Interventionen zur Prävention und Behandlung von kortikosteroidbedingter Osteoporose und zur Vorbeugung osteoporotischer Frakturen bei der Duchenne‐Muskeldystrophie
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Prospective, double‐blind, randomised, placebo‐controlled study | |
| Participants | Ambulant children with DMD (N = 21) (mean age: 9.3 ± 3.9 years) | |
| Interventions | For at least 6 months before the study, all children were treated with corticosteroids and calcifediol (0.7 mcg/kg/day) and took 100% of the calcium recommended daily allowance. Randomised intervention: weight‐bearing activity (low‐magnitude high‐frequency vibration (delivering 0.3 g, 30 Hz; N = 11)) or placebo devices (N = 10) for 10 minutes per day for 1 year | |
| Outcomes |
| |
| Funding sources | Not stated ‐ the abstract did not provide information on funding sources. | |
| Declarations of interest | Not stated | |
| Notes | The full trial is not yet published. The conference abstract was published in 2013, but a trial start date and end date has not been provided. An email response from Bianchi 2013 confirmed that there was as yet no more published information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This was a prospective, double‐blind, randomised, placebo‐controlled study. |
| Allocation concealment (selection bias) | Unclear risk | The study was reported as an abstract only. |
| Blinding of participants and personnel (performance bias) | Low risk | This was a double‐blind, randomised, placebo‐controlled study. |
| Blinding of outcome assessment (detection bias) | Low risk | This was a double‐blind, randomised, placebo‐controlled study. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study was reported as an abstract only. |
| Selective reporting (reporting bias) | Unclear risk | The study was reported as an abstract only. |
| Other bias | Unclear risk | The study was reported as an abstract only. |
| Methods | Randomised controlled trial | |
| Participants | Boys (N = 13) affected with DMD (median age (years): 8.5, range: 5.4 to 15.5) and spine Z‐score ≤ 1.0 | |
| Interventions | Risedronate group: risedronate 1 mg/kg per week (maximum 35 mg), calcium (500 mg/day), and vitamin D (10 mg/day) (N = 6) Control group: calcium and vitamin D (N = 7) 9 participants were ambulant and on corticosteroid therapy. The effects were determined after 1 year of treatment with risedronate. There was not enough detail in the abstract on timing of risedronate treatment with reference to taking steroids. | |
| Outcomes |
| |
| Funding sources | Not stated ‐ the abstract did not provide information on funding sources. | |
| Declarations of interest | Not stated | |
| Notes | The full trial is not published. A conference abstract was published in 2014. The trial start date was 22 June 2010 with no end date provided. EudraCT Number: 2009‐017649‐67 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants (spine Z‐score less than ‐1.0) were randomised to each treatment arm. |
| Allocation concealment (selection bias) | Unclear risk | The study was reported as an abstract only. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study was reported as an abstract only. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The study was reported as an abstract only. |
| Incomplete outcome data (attrition bias) | Unclear risk | The study was reported as an abstract only. |
| Selective reporting (reporting bias) | Unclear risk | The study was reported as an abstract only. |
| Other bias | Unclear risk | The study was reported as an abstract only. |
DMD: Duchenne muscular dystrophy.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This study did not include participants with DMD. | |
| This study was not a RCT. | |
| This study was not a RCT. The author confirmed this. | |
| This study was not a RCT. | |
| This study did not include participants with DMD. | |
| This controlled study did not assess the effect of exercise on bone health. | |
| This study was a before and after trial, not a RCT. | |
| This was a retrospective cohort study, not a RCT. | |
| This was a prospective observational study, not a RCT. | |
| This study was not a RCT. | |
| This controlled study did not assess the effect of exercise on bone health. | |
| This was a retrospective cohort study, not a RCT. | |
| This study was not a RCT. | |
| This study did not assess the effect of the intervention on bone health. |
DMD: Duchenne muscular dystrophy.
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Clinical trial of zoledronic acid in children and adolescents with Duchenne muscular dystrophy |
| Methods | Open‐label RCT |
| Participants | Children and adolescents with DMD |
| Interventions | Zoledronic acid (Aclasta) versus vitamin D plus calcium |
| Outcomes |
|
| Starting date | 1 September 2010 for the 1st recruited participant |
| Contact information | email: [email protected] |
| Notes | The trial start date was 13 September 2013, and the estimated final collection date was 31 December 2015. Source of monetary support: Altum Novartis |
| Trial name or title | Whole body vibration therapy in boys with Duchenne muscular dystrophy |
| Methods | RCT, parallel assignment, open‐label interventional |
| Participants | Boys with DMD |
| Interventions | Whole‐body vibration therapy |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | March 2014 |
| Contact information | Hugh McMillan, MD [email protected] |
| Notes | The trial start date was stated as 13 September 2013, with an estimated final collection date of March 2015. |
DMD: Duchenne muscular dystrophy.
RCT: randomised controlled trial.

A flow diagram illustrating the study selection process.
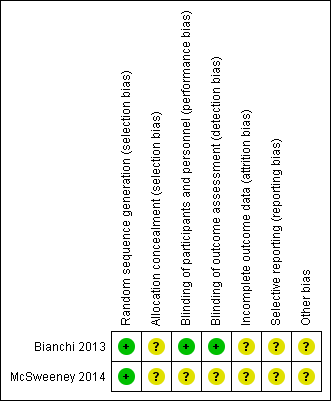
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study. Green = low risk of bias; yellow = unclear risk of bias; red (not shown) = high risk of bias.
| Risedronate (plus calcium and vitamin D supplementation) | Control (calcium and vitamin D supplementation alone) | |||
| Baseline | 12‐month | Baseline | 12 months | |
| Median spine Z‐score (range) | ‐1.75 (‐1.2 to ‐3.5) | ‐0.8 (‐1.7 to 0) | ‐2.2 (‐4.1 to ‐1.2) | ‐1.6 (‐8.4 to ‐0.8) |
| Median whole‐body Z‐score (range) | ‐1.95 (‐0.5 to 2.7)* | 1.3 (‐1.0 to 2.5) | ‐1.2 (‐1.6 to 4.7) | ‐0.8 (‐3.1 to 3) |
| A median of ‐1.95 is outside the range given, but we were unable to confirm correct figures with the study author. | ||||

