Oxigenoterapia hiperbárica para personas con trastorno del espectro autista (TEA)
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library
CENTRAL 2015, Issue 11. Searched 14 December 2015, limited to publication years 2014‐2015 [0 records].
CENTRAL 2014, Issue 10. Searched 25 November 2014, limited to publication years 2013‐2014 [1 record].
CENTRAL 2013, Issue 10. Searched 20 November 2013 [6 records].
#1 [mh ^"child development disorders, pervasive"]
#2 [mh "Developmental Disabilities"]
#3 pervasive development* disorder*
#4 (pervasive near/3 child*)
#5 (PDD or PDDs or PDD‐NOS or ASD or ASDs)
#6 autis*
#7 asperger*
#8 kanner*
#9 childhood next schizophrenia
#10 {or #1‐#9}
#11 [mh "Hyperbaric Oxygenation"]
#12 (Hyperbaric near/3 oxygen*)
#13 oxygen next therap*
#14 (Hyperbaric near/3 therap*)
#15 HBO
#16 HBOP
#17 {or #11‐#16}
#18 #10 and #17
Ovid MEDLINE
Ovid MEDLINE 1946 to November week 3 2015. Searched 14 December 2015, limited to ed=20141101‐20151117 [2 records].
Ovid MEDLINE 1946 to November week 2 2014. Searched 25 November 2014, limited to ed=20131101 to 20141125 [4 records].
Ovid MEDLINE 1946 to November week 1 2013. Searched 19 November 2013 [44 records].
1 exp child development disorders, pervasive/
2 Developmental Disabilities/
3 pervasive development$ disorder$.tw.
4 (pervasive adj3 child$).tw.
5 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw.
6 autis$.tw.
7 asperger$.tw.
8 kanner$.tw.
9 childhood schizophrenia.tw.
10 or/1‐9
11 Hyperbaric Oxygenation/
12 (Hyperbaric adj3 oxygen$).tw.
13 oxygen therap$.tw.
14 (Hyperbaric adj3 therap$).tw.
15 HBO.tw.
16 HBOP.tw.
17 or/11‐16
18 10 and 17
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, 10 December 2015. Searched 14 December 2015 [5 records].
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, 24 November 2014. Searched 25 November 2014 [2 records].
Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, 18 November 2013. Searched 19 November 2013 [2 records].
1 pervasive development$ disorder$.tw.
2 (pervasive adj3 child$).tw.
3 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw.
4 autis$.tw.
5 asperger$.tw.
6 kanner$.tw.
7 childhood schizophrenia.tw.
8 or/1‐7
9 (Hyperbaric adj3 oxygen$).tw.
10 oxygen therap$.tw.
11 (Hyperbaric adj3 therap$).tw.
12 HBO.tw.
13 HBOP.tw.
14 or/9‐13
15 8 and 14
Embase (Ovid)
Embase 1980 to 2015 Week 50. Searched 14 December 2015, limited to ed=201447‐201550 [12 records].
Embase 1980 to 2014 Week 47. Searched 25 November 2014, limited to ed=201346 to 201447 [10 records].
Embase 1980 to 2013 Week 46. Searched 19 November 2013 [82 records].
1 exp autism/
2 developmental disorder/
3 autis*.tw.
4 pervasive development$ disorder$.tw.
5 (pervasive adj3 child$).tw.
6 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw.
7 childhood schizophrenia.tw.
8 asperger$.tw.
9 kanner$.tw.
10 or/1‐9
11 hyperbaric oxygen/
12 (Hyperbaric adj3 oxygen$).tw.
13 oxygen therap$.tw.
14 (Hyperbaric adj3 therap$).tw.
15 HBO.tw.
16 HBOP.tw.
17 or/11‐16
18 10 and 17
19 limit 18 to em=201346‐201447
20 from 19 keep 1‐10
PsycINFO (Ovid)
PsycINFO 1667 to December Week 2 2015. Searched 14 December 2015, limited to up=20141101‐20151207 [1 record].
PsycINFO 1967 to November Week 3 2014. Searched 25 November 2014, limited to up=20131118 to 20141125 [0 records].
PsycINFO 1967 to November Week 2 2013. Searched 19 November 2013 [18 records].
1 exp pervasive developmental disorders/
2 developmental disabilities/
3 pervasive development$ disorder$.tw.
4 (pervasive adj3 child$).tw.
5 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw.
6 autis$.tw.
7 asperger$.tw.
8 kanner$.tw.
9 childhood schizophrenia.tw.
