Tests génétiques pour la prévention des éruptions cutanées graves causées par des médicaments
Résumé scientifique
Contexte
Les réactions cutanées d'origine médicamenteuse se manifestent par un éventail de symptômes cliniques, allant d'éruptions cutanées maculopapuleuses légères à des éruptions cutanées potentiellement mortelles formant des cloques ‐ comme le syndrome de Stevens‐Johnson (SJS) ou la nécrolyse épidermique toxique (NET) ‐ qui peuvent entraîner la mort. Des réactions plus légères peuvent être gênantes et entraîner une faible observance du traitement. La pathogenèse de ces réactions médicamenteuses n'est pas encore entièrement comprise ; cependant, il existe des preuves que les tests génétiques effectués avant le traitement peuvent aider à prévoir et à éviter ces réactions dans certains cas.
Objectifs
Évaluer les effets d'un dépistage pharmacogénétique prospectif visant à réduire les réactions cutanées associées à des médicaments chez une population de patients.
Stratégie de recherche documentaire
Jusqu'en juillet 2018, nous avons effectué des recherches dans les bases de données suivantes : le Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase et LILACS. Nous avons également effectué des recherches dans cinq registres d'essais cliniques et vérifié les listes de références des études analysées et des revues pertinentes pour trouver d'autres références à des essais cliniques comparatifs et randomisés (ECR) pertinents.
Critères de sélection
Nous avons inclus les ECR de participants qui avaient fait l'objet d'un dépistage pharmacogénétique prospectif pour déterminer les variantes génétiques associées aux réactions d'hypersensibilité, comparativement à ceux qui n'avaient pas fait l'objet d'un dépistage pharmacogénétique prospectif. Nous avons inclus des participants de tous les milieux, quels que soient leur âge, leur sexe et leur origine ethnique, à qui on avait prescrit des médicaments connus pour causer des réactions d'hypersensibilité de type retardé.
Recueil et analyse des données
Nous avons utilisé les procédures méthodologiques standard définies par Cochrane. Afin d'évaluer les études en vue de leur inclusion à l’analyse, deux auteurs ont examiné indépendamment tous les titres et résumés des publications identifiées lors des recherches. Étant donné qu'une seule étude a été incluse, bon nombre des analyses de données prévues ne s'appliquaient pas à l’étude. Nous avons utilisé GRADE pour évaluer la qualité de l'étude analysée.
Les principaux critères de jugement de l’analyse étaient le nombre d'éruptions cutanées graves accompagnées de symptômes systémiques (comme la fièvre et l'atteinte de plusieurs organes) et les effets à long terme (comme les cicatrices des paupières ou du tissu pulmonaire). Les critères de jugement secondaires étaient l'hospitalisation pour des réactions cutanées dues aux médicaments, des éruptions cutanées formant des cloques (comme le SJS, le syndrome d'hypersensibilité (HSS)) et la mort.
Résultats principaux
Une étude, qui était un essai randomisé, en double aveugle, contrôlé et multicentrique, répondait à nos critères d'inclusion. L'essai comprenait 1956 participants adultes (74% d'hommes, avec une moyenne d'âge de 42 ans) dans 265 centres (centres médicaux, hôpitaux, dispensaires) dans 19 pays à travers le monde qui étaient infectés par le VIH de type 1 et qui n'avaient jamais reçu d'abacavir auparavant. Les participants, qui avaient un besoin clinique de traitement par un antirétroviral contenant de l'abacavir, ont été répartis au hasard pour subir un dépistage prospectif de l'antigène leucocytaire humain (HLA) de classe I, locus B, allèle 57:01 (HLA‐B*57:01) (groupe dépistage prospectif) avant ce traitement ou pour suivre une approche standard en utilisant l'abacavir sans dépistage prospectif HLA‐B*57:01 (groupe témoin). Les participants qui ont obtenu un résultat positif au test HLA‐B*57:01 n'ont pas reçu d'abacavir ; ils ont plutôt reçu un traitement antirétroviral qui ne comprenait pas d'abacavir. Le groupe témoin a fait l'objet d'essais pharmacogénétiques HLA‐B*57:01 rétrospectivement. La durée de l’essai était de six mois. Chaque participant a été observé pendant six semaines. Les évaluations ont été effectuées au moment de l'admission à l'étude, au départ (le premier jour du traitement par l'abacavir) et aux semaines 1, 2 et 6. Cette étude a été financée par le fabricant de l'abacavir, GlaxoSmithKline.
L'étude n'a évalué aucun de nos principaux critères de jugement, et elle n'a mesuré aucun de nos critères de jugement secondaires isolément. Cependant, elle a évalué un critère de réaction d'hypersensibilité (caractérisée comme sévère) qui comprenait (sans toutefois s'y limiter) nos critères de jugement secondaires tels que l’HSS et le SJS/NET.
L'étude a démontré que le dépistage prospectif du HLA‐B*57:01 réduit probablement le nombre des réactions d'hypersensibilité à l'abacavir. Le nombre de réactions d’HSS diagnostiquées cliniquement à l'abacavir était plus faible dans le groupe de dépistage prospectif (risque relatif (RR) de 0,43, intervalle de confiance à 95 % (IC) de 0,28 à 0,67 ; 1 650 participants ; données de qualité moyenne), tout comme le nombre de réactions d’HSS confirmées immunologiquement (RR 0,02, 95 % 0,00 à 0,37 ; 1644 participants ; données de qualité moyenne). Un résultat positif d'un test épicutané réalisé six à dix semaines après le diagnostic clinique a fourni une confirmation immunologique.
Dans l'ensemble, l'étude démontre un faible risque de biais dans cinq des sept domaines. Le risque de biais de détection était élevé car les réactions d'hypersensibilité ont été diagnostiquées par le chercheur principal sur le site de recrutement sans l'utilisation de critères cliniques prédéfinis. Bien qu'il y ait également un risque élevé de biais d'attrition en raison de l'exclusion des participants dont le suivi des analyses était incomplet, les auteurs ont entrepris une série d'analyses de sensibilité sur la population visée par le traitement, qui ont donné des résultats cohérents avec la première analyse. Nous avons évalué la qualité de l'étude comme étant de qualité moyenne selon les critères de GRADE.
Conclusions des auteurs
Le dépistage prospectif de HLA‐B*57:01 réduit probablement les réactions cutanées d'hypersensibilité grave à l'abacavir chez les patients séropositifs pour le VIH de type 1. Toutefois, ces résultats ne reposent que sur une seule étude, qui présentait un risque élevé de biais d'attrition et de détection.
Nos principaux critères de jugement (nombre des éruptions cutanées graves avec symptômes systémiques et effets à long terme) n'ont pas été évalués dans le cadre de l'essai, et un seul des critères de jugement secondaires a été mesuré (réaction d'hypersensibilité) ; nous n'avons donc trouvé aucune donnée relative à une hospitalisation, à un décès ou à des situations à long terme résultant de lésions liées au médicament.
