Oxigenoterapia hiperbárica para personas con trastorno del espectro autista (TEA)
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010922.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 13 octubre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Problemas de desarrollo, psicosociales y de aprendizaje
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Conceiving the review: Tao Xiong.
Designing the review: Tao Xiong.
Coordinating the review: Tao Xiong and Dezhi Mu.
Designing search strategies: Tao Xiong, Hongju Chen, and Rong Luo.
Extracting data: Tao Xiong, Hongju Chen, and Rong Luo.
Writing the review: Tao Xiong and Hongju Chen.
Providing general advice on the review: Dezhi Mu.
Securing funding for the review: Tao Xiong and Dezhi Mu.
Sources of support
Internal sources
-
Department of Pediatrics, West China, Second University Hospital, Sichuan University, Chengdu, China.
Salary support for Tao Xiong, Hongju Chen, and Dezhi Mu
External sources
-
Chinese Cochrane Center, West China, Second University Hospital, Sichuan University, Chengdu, China.
Academic support to the team
-
The National Natural Science Foundation of China (No.81330016, 81630038 to Dezhi Mu; No. 81300525 to Tao Xiong); the Foundation of Health and Family Planning Commission of Sichuan Province (No.140044 to Tao Xiong), China.
Financial support to Dezhi Mu and Tao Xiong
-
Cochrane Developmental, Psychosocial and Learning Problems Group, Other.
Academic support to the team
Declarations of interest
Tao Xiong ‐ none known.
Hongju Chen ‐ none known.
Rong Luo ‐ none known.
Dezhi Mu ‐ none known.
Acknowledgements
We thank the staff at the Cochrane Developmental, Psychosocial and Learning Problems Group for assistance in preparation of the review: Professor Geraldine Macdonald, Dr Joanne Wilson, Margaret Anderson, and Gemma O'Loughlin.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Oct 13 | Hyperbaric oxygen therapy for people with autism spectrum disorder (ASD) | Review | Tao Xiong, Hongju Chen, Rong Luo, Dezhi Mu | |
| 2014 Jan 17 | Hyperbaric oxygen therapy for autism spectrum disorder (ASD) in children and adults | Protocol | Tao Xiong, Hongju Chen, Rong Luo, Dezhi Mu | |
Differences between protocol and review
We created a table to detail the differences between the protocol and the review (see Table 1).
| Review section | Change |
| Description of the intervention | We added the paragraph below on 'nonclassical' hyperbaric oxygen therapy: In addition to the 'classical' hyperbaric oxygen therapy defined by the Undersea and Hyperbaric Medical Society (UHMS 2016), some ASD trials have used nonclassical hyperbaric oxygen therapy (Granpeesheh 2010; Rossignol 2009). In these trials, nonclassical hyperbaric oxygen therapy consisted of a chamber pressurized to greater than one ATA with less than 100% oxygen concentration. In one study, both classical and nonclassical hyperbaric oxygen therapies produced significant improvement in children with autism, as evidenced by normal levels of oxidative stress and inflammation markers (Rossignol 2007a). As outlined in our protocol (Xiong 2014), we assessed only the use of classical hyperbaric oxygen therapy in this review. |
| Types of participants | We revised this section as follows: Participants of any age with a diagnosis of autism spectrum disorder (ASD) based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) (APA 2013); or individuals with a diagnosis of one of the four pervasive developmental disorders from fourth edition text revision version of the DSM (DSM‐IV‐TR) (APA 2000), including autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD‐NOS); or from theInternational Classification of Mental and Behavioural Disorders, 10th Edition (ICD‐10; WHO 1993). We accepted diagnoses that were derived following use of assessment tools, such as the Autism Diagnostic Observation Scale (ADOS) (Lord 1997) and the Autism Diagnostic Interview ‐ Revised (ADI‐R) (Lord 1994). |
| Electronic searches | We have specified that, when searching online clinical trial registries such as ClinicalTrials.gov and WHO ICTRP, we checked the publication status of each trial identified and contacted study authors for results of finished unpublished trials. |
| Searching other resources | We have clarified that we handsearched reference lists of relevant studies. We also searched the gray literature from the Internet using the academic search engine "Baidu Scholar." |
| Measures of treatment effect | We have clarified how we will handle final values and changes from baseline data, as follows: When final values and changes from baseline data are available in included trials, we shall analyse them separately. Apart from analysing those values separately, we will combine final values and changes from baseline data using the MD when both types of data are available for the same scale. We will not incorporate skewed data in future analyses. |
| Unit of analysis issues | We explained how we would handle multiple intervention groups. See Appendix 2. |
| Assessment of heterogeneity | We regraded the degree of heterogeneity, as follows (Deeks 2011).
