zero to 23 hours, 24 to 47 hours, 48 to 72 hours) and median time to ambulation.
-
Length of hospital stay and hospital costs.
-
Participant satisfaction.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases from the date of inception:
-
The Cochrane Central Register of Controlled Trials (CENTRAL), on The Cochrane Library Issue 1 of 12, 2014
-
MEDLINE & MEDLINE in Process (OVID) 1946 to 10th February 2014
-
EMBASE (OVID) 1974 to 10th February 2014
See Appendix 1 for the search strategies. We applied no language restrictions.
Searching other resources
We carried out handsearching of the reference lists of retrieved articles for relevant trials not identified by the electronic searches. We identified any ongoing trials by searching trial registries on 10th February 2014, including the metaRegister of controlled trials (mRCT), clinicaltrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
Data collection and analysis
Selection of studies
We initially determined eligibility by reading the titles retrieved from each search. We then screened all remaining articles by reading each abstract. Two review authors (JX, XC) evaluated the studies independently, resolving disagreements by discussion or, if necessary, by recourse to a third review author (XW). We documented the selection process in sufficient detail to complete a PRISMA flowchart (Liberati 2009; Figure 1). We created a table of Characteristics of included studies.
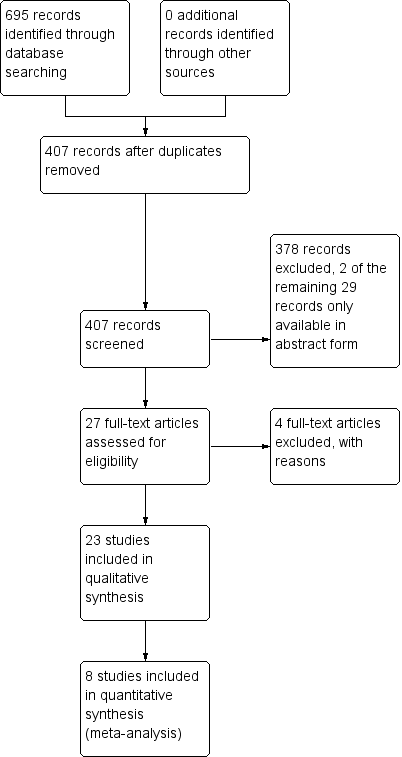
Study flow diagram.
Data extraction and management
We extracted data from the included trials (JX, XC), resolving disagreements through recourse to a third review author (XW). We used a standard data extraction form to tabulate the extracted data. This form included:
-
participant characteristics (total numbers, age and gender of each person);
-
type of knee surgery;
-
type of analgesia and related details;
-
pain intensity, both at rest and on movement, from the day of surgery to 72 hours after surgery;
-
additional analgesic requirements (name and amount of analgesic consumed, median time to remedication);
-
safety (incidence and degree of adverse events);
-
rehabilitation indices (median time to ambulation and knee range of motion);
-
length of hospital stay and hospital costs;
-
participant satisfaction.
Assessment of risk of bias in included studies
We used the Oxford quality and validity scales to assess the risk of bias in included studies (Jadad 1996). Two review authors (JX, XC) assessed each study independently and resolved any disagreement through discussion, with recourse to a third review author (XW) if necessary.
The scales are as follows.
-
Is the study randomized? If yes add one point.
-
Is the randomization procedure reported and is it appropriate? If yes add one point, if no deduct one point.
-
Is the study double‐blind? If yes add one point.
-
Is the double‐blind method reported and is it appropriate? If yes add one point, if no deduct one point.
-
Are the reasons for participant withdrawals and drop‐outs described? If yes add one point.
We also used the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to further assess the risk of bias.
The criteria are as follows:
Sequence generation (checking for possible selection bias)
We assessed the method used in each included study to generate the allocation sequence. The judgment was as follows: low risk of bias (a random component was described in the process, e.g. random number table; computer random number generator; coin tossing); high risk of bias (a non‐random component is described in the process, e.g. odd or even date of birth; date of admission; hospital or clinical record number); unclear risk of bias.
Allocation concealment (checking for possible selection bias)
We assessed the method used in each included study to conceal the allocation sequence. The judgment was as follows: low risk of bias (assignment could not be foreseen, e.g. central allocation; sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes); high risk of bias (assignment could possibly be foreseen, e.g. using an open random allocation schedule; assignment envelopes were used without appropriate safeguards; alternation or rotation); unclear risk of bias.
Blinding of participants and personnel (checking for possible performance bias)
We assessed the method used in each included study to blind study participants and personnel from knowledge of which intervention a participant received. The judgment was as follows: low risk of bias (no blinding or incomplete blinding, but the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken); high risk of bias (no blinding or incomplete blinding, or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding); unclear risk of bias.
Blinding of outcome assessment (checking for possible detection bias)
We assessed the method used in each included study to blind outcome assessors from knowledge of which intervention a participant received. The judgment was as follows: low risk of bias (no blinding of outcome assessment, but the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken); high risk of bias (no blinding of outcome assessment, or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding); unclear risk of bias.
Incomplete outcome data (checking for possible attrition bias)
We assessed the completeness of outcome data in each included study. The judgment was as follows: low risk of bias (e.g. no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome; missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups); high risk of bias (e.g. reasons for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups); unclear risk of bias.
Selective reporting (checking for possible reporting bias)
We stated how the possibility of selective outcome reporting is examined and what is found. The judgment was as follows: low risk of bias (the study protocol is available and all of the prespecified outcomes that are of interest in the review are reported in the prespecified way; the study protocol is not available but the published report includes all expected outcomes); high risk of bias (e.g. not all of the prespecified primary outcomes are reported; one or more primary outcomes are reported using measurements, analysis methods or subsets of the data that were not prespecified; one or more reported primary outcomes are not prespecified); unclear risk of bias.
