Intervenciones para la prevención y el tratamiento de la osteoporosis inducida por corticosteroides y la prevención de fracturas osteoporóticas en la distrofia muscular de Duchenne
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010899.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 24 enero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neuromuscular
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Jennifer Bell prepared the protocol with discussion and comments from review authors. Dr Janet Watters reviewed the text from the perspective of a healthcare consumer as the mother of a boy with Duchenne muscular dystrophy.
Conceiving the review: MS; BB
Co‐ordinating the review: JMB
Undertaking manual searches: JMB
Organising retrieval of papers: JMB
Screening retrieved papers against inclusion criteria: JMB; MS
Writing to authors of papers for additional information: JMB
Data management for the review: JMB
Data extraction and quality assessment: JMB; MS
Interpretation of data: JMB; MS; BB
Writing the review: JMB with contributions from all authors
Guarantor for the review (one author): MS
Person responsible for reading and checking the review before submission: JMB and all authors
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Health Research Board/HSC R&D Division Cochrane Fellowship Award, Ireland.
Declarations of interest
JMB: none known
TB: acknowledges financial support received from Servier Laboratories Ltd and Amgen UK Ltd to attend national conferences and payment with respect to chairman and speakers fees.
BB: none known
ME: none known
AH: none known
RQ: is Joint Co‐ordinating Editor of Cochrane Neuromuscular and is the author of two consensus workshop reports for bone health in Duchenne muscular dystrophy. Dr Quinlivan has received an honorarium from Genzyme for lecturing on metabolic muscle disease, as well as consultancy fees from Novartis and Guidepoint Global.
MDS: has received honoraria from GlaxoSmithKline, AstraZeneca, Novartis, Merck Sharp & Dohme, and ALK‐Abelló for lectures given at educational meetings and received hospitality to attend the European Respiratory Society and British Thoracic Society annual meetings.
ST: none known
JW: a member of charities Action Duchenne and the Muscular Dystrophy UK.
Acknowledgements
We wish to thank Angela Gunn, Information Specialist, Cochrane Neuromuscular, for assistance with the development of the search strategy.
We thank Dr Ruth Brassington, Managing Editor, Cochrane Neuromuscular, for support and guidance during the writing process.
This review was supported by funding provided from the Cochrane Fellowship scheme, HSC R&D Division, Public Health Agency Northern Ireland.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Diseases.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jan 24 | Interventions to prevent and treat corticosteroid‐induced osteoporosis and prevent osteoporotic fractures in Duchenne muscular dystrophy | Review | Jennifer M Bell, Michael D Shields, Janet Watters, Alistair Hamilton, Timothy Beringer, Mark Elliott, Rosaline Quinlivan, Sandya Tirupathi, Bronagh Blackwood | |
| 2014 Mar 19 | Interventions to prevent steroid‐induced osteoporosis and osteoporotic fractures in Duchenne muscular dystrophy | Protocol | Jennifer M Bell, Bronagh Blackwood, Michael D Shields, Janet Watters, Alistair Hamilton, Timothy Beringer, Mark Elliott, Rosaline Quinlivan, Sandya Tirupathi | |
Differences between protocol and review
Lack of data prevented full implementation of our protocol (Bell 2014).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Weight‐Bearing;
- Adrenal Cortex Hormones [adverse effects];
- Bone Density [drug effects];
- Bone Density Conservation Agents [*therapeutic use];
- Calcium [therapeutic use];
- Diphosphonates [therapeutic use];
- Imidazoles [therapeutic use];
- Muscular Dystrophy, Duchenne [*drug therapy];
- Osteoporosis [chemically induced, complications, *drug therapy, prevention & control];
- Osteoporotic Fractures [etiology, *prevention & control];
- Randomized Controlled Trials as Topic;
- Risedronic Acid [therapeutic use];
- Spinal Fractures [etiology, *prevention & control];
- Vibration [*therapeutic use];
- Vitamin D [therapeutic use];
- Zoledronic Acid;
Medical Subject Headings Check Words
Adolescent; Child; Child, Preschool; Humans; Male;
PICO

A flow diagram illustrating the study selection process.
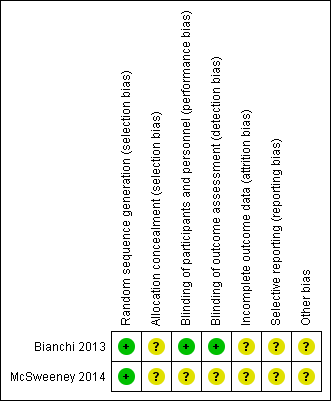
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study. Green = low risk of bias; yellow = unclear risk of bias; red (not shown) = high risk of bias.
| Risedronate (plus calcium and vitamin D supplementation) | Control (calcium and vitamin D supplementation alone) | |||
| Baseline | 12‐month | Baseline | 12 months | |
| Median spine Z‐score (range) | ‐1.75 (‐1.2 to ‐3.5) | ‐0.8 (‐1.7 to 0) | ‐2.2 (‐4.1 to ‐1.2) | ‐1.6 (‐8.4 to ‐0.8) |
| Median whole‐body Z‐score (range) | ‐1.95 (‐0.5 to 2.7)* | 1.3 (‐1.0 to 2.5) | ‐1.2 (‐1.6 to 4.7) | ‐0.8 (‐3.1 to 3) |
| A median of ‐1.95 is outside the range given, but we were unable to confirm correct figures with the study author. | ||||