10 or/1‐9
11 oxygenation/
12 (Hyperbaric adj3 oxygen$).tw.
13 oxygen therap$.tw.
14 (Hyperbaric adj3 therap$).tw.
15 HBO.tw.
16 HBOP.tw.
17 or/11‐16
18 10 and 17
19 limit 18 to up=20131118‐20141125
Web of Science databases
Science Citation Index (SCI) 1970 to 14 December 2015. Searched 14 December 2015, limited to publication years 2014‐2015 [1 record].
SCI 1970 to 21 November 2014. Searched 25 November 2014, limited to publication years 2013‐2014 [3 records].
SCI 1970 to 15 November 2013. Searched 20 November 2013 [21 records].
Social Sciences Citation Index (SSCI) 1970 to 14 December 2015. Searched 14 December 2015, limited to publication years 2014‐2015 [0 records].
SSCI 1970 to 21 November 2014. Searched 25 November 2014, limited to publication years 2013 to 2014 [0 records].
SSCI 1970 to 15 November 2013. Searched 20 November 2013 [13 records].
Conference Proceedings Citation Index ‐ Science (CPCI‐S) and Conference Proceedings Citation Index ‐ Social Sciences & Humanities 1990 to 14 December 2015. Searched 14 December 2015, limited to publication years 2014‐2015 [0 records].
CPCI‐S & CPCI‐SS&H 1990 to 21 November 2014. Searched 25 November 2014, limited to publication years 2013 to 2014 [0 records].
CPCI‐S & CPCI‐SS&H 1990 to 15 November 2013. Searched 20 November 2013 [2 records].
#12 #11 AND #6
DocType=All document types; Language=All languages;
#11 #10 OR #9 OR #8 OR #7
DocType=All document types; Language=All languages;
#10 TS= (HBO OR HBOP)
DocType=All document types; Language=All languages;
#9 TS=(Hyperbaric near/3 therap*)
DocType=All document types; Language=All languages;
#8 TS=("oxygen therap*")
DocType=All document types; Language=All languages;
#7 TS=(Hyperbaric near/3 oxygen*)
DocType=All document types; Language=All languages;
#6 #5 OR #4 OR #3 OR #2 OR #1
DocType=All document types; Language=All languages;
#5 TS=(PDD or PDDs or PDD‐NOS or ASD or ASDs)
DocType=All document types; Language=All languages;
#4 TS=("childhood schizophrenia")
DocType=All document types; Language=All languages;
#3 TS= (pervasive near/3 child*)
DocType=All document types; Language=All languages;
#2 TS= ("pervasive development* disorder*")
DocType=All document types; Language=All languages;
#1 TS=(autis* or asperger* or kanner*)
DocType=All document types; Language=All languages;
WorldCat
14 December 2015 [0 records].
25 November 2014 [0 records].
21 November 2013 [1 record].
Advanced Search; kw:autis* AND hyperbaric then limited to Theses
Cochrane Database of Systematic Reviews (CDSR) and Database of Abstracts of Reviews of Effects (DARE), in the Cochrane Library
CDSR 2015, Issue 12. Searched 14 December 2015 [0 records].
CDSR 2014, Issue 11. Searched 25 November 2014 [0 records].
CDSR 2013, Issue 11. Searched 20 November 2013 [1 record].
DARE 2015, Issue 2. Searched 14 December 2015 [0 records].
DARE 2014, Issue 4. Searched 25 November 2014 [0 records].
DARE 2013, Issue 4. Searched 20 November 2013 [0 records].
#1[mh ^"child development disorders, pervasive"]
#2[mh "Developmental Disabilities"]
#3(pervasive development* disorder*):ti,ab
#4(pervasive near/3 child*):ti,ab
#5(PDD or PDDs or PDD‐NOS or ASD or ASDs):ti,ab
#6(autis*):ti,ab
#7(asperger*):ti,ab
#8(kanner*):ti,ab
#9(childhood next schizophrenia):ti,ab
#10{or #1‐#9}
#11[mh "Hyperbaric Oxygenation"]
#12(Hyperbaric near/3 oxygen*):ti,ab
#13(oxygen next therap*):ti,ab
#14(Hyperbaric near/3 therap*):ti,ab
#15"HBO":ti,ab
#16HBOP:ti,ab
#17{or #11‐#16}
#18#10 and #17 in Cochrane Reviews (Reviews only) and Other Reviews
CINAHL Plus EBSCO (Cumulative Index to Nursing and Allied Health Literature)
CINAHL 1937 to current. Searched 14 December 2015, limited to EM 20141101‐current [4 records].