Size of study (checking for possible biases related to small size)
We assessed studies as follows: low risk of bias (more than or equal to 200 participants per treatment arm); high risk of bias (fewer than 50 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm).
Other sources of bias
We stated any important concerns about bias that are not addressed by the other domains. The judgment was as follows: low risk of bias (the study appears to be free of other sources of bias); high risk of bias (at least one important risk of bias due to problems not covered elsewhere in the criteria above); unclear risk of bias.
Measures of treatment effect
We carried out statistical analysis of treatment effects using the Review Manager 5 software (RevMan 2014) where two or more studies investigated the same outcome.
For dichotomous data, we present results as a summary risk ratio (RR) and 95% confidence interval (95% CI). Where possible, we calculated number needed to treat for an additional beneficial outcome (NNTB) or for an additional harmful outcome (NNTH), together with 95% CIs. For continuous data, we used the mean difference (MD) and the 95% CI for similar outcome measures. When the continuous data were presented as the median and interquartile range, we estimated the mean as equivalent to the median and the standard deviation as a quarter of the interquartile range. When the continuous data were presented as a 95% CI, we calculated the standard deviation from the formula SD = √ ̄N ×(upper limit ‐ lower limit)/3.92 (Higgins 2011). We report individual study results if pooling of data was not possible.
Unit of analysis issues
We had intended to describe special issues in the analysis of studies with non‐standard designs, but we found no such studies.
Dealing with missing data
If some data were not reported or not clearly reported for some outcomes or groups, we attempted to contact the study authors for further information.
We had intended to conduct an intention‐to‐treat (ITT) analysis to estimate whether the intervention effect was biased if some participants were excluded from analysis in the randomized trials, or if they were lost to follow‐up. We would have analyzed available data on all participants in each arm, regardless of what happened subsequently (Newell 1992).
We had intended to use the baseline observation carried forward (BOCF) approach to avoid the analgesic effect calculation of the original intervention being affected by remedication. After remedication, pain intensity would revert to its initial value and pain relief would become zero for all subsequent time points (Moore 2005). However, there was insufficient information to implement this.
Assessment of heterogeneity
We used the I² statistic (Higgins 2003) to identify whether there was statistical heterogeneity. If there was no significant heterogeneity (I² value 0% to 40%), we used a fixed‐effect model for meta‐analysis. We used a random‐effects model if the I² value was above 40%.
Assessment of reporting biases
The methods of assessing the risk of reporting bias are described above in 'Selective reporting (checking for possible reporting bias)' (Assessment of risk of bias in included studies). When we suspected reporting bias, we performed a sensitivity analysis to explore whether the related studies contributed severe bias. We intended to use funnel plots to explore publication bias. However, all analyses contained fewer than 10 studies, so the power of the tests was too low to distinguish chance from real asymmetry (Higgins 2011).
Data synthesis
We used a fixed‐effect model to conduct meta‐analysis if data were homogeneous, but otherwise a random‐effects model. Where the data were unsuitable for meta‐analysis, we described the findings of multiple studies separately.
Subgroup analysis and investigation of heterogeneity
We took into consideration several potential sources of heterogeneity. Firstly, the types and doses of analgesics applied in each intervention were not standardized between studies. Secondly, peripheral nerve blocks may be administered as single‐shot or continuous doses, and continuous peripheral nerve blocks increased the duration of the analgesic effect beyond that of single‐shot peripheral nerve blocks. Thirdly, applying the intervention to different locations, such as femoral nerve block only, or combining a femoral with a sciatic or obturator nerve block, may have different analgesic effects. Fourthly, major knee surgery included various types of operation. Peripheral nerve blocks may exert different effects on the participants undergoing different surgeries. However, we only managed to conduct subgroup analysis according to the types of surgery. There was insufficient information (a minimum of two studies and 200 participants) (Moore 1998) to carry out subgroup analysis to check whether other differences affect the results.
Sensitivity analysis
We intended to perform sensitivity analyses to explore the effect of quality score (two versus three or more) and trial size (39 or fewer versus 40 or more per treatment arm) for important outcomes in the review.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies
Results of the search
We screened 407 papers from CENTRAL, MEDLINE, and EMBASE. After reading the titles and abstracts, we identified 27 papers as potentially eligible for inclusion. We excluded four papers after reading the full text. Two studies were only available in the form of abstract and we failed to get further information from the authors. See: Characteristics of studies awaiting classification. Finally, we identified 23 studies that met the inclusion criteria. The study selection process is summarized in Figure 1. We also found three ongoing studies, which will be considered for inclusion in the update of this review. See: Characteristics of ongoing studies.
Included studies
All of the included studies recruited participants older than 15 years and the sex ratio was balanced. Participants were in good general health, and were excluded if they had an American Society of Anesthesiologists (ASA) score worse than Ⅲ. Two studies (Good 2007; Widmer 2012) did not report the ASA score of their participants. With regard to the types of surgery, 14 studies (Allen 1998; Carli 2010; Chan 2012; Ganapathy 1999; Good 2007; Hirst 1996; Ilfeld 2010; Jenstrup 2012; Moghtadaei 2014; Ng 2001; Szczukowski 2004; Wang 2002; Widmer 2012; Xie 2012) evaluated people undergoing total knee arthroplasty. Six studies (Espelund 2013; Lundblad 2011; Matava 2009; Mulroy 2001; Williams 2006; Wulf 2010) evaluated people undergoing anterior cruciate ligament reconstruction. Two studies (Schultz 1991; Tierney 1987) evaluated those undergoing open knee surgery, and one study (Akkaya 2008) evaluated people undergoing partial menisectomy arthroscopically.
