Different infusion durations for preventing platinum‐induced hearing loss in children with cancer
Abstract
Background
Platinum‐based therapy, including cisplatin, carboplatin or oxaliplatin, or a combination of these, is used to treat a variety of paediatric malignancies. Unfortunately, one of the most important adverse effects is the occurrence of hearing loss or ototoxicity. In an effort to prevent this ototoxicity, different platinum infusion durations have been studied. This review is the third update of a previously published Cochrane Review.
Objectives
To assess the effects of different durations of platinum infusion to prevent hearing loss or tinnitus, or both, in children with cancer. Secondary objectives were to assess possible effects of these infusion durations on: a) anti‐tumour efficacy of platinum‐based therapy, b) adverse effects other than hearing loss or tinnitus, and c) quality of life.
Search methods
We searched the electronic databases Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library 14 November 2019), MEDLINE (PubMed) (1945 to 14 November 2019) and Embase (Ovid) (1980 to 14 November 2019). In addition, we handsearched reference lists of relevant articles and we assessed the conference proceedings of the International Society for Paediatric Oncology (2009 up to and including 2019) and the American Society of Pediatric Hematology/Oncology (2014 up to and including 2019). We scanned ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch) for ongoing trials (both searched on 4 November 2019).
Selection criteria
Randomised controlled trials (RCTs) or controlled clinical trials (CCTs) comparing different platinum infusion durations in children with cancer. Only the platinum infusion duration could differ between the treatment groups.
Data collection and analysis
Two review authors independently performed the study selection, 'Risk of bias' assessment and GRADE assessment of included studies, and data extraction including adverse effects. Analyses were performed according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
We identified one RCT and no CCTs; in this update no additional eligible studies were identified. The RCT (total number of children = 91) evaluated the use of a continuous cisplatin infusion (N = 43) versus a one‐hour bolus cisplatin infusion (N = 48) in children with neuroblastoma. For the continuous infusion, cisplatin was administered on days one to five of the cycle, but it is unclear if the infusion duration was a total of five days. Risk of bias was present. Only results from shortly after induction therapy were provided. No clear evidence of a difference in hearing loss (defined as asymptomatic and symptomatic disease combined) between the different infusion durations was identified as results were imprecise (risk ratio (RR) 1.39, 95% confidence interval (CI) 0.47 to 4.13, low‐quality evidence). Although the numbers of children were not provided, it was stated that tumour response was equivalent in both treatment arms. With regard to adverse effects other than ototoxicity, we were only able to assess toxic deaths. Again, the confidence interval of the estimated effect was too wide to exclude differences between the treatment groups (RR 1.12, 95% CI 0.07 to 17.31, low‐quality evidence). No data were available for the other outcomes of interest (i.e. tinnitus, overall survival, event‐free survival and quality of life) or for other (combinations of) infusion durations or other platinum analogues.
Authors' conclusions
Since only one eligible RCT evaluating the use of a continuous cisplatin infusion versus a one‐hour bolus cisplatin infusion was found, and that had methodological limitations, no definitive conclusions can be made. It should be noted that 'no evidence of effect', as identified in this review, is not the same as 'evidence of no effect'. For other (combinations of) infusion durations and other platinum analogues no eligible studies were identified. More high‐quality research is needed.
PICO
Plain language summary
Different infusion durations for preventing platinum‐induced hearing loss in children with cancer
Review question
We reviewed the evidence of the effects of different durations of platinum infusion to prevent hearing loss or tinnitus, or both, in children with cancer. We also looked at anti‐tumour efficacy, adverse effects other than hearing loss and quality of life.
Background
Platinum‐based chemotherapy, including cisplatin, carboplatin or oxaliplatin, or a combination of these, is used to treat different types of childhood cancer. Unfortunately, one of the most important adverse effects of platinum chemotherapy is hearing loss. This can occur not only during treatment but also years after the end of treatment. Although it is not life‐threatening, the loss of hearing, especially during the first three years of life, may lead to difficulties with school performance and psychosocial functioning. Therefore, prevention of platinum‐induced hearing loss is very important and might improve the quality of life of children undergoing cancer treatment and those who have survived treatment with platinum‐based chemotherapy.