CINAHL 1937 to current. Searched 26 November 2014, limited to EM=20131101‐current [1 record].
CINAHL 1937 to current. Searched 20 November 2013 [31 records].
S17 S10 AND S16
S16 S11 OR S12 OR S13 OR S14 OR S15
S15 HBO or HBOP
S14 (Hyperbaric N3 therap*)
S13 oxygen therap*
S12 (Hyperbaric N3 oxygen*)
S11 (MH "Hyperbaric Oxygenation")
S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9
S9 (PDD or PDDs or PDD‐NOS or ASD or ASDs)
S8 childhood schizophren*
S7 kanner*
S6 asperger*
S5 autis*
S4 (pervasive N3 child*)
S3 pervasive development* disorder*
S2 (MH "Developmental Disabilities")
S1 (MH "Child Development Disorders, Pervasive+")
ERIC EBSCOhost (Education Resources Information Center)
ERIC 1966 to current. Searched 14 December 2015 [0 records].
ERIC 1966 to current. Searched 25 November 2014, limited by publication year 2013 to current [0 records].
S17 S10 AND S16
S16 S11 OR S12 OR S13 OR S14 OR S15
S15 HBO or HBOP
S14 (Hyperbaric N3 therap*)
S13 oxygen therap*
S12 (Hyperbaric N3 oxygen*)
S11 (MH "Hyperbaric Oxygenation")
S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9
S9 pervasive development* disorder*
S8 (PDD or PDDs or PDD‐NOS or ASD or ASDs)
S7 childhood schizophren*
S6 kanner*
S5 asperger*
S4 autis*
S3 (pervasive N3 child*)
S2 (MH "Developmental Disabilities")
S1 (MH "Child Development Disorders, Pervasive+")
ERIC (ProQuest)
ERIC 1966 to current. Searched 21 November 2013 [15 records].
Set#: S1
Searched for: SU.EXACT.EXPLODE("Pervasive Developmental Disorders") or autis* or Asperger* or kanner* or "pervasive development* disorder*" or "childhood schizophrenia" or pervasive near/3 child* or pdd or pdds or asd or asds or pdd‐nos
Databases: ERICSet#: S5
Searched for: Hyperbaric or oxygen* or HBO OR HBOP
Databases: ERIC
Set#: S6
Searched for: (SU.EXACT.EXPLODE("Pervasive Developmental Disorders") OR autis* OR Asperger* OR kanner* OR "pervasive development* disorder*" OR "childhood schizophrenia" OR pervasive NEAR/3 child* OR pdd OR pdds OR asd OR asds OR pdd‐nos) AND (Hyperbaric OR oxygen* OR HBO OR HBOP)
Databases: ERIC
HBO Evidence. Database of Randomised Controlled Trials in Diving and Hyperbaric Medicine
(hboevidence.unsw.wikispaces.net)
HBO accessed 14 December 2015 [0 records].
HBO accessed 26 November 2014 [0 records].
HBO accessed 21 November 2013 [2 records].
Search term: autism
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP)
15 December 2015 [10 records].
25 November 2014 [0 records].
21 November 2013 [10 records].
Basic search : autism AND oxygen OR autism AND hyperbaric
ClinicalTrials.gov
15 December 2015 [8 records].
25 November 2014 [0 records].
21 November 2013 [8 records].
Basic search: hyperbaric AND autism
metaRegister of Controlled Trials (mRCT)
14 December 2015. Not searched. Website message " service under review".
26 November 2014. Not searched. Website message "service under review".
21 November 2013 [4 records].
Selected: All registers
Search terms: hyperbaric AND autism
Research Autism
14 December 2015 [3 records].
26 November 2014 [0 records).
21 November 2013 [9 records].
Browsed the alpabetical list from the Interventions tab for "hyperbaric" and downoaded the webpage.
Autism Data
14 December 2015 [0 records].
25 November 2014 [1 record].
21 November 2013 [15 records].
Australian New Zealand Clinical Trials Registry (ANZCTR)
Searched 26 November 2014 [0 records]. This registry was not searched separately after this date as the content feeds into WHO ICTRP.
Chinese databases
China National Knowledge Infrastructure (CNKI) (cnki.net). Searched 18 December 2013, 5 December 2014 and 10 December 2015.
WEIPU periodical database (cqvip.com). Searched 18 December 2013, 5 December 2014 and 10 December 2015.
Wan Fang Data (wanfangdata.com.cn). Searched 18 December 2013, 5 December 2014 and 10 December 2015.
