防治皮质类固醇引起的骨质疏松症和预防杜氏肌营养不良症骨质疏松性骨折的干预措施
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register (CRS) search strategy
#1 muscular NEAR5 dystroph* [REFERENCE] [STANDARD]
#2 duchenne or dystrophinopathy [REFERENCE] [STANDARD]
#3 nonambula* or "non ambula*" [REFERENCE] [STANDARD]
#4 #1 or #2 or #3 [REFERENCE] [STANDARD]
#5 "vertebral deformity" or fracture or compression or crush or wedging or biconcavity [REFERENCE] [STANDARD]
#6 osteoporosis [REFERENCE] [STANDARD]
#7 bone or bmc [REFERENCE] [STANDARD]
#8 (bone or bmc):so [REFERENCE] [STANDARD]
#9 #7 not #8 [REFERENCE] [STANDARD]
#10 lumbar or spine [REFERENCE] [STANDARD]
#11 MeSH DESCRIPTOR Diphosphonates Explode All [REFERENCE] [STANDARD]
#12 bisphosphonate* or diphosphonate* [REFERENCE] [STANDARD]
#13 alendronate or clodronate or etidronate or ibandronate or incadronate or opadronate or pamidronate or risedronate or tiludronate or zoledronate [REFERENCE] [STANDARD]
#14 calcitonin or calcium or calcitriol or cholecalciferol* or Ergocalciferol* [REFERENCE] [STANDARD]
#15 "vitamin D" [REFERENCE] [STANDARD]
#16 "dietary supplements" or testosterone [REFERENCE] [STANDARD]
#17 Teriparatide or "weight bearing" or diet or exercise [REFERENCE] [STANDARD]
#18 "non drug" NEXT therapy [REFERENCE] [STANDARD]
#19 #5 or #6 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17
or #18 [REFERENCE] [STANDARD]
#20 #4 and #19 [REFERENCE] [STANDARD]
#21 (#4 and #19) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 2. CENTRAL search strategy
Search run on Mon Sep 12 2016
#1(muscular near dystrophy or duchenne near dystrophy or dystrophinopathy or non next ambula* or nonambula*):TI,AB,KY520
#2("vertebral deformity" or fracture or compression or crush or wedging or biconcavity):TI,AB,KY11862
#3(steroid near1 induced and osteoporosis):TI,AB,KY34
#4(steroid near1 induced and "bone loss"):TI,AB,KY18
#5(steroid near1 induced and osteopenia):TI,AB,KY6
#6MESH DESCRIPTOR Osteoporosis WITH QUALIFIERS CI205
#7(secondary next osteoporosis):TI,AB,KY41
#8(bone next strength):TI,AB,KY248
#9(bone next fragil*):TI,AB,KY51
#10(bone next loss):TI,AB,KY2938
#11("bone density" or "bone mineral density" or "bone mineral content" or bmc):TI,AB,KY7029
#12(bone next deformity or lumbar next spine):TI,AB,KY3340
#13MESH DESCRIPTOR Spine EXPLODE ALL TREES3740
#14MESH DESCRIPTOR Diphosphonates EXPLODE ALL TREES2659
#15(bisphosphonate* or diphosphonate*):TI,AB,KY1765
#16(alendronate or clodronate or etidronate or ibandronate or incadronate or opadronate or pamidronate or risedronate or tiludronate or zoledronate):TI,AB,KY2255
#17(calcitonin or calcium or calcitriol or ergocalciferol* or "vitamin D"):TI,AB,KY23566
#18MESH DESCRIPTOR Calcifediol EXPLODE ALL TREES215
#19("dietary supplements" or teriparatide):TI,AB,KY7410
#20("non drug" next therapy):TI,AB,KY16
#21(diet or exercise or "weight bearing" or testosterone):TI,AB,KY79090
#22#2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21119677
#23#1 and #22119
#24sr‐neuromusc:cc5974
#25#23 not #2478
Appendix 3. MEDLINE (OvidSP) search strategy
Ovid MEDLINE(R) 1946 to August Week 5 2016
Database: Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 randomized controlled trial.pt. (430785)
2 controlled clinical trial.pt. (91698)
3 randomized.ab. (370085)
4 placebo.ab. (178870)
5 drug therapy.fs. (1906928)
6 randomly.ab. (263462)
7 trial.ab. (384951)
8 groups.ab. (1638648)
9 or/1‐8 (3900462)
10 exp animals/ not humans.sh. (4315089)
11 9 not 10 (3364542)
12 exp muscular dystrophy/ (23751)
13 ((muscular adj5 dystrophy) or duchenne or dystrophinopathy).tw. (19784)
14 (non ambula* or nonambula*).mp. (1602)
15 or/12‐14 (30865)