We added the following information: Studies have shown that different estimation methods may lead to different results and conclusions. For example, the DerSimonian and Laird (DL) estimator, which is currently widely used by default to estimate between‐study variance, has been long challenged (Veroniki 2016). The DL estimator can lead to erroneous conclusions (Cornell 2014) or can largely underestimate the true value for dichotomous outcomes (Novianti 2014). For continuous data, the restricted maximum likelihood estimator is a better alternative for estimating between‐study variance when compared with other estimators (Veroniki 2016). We also specified that: We plan to assess heterogeneity by comparing the estimated magnitude of the heterogeneity variance with the empirical distribution of Turner 2012 for dichotomous data and Rhodes 2015 for continuous data. |
| Data synthesis | We have clarified when we will report results of the fixed‐effect model and when we will report results of the random‐effects model, as follows: If no significant heterogeneity is present, we will report the results of the fixed‐effect model only. If significant heterogeneity or severe asymmetry of the funnel plot is observed, we will report the results of the random‐effects model. |
ASD: autism spectrum disorder.
ATA: atmosphere absolute.
DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision.
MD: mean difference.
WHO ICTRP: World Health Organisation International Clinical Trials Registry Platform.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Child; Child, Preschool; Humans;
PICO

Flow diagram.
Footnotes
RCT: randomized controlled trial.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Primary outcome, Outcome 1 Social interaction and communication.
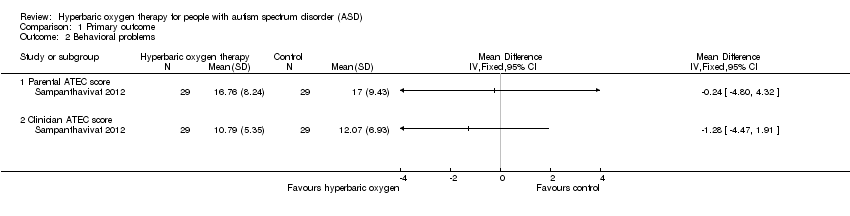
Comparison 1 Primary outcome, Outcome 2 Behavioral problems.

Comparison 2 Second outcome, Outcome 1 Communication and linguistic abilities.

Comparison 2 Second outcome, Outcome 2 Cognitive function.

Comparison 2 Second outcome, Outcome 3 Safety of hyperbaric oxygen therapy.
