Postupci za poticanje zajedničkog odlučivanja o uporabi antibiotika za akutne infekcije dišnog sustava u općoj praksi
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: cluster‐randomised controlled trial Unit of randomisation: general practitioner (GP) Trial duration: November 2003 to March 2005 Recruitment: 2036 GPs from 9 regions in North‐Rhine and Westphalia‐Lippe, Germany, invited to participate (blinded to the primary outcome); of 239 GPs willing to participate and receiving baseline materials, 104 completed reliable baseline study documentation and were randomised (10 practice partners randomised as pairs) into intervention (GPs = 52, patients = 1389) and control groups (GPs = 52, patients = 1398) Methods of data collection: GPs recorded all consecutive and eligible patients during each documentation period on study specific paper documentation Data collection time points: 3 documentation intervals of 6 weeks each: baseline (before randomisation), and 6 weeks and 12 months post‐intervention Length of follow‐up: 12 months | |
| Participants | GPs documented all consecutive and eligible patients: ≥ 16 years of age with an initial episode of acute cough (without prior episode < 8 weeks) and could comprehend German Exclusion: patients with underlying chronic lung diseases (e.g. asthma, chronic obstructive pulmonary disease), immune deficiency or malignant diseases | |
| Interventions | Brief intervention name: complex, peer‐led, educational intervention Recipients: GPs and patients (passive) Providers: GP peers were trained to provide (in 3 sessions) the outreach visits in clinics during normal working hours (methods of training these GP peers were not specified) Health professional components: focused on antibiotic 'misunderstanding' during a consultation, and aimed to motivate GPs to change attitudes to communication and empower patients. Peers addressed GP beliefs and attitudes by exploring and evaluating GPs 'opposite' motivational background using a standardised dialogue script and communication techniques derived from the elaboration likelihood model. Aspects of the intervention were also informed by previous qualitative work Patients: waiting room poster and leaflet focusing on the patients' role within the antibiotic misunderstanding (e.g. GP perceptions that patients expect an antibiotic) and also brief evidence‐based information about acute cough and antibiotics to enable patients to raise and clarify issues and make a joint decision about antibiotic use with their doctor Materials: waiting room poster and leaflet (patient only); script used by GP peers Mode of delivery: face‐to‐face (GPs) and waiting room posters and leaflets (patients) Duration and intensity: 1 peer outreach visit per GP (duration not specified) Comparator: nil active comparator; GPs provided usual care | |
| Outcomes | Primary: rate of antibiotic prescriptions per acute cough and by GP (study specific paper documentation) Secondary: nil | |
| Notes | Funding: yes Conflict of interest: none disclosed Published trial protocol: no Trial registration: yes Ethics approval: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Program‐generated complete randomisation list |
| Allocation concealment (selection bias) | Low risk | Not described. However, GPs recruited prior to randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Not possible (complex peer‐led educational intervention) |
| Blinding of outcome assessment (detection bias) | Low risk | Participating GPs sent data to researchers. Each patient was assigned a unique identification number that could be connected with the patient only by the participating GP |
| Incomplete outcome data (attrition bias) | High risk | Randomised: 104 GPs (intervention = 52, 1389 patients; control = 52, 1398 patients) 6 weeks post‐intervention: 86 GPs (intervention = 42 (80%), patients = 1021; control = 44 (84%), patients = 1143) 12 months post‐intervention: 61 GPs (intervention = 28 (54%); 787 patients; control = 33 (63%); 920 patients) 17% (18/104) dropped out at 6 weeks and 41% (43/104) by 12 months (reasons for GPs' exclusion from analysis: poor data quality or did not return data) Cluster‐level sensitivity analysis performed to explore effect of differential missing values |
| Selective reporting (reporting bias) | Low risk | All indicated results reported. Prospective trial registration: Projektdatenbank Versorgungsforschung NRW, ID: 90/34/CHANGE |
| Other bias | Unclear risk | Sample size (power) calculation: yes. Sample size calculated on number of patients to detect a 10% difference in 6‐month prescription rates (50% control, 40% intervention). Allowing for 20% drop‐out rate, it was estimated 200 GPs would be required to contribute 20 patients during each observation period (i.e. 4000 at each of the 3 documentation periods) ITT or per protocol analysis: no, all analysis (with exception of sensitivity analyses) included only general practices with complete follow‐up Large baseline difference found in antibiotic prescription rates between intervention and control groups (36.4% versus 54.7%) (unadjusted and adjusted analysis performed) GPs were not monitored during the trial period and may have under‐reported patients who received an antibiotic Government regulatory change during study to exclude OTC medicines from reimbursement by German statutory health insurance funds may have increased antibiotic prescribing decisions to minimise patient out‐of‐pocket cost Generalised estimating equation (GEE) models applied Intraclass correlation (coefficient): 0.20 |
| Methods | Study design: cluster‐randomised controlled trial Unit of randomisation: general practitioner (GP) Trial duration: January to May 2004 Recruitment: 345 eligible GPs (criteria undefined) from 2 Swiss cantons (Basel‐Stadt and Aargau), where self dispensation of drugs is not allowed. 30 GPs (providing written consent by 1 December 2003) were randomised to limited or full intervention groups (15 GPs each); the remaining 15 GPs (providing written consent by 1 January 2004) formed the non‐randomised control group Methods of data collection: baseline data for eligible GPs obtained from the registry of the Swiss Medical Association; GPs recorded patient baseline data; medical students conducted standardised patient follow‐up interviews at 7 and 14 days by telephone; pharmacists faxed all prescriptions with study labels to the study centre Length of follow‐up: 14 days | |
| Participants | GPs recruited all consecutive and eligible adult patients: ≥ 18 years with symptoms of acute infections of the respiratory system (first experienced within the previous 28 days; including common cold, rhinosinusitis, pharyngitis, exudative tonsillitis, laryngitis, otitis media, bronchitis, exacerbated COPD or influenza) Exclusion: patients with pneumonia, not fluent in German, with intravenous drug use or psychiatric disorders, and not available for phone interviews or unable to give written informed consent | |
| Interventions | Brief intervention name: patient‐centred communication training Recipients: GPs Providers: unclear Health professional components: evidence‐based guidelines (developed by 3 trial authors based on existing US guidelines, adapted to local conditions and reviewed by local experts) presented as a booklet and in a 2‐hour interactive seminar, plus a 6‐hour patient‐centred communication seminar in small groups (number not defined) and 2 hours of personal feedback by phone prior to the start of the trial. Training aimed to teach GPs how to understand and modify patients' concepts and beliefs about the use of antibiotics for ARIs. Physicians were taught to practice elements of active listening, to respond to emotional clues and tailor information given to patients. GPs identified patients' attitudes and readiness for behaviour change using a theoretical model (Prochaska and DiClemente 1992) Patient components: nil Materials: evidence‐based guidelines for the treatment of ARIs distributed as a booklet (http://www.bice.ch/publications/reports) Mode of delivery: booklet and face‐to‐face small‐group interactive patient‐centred communication seminar Duration and intensity: GPs attended 1 x 2‐hour interactive evidence‐based guidelines seminar and 1 x 6‐hour small group interactive patient‐centred communication seminar Comparator 1 (Limited intervention): evidence‐based guidelines presented as a booklet and in a 2‐hour interactive seminar alone Comparator 2 (Non‐randomised control): usual care (data not extracted) | |
| Outcomes | Primary: antibiotic prescriptions dispensed by pharmacists < 2 weeks following initial consultation (prescriptions with study labels faxed by pharmacists to the study centre) Secondary: rates of different diagnoses of respiratory infections (GP records) Adherence to guidelines for antibiotic prescription (GP records) Days with restrictions from respiratory infection (patient follow‐up interview at 7 and 14 days) Days off work (patient follow‐up interview at 7 and 14 days) Re‐consultation rates (patient follow‐up interview at 7 and 14 days) Patient satisfaction (Patient Satisfaction Questionnaire; patient follow‐up interview at 7 and 14 days) Patient enablement (Patient Enablement Instrument; patient follow‐up interview at 7 and 14 days) Other: serious adverse events (independent monitoring board review of serious adverse events that occurred < 28 days of study enrolment) | |
| Notes | Funding: yes Conflict of interest: none disclosed Published trial protocol: no Trial registration: not stated Ethics approval: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list created by an independent institution |
| Allocation concealment (selection bias) | Low risk | Allocation to either intervention was concealed. However, method not stated. However, GPs recruited prior to randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of general practitioners and trial staff reported. As this trial had 3 arms (2 intervention arms where the intervention in each involved a seminar and distribution of evidence guidelines; 1 usual care arm), it is possible that the GPs in the intervention arms would not have known which intervention group they were in |
| Blinding of outcome assessment (detection bias) | Low risk | Medical students, blinded to the goal of the trial, were trained to conduct standardised follow‐up interviews at 7 and 14 days by phone Prescriptions with study labels faxed by pharmacists to the study centre were checked and entered into the database by a person blinded to the intervention group Trial authors assessed adherence of all prescriptions to guidelines independently and blinded to the intervention group |
| Incomplete outcome data (attrition bias) | Low risk | GPs randomised into limited intervention (GPs = 15; patients = 293) and full intervention groups (GPs = 15; patients = 259); 15 GPs (285 patients) participated as non‐randomised controls (data not extracted). All GPs completed the trial. There were 290, 253 and a convenience sample of 93 patients (stratified by physician), respectively, interviewed at 7 days; and 287, 245 and 92 patients interviewed at 14 days. Reasons for loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | All indicated results reported. Trial registration or published trial protocol not stated |
| Other bias | Unclear risk | Sample size (power) calculation: yes ITT or per protocol analysis: ITT Intraclass correlation (co‐efficient) reported: 4.0% and a design effect of 1.6% Low study baseline prescribing rates – full intervention (13.5%), limited intervention (15.7%) and non‐randomised control (21.4%) Highly motivated GPs: recruitment coincided with introduction of a new nation‐wide computer‐based reimbursement system and due to increased workload participating GPs considered to be highly motivated |