Nous n'avons trouvé aucune donnée éligible sur les tests génétiques de dépistage lors d’éruptions cutanées graves causées par différents médicaments et différentes classes de médicaments. D'autres essais cliniques portant sur d'autres médicaments et sur différentes populations de patients seraient utiles pour conseiller des changements de politique afin d’améliorer la prévention des réactions cutanées indésirables dues aux traitements médicamenteux.
PICOs
Résumé simplifié
Tests génétiques pour prédire et éviter les éruptions cutanées graves causées par des médicaments
Problématique de la revue
Le but de cet revue Cochrane était de déterminer si le dépistage génétique de certains génotypes (c.‐à‐d. la présence ou l'absence d'une variation génétique particulière) avant de prescrire des médicaments connus pour causer une hypersensibilité de type retardé (c.‐à‐d. une éruption cutanée allergique jusqu'à six semaines après avoir pris le médicament prescrit) peut éviter des réactions cutanées graves chez les personnes ayant une prescription de ce type de médicaments. Les personnes qui ont subi ce type de test ont été comparées à celles qui n'en ont pas subi. Nous avons cherché toutes les études pertinentes afin de pouvoir les analyser pour répondre à cette question, mais nous n’en avons trouvé qu'une seule.
Contexte
Différents types de médicaments peuvent avoir des effets indésirables, dont des éruptions cutanées. Ces réactions sont souvent une éruption cutanée légère ; cependant, le médicament peut rarement (mais possiblement) causer un décollement de la peau, de la fièvre et une atteinte des organes internes, ce qui peut mettre la vie des patients en danger. Les cas graves peuvent nécessiter une hospitalisation et un traitement dans des unités spécialisées de traitement des brûlés. On ne comprend pas tout à fait comment ces réactions se produisent et quelles sont les personnes qui courent un risque accru de développer ces réactions, mais il est connu que des facteurs génétiques peuvent jouer un rôle. Des recherches ont été menées sur l'utilisation de tests génétiques simples pour prédire ces réactions et ainsi les éviter.
Caractéristiques des études
Nous n'avons trouvé qu'une seule étude pertinente. Elle comprenait 1 956 participants adultes, dont 74 % étaient des hommes, qui avaient été testés positifs pour le VIH de type 1 et étaient éligibles pour un traitement antirétroviral très efficace, incluant un médicament appelé abacavir. (Les antirétroviraux sont une classe de médicaments utilisés pour traiter les patients infectés par le VIH.) L'âge des participants à l'étude variait de 18 à 77 ans ; l'âge moyen était de 42 ans. Les participants venaient de 265 centres de santé (hôpitaux, cliniques, etc.) répartis dans 19 pays du monde entier. Cette étude visait à déterminer si les éléments suivants peuvent réduire le taux de réactions cutanées graves à l'abacavir : un test génétique pour le marqueur génétique HLA‐B*57:01 avant de prescrire l'abacavir (c.‐à‐d. un test prospectif) par opposition à aucun test génétique prospectif pour le HLA‐B*57:01. Le marqueur HLA‐B*57:01 est connu pour jouer un rôle clé dans le développement de réactions cutanées graves à l'abacavir, médicament antirétroviral contre le VIH. Les participants dont le test de dépistage du génotype était positif n'ont pas reçu d'abacavir, mais un autre traitement antirétroviral à la place. L'étude a duré six mois et chaque participant a été observé pendant six semaines. GlaxoSmithKline, l'entreprise qui produit l'abacavir, a financé l'étude. Les participants témoins ont subi un test pharmacogénétique HLA‐B*57:01 après avoir reçu l'abacavir comme traitement standard.
Principaux résultats
Notre unique étude ne rapporte pas les résultats de tous les participants et l'évaluation clinique de l'hypersensibilité (HSS) a été effectuée au moment de l'inscription à l'étude, au départ (jour 1) et aux semaines 1, 2 et 6, sans utiliser de critères prédéfinis. Cela peut être une source importante de biais et la qualité des données a donc été jugée modérée. Les données disponibles montrent que le dépistage prospectif du HLA‐B*57:01 réduit probablement le nombre de réactions d'hypersensibilité à l'abacavir (cela comprend, mais sans s'y limiter, les réactions secondaires comme le syndrome d'hypersensibilité, le syndrome de Stevens‐Johnson (SJS) ou la nécrolyse épidermique toxique (NET) (i.e des éruptions cutanées graves et des cloques sur la peau causées par les médicaments)).
D'après ces résultats, on s'attendrait à ce que 78 des 1 000 personnes qui n'ont pas fait l'objet d'un dépistage pharmacogénétique prospectif (c.‐à‐d. un dépistage visant à évaluer comment une personne pourrait répondre à un médicament particulier) connaissent une réaction d'hypersensibilité, y compris un SJS/NET (évaluation clinique), comparativement à 22 à 52 personnes qui ont fait un dépistage pharmacogénétique prospectif pour le HLA‐B*57:01.
De plus, on s'attendrait à ce que 27 des 1 000 personnes qui n'ont pas fait l'objet d'un dépistage pharmacogénétique prospectif (traitement standard) présentent une réaction d'hypersensibilité, y compris une réaction SJS/TEN (confirmée par immunologie), comparativement à aucun participant qui a subi un dépistage pharmacogénétique prospectif pour tester le marqueur HLA‐B*57:01. Un test épicutané a fourni une confirmation immunologique 6 à 10 semaines après le diagnostic clinique.
L'étude n'a pas évalué les autres critères de jugement de cette revue.
Les données sont à jour en juillet 2018.
Qualité des données probantes
La qualité de l'étude incluse a été jugée moyenne. Certains patients ont été retirés de l'étude et n'ont pas été inclus dans les analyses, et le chercheur qui a diagnostiqué des réactions de HSS n'a pas utilisé de critères cliniques prédéfinis de l'hypersensibilité. Ces questions nous ont amenés à déclasser la valeur probante des données.
Authors' conclusions
Summary of findings
| Prospective genetic HLA‐B*57:01 screening compared with standard care for drug‐induced skin rash | ||||||
| Patient or population: patients with HIV‐1 infection and a pre‐established clinical need for treatment with an antiretroviral drug regimen containing abacavir but with an unknown HLA‐B*57:01 status. Settings: secondary care clinics Intervention: prospective genetic screening for the HLA‐B*57:01 allele Comparison: no prospective genotyping, standard‐of‐care treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard Carea | Prospective genetic HLA‐B*57:01 screening | |||||
| Severe skin drug‐induced rash | No data | ‐ | ‐ | ‐ | Not assessed | |
| Long‐term sequelae | No data | ‐ | ‐ | ‐ | Not assessed | |
| Hospitalisation for drug‐induced skin reaction | No data | ‐ | ‐ | ‐ | Not assessed | |
| SJS/TEN (Stevens‐Johnson syndrome/toxic epidermal necrolysis) | See hypersensitivity | ‐ | ‐ | ‐ | Not assessable | |
| AGEP (acute generalised exanthematous pustulosis) | No data | ‐ | ‐ | ‐ | Not assessed | |
| HSS (hypersensitivity) reaction including SJS/TEN (clinically diagnosed) (6 weeks clinical assessment) | 78 per 1000 | 34 per 1000 | RR 0.43 (0.28 to 0.67) | 1650 participants | ⊕⊕⊕⊝ | ‐ |
| HSS reaction including SJS/TEN (immunologically confirmed) (6 weeks clinical assessment) | 27 per 1000 | 0 per 1000 | RR 0.02 (0.00 to 0.37) | 1644 participants (1 study) | ⊕⊕⊕⊝ moderateb | ‐ |
| Death | No data | ‐ | ‐ | ‐ | Not assessed | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a The assumed risk is estimated from the event rate (confirmed clinically diagnosed occurrences of HSS including SJS/TEN or immunologically confirmed) in the control arm of the included study. b We downgraded the evidence to moderate quality, due to study limitations (high risk of detection and attrition bias). | ||||||
Background
We have explained some terms we have used in a glossary. Please see Table 1.
