Вмешательства для предотвращения и лечения кортикостероид‐индуцированного остеопороза и для предотвращения переломов, связанных с остеопорозом, при мышечной дистрофии Дюшенна
Abstract
Background
Corticosteroid treatment is considered the 'gold standard' for Duchenne muscular dystrophy (DMD); however, it is also known to induce osteoporosis and thus increase the risk of vertebral fragility fractures. Good practice in the care of those with DMD requires prevention of these adverse effects. Treatments to increase bone mineral density include bisphosphonates and vitamin D and calcium supplements, and in adolescents with pubertal delay, testosterone. Bone health management is an important part of lifelong care for patients with DMD.
Objectives
To assess the effects of interventions to prevent or treat osteoporosis in children and adults with DMD taking long‐term corticosteroids; to assess the effects of these interventions on the frequency of vertebral fragility fractures and long‐bone fractures, and on quality of life; and to assess adverse events.
Search methods
On 12 September 2016, we searched the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE, Embase, and CINAHL Plus to identify potentially eligible trials. We also searched the Web of Science ISI Proceedings (2001 to September 2016) and three clinical trials registries to identify unpublished studies and ongoing trials. We contacted correspondence authors of the included studies in the review to obtain information on unpublished studies or work in progress.
Selection criteria
We considered for inclusion in the review randomised controlled trials (RCTs) and quasi‐RCTs involving any bone health intervention for corticosteroid‐induced osteoporosis and fragility fractures in children, adolescents, and adults with a confirmed diagnosis of DMD. The interventions might have included oral and intravenous bisphosphonates, vitamin D supplements, calcium supplements, dietary calcium, testosterone, and weight‐bearing activity.
Data collection and analysis
Two review authors independently assessed reports and selected potential studies for inclusion, following standard Cochrane methodology. We contacted study authors to obtain further information for clarification on published work, unpublished studies, and work in progress.
Main results
We identified 18 potential studies, of which two, currently reported only as abstracts, met the inclusion criteria for this review. Too little information was available for us to present full results or adequately assess risk of bias. The participants were children aged five to 15 years with DMD, ambulant and non‐ambulant. The interventions were risedronate versus no treatment in one trial (13 participants) and whole‐body vibration versus a placebo device in the second (21 participants). Both studies reported improved bone mineral density with the active treatments, with no improvement in the control groups, but the abstracts did not compare treatment and control conditions. All children tolerated whole‐body vibration treatment. No study provided information on adverse events. Two studies are ongoing: one investigating whole‐body vibration, the other investigating zoledronic acid.
Authors' conclusions
We know of no high‐quality evidence from RCTs to guide use of treatments to prevent or treat corticosteroid‐induced osteoporosis and reduce the risk of fragility fractures in children and adults with DMD; only limited results from two trials reported in abstracts were available. We await formal trial reports. Findings from two ongoing relevant studies and two trials, for which only abstracts are available, will be important in future updates of this review.
PICO
Резюме на простом языке
Виды лечения для предотвращения и лечения истончения костей и предотвращения переломов, вызванных кортикостероидами, при мышечной дистрофии Дюшенна
Вопрос обзора
Каковы эффекты лечения для предотвращения истончения костей (остеопороз), а также предотвращения хрупкости костей, приводящей к их переломам, у взрослых и детей с мышечной дистрофией Дюшенна (МДД), длительно принимающих кортикостероиды?
Актуальность
МДД ‐ это наследственное заболевание, поражающее примерно одного из 3500 ‐ 6000 новорожденных мальчиков. МДД является причиной слабости мышц, ухудшающейся с годами. Мышечная слабость имеет распространенный характер, влияет на движения, пищеварительную систему, дыхание и сердце. Слабость имеет следующий характер: в основном, затрагивает мышцы туловища, вокруг плеч и бедер. При отсутствии лечения МДД приводит к смерти в позднем подростковом возрасте от дыхательных или сердечных осложнений. Первые признаки МДД проявляются у детей в дошкольном возрасте. При отсутствии лечения к 13 годам больной теряет возможность ходить и будет нуждаться в инвалидном кресле. Вылечить МДД невозможно, но существуют определенные лекарства называемые глюкокортикостероидами, которые замедляют повреждение мышц и позволяют мальчикам с МДД продлить их возможность ходить. Мальчики с МДД также, как правило, имеют слабые кости из‐за ослабленных мышц и сниженной подвижности.
Кортикостероиды приводят к остеопорозу (истончению костей), что делает кости более хрупкими и подверженными к перелому. Такие методы лечения, как добавки витамина D и кальция, увеличение кальция в рационе человека, бисфосфонаты, тестостерон и упражнения с весовой нагрузкой вероятно могут укрепить кости, предотвратить и лечить остеопороз, а также предотвратить переломы у людей,страдающих МДД и принимающих кортикостероиды. Исследователи использовали плотность костной ткани как показатель, насколько кость крепкая, а также для оценки результатов методов лечения для предотвращения и лечения остеопороза.
Характеристика исследований
Мы провели всесторонний поиск медицинской литературы и нашли два завершенных клинических испытания. Только краткие отчеты (тезисы) об этих испытаниях были доступны. Участниками были дети в возрасте от 5 до 15 лет с синдромом МДД, которые еще имели возможность ходить и те, кто не мог передвигаться. В одном испытании (13 участников) лечение ризендронатом сравнивали с отсутствием лечения, в другом испытании (21 участник) вибрацию всего тела сравнивали с плацебо (неактивным устройством).
Основные результаты и качество доказательств.
Мы обнаружили два завершенных исследования, которые потенциально отвечали критериям этого обзора, полные результаты которых еще не были опубликованы, и два продолжающихся исследования на момент поиска. В этих исследованиях изучаются эффекты вибрации всего тела (два исследования), ризендроната и золедроновой кислоты. В обоих завершенных испытаниях, по которым были представлены только тезисы (абстракты), сообщали об улучшении минеральной плотности кости у детей, получавших активное лечение, и отсутствии улучшения состояния у детей в группе контроля (плацебо или отсутствие лечения). Однако, не было информации о результатах сравнения группы лечения и контрольной группы, что означает, что невозможно сделать выводы об эффективности какого‐либо лечения. У всех детей была отмечена переносимость лечения вибрацией всего тела. Ни одно из исследований не предоставило информацию о неблагоприятных событиях.
Таким образом, в настоящее время отсутствуют доказательства из рандомизированных клинических испытаний, которые были бы руководством в применении лечения, направленного на предотвращение или лечение остеопороза и предотвращение переломов у людей с МДДМ, принимающих кортикостероиды.
Поиск актуален на сентябрь 2016 года.