The included studies varied in the types of peripheral nerve blocks. There was respectively one included study used saphenous nerve block (Akkaya 2008), infrapatellar nerve block (Lundblad 2011), and femoral nerve block plus sciatic nerve block (Allen 1998). Two studies (Espelund 2013; Jenstrup 2012) used adductor canal block. Five studies (Ganapathy 1999; Hirst 1996; Ng 2001; Schultz 1991; Xie 2012) used three‐in‐one block. Thirteen studies used femoral nerve block, among which, three studies (Carli 2010; Ilfeld 2010; Williams 2006) administered block continuously via a catheter and a pump, while the other 10 studies (Chan 2012; Good 2007; Matava 2009; Moghtadaei 2014; Mulroy 2001; Szczukowski 2004; Tierney 1987; Wang 2002; Widmer 2012; Wulf 2010) used a single shot. Anesthetic used in the blocks was also variable. Fourteen studies (Allen 1998; Chan 2012; Ganapathy 1999; Good 2007; Hirst 1996; Matava 2009; Mulroy 2001; Ng 2001; Schultz 1991; Szczukowski 2004; Tierney 1987; Wang 2002; Wulf 2010; Xie 2012) used bupivacaine; three studies (Akkaya 2008; Lundblad 2011; Williams 2006) used levo bupivacaine; eight studies (Carli 2010; Espelund 2013; Ilfeld 2010; Jenstrup 2012; Moghtadaei 2014; Ng 2001; Widmer 2012; Wulf 2010) used ropivacaine. To locate the nerve, 16 studies (Akkaya 2008; Allen 1998; Carli 2010; Ganapathy 1999; Good 2007; Hirst 1996; Ilfeld 2010; Matava 2009; Moghtadaei 2014; Mulroy 2001; Ng 2001; Schultz 1991; Szczukowski 2004; Wang 2002; Widmer 2012; Wulf 2010) used nerve stimulators; three studies (Espelund 2013; Jenstrup 2012; Lundblad 2011) used ultrasound guidance. There was one study (Chan 2012) combining ultrasound imaging and nerve stimulators. One study (Tierney 1987) located the nerve without the aid of any instrument. The remaining two studies (Williams 2006; Xie 2012) did not describe the exact methods. In terms of block timing, eight studies (Akkaya 2008; Allen 1998; Good 2007; Hirst 1996; Ilfeld 2010; Lundblad 2011; Widmer 2012; Xie 2012) performed this before the induction of anesthesia; two studies (Matava 2009; Wulf 2010) performed this after anesthetic induction but before surgical incision; and 12 studies (Carli 2010; Espelund 2013; Ganapathy 1999; Jenstrup 2012; Moghtadaei 2014; Mulroy 2001; Ng 2001; Schultz 1991; Szczukowski 2004; Tierney 1987; Wang 2002; Williams 2006) performed this at the end of surgery. Chan 2012 had two intervention groups, with one performing block after anesthesia but before the surgical procedure, and the other group performing this postoperatively.
Nineteen studies (Akkaya 2008; Allen 1998; Chan 2012; Espelund 2013; Ganapathy 1999; Good 2007; Hirst 1996; Ilfeld 2010; Jenstrup 2012; Lundblad 2011; Matava 2009; Mulroy 2001; Ng 2001; Szczukowski 2004; Tierney 1987; Wang 2002; Williams 2006; Wulf 2010; Xie 2012) compared peripheral nerve blocks as adjunctive techniques to systemic analgesia with systemic analgesia alone. Three studies (Carli 2010; Moghtadaei 2014; Widmer 2012) compared peripheral nerve blocks with local infiltration and one study (Schultz 1991) compared peripheral nerve blocks with epidural analgesia; all four used systemic analgesia as remedication.
Ten included studies (Allen 1998; Ganapathy 1999; Hirst 1996; Ilfeld 2010; Jenstrup 2012; Mulroy 2001; Schultz 1991; Szczukowski 2004; Tierney 1987; Wulf 2010) presented the outcomes in the form of graphs without exact data. We attempted to acquire the data through email and acquired the data for one study (Jenstrup 2012).
We describe the details of the included studies in the 'Characteristics of included studies' table.
Excluded studies
All four excluded studies (Bogoch 2002; De Lima 2008; Frassanito 2010; Hunt 2009) were randomized trials comparing peripheral nerve blocks with other analgesia methods. Among them, Bogoch 2002 recruited participants undergoing either total hip arthroplasty or total knee arthroplasty, but with the outcomes for participants undergoing total knee arthroplasty not presented separately. The remaining three studies claimed to be participant‐blind. However, sham block was not performed in the comparator treatment group, and the measures to maintain participant blinding were not described.
We describe the details of the excluded studies in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
All 23 included studies were randomized and either double‐blind or participant‐blind. Two review authors scored each study independently for quality, using the Oxford quality and validity scale, with a maximum possible score of five. Nine included studies (Carli 2010; Espelund 2013; Ilfeld 2010; Jenstrup 2012; Lundblad 2011; Matava 2009; Moghtadaei 2014; Szczukowski 2004; Williams 2006) scored five, nine studies (Chan 2012; Good 2007; Mulroy 2001; Ng 2001; Schultz 1991; Tierney 1987; Wang 2002; Widmer 2012; Xie 2012) scored four, and the remaining five studies (Akkaya 2008; Allen 1998; Ganapathy 1999; Hirst 1996; Wulf 2010) scored three. The quality scores for individual studies are recorded in the 'Characteristics of included studies' table.
Risk of bias is also presented in the 'Risk of bias' graph and 'Risk of bias' summary (Figure 2; Figure 3).