Study characteristics
The evidence is current to November 2019.
We found one study (91 participants) comparing a continuous cisplatin infusion with a one‐hour cisplatin bolus infusion in children with neuroblastoma. For the continuous infusion, cisplatin was administered on days one to five of the treatment cycle but it is not clear if the infusion duration was a total of five days. Only results from shortly after induction therapy were available.
Key results
At the moment there is no evidence showing that the use of a different cisplatin infusion duration prevents hearing loss or adversely affects tumour response and adverse effects. No data were available for the other outcomes of interest (i.e. tinnitus, overall survival, event‐free survival and quality of life) or for other (combinations of) infusion durations or other platinum analogues. We need more high‐quality research before definite conclusions can be made about the usefulness of different platinum infusion durations to prevent hearing loss in children with cancer.
Quality of the evidence
The quality of the evidence was low.
Authors' conclusions
Summary of findings
| Continuous platinum infusion compared to bolus platinum infusion for children with cancer treated with platinum‐based therapy | ||||||
| Patient or population: children with cancer treated with platinum‐based therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Bolus platinum infusion | Continuous platinum infusion | |||||
| Hearing loss (asymptomatic and symptomatic disease according to Brock criteria) | 139 per 10001 | 193 per 1000 | RR 1.39 | 67 | ⊕⊕⊝⊝ | Length of follow‐up was not mentioned, but the median duration of induction was 102 days in the continuous infusion arm and 107 days in the bolus infusion arm (no significant difference) and only results from shortly after induction therapy were provided. For 24 of the 91 children included in the study no data on hearing loss were available (12 in each treatment group). The RR presented here results from the 'available data' analysis. Intention‐to‐treat analyses (i.e. worst‐ and best‐case scenarios) also showed no significant difference between the treatment groups. The GRADE assessment for the worst‐ and best‐case scenarios was identical to that of the 'available data' analysis. |
| Tinnitus ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on tinnitus was provided. |
| Overall survival ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on overall survival was provided. |
| Event‐free survival ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on event‐free survival was provided. |
| Tumour response (complete or partial remission according to INRC) | See comment | See comment | Not estimable (see comments) | Unclear | ⊕⊕⊝⊝ | The number of children with a complete or partial remission was not provided, but it was stated that tumour response was equivalent in both treatment arms. Length of follow‐up was not mentioned, but the median duration of induction was 102 days in the continuous infusion arm and 107 days in the bolus infusion arm (no significant difference) and only results from shortly after induction therapy were provided. |
| Adverse effects: toxic death (no definition provided) | 21 per 10001 | 23 per 1000 | RR 1.12 | 91 | ⊕⊕⊝⊝ | Length of follow‐up was not mentioned, but the median duration of induction was 102 days in the continuous infusion arm and 107 days in the bolus infusion arm (no significant difference) and only results from shortly after induction therapy were provided. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on quality of life was provided. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is based on the prevalence in the control group of the included study. 4 We downgraded 1 level based on the fact that the presence of selection bias, performance bias, detection bias, attrition bias and other bias is unclear. 5 We downgraded 1 level based on the fact that the presence of selection bias, performance bias, detection bias and other bias is unclear. | ||||||
Background
Description of the condition
Platinum‐based therapy, that is therapy including cisplatin, carboplatin or oxaliplatin, or a combination of these, is used to treat a variety of paediatric cancers. Unfortunately, one of the most important adverse effects is the occurrence of hearing loss or ototoxicity. This usually manifests itself by bilateral, symmetrical, sensorineural hearing loss first affecting the higher frequencies (≥ 6000 Hz) (McHaney 1983); and it is often accompanied by tinnitus (Reddel 1982).
There is a wide variation in the reported frequency of platinum‐induced hearing loss, but the frequency has been described to be as high as 90.1% (Van As 2016a). Hearing loss not only develops during platinum‐based therapy but also years after completion of the therapy (Bertolini 2004; Knight 2005). This might be explained by the prolonged retention of platinum in the body; up to 20 years after treatment circulating platinum is still detectable in plasma (Gietema 2000). Platinum‐induced hearing loss seems to be irreversible, and worsening of hearing loss occurs during follow‐up (McHaney 1983; Bertolini 2004).