| Term | Explanation |
| Allele | One of two or more alternative forms of a gene at corresponding sites (loci) on homologous chromosomes |
| Antiretroviral | A class of drugs that inhibit the activity of retroviruses that cause HIV infection |
| Hardy‐Weinberg equilibrium | This states that allele and genotype frequencies in a population will remain constant from generation to generation in the absence of other evolutionary influences |
| HLA | Human leukocyte antigen: a group of protein molecules located on bone marrow and other cells that can provoke an immune response |
| Hypersensitivity | A state of altered reactivity in which the body reacts with an exaggerated immune response to a foreign substance, such as a drug |
| Immunologically confirmed | Patch testing is done to see whether a particular drug is causing allergic skin reaction. Patch test can detect delayed allergic or immunological reaction and confirm the diagnosis of hypersensitivity. |
| Phenotypes | The set of observable characteristics of an individual resulting from the interaction of its genotype with the environment |
| Polymorphic | A variation in the DNA that is too common to be due merely to new mutation. A polymorphism must have a frequency of at least 1% in a population |
| Maculopapular rash | A rash with both macules (flat and coloured like a freckle) and papules (a small raised spot) |
| Sequelae | A condition that is a consequence of a previous disease or injury |
| T‐cells | Another term for T‐lymphocyte, a type of cell that participates in immune response |
Description of the condition
Some drugs may cause skin rashes that vary in their severity and incidence. Skin reactions caused by drugs, often termed 'drug‐induced skin injury', are common (carbamazepine‐induced skin rash has a 10% incidence rate (Marson 2007)); they can present with a range of clinical manifestations, from a mild maculopapular skin rash to life‐threatening skin rashes such as Stevens‐Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) (Pirmohamed 2004; Roujeau 1987). The most severe forms of hypersensitivity are very rare, but these may result in up to 30% mortality (Roujeau 1994). Less severe forms of hypersensitivity reactions are still troublesome and may prevent people from taking medications that are otherwise effective. Delayed type hypersensitivity reactions are T‐cell mediated: they usually occur 24 to 48 hours, and up to six weeks, after exposure to culprit drugs.
The mechanisms involved in the pathogenesis of these drug‐induced reactions are still poorly understood; however, immunogenetic and non‐immune factors have been implicated (Chung 2004; Pirmohamed 2004; Vitezica 2008; Watanabe 2010). Recent evidence suggests that drug‐specific T‐cells can be identified in individuals who previously experienced adverse drug reactions to the culprit drug (Illing 2012).
Description of the intervention
There is increasing evidence from clinical trials that pretreatment genetic testing may reduce the possibility of severe drug‐induced hypersensitivity (Chen 2011; Mallal 2008).
Figure 1 represents a diagram of decision‐making informed by genetic testing.
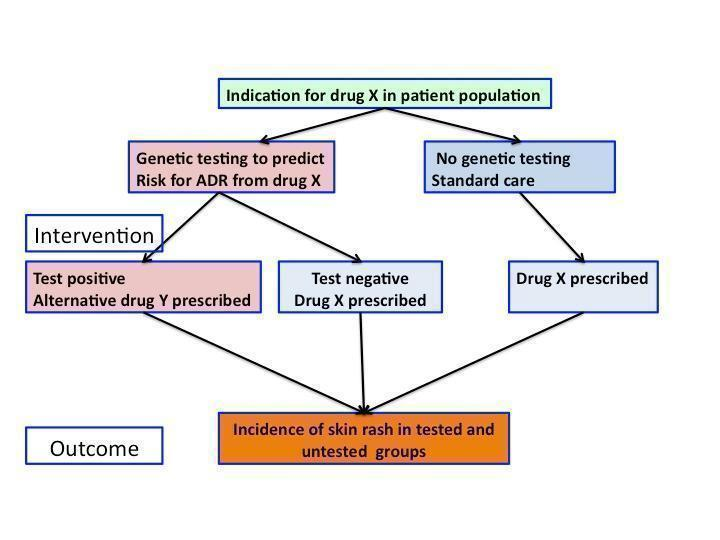
Flowchart of interventions (genetic testing) and outcomes (skin rash) in a patient population prescribed drug X
To date, the strongest association with drug‐induced skin injury has been reported with genetic variants in the human leukocyte antigens (HLA) (Amstutz 2013; Chung 2004; Hetherington 2002; Hung 2005; Mallal 2002; McCormack 2011; Ozkaya‐Bayazit 2001). HLA are cell surface proteins involved in presenting antigens to the immune system. They are encoded by most polymorphic genes in the human genome. However, different genetic markers are associated with hypersensitivity in different populations, and the effect size varies in different ethnicities. Also, there is evidence that some genetic factors could predispose to drug‐induced skin injury irrespective of the underlying drug aetiology. In addition, it is possible that different severity phenotypes can share the same predisposing factor (McCormack 2011). Table 2 shows reported associations between hypersensitivity reactions, which include skin injury and genetic variants in HLA genes.