| Methods | Study design: cluster‐randomised controlled trial Unit of randomisation: general practices Trial duration: conducted during 2007 and 2008 Recruitment: 212 general practices approached at random from 454 eligible practices in Wales, UK. 102 practices expressed interest to participate; 70 recruited; 68 practices (˜480,000 patients) randomised to intervention or control groups (34 each) Methods of data collection: routine administrative systems (see 'Outcomes') Data collection time points: total numbers of dispensed oral antibiotic items (primary) and hospital admissions for possible RTIs and their complications (secondary): rate per 1000 patients for the year after the intervention practices were exposed to the intervention; re‐consultation for RTIs: (secondary; 7, 14 and 31 days after initial consultation). Cost data not extracted Length of follow‐up: 12 months | |
| Participants | Clinicians (general practitioners (GPs) and nurse practitioners) and all patients registered with and consulting a participating general practice in Wales (practice list) | |
| Interventions | Brief intervention name: Stemming the Tide of Antibiotic Resistance (STAR) educational programme: multifaceted flexible blended learning approach to continuing education for clinicians Recipients: clinicians (GPs and nurse practitioners) Providers: web‐based modules and practice‐based seminar led by a facilitator Health professional components: the programme is a blended learning experience, and based on Social Learning Theory to develop GPs sense of importance about change (the 'why' of change) and confidence in their ability to achieve change (the 'how' of change). The intervention consist of 7 parts (5 online, 1 face‐to‐face and 1 facilitator‐led practice‐based seminar): case‐scenarios and updated summaries of research evidence and guidelines; reflections on clinical judgement on antibiotic prescribing; a facilitator‐led practice‐based seminar presenting regional, local and practice‐level antibiotic prescribing and resistance data; novel communicative consulting skills and information exchange based on motivational interviewing; personal reflections on clinical practice; web‐based forum to share experiences and views; and a booster module completed 6 to 8 months after completion of the initial training to reinforce previously outlined communication skills. GPs had to complete each online learning component before the software would allow them access to the next. The intervention was flexible to allow GPs to access online components and try out new skills with patients at their convenience Patient components: nil Materials: web‐based materials Mode of delivery: interactive web‐based modules (including online videos in addition to a facilitator‐led practice‐based seminar Duration and intensity: not specified Comparator: usual care | |
| Outcomes | Primary: total number of dispensed oral antibiotic items per 1000 registered patients for the year after practices were exposed to the STAR programme (Prescribing Audit Reports and Prescribing Catalogues; www.nhsbsa.nhs.uk/prescriptions) Secondary: hospital admission rates for possible RTIs and their complications per 1000 registered patients for the year after practices were exposed to the STAR programme.(Patient Episode Database for Wales); and practice re‐consultation rates (for patients with RTIs, practice re‐consultation rates were identified using diagnostic READ codes recorded by the general practitioner over 7, 14 and 31 days after an initial consultation) Costs data not extracted | |
| Notes | Funding: yes Conflict of interest: none disclosed Published trial protocol: yes Trial registration: yes Ethics approval: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted once all practices were recruited and all participating physicians had provided written consent. Dynamic block allocation was used to achieve balance between groups of practices for the potential confounders of previous rate of antibiotic dispensing (averaged over the past year), practice size (number of whole time equivalent staff at recruitment), and proportion of clinicians in the practice registered for the study. The practices were divided into 3 sets of 24, 22 and 22 practices; within each set we generated all possible allocations into 2 groups and selected the 1000 allocations within each set with the best balance with respect to the specified confounders. The independent statistician on the trial steering committee selected 1 allocation at random for each set and randomly assigned intervention or control to the 2 groups in each set to construct the final allocation |
| Allocation concealment (selection bias) | Low risk | Clinicians and researchers were blinded to group allocation until after randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Not possible (multifaceted intervention programme) |
| Blinding of outcome assessment (detection bias) | Low risk | Data on antibiotic dispensing, hospital admissions and re‐consultations were collected through routine administrative systems that were not influenced by the study research process |
| Incomplete outcome data (attrition bias) | Low risk | 68 practices (˜480,000 patients) randomised to intervention (34 practices; 137 GPs, 2 nurse practitioners) or control (34 practices; 122 GPS, 2 nurse practitioners) groups. 2 practices (one in each group; including 12 intervention GPs and 7 control GPs) withdrew after randomisation but were included in the ITT analyses |
| Selective reporting (reporting bias) | Low risk | All indicated results reported. Published trial protocol available |
| Other bias | Low risk | Sample size (power) calculation: yes ITT or per protocol analysis: ITT analysis for primary outcome |
| Methods | Study design: cluster‐randomised controlled trial (factorial design). Unit of randomisation: general practices (cluster of 2 general practitioners (GPs) per practice) Trial duration: conducted during the winters of 2005 to 2006 and 2006 to 2007 Recruitment: 54 general practices within a large suburban region of the Netherlands were assessed for eligibility; 20 eligible general practices (with 2 participating GPs per practice) were randomised into groups of 10 practices per intervention (resulting in 4 trial arms of 5 general practices and 10 GPs): ‐ use of C‐reactive protein (CRP) testing; ‐ training in enhanced communication skills; ‐ use of CRP and training in enhanced communication skills; ‐ control (usual care) Methods of data collection: antibiotic prescribing and re‐consultation data obtained from patient medical records. Patients rated symptoms (cough, phlegm, shortness of breath, disturbance of daily activities, sleeping problems and generally feeling unwell), satisfaction and enablement, on a 28‐day daily diary validated for use in a RCT on management of LRTI in primary care Data collection time points: index consultation and 28‐day follow‐up | |
| Participants | General practitioners recruited sequential eligible adults within regular consultation hours during the winters of 2005 to 2006 and 2006 to 2007 | |
| Interventions | Brief intervention name: enhanced communication skills training Recipients: GPs Providers: seminars led by a moderator Health professional components: enhanced communication skills training involved 1 x 2‐hour training seminar at a central location, preceded and followed by consulting with simulated patients in routine surgeries and peer‐review of transcripts. The moderator‐led seminar on shared decision making (within 1 week of simulated patient consultation) comprised GPs' reflection on simulated patient transcript, current views and insights on LRTI (highlighting contrast between research and practice), outline of elicit‐provide‐elicit framework (elicit patient's main worries and expectations and conveying the balance of possible antibiotic benefits and harms, provide information relevant to the patients' individual understanding and interest, and elicit patients' interpretation about what has been said and done and discusses implications for help seeking behaviour), videos presenting practice‐based examples and GPs identifying specific aspects during their consultations that need most attention Patient components: nil Materials: desk reminder for GPs Mode of delivery: face‐to‐face seminar and simulated patient consultations with peer‐review of transcripts Duration and intensity: 1 x 2‐hour moderator‐led training seminar; pre‐ and post‐seminar simulated patient consultations with peer‐review of transcripts Comparator 1: C‐reactive protein point of care testing (date not extracted) Comparator 2: enhanced communication skills training plus C‐reactive protein point of care testing (date not extracted) Comparator 4: usual care (Dutch guidelines for managing acute cough, including diagnostic and therapeutic advice for lower respiratory tract infection are distributed to all GPs in the Netherlands) | |
| Outcomes | Primary: antibiotic prescribing in the index consultation (medical records) Re‐consultation (medical records) Clinical recovery data not extracted Patients' satisfaction (Likert scale; 28‐day daily diary) Patients' enablement (Patient Enablement Index; 28‐day daily diary) | |
| Notes | Funding: yes Conflict of interest: none declared Published trial protocol: yes Trial registration: yes Ethics approval: yes Main comparator reported in this review: communication skills training (n = 201) versus no communication skills training (n = 230) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | General practices randomised into 2 groups of 10 practices per intervention, balanced for recruitment potential, resulting in the 4 trial arms. The balancing factor used for randomisation was the amount of GP's consultation time (expressed as full time equivalent (FTE)) that the practice was contributing to the study (which equated to between1 and 2 FTEs for clinical contact time. The randomisation was balanced for those with 1.5 or less FTEs and those with more than 1.5 FTEs |
| Allocation concealment (selection bias) | Low risk | All practices and general practitioners were recruited before randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Not possible (due to the nature of the intervention) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | 20 practices (40 GPs) randomised to each of the 4 trial arms (5 practices, 10 GPs each) and recruited 431 patients. 37 GPs completed the trial (3 left on maternity leave in the enhanced communication skills group). All patients (100%) had data for the primary outcome, 90% (mean) had 28‐day diary data For the communication skills training group (10 GPs, 84 patients), there was 100% prescribing data and 88% returned diaries. |
| Selective reporting (reporting bias) | Low risk | All indicative results reported. Published study protocol. Prospective trial registration |
| Other bias | Low risk | Sample size (power) calculation: yes ITT or per protocol analysis: the primary analysis was ITT |
| Methods | Study design: 3.5 year follow‐up of a cluster‐randomised controlled trial (factorial design) (Cals 2009) Trial duration: 3.5 years (mean 3.67 years) Recruitment: patients recruited in the winter periods from September 2005 until March 2007 (Cals 2009), were observed until July 2010 Methods of data collection: medical records Data collection time points: recorded consultations for RTI from original 28‐day follow‐up period until July 2010 (follow‐up period); recorded consultation for RTI for the exact same period preceding the consultation in which the patient was recruited in the original trial (baseline period). Deceased patients and patients that moved practices and whose medical records could not be retrieved were excluded Length of follow‐up: mean 3.67 years | |