Different risk factors for hearing loss have been identified, such as the type of platinum analogue used. Cisplatin seems to cause substantially more hearing loss than carboplatin, and the highest incidence of hearing loss has been found in patients who received both cisplatin and carboplatin (Bertolini 2004; Dean 2008). The ototoxicity of oxaliplatin compared to the other platinum analogues is not as well established, but oxaliplatin seems to be the least ototoxic (Eloxatin SPC). Furthermore, the incidence of platinum‐induced hearing loss seems to be dose‐dependent, increasing with higher cumulative doses (McHaney 1983; Schell 1989; Bertolini 2004; Li 2004) and with higher individual doses (Reddel 1982; Li 2004). Different dosing formulae, like dose per body surface area or per kilogram bodyweight, can influence the platinum doses actually received, especially in infants (Leahey 2012; Qaddoumi 2012). Cranial radiotherapy (Schell 1989), younger age (Schell 1989; Li 2004; Qaddoumi 2012), genetic variants (Ross 2009; Grewal 2010; Langer 2013), and other host‐specific factors (Veal 2001), impaired renal function at the time of platinum treatment (Skinner 2004), and other ototoxic drugs like aminoglycosides (Skinner 2004; Jenney 2005), and furosemide (Gallagher 1979) have been reported as additional risk factors. Finally, it has been suggested that different infusion durations (such as bolus and continuous infusions) have different levels of ototoxicity (Reddel 1982).
Why it is important to do this review
Although platinum‐induced hearing loss is not life‐threatening, loss of hearing, especially during the first three years of life and even when only borderline to mild, can have important implications. It can negatively impact speech and language development, which may lead to difficulties with school performance and psychosocial functioning (Gregg 2004; Skinner 2004; Dean 2008). This is even more true for children who suffer dual sensory loss, such as retinoblastoma or optic pathway glioma patients.
Prevention of platinum‐induced hearing loss is, thus, very important and might improve the quality of life of childhood cancer patients and survivors treated with platinum‐based therapy. A recent systematic review has shown that at present no definitive conclusions on benefits and harms of the use of medical interventions to prevent the occurrence of platinum‐induced ototoxicity, such as amifostine and sodium thiosulphate, can be made (Van As 2012; Van As 2014a; Van As 2016b; Van As 2019). Therefore, it is important to identify other options, such as different platinum infusion durations.
This is the third update of the first systematic review evaluating all evidence on the use of different platinum infusion durations for the prevention of platinum‐induced hearing loss in children with cancer (Van As 2014b; Van As 2016c; Van As 2018).
Objectives
To assess the effects of different durations of platinum infusion to prevent hearing loss or tinnitus, or both, in children with cancer. Secondary objectives were to assess possible effects of these infusion durations on: a) anti‐tumour efficacy of platinum‐based therapy, b) adverse effects other than hearing loss or tinnitus, and c) quality of life.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or controlled clinical trials (CCTs).
Types of participants
Children (aged 0 to 18 years at diagnosis) with any type of childhood malignancy treated with a platinum analogue.
Studies including both children and adults were only eligible for inclusion in this review if the majority of participants were children (that is either more than 90% children or the maximal age of participants did not exceed 22 years).
Types of interventions
Platinum‐based therapy using one platinum infusion duration compared with the same platinum‐based therapy using another platinum infusion duration.
Only the platinum infusion duration could differ between the treatment groups; all other treatment, including type(s) of platinum analogue(s), the individual platinum dose, and radiotherapy to the head and neck should have been the same in both treatment groups. In the design of the study it should have been the intention to treat both groups with the same cumulative dose of cisplatin, carboplatin or oxaliplatin, or combination of these drugs.
Types of outcome measures
Outcomes listed here were not used as criteria for including studies, but are the outcomes of interest within studies identified for inclusion. Hearing loss and tinnitus were included irrespective of time of occurrence after platinum‐based therapy.
Primary outcomes
-
Hearing loss (as defined by the authors of the original study).
-
Tinnitus (as defined by the authors of the original study).
-
Overall survival (as defined by the authors of the original study).
Secondary outcomes
-
Event‐free survival (as defined by the authors of the original study).