| Drugs associated with skin injury | Class of drug | HLA allele | Population | Reference |
| Stevens‐Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) | ||||
| Allopurinol | Antiuric acid | B*5801 | Han Chinese | |
| ‐ | ‐ | ‐ | Thai | |
| ‐ | ‐ | ‐ | Japanese | |
| ‐ | ‐ | ‐ | Malay | |
| Carbamazepine | Antiepileptic | B*1502 | Han Chinese | |
| ‐ | ‐ | ‐ | Thai | Kulkantrakorn 2012; Locharernkul 2008; Tassaneeyakul 2010; Tangamornsuksan 2013 |
| ‐ | ‐ | ‐ | Malay | |
| ‐ | ‐ | ‐ | Indian | |
| ‐ | ‐ | A*3101 | White | |
| ‐ | ‐ | A*3101 | Japanese | |
| Phenytoin | Antiepileptic | B*1502 | Han Chinese | |
| ‐ | ‐ | ‐ | Thai | |
| Oxicam | Non‐steroidal anti‐inflammatory drug (NSAID) | A2, B12 | White | |
| Sulphamethoxazole | Antibiotic | A29, B12, DR7 | White | |
| Hypersensitivity syndrome (drug‐induced hypersensitivity syndrome (DIHS) or drug reaction with eosinophilia and systemic symptoms (DRESS)) | ||||
| Abacavir | Antiretroviral | B*57:01 | White | Hetherington 2002; Hughes 2004a; Mallal 2002; Mallal 2008; Martin 2004 |
| ‐ | ‐ | ‐ | African American | |
| Aminopenicillins | Antibiotic | A2, Drw52 | White | |
| Nevirapine | Antiretroviral | DRB1*01 | White ‐ Australian | |
| ‐ | ‐ | DRB1*01 | White ‐ French | |
| ‐ | ‐ | Cw8, B14 | White ‐ Italian | |
| ‐ | ‐ | Cw8 | Japanese | |
| ‐ | ‐ | B*3505 | Thai | |
| ‐ | ‐ | Cw4 | Thai | |
| ‐ | ‐ | C*0404 | Black African | |
| ‐ | ‐ | Cw*04 | Chinese | |
| Aspirin | NSAIDs | DRB1*1302, DQB1*0609 | ‐ | |
| ‐ | NSAIDs | DR11 | ‐ | |
| Iodine contrast media | ‐ | DR | White ‐ Spanish | |
| Paraphenylenediamine | Hair dye | DP | White ‐ German | |
| Gold sodium thiomalate | Treatment of rheumatoid arthritis | DR5 | White ‐ Spanish | |
| Lamotrigine | Antiepileptic | B*5801, A*6801 | White | |
| Trichloroethylene | Industrial solvent, dry cleaning | B*1301 | Japanese | |
| Fixed drug eruptions | ||||
| Co‐trimoxazole | Antibiotic | A30, B13, Cw6 | White ‐ Turkish | |
| Feprazone | Analgesic | B22 | ‐ | |
Standard clinical practice does not include genetic testing for most drugs, despite some strong evidence on the benefits of pretreatment genotyping (Kim 2005; McCormack 2011; Ozeki 2011). There are potential ethical issues with genetic tests, such as the effect on family members, unintended findings, and storage and access of data.
How the intervention might work
Two recent clinical trials suggested that pretreatment genetic testing could reduce the possibility of severe hypersensitivity induced with an anti‐AIDS drug, abacavir (Mallal 2008), and an antiepileptic drug, carbamazepine (Chen 2011).
Patients who have a clinical requirement for a particular drug treatment can be stratified on the basis of a genetic test. Those who test positive for the risk marker are not prescribed the culprit drug, while those who test negative are safe to take the medicine of interest. In this way, it may be possible to reduce the incidence of severe drug skin reactions in the genotyped group compared to the randomly assigned group of patients who are not offered genetic testing, but for whom decision on drug choice is based on traditional clinical and biochemical parameters (Chen 2011; Mallal 2008).
Why it is important to do this review
Adverse drug reactions affecting the skin are common; they can have high morbidity and mortality and are a burden for healthcare systems around the world. If we were able to predict these reactions using a simple genetic test, it should be possible to prevent them with one of the following approaches:
-
by prescribing alternative therapy, if available;
-
by informing patients and healthcare providers so the patients could be monitored more closely if they are at an increased risk;
-
by informing drug developers, in order to improve drug design and future drug development.
We aimed to assess current research evidence to determine whether prospective pharmacogenetic screening is effective in reducing drug‐associated skin reactions. The planned methods for this review were published as a protocol (Alfirevic 2014).
Objectives
To assess the effects of prospective pharmacogenetic screening to reduce drug‐associated skin reactions in a patient population.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only.
Types of participants
We included participants who were prescribed drugs known to cause delayed type hypersensitivity reactions with skin involvement. We accepted participants in any setting, who were of any age, gender, and ethnicity.
Prescribed drugs included, but were not limited to: antiepileptic drugs, antiretroviral drugs, antigout drugs, and antibiotics such as beta‐lactams (penicillin, amoxicillin, piperacillin, cephalosporins) and sulphonamides (sulphamethoxazole and trimethoprim). We would have included studies that described only a subset of relevant participants. Decisions to include studies that only partially addressed the population of interest would have been documented in the review, and we would have conducted a sensitivity analysis to assess the impact of the decisions on the review's findings.
Types of interventions
We considered genetic testing for any genetic variants associated with hypersensitivity reactions using all available techniques to determine individual genotypes, and compared to standard clinical practice, which does not include a genetic test. The intervention was a randomly allocated genetic test; if the test was positive, a drug that can cause hypersensitivity was avoided. We included studies whose purpose was to genotype on the basis of likely adverse skin reaction. We excluded any participants who had previously been administered the study drugs.
Types of outcome measures
We based core outcome measures on several papers describing clinical classification of drug‐induced skin reactions, including a paper entitled 'Phenotype standardisation for immune‐mediated drug‐induced skin injury' (Pirmohamed 2011), as well as papers by the RegiSCAR (European Registry of Severe Cutaneous Adverse Reactions) consortium (Bouvresse 2012; Kardaun 2013; Sekula 2011).
We assessed clinically defined hypersensitivity reaction, immunologically confirmed hypersensitivity reactions (if skin patch testing or lymphocyte proliferation assay data were available), and long‐term sequelae (including ophthalmologic, cutaneous, or liver damage, etc.).
We have provided a full list of clinically relevant outcomes, and distinction between primary and secondary outcomes, in Appendix 1.
Primary outcomes
-
The incidence of severe drug‐induced skin rash (defined as skin rash with systemic symptoms including fever and multiple organ involvement).
-
Long‐term sequelae (any of the following: ophthalmologic, cutaneous, or liver damage, etc.) up to 12 months after the severe drug‐induced skin rash.
Secondary outcomes
-
Hospitalisation for drug‐induced skin reaction within three months of exposure to the drug.
-
SJS/TEN (Stevens‐Johnson syndrome, toxic epidermal necrolysis).
-
AGEP (acute generalised exanthematous pustulosis).
-
HSS (hypersensitivity syndrome).
-
Death.
Additional terminology for HSS includes the following: drug‐induced hypersensitivity syndrome (DIHS), drug reaction with eosinophilia and systemic symptoms (DRESS), drug‐induced delayed multiorgan hypersensitivity syndrome, and hypersensitivity reaction.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
The Cochrane Skin Information Specialist searched the following databases up to 9 July 2018 using strategies based on the draft strategy for MEDLINE in our published protocol (Alfirevic 2014):
-
the Cochrane Skin Specialised Register using the search strategy in Appendix 2;
-
the Cochrane Central Register of Controlled Trials (CENTRAL) 2018, Issue 6, in the Cochrane Library using the strategy in Appendix 3;
-
MEDLINE via Ovid (from 1946) using the strategy in Appendix 4;
-
Embase via Ovid (from 1974) using the strategy in Appendix 5; and
-
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 6.