| Participants | General practices: see Cals 2009 Patients: of the original 431 patients enrolled in the trial, 379 patients (87.9%) had accessible medical records for the follow‐up period. Only data for the enhanced communication training (178) versus no enhanced communication skills training (201) extracted | |
| Interventions | See Cals 2009 | |
| Outcomes | Primary outcome: average number of episodes of RTIs during the follow‐up period for which patients consulted their physician per patient per year (PPPY) and the proportion of these episodes that resulted in an antibiotic prescription Secondary outcome: nil | |
| Notes | Funding: yes Conflict of interest: RH received travel/lecture funds from Axis‐shield (Norway) and Orion Diagnostica (Finland), both manufacturers of C‐reactive protein devices Trial registration: yes Ethics approval: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Cals 2009 |
| Allocation concealment (selection bias) | Low risk | See Cals 2009 |
| Blinding of participants and personnel (performance bias) | High risk | See Cals 2009 |
| Blinding of outcome assessment (detection bias) | Unclear risk | Data were extracted, by 2 researchers, from the patients' medical records system. No mention if these researchers were blind to the practices' original allocation |
| Incomplete outcome data (attrition bias) | Low risk | 379 of 431 patients enrolled in the original trial (87.9%) had accessible medical records for the follow‐up period |
| Selective reporting (reporting bias) | Low risk | See Cals 2009 |
| Other bias | Low risk | Sample size (power) calculation: see Cals 2009 ITT or per protocol analysis: see Cals 2009 |
| Methods | Study design: cluster‐randomised controlled trial Unit of randomisation: general practices Trial duration: October 2006 to April 2008 Recruitment: half of all general practices from 9 local health boards in Wales (n = 147) were randomly selected to be sent study information (the other half were provided information about a related RCT conducted in parallel); 49 returned a practice agreement and were randomised. 4 primary care research networks in England also recruited practices; 34 returned practice agreement and were randomised. All randomised practise (83) were allocated to intervention (41 practices; 30 recruited patients; patients = 274) or control (42 practices; 31 recruited patients; patients = 284) Methods of data collection: baseline data (age, duration of illness, symptoms) collected by GPs. Follow‐up via a telephone administered questionnaire (or self completion questionnaire contact unsuccessful by telephone) with child's parent or guardian Data collection time points: index consultation and 14 days after recruitment Length of follow‐up: 14 days | |
| Participants | Participating clinicians recruited sequential eligible children (6 months to 14 years) consulting with a respiratory tract infection (cough, cold, sore throat, earache for 7 days or less) and their parents Exclusion: children with asthma and those with serious ongoing medical conditions such as malignancy or cystic fibrosis | |
| Interventions | Brief intervention name: interactive booklet on respiratory tract infections in children for use within the consultation and provided as a take home resource Recipients: parents and clinicians Providers: not stated Health professional components: the online training described the content and aims of the booklet, and encouraged its use within the consultation to facilitate the use of certain communication skills, mainly exploring the parent's main concerns, asking about their expectations, and discussing prognosis, treatment options and any reasons that should prompt re‐consultation Patient components: use of the booklet in the consultation and as a take home resource Materials: 8‐page interactive booklet (see www.whenshouldiworry.com) Mode of delivery: 8‐page interactive booklet and online training for clinicians in use of the booklet Duration and intensity: not stated Comparator: usual care (clinicians were asked to conduct consultations in usual manner) | |
| Outcomes | Primary: re‐consultation (primary or secondary care) during the 2 weeks after the index consultation (telephone administered questionnaire) Secondary: antibiotic prescriptions (telephone administered questionnaire) Antibiotic consumption (telephone administered questionnaire) Future consulting intention (telephone administered questionnaire) Parental satisfaction with the index consultation (5‐point Likert; telephone administered questionnaire) Parental enablement (modified Patient Enablement Instrument; telephone administered questionnaire) Perception of the usefulness (value) of the information received during the index consultation (5‐point Likert; telephone administered questionnaire) Parental reassurance (3‐point Likert; telephone administered questionnaire) | |
| Notes | Funding: yes Conflict of interest: none disclosed Published trial protocol: yes Trial registration: yes Ethics approval: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Practices were randomised by a statistician using block randomisation with random block sizes and stratification by practice list size, antibiotic prescribing rate for 2005, and country |
| Allocation concealment (selection bias) | Unclear risk | It is reported that practices were randomised after agreeing to take part, but no other details are provided |
| Blinding of participants and personnel (performance bias) | High risk | Not possible (training and use of an interactive booklet for use within consultations and as a take home resource) |
| Blinding of outcome assessment (detection bias) | Low risk | Telephone interviewers were blinded to treatment group and asked to record any subsequent unblinding of allocation (e.g. parent talking about receiving a booklet). Interviewers reported becoming aware of participants treatment group in 34/509 (6.7%) of interviews |
| Incomplete outcome data (attrition bias) | Low risk | 83 practices were randomised to intervention (41) or control (42) groups; 61 practices, 30 intervention and 31 control practices, recruited 274 and 284 patients, respectively. Primary outcome data were available for 256 patients (93%) in the intervention group (246 completed telephone interviews, 10 postal questionnaire returned) and 272 (96%) control group patients (262 completing telephone interviews, 9 postal questionnaires returned) |
| Selective reporting (reporting bias) | Low risk | All indicted outcomes reported. Published trial protocol |
| Other bias | Low risk | Sample size (power) calculation: yes ITT or per protocol analysis: primary analysis was ITT |
| Methods | Study design: cluster‐randomised controlled trial (factorial design) Unit of randomisation: general practices Trial duration: October 2010 to May 2011 Recruitment: all general practices (n = 440) in the localities of study centres were approached, and all clinicians (and nurse prescribers in the UK) in eligible practices who prescribed antibiotics for respiratory tract infections were invited to participate Eligibility: practices that had not previously used interventions to reduce antibiotic prescribing and could include > 10 patients at baseline audit. Networks of at least 2 practices were selected separately in Antwerp (Belgium), Barcelona (Spain), Cardiff (Wales), Łódź (Poland), Southampton (UK), Szczecin (Poland), Utrecht (Netherlands) and the Spanish Society of Family Medicine (Spain) to ensure a range of cultures, languages and regions of Europe (north, south and east) were represented). Of the 259 eligible practices enrolled; 246 were randomised to usual care (n = 61), training in the use of a C‐reactive protein (CRP) test at point of care (n = 62), training in enhanced communication skills (n = 61), or in both CRP and enhanced communication skills training (n = 62) Methods of data collection: case report forms (index consultation and follow‐up) Data collection time points: index consultation and follow‐up (until resolution of symptoms) | |
| Participants | General practitioners (GPs and nurse prescribers in the UK) who prescribed antibiotics for RTIs consecutively recruited up to the first 30 patients with LRTI and up to the first 5 with URTI presenting at each practice. Eligible patients were ≥ 18 years of age, attending a first consultation for acute cough of up to 28 days' duration or what the clinician believed to be an acute LRTI as the main diagnosis, despite cough not being the most prominent symptom; and diagnosis judged by the physician to be an acute upper respiratory tract infection (e.g. sore throat, otitis media, sinusitis, influenza and coryzal illness) Exclusion: patients with a working diagnosis of a non‐infective disorder (e.g. pulmonary embolus, heart failure, oesophageal reflux, or allergy); use of antibiotics in the previous month; inability to provide informed consent (e.g. due to dementia, psychosis or severe depression); pregnancy; and immunological deficiencies. Pneumonia was not an exclusion criterion | |
| Interventions | Brief intervention name: enhanced communication skills training Recipients: GPs Providers: n/a Health professional components: training focused on the gathering of information on patients' concerns and expectations; exchange of information on symptoms, natural disease course and treatments; agreement of a management plan, summing up and providing guidance about when to re‐consult. Physicians were provided with an interactive booklet to use during consultations that included information on symptoms, use of antibiotics and antibiotic resistance, self help measures, and when to re‐consult. The training was supported by video demonstrations of consultation techniques. The Internet modules and materials were translated into the relevant national language and mainly addressed lower respiratory tract infections, although many of the issues were relevant to all respiratory tract infections Patient components: interactive booklet used within consultations Materials: interactive booklet for use within consultations Mode of delivery: Internet training supported by video demonstrations of consultation techniques Duration and intensity: not described Comparator: 1. Usual care 2. Training in use of C‐reactive protein (CRP) test at point of care (data not extracted for this review) 3. Both CRP and enhanced communication skills training (data not extracted for this review) | |
| Outcomes | Primary: antibiotic use (index consultation; case‐report form) Secondary: new or worsening symptoms defined as re‐consultation for new or worsening symptoms < 4 weeks, new signs or hospital admission (review of medical notes) Symptom severity and duration defined as the severity of symptoms in the 2 to 4 days after seeing the physician (case report form; 0 = no problem to 4 = severe problem) | |
| Notes | Funding: yes Conflict of interest: none disclosed Published trial protocol: no Trial registration: yes Ethics approval: yes ITT or per protocol analysis: ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of practices was done by 2 study authors, and was achieved by computer generation of random numbers, stratified by network. Minimisation was applied, on the basis of the proportion of patients prescribed antibiotics from the baseline audit, the number of participating physicians per practice, and the number of patients recruited |
| Allocation concealment (selection bias) | Low risk | Physicians and patients were unaware of initial group allocation |
| Blinding of participants and personnel (performance bias) | High risk | Not possible (due to the nature of the intervention) |
| Blinding of outcome assessment (detection bias) | High risk | GPs recorded data on a case‐report from, during the index consultation |