Chinese Biologic Medical Database (CBM) (sinomed.imicams.ac.cn). Searched 18 December 2013, 5 December 2014 and 10 December 2015.
1 高压氧
2 孤独症 或 自闭症
3 随机
4 1 and 2 and 3
Appendix 2. Additional methods archived for future updates of this review
| Analysis | Methods |
| Measures of treatment effect | Continuous data For continuous data, we will calculate the mean difference (MD) and 95% confidence intervals (CIs). Because different scales may be used to measure the same outcomes in trials on autism spectrum disorder (ASD), standardized mean differences (SMDs) may be used widely in our review. Final values and changes from baseline data should not be combined together as SMDs. When final values and changes from baseline data are available in included trials, we shall analyze them separately. Apart from analyzing those values separately, we will combine final values and changes from baseline data using the MD when both types of data are available for the same scale. We will not incorporate skewed data in future analyses. Dichotomous data For dichotomous data, we will calculate the risk ratio (RR), odds ratio (OR), and risk difference (RD) with 95% CIs. |
| Unit of analysis issues | For most outcomes, the unit of analysis will be the individual participant. Cluster‐randomized trials We will include cluster‐randomized trials along with individually randomized trials in the analysis. We will analyze them, as detailed in section 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible) or from another source. If we use ICCs from other sources, we will report this fact and we will conduct sensitivity analyses to investigate effects of variation in the ICC. If we identify both cluster‐randomized trials and individually randomized trials, we will synthesize relevant information. We will consider it reasonable to combine the results derived from both if little heterogeneity between the study designs is noted, and if interaction between the effect of the intervention and the choice of randomization unit is considered unlikely. We will also acknowledge heterogeneity in the randomization unit, and we will perform a separate meta‐analysis. Multiple intervention groups We were not faced with multiple intervention studies until now. In the future, for a study with multiple intervention groups, if appropriate, we will combine groups to create a single pairwise comparison. The recommended method in most situations is to combine all relevant experimental intervention groups of the study into a single group, and to combine all relevant control intervention groups into a single control group (Higgins 2011a). Indirect comparisons are not randomized comparisons. They are observational findings across trials and may suffer the biases of observational studies (Higgins 2011a). Thus, we will exclude indirect comparisons. |
| Dealing with missing data | When published data are incomplete, we will try to obtain the missing data from the primary investigator, if possible. If this approach is unsuccessful, we will restrict the analyses to available data. We will use sensitivity analyses to examine whether overall findings are robust to the potential influence of missing data. We will assess how sensitive results are to reasonable changes in the assumptions made. We will critically appraise issues of the intention‐to‐treat (ITT) analysis and will compare them with specifications of primary outcome parameters and power calculations. |
| Assessment of heterogeneity | We will consider 3 types of heterogeneity: clinical, methodological, and statistical. We will assess clinical heterogeneity by comparing the distribution of important participant factors between trials such as age, gender, specific diagnoses or diagnostic subtypes (or both), duration of the disorder, and associated neuropsychiatric diseases. We will assess methodological heterogeneity by comparing trial characteristics such as randomization concealment, blinding, and losses to follow‐up (see Quality of the evidence). We will assess statistical heterogeneity by examining Chi² and I². We will use the Chi² test (P ≤ 0.10 shows substantial or considerable heterogeneity) to determine whether statistically significant heterogeneity is present. The Chi² test is not very reliable when a few studies or small sample sizes form the dataset. This means that a nonsignificant result cannot be taken as evidence of no heterogeneity. We will also assess the degree of statistical heterogeneity by examining I². We will grade the degree of heterogeneity as follows (Deeks 2011):