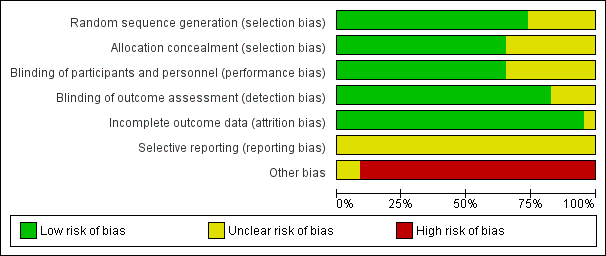
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
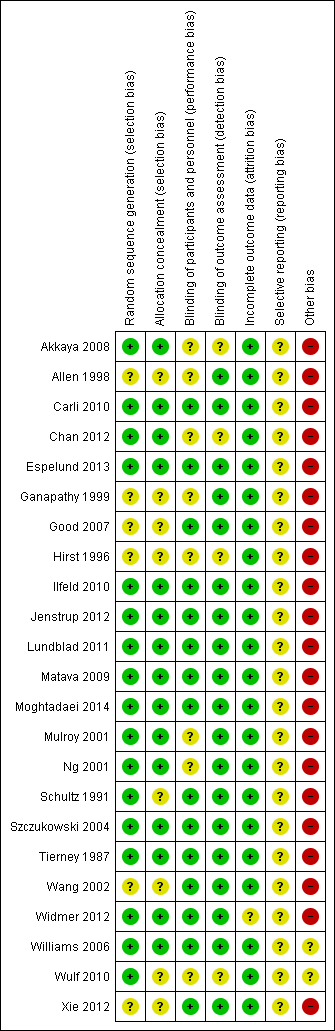
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All 23 included studies reported that they were randomized, while only 15 of them (Akkaya 2008; Carli 2010; Chan 2012; Espelund 2013; Ilfeld 2010; Jenstrup 2012; Lundblad 2011; Matava 2009; Moghtadaei 2014; Mulroy 2001; Ng 2001; Szczukowski 2004; Tierney 1987; Widmer 2012; Williams 2006) described a detailed method to generate a random sequence and to conceal allocation.
Blinding
Fifteen of the included studies (Carli 2010; Espelund 2013; Good 2007; Ilfeld 2010; Jenstrup 2012; Lundblad 2011; Matava 2009; Moghtadaei 2014; Schultz 1991; Szczukowski 2004; Tierney 1987; Wang 2002; Widmer 2012; Williams 2006; Xie 2012) were double‐blind and adequately described how this was achieved. Although three studies (Chan 2012; Hirst 1996; Ng 2001) were stated to be double‐blind, they only adequately described how they blinded the participants, but did not describe the exact method to blind the investigators. Five studies (Akkaya 2008; Allen 1998; Ganapathy 1999; Mulroy 2001; Wulf 2010) achieved participant‐blinding through use of a sham block with saline or by performing nerve block behind a drape to block the participant's view. But these five studies did not report whether or not the investigators were blinded to the treatment assignment. For the blinding of outcome assessment, 19 of the included studies (Allen 1998; Carli 2010; Espelund 2013; Ganapathy 1999; Good 2007; Ilfeld 2010; Jenstrup 2012; Lundblad 2011; Matava 2009; Moghtadaei 2014; Mulroy 2001; Ng 2001; Schultz 1991; Szczukowski 2004; Tierney 1987; Wang 2002; Widmer 2012; Williams 2006; Xie 2012) stated that outcomes were assessed by blinded assessors, while the remaining four studies (Akkaya 2008; Chan 2012; Hirst 1996; Wulf 2010) did not describe this.
Incomplete outcome data
Eleven of the included studies (Akkaya 2008; Allen 1998; Carli 2010; Ganapathy 1999; Hirst 1996; Matava 2009; Ng 2001; Schultz 1991; Szczukowski 2004; Tierney 1987; Wang 2002) reported no missing outcome data. Another 11 studies (Chan 2012; Espelund 2013; Good 2007; Ilfeld 2010; Jenstrup 2012; Lundblad 2011; Moghtadaei 2014; Mulroy 2001; Williams 2006; Wulf 2010; Xie 2012) reported missing outcome data. Reasons for the missing data were acceptable, and did not seem to have potential impact on outcomes. One study (Widmer 2012) had 55 participants who met the inclusion criteria and were allocated, but analyzed only 54 participants. The reason for lost follow‐up was not described.
Selective reporting
All the included studies reported our main outcome of pain intensity but did not cover all the secondary outcomes of interest. We rated all of them as unclear for risks of selective reporting.
Other potential sources of bias
According to the 'Checking for possible biases related to small size' section of Assessment of risk of bias in included studies, only two included studies had 50 to 199 participants per treatment arm, and were therefore assessed as being at unclear risk of bias. The remaining 21 studies had fewer than 50 participants per treatment arm, and were assessed as being at high risk of bias.
Effects of interventions
1. Peripheral nerve blocks adjunctive to systemic analgesia versus systemic analgesia alone
We include 19 studies comparing peripheral nerve blocks as adjunctive techniques to systemic analgesia with systemic analgesia alone (Akkaya 2008; Allen 1998; Chan 2012; Espelund 2013; Ganapathy 1999; Good 2007; Hirst 1996; Ilfeld 2010; Jenstrup 2012; Lundblad 2011; Matava 2009; Mulroy 2001; Ng 2001; Szczukowski 2004; Tierney 1987; Wang 2002; Williams 2006; Wulf 2010; Xie 2012). Some trials did not report every outcome prespecified in our protocol, and some trials did not report the outcome at every time interval of interest. Some trials presented data in graph form that we could not accurately extract. We were therefore only able to include eight of the 19 studies in any of our meta‐analyses. All meta‐analyses were for the primary outcome of pain intensity.