Trials registers
We (AA and AJ) searched the following trials registers on 15 July 2018 using the following terms: skin rash, genetic test, drug:
-
the ISRCTN registry (www.isrctn.com);
-
ClinicalTrials.gov (www.clinicaltrials.gov);
-
the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
-
the World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch); and
-
the EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
Searching other resources
References from included studies
We checked the bibliographies of the included studies and published reviews for further references to relevant trials.
Correspondence
We requested relevant information from one author of an included study, but they did not reply to us (Appendix 7).
Adverse effects
We did not perform a separate search for adverse effects of the target intervention. We examined data on adverse effects of genotyping from the included study we identified, but we did not find evidence of adverse effects of genotyping.
Data collection and analysis
We included a 'Summary of findings' table in our review which summarised our primary and secondary outcomes for the comparison of genetic testing versus no genetic testing.
Selection of studies
Two review authors (AA and AJ) independently assessed all the titles and abstracts of publications identified by the searches to assess their eligibility. The full texts of all papers found to be eligible at this initial stage were then assessed for inclusion. Publications found to be irrelevant after reading their full text were excluded, and 'Characteristics of excluded studies' tables were prepared to identify the reasons for exclusion. Consensus on the final list of trials to include was reached by discussion.
Data extraction and management
Two review authors (AA and AJ) independently extracted data from the included studies and resolved disagreements by discussion with a third author (TC). The following information on study characteristics and methods were collected into a standardised data extraction form:
-
study design;
-
inclusion and exclusion criteria;
-
setting;
-
country;
-
language of publication;
-
ethnicity of participants;
-
control population (comprising individuals exposed to the culprit drug with no adverse effects or individuals from a healthy population who were not exposed to medications used in the trial);
-
description of genotyping techniques used;
-
genotyping quality control, which included deviation from Hardy‐Weinberg equilibrium (Hardy‐Weinberg equilibrium is a crucial concept in population genetics; it predicts how gene frequencies will be inherited from generation to generation and is used as a measure of quality of genetic tests) and genotype call rate;
-
age;
-
gender;
-
concomitant medications;
-
time from exposure to the culprit drug to skin reaction;
-
type of skin reaction;
-
location of skin lesion;
-
sequelae of adverse reactions;
-
other manifestations indicating systemic involvement; and
-
clinical laboratory tests.
No language translations of papers were required.
Assessment of risk of bias in included studies
Two review authors (AA and AJ) independently assessed the risk of bias in each trial. We used Cochrane's tool for assessing risk of bias (Table 8.5.a in the Cochrane Handbook for Systematic Reviews of Interventions), which is based on seven domains (Higgins 2011):
-
random sequence generation (selection bias);
-
allocation concealment (selection bias);
-
blinding of participants and personnel (performance bias);
-
blinding of outcome assessment (detection bias);
-
incomplete outcome data (attrition bias);
-
selective outcome reporting (reporting bias); and
-
other risk of bias.
The tool allows for the risk of bias for each domain to be assessed as 'low', 'high', or 'unclear' (indicating lack of information or uncertainty over the potential for bias). An additional review author (MP) was consulted in the case of disagreements.
Measures of treatment effect
We planned to use statistical methods in accordance with theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to calculate mean difference (MD) with 95% confidence interval (CI) for continuous data or indeed standardised mean difference (SMD) with 95% CI where all studies reported an outcome using different but similar scales. A risk ratio (RR) with 95% CI was calculated for dichotomous data.
Unit of analysis issues
There were no unit of analysis issues. If such issues arise in an update of this review, we plan to take the appropriate approach to analysis to avoid a unit of analysis error due to repeated observations on participants, multiple treatments, or re‐occurring events. In future updates, if we include cluster‐randomised trials, we will take into account issues such as recruitment bias, baseline comparability of clusters, and number of clusters, and make sure that appropriate statistical methods are used that take into account weighting, etc. (Higgins 2011).
Dealing with missing data
We considered the possible different types of missing data. We planned to deal with missing studies (and the associated risk of bias) by assessing for publication bias, and to deal with missing outcomes (and the associated risk of bias) by assessing for selective reporting (see Assessment of reporting biases). However this was not applicable, due to there only being one included study in this review.
The included study did not report on either of our primary outcomes, but instead reported on an outcome of hypersensitivity reaction which included (but was not limited to) our secondary outcomes HSS and SJS/TEN. So in order to deal with missing data we contacted the study author for additional data on the outcomes of our review. We did not receive a response and therefore we are unaware if the outcomes were measured, and were unable to include these data in our review.
Assessment of heterogeneity
Had a meta‐analysis been possible, we planned to assess the extent of heterogeneity using the I² statistic. We would have used the following thresholds for the interpretation of the I² statistic:
-
0% to 40% = might not be important;
-
30% to 60% = moderate heterogeneity;
-
50% to 90% = may represent substantial heterogeneity; and
-
75% to 100% = considerable heterogeneity.
Due to there being only one included study, we did not need to assess heterogeneity. If eligible studies become available in the future during an update of this review, we will perform a meta‐analysis, report the I² statistic, and interpret the two data together.
Assessment of reporting biases
We planned to assess publication bias by using a funnel plot, Begg test and Egger test (Begg 1989; Egger 1998). Tests for funnel plot asymmetry would only be undertaken when at least 10 studies could be included in the meta‐analysis. Due to the fact that there was only one included study, the power of the tests is too low to distinguish chance from real asymmetry; therefore, this was not assessed.
Data synthesis
Had there been sufficient studies and no significant clinical heterogeneity, we planned to synthesise the results in meta‐analyses, stratified according to type of intervention (e.g. type of genetic test). A random‐effects model would have been assumed.
Where events are rare, a random‐effects approach may be inappropriate. Where events were rare, we planned to take extra care to adopt appropriate methods of meta‐analysis, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (section 16.9) (Higgins 2011), since many meta‐analysis methods are suboptimal where events are rare through results being biased, confidence intervals being too wide, or power being too low. Choice of method would have been guided by control group risk, likely treatment effect size, and consideration of balance in numbers of treated and control participants in the constituent studies. Had the control groups differed — e.g. if they were drawn from a healthy population or from a population of people treated with the culprit drug but without any adverse effects — we planned to conduct separate analyses. We planned to use Review Manager 5 software to undertake the meta‐analyses (Review Manager 2014).
As we only included one study, it was not possible to undertake a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned to assess heterogeneity using the I² statistic and in the event of substantial heterogeneity, we planned to explore the causes by way of subgroup analyses. We planned to consider the following:
-
participant factors (age, ethnicity, classification of adverse drug reactions, and comparability of participant groups); and
-
trial design issues (genotyping methodology and quality control, blinding, drugs included, and drug dosage and duration of use).
As we only included one study, it was not possible to assess for heterogeneity and therefore a subgroup analysis was not relevant.
Sensitivity analysis
We planned to evaluate the robustness of the results of the meta‐analyses by removing trials of low methodological quality as identified by their risk of bias. As we only included one study, it was not possible to undertake a meta‐analysis, or indeed sensitivity analyses.