| Incomplete outcome data (attrition bias) | Low risk | 259 practices enrolled and provided baseline data (6771 patients); 13 practices recruited < 10 patients each) were excluded Remaining were 246 practices randomised to CRP (62), enhanced communication training (61), both interventions combined (62), or usual care (61) Antibiotic prescription documentation was available for 58 CRP practices (1062 patients), 55 (90%) enhanced communication skills practices (1170 patients), 62 combined intervention practices (1162 patients) and 53 (87%) usual care practices (870 patients). Reasons for exclusion were reported as recruiting no patients |
| Selective reporting (reporting bias) | Low risk | All indicated outcomes are reported |
| Other bias | Low risk | Sample size (power) calculation: not stated ITT analysis: yes |
| Methods | Study design: cluster‐randomised controlled trial (pilot) Unit of randomisation: family medicine groups (FMGs) Trial duration: during November 2007 and March 2008 Recruitment: 24 FMGs (group of family physicians who work closely with nurses to offer family medicine services to registered individuals) from the greater urban area of Quebec City, Canada, were invited to participate; 4 participating FMGs were randomised either to a group immediately exposed to the DECISION+ program (n = 2) or to a control group which exposure to DECISION+ program was delayed for 6 months (n = 2) Methods of data collection: self administered questionnaire completed following the consultation at each time point Data collection time points: baseline, following exposure of the intervention group to DECISION+ (˜ 6 months), and following delayed exposure of DECISION+ to controls (˜ 12 months) Length of follow‐up: 12 months | |
| Participants | Eligible general practitioners (no previous participation in an implementation trial of SDM and planned to remain in clinical practice for the trial duration) recruited eligibility patients consulting their GP for an ARI: no age restriction, patients or their guardians had to be able to read, understand and write French and had to give informed consent to participate in the trial Exclusion: patients with a condition requiring emergency care. A research professional waited in the FMG's waiting room and recruited patients of enrolled FPs during walk‐in clinic hours; 15 patients were recruited per GP: 5 at baseline, 5 after the GPs in the experimental group were exposed to DECISION +, and 5 after the FPs in the control group were exposed to DECISION+ | |
| Interventions | Brief intervention name: DECISION+ Recipients: GPs Providers: principal investigators (or co‐trainers) Health professional components: DECISION+ is made up of 3 main components 1. Interactive workshops addressed the probability of bacterial versus viral ARIs in primary care, evidence of the benefit/risk of the various treatment options, risk communication techniques and strategies for fostering patient participation in the decision making process. Workshops included videos of simulated patient‐GP consultations for each ARI and distinguished 2 approaches (usual care or SDM), and exercises to facilitate group discussion about facilitators and barriers to SDM. GPs were trained to use decision support tools (though video examples and group exercises) developed for each of the 4 targeted ARIs (rhinosinusitis, pharyngitis, bronchitis and acute otitis media) and 1 integrating all 4 ARIs 2. Reminders of expected behaviours: a reminder printed on a letter‐sized piece of paper emphasised the use of the decision support tools, reiterated the expected SDM‐related behaviours, and highlighted new studies relevant to the pilot trial topics (e.g. new evidence on the risks and benefits of antibiotics). These reminders were mailed to GPs between each workshop. A second reminder was postcards that participants had written to themselves in the last workshop to remind themselves of what they needed to implement in their practice. The research team collected the postcards and mailed them 6 to 8 weeks later 3. Feedback to GPs on the agreement between their decisional conflict scores and that of their first 5 patients Patient components: decision support tools Materials: a booklet summarising the content of the workshop and decision support tools was developed for physician participants and training manuals for the co‐trainers Mode of delivery: interactive workshops led by 2 study principal investigators (or co‐trainers) and conducted face‐to‐face in a group format, and using videos and group exercises Duration and intensity: DECISION+: 3 x 3 3‐hour interactive workshops, reminders and feedback conducted over a 4‐ to 6‐month period Comparator: Usual care (delayed exposure to the DECISION+ intervention) | |
| Outcomes | Primary: decision about using antibiotics (immediate use, delayed use or no use) (GP/patient; self administered questionnaire) Secondary: Perception of the quality of the decision (GP/patient; single item on a 10‐point Likert scale; self administered questionnaire) Decisional conflict (GP/patient; Decisional Conflict Scale) Patients' intention to engage in SDM in future consultations concerning antibiotics for ARIs (3‐item, 7‐point Likert scale; self administered questionnaire) GPs' intentions to engage in SDM and comply with clinical practice guidelines regarding prescribing antibiotics for ARIs (3‐item, 7‐point Likert scale) Decision Regret Scale (patients; telephone interview; 2 weeks following consultation) Perception of health changes since the consultation (patients; telephone interview; 2 weeks following consultation) Number of prescriptions filled by patients covered by Quebec's public drug insurance plan (Regie de l'Assurance‐Maladie du Quebec medication claims database) (during the 3 months preceding baseline and during the 3 months after FPs in the experimental group were exposed to DECISION+) Script concordance test (probes whether respondents' knowledge is efficiently organised to take appropriate clinical action by placing respondents in written, but authentic, clinical situations in which they must interpret data to make decisions. It measures the concordance between respondents' scripts and the scripts of a panel of experts (administered to GPs at each data collection point) | |
| Notes | Funding: yes Conflict of interest: none disclosed Published trial protocol: yes Trial registration: not reported Ethics approval: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A biostatistician simultaneously randomised all FMGs using Internet‐based software |
| Allocation concealment (selection bias) | High risk | A biostatistician allocated FMGs to groups using Internet‐based software. There was concealed allocation of the Family Medicine Groups, but not the family physicians |
| Blinding of participants and personnel (performance bias) | High risk | Not possible (multiple‐component, continuing professional development programme in shared decision making) |
| Blinding of outcome assessment (detection bias) | Low risk | Codes were attributed to the trial groups and the bio‐statistician analysed the data blindly. Team members accessed the codes only after having completed the analyses and interpreting the results |
| Incomplete outcome data (attrition bias) | Low risk | 4 FMGs randomised to intervention (2; GPs = 18; patients = 245) or control groups (2; GPs = 15; patients = 214). 3/33 (9%) GPs dropped out of the trial 20/245 patients in the intervention group and 14/214 controls could not be contacted over the 2‐week follow‐up |
| Selective reporting (reporting bias) | Low risk | All indicated outcomes reported. Published trial protocol |
| Other bias | Low risk | Sample size (power) calculation: no Primary analysis was ITT |
| Methods | Study design: cluster‐randomised controlled trial Unit of randomisation: family practice teaching units Trial duration: July 2010 to April 2011 Recruitment: the network of 12 family practice teaching units in 6 regions of Quebec, Canada, were randomised to intervention (6) or control (6) groups Methods of data collection: following the consultation, patients and GPs independently completed self administered questionnaires (primary and secondary outcomes). 2 weeks later, a telephone follow‐up interview was conducted by a research assistant (secondary outcomes) Data collection time points: immediately following consultation and 14 days | |
| Participants | GPs, including physician teachers and residents, who provide care in the walk‐in clinics of the 12 family practice teaching units. GPs participating in the pilot trial (Légaré 2011) or those not expecting to practice in the teaching unit during the trial period were excluded. Patients with symptoms suggestive of an ARI were recruited by a research assistant in the waiting room prior to consultation with a physician. Eligible patients were adults (and children who were accompanied by a parent/legal guardian) with a diagnosis of ARI (e.g. bronchitis, otitis media, pharyngitis or rhinosinusitis) and for which the use of antibiotics was subsequently considered either by the patient or physician during the visit. The patient, parent or legal guardian had to be able to read, understand and write French | |
| Interventions | Brief intervention name: DECISION+2 shared decision making program Recipients: GPs Providers: trained facilitators Health professional components: an online tutorial comprised of 5 modules addressing key components of the clinical decision making process about antibiotic treatment for ARI in primary care: introduction to shared decision making and ARIs, estimating diagnostic probabilities for ARIs, therapeutic options, effective strategies to communicate risk and benefits, identify patients' values and preferences; and use of decision support tools that promote shared decision making. Participants had 1 month to complete the online tutorial. The on‐site facilitator‐led interactive workshop aimed to help physicians review and integrate the concepts they acquired during the online training Patient components: decision support tools Materials: both the online tutorial and workshop included videos, exercises and decision aids to help physicians communicate to their patients the probability of a bacterial acute respiratory infection and the benefits and harms associated with the use of antibiotics Mode of delivery: online tutorial and facilitator‐led interactive workshop Duration and intensity: 2‐hour online tutorial followed by a 2‐hour on‐site interactive workshop Comparator: usual care | |
| Outcomes | Primary: proportion of patients who decided to use antibiotics immediately after consultation (GP and patient self administered questionnaire) Secondary: decisional conflict (GP/patient; Decisional Conflict Scale) Perception that shared decision making occurred (GP/patient; modified Control Preference Scale) Quality of decision made (GP/patient; single question Likert scale) Adherence to the decision (patient; single‐item asking if decision made was maintained) Repeat consultation (for the same reason) (patient) Decisional Regret (patient; Decisional regret Scale) Quality of life (patient; SF‐12) Intention to engage in SDM in future consultations regarding the use of antibiotics for ARIs (patients; questions based on Theory of Planned Behaviour) Intentions to engage in shared decision making (GP) Intention to adhere to clinical practice guidelines (GP) Preferred role in decision making (Control Preference Scale) | |
| Notes | Funding: yes Conflict of interest: none disclosed Published trial protocol: yes Trial registration: yes Ethics approval: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A biostatistician used Internet‐based software to simultaneously randomise all 12 family practice teaching units to either the intervention group (DECISION+2) or control group. The teaching units were stratified according to rural or urban location |
| Allocation concealment (selection bias) | Unclear risk | The family practice teaching unites were recruited prior to randomisation, but it is not clear when the physicians in the units were recruited |