Primary outcomes:
Pain intensity (Analyses 1.1 to 1.6)
Pain intensity was assessed on a visual analogue scale (VAS) by participants themselves or by blinded assessors. Different authors reported this outcome on different scales. We standardized all of them to a 100 mm VAS so that we could analyze them in the review. Where the pain score was given but was not defined as measuring pain at rest or on movement, we considered it to be at rest.
Data were generally reported as the average pain intensity of a time period. However, in studies in which the only data available were measured at a single time point within the interval of interest, we used this measurement to represent the average pain intensity of that time period. Where measurements were reported at several time points within the interval of interest, we selected the one that measured at the time point closest to the mid‐point of that interval. To clarify, an outcome measured at the time point closest to the 12th hour represented the 'zero to 23 hours' interval, the time point closest to the 36th hour represented the '24 to 47 hours' interval, and the time point closest to the 60th hour represented the '48 to 72 hours' interval.
Seven studies examined pain scores at rest in the interval from zero to 23 hours, involving 390 participants with 196 in the peripheral nerve blocks adjunctive to systemic analgesia group and 194 in the systemic analgesia alone group, respectively. Meta‐analysis showed that the former group had a mean VAS score 11.85 points lower than the latter group (95% confidence interval (CI) ‐20.45 to ‐3.25, I² value 87%, random‐effects model) (Analysis 1.1). Pain intensity at rest in the 24 to 47 hours interval was reported in six studies (320 participants, 162 with peripheral nerve blocks adjunctive to systemic analgesia and 158 with systemic analgesia alone, respectively), and showed significantly lower mean VAS in the adjunctive group than that in the control group (mean difference (MD) ‐12.92, 95% CI ‐19.82 to ‐6.02, I² value 72%, random‐effects model) (Analysis 1.2). Four studies (210 patients, 109 with peripheral nerve blocks adjunctive to systemic analgesia and 101 with systemic analgesia alone, respectively) analyzed pain intensity at rest in the 48 to 72 hours interval. Mean VAS of the adjunctive group was reported to be 9.72 points lower than that of the control group (95% CI ‐16.75 to ‐2.70, I² value 54%, random‐effects model) (Analysis 1.3). Five studies reported pain scores on movement in the zero to 23 hours interval (304 patients, 150 with peripheral nerve blocks adjunctive to systemic analgesia and 154 with systemic analgesia alone, respectively) and there appeared to be no significant difference between these two groups (Analysis 1.4). Only three studies (182 participants) reported results of VAS on movement in the 24 to 47 hours, and two studies (112 participants) reported results of VAS in the 48 to 72 hours interval. For the former comparison (24 to 47 hours), there was no significant difference between the groups (Analysis 1.5), while the latter comparison (48 to 72 hours) showed that the adjunctive group had a 6.19 points lower mean VAS than the control group (95% CI ‐11.76 to ‐0.62, I² value 0%, fixed‐effect model) (Analysis 1.6). There was substantial heterogeneity between studies in most of the analyses, except for Analysis 1.6. The heterogeneity was likely due to different study protocols, different types of surgery, different times at which blocks were performed, and different time points of postoperative pain assessment. The results should therefore be interpreted cautiously.
We stratified pain intensity by the type of surgery, and were able to create two subgroups: total knee arthroplasty with eight studies, and anterior cruciate ligament reconstruction with four studies. The number of studies for the remaining types of surgery was insufficient to create other subgroups, because only one study investigated partial menisectomy arthroscopically. In the sub‐category of total knee arthroplasty, peripheral nerve blocks adjunctive to systemic analgesia showed significantly superior pain relief to systemic analgesia alone at rest in all three time intervals within 72 hours (MD ‐19.50, 95% CI ‐35.01 to ‐3.99 ( three studies, 183 participants, Analysis 1.1); MD ‐15.05, 95% CI ‐24.11 to ‐5.98 (four studies, 224 participants, Analysis 1.2); MD ‐13.73, 95% CI ‐18.21 to ‐9.26 (three studies, 154 participants, Analysis 1.3) respectively). In the zero to 23 hours postoperative interval, there was no significant difference in the VAS for participants undergoing total knee arthroplasty on movement (MD ‐12.93, 95% CI ‐26.62 to 0.75, two studies, 153 participants, Analysis 1.4) or for participants undergoing anterior cruciate ligament reconstruction at rest (MD ‐2.82, 95% CI ‐8.03 to 2.38, three studies, 167 participants, Analysis 1.1) or on movement (MD 0.10, 95% CI ‐7.49 to 7.70, two studies, 111 participants, Analysis 1.4). There were insufficient data to conduct a subanalysis in the 24 to 47 hours and 48 to 72 hours intervals postoperatively for anterior cruciate ligament reconstruction.
Sensitivity analysis
All of the included studies were ranked as equal to or better than 3/5 on the Oxford quality and validity scale. Only one study (Chan 2012) in the meta‐analysis had 40 or more participants per treatment arm, and removing the outcome of this study did not affect the results.
Secondary outcomes:
Proportion of participants with 'no worse than mild pain'
Two studies (Lundblad 2011; Williams 2006) reported the proportion of participants with 'no worse than mild pain'. Lundblad 2011 observed that in the zero to 23 hours interval postoperatively, the percentage of participants with 'no worse than mild pain' was significantly higher in the adjunctive group as compared to the control group at rest but not on movement. Data were only available in graph form. Williams 2006 recorded that in the 24 to 47 hours interval postoperatively, the proportion of 'no worse than mild pain' in the systemic analgesia alone group, single peripheral nerve block adjunctive to systemic analgesia group, and continuous peripheral nerve block adjunctive to systemic analgesia group was 36.8%, 52.6%, and 73.7% respectively. In the 48 to 72 hours interval postoperatively, the proportions were 39.5%, 46.8%, and 68% respectively.