Results
Description of studies
Results of the search
The searches of the five databases (see Electronic searches) retrieved 1790 records. Our checks of the trials registers and bibliographies of included studies and relevant reviews did not identify any further relevant studies. Once duplicates were removed we had a total of 1786 records. We excluded 1774 records based on titles and abstracts. We obtained the full text of the remaining 12 records. We excluded nine studies (see Characteristics of excluded studies). One study is awaiting classification (see Characteristics of studies awaiting classification). We did not identify any ongoing studies. We included one study (Mallal 2008), which was reported in two papers.
For a further description of our screening process, see the study flow diagram in Figure 2.
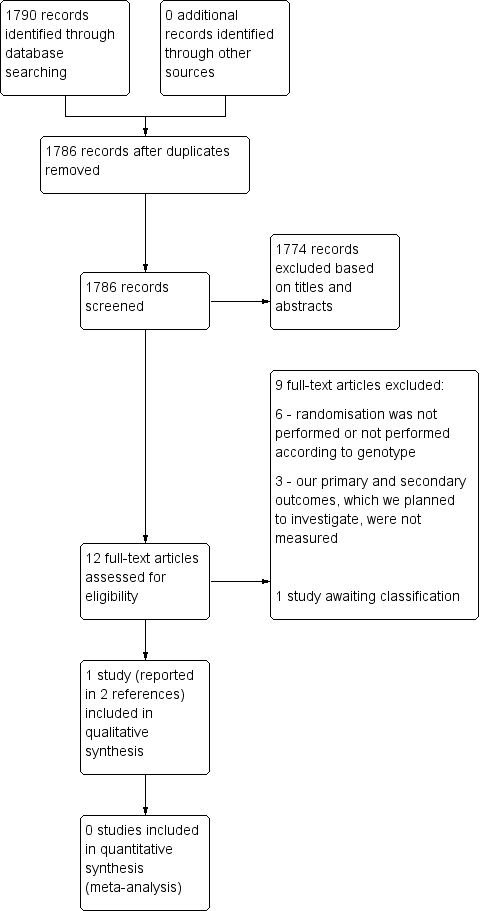
Study flow diagram.
Included studies
Only one study fulfilled our inclusion criteria (Mallal 2008). The summary information can be found in Characteristics of included studies.
This study was a randomised, prospective, double‐blind, multicentre controlled trial, which included 1956 participants from 265 centres (medical centres, hospitals, outpatient clinics) in 19 countries. The participants were infected with HIV‐type 1 (HIV‐1) and had not previously been exposed to the antiretroviral drug abacavir. The study population consisted of men and women (74% men) between the ages of 18 and 77 years old (mean age: 42 years) who were predominantly white. The initial target sample size was 1578 participants. The study was supported by GlaxoSmithKline.
Participants were randomly assigned to undergo prospective pharmacogenetic screening for the human leukocyte antigen class I gene HLA‐B*57:01 (prospective screening group, i.e. the treatment group) or to undergo a standard care approach without genetic testing (control group). In the prospective screening group, HLA‐B*57:01‐positive participants were excluded from abacavir treatment to prevent skin rash. These individuals were given a combination of active antiretroviral therapy that did not include abacavir. (Those that tested negative for HLA‐B*57:01 were given a combination of active antiretroviral therapy including abacavir.)
All those in the control group were given a combination of active antiretroviral therapy including abacavir without prospective screening for HLA‐B*57:01. These participants had retrospective HLA‐B*57:01 pharmacogenetic testing.
The study encompassed six‐week observation periods conducted over a six‐month trial period from April to September 2006.
The primary outcomes reported by the study were to investigate whether there was a reduction in clinically diagnosed hypersensitivity reactions to abacavir in the genotyped group compared to the control group, and to explore the rate of immunologically confirmed hypersensitivity reaction (defined as "clinically diagnosed reaction that was confirmed by a positive result on epicutaneous patch testing 6 to 10 weeks after clinical diagnosis" (Mallal 2008)). Assessment of hypersensitivity was performed at the time of study entry at baseline (day one) and at weeks one, two and six. None of the review's primary outcomes were reported in this trial. In terms of the review's secondary outcomes, HSS and SJS/TEN were reported, but only as a combined outcome under 'hypersensitivity reactions'.
Excluded studies
We excluded nine studies following full‐text review. Reasons for exclusion were as follows:
-
randomisation was not performed, or not performed according to genotype (Bonnefoi 2011; Cheng 2009; Pusztai 2014; Sequist 2013; Seymour 2013; Young 2008); and
-
our primary and secondary outcomes, which we planned to investigate, were not measured (Azuma 2013; Damronglerd 2015; Newman 2011).
Full reasons for exclusion are listed in Characteristics of excluded studies.
Risk of bias in included studies
The overall risk of bias was low, with the exception of a notably high risk of attrition and detection bias (Figure 3; Figure 4).
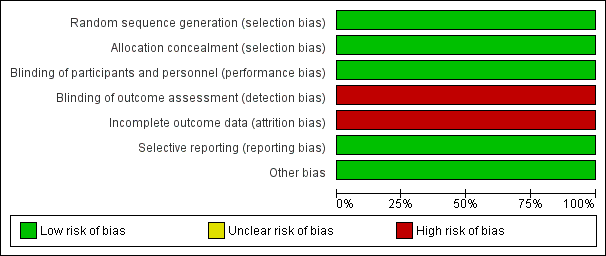
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
All 1956 participants in the study were adequately randomised based on a computer‐generated, centralised schedule; thus, allocation to the treatment and control groups (prospective and retrospective testing) indicates a low risk of selection bias.
Allocation concealment
The method of allocation concealment represents low risk of bias because it was performed by the central study‐management group without foreknowledge of intervention assignments. The sequence was implemented in the block sizes of four. Stratification was done according to ethnicity to ensure a good balance of ethnic subgroups between the two trial arms, since risk of outcomes can vary across ethnic groups.
Blinding
Blinding was undertaken sufficiently, with the investigators, participants and study management team remaining unaware of the participant assignments to the treatment or control group. Therefore we deemed the study to have a low risk of performance bias. In terms of detection bias, the assessors were unaware of the genetic test results and investigators were trained using illustrated guides and an informational video. However, hypersensitivity reactions were diagnosed by the principal investigator at the recruitment site without the use of predefined clinical criteria and this poses a risk of detection bias.
Incomplete outcome data
Participants treated with abacavir, but for whom there were incomplete follow‐up data for various detailed reasons, were excluded from the primary analyses; therefore these analyses are at risk of attrition bias. However, potential bias introduced by the exclusion of the participants who could not be evaluated was addressed by several sensitivity analyses of data from the full intention‐to‐treat population that had received abacavir, with assumptions about the missing data ranging from 0% to 100% of the exclusions being associated with a hypersensitivity reaction. The results of the sensitivity analyses were in line with the results of the primary analyses.