| Blinding of participants and personnel (performance bias) | High risk | Not possible (due to the nature of the intervention and the self administered outcomes) |
| Blinding of outcome assessment (detection bias) | Low risk | Statistical analysis was performed by a statistician who was unaware of the teaching unit allocations |
| Incomplete outcome data (attrition bias) | Low risk | 12 family practice teaching units randomised; 9 participated in the study and all clusters completed the trial |
| Selective reporting (reporting bias) | Low risk | All indicated outcome reported. Prospective trial registration. Published trial protocol |
| Other bias | Unclear risk | Sample size calculation: yes ITT or per protocol analysis: not stated |
| Methods | Study design: cluster‐randomised controlled trial Unit of randomisation: GP peer review group Trial duration: 2000 to 2002 Recruitment: general practitioners' (GP) peer review groups, with collaborating pharmacists (which aim to promote rational prescribing through audit and feedback), in the region of Utrecht, Netherlands, if the group consisted of ≥ 4 GPs and all agreed to participate Methods of data collection: during a 3‐week period during 2000 and 2001 Data collection time points: index consultation Length of follow‐up: nil | |
| Participants | Primary care setting type: recruited from general practitioner (GP) peer review groups General practitioners: 100 GPs Patients: all registered patients presenting with acute symptoms of the respiratory tract *Relatively low prescription rates in the Netherlands | |
| Interventions | Brief intervention name: multiple intervention Recipients: GPs and patients Providers: GP peer facilitators Health professional components: a) Group education meeting (jointly led a GP and pharmacist in each peer review group) included a review of previous years claims data, discussion of evidence‐based medicine and communication of evidence for treatment benefit and risk to inform group consensus about the indication and first choice of antibiotics per indication (AOM, sinusitis, tonsillitis and acute cough); communication skills training (how to explore patients' worries and expectations and to inform patients about the natural course of the symptoms, self medication and alarm symptoms). GPs received a summary of their group's guidelines by mail 1 week after the meeting, and received the results of the baseline measurement (to reinforce the consensus reached) after 2 months b) Monitoring and feedback on prescribing behaviour (6 months post‐intervention) based on insurance claims data comparing the period after the intervention (March to May 2001) with the same period before the intervention (March to May 2000). Volumes of different kinds of antibiotics and the extent to which prescribed antibiotics were in line with the consensus about first choice antibiotics were presented at practice level c) Group education for assistants of GPs and pharmacists attended a 2‐hour group education session informing them about Dutch guidelines for GPs, followed by skills training in educating patients Patient components: education material for patients consisted of a brochure and accompanying posters (also translated into Turkish and Arabic) available in waiting rooms of intervention group general practices, pharmacies and municipal health services, aiming to inform patients about the self limiting character of most respiratory tract symptoms, self medication and serious symptoms ("alarm signals") necessitating a consultation with the GP Materials: consensus guidelines for GPs and education material for patients Mode of delivery: GP and pharmacist‐led group education meeting for GPs and assistants, and patient education brochure and posters Duration and intensity: 1 x group education meetings for GPs (duration not stated) and 1 x 2‐hour group education meetings for assistants Comparator: usual care | |
| Outcomes | Primary: proportion of practice encounters for acute symptoms of the respiratory tract for which antibiotics were prescribed (patient records) Patient satisfaction (self reported questionnaire; 1 = very dissatisfied to 5 = very satisfied) Secondary: administrative claims data (from regional health insurance company, Agis, over the period 2000 to 2002) (March to May, 2000 and March to May, 2001) | |
| Notes | Funding: yes Conflict of interest: none declared Published trial protocol: not reported Trial registration: not reported Ethics approval: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 12 peer review groups were allocated to groups A or B. All possible compositions of groups A and B were considered and the option chosen of those groups resulting in comparability between group A and B in groups with a high or low volume of antibiotic prescribing (above or below the median), rural or urban working groups, and number of general practitioners per group (above or below the median). MMK, who was blinded to the composition of the groups, flipped a coin to determine whether group A became the intervention or control group |
| Allocation concealment (selection bias) | Low risk | Not stated. However, practices recruited prior to randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Not possible (multiple intervention) |
| Blinding of outcome assessment (detection bias) | Low risk | Research assistants blinded to the intervention status of the practices extracted information from patient records (age, sex, diagnoses, antibiotic prescriptions and referrals to hospital doctors) Patient satisfaction questionnaires returned directly to the investigators without being shown to the GP |
| Incomplete outcome data (attrition bias) | Low risk | Of the 42 of 48 peer‐review groups in the Utrecht region that were invited to participate, 30 groups refused or were unable to participate. The 12 remaining peer‐review groups were randomised to intervention (6 groups, 46 GPs) or control (6 groups, 54 GPs). All clusters and 89/100 GPs completed the study (intervention = 42, control = 49), with loss to follow‐up due to retirement (n = 1), removal outside the region (n = 3), illness (n = 3), motivational problems (n = 2) or technical problems (n = 2) |
| Selective reporting (reporting bias) | Low risk | All indicative results reported |
| Other bias | Low risk | Sample size (power) calculation: yes ITT of per protocol analysis: yes |
AOM: acute otitis media
ARI: acute respiratory infection
COPD: chronic obstructive pulmonary disease
CRP: C‐reactive protein
FP: family physician
GP: general practitioner
ITT: intention‐to‐treat
LRTI: lower respiratory tract infection
n/a: not applicable
OTC: over‐the‐counter
RCT: randomised controlled trial
RTI: respiratory tract infection
SDM: shared decision making
URTI: upper respiratory tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Shared decision making not explicit or inferred | |
| Shared decision making not explicit or inferred | |
| Shared decision making not explicit or inferred | |
| Shared decision making not explicit or inferred | |
| Shared decision making not explicit or inferred | |
| Shared decision making not explicit or inferred |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Converting habits of antibiotic prescribing for respiratory tract infections in German primary care ‐ the cluster‐randomised controlled (CHANGE‐2) trial |
| Methods | 3‐arm cluster‐randomised controlled trial |
| Participants | GPs (n = 94) or practice‐based paediatricians (n = 94) and their patients (˜ 30,000 children and adults) who consult in general practices located in 2 German regions (Baden‐Württemberg and Mecklenburg‐Western Pomerania) for an ARI |
| Interventions | Communication training versus communication training and point of care testing (C‐reactive protein and rapid antigen detection testing) versus control |
| Outcomes | Primary: physician antibiotic prescription rate for ARI at 2‐year follow‐up (post‐intervention) derived from data of the statutory health insurance company Secondary: 1. Re‐consultation rate 2. Use of medical services 3. Hospital admissions |
| Starting date | GP and paediatrician recruitment commenced October 2012; patient recruitment over 3 successive winter periods |
| Contact information | Prof Attila Altiner; Institute for General Practice, Rostock University Medical Center; POB 100888; Rostock 18055 Germany Phone: +49 (0)381 494 2481 Fax: +49 (0)381 494 2482 Email: [email protected]‐rostock.de |
| Notes | — |
ARI: acute respiratory infection
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
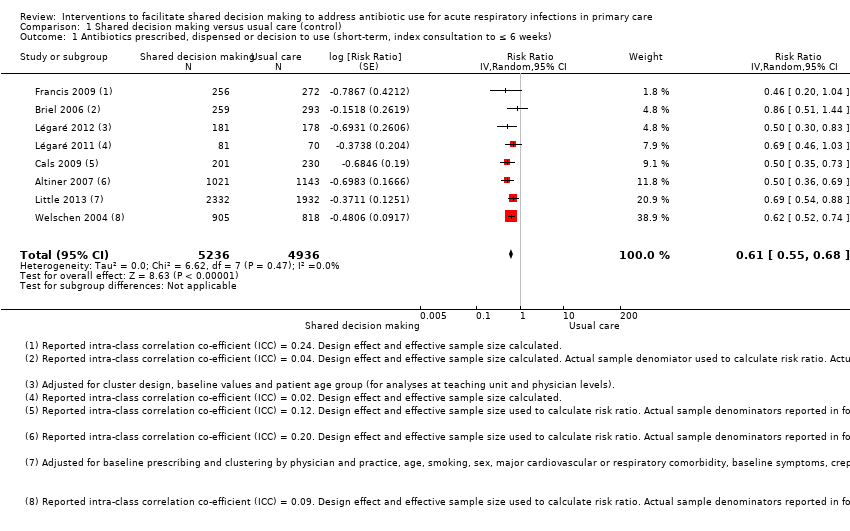
| 1 Antibiotics prescribed, dispensed or decision to use (short‐term, index consultation to ≤ 6 weeks) Show forest plot | 8 | 10172 | Risk Ratio (Random, 95% CI) | 0.61 [0.55, 0.68] |
| Analysis 1.1  Comparison 1 Shared decision making versus usual care (control), Outcome 1 Antibiotics prescribed, dispensed or decision to use (short‐term, index consultation to ≤ 6 weeks). | ||||
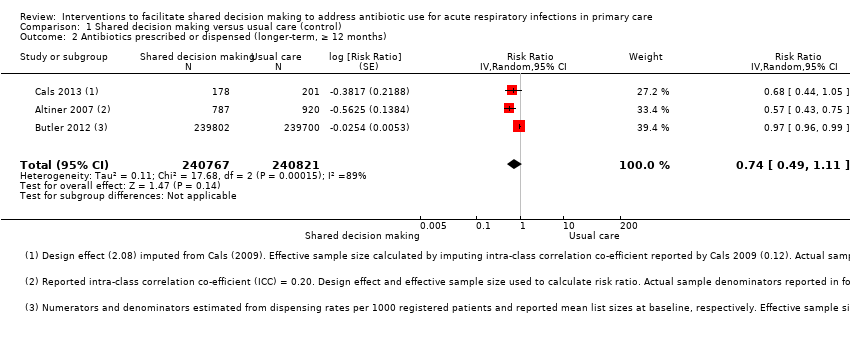
| 2 Antibiotics prescribed or dispensed (longer‐term, ≥ 12 months) Show forest plot | 3 | 481588 | Risk Ratio (Random, 95% CI) | 0.74 [0.49, 1.11] |
| Analysis 1.2  Comparison 1 Shared decision making versus usual care (control), Outcome 2 Antibiotics prescribed or dispensed (longer‐term, ≥ 12 months). | ||||
| 3 Antibiotic prescriptions (index consultation) (adjusted odds ratio) Show forest plot | 3 | 3244 | Odds Ratio (Random, 95% CI) | 0.44 [0.26, 0.75] |
| Analysis 1.3  Comparison 1 Shared decision making versus usual care (control), Outcome 3 Antibiotic prescriptions (index consultation) (adjusted odds ratio). | ||||
| 4 Antibiotic prescriptions (index consultation) (adjusted risk ratio) Show forest plot | 2 | 4623 | Risk Ratio (Random, 95% CI) | 0.64 [0.49, 0.84] |
| Analysis 1.4  Comparison 1 Shared decision making versus usual care (control), Outcome 4 Antibiotic prescriptions (index consultation) (adjusted risk ratio). | ||||
| 5 Antibiotic prescriptions (index consultation or population rate per unit of time) (adjusted risk difference) Show forest plot | 4 | 481807 | Mean Difference (Random, 95% CI) | ‐18.44 [‐27.24, ‐9.65] |
| Analysis 1.5  Comparison 1 Shared decision making versus usual care (control), Outcome 5 Antibiotic prescriptions (index consultation or population rate per unit of time) (adjusted risk difference). | ||||
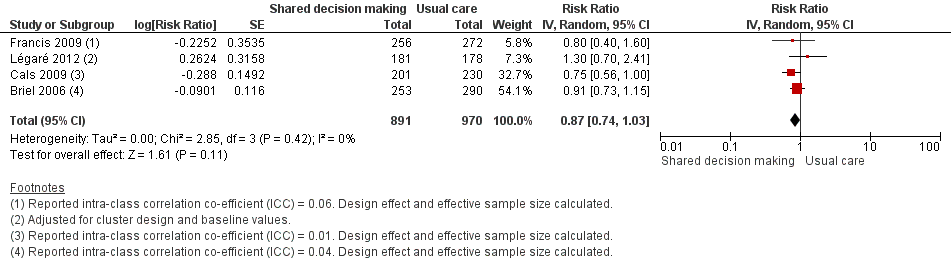
| 6 Number or rate of re‐consultations (risk ratio) Show forest plot | 4 | 1861 | Risk Ratio (Random, 95% CI) | 0.87 [0.74, 1.03] |
| Analysis 1.6  Comparison 1 Shared decision making versus usual care (control), Outcome 6 Number or rate of re‐consultations (risk ratio). | ||||
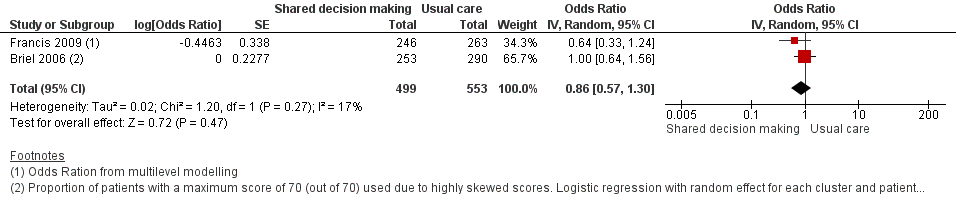
| 7 Patient satisfaction with the consultation Show forest plot | 2 | 1052 | Odds Ratio (Random, 95% CI) | 0.86 [0.57, 1.30] |
| Analysis 1.7  Comparison 1 Shared decision making versus usual care (control), Outcome 7 Patient satisfaction with the consultation. | ||||
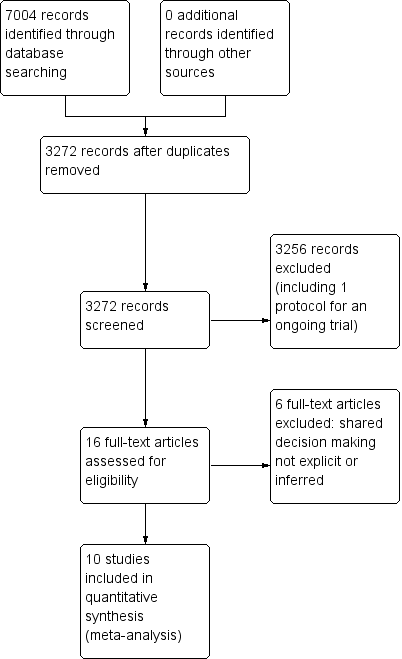
PRISMA study flow diagram.
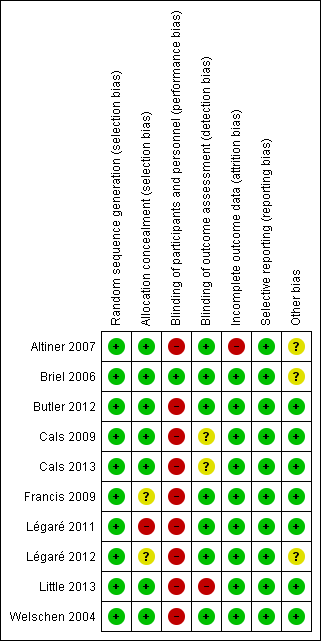
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
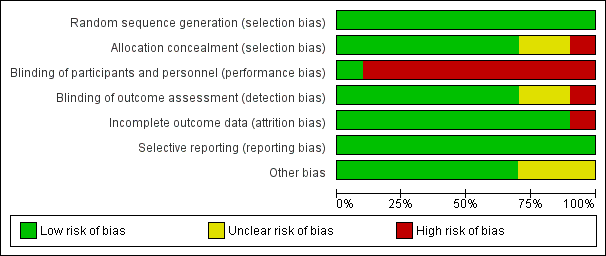
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
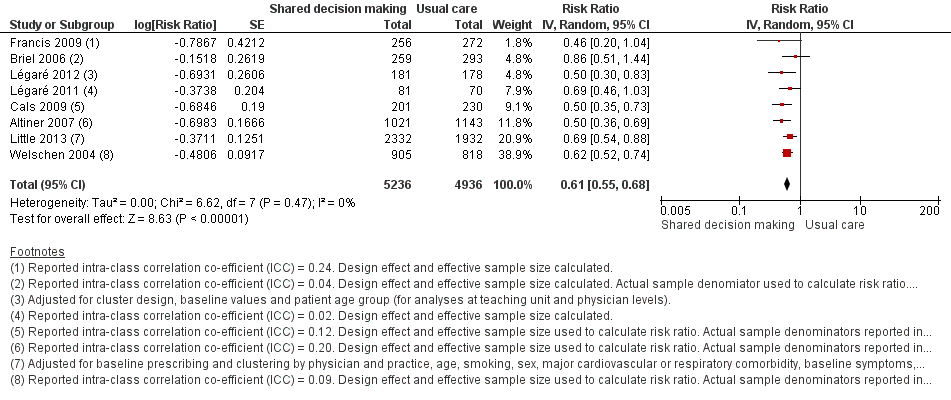
Forest plot of comparison: 1 Shared decision making versus usual care (control), outcome: 1.1 Antibiotics prescribed, dispensed or decision to use (short‐term, index consultation to ≤ 6 weeks).
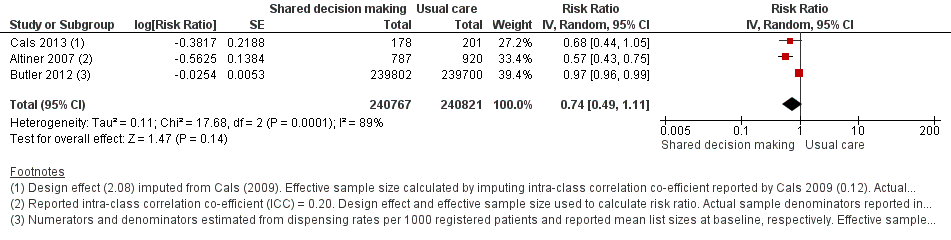
Forest plot of comparison: 1 Shared decision making versus usual care (control), outcome: 1.2 Antibiotics prescribed or dispensed (longer‐term, ≥ 12 months).
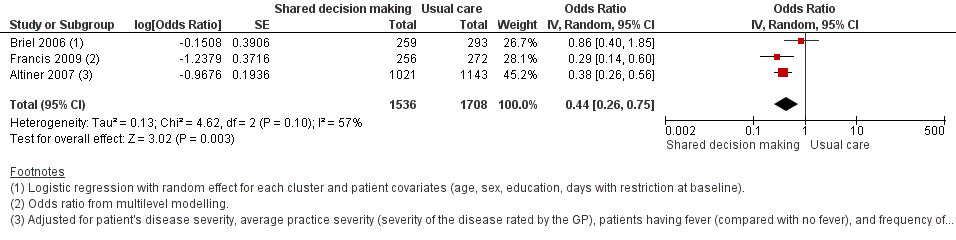
Forest plot of comparison: 1 Shared decision making versus usual care (control), outcome: 1.3 Antibiotic prescriptions (index consultation) (adjusted odds ratio).