Additional analgesic consumption within 72 hours after surgery and median time to remedication
Eighteen included studies recorded additional analgesic consumption within 72 hours postoperatively. Only three of them (Akkaya 2008; Chan 2012; Jenstrup 2012) presented data in the form of a mean and standard deviation; Akkaya 2008 reported less tramadol consumption in the adjunctive group in the zero to 23 hours interval (mean consumption: 108.5 mg versus 217.1 mg). Chan 2012 and Jenstrup 2012 reported less morphine consumption in the adjunctive group in the zero to 23 hours interval (mean consumption:15.71 mg versus 29.82 mg, and 40 mg versus 56 mg, respectively). Among the remaining 15 studies, four studies (Espelund 2013; Hirst 1996; Ilfeld 2010; Matava 2009) reported additional analgesic use did not differ between the two groups, while 11 studies (Allen 1998; Ganapathy 1999; Good 2007; Mulroy 2001; Ng 2001; Szczukowski 2004; Tierney 1987; Wang 2002; Williams 2006; Wulf 2010; Xie 2012) found less additional analgesic consumption in the adjunctive group than in the control group. In addition, three studies (Chan 2012; Tierney 1987; Wulf 2010) reported that participants in the adjunctive group had a significantly longer time to first request for additional analgesic than the control group.
Adverse events
Seventeen of the included studies reported various types of adverse events, including nausea, vomiting, sedation, pruritus, urinary retention, fever, and motor block. We were unable to conduct a meta‐analysis on specific type of adverse event. Among them, 11 studies (Akkaya 2008; Allen 1998; Espelund 2013; Ganapathy 1999; Good 2007; Jenstrup 2012; Matava 2009; Mulroy 2001; Ng 2001; Williams 2006; Xie 2012) observed no significant difference in the incidence of adverse events between the adjunctive group and the control group. Four studies (Chan 2012; Hirst 1996; Szczukowski 2004; Wang 2002) found that those in the adjunctive group had significant reductions in adverse events compared with the control group. Two studies (Ilfeld 2010; Wulf 2010) reported that motor block was significantly increased in the adjunctive group compared with the control group, and Ilfeld 2010 observed that three of the 39 participants in the adjunctive group fell within 72 hours postoperatively. None of the included studies reported any withdrawal of cases due to the adverse events.
Knee range of motion within 72 hours after surgery
Knee range of motion included range of flexion and range of extension, which was reported in six studies (Chan 2012; Ganapathy 1999; Good 2007; Ilfeld 2010; Szczukowski 2004; Wang 2002) within 72 hours postoperatively. Most of them did not mention whether the knee range of motion was active or passive, except for Ilfeld 2010, who recorded that the knee range of motion was achieved in a passive way. Among all six studies, only Wang 2002 reported knee range of flexion, and found a significantly higher degree in the adjunctive group than in the control group in the 48 to 72 hours interval postoperatively (mean degree: 70° versus 60°). Ganapathy 1999 observed increased range of motion achieved by the participants in the adjunctive group over 24 to 47 hours postoperatively, but without exact data. Chan 2012, Ilfeld 2010 and Szczukowski 2004 reported that there was no significant difference in range of knee motion over 24 to 47 hours or over 48 to 72 hours postoperatively. Good 2007 also reported no significant difference in range of knee motion over 48 to 72 hours postoperatively. However, Ilfeld 2010 and Szczukowski 2004 presented the outcomes in a form that could not be standardized to the mean and the standard deviation; pooling of data was therefore not possible.
Length of hospital stay and hospital costs
Four included studies (Matava 2009; Ng 2001; Szczukowski 2004; Wang 2002) reported the length of hospital stay, while two of them presented the outcomes in a form that could not be standardized to the mean and the standard deviation. Wang 2002 showed that length of hospital stay was significantly shorter in the adjunctive group than in the control group (mean length: 3 days versus 4 days). However, Matava 2009, Ng 2001 and Szczukowski 2004 reported no significant difference between the two groups. Only one study (Matava 2009) reported hospital costs, and found no significant difference between the two groups.
Participant satisfaction
Four studies (Allen 1998; Hirst 1996; Matava 2009; Xie 2012) recorded overall satisfaction with the pain management on a 5‐point scale or a 10‐point scale ranked by participants, while three of them presented the outcomes in a form that could not be standardized to the mean and the standard deviation. Allen 1998, Hirst 1996 and Matava 2009 reported no difference between the adjunctive group and the control group. Xie 2012 reported that participants in the low‐dose block adjunctive to systemic analgesia group were more likely to have higher satisfaction than those in the systemic analgesia alone group (odds ratio (OR) 5.64, 95% CI 1.60 to 19.8) and than participants in high‐dose block adjunctive to systemic analgesia group (OR 5.34, 95% CI 1.53 to 18.7).
2. Peripheral nerve blocks versus local infiltration
We include three studies comparing peripheral nerve blocks with local infiltration (Carli 2010; Moghtadaei 2014; Widmer 2012). There were too few data to conduct a meta‐analysis. We therefore describe the findings of the individual studies separately.