Authors' conclusions
Background
Description of the condition
Duchenne muscular dystrophy (DMD)
Duchenne muscular dystrophy (DMD) is a progressive disorder affecting skeletal, cardiac, and smooth muscle (Emery 1998; Voisin 2004). Inheritance is sex‐linked recessive, and the condition has an incidence of one in 3500 to one in 6000 male live births (Emery 1991; Emery 2003; Flanigan 2012). DMD is caused by deletions, duplications or mutations in the dystrophin gene located at Xp21, resulting in the absence or low levels of the protein known as dystrophin (Chaturvedi 2001; Flanigan 2009; Lee 2012; Muntoni 2003; Soltanzadeh 2010). Dystrophin is a large protein located on the sarcolemmal membrane. It forms part of the dystrophin‐associated protein complex and is involved in membrane integrity. Absence of dystrophin leads to membrane damage and progressive dystrophic changes within the muscle (Blake 2002; Deconinck 2007). Affected boys appear normal at birth and first show signs of muscle weakness in early childhood, often as a toddler. Without treatment, they become wheelchair dependent by 13 years of age. Without respiratory support, death occurs in the late teens or early twenties. Because of postural imbalance and reduced muscular support to the spine, scoliosis (spinal deformity) typically develops. Scoliosis of the thoracic region restricts movement of the ribs making breathing even more difficult for already weakened respiratory muscles. However, in recent years, prognosis has improved substantially with the introduction of non‐invasive ventilation support along with cough‐assist technology and co‐ordinated multidisciplinary care (Bushby 2010; Passamano 2012).
Corticosteroid treatment is now considered the 'gold standard' for DMD, and corticosteroid‐induced osteoporosis is an important emerging problem (Bushby 2010). Corticosteroids have been shown to slow the decline in muscle function and may protect cardiac and respiratory function. Corticosteroid‐treated boys lose the ability to walk at around 12 to 14 years of age, compared with seven to 13 years for non‐treated boys (Matthews 2010; Ricotti 2012). Physicians usually start treatment during the 'plateau phase' of the condition (usually between four and six years of age) (Bushby 2010). Doses of prednisone, prednisolone, or deflazacort are either taken daily, Biggar 2004; Fenichel 1991; Griggs 1991; Matthews 2010; Mesa 1991, or intermittently, Dubowitz 2002; Kinali 2002. Treatment that is continued after the loss of ambulation may preserve upper body strength, respiratory and cardiac function, and delay the onset of scoliosis (Balaban 2005; Biggar 2004; Biggar 2006; Daftary 2007; Hussein 2014; King 2007; Lebel 2013; Manzur 2008; Schram 2013). Corticosteroids have significant side‐effects, which require proactive management (Angelini 2007; Balaban 2005; Biggar 2006; Bonifati 2000; Bushby 2004; Bushby 2010; Houde 2008; King 2007; Manzur 2008; Matthews 2010; Merlini 2012; Moxley 2010). One such side‐effect is corticosteroid‐induced osteoporosis, which includes the increased risk of vertebral fractures (Angelini 2012; Bachrach 2005; Balaban 2005; Bothwell 2003; Bianchi 2003; Canalis 2004; Houde 2008; King 2007; Mayo 2012; Quinlivan 2010; Soderpalm 2007; Van Staa 2003; Weldon 2009).
Osteoporosis
Osteoporosis is a progressive, systemic skeletal disease, characterised by reduced bone mass and micro‐architectural deterioration of bone tissue. As a result, bone is increasingly fragile and more susceptible to fracture (NICE 2012). Fragility fractures are fractures that result from mechanical forces that would not ordinarily result in fracture. Osteoporotic fragility fractures are defined as fractures associated with low bone mineral density and include spine, forearm, hip, and shoulder fractures.
In children and adolescents, osteoporosis is defined as low bone mineral content or bone mineral density for chronological age. The diagnosis of osteoporosis requires the presence of both a clinically significant fracture history, for example, vertebral compression fracture or long‐bone fractures, and low bone mineral content or bone mineral density. Low bone mineral content or bone mineral density is defined as bones with a Z‐score that is less than or equal to ‐2.0, adjusted for age, gender, and body size, as appropriate (ISCD 2007).
Bone mineral content and bone mineral density are assessed in children by dual‐energy X‐ray absorptiometry (DXA) (Bianchi 2010). Quantitative‐computed tomography, peripheral quantitative‐computed tomography, quantitative ultrasonography, or magnetic resonance imaging (MRI) are also used (Bachrach 2011; Buckner 2015). To avoid incorrect estimations, conventional DXA measurements are adjusted according to size for use in children, those with deteriorating bone, and people of shorter stature (Bianchi 2014). Bone mineral density is measured as age‐based and height‐adjusted Z‐scores (Bachrach 2011; Baim 2008; Bianchi 2010; Crabtree 2014; Fewtrell 2003). DXA can also predict the risk of fractures before they occur. Its use is based on a relationship between low bone mineral density and risk of fracture in adults, which also exists in children (Clark 2006; Goulding 1998). This is an important application of DXA in patients with DMD, as part of bone health evaluation (Bianchi 2011a).
Bone is a living tissue, which bone formation and bone resorption constantly remodels (Joyce 2012). Factors that affect bone health include genetics, nutrition, physical activity (loading), age, and hormones. Corticosteroid treatment affects endocrine function, suppressing growth, delaying puberty, reducing bone mineral density, and increasing body weight (Bianchi 2011a; Dooley 2013; Wood 2015). Corticosteroids induce bone loss by hindering bone formation through osteoblastic apoptosis (the death of cells that make bone) and by stimulating bone mineral resorption through an increase in the number of osteoclasts (cells that break down bone) (Popp 2006). The reduction in bone mineral density from changes in bone metabolism is not fully understood (Bianchi 2011a). Deficiencies in testosterone (delayed puberty) and growth hormone, or resistance to an insulin‐like growth hormone, and limited mobility with excessive weight gain contribute to a reduction in bone mineral density (Allen 1998; Bianchi 2011a; Dooley 2013). Bone mineral density is difficult to assess in DMD due to lack of normative curves, short stature, delayed bone age, etc. Corticosteroid treatment may not appear to reduce bone mineral density to the same extent as in adults. Soderpalm 2008 commented that lower bone mineral density in boys with DMD is likely to be the combined result of corticosteroid treatment, muscle loss from the disease, and reduced mobility. Low bone mineral density increases the risk of bone fragility fractures (Bachrach 2005; Douvillez 2005; Joyce 2012; Quinlivan 2005; Talim 2002). There are some data to suggest that boys with DMD who are corticosteroid‐naive (that is, those who have not previously been exposed to corticosteroids) are at increased risk of long‐bone fractures (Biggar 2006; Granata 1991; Houde 2008; Hsu 1979; Larson 2000; McDonald 2002; Vestergaard 2001), implying that corticosteroids are not the only cause for these fractures and causation is complex.
Osteoporotic fragility fractures
Vertebral fragility fractures are recognised as the most common manifestation of corticosteroid‐induced osteoporosis (Al‐Osail 2010; Rodd 2012; Sugiyama 2011; Van Staa 2000; Van Staa 2001; Van Staa 2003; Varonos 1987). Vertebral fragility fractures have not been reported in corticosteroid‐naive patients with DMD of any age (Alman 2004; Houde 2008; King 2007), and very often, vertebral fractures are not tracked in children with DMD (especially those not treated with steroids). In boys with DMD taking corticosteroids, the occurrence of vertebral fractures ranges from 15% to 75% after a mean duration of 6.3 years on treatment (ranging from 4.5 to 8.0 years) (Bothwell 2003; Houde 2008; King 2007; Mayo 2012). The incidence of long‐bone fractures was, however, similar in treated and untreated patients (Houde 2008; McDonald 2002) (24% and 26%, respectively, in Houde 2008).