Selective reporting
We judged the study to be at low risk of reporting bias as both prespecified outcomes from the protocol (ClinicalTrials.gov identifier: NCT00340080) were reported. It was not possible to assess for between‐study selective reporting as there was only one included study.
Other potential sources of bias
No other potential sources of bias appear within the study.
Effects of interventions
See: Summary of findings for the main comparison
The study did not report on either of our primary outcomes (the incidence of severe drug‐induced skin rash; or long‐term sequelae). The study did report 'hypersensitivity reactions' which included, but was not limited to, our secondary outcomes HSS (hypersensitivity syndrome) and SJS/TEN (Stevens‐Johnson syndrome, toxic epidermal necrolysis).
The study demonstrated that prospective HLA‐B*57:01 screening can reduce the incidence of hypersensitivity reaction to abacavir. The incidence of immunologically confirmed hypersensitivity reaction to abacavir was lower in the screening arm (risk ratio 0.02; 95% CI 0.00 to 0.37; P < 0.001; 1644 participants; Analysis 1.1), as was the incidence of clinically diagnosed hypersensitivity reaction to abacavir (risk ratio 0.43; 95% CI 0.28 to 0.67; P < 0.001; 1650 participants; Analysis 2.1). These results are presented in summary of findings Table for the main comparison, Figure 5 and Figure 6.

Forest plot of comparison: 1 Genetic testing vs no testing, outcome: 1.1 Hypersensitivity (HSS).

Forest plot of comparison: 1 Genetic testing with skin patch testing versus no genetic testing with skin patch testing, outcome: 1.1 Hypersensitivity (HSS) immunologically confirmed.
However, we are aware of some potential long‐term effects of abacavir. In order to determine any further effects of the intervention, we made an attempt to contact the study authors for information on our remaining primary and secondary outcomes (as mentioned above in Dealing with missing data). We did not receive a response, and therefore are unable to estimate any further effects of the intervention.
Discussion
Summary of main results
Our one included study (with a total of 1956 participants) did not report on either of our primary outcomes of interest, which were:
-
the incidence of severe drug‐induced skin rash (defined as skin rash with systemic symptoms including fever and multiple organ involvement); and
-
long‐term sequelae (any of the following: ophthalmologic, cutaneous, or liver damage, etc.) up to 12 months after the severe drug‐induced skin rash.
The study addressed a combination of our second and fourth secondary outcomes, which were: SJS/TEN (Stevens‐Johnson syndrome, toxic epidermal necrolysis) and HSS (hypersensitivity syndrome). Based on moderate‐quality evidence, prospective HLA‐B*57:01 screening probably reduces the incidence of hypersensitivity reaction to abacavir compared to when prospective pharmacogenetic screening is not performed. However, hypersensitivity reactions were diagnosed by the principal investigator at the recruitment site without the use of predefined clinical criteria and therefore there is a high risk of detection bias.
Carriers of HLA‐B*57:01 were excluded from receiving abacavir; the rate of clinical diagnosis of hypersensitivity reaction was 7.8% in the control group (who received no screening, but underwent HLA typing retrospectively after abacavir exposure) compared to 3.4% (95% CI 2.2% to 5.2%) in the prospective‐screening group (risk ratio (RR) 0.43, 95% CI 0.28 to 0.67) (summary of findings Table for the main comparison; Figure 5).
In addition, undertaking skin patch testing to confirm the diagnosis of hypersensitivity and excluding carriers of HLA‐B*57:01 from receiving abacavir, reduced the rate of immunologically confirmed diagnosis of hypersensitivity from 2.7% in the control group (no screening) to zero in the prospective‐screening group (RR 0.02, 95% CI 0.00 to 0.37) (summary of findings Table for the main comparison; Figure 6).
Based on this trial, pretreatment genetic testing for the HLA‐B*57:01 allele has been recommended by the Clinical Pharmacogenetics Implementation Consortium (Martin 2014) and several regulating agencies, including the Food and Drug Administration (FDA), European Medicines Agency (EMA) and Pharmaceuticals and Medical Devices Agency (PMDA).
Overall completeness and applicability of evidence
The included study is not sufficient to address all the objectives of this review (Mallal 2008). Whilst it did provide some data related to two secondary outcomes, and was generally at low risk of bias, more studies are required if our objectives are to be explored fully. The study is the only randomised controlled trial that assessed efficacy and safety of genetic testing in prediction of adverse drug reactions. Included individuals were representative of the study population of interest. However, it will be important in future studies to explore ethnic variability as this study has been conducted in a predominantly white population. The follow‐up period was short and there was no information on long‐term sequelae in drug‐hypersensitivity survivors. The study did not report on either of our primary outcomes of interest. In addition, information on hospitalisation, AGEP (acute generalised exanthematous pustulosis), or death were not reported either. We found no eligible evidence with regard to genetic testing for severe drug‐induced skin rash in relation to different drugs and classes of drugs.
Quality of the evidence
The study included in this review has adequate randomisation methods, as well as methods of allocation concealment. However, the included trial did not report on the characteristics of the 55 participants (5.6%) who were positive for HLA‐B*57:01 and therefore did not receive abacavir treatment and were excluded from data analysis. Information on outcomes within this patient group would have been informative. There is also potential for detection bias as the principal investigator diagnosed hypersensitivity reactions without the use of predefined clinical criteria. Therefore, we downgraded the quality of evidence by one level due to study limitations (risk of bias). We did not find any reason to downgrade the evidence for the other GRADE domains (inconsistency, imprecision, indirectness, or publication bias). Therefore, quality of evidence was rated as moderate using the GRADE criteria (Schünemann 2013).
Potential biases in the review process
We did not identify any sources of potential bias in the review process. We carefully assessed diverse terminology used to describe drug‐induced hypersensitivity and included alternative search terms in our literature searches. We clearly defined the outcomes and patient subgroups. As we included only RCTs in our protocol, no other study designs were considered. We did not impose any date restrictions on the search. We contacted the study authors to provide additional data, but that did not generate any additional information.
Agreements and disagreements with other studies or reviews
To our knowledge there have been no previous systematic reviews addressing this research question, and the only previous study addressing the research question is included as the only study in this review (Mallal 2008). Due to there only being one identified RCT, there are remaining uncertainties about the effects of prospective pharmacogenetic screening to reduce drug‐associated skin reactions in a patient population. Several studies have recently reported clinical utility of pretreatment genetic testing that can predict and prevent serious cutaneous adverse drug reactions (Chen 2011; Fang 2019; Liu 2019; Mushiroda 2018; Park 2019; Stainsby 2019). However, these studies are not RCTs and therefore were not included in our review; they use historical frequency data on adverse drug reactions over a period of several years to control for HLA allele screening that helps to predict and prevent adverse drug reactions in carriers of risk alleles. The findings in these studies agree with the conclusions of our review.

Flowchart of interventions (genetic testing) and outcomes (skin rash) in a patient population prescribed drug X

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Genetic testing vs no testing, outcome: 1.1 Hypersensitivity (HSS).