Primary outcomes:
Pain intensity
Two studies (Carli 2010; Moghtadaei 2014) reported pain intensity within 72 hours postoperatively. Carli 2010 reported a trend of less pain at rest in the block group (mean VAS: 20 mm versus 50 mm in the 24 to 47 hours interval, 10 mm versus 30 mm in the 48 to 72 hours interval), but on walking, there was no significant difference in pain intensity between the two groups. Pain intensity at greatest knee flexion was also similar between these two groups in the 24 to 47 hours interval and the 48 to 72 hours interval postoperatively. Moghtadaei 2014 reported that participants in the local infiltration group suffered less pain in the first six hours after surgery (mean VAS: 30 mm versus 40 mm), while the block group reported better pain relief within 12 hours postoperatively (mean VAS: 50 mm versus 60 mm). Eventually, there appeared to be no significant difference in pain score between the two groups 24 hours after surgery.
Secondary outcomes:
Additional analgesic consumption within 72 hours after surgery
Two studies (Moghtadaei 2014; Widmer 2012) reported additional analgesic consumption. Moghtadaei 2014 reported consumption of morphine or tramadol, and found significantly less consumption in the local infiltration group than in the block group in the first 24 hours after surgery (mean consumption:10 mg versus 12.5 mg). However, no difference was demonstrated between the two groups within 48 hours postoperatively. Widmer 2012 reported consumption of fentanyl, and observed that the block group consumed significantly less fentanyl than the local infiltration group in the first 24 hours postoperatively (mean consumption: 973 mg versus 1502 mg).
Knee range of motion within 72 hours after surgery and median time to ambulation
Two studies (Carli 2010; Moghtadaei 2014) reported median time to ambulation. Median time for each participant to have the first walk was about 24 hours in Carli 2010 and 12 hours in Moghtadaei 2014, with no significant difference between the block group and the local infiltration group. Carli 2010 reported knee range of motion within 72 hours postoperatively and observed no significant difference between the two groups over 24 to 47 hours and 48 to 72 hours postoperatively. Whether the knee range of motion was achieved actively or passively was not mentioned.
Length of hospital stay
Two studies (Carli 2010; Moghtadaei 2014) reported length of hospital stay. Both studies showed no significant difference between the two groups.
Participant satisfaction
Only one study (Moghtadaei 2014) reported a satisfaction scale ranked by participants, which did not reveal a significant difference between the two groups within 48 hours after surgery.
3. Peripheral nerve blocks versus epidural analgesia
We include only one study comparing peripheral nerve blocks with epidural analgesia (Schultz 1991). This study observed the pain intensity and supplemental morphine consumption only during the first 18 hours postoperatively, and did not find a significant difference between the three‐in‐one nerve block group and the epidural analgesia group. However, the block group had a statistically significantly lower incidence of nausea, vomiting, pruritus and urinary retention than the epidural analgesia group.
Discussion
We included 23 studies (1571 participants) examining the effectiveness and safety of peripheral nerve blocks on pain relief after major knee surgery. Nineteen of the 23 studies compared peripheral nerve blocks as adjunctive techniques to systemic analgesia with systemic analgesia alone, three studies compared peripheral nerve blocks with local infiltration, and one study compared peripheral nerve blocks with epidural analgesia. The studies were of high methodological quality overall. Given the heterogeneity and the small numbers of studies and participants, evidence for or against the efficacy and safety of peripheral nerve blocks is currently inconclusive, and should be interpreted cautiously.
Summary of main results
Compared with systemic analgesia alone, peripheral nerve blocks as adjunctive techniques to systemic analgesia resulted in a lower pain intensity score at rest in the zero to 23 hours interval, the 24 to 47 hours interval and the 48 to 72 hours interval postoperatively. Pain intensity was also reduced on movement in the 48 to 72 hours interval. There appeared to be no significant differences for pain on movement over the time period of zero to 23 hours and 24 to 47 hours. In the subcategory of total knee arthroplasty, meta‐analysis favored peripheral nerve blocks adjunctive to systemic analgesia at rest in all three intervals within 72 hours postoperatively. In the zero to 23 hours postoperative interval, there was no significant difference in the mean visual analogue scale (VAS) for participants undergoing total knee arthroplasty on movement or for participants undergoing anterior cruciate ligament reconstruction at rest and on movement.
Overall completeness and applicability of evidence
Studies were powered to identify the pain relief efficacy of peripheral nerve blocks as adjunctive techniques to systemic analgesia when compared with systemic analgesia alone following major knee surgery, but the numbers of studies and participants were too few to draw conclusions on other outcomes prespecified in our review. Three studies met the inclusion criteria comparing peripheral nerve blocks with local infiltration, and only one study met the inclusion criteria comparing peripheral nerve blocks with epidural analgesia. Data from these four studies were inadequate to be pooled for analysis. We have therefore described the findings of individual studies separately. None of the studies comparing peripheral nerve blocks with spinal analgesia was included in our review.
Quality of the evidence
The evidence for the analgesic efficacy of peripheral nerve blocks following major knee surgery was based on studies of high methodological quality, but sample sizes were somewhat limited (21 of the 23 included studies had fewer than 50 participants per treatment arm). Moreover, there was considerable heterogeneity among studies, likely due to different study protocols, different types of surgery, different times at which blocks were performed, and different time points and scales of postoperative pain assessment. The results should therefore be interpreted with caution.
Potential biases in the review process
We carried out extensive searches to identify relevant studies, and minimised the potential biases by having two review authors complete each assessment of eligibility and data extraction. However, there always remains the possibility of unidentified studies. In addition, all of the included studies used remedication once the pain relief effect of the original intervention seemed insufficient; any benefit seen for peripheral nerve blocks may therefore be a combination of the benefit of peripheral nerve blocks and remedication used. We had intended to use the 'baseline observation carried forward' approach to avoid interference by the effect of remedication, but there was insufficient information to do so. We found no other potential bias in the review process.