16 (vertebral deformity or fracture or compression or crush or wedging or biconcavity).mp. (270009)
17 ((steroid adj1 induced) and osteoporosis).tw. (252)
18 ((steroid adj1 induced) and bone loss).tw. (75)
19 ((steroid adj1 induced) and osteopenia).tw. (37)
20 osteoporosis/ci (2760)
21 secondary osteoporosis.tw. (744)
22 (bone adj1 strength).tw. (4188)
23 (bone adj1 fragil$).tw. (1680)
24 (bone adj1 loss).tw. (23360)
25 (bone mineral density or bone mineral content or bmc).mp. (38924)
26 Bone Density/ (45641)
27 (bone adj1 deformity).tw. (313)
28 (lumbar adj1 spine).tw. (24195)
29 Lumbar Vertebrae/ (44335)
30 exp Spine/ (125272)
31 exp Diphosphonates/ (22891)
32 (bisphosphonate$ or diphosphonate$).tw. (17118)
33 (alendronate or clodronate or etidronate or ibandronate or incadronate or opadronate or pamidronate or risedronate or tiludronate or zoledronate).tw. (9832)
34 Calcitonin/ (14866)
35 calcium/ (253994)
36 Calcitriol/ (13310)
37 exp Cholecalciferol/ (24172)
38 Ergocalciferols/ (2943)
39 Vitamin D/ (27463)
40 Dietary Supplements/ (41269)
41 Teriparatide/ (1589)
42 Weight‐Bearing/ (17139)
43 (non‐drug adj1 therapy).tw. (103)
44 (diet or exercise).tw. (454348)
45 exp Testosterone/ (65868)
46 testosterone.mp. (91168)
47 or/16‐46 (1299956)
48 11 and 15 and 47 (319)
49 remove duplicates from 48 (303)
Appendix 4. Embase (OvidSP) search strategy
Database: Embase <1980 to 2016 Week 37>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 crossover‐procedure.sh. (48595)
2 double‐blind procedure.sh. (131481)
3 single‐blind procedure.sh. (23071)
4 randomized controlled trial.sh. (416900)
5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1294764)
6 trial.ti. (207191)
7 or/1‐6 (1447525)
8 exp animal/ or exp invertebrate/ or animal.hw. or non human/ or nonhuman/ (23158822)
9 human/ or human cell/ or human tissue/ or normal human/ (17529785)
10 8 not 9 (5661779)
11 7 not 10 (1276235)
12 limit 11 to embase (1041572)
13 exp muscular dystrophy/ (36206)
14 ((muscular adj5 dystrophy) or duchenne or dystrophinopathy).tw. (23473)
15 (non ambula* or nonambula*).mp. (2209)
16 or/13‐15 (41054)
17 vertebra malformation/ (2625)
18 (vertebral deformity or fracture or compression or crush or wedging or biconcavity).mp. (420882)
19 corticosteroid induced osteoporosis/ (1750)
20 ((steroid adj1 induced) and osteoporosis).tw. (356)
21 ((steroid adj1 induced) and bone loss).tw. (104)
22 ((steroid adj1 induced) and osteopenia).tw. (52)
23 osteoporosis/pc [Prevention] (9654)
24 secondary osteoporosis.tw. (1320)
25 (bone adj1 strength).tw. (5666)
26 (bone adj1 fragil$).tw. (2437)
27 (bone adj1 loss).tw. (27779)
28 (bone mineral density or bone mineral content or bmc).mp. (52199)
29 bone density/ (70004)
30 bone malformation/ (4902)
31 (((bone adj1 density) or bone) adj1 deformity).tw. (404)
32 exp spine/ (149050)
33 (lumbar vertebra$ or lumbar spine).tw. (39235)
34 bisphosphonic acid derivative/ (28745)
35 (bisphosphonate$ or diphosphonate$).tw. (23178)
36 (alendronate or clodronate or etidronate or ibandronate or incadronate or opadronate or pamidronate or risedronate or tiludronate or zoledronate).tw. (13945)
37 calcitonin/ (21362)
38 calcium/ (242503)
39 calcitriol/ (25813)
40 colecalciferol/ (14523)
41 ergocalciferol/ (6751)
42 vitamin D/ (59328)
43 diet supplementation/ (75411)
44 "parathyroid hormone[1‐34]"/ (5412)
45 resistance training/ (9634)
46 weight bearing.mp. (29389)
47 (non‐drug adj1 therapy).tw. (166)
48 (diet or exercise).tw. (550445)
49 testosterone.mp. (114818)
50 or/17‐49 (1607048)
51 12 and 16 and 50 (162)
52 remove duplicates from 51 (159)
Appendix 5. CINAHL Plus (EBSCOhost) search strategy
Monday, September 12, 2016 9:27:36 AM
S48 S46 AND S47 5