Forest plot of comparison: 1 Genetic testing with skin patch testing versus no genetic testing with skin patch testing, outcome: 1.1 Hypersensitivity (HSS) immunologically confirmed.

Drug‐induced skin rash: top panel = maculopapular exanthema, bottom panel = Steven Johnson Syndrome (blistering skin rash with skin detachment)

Comparison 1 Genetic testing with skin patch testing versus no genetic testing with skin patch testing, Outcome 1 Hypersensitivity (HSS), immunologically confirmed.

Comparison 2 Genetic testing versus no testing, Outcome 1 Hypersensitivity (HSS).
| Prospective genetic HLA‐B*57:01 screening compared with standard care for drug‐induced skin rash | ||||||
| Patient or population: patients with HIV‐1 infection and a pre‐established clinical need for treatment with an antiretroviral drug regimen containing abacavir but with an unknown HLA‐B*57:01 status. Settings: secondary care clinics Intervention: prospective genetic screening for the HLA‐B*57:01 allele Comparison: no prospective genotyping, standard‐of‐care treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard Carea | Prospective genetic HLA‐B*57:01 screening | |||||
| Severe skin drug‐induced rash | No data | ‐ | ‐ | ‐ | Not assessed | |
| Long‐term sequelae | No data | ‐ | ‐ | ‐ | Not assessed | |
| Hospitalisation for drug‐induced skin reaction | No data | ‐ | ‐ | ‐ | Not assessed | |
| SJS/TEN (Stevens‐Johnson syndrome/toxic epidermal necrolysis) | See hypersensitivity | ‐ | ‐ | ‐ | Not assessable | |
| AGEP (acute generalised exanthematous pustulosis) | No data | ‐ | ‐ | ‐ | Not assessed | |
| HSS (hypersensitivity) reaction including SJS/TEN (clinically diagnosed) (6 weeks clinical assessment) | 78 per 1000 | 34 per 1000 | RR 0.43 (0.28 to 0.67) | 1650 participants | ⊕⊕⊕⊝ | ‐ |
| HSS reaction including SJS/TEN (immunologically confirmed) (6 weeks clinical assessment) | 27 per 1000 | 0 per 1000 | RR 0.02 (0.00 to 0.37) | 1644 participants (1 study) | ⊕⊕⊕⊝ moderateb | ‐ |
| Death | No data | ‐ | ‐ | ‐ | Not assessed | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a The assumed risk is estimated from the event rate (confirmed clinically diagnosed occurrences of HSS including SJS/TEN or immunologically confirmed) in the control arm of the included study. b We downgraded the evidence to moderate quality, due to study limitations (high risk of detection and attrition bias). | ||||||
| Term | Explanation |
| Allele | One of two or more alternative forms of a gene at corresponding sites (loci) on homologous chromosomes |
| Antiretroviral | A class of drugs that inhibit the activity of retroviruses that cause HIV infection |
| Hardy‐Weinberg equilibrium | This states that allele and genotype frequencies in a population will remain constant from generation to generation in the absence of other evolutionary influences |
| HLA | Human leukocyte antigen: a group of protein molecules located on bone marrow and other cells that can provoke an immune response |
| Hypersensitivity | A state of altered reactivity in which the body reacts with an exaggerated immune response to a foreign substance, such as a drug |
| Immunologically confirmed | Patch testing is done to see whether a particular drug is causing allergic skin reaction. Patch test can detect delayed allergic or immunological reaction and confirm the diagnosis of hypersensitivity. |
| Phenotypes | The set of observable characteristics of an individual resulting from the interaction of its genotype with the environment |
| Polymorphic | A variation in the DNA that is too common to be due merely to new mutation. A polymorphism must have a frequency of at least 1% in a population |
| Maculopapular rash | A rash with both macules (flat and coloured like a freckle) and papules (a small raised spot) |
| Sequelae | A condition that is a consequence of a previous disease or injury |
| T‐cells | Another term for T‐lymphocyte, a type of cell that participates in immune response |
| Drugs associated with skin injury | Class of drug | HLA allele | Population | Reference |
| Stevens‐Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) | ||||
| Allopurinol | Antiuric acid | B*5801 | Han Chinese | |
| ‐ | ‐ | ‐ | Thai | |
| ‐ | ‐ | ‐ | Japanese | |
| ‐ | ‐ | ‐ | Malay | |
| Carbamazepine | Antiepileptic | B*1502 | Han Chinese | |
| ‐ | ‐ | ‐ | Thai | Kulkantrakorn 2012; Locharernkul 2008; Tassaneeyakul 2010; Tangamornsuksan 2013 |
| ‐ | ‐ | ‐ | Malay | |
| ‐ | ‐ | ‐ | Indian | |
| ‐ | ‐ | A*3101 | White | |
| ‐ | ‐ | A*3101 | Japanese | |
| Phenytoin | Antiepileptic | B*1502 | Han Chinese | |
| ‐ | ‐ | ‐ | Thai | |
| Oxicam | Non‐steroidal anti‐inflammatory drug (NSAID) | A2, B12 | White | |
| Sulphamethoxazole | Antibiotic | A29, B12, DR7 | White | |
| Hypersensitivity syndrome (drug‐induced hypersensitivity syndrome (DIHS) or drug reaction with eosinophilia and systemic symptoms (DRESS)) | ||||
| Abacavir | Antiretroviral | B*57:01 | White | Hetherington 2002; Hughes 2004a; Mallal 2002; Mallal 2008; Martin 2004 |
| ‐ | ‐ | ‐ | African American | |
| Aminopenicillins | Antibiotic | A2, Drw52 | White | |
| Nevirapine | Antiretroviral | DRB1*01 | White ‐ Australian | |
| ‐ | ‐ | DRB1*01 | White ‐ French | |
| ‐ | ‐ | Cw8, B14 | White ‐ Italian | |
| ‐ | ‐ | Cw8 | Japanese | |
| ‐ | ‐ | B*3505 | Thai | |
| ‐ | ‐ | Cw4 | Thai | |
| ‐ | ‐ | C*0404 | Black African | |
| ‐ | ‐ | Cw*04 | Chinese | |
| Aspirin | NSAIDs | DRB1*1302, DQB1*0609 | ‐ | |
| ‐ | NSAIDs | DR11 | ‐ | |
| Iodine contrast media | ‐ | DR | White ‐ Spanish | |
| Paraphenylenediamine | Hair dye | DP | White ‐ German | |
| Gold sodium thiomalate | Treatment of rheumatoid arthritis | DR5 | White ‐ Spanish | |
| Lamotrigine | Antiepileptic | B*5801, A*6801 | White | |
| Trichloroethylene | Industrial solvent, dry cleaning | B*1301 | Japanese | |
| Fixed drug eruptions | ||||
| Co‐trimoxazole | Antibiotic | A30, B13, Cw6 | White ‐ Turkish | |
| Feprazone | Analgesic | B22 | ‐ | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hypersensitivity (HSS), immunologically confirmed Show forest plot | 1 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hypersensitivity (HSS) Show forest plot | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.28, 0.67] |