| Hyperbaric oxygen therapy for autism spectrum disorder (ASD) in children | ||||||
| Patient or population: children with ASD Comparison: control group (same chambers as those in hyperbaric oxygen therapy group with an oxygen concentration of 21%) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Primary outcome | |||||
| Social interaction and communication ‐ Parental ATEC score (scale from 0 to 40) | Mean score in the control groups was 14.28 | Mean score in the intervention groups was 1.21 higher (2.21 lower to 4.63 higher) | Not estimable | 60 | ⊕⊕⊝⊝ | — |
| Social interaction and communication ‐ Clinician ATEC score (scale from 0 to 40) | Mean score in the control groups was 14.28 | Mean score in the intervention groups was 1.55 higher (1.35 lower to 4.45 higher) | Not estimable | 60 | ⊕⊕⊝⊝ | — |
| Behavioral problems ‐ Parental ATEC score | Mean score in the control groups was 20.41 | Mean score in the intervention groups was 0.24 lower (4.80 lower to 4.32 higher) | Not estimable | 60 | ⊕⊕⊝⊝ | — |
| Behavioral problems ‐ Clinician ATEC score (scale from 0 to 40) | Mean score in the control groups was 13.52 | Mean score in the intervention groups was 1.28 lower (4.47 lower to 1.91 higher) | Not estimable | 60 | ⊕⊕⊝⊝ | — |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSmall sample size (60 participants). | ||||||
| Review section | Change |
| Description of the intervention | We added the paragraph below on 'nonclassical' hyperbaric oxygen therapy: In addition to the 'classical' hyperbaric oxygen therapy defined by the Undersea and Hyperbaric Medical Society (UHMS 2016), some ASD trials have used nonclassical hyperbaric oxygen therapy (Granpeesheh 2010; Rossignol 2009). In these trials, nonclassical hyperbaric oxygen therapy consisted of a chamber pressurized to greater than one ATA with less than 100% oxygen concentration. In one study, both classical and nonclassical hyperbaric oxygen therapies produced significant improvement in children with autism, as evidenced by normal levels of oxidative stress and inflammation markers (Rossignol 2007a). As outlined in our protocol (Xiong 2014), we assessed only the use of classical hyperbaric oxygen therapy in this review. |
| Types of participants | We revised this section as follows: Participants of any age with a diagnosis of autism spectrum disorder (ASD) based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) (APA 2013); or individuals with a diagnosis of one of the four pervasive developmental disorders from fourth edition text revision version of the DSM (DSM‐IV‐TR) (APA 2000), including autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD‐NOS); or from theInternational Classification of Mental and Behavioural Disorders, 10th Edition (ICD‐10; WHO 1993). We accepted diagnoses that were derived following use of assessment tools, such as the Autism Diagnostic Observation Scale (ADOS) (Lord 1997) and the Autism Diagnostic Interview ‐ Revised (ADI‐R) (Lord 1994). |
| Electronic searches | We have specified that, when searching online clinical trial registries such as ClinicalTrials.gov and WHO ICTRP, we checked the publication status of each trial identified and contacted study authors for results of finished unpublished trials. |
| Searching other resources | We have clarified that we handsearched reference lists of relevant studies. We also searched the gray literature from the Internet using the academic search engine "Baidu Scholar." |
| Measures of treatment effect | We have clarified how we will handle final values and changes from baseline data, as follows: When final values and changes from baseline data are available in included trials, we shall analyse them separately. Apart from analysing those values separately, we will combine final values and changes from baseline data using the MD when both types of data are available for the same scale. We will not incorporate skewed data in future analyses. |
| Unit of analysis issues | We explained how we would handle multiple intervention groups. See Appendix 2. |
| Assessment of heterogeneity | We regraded the degree of heterogeneity, as follows (Deeks 2011).
We added the following information: Studies have shown that different estimation methods may lead to different results and conclusions. For example, the DerSimonian and Laird (DL) estimator, which is currently widely used by default to estimate between‐study variance, has been long challenged (Veroniki 2016). The DL estimator can lead to erroneous conclusions (Cornell 2014) or can largely underestimate the true value for dichotomous outcomes (Novianti 2014). For continuous data, the restricted maximum likelihood estimator is a better alternative for estimating between‐study variance when compared with other estimators (Veroniki 2016). We also specified that: We plan to assess heterogeneity by comparing the estimated magnitude of the heterogeneity variance with the empirical distribution of Turner 2012 for dichotomous data and Rhodes 2015 for continuous data. |
| Data synthesis | We have clarified when we will report results of the fixed‐effect model and when we will report results of the random‐effects model, as follows: If no significant heterogeneity is present, we will report the results of the fixed‐effect model only. If significant heterogeneity or severe asymmetry of the funnel plot is observed, we will report the results of the random‐effects model. |
| ASD: autism spectrum disorder. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Social interaction and communication Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Parental ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Clinician ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Behavioral problems Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Parental ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clinician ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Communication and linguistic abilities Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Parental ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Clinician ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Cognitive function Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Parental ATEC score | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clinician ATEC score | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Safety of hyperbaric oxygen therapy Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3.1 Side effect (barotrauma) events in all sessions | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 The number of children who had side effects (barotrauma) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