S47 EM 20150601‐ 413,167
S46 s45 Limiters ‐ Exclude MEDLINE records
Search modes ‐ Boolean/Phrase 22
S45 S18 AND S19 AND S44 199
S44 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 334,135
S43 diet or exercise or testosterone replacement therapy 197,974
S42 "non drug" W1 therapy 37
S41 weight W1 bearing 6,645
S40 teriparatide 392
S39 vitamin W1 D 15,233
S38 Cholecalciferol* or Ergocalciferol* 886
S37 calcitonin or calcium or calcitriol 29,703
S36 alendronate or clodronate or etidronate or ibandronate or incadronate or opadronate or pamidronate or risedronate or tiludronate or zoledronate 2,128
S35 bisphosphonate* or diphosphonate* 6,770
S34 (MH "Diphosphonates+") 6,762
S33 spine 24,224
S32 lumbar N4 vertebrae 10,854
S31 bone N4 deformity OR lumbar spine 4,994
S30 bone density 14,412
S29 bone mineral density or bone mineral content or bmc 6,242
S28 bone loss 4,602
S27 bone fragil* 260
S26 bone strength 1,008
S25 secondary osteoporosis 203
S24 (MH "Osteoporosis/CI") 576
S23 steroid W1 induced AND osteopenia 2
S22 steroid W1 induced AND bone loss 8
S21 steroid W1 induced AND osteoporosis 32
S20 vertebral W1 deformity OR ( fracture or compression or crush or wedging or biconcavity ) 67,110
S19 muscular W5 dystrophy OR ( duchenne or dystrophinopathy or "non ambula*" or nonambula*) 3,104
S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 855,321
S17 ABAB design* 92
S16 TI random* or AB random* 178,868
S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 355,248
S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 129,453
S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) 48,376
S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 27,566
S11 PT ("clinical trial" or "systematic review") 131,822
S10 (MH "Factorial Design") 977
S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 288,464
S8 (MH "Meta Analysis") 25,156
S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 49
S6 (MH "Quasi‐Experimental Studies") 7,981
S5 (MH "Placebos") 9,796
S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 33,944
S3 (MH "Clinical Trials+") 202,467
S2 (MH "Crossover Design") 13,928
S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 73,492
Appendix 6. Web of Science Core Collection ‐ Indexes=CPCI‐S Timespan=1900‐2016
2001‐2016
#19 #17 AND #16
Refined by: DOCUMENT TYPES: (PROCEEDINGS PAPER OR MEETING ABSTRACT)
DocType=All document types; Language=All languages;
#18 #17 AND #16
#17 TS=(random* or "clinical trial" or group or groups or blind* or crossover or "crossover" or "controlled trial")
#16 #15 AND #1
#15 #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2
#14 TS=(bisphosphonate* or diphosphonate* or alendronate or clodronate or etidronate or ibandronate or incadronate or opadronate or pamidronate or risedronate or tiludronate or zoledronate or calcitonin or calcium or calcitriol or cholecalciferol* or Ergocalciferol* or "vitamin D" or "dietary supplements" or Teriparatide or "weight bearing" or diet or exercise or ("non drug" NEAR therapy) or bone or testosterone)
#13 TS=((bone NEAR/3 deformity) or (lumbar NEAR/3 spine))
#12 TS=("bone density" or "lumbar vertebra*")
#11 TS=("bone mineral density" or "bone mineral content" or bmc)
#10 TS=("bone loss")
#9 TS=(bond NEAR/3 fragil*)
#8 TS=(bone NEAR/3 strength)
#7 ts=("secondary osteoporosis")
#6 TS=(induced and osteopenia)
#5 TS=(induced and "bone loss")
#4 TS=(induced and osteoporosis)
#3 TOPIC: (fracture or compression or crush or wedging or biconcavity or lumbar or spine)
#2 TOPIC: ("vertebral deformity" or fracture or compression or crush or wedging or biconcavity or "vertebral deformity")
#1 TOPIC: ((muscular NEAR/5 dystroph*) or duchenne or dystrinopathy or nonambula* or "non ambula*")