Those treated with corticosteroids tend to be more ambulant, and patients who are more ambulant are more likely to fall and sustain a long‐bone fracture. Non‐ambulatory patients appear to be at an increased risk of vertebral fractures (Larson 2000; Mayo 2012). Previous surgical intervention for scoliosis may also prevent vertebral fragility fractures (Mayo 2012). Lack of ambulation, taking steroids previously, or both, may lead to a greater body fat composition in patients with DMD. Body fat percentage was reported as significantly higher in patients with vertebral fractures (47.8% ± 12%) than in those with long‐bone fractures (33% ± 14.2%) (P < 0.05) (Mayo 2012).
Vertebral fractures can be symptomatic or asymptomatic. Acute back pain is closely associated with symptomatic vertebral fragility fractures (odds ratio (OR) 10.6, 95% confidence interval (CI) 2.1 to 53.8, P = 0.004) and requires radiological investigation to confirm the diagnosis (Huber 2010; Talim 2002), but an estimated 20% of vertebral fractures are asymptomatic (Manzur 2010). Asymptomatic fractures are thought to be precursors of painful symptomatic fragility fractures (Leung 2011). Asymptomatic fractures may be undetected because radiological or densitometric monitoring is not conducted routinely (Quinlivan 2010). Diagnosis and confirmation of a vertebral fracture requires visual examination of lateral X‐ray or magnetic resonance imaging (MRI) images to assess the change in shape of the vertebrae as biconcave, wedge, or crush, as well as the degree of deformity (Genant 1993; Lenchik 2004; Quinlivan 2010).
The occurrence of fractures is significantly related to low bone mineral density in DMD (Aparicio 2002; Khatri 2008; Larson 2000). Bone mineral density is often lower than normal for the age of the patient (Bianchi 2003; Crabtree 2010; Mayo 2012; Soderpalm 2012). In children and adolescents with DMD, the baseline bone mineral density is low due to lack of weight‐bearing activity. It is probably lowered further by corticosteroids. While some studies have shown no reduction in spinal bone mineral density after 30 months of corticosteroid therapy for DMD in prepubertal boys (Crabtree 2010), or only a marginal reduction in children and adolescents taking corticosteroids (Cohran 2008), others reported a substantially lowered bone mineral density in boys (children and adolescents) taking corticosteroids, particularly those with reduced mobility (Bianchi 2003; Hawker 2005; Mayo 2012).
International consensus workshops have taken place to prioritise poor bone health in DMD patients and to improve clinical outcomes through education (Biggar 2005; Leung 2011; Muntoni 2006; Quinlivan 2005; Quinlivan 2010). Recent advances in treatments for muscle loss have enabled people with DMD to actively participate in life for longer. Bone health in patients with DMD is an important issue because long‐bone fractures seriously harm mobility: approximately 20% to 50% of independently mobile patients lose the ability to walk unaided following a long‐bone fracture (Larson 2000; Manzur 2010; Mayo 2012; McDonald 2002; Vestergaard 2001). Identifying treatment strategies to prevent corticosteroid‐induced osteoporosis and prevent often painful fragility fractures in DMD may have a significant effect in improving quality of life for these patients.
Description of the intervention
The following pharmacological and non‐pharmacological interventions are thought to have the potential to prevent or treat corticosteroid‐induced osteoporosis by increasing bone mineral density and reduce the risk of fractures in patients with DMD.
Bisphosphonates
Intravenous bisphosphonates are used to treat painful vertebral fractures in boys with DMD and are associated with improvements in back pain (Sbrocchi 2012). The use of bisphosphonate treatment in children is relatively new, but there are growing numbers of trials suggesting benefit, particularly in children with osteogenesis imperfecta (bone fragility) (Allington 2005; Bianchi 2000; Brumsen 1997; Glorieux 1998; Hawker 2005; Henderson 2002; Houston 2014; Palomo 2011; Plotkin 2000; Plotkin 2006; Rauch 2002; Rudge 2005; Sbrocchi 2012; Shaw 2000; Sholas 2005; Wagner 2011; Ward 2011). A randomised controlled trial (RCT) to investigate the efficacy and safety of daily oral alendronate in children and adolescents with osteogenesis imperfecta over two years found that the mean spine area Z‐score increased from ‐4.6 to ‐3.3 compared to ‐4.6 to ‐4.5 in the placebo group. Long‐bone fracture incidence and bone pain were similar between groups (Ward 2011).
Bisphosphonates appear to be well tolerated by children for periods of up to three years (Shaw 2005; Ward 2007b). Dosing regimes differ among paediatric studies (Ward 2007b). Oral bisphosphonates are prescribed according to body weight (mg/kg) (Bianchi 2000; Hawker 2005; Rudge 2005). Intravenous bisphosphonates are usually administered at a dose of 1 mg/kg to 1.5 mg/kg (Acott 2005; Shaw 2000).
Treatment is usually combined with vitamin D and calcium supplements to minimise the risk of hypocalcaemia (Maalouf 2006; Poole 2012); however, calcium supplements reduce the absorption of bisphosphonates. Adverse effects are more common with intravenous than oral bisphosphonates (Somalo 2007). The U.S. Food and Drug Adminstration (FDA) is reviewing long‐term use (FDA 2011). Atypical fractures occurring with prolonged bisphosphonate therapy have been a featured concern in recent publications (Compston 2011; Edwards 2010; Girgis 2010; Isaacs 2010; Shane 2010), and there have been cautions that high and prolonged doses may paradoxically induce osteoporosis (Whyte 2008).
Calcium
A balanced calcium‐rich diet is recommended as the best long‐term strategy to maintain adequate calcium levels (Bacciottini 2004; Biggar 2005; Sunyecz 2008). Calcium supplements in healthy children have shown short‐term improvements in bone mineral density (Winzenberg 2006). Intestinal calcium absorption is dependent on other factors such as vitamin D levels.
Genetics determine 60% to 80% of attained peak bone mass, and environmental factors, such as nutrition, exercise, and disease, determine the remainder (Quinlivan 2010). Bone mineral density can vary between individuals from early childhood and be predictive of a potential fracture risk in children with medical conditions affecting bone health (Ferrari 1998; Goulding 2005; Harvey 2012; Quinlivan 2010; Winzenberg 2006). A review of calcium supplementation for improving bone mineral density in children showed no effect on spinal bone mineral density, but a small effect on total body bone mineral content (BMC) (Winzenberg 2006). A double‐blind, placebo‐controlled study of 149 girls showed an increase in bone mineral density in those taking calcium‐supplemented food compared to those who did not (Bonjour 1997). There is poor evidence to indicate whether calcium supplementation alone reduces the risk of fractures (Black 2002; Bolland 2015; Goulding 1998; Goulding 2004; Petridou 1997; Winzenberg 2006). The consumption of adequate dietary calcium and vitamin D can increase bone mineral density in patients with DMD (Bianchi 2011). Evidence that calcium alone has a positive effect on bone health in boys with DMD is limited.