We will examine the trials to investigate possible explanations for heterogeneity. If heterogeneity is identified among a group of studies, we will check the data and establish potential reasons for the observed heterogeneity. For heterogeneity that cannot be readily explained, we intend to divide the data into subgroups if an appropriate basis is identified. Studies have shown that different estimation methods may lead to different results and conclusions. For example, the DerSimonian and Laird (DL) estimator, which is currently widely used by default to estimate between‐study variance, has long been challenged (Veroniki 2016). The DL estimator can lead to erroneous conclusions (Cornell 2014), or can largely underestimate the true value for dichotomous outcomes (Novianti 2014). For continuous data, the restricted maximum likelihood estimator is a better alternative for estimating between‐study variance when compared with other estimators (Veroniki 2016). We plan to assess heterogeneity by comparing the estimated magnitude of the heterogeneity variance with the empirical distribution of Turner 2012 for dichotomous data and Rhodes 2015 for continuous data. |
| Assessment of reporting biases | We will try to obtain the study protocols of all included studies so that we can compare outcomes reported in the protocol versus those reported in the findings. When we suspect reporting bias, we will attempt to contact study authors to ask them to provide missing outcome data. When this is not possible, and the missing data are thought to introduce serious bias, we will conduct a sensitivity analysis to evaluate the impact of including such studies in the overall assessment of results. We will assess publication bias by using funnel plots or the Egger test (Egger 1997), depending on the number of clinical trials included in the systematic review. The funnel plot should be seen as a generic means of displaying small‐study effects. Asymmetry may arise as a result of publication bias or a relationship between trial size and effect size. True heterogeneity in intervention effects is only one cause of funnel plot asymmetry (Egger 1997; Sterne 2011). |
| Data synthesis | If more than one eligible trial is identified and sufficient homogeneity is observed among studies with respect to participants and reported outcomes, we will perform meta‐analyses using Review Manager 5 (RevMan 5) (RevMan 5 2014). We will use both the fixed‐effect model and random‐effects model in the meta‐analysis. Both models will yield similar results if no significant heterogeneity and no publication bias are noted among the trials. If no significant heterogeneity is present, we will report results of the fixed‐effect model only. However, the asymmetry of the funnel plot may be due to true heterogeneity. If significant heterogeneity or severe asymmetry of the funnel plot is observed, we will report the results of the random‐effect model. For continuous data, we will use the inverse variance method, which is available in RevMan 5 2014. When data are sparse, in terms of low event rates or small study size, the Mantel‐Haenszel methods have better statistical properties than the inverse variance method for dichotomous data (Deeks 2011). In such cases, we will choose the Mantel‐Haenszel method for calculating the RR and RD for dichotomous data. Both Mantel‐Haenszel and inverse variance methods are poor when event rates are very low. In such cases, Peto's method works well; however, Peto's method can be used only to pool odds ratios (Deeks 2011). |
| Subgroup analysis and investigation of heterogeneity | We will perform subgroup analysis based on the factors below.
|
| Sensitivity analysis | We will perform sensitivity analyses for missing data and for study risk of bias. We will employ sensitivity analysis using different approaches to impute missing data. We will critically appraise last observation carried forward (LOCF), ITT, and per‐protocol (PP) analysis and will compare them with primary outcome parameters and power calculations. If appropriate, we will conduct sensitivity analyses by study risk of bias based on the presence or absence of a reliable random allocation method, concealment of allocation, and blinding of participants or outcome assessors. We will test robustness of the results by including or excluding studies of poor quality. |

Flow diagram.
Footnotes
RCT: randomized controlled trial.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Primary outcome, Outcome 1 Social interaction and communication.
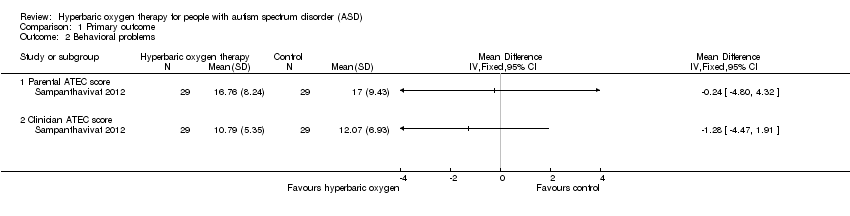
Comparison 1 Primary outcome, Outcome 2 Behavioral problems.

Comparison 2 Second outcome, Outcome 1 Communication and linguistic abilities.

Comparison 2 Second outcome, Outcome 2 Cognitive function.

Comparison 2 Second outcome, Outcome 3 Safety of hyperbaric oxygen therapy.