Agreements and disagreements with other studies or reviews
Two previous systematic reviews compared femoral nerve block with an intravenous patient‐controlled analgesia opioid, epidural analgesia, local infiltration analgesia, and oral analgesia after total knee arthroplasty (Chan 2014; Paul 2010). We excluded several studies covered by these reviews, because of methodological issues. Despite some differences in approach, the findings of these two systematic reviews were similar to our review in terms of pain relief efficacy of peripheral nerve blocks adjunctive to systemic analgesia compared with systemic analgesia alone. Another previous review (Fowler 2008), comparing peripheral nerve blocks with epidural analgesia after major knee surgery, showed that peripheral nerve blocks provided comparable postoperative analgesia with epidural techniques, and caused neuraxial complication less frequently than epidural analgesia. However, none of the eight included studies was participant‐blind, and therefore did not meet the inclusion criteria of this review.
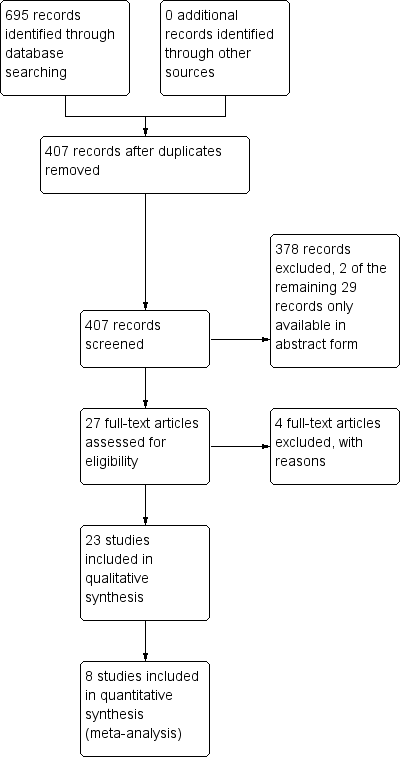
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Peripheral nerve blocks adjunctive to systemic analgesia versus systemic analgesia alone, Outcome 1 Pain intensity at rest (0 ‐ 23 hours postoperatively).

Comparison 1 Peripheral nerve blocks adjunctive to systemic analgesia versus systemic analgesia alone, Outcome 2 Pain intensity at rest (24 ‐ 47 hours postoperatively).

Comparison 1 Peripheral nerve blocks adjunctive to systemic analgesia versus systemic analgesia alone, Outcome 3 Pain intensity at rest (48 ‐ 72 hours postoperatively).

Comparison 1 Peripheral nerve blocks adjunctive to systemic analgesia versus systemic analgesia alone, Outcome 4 Pain intensity on movement (0 ‐ 23 hours postoperatively).

Comparison 1 Peripheral nerve blocks adjunctive to systemic analgesia versus systemic analgesia alone, Outcome 5 Pain intensity on movement (24 ‐ 47 hours postoperatively).

Comparison 1 Peripheral nerve blocks adjunctive to systemic analgesia versus systemic analgesia alone, Outcome 6 Pain intensity on movement (48 ‐ 72 hours postoperatively).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain intensity at rest (0 ‐ 23 hours postoperatively) Show forest plot | 7 | 390 | Mean Difference (IV, Random, 95% CI) | ‐11.85 [‐20.45, ‐3.25] |
| 1.1 Total knee arthroplasty | 3 | 183 | Mean Difference (IV, Random, 95% CI) | ‐19.50 [‐35.01, ‐3.99] |
| 1.2 Anterior cruciate ligament reconstruction | 3 | 167 | Mean Difference (IV, Random, 95% CI) | ‐2.82 [‐8.03, 2.38] |
| 1.3 Partial menisectomy arthroscopically | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐15.60 [‐22.84, ‐8.36] |
| 2 Pain intensity at rest (24 ‐ 47 hours postoperatively) Show forest plot | 6 | 320 | Mean Difference (IV, Random, 95% CI) | ‐12.92 [‐19.82, ‐6.02] |
| 2.1 Total knee arthroplasty | 4 | 224 | Mean Difference (IV, Random, 95% CI) | ‐15.05 [‐24.11, ‐5.98] |
| 2.2 Anterior cruciate ligament reconstruction | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐11.59, 11.39] |
| 2.3 Partial menisectomy arthroscopically | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐15.50 [‐22.27, ‐8.73] |
| 3 Pain intensity at rest (48 ‐ 72 hours postoperatively) Show forest plot | 4 | 210 | Mean Difference (IV, Random, 95% CI) | ‐9.72 [‐16.75, ‐2.70] |
| 3.1 Total knee arthroplasty | 3 | 154 | Mean Difference (IV, Random, 95% CI) | ‐13.73 [‐18.21, ‐9.26] |
| 3.2 Anterior cruciate ligament reconstruction | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐10.60, 9.60] |
| 4 Pain intensity on movement (0 ‐ 23 hours postoperatively) Show forest plot | 5 | 304 | Mean Difference (IV, Random, 95% CI) | ‐6.95 [‐15.92, 2.01] |
| 4.1 Total knee arthroplasty | 2 | 153 | Mean Difference (IV, Random, 95% CI) | ‐12.93 [‐26.62, 0.75] |
| 4.2 Anterior cruciate ligament reconstruction | 2 | 111 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐7.49, 7.70] |
| 4.3 Partial menisectomy arthroscopically | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐7.5 [‐19.91, 4.91] |
| 5 Pain intensity on movement (24 ‐ 47 hours postoperatively) Show forest plot | 3 | 182 | Mean Difference (IV, Random, 95% CI) | ‐8.87 [‐27.77, 10.03] |
| 6 Pain intensity on movement (48 ‐ 72 hours postoperatively) Show forest plot | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐6.19 [‐11.76, ‐0.62] |