Vitamin D
Significantly lower serum 25OHD levels have been highlighted in boys with DMD, especially in those taking corticosteroids (Bianchi 2003; Bianchi 2011). People taking corticosteroids are twice as likely to have low 25OHD levels as those not on corticosteroids (OR 2.36, 95% CI 1.25 to 4.45) (Skversky 2011). Dhawan 2010 proposed that corticosteroids may inactivate 25OHD by increasing the activity of the enzyme 24‐hydroxylase.
Vitamin D levels collected in boys with DMD aged from 1.5 to 15.5 years prior to starting corticosteroid treatment revealed that 78% had inadequate levels and 15% qualified as deficient (Munot 2010). In two other studies, 54% to 70% of those with DMD on corticosteroids were vitamin D deficient (Manzur 2010; Wong 2010).
In a Cochrane Review, the bone mineral density in healthy children taking vitamin D supplements did not change, but bone mineral density did increase in those with low 25OHD levels (Winzenberg 2010). When investigators examined vitamin D levels at the time of a vertebral fracture in boys with DMD, they categorised the mean serum vitamin D levels as insufficient (Manzur 2010).
Testosterone
Testosterone is the primary sex hormone for the maturation of sex characteristics in adolescent males. It is also involved in the increase in muscle strength and bone mineral content during puberty (Seeman 2001; Wood 2015). Testosterone is a steroid hormone produced by the testes and the adrenal glands and belongs to a group of male hormones known as androgens. The production of this hormone is regulated by the hypothalamus and pituitary gland.
Long‐term corticosteroid treatment in prepubertal boys can slow down growth and delay puberty compared to their healthy peers (Wood 2015). All but one of 44 boys with DMD aged 13 years and upwards assessed at Cincinnati Children's Hospital were prepubertal, compared to the 50% of healthy UK boys who are in puberty by 12 years of age (Bianchi 2011a; Bonifati 2000). Although not clearly understood, high doses of corticosteroids inhibit or reduce the secretion of gonadotrophin‐releasing hormone by the hypothalamus, which in turn down‐regulates pituitary gonadotrophin production. Corticosteroids may also have a direct effect on the testes to reduce testosterone production. A deficiency in testosterone can delay the onset of puberty (Al‐Harbi 2008; Eastell 1998; Hampson 2002; Kamischke 1998a; Reid 1985).
The absence or delay of puberty has a significant adverse effect on bone health, which is already poor in those with DMD due to corticosteroid treatment, diminishing muscle mass, and reduced mobility (Bianchi 2011a; Fairfield 2001; Guzman 2012; Reid 1985). Hypogonadism increases the risk of osteoporosis and fractures. Testosterone is an important hormone involved in the development of bone and in the maintenance of bone mineral density in adolescence and adulthood (Amory 2004; Devogelaer 1992; Katznelson 1996; Kenny 2001; Leder 2002; Tenover 1992).
Weight‐bearing exercise
In DMD patients over eight years of age, there is a gradual decline in physical activity due to natural disease progression (Mazzone 2011; McDonald 2010). Weight‐bearing exercise, for example, walking, stimulates bone regeneration. Dynamic‐loading activity achieves greater gains in bone tissue than static activity (Lanyon 1984). Muscle weakness hinders dynamic weight‐bearing exercise and has a negative impact on bone development. Disuse of the skeleton as a result of prolonged inactivity degenerates bone tissue further.
How the intervention might work
In children with corticosteroid‐induced osteoporosis, the rate of bone resorption exceeds that of bone formation. The main focus of interventions is either to slow bone resorption or promote bone formation to increase bone mineral density whilst receiving corticosteroid therapy. These interventions can be used alone or in combination to prevent or treat bone loss.
Bisphosphonates
Bisphosphonates are drugs that inhibit bone resorption by causing apoptosis of osteoclasts (Fleisch 2003; Poole 2012; Rogers 2011; Russell 2008). They are inorganic analogues of naturally occurring pyrophosphates, which prevent calcification by binding to hydroxyapatite salt in bone (Nancollas 2006; Russell 2011; Sebestyen 2012). Bisphosphonates accumulate at bone resorption sites in the skeleton and stop osteoclast‐mediated activity (Dominguez 2011; Drake 2008; Fleisch 2003; Hughes 1989; Hughes 1995; Sato 1991; Sebestyen 2012; Somalo 2007). There are two groups of bisphosphonates: non‐nitrogen‐containing bisphosphonates (etidronate, clodronate, and tiludronate) and nitrogen‐containing bisphosphonates (alendronate, risedronate, ibandronate, pamidronate, and zoledronate). The presence of a nitrogen or amino group in the chemical structure of bisphosphonates increases the potency of the drugs for osteoclastic activity (Drake 2008; Ebetino 2011; Reszka 2004). These more potent nitrogen‐containing bisphosphonates are often used to treat children with corticosteroid‐induced osteoporosis and osteopenia (Allington 2005; Black 2012; Brumsen 1997; Hawker 2005; Poole 2012; Rudge 2005; Schwartz 2010; Sholas 2005).
Calcium
Calcium is essential to enable normal bone growth and development in children and adolescents (Flynn 2003; Peacock 2010). When combined with phosphate, calcium has a structural role in bone health as a component of bone matrix hydroxyapatite, which provides compressional strength in bone (Bonjour 2011; Peacock 2010). During remodelling, bone turns over calcium. Remodelling activity determines the amount of calcium required to maintain bone health, and this varies throughout life (Mesias 2011). Calcium requirements are greater during adolescence as growth peaks, and approximately 40% of total skeletal bone mass is acquired (Flynn 2003; Greer 2006; Mesias 2011; Weaver 2006). Calcium deficiency can contribute to the development of osteoporosis. Not only is there inadequate calcium for bone mineralisation, but bone calcium stores are deleted to maintain blood calcium levels. Reduced blood calcium levels triggers parathyroid hormone (PTH) release (Peacock 2010). PTH stimulates osteoclasts and bone resorption. High blood calcium levels result in calcitonin release, which inhibits osteoclast activity, promoting bone formation and storage of excess calcium in the bone matrix.
The relationship between calcium intake and bone mass is complicated in DMD patients because corticosteroid treatment induces bone resorption, transferring calcium from bone to blood. High blood calcium levels prevent further intestinal calcium absorption to build bone. However, adequate calcium intake could slow bone resorption and contribute to improving bone health in boys and adolescents with DMD (Bianchi 2011; Quinlivan 2010). Many intervention studies in DMD patients have advised a daily calcium supplement of 750 mg (Hawker 2005; Mayo 2012).
Vitamin D
Vitamin D is vital for intestinal calcium absorption (Frolik 1971; Wei 2010). It may also have its own protective effect on bone (Bartoszewska 2010; Peppone 2010). Vitamin D₂ (ergocalciferol) is largely man‐made and present in supplemented food. The skin synthesises vitamin Dɜ (cholecalciferol) after exposure to sunlight, and it is also available in the diet (Misra 2008). The liver metabolises vitamin D into calcifediol (25‐dihydroxyvitamin Dɜ (25OHD)), which the kidneys convert to the active calcitriol (1,25‐dihydroxyvitamin Dɜ) (Peppone 2010). Vitamin D deficiency can cause rickets in children and osteomalacia in adults from poor bone mineralisation (Holick 2007; Pela 2012; Misra 2008; Wagner 2008; Ward 2007a). To a lesser extent, vitamin D deficiency contributes to bone resorption and osteoporosis (Joyce 2012; Lips 2006). Many children and young adults in the UK have vitamin D insufficiency from increased use of sunscreens and reduced outdoor activities (Biggar 2005; Davies 2011; Lips 2012; Misra 2008; Pela 2012). Its prevalence, especially in non‐white families, is underappreciated (Ahmed 2011; Kehler 2013; Lips 2010; Shaw 2011; Zipitis 2006).