Appendix 7. clinicaltrials.gov search strategy
Search performed 11 October 2016
Duchenne muscular dystrophy AND (bone OR bone mineral density OR fractures) AND (vitamin D OR calcium OR testosterone OR physical activity OR exercise)
Appendix 8. apps.who.int/trialsearch/ search strategy
Search performed 11 October 2016.
The following terms were used:
Duchenne muscular dystrophy AND bone 8
Duchenne muscular dystrophy AND osteoporosis 1
Duchenne muscular dystrophy AND bisphosphonates 1
Duchenne muscular dystrophy AND vitamin D 2
Duchenne muscular dystrophy AND calcium 3
Duchenne muscular dystrophy AND testosterone 1
Duchenne muscular dystrophy AND whole body vibration 1
Appendix 9. www.isrctn.com search strategy
Search performed 11 October 2016
Duchenne muscular dystrophy
Appendix 10. Additional methods described in the protocol
Data extraction and management
Two review authors (JMB and MS) will independently extract data using a standard data extraction form. One author (JMB) will enter data into the Cochrane software Review Manager 5 (RevMan) (RevMan 2014), and a second author (MS) will check data entry. JMB will contact study authors directly for any additional or missing data required. We will note in the 'Characteristics of included studies' table if outcome data are not reported in a usable way.
Where studies provide data on our outcomes at time points less than or more than 24 months, we will extrapolate the data to 24 months when it is reasonable to do so. We will assume a linear response rate but carry out a sensitivity analysis to assess the effects of the imputation, by re‐analysis using alternative imputed values.
Measures of treatment effect
Statistical methods used to measure treatment effects will be in accordance with theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
We will analyse dichotomous data as risk ratios (RR) and continuous data as mean difference, or standardised mean difference for results across studies with outcomes that are conceptually the same but measured in different ways. We will report these measures of effect with 95% confidence intervals (CI).
We will undertake meta‐analyses, using RevMan, only where this is meaningful, that is, if the treatments, participants, and the underlying clinical question are similar enough for pooling to make sense.
Unit of analysis issues
Where a single trial includes multiple trial arms, we will include only the relevant arms. If we combine two comparisons (e.g. drug A versus placebo and drug B versus placebo) in the same meta‐analysis, we will halve the control group to avoid double‐counting. For each participant, there may be multiple observations for the same outcome.
Dealing with missing data
We will attempt to contact the trial correspondence author to obtain any missing data. If we are unable to obtain data, we will try to find out why the data are missing and decide, based on whether they are missing at random or not, whether to analyse available data or impute missing values using a statistical model. We will consider how best to include missing data where baseline‐observation‐carried‐forward (BOCF) or last‐observation‐carried‐forward (LOCF) methods have been used for imputation and attempt to standardise these between studies (LOCF being preferable).
We will address the potential implications of missing data (for example, loss to follow ‐up and no outcome obtained, lack of compliance) in the 'Discussion'.
Assessment of heterogeneity
We will assess clinical heterogeneity by judging, qualitatively, the differences between studies regarding the participants, therapies, and reporting of important study outcomes.