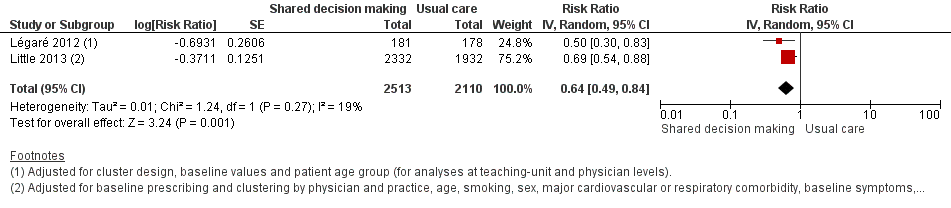
Forest plot of comparison: 1 Shared decision making versus usual care (control), outcome: 1.4 Antibiotic prescriptions (index consultation) (adjusted risk ratio).
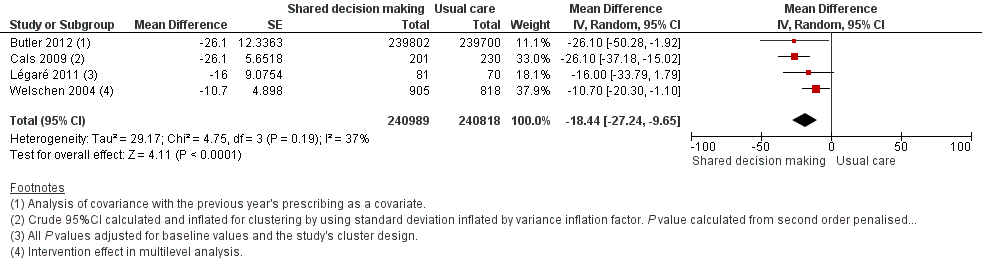
Forest plot of comparison: 1 Shared decision making versus usual care (control), outcome: 1.5 Antibiotic prescriptions (index consultation or population rate per unit of time) (adjusted risk difference).
Forest plot of comparison: 1 Shared decision making versus usual care (control), outcome: 1.6 Number or rate of re‐consultations (risk ratio).
Forest plot of comparison: 1 Shared decision making versus usual care (control), outcome: 1.7 Patient satisfaction with the consultation.
Comparison 1 Shared decision making versus usual care (control), Outcome 1 Antibiotics prescribed, dispensed or decision to use (short‐term, index consultation to ≤ 6 weeks).
Comparison 1 Shared decision making versus usual care (control), Outcome 2 Antibiotics prescribed or dispensed (longer‐term, ≥ 12 months).
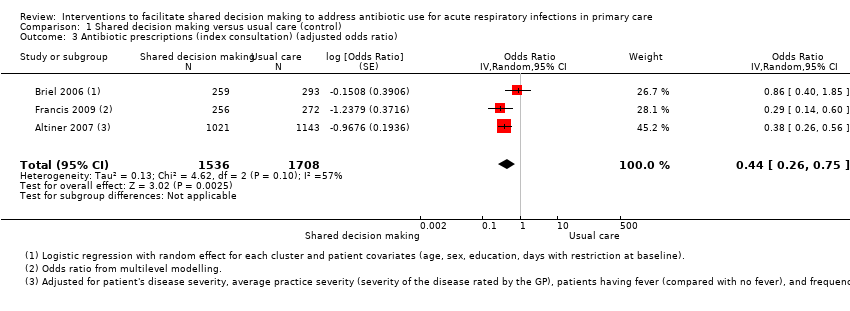
Comparison 1 Shared decision making versus usual care (control), Outcome 3 Antibiotic prescriptions (index consultation) (adjusted odds ratio).
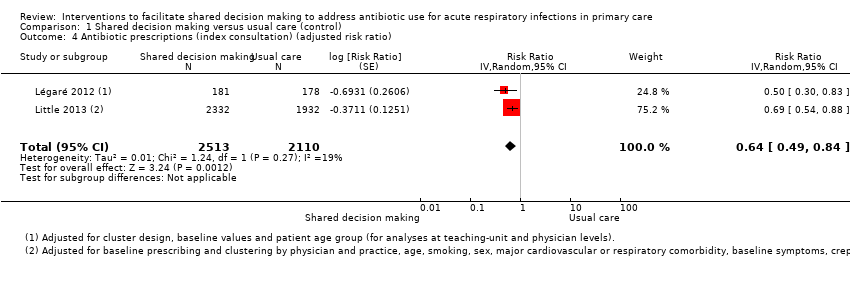
Comparison 1 Shared decision making versus usual care (control), Outcome 4 Antibiotic prescriptions (index consultation) (adjusted risk ratio).
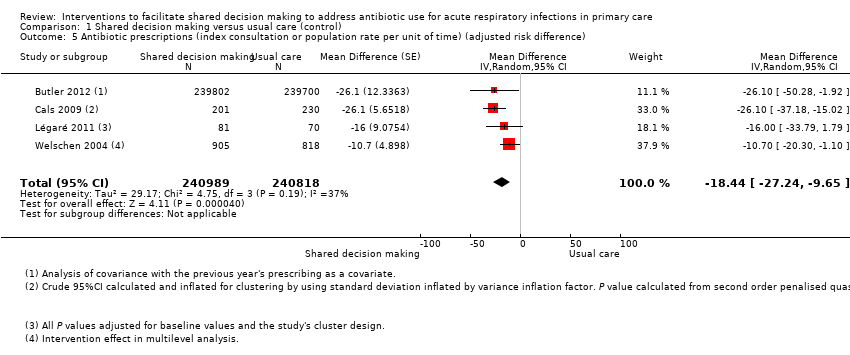
Comparison 1 Shared decision making versus usual care (control), Outcome 5 Antibiotic prescriptions (index consultation or population rate per unit of time) (adjusted risk difference).
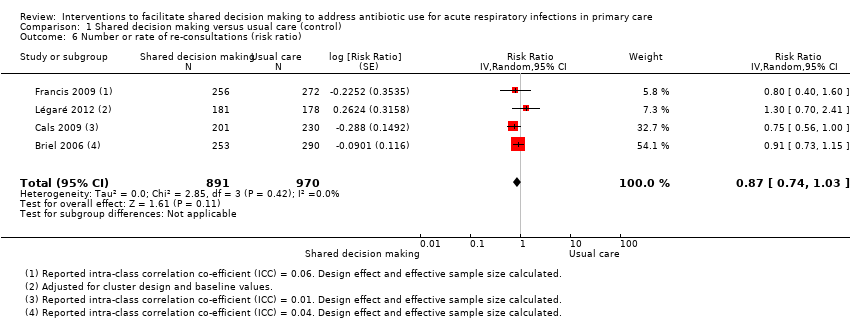
Comparison 1 Shared decision making versus usual care (control), Outcome 6 Number or rate of re‐consultations (risk ratio).
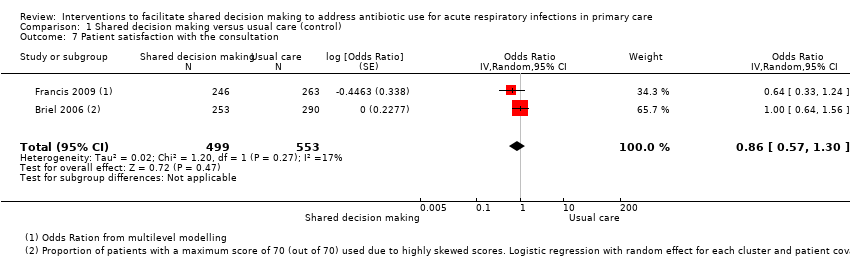
Comparison 1 Shared decision making versus usual care (control), Outcome 7 Patient satisfaction with the consultation.
| Shared decision making compared to usual care for acute respiratory infections in primary care | ||||||
| Patient or population: antibiotic use in acute respiratory infections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with Interventions to facilitate shared decision making | |||||
| Antibiotics prescribed or dispensed (6 weeks or less) | Moderate | RR 0.61 | 10172 | ⊕⊕⊕⊝ | ||
| 47 per 100 | 29 per 100 | |||||
| Antibiotics prescribed or dispensed (12 months or greater) | Moderate | RR 0.74 | 481588 | ⊕⊕⊝⊝ | ||
| 47 per 100 | 35 per 100 | |||||
| Patient initiated re‐consultations for the same illness episode | Moderate | RR 0.87 | 1861 | ⊕⊕⊕⊝ | ||
| 40 per 100 | 35 per 100 | |||||
| Patient satisfaction with the consultation | Moderate | OR 0.86 | 1052 | ⊕⊕⊝⊝ | ||
| 71 per 100 | 68 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level because of risk of bias: participants in most studies were aware of whether they had received the intervention or not. 2 Downgraded one level because of imprecision: confidence interval includes reduction and possible increase in use of antibiotics. There was considerable heterogeneity in the rates of antibiotic prescribing during longer‐term follow‐up (12 months or greater). 3 Sample numbers in one trial, Butler 2012, were calculated from mean list size at baseline multiplied by the number of participating practices in each group (practice list sizes vary over time and no denominator data were available). 4 Downgraded one level due to imprecision: confidence interval includes both satisfaction and lack of satisfaction of patients with the consultation. | ||||||
| Author year | Brief name | Recipient | Why | What (materials) | What (procedures) | Who provided | How | Where | When and how much | Tailoring | Modification of intervention throughout trial | Strategies to improve or maintain intervention fidelity | Extent of intervention fidelity |
| Altiner 2007 | Complex GP peer‐led educational intervention | GPs and patients | Focused on communication within a consultation and the mutual discordance between patients' expectations and doctors' perceived patient expectations, empowering patients to raise the issue within the consultation. By 'informing' both sides in the consultation, it is hoped that doctors and patients would openly talk about the issue and thus reduce unnecessary antibiotic prescriptions | Peers used a semi‐structured dialogue script for outreach visits Patient materials (leaflet and poster) provided in waiting room primarily focused on the patients' role doctor‐patient 'antibiotic misunderstanding' and brief evidence‐based information on acute cough and antibiotics | GP peer‐led outreach visits. Peers were trained to explore GPs' 'opposite' motivational background to address their beliefs and attitudes. GPs were motivated to explore patient expectations and demands, to elicit anxieties and make antibiotic prescribing a subject in the consultation Patient materials were aimed at empowering patients to raise and clarify issues within the consultation | 5 practising GPs and teaching academics in the lead authors' department (2 female, 33 to 63 years of age); trained in 3 sessions for outreach visits | Face‐to‐face outreach visits to GPs | GP clinics during normal working hours | 1 outreach visit performed per GP (duration not specified) | Not described | Not described | Not described | 51/52 GPs received intervention |
| Briel 2006 | Brief training programme in patient‐centred communication | GPs | Focused on teaching GPs how to understand and modify patients' concepts and beliefs about the use of antibiotics for ARIs. GPs were introduced to a model (Prochaska 1992) for identifying patients' attitude and readiness for behaviour change | Evidence‐based guidelines for diagnosis and treatment of ARIs (updated, locally adapted and reviewed by local experts) distributed as a booklet [URL provided is no longer active] | GPs were trained in elements of active listening, to respond to emotional cues, and to tailor information given to patients. Physicians used a model were introduced to a model (Prochaska 1992) to identify patients' attitude and readiness for behaviour change | Not specified | Seminar in small groups (number not specified) and personal feedback by telephone prior to the start of the trial. Evidence‐based guidelines were distributed as a booklet | Not specified | Attendance at 1 x 6‐hour seminar and 1 x 2‐hour telephone call to give personal feedback prior to the trial start | Not described | Not described | Not described | Not described |
| Butler 2012 | Multifaceted flexible blended learning approach for clinicians | GPs and nurse practitioners | Blended learning experience to develop clinicians' sense of the importance about change and their confidence in their ability to achieve change based on Social Learning Theory Clinicians reflected on practice‐level antibiotic dispensing and resistance data, reflected on own clinical practice (context‐bound learning), and were trained in novel communication skills derived from principles of motivational interviewing | Summaries of research evidence and guidelines, web‐based modules using video‐rich material presenting novel communication skills, and a web‐based forum to share experiences and views (see for online component) | Intervention consist of 7 components: experiential learning, updated summaries of research evidence and guidelines; web‐based learning in novel communication skills; practising consulting skills in routine care; facilitator‐led practice‐based seminar on practice‐level data on antibiotic prescribing and resistance; reflections on own clinical practice, and a web‐based forum to share experiences and views | A facilitator conducted the face‐to‐face seminar | Intervention consisted of 7 parts (5 online modules, 1 face‐to‐face seminar and 1 facilitator‐led practice‐based seminar) | The face‐to‐face and facilitator‐led seminars were presented at the general practice | 7 components (5 online, 1 face‐to‐face and 1 facilitator‐led practice‐based seminar) A booster module (6 to 8 months after completion of initial training) reinforced these skills | Intervention was flexible so clinicians could access the online components and try out new skills with their patients at their convenience | Not described | Not described | 138/139 completed all online training and uploaded descriptions of consultations for the portfolio tasks; 129/139 attended the practice‐based seminars; 76/139 completed the optional booster session at 6 months; 11/139 entered new threads on the online forum with 81 posts and 1485 viewings of posts and threads |
| Cals 2009 | Enhanced communication skills training | GPs | Focused on information exchange based on the elicit‐provide‐elicit framework from counselling in behaviour change ‐ exploring patients' fears and expectations, patients' opinion on antibiotics and outlining the natural duration of cough in lower respiratory tract infections | Pre and post‐workshop transcripts of simulated patients | Brief context‐learning based workshop in small groups (5 to 8 GPs), preceded and followed by practice‐based consultations with simulated patients. GPs reflected on own transcripts of consultations with simulated patients, which were also peer‐reviewed by colleagues | Experienced moderator to lead seminars | Brief workshop (5 to 8 GPs), preceded and followed by practice‐based consultation with simulated patients | General practice | 1 x 2‐hour moderator‐led small groups workshop, preceded and followed by practice‐based consultation with simulated patients | Not described | Not described | Not described | 66% of patients recruited by GPs allocated to training in enhanced communication skills recalled their GP's use at least 3 of 4 specific communication skills compared with 19% in the no training group |