-
Tumour response (complete and partial remission as defined by the authors of the original study).
-
Adverse effects (grade three or higher according to the criteria used by the authors of the original study), other than hearing loss and tinnitus.
-
Quality of life (as defined by the authors of the original study).
Search methods for identification of studies
We did not impose language restrictions. Cochrane Childhood Cancer ran the searches in CENTRAL, MEDLINE and Embase for the original version of the review and the first update, the clinical librarian at the medical library of the Academic Medical Center, Amsterdam, the Netherlands ran the searches in CENTRAL, MEDLINE and Embase for the second and third updates; all other searches were run by the review authors.
Electronic searches
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, on 14 November 2019), MEDLINE in PubMed (from 1945 to 14 November 2019) and Embase in Ovid (from 1980 to 14 November 2019).
The search strategies for the different electronic databases (using a combination of controlled vocabulary and text words) are shown in the appendices (Appendix 1; Appendix 2; Appendix 3).
Searching other resources
We located information about trials not registered in CENTRAL, MEDLINE or Embase, either published or unpublished, by searching the reference lists of included studies and review articles. We assessed the conference proceedings of the International Society for Paediatric Oncology (SIOP) (from 2009 up to and including 2019) and the American Society of Pediatric Hematology/Oncology (2014 up to and including 2019) (search strategies are shown in Appendix 4). We scanned ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch) for ongoing trials (both searched on 4 November 2019; search strategies are shown in Appendix 5).
Data collection and analysis
Selection of studies
After employing the search strategy described previously, two review authors independently identified studies meeting the inclusion criteria for this review. Discrepancies between authors were resolved by discussion. No third‐party arbitration was needed. We obtained in full any study which seemed to meet the inclusion criteria on the grounds of the title or abstract, or both, for closer inspection. We clearly stated details of the reasons for exclusion of any study considered for the review. We have included a flow chart of the selection of studies in the review (Figure 1). Had we identified multiple reports of one study, we would have collated the full‐text results.
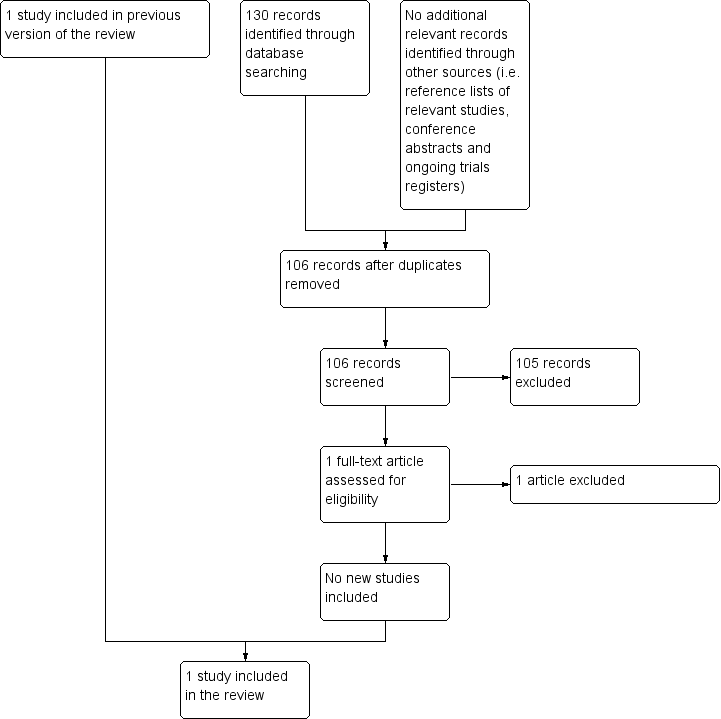
Flow diagram of selection of studies
Data extraction and management
Two review authors independently performed data extraction using standardised forms. We extracted data on the characteristics of participants (such as age, sex, type of malignancy, stage of disease, prior hearing loss, genetic variants and renal function at time of platinum treatment), interventions (such as information on the received antineoplastic treatment and possible other ototoxic drugs such as aminoglycosides and furosemide), outcome measures, length of follow‐up, details of funding sources and the declaration of interests for each included study. Discrepancies between authors were resolved by discussion. No third‐party arbitration was needed.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in included studies (that is selection bias, performance bias, detection bias (for each outcome separately), attrition bias (for each outcome separately), reporting bias and other bias). We used the 'Risk of bias' items and definitions of low risk, unclear risk and high risk as described in the module of Cochrane Childhood Cancer (Kremer 2016), which is based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Discrepancies between authors were resolved by discussion. No third‐party arbitration was needed. The risk of bias in included studies was taken into account in the interpretation of the review's results.