Calcitriol slows bone resorption and enhances bone mineralisation because it aids calcium absorption. Calcitriol influences the genes that control neuromuscular cell proliferation, differentiation, and apoptosis (Banerjee 2003; Carlberg 2003; Garcia 2011). There is little evidence that increasing vitamin D intake alone reduces the risk of fractures (Bischoff‐Ferrari 2005; DIPART 2010; Looker 2008).
Testosterone
Testosterone therapy increases trabecular bone density (Katznelson 1996). Testosterone is used to reverse delayed puberty, reduced growth, and osteoporosis exacerbated by corticosteroid use in DMD (Bianchi 2011a).
Testosterone replacement therapy is started in low doses at around 14 years of age and increased over three to four years until adult levels are reached. The expected benefits include an increase in bone density, muscle strength, and energy levels from a pubertal growth spurt.
Testosterone supplementation can also increase height and improve quality of life during adolescence and young adulthood from an improved body image (Landon 1984; Mason 2011). A meta‐analysis of eight RCTs examining the use of testosterone in 365 men found moderate gains in lumbar bone density (Tracz 2006). Three of the eight studies included men taking corticosteroids (N = 87). The meta‐analysis concluded that the effect of testosterone was weak with regard to osteoporosis prevention and treatment in men, as no bone fracture data were available. Another review of RCTs for the effects of testosterone on body composition in middle‐aged men found that testosterone improved bone mineral density at the lumbar spine by 3.7% (CI 1.0% to 6.4%) compared to placebo (Isidori 2005).
Weight‐bearing exercise
The most evident decline in vertebral bone mineral density in DMD appears to occur with the loss of independent ambulation, regardless of whether or not the patient is taking corticosteroids (Biggar 2004; Crabtree 2010; Mayo 2012). A reduction in mechanical loading reduces osteoblast bone formation and promotes osteoclast‐mediated bone resorption (Takata 2001). A study revealed that bone mineral density at a number of sites including the lumbar spine improved with calcium and exercise in prepubertal and early pubertal boys (Bass 2007; Iuliano‐Burns 2003; Stear 2003). The Iowa Bone Development Study found significant longitudinal associations between physical activity that increased the mechanical loading of the skeleton in boys aged five years and bone mineral content of the hip at eight and 11 years of age (Janz 2010). Growing bone adapts to withstand mechanical loading. In boys with DMD, standing programs and prolongation of walking using mechanical walking aids are common components of management (Pedlow 2015), and boys have shown improvement in vertebral bone mineral density and function capacity (Bianchi 2013; Spencer 1962). More recently, high‐frequency, low‐intensity whole‐body vibration appeared to improve bone health in children and adolescents with a disability (Matute‐Llorente 2015; Reyes 2011;Ruck 2010), but was inconclusive in people with DMD (Söderpalm 2013). Regular low‐intensity and appropriate exercise strengthens bone tissue, as well as improving the balance of action between opposing muscle groups and balance while standing and moving (Bushby 2010; Grange 2007; Jansen 2010). These interventions could reduce the risk of fractures in non‐ambulant boys (Ward 2004).
Why it is important to do this review
Corticosteroids delay the loss of ambulation and cardiorespiratory failure in patients with DMD. However, boys require treatment for many years, and the risk of vertebral fragility and possibly long‐bone fractures is high. Identifying treatment strategies to prevent and treat osteoporosis and prevent fragility fractures will improve the quality of life for these patients.
Objectives
To assess the effects of interventions to delay or treat osteoporosis in children and adults with DMD taking long‐term corticosteroids; to assess the effects of these interventions on the frequency of vertebral fragility fractures and long‐bone fractures, and on quality of life; and to assess adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We searched for all randomised controlled trials (RCTs) and quasi‐RCTs involving treatment or prevention of corticosteroid‐induced osteoporosis and prevention of fragility fractures in patients with DMD who were taking corticosteroids. Quasi‐RCTs employ methods of allocation that are not truly randomised (for example, by alternation or using date of birth or hospital number).
Types of participants
We considered for inclusion in the review all children, adolescents, and adults with a diagnosis of DMD, with any pathogenic mutation, deletion, or duplication confirmed by genetic testing or muscle biopsy showing the complete absence of dystrophin.
Types of interventions
We considered for inclusion in the review any intervention to prevent or treat corticosteroid‐induced osteoporosis or prevent fragility fractures in patients with DMD compared with placebo, another intervention, or no treatment (no available standard care).
The interventions included oral and intravenous bisphosphonates (including both non‐amino and amino groups), vitamin D supplements, calcium supplements, dietary calcium, testosterone, and weight‐bearing activity.
We planned to analyse oral and intravenous bisphosphonates separately.
Types of outcome measures
Primary outcomes
-
Change in vertebral bone mineral density measured by dual‐energy X‐ray absorptiometry (DXA) and expressed as a Z‐score (which is based on age‐matched results with a correction for body height) between baseline and 24 months of intervention.
The presence of vertebral compression fractures could artificially increase bone mineral density and produce higher Z‐scores in an individual. However, we considered this effect unlikely to influence the outcome of a meta‐analysis.
Secondary outcomes
Secondary outcomes measured between baseline and 24 months of intervention.
-
The number of vertebral fragility fractures, defined as one or a combination of the following: end‐plate deformity with a reduction in the mid‐vertebral height (biconcavity), decrease in the anterior vertebral height (wedging), or reduction in the anterior and posterior vertebral height (compression or crush). Identification of such vertebral fractures was based on visual evaluation with an assessment of grade or severity linked to vertebral height, shape, and appearance (Baim 2008), for example, using the Genant visual semi‐quantitative method (Genant 1993). Fractures were detected using lateral spinal X‐ray, DXA vertebral fracture assessment (VFA), computed‐tomography (CT) scans, or magnetic resonance imaging (MRI).
-
The number of long‐bone fractures.
-
Change in reported bone pain measured using a standardised pain score (McGrath 2008), such as the visual analogue scale (VAS) (Scott 1979; Zebracki 2008).
-
Change in quality of life, measured using a validated rating scale, for example, the Pediatric Quality of Life Inventory (PedsQL) (Varni 2001), Neuromuscular Module and Generic Core Scales (Davis 2010).
-
Any adverse events; adverse events that led to the withdrawal of treatment; serious adverse events that are fatal, life‐threatening, or led to prolonged hospitalisation; and specific adverse events, such as jaw osteonecrosis and atypical femur fractures.