| Francis 2009 | Interactive booklet for parents and clinician training in its use | GPs and patients | Focused on specific communication skills, such as exploring parent's main concerns, asking about their expectations, and discussing prognosis, treatment options and reasons that should prompt re‐consultation | 8‐page booklet (now at ); online training in use of the booklet included videos to demonstrate use of the booklet within a consultation, as well as audio feeds, pictures and links to study materials [original URL no longer active] | Booklet given to parents to use in the consultation and as a take‐home resource (no further details provided) Online training on the use of the booklet was provided to GPs: describing the content and aims of the booklet, and encouraging use within the consultation to facilitate use of specific communication skills | N/A (online training) | Parents used the booklet face‐to‐face in the consultation with GPs and took it home; GP training in use of booklet was online | General practice; parents' homes | 1 x 40‐minute online training module | Not described | Not described | Online clinician training monitored through study website: whether a GP has logged on to the site, how much time spent on it and which pages were viewed | Stated that treatment fidelity was not measured so that assessors could remain blind to the study group |
| Légaré 2012 | Shared decision making training program (DECISION+2) | Family physicians (including teachers and residents) | A shared decision making training program that aimed to help physicians communicate to patients the probability of a bacterial ARI and the benefits and harms associated with the use of antibiotics | Online tutorial and workshop included videos, exercises and decision aids to help physicians communicate to their patients the probability of bacterial ARIs and benefits/harms of antibiotic use. Decision aids were available in the consultation rooms in all family practice teaching units | Online self tutorial comprising 5 modules 2‐hour online tutorial followed by a facilitator‐led on‐site interactive workshops aimed to help physicians review and integrate concepts acquired during online training | Trained facilitators | Online tutorial and face‐to‐face workshop | Family practice teaching units | 1 x 2‐hour online tutorial, followed by 1 x 2‐hour on‐site interactive workshop. Participants had 1 month to complete the programme | Not described | Not described | Not described | Of the 162 physicians, 103 completed both the online tutorial and workshop; 16 completed only the workshop; 15 only the tutorial; and 28 completed none of the training components |
| Légaré 2011 | Multiple‐component, continuing professional development program in shared decision making (DECISION+) | Family medicine groups (physicians and nurses) | Aimed to help family physicians communicate to patients the probability of bacterial ARI and benefits and harms of antibiotic use | Workshops included videos (simulated consultations of usual care and SDM) and exercises (facilitators and barriers to SDM). GPs trained in the use of 5 decision support tools using video examples and group exercises. A booklet summarising workshop content provided to participants. Postcard reminders sent | Interactive workshops and related material, reminders of expected behaviours and GP feedback on agreement between their decisional conflict and that of their patients | Trained facilitators | Face‐to‐face workshop | Family medicine groups | 3 x 3‐hour interactive workshops and related material, in addition to reminders of expected behaviours and GP feedback on agreement between their decisional conflict and that of their patients. DECISION+ conducted over 4 to 6 months | Not described | 4 pilot workshops held rather than 3 as the second workshop was redesigned and re‐piloted after feedback on its first testing | Not described | Not described |
| Little 2013 | Internet‐based training in enhanced communication skills | GPs | Rationale was that Internet‐based training can be more widely disseminated than face‐to‐face training. Training focused on eliciting patients' expectations and concerns, natural disease course, treatments, agreement on a management plan, summing up and guidance on when to re‐consult | Interactive booklet for use by GPs within consultations Training supported by video demonstrations of consultation techniques | Online modules and an interactive booklet for use within consultations. (Group practices also appointed a lead GP to organise a structured meeting on prescribing issues) | N/A (online modules) other than lead GP at each practice to organise a meeting (not specific to just this arm of the intervention though) | Online modules (and GP‐led structured practice‐based meeting) | General practice | Internet modules completed alone or in a group | Not described | Not described | Not described | 94/108 practices (87%) completed the communication training. Mean (SD) time spent on the website was 37 (29) minutes |
| Welschen 2004 | Group education meeting with consensus procedure and communication skills training | GPs/pharmacists and their assistants, and patients | GPs discussed evidence for antibiotic benefit/risk, and learned communication techniques to explore patients' expectations and concerns, inform about natural course of symptoms, self‐ medication and alarm symptoms. Patient education provided information on the self‐ limiting nature or ARIs, self‐medication and alarm symptoms requiring re‐consultation | Group consensus guidelines and patient waiting room materials (poster/leaflets) | Group education meeting with consensus procedure, with a summary, and guidelines mailed 1 month later to reinforce consensus reached; feedback on prescribing behaviour (post‐ and pre‐intervention insurance claims data) and practice‐level reporting of extent prescribing behaviours aligned with consensus reached; group education session for GP and pharmacists assistants (Dutch guidelines and skills training in patient education); waiting room education al material for patients | Jointly led by GP and pharmacist | Group education meeting for GPs with consensus procedure and communication skills training, Group education for GPs' and pharmacists' assistants, monitoring and feedback on prescribing behaviour, and patient education materials | Not described | 1 x group education meeting with consensus procedure; 1 x 2‐hour group education session for GP and pharmacists' assistants; monitoring and feedback of prescribing behaviour at 6 months post‐intervention | Not described | Not described | Not described | Not described |
| ARI: acute respiratory infection | |||||||||||||
| Author | Outcome | Measurement time point | Intervention (n) | Control | Effect estimate | P value | Notes |
| Adjusted odds ratio (95% CI) | |||||||
| Francis (2009) | Antibiotics prescribed at the index consultation | 14 days | (30 practices) Patients = 50/256 (19.5%) | (31 practices) | 0.29 (0.14 to 0.60)a | NR | ICC = 0.24 |
| Altiner (2007) | Rate of antibiotic prescriptions (per acute cough and per GP) | 6 weeks | GPs = 42 | GPs = 44 | 0.38 (0.26 to 0.56)b | < 0.001 | ICC=0.20 |
| 12 months | GPs = 28 | GPs = 33 | 0.55 (0.38 to 0.80)b | 0.002 | |||
| Briel (2006) | Uptake of antibiotic prescriptions as reported by pharmacists < 2 weeks after the consultation | 14 days | GPs = 15 | GPs = 15 | 0.86 (0.40 to 1.93)c | NR | ICC = 0.04 Design effect = 1.6 |
| Adjusted risk ratio (95% CI) | |||||||
| Little (2013) | Antibiotic prescription | index consultation | Practices = 61 | Practices = 61 | 0.69 (0.54 to 0.87)d | < 0.0001 | — |
| Légaré (2012) | % patients who decided to use antibiotics immediately after the consultation | Index consultation | Practice units = 6 | Practice units = 6 | 0.50 (0.30 to 0.70)e | — | — |
| Adjusted risk difference (95% CI) | |||||||
| Légaré (2011) | % patients who decided to use antibiotics immediately after the consultation | Index consultation | Medicine groups = 2 | Medicine groups | ‐16 (‐31 to 1)f | 0.08 | — |
| Butler (2012) | Total no. dispensed oral antibiotic items per 1000 registered patients for the year after the intervention | 12‐month period | Practices = 34 Patients = 7053 | Practices = 34 Patients = 7050 | ‐4.2 (‐0.6 to ‐7.7) | 0.02 | — |
| Cals (2009) | Antibiotic prescribing at the index consultation | Index consultation | n/N = 55/201 % crude (95% CI)G 27.4 (25.6 to 36.6) | n/N = 123/230 % crude (95% CI)g 53.5 (43.8 to 63.2) | ‐26.1 (% crude) | < 0.01h | ICC = 0.12 |
| Cals (2013) | Proportion of episodes of respiratory tract infections during follow‐up for which a GP was seen and that antibiotics were prescribed for | Mean 3.67 years follow‐up | n = 178 % (95% CI) 26.3 (20.6 to 32.0) | n = 201 % (95% CI) 39.1 (33.1 to 45.1) | ‐10.4i | 0.02i | — |
| Welschen (2006) | % practice encounters for acute symptoms of the respiratory tract for which antibiotics were prescribed | Index consultation | Review groups = 6 | Review groups = 6 | –10.7 (–20.3 to –1.0)j | — | Practice = 0.17 Review group = 0.09 |
| aTwo level (practice and patient) random intercept logistic regression models. CI: confidence interval | |||||||