We will statistically test heterogeneity of intervention effects among trials using the standard Chi² statistic (P value) and the Higgins I² statistic expressed as a percentage. We will take P values of less than 0.05 as evidence of heterogeneity. We will interpret I² for heterogeneity as follows:
-
0% to 40%: may not be important;
-
30% to 60%: may represent moderate heterogeneity;
-
50% to 90%: may represent substantial heterogeneity; and
-
75% to 100%: considerable heterogeneity.
If we identify substantial unexplained heterogeneity, we will report it and explore possible causes by prespecified subgroup analysis (Higgins 2011b).
Assessment of reporting biases
To detect the presence of publication bias, we will construct a funnel plot using RevMan if there are a reasonable number of studies (at least 10 in the same meta‐analysis). We will use Beggs's and Egger’s tests to verify the bias (Begg 1994; Egger 1997).
Data synthesis
If there is no substantial or considerable heterogeneity, we will synthesise the data in a meta‐analysis using RevMan. We will perform both fixed‐effect and random‐effects models for comparison purposes and use the most appropriate, depending upon the degree of heterogeneity.
If the review includes more than one comparison, and we cannot include them in the same analysis, we will report the results for each comparison separately.
Cost‐utility analyses
We will consider cost‐effectiveness of interventions per QALY (quality‐adjusted‐life‐year) in the Discussion, where data are available.
Subgroup analysis and investigation of heterogeneity
If there are sufficient data, we plan to undertake the following subgroup analyses using the outcome vertebral fractures:
-
ambulant versus non‐ambulant (due to disease progression, these groups will reflect age groups); and
-
intervention versus intervention combination.
Within each group, we will use the I² statistic for heterogeneity, and if its value is greater than 50%, we will scrutinise the trials and forest plots for differences to explain the heterogeneity. If we find no explanation, we will repeat the analysis using a random‐effects model.
'Summary of findings' table
We will create a 'Summary of findings' table using the following outcomes:
-
change in bone mineral density as assessed by Z‐score;
-
frequency of vertebral fragility fractures; and
-
adverse events.
We will use the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence (studies that contribute data for the prespecified outcomes). We will use methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions, Higgins 2011a, using GRADEpro software. We will justify all decisions to downgrade or upgrade the quality of studies using footnotes, and we will make comments to aid readers' understanding of the review where necessary.
Sensitivity analysis
We will perform a sensitivity analysis to determine whether conclusions are robust by undertaking both fixed‐effect and random‐effects meta‐analysis. We will perform sensitivity analyses to assess the effect of including studies at high risk of bias on the change in vertebral bone mineral density (body height corrected Z‐scores), and by repeating the meta‐analysis excluding any studies at high risk of bias. If we have imputed missing data, we will assess the effects of the imputation by re‐analysis using several alternative imputed values.

A flow diagram illustrating the study selection process.
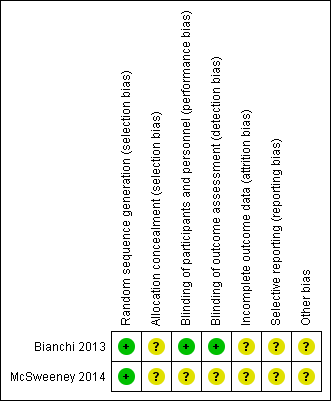
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study. Green = low risk of bias; yellow = unclear risk of bias; red (not shown) = high risk of bias.
| Risedronate (plus calcium and vitamin D supplementation) | Control (calcium and vitamin D supplementation alone) | |||
| Baseline | 12‐month | Baseline | 12 months | |
| Median spine Z‐score (range) | ‐1.75 (‐1.2 to ‐3.5) | ‐0.8 (‐1.7 to 0) | ‐2.2 (‐4.1 to ‐1.2) | ‐1.6 (‐8.4 to ‐0.8) |
| Median whole‐body Z‐score (range) | ‐1.95 (‐0.5 to 2.7)* | 1.3 (‐1.0 to 2.5) | ‐1.2 (‐1.6 to 4.7) | ‐0.8 (‐3.1 to 3) |
| A median of ‐1.95 is outside the range given, but we were unable to confirm correct figures with the study author. | ||||