Search methods for identification of studies
Electronic searches
On 12 September 2016, we searched the following databases to identify potentially eligible trials:
-
the Cochrane Neuromuscular Specialised Register;
-
CENTRAL (the Cochrane Central Register of Controlled Trials) in the Cochrane Register of Studies Online;
-
MEDLINE OvidSP (1966 to September 2016);
-
Embase OvidSP (1980 to September 2016);
-
CINAHL (Cumulative Index to Nursing and Allied Health Literature) Plus (1982 to September 2016); and
-
Web of Science Core Collection (Proceedings paper or Meeting abstract) (2001 to September 2016).
We did not limit our search by language or publication status.
The detailed search strategies are in the appendices.
-
Cochrane Neuromuscular Specialised Register (Appendix 1)
-
CENTRAL (Appendix 2)
-
MEDLINE (Appendix 3)
-
Embase (Appendix 4)
-
CINAHL Plus (Appendix 5)
-
WOS (Appendix 6)
Searching other resources
In addition, we searched the following registries to identify unpublished data, unpublished studies, and ongoing trials:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 11 October 2011);
-
the World Health Organization International Clinical Trials Registry Platform (ICTRP, apps.who.int/trialsearch; searched 11 October 2011); and
-
the ISRCTN registry (www.isrctn.com; searched 11 October 2011).
We presented detailed search strategies in the appendices.
-
ClinicalTrials.gov (Appendix 7)
-
ICTRP (Appendix 8)
-
ISRCTN registry (Appendix 9)
Data collection and analysis
Selection of studies
Two review authors (JMB and MS) independently reviewed titles and abstracts from the electronic search results and selected papers for full‐text review according to the inclusion criteria. We obtained the full text of all potentially relevant studies for further assessment and selected studies that met the inclusion criteria. We would have resolved disagreements by discussion, but there were no disagreements. We agreed by discussion to contact five study authors to obtain further information for clarification on published work, unpublished studies, and work in progress.
We collated multiple reports of the same study under the same study ID. We completed a PRISMA flow diagram, 'Characteristics of excluded studies' tables, and 'Characteristics of ongoing studies' tables to detail the selection process.
A lack of randomised trials, other than two abstracts, meant that the two review authors (JMB and MS) were unable to extract data.
Assessment of risk of bias in included studies
Two review authors (JMB and MS) independently assessed the risk of bias in the included studies, and a third (BB) verified the assessments. We used the domain‐based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The domains included the following:
-
random sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessors;
-
incomplete outcome data;
-
selective reporting; and
-
other bias.
For each domain, we graded the risk of bias as high, low, or unclear (Higgins 2011a). We contacted the trial's corresponding authors for clarification of insufficient detail in study reports. We resolved disagreements through discussion with the other review authors, and we assigned studies to the following categories.
-
Low risk of bias: all domains at low risk of bias.
-
High risk of bias: two or more domains at high risk of bias.
-
Unclear risk of bias: one or more domains at unclear risk of bias.
We constructed a 'Risk of bias' table using RevMan to present the results. When considering treatment effects, we planned to take into account the risk of bias in the studies that contributed to the outcome.
We would have used the 'Risk of bias' assessments to perform sensitivity analyses, if sufficient data had been available.
Information on risk of bias related to unpublished data or correspondence with a trialist was noted in the 'Risk of bias' table.
Assesment of bias in conducting the systematic review
We conducted the review according to the published protocol (Bell 2014). We would have reported any deviations from protocol in Differences between protocol and review.
Appendix 10 details methods described in the protocol to be followed if data become available for analysis in future.
Data synthesis
In the absence of meta‐analysis or full‐text trial reports, we presented the limited data available narratively, in the Results section.
Results
Description of studies
Results of the search
The total number of potentially relevant references retrieved from each database was 645: 58 from the Cochrane Neuromuscular Specialised Register, 78 from the Cochrane Central Register of Controlled Trials (CENTRAL), 303 from MEDLINE, 159 from Embase, 22 from CINAHL (Cumulative Index to Nursing and Allied Health Literature) Plus, and 25 from Web of Science ISI Proceedings. The number of references from database searches after deduplication was 506.
We identified 30 additional references from other sources: four from the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov), seven from the ISRCTN registry, 17 from ICTRP, one published study from correspondence with a co‐author (AH), and one unpublished study from correspondence with an author of related studies. The number of studies from these sources after deduplication was 26.
The number of references selected for further review based on their titles and abstracts was 18. Of these, only two met the inclusion criteria for the review, but only conference abstracts were available. We requested unpublished data from the authors of both studies; one responded with further information, and the second did not respond. We categorised two further studies as Ongoing studies.
We illustrated the overall study selection process in a flow diagram (Figure 1).

A flow diagram illustrating the study selection process.
Included studies
We found two randomised clinical trials that appeared relevant for inclusion in this review (see Characteristics of included studies).
McSweeney 2014 assessed the effects of bisphosphonate therapy (risedronate 1 mg/kg, maximum 35 mg) combined with calcium (500 mg/day) and vitamin D (10 mg/day) compared to calcium and vitamin D alone in a randomised controlled trial (RCT) involving 13 boys with Duchenne muscular dystrophy (DMD). The median age of participants was 8.5 years (range 5.4 to 15.5 years); nine boys were ambulant and on corticosteroid therapy. The abstract did not provide information on funding sources.
Bianchi 2013 investigated weight‐bearing activity (low‐magnitude high‐frequency vibration) compared to the effects of a placebo device on bone mineral density in 21 ambulant boys who had received corticosteroids for at least six months. The trial had a prospective, double‐blind, randomised, placebo‐controlled design. The abstract did not provide information on funding sources.
Both studies were reported in conference abstracts, but there was insufficient information available to collect outcome data or assess risk of bias. We contacted both primary authors for further information: Bianchi 2013 had no further information to offer until publication, and we received no response from McSweeney 2014. We do not have information on when we can expect full publication of the results.
Excluded studies
We excluded 14 studies that did not meet the inclusion criteria for this review (see Characteristics of excluded studies). The reasons for rejection were that the studies did not include patients with DMD in the study population (Adachi 2001; Cohran 2013), were not randomised (Bianchi 2011; Biggar 2004; Cohran 2008, Hawker 2005; Houston 2014; Mayo 2012; Srinivasan 2016; Sbrocchi 2012; Söderpalm 2013), or did not assess the effects of the intervention on bone health (Dubowitz 1984; Scott 1981; Yilmaz 2004).
Ongoing studies
We found two ongoing clinical studies (see Characteristics of ongoing studies). The first was a RCT to assess the effect of a novel whole‐body vibration therapy on bone health as well as muscle strength (NCT01954940). This trial was registered on 13 September 2013, with an estimated recruitment end date in March 2015. The study is still recruiting participants (last verified 26 July 2016). The second was a RCT of zoledronic acid (Aclasta) versus vitamin D plus calcium in children and adolescents with DMD, to assess change in lumbar spine bone density over 12 months (ACTRN12610000507088). The trial was registered on 18 June 2010, and the anticipated date of the enrollment of the last participant is 31 December 2016.