| Author | Outcome | Measurement time point | Intervention | Control | Effect estimate | P value | Notes |
| Briel (2006) | Re‐consultations | Within 14 days | n/N (%) 113/253 (44.7) | n/N (%) 143/290 (49.3) | Adjusted rate ratio (95% CI)a 0.97 (0.78 to 1.21) | NR | — |
| Butler (2013) | Re‐consultations after index consultation)b | Within 7 days Within 14 days Within 31 days | Median (IQR) 2.66 (1.88 to 4.25) 5.10 (4.70 to 7.92) 9.06 (7.53 to 12.62) | Median (IQR) 3.35 (2.16 to 4.31) 6.43 (4.04 to 7.84) 11.38 (7.39 to 14.05) | Median difference (95% CI)c ‐0.65 (‐1.69 to 0.55) ‐1.33 (‐2.12 to 0.74) ‐2.32 (‐4.76 to 1.95) | P value = 0.446d P value = 0.411d P value = 0.503d | — |
| Cals (2009) | Re‐consultations | Within 28 days | n/N = 56/201 % crude (95% CI)e 27.9 (21.4 to 34.4) | n/N = 85/230 % crude (95% CI)e 37.0 (30.4, 43.6) | Absolute difference 9.1 (% crude) | 0.14f | ICC = 0.01 |
| Francis (2009) | Re‐consultationg | Within 14 days | n/N (%) 33/256 (12.9) | n/N (%) 44/272 (16.2) | Adjusted odds ratio (95% CI) 0.75 (0.41 to 1.38) | NR | ICC = 0.06 |
| Légaré (2012) | Re‐consultation | Baseline (pre) | 21.6 (12.1 to 29.7) | 22.7 (10.3 to 27.3) | Adjusted risk ratio (95% CI)h 1.3 (0.7 to 2.3) Absolute difference = 7.5 | NR | — |
| Within 14 days (post) | 13.4 (9.9 to 15.9) | 15.2 (11.9 to 19.4) | |||||
| Little (2013) | New or worsening symptomsi | — | n/N (%) 451/2242 (20%) | n/N (%) 309/1879 (16%) | Adjusted risk ratio (95% CI)j 1.33 (0.99 to 1.74) | P value = 0.055 | — |
| aPoisson regression with random effects for each cluster and patient covariates (age, sex, education, days with restrictions at baseline). CI: confidence interval ICC: intra‐class correlation co‐efficient | |||||||
| Author | Outcome | Measurement time point | Intervention | Control | Effect estimate | P value | Notes |
| Briel (2006) | Hospital admissions | < 28 days of study enrolment | n/N = 2/253 | n/N = 1/290 | NR | NR | — |
| Butler (2012) | Hospital admissionsa | Baseline Follow‐up | Mean 7.7 7.5 | Mean 8.7 8.0 | % reduction (intervention relative to controlsb (95% CI) ‐1.9 (‐13.2 to 8.2) | P value = 0.72 | — |
| Cals (2013) | Hospital admissions | Mean 3.67 year follow‐up | n/N 0/178 | n/N 5/201 | NR | NR | — |
| Francis (2009) | Hospital admissions (or observed in a paediatric assessment unit) | < 14 days | n/N 3/256 | n/N 4/272 | NR | NR | — |
| Little (2013) | Hospital admissionsc | < 4 weeks | n/N 6/1170 | n/N 2/870 | NR | — | — |
| aAnnual number of hospital episodes for possible respiratory tract infections and complications of common infections per 1000 registered patients. A single admission occurred if patient admitted to hospital for a possible RTI or complication. If patient admitted more than once, and gap between admissions was 30 days or more, this was considered a separate complication episode. bDifference between means in intervention group and control group as percentage of mean control group. cFactorial analysis data not reported NR: not reported | |||||||
| Author | Outcome | Measurement time point | Intervention | Control | Effect estimate | P value | Notes |
| Briel (2006) | Pneumonia | < 28 days | n/N = 0/253 | 1/290 | NR | NR | — |
| Cals (2013) | Pneumonia | Mean 3.67 year follow‐up | n/N = 0/178 | n/N = 1/201 | NR | NR | — |
| NR: not reported | |||||||
| Author | Outcome | Measurement time point | Intervention | Control | Effect estimate | P value | Notes |
| Briel (2006) | Patient satisfaction (Patient Satisfaction Questionnaire)a | 7 and 14 days | 121/253 (47.8) | 142/290 (49.0) | Adjusted OR (95% CI)b 1.00 (0.64 to 1.31) | NR | — |
| Cals (2009) | Patient satisfaction (% at least 'very satisfied' on Likert scale)c | 28 days | n/N = 144/201 % (crude 95% CI)d 78.7 (72.5 to 84.9) | n/N = 151/230 % (crude 95% CI)d 74.4 (68.2 to 80.6) | 4.3 | P value = 0.88e | — |
| Francis (2009) | Parent satisfaction (Likert scale)f | 14 days | n/N (%) = 222/246 (90.2) | n/N (%) = 246/263 (93.5) | Adjusted OR (95% CI)g 0.6 (0.3 to 1.2) | NR | — |
| Welschen (2006) | Patient satisfaction (Likert scale)h | Index consultation | Patient satisfaction (%) Baseline (pre) = 4.3 (0.3) Follow‐up (post) = 4.3 (0.3) % change (SD) = 0 (0.4) | Patient satisfaction (%) Baseline (pre) = 4.2 (0.4) Follow‐up (post) = 4.2 (0.3) % change (SD): 0 (0.4) | Mean difference of changes (95% CI) 0 (–0.2 to 0.1) i | NR | — |
| a% patients with a maximum score of 70 reported, as satisfaction scores (scale 14 to 70; median 68/70) were highly skewed. CI: confidence interval | |||||||
| Author | Outcome | Measurement time point | Intervention | Control | Effect estimate | P value | Notes |
| Légaré (2012) | Decisional conflict (GPs)a | Immediately after consultation | Baseline: 4.5 (0 to 9.0) Follow‐up: 4.6 (0 to 6.1) | Baseline: 3.0 (0 to 5.9) Follow‐up:1.1 (0 to 2.4) | Adjusted RR 3.4 (0.3 to 38.0) | NR | — |
| Légaré (2012) | Decisional conflict (patients)a | Immediately after consultation | Baseline: 5.1 (0 to 13.5) Follow‐up: 4.6 (2.6 to 7.4) | Baseline: 4.2 (0 to 8.9) Follow‐up: 6.3 (0 to 12.8) | Adjusted RR: 0.8 (0.2 to 2.4) | NR | — |
| Légaré (2011) | Correlation of decisional conflict between GPs and patientsa | Immediately after consultation | Baseline: 0.14 Follow‐up: 0.24 | Baseline: ‐0.05 Follow‐up: 0.02 | Difference at follow‐up (95% CI) 0.26 (‐0.06 to 0.53) | 0.06 | — |
| aProportion of participants who had a value of 2.5 or more on the Decision Conflict Scale (where 1 = low decisional conflict and 5 = very high decisional conflict). CI: confidence interval | |||||||
| Author | Outcome | Measurement time point | Intervention | Control | Effect estimate | P value | Notes |
| Légaré (2012) | Decisional regret a | 2 weeks after consultation | Baseline: 10.5 ± 15.4 Follow‐up: 12.4 ± 19.1 | Baseline: 10.8 ± 20.8 Follow‐up: 7.6 ± 13.7 | Adjusted mean difference 4.8 (0.9 to 8.7) | — | — |
| Légaré (2011) | Patients (%) with decisional regret | 2 weeks after consultation | Baseline: 1 Follow‐up: 7 | Baseline: 1 Follow‐up: 9 | Difference at follow‐up (95% CI) ‐2 (‐12 to 5) | 0.91 | — |
| a = Decisional Regret Scale used, where 0 = very low regret and 100 = very high regret CI: confidence interval | |||||||
| Author | Outcome | Measurement time point | Intervention | Control | Effect estimate | P value | Notes |
| Briel (2006) | Patient enablement (Patient Enablement Instrument; scale 0 to 12) | 7 and 14 days | Mean (SD): 8.49 (1.98) | Mean (SD): 8.15 (2.03) | Adjusted coefficient (95% CI)a 0.35 (‐0.05 to 0.75) | NR | — |
| Cals (2009) | Patient enablement (Patient Enablement Instrument; max score is 12) | 28 days | Median (IQR) score: 3 (4) Mean (SD) score: 3.29 (2.52) | Median (IQR) score: 3 (4)d Mean (SD) score: 3.06 (2.54) | — | NR 0.70b | — |
| Francis (2009) | Parent enablement (Modified Patient Enablement Instrument, scale 1 to 10)c | 14 days | n/N (%): 99/246 (40.2) | n/N (%): 94/262 (35.9) | Adjusted OR (95% CI) 1.20 (0.84 to 1.73) | NR | — |
| aLinear regression with random effects for each cluster and patient covariates (age, sex, education, days with restrictions at baseline). CI: confidence interval | |||||||
| Author | Outcome | Measurement time point | Intervention | Control | Effect estimate | P value | Notes |
| Légaré (2012) | Quality of decision made (GPs) (0 to 10 Likert scale) | After consultation | Baseline: 8.7 ± 1.5 Follow‐up: 8.5 ± 1.6 | Baseline: 8.7 ± 1.5 Follow‐up: 8.5 ± 1.5 | Adjusted mean difference 0.0 (‐0.4 to 0.4) | NR | — |
| Légaré (2011) | Quality of decision made (GPs) (0 to 10 Likert scale) | After consultation | Baseline: 8.8 ± 1.1 Follow‐up: 8.7 ± 1.2 | Baseline: 8.3 ± 1.4 Follow‐up: 8.5 ± 1.3 | Difference at follow‐up (95% CI) 0.2 (‐0.34 to 0.89) | 0.29 | — |
| CI: confidence interval | |||||||
| Author | Outcome | Measurement time point | Intervention | Control | Effect estimate | P value | Notes |
| Légaré (2012) | Quality of decision made (patients) (0 to 10 Likert scale) a | After consultation | Baseline: 8.2 ± 1.1 Follow‐up: 8.2 ± 1.3 | Baseline: 8.2 ± 1.4 Follow‐up: 8.4 ± 1.0 | Adjusted mean difference 0.2 (‐0.6 to 0.2) | NR | — |
| Légaré (2011) | Quality of the decision made (patients) (0 to 10 Likert scale) a | After consultation | Baseline: 8.2 ± 2.1 Follow‐up: 8.7 ± 1.9 | Baseline: 8.4 ± 1.9 Follow‐up: 8.6 ± 1.9 | Difference at follow‐up (95% CI) 0.1 (‐0.88 to 0.94) | 0.57 | — |
| aLikert scale where 0 = very low quality and 10 = very high quality. CI: confidence interval | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antibiotics prescribed, dispensed or decision to use (short‐term, index consultation to ≤ 6 weeks) Show forest plot | 8 | 10172 | Risk Ratio (Random, 95% CI) | 0.61 [0.55, 0.68] |
| 2 Antibiotics prescribed or dispensed (longer‐term, ≥ 12 months) Show forest plot | 3 | 481588 | Risk Ratio (Random, 95% CI) | 0.74 [0.49, 1.11] |
| 3 Antibiotic prescriptions (index consultation) (adjusted odds ratio) Show forest plot | 3 | 3244 | Odds Ratio (Random, 95% CI) | 0.44 [0.26, 0.75] |
| 4 Antibiotic prescriptions (index consultation) (adjusted risk ratio) Show forest plot | 2 | 4623 | Risk Ratio (Random, 95% CI) | 0.64 [0.49, 0.84] |
| 5 Antibiotic prescriptions (index consultation or population rate per unit of time) (adjusted risk difference) Show forest plot | 4 | 481807 | Mean Difference (Random, 95% CI) | ‐18.44 [‐27.24, ‐9.65] |
| 6 Number or rate of re‐consultations (risk ratio) Show forest plot | 4 | 1861 | Risk Ratio (Random, 95% CI) | 0.87 [0.74, 1.03] |
| 7 Patient satisfaction with the consultation Show forest plot | 2 | 1052 | Odds Ratio (Random, 95% CI) | 0.86 [0.57, 1.30] |