| Hyperbaric oxygen therapy for autism spectrum disorder (ASD) in children | ||||||
| Patient or population: children with ASD Comparison: control group (same chambers as those in hyperbaric oxygen therapy group with an oxygen concentration of 21%) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Primary outcome | |||||
| Social interaction and communication ‐ Parental ATEC score (scale from 0 to 40) | Mean score in the control groups was 14.28 | Mean score in the intervention groups was 1.21 higher (2.21 lower to 4.63 higher) | Not estimable | 60 | ⊕⊕⊝⊝ | — |
| Social interaction and communication ‐ Clinician ATEC score (scale from 0 to 40) | Mean score in the control groups was 14.28 | Mean score in the intervention groups was 1.55 higher (1.35 lower to 4.45 higher) | Not estimable | 60 | ⊕⊕⊝⊝ | — |
| Behavioral problems ‐ Parental ATEC score | Mean score in the control groups was 20.41 | Mean score in the intervention groups was 0.24 lower (4.80 lower to 4.32 higher) | Not estimable | 60 | ⊕⊕⊝⊝ | — |
| Behavioral problems ‐ Clinician ATEC score (scale from 0 to 40) | Mean score in the control groups was 13.52 | Mean score in the intervention groups was 1.28 lower (4.47 lower to 1.91 higher) | Not estimable | 60 | ⊕⊕⊝⊝ | — |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSmall sample size (60 participants). | ||||||
| Review section | Change |
| Description of the intervention | We added the paragraph below on 'nonclassical' hyperbaric oxygen therapy: In addition to the 'classical' hyperbaric oxygen therapy defined by the Undersea and Hyperbaric Medical Society (UHMS 2016), some ASD trials have used nonclassical hyperbaric oxygen therapy (Granpeesheh 2010; Rossignol 2009). In these trials, nonclassical hyperbaric oxygen therapy consisted of a chamber pressurized to greater than one ATA with less than 100% oxygen concentration. In one study, both classical and nonclassical hyperbaric oxygen therapies produced significant improvement in children with autism, as evidenced by normal levels of oxidative stress and inflammation markers (Rossignol 2007a). As outlined in our protocol (Xiong 2014), we assessed only the use of classical hyperbaric oxygen therapy in this review. |
| Types of participants | We revised this section as follows: Participants of any age with a diagnosis of autism spectrum disorder (ASD) based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) (APA 2013); or individuals with a diagnosis of one of the four pervasive developmental disorders from fourth edition text revision version of the DSM (DSM‐IV‐TR) (APA 2000), including autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD‐NOS); or from theInternational Classification of Mental and Behavioural Disorders, 10th Edition (ICD‐10; WHO 1993). We accepted diagnoses that were derived following use of assessment tools, such as the Autism Diagnostic Observation Scale (ADOS) (Lord 1997) and the Autism Diagnostic Interview ‐ Revised (ADI‐R) (Lord 1994). |
| Electronic searches | We have specified that, when searching online clinical trial registries such as ClinicalTrials.gov and WHO ICTRP, we checked the publication status of each trial identified and contacted study authors for results of finished unpublished trials. |
| Searching other resources | We have clarified that we handsearched reference lists of relevant studies. We also searched the gray literature from the Internet using the academic search engine "Baidu Scholar." |
| Measures of treatment effect | We have clarified how we will handle final values and changes from baseline data, as follows: When final values and changes from baseline data are available in included trials, we shall analyse them separately. Apart from analysing those values separately, we will combine final values and changes from baseline data using the MD when both types of data are available for the same scale. We will not incorporate skewed data in future analyses. |
| Unit of analysis issues | We explained how we would handle multiple intervention groups. See Appendix 2. |
| Assessment of heterogeneity | We regraded the degree of heterogeneity, as follows (Deeks 2011).
We added the following information: Studies have shown that different estimation methods may lead to different results and conclusions. For example, the DerSimonian and Laird (DL) estimator, which is currently widely used by default to estimate between‐study variance, has been long challenged (Veroniki 2016). The DL estimator can lead to erroneous conclusions (Cornell 2014) or can largely underestimate the true value for dichotomous outcomes (Novianti 2014). For continuous data, the restricted maximum likelihood estimator is a better alternative for estimating between‐study variance when compared with other estimators (Veroniki 2016). We also specified that: We plan to assess heterogeneity by comparing the estimated magnitude of the heterogeneity variance with the empirical distribution of Turner 2012 for dichotomous data and Rhodes 2015 for continuous data. |
| Data synthesis | We have clarified when we will report results of the fixed‐effect model and when we will report results of the random‐effects model, as follows: If no significant heterogeneity is present, we will report the results of the fixed‐effect model only. If significant heterogeneity or severe asymmetry of the funnel plot is observed, we will report the results of the random‐effects model. |
| ASD: autism spectrum disorder. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Social interaction and communication Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Parental ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Clinician ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Behavioral problems Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Parental ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clinician ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Communication and linguistic abilities Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Parental ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Clinician ATEC score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Cognitive function Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Parental ATEC score | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clinician ATEC score | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Safety of hyperbaric oxygen therapy Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3.1 Side effect (barotrauma) events in all sessions | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 The number of children who had side effects (barotrauma) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