Risk of bias in included studies
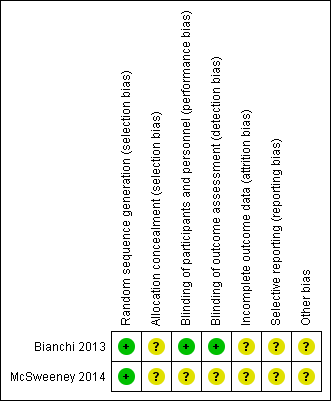
See Characteristics of included studies and Figure 1 and Figure 2 for 'Risk of bias' assessments. As information was only available from abstracts, we were limited in our ability to fully assess risk of bias. We assessed random sequence generation as low risk for both studies (Bianchi 2013; McSweeney 2014) and blinding as low risk for (Bianchi 2013). We assessed all other domains as unclear.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study. Green = low risk of bias; yellow = unclear risk of bias; red (not shown) = high risk of bias.
Effects of interventions
Risedronate, calcium, and vitamin D versus calcium and vitamin D alone
Studied in McSweeney 2014
McSweeney 2014 (N = 13) randomised participants with a spine Z‐score less than ‐1.0 to risedronate plus calcium and vitamin D (N = 6) or to calcium and vitamin D alone (N = 7). At baseline and 12 months, investigators measured the spine and whole body bone mineral density Z‐score in both groups (Table 1). The study authors reported a significant improvement in bone mineral density of the spine and whole body at 12 months compared to baseline in the risedronate group. The published abstract reported no information on fractures. Nine participants were ambulant and taking corticosteroid therapy, but it is unclear whether those taking risdronate were ambulant. If current practice was applied, all participants given risdronate would be taking corticosteroids. The abstract did not provide information on adverse events.
| Risedronate (plus calcium and vitamin D supplementation) | Control (calcium and vitamin D supplementation alone) | |||
| Baseline | 12‐month | Baseline | 12 months | |
| Median spine Z‐score (range) | ‐1.75 (‐1.2 to ‐3.5) | ‐0.8 (‐1.7 to 0) | ‐2.2 (‐4.1 to ‐1.2) | ‐1.6 (‐8.4 to ‐0.8) |
| Median whole‐body Z‐score (range) | ‐1.95 (‐0.5 to 2.7)* | 1.3 (‐1.0 to 2.5) | ‐1.2 (‐1.6 to 4.7) | ‐0.8 (‐3.1 to 3) |
A median of ‐1.95 is outside the range given, but we were unable to confirm correct figures with the study author.
Weight‐bearing activity (low‐magnitude high‐frequency vibration) versus placebo device
Studied in Bianchi 2013
Bianchi 2013 examined the benefits of low‐magnitude high‐frequency vibration by randomising 21 ambulant children with DMD taking corticosteroids (mean age 9.3 ± 3.9 years) to either vibration devices (N = 11) or placebo devices (N = 10). Spine, hip, and whole‐body bone mineral density measurements were taken at baseline, six, and 12 months to monitor change in each treatment group. Bone mineral density significantly increased at 12 months versus baseline in the active group only (spine bone mineral apparent density (BMAD): +7.9%, P < 0.02; total body (TB): +6.8%, P < 0.02; femoral neck: +9.8%, P < 0.01). There was "no change or a decrease" in bone mineral density versus baseline in the placebo group (spine BMAD: ‐2.9%; TB: ‐3.9%; femoral neck: ‐4.8%). All children tolerated whole‐body vibration treatment. The abstract provided no information on fractures.
Discussion
Summary of main results
This review was only able to include two randomised studies involving a total of 34 boys with Duchenne muscular dystrophy (DMD) (Bianchi 2013; McSweeney 2014). One study compared risedronate plus calcium and vitamin D with calcium and vitamin D alone; the other compared the effects of whole‐body vibration with a placebo intervention. Neither study was published except in abstract form. Although each trial reported improvements in bone mineral density with active treatment over baseline, the abstracts provide insufficient data for analysis or to assess the quality of the evidence.
Evidence from non‐randomised studies
In the absence of data from studies meeting the inclusion criteria for this review, we detailed results of non‐randomised studies to inform future trial design and provide a platform for new RCTs of needed interventions to improve or stabilise bone health in DMD.
Bisphosphonates
No RCTs of alendronate in boys with DMD have been conducted.
A small retrospective study assessed the effects of alendronate in 29 participants with DMD with a mean age of 12 years (23/29 participants had been treated with corticosteroids) (Houston 2014). Nineteen boys had dual‐energy X‐ray absorptiometry (DXA) measurements taken during alendronate treatment over a mean of 3.7 years. This study reported a non‐significant effect in favour of alendronate on lumbar spine bone mineral density Z‐score (Houston 2014). The lumbar spine bone mineral density Z‐score increased from ‐2.09 to ‐1.65 (P = 0.38). In comparison, seven participants not taking alendronate but taking corticosteroids only also showed an increase in the mean lumbar spine Z‐score (from ‐1.71 to ‐1.44 (P = 0.30). This increase could have been caused artificially by scoliosis. In another study, 16 boys taking alendronate over two years maintained a mean lumbar spine Z‐score of less than ‐1 (Hawker 2005). Each boy was taking deflazacort and bone protection (calcium and vitamin D supplements). Improvements in spinal Z‐scores were significantly associated with starting bisphosphonates at a younger age (P = 0.01). In both of the above studies a mild gastrointestinal side‐effect was the major complaint associated with alendronate.
A retrospective study examined 52 children with DMD taking oral risedronate treatment (treated) for a mean duration of 3.6 years while taking long‐term corticosteroids (Srinivasan 2016). The investigators also collected data from a historical comparison group of children with DMD who took corticosteroids alone (N = 15). Risedronate‐treated children also received vitamin D supplementation of 800 IU/day to 1000 IU/day; it is not clear if the untreated children also received vitamin D. Risedronate was stopped in 9/52 children due to side‐effects (abdominal pain, vomiting, constipation, joint pain, flu‐like symptoms, and dizziness). DXA results were available in 35 children who continued risedronate treatment for over 12 months. The treated group DXA scores remained stable (mean 0.04 (standard deviation (SD) 1.3) at baseline and ‐0.04 (1.2) at final assessment, mean follow‐up: 4.9 years). The untreated group mean (SD)‐adjusted Z‐score decreased from ‐0.1 (1.3) to ‐1.00 (1.3) (mean follow‐up: 6.2 years). During this study, three 'treated' children, all with baseline Z‐scores of less than ‐1 developed lumbar vertebral fractures. This study did not assess the presence of asymptomatic vertebral fractures. Given that it was not clear if the untreated group received vitamin D, it is unclear whether or not the improvement in bone mineral density was due to risedronate or vitamin D or a combination of both. Although no child experienced osteonecrosis of the jaw, a significant proportion of participants stopped taking risedronate because of side‐effects.
Sbrocchi 2012 retrospectively studied intravenous bisphosphonate (pamidronate or zoledronic acid) treatment for painful vertebral fractures in boys with DMD. At baseline, there were 27 vertebral fractures among the seven boys. After two years of bisphosphonate treatment, 17 of the fractures showed an improved vertebral height ratio, 10 vertebrae were stabilised, and no vertebrae showed a reduced vertebral height ratio. Treatment did not prevent the development of new vertebral fractures in some participants, but it did remove the associated back pain completely in three boys and improved back pain in the remaining four. The median change in lumbar spine bone mineral density Z‐score was 0.5 SD (interquartile range: ‐0.3 to 1.7). Side‐effects occurred mostly with the first dose of intravenous bisphosphonate treatment.
From the non‐randomised studies available, bisphosphonate treatment may stabilise bone mineral density. It is not clear if bisphosphonates prevent fragility fractures from the data available. The effect of bisphosphonates may well depend upon the time they are started in relation to bone fragility appearing.
Vitamin D and calcium
A prospective study in DMD involving 33 participants taking corticosteroids for three years reported that 67% (22 of 33) had an increase in bone mineral density in response to vitamin D (calcifediol and 25‐dihydroxyvitamin Dɜ (25OHD)) and increased dietary calcium (Bianchi 2011). During the first year of the study, participants had increased dietary calcium but no additional vitamin D, and the bone mineral density decreased at the lumbar spine in all participants, with measured urinary calcium and serum 25OHD levels below normal. In 20 participants, serum 25OHD levels were below 20 ng/ml. During the following years, participants took calcifediol (0.8 mcg/kg/day) and increased dietary calcium (11,236 mg/day, P < 0.001). The bone mineral density at the spine significantly increased in 22 participants, urinary calcium increased to normal levels, and serum calcium and 25OHD returned to normal levels. No vertebral fractures occurred during the three‐year study, but seven fractures of the leg and foot occurred: four in the non‐treatment year and three in the following treatment years. The overall increase in both lumbar spine and total body bone mineral density were not thought to be associated with an increase in body (bone) size and pubertal development, as most participants were prepubertal.
Weight‐bearing exercise
Six participants with DMD (median age: 6.8 years) walking without assistance and taking corticosteroids had whole‐body vibration therapy two or three times a week for 10 weeks and were assessed for one year (Söderpalm 2013). No significant changes occurred in bone mineral density in total body, spine, hip, or heel bone or bone markers after the intervention treatment period. No fractures occurred before or during the study. This was a very small observational study with no controls; hence, we could draw no clinical conclusions.
Overall completeness and applicability of evidence
Limited reports from two small studies are only available in published conference proceedings at this time. There is as yet no fully published evidence from randomised trials.
Vitamin D, calcium, and testosterone
We identified no randomised or controlled trials to assess vitamin D, calcium, and testosterone treatment for corticosteroid‐induced osteoporosis and fragility fractures in DMD.
Quality of the evidence
We cannot fully assess the quality of the studies from the abstract publications. The two included studies met prespecified eligibility criteria and had small sample sizes (N = 13 and N = 21). Both trials reported that they randomised participants to an intervention group or no intervention or placebo group. The low‐magnitude, high‐frequency vibration study reported blinding (Bianchi 2013), but this was unclear in the bisphosphonate study (McSweeney 2014). From the McSweeney 2014 abstract, it was unclear if participants in the intervention group were taking corticosteroids or not.
Of the excluded studies, four were retrospective cohort studies or uncontrolled studies with a small sample size (Biggar 2004; Cohran 2008; Hawker 2005; Srinivasan 2016). These studies had many limitations and provided very low quality evidence.
Potential biases in the review process
There were few limitations to this process. The small randomised controlled trials performed in DMD are unlikely to capture and provide high‐quality adverse event data, especially for rare events. The comprehensive searches were updated throughout the review process when necessary.
Agreements and disagreements with other studies or reviews
We examined published systematic reviews that analysed interventions to prevent corticosteroid‐induced osteoporosis and fragility fractures in other conditions.
Bisphosphonates
Reviews of treatments for corticosteroid‐induced osteoporosis in cystic fibrosis, neuromuscular disease, and chronic illnesses found extensive variation in the choice of bisphosphonate, dosing regimen, and outcome measurement (Conwell 2014; Ward 2007b). Bisphosphonates had a positive effect on the lumbar spine bone mineral density, especially in the short term, although there was some heterogeneity.
The trials reviewed, Conwell 2014 and Ward 2011, reported no new fractures in treatment groups over a two‐year period.
In Ward 2007b, although bisphosphonates were well tolerated, the major complaint was bone pain in those taking bisphosphonates but not taking corticosteroids (odds ratio (OR) 18.52, 95% confidence interval (CI) 5.39 to 63.57, six trials). This effect was mostly related to intravenous bisphosphonates (OR 14.17, 95% CI 3.64 to 55.17) and oral risedronate (OR 43.59, 95% CI 2.27 to 837.56). Conwell 2014 listed gastrointestinal effects as a major side‐effect.
Overall, both reviews found insufficient evidence to justify the routine use of the bisphosphonates in clinical care to prevent or treat corticosteroid‐induced osteoporosis and fractures (Conwell 2014; Ward 2007b). The included trials were too short to monitor vertebral fracture events and treatment adequately.
Vitamin D
Participants with cystic fibrosis taking vitamin D supplements had significantly higher serum levels (mean difference (MD) 7.24 ng/ml, 95% confidence interval 5.01 to 9.46 ng/ml) than those taking a placebo (Ferguson 2014). Vitamin D supplementation was found not to improve bone density in healthy children with normal vitamin D levels, but was found to be clinically useful in children with low serum vitamin D levels by preventing bone loss at the lumbar spine (Homik 1998; Winzenberg 2010).
The vitamin D Individual Patient Analysis of Randomized Trials showed that vitamin D given alone was not effective in preventing fractures (risk ratio (RR) 0.92, 95% CI 0.86 to 0.99, P = 0.025), but if taken with calcium, the combination reduced the risk of vertebral fragility fractures (DIPART 2010). Evidence from randomised and cross‐over design trials and meta‐analysis supports the use of calcium in combination with vitamin D as a preventive treatment for bone loss in the spine of people treated with corticosteroids and adults over 50 years of age (Amin 1999; Homik 1998; Rianthavorn 2012; Tang 2007; Warady 1994). Further research is required to investigate the effect of vitamin D levels on bone strength of patients with DMD (Wong 2010).
Weight‐bearing exercise
No useful data were available from studies of weight‐bearing exercise in people over 40 with vertebral fragility fractures (Papaioannou 2003). A systematic review of vibration treatment for osteoporosis is published only as a protocol (Lorenzen 2010).

A flow diagram illustrating the study selection process.
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study. Green = low risk of bias; yellow = unclear risk of bias; red (not shown) = high risk of bias.
| Risedronate (plus calcium and vitamin D supplementation) | Control (calcium and vitamin D supplementation alone) | |||
| Baseline | 12‐month | Baseline | 12 months | |
| Median spine Z‐score (range) | ‐1.75 (‐1.2 to ‐3.5) | ‐0.8 (‐1.7 to 0) | ‐2.2 (‐4.1 to ‐1.2) | ‐1.6 (‐8.4 to ‐0.8) |
| Median whole‐body Z‐score (range) | ‐1.95 (‐0.5 to 2.7)* | 1.3 (‐1.0 to 2.5) | ‐1.2 (‐1.6 to 4.7) | ‐0.8 (‐3.1 to 3) |
| A median of ‐1.95 is outside the range given, but we were unable to confirm correct figures with the study author. | ||||