Measures of treatment effect
Dichotomous variables were analysed using risk ratios (RR). We presented all results with the corresponding 95% confidence intervals (CIs).
Dealing with missing data
During study selection no relevant data were missing. We attempted to contact the authors of Coze 1997 with regard to missing data for data extraction and 'Risk of bias' assessment, but unfortunately we did not receive a response. We extracted data by the allocated intervention, irrespective of compliance, in order to allow an intention‐to‐treat analysis. If this was not possible, this was stated and an 'available data' analysis was performed.
Assessment of heterogeneity
Since only one study was included in the review, the assessment of heterogeneity (both by visual inspection of the forest plots and by a formal statistical test for heterogeneity, that is the I² statistic (Higgins 2011)) was not applicable.
Assessment of reporting biases
In addition to the evaluation of reporting bias, as described in the Assessment of risk of bias in included studies section, we planned to assess reporting bias by constructing a funnel plot. This is only really possible when there are at least 10 studies included in a meta‐analysis because otherwise the power of the tests is too low to distinguish chance from real asymmetry (Higgins 2011). Since only one study was included in the review, this was not possible.
Data synthesis
We entered data into Cochrane Review Manager 5 software (RevMan 2014) and undertook analyses according to the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included outcome measures only if it was the intention of the study to perform the necessary assessments in all randomised participants (that is, not only optional or only performed in some centres). When the results of a particular outcome measure were available for less than 50% of the participants of a study, due to the associated high risk of attrition bias, we did not report the results of this outcome measure. Since only one study was included in the review, pooling of results was not applicable; otherwise we would have pooled results only when treatment groups were comparable, including the definition of outcomes used. We summarised results descriptively. We used a fixed‐effect model throughout the review.
For each comparison we prepared a 'Summary of findings' table using the GRADEprofiler software (GRADEpro GDT), in which we presented the following outcomes: hearing loss, tinnitus, overall survival, event‐free survival, tumour response, adverse effects other than ototoxicity (grade three or higher) and quality of life. The quality of the evidence for each outcome (i.e. very low, low, moderate or high quality) was assessed independently by two review authors using the five GRADE considerations, i.e. study limitations, inconsistency, indirectness, imprecision and publication bias.
Subgroup analysis and investigation of heterogeneity
We planned to analyse data separately for children treated with cisplatin, carboplatin, oxaliplatin or combinations of these platinum analogues. However, all children included in the review were treated with cisplatin and as a result subgroup analyses were not possible.
Sensitivity analysis
Since only one study was included in the review, sensitivity analyses for 'Risk of bias' items (that is, excluding studies with a high risk of bias and studies for which the risk of bias was unclear and comparing the results of studies with a low risk of bias with the results of all available studies; sensitivity analyses would only have been performed if at least two studies remained in the analysis after exclusion of the studies with a high or unclear risk of bias) were not applicable.
Results
Description of studies
Results of the search
For the original review, running the searches in the electronic databases of CENTRAL, MEDLINE (PubMed) and Embase (Ovid) (4 December 2013) yielded a total of 681 references. Following initial screening of the titles, abstracts, or both, we excluded 678 references which clearly did not meet all criteria required for considering studies for this review. The three remaining references were assessed in full, of which one fulfilled all the criteria for considering studies for this review and was thus eligible for inclusion. The other two references were excluded for the reasons described in the Characteristics of excluded studies table. Scanning the reference lists of the included article and reviews, conference proceedings and ongoing trials databases did not identify any additional eligible (ongoing) studies.
For the first update, running the searches in the electronic databases of CENTRAL, MEDLINE (PubMed) and Embase (Ovid) (18 May 2016) yielded a total of 90 references after duplicates were removed. Following initial screening of the titles, abstracts, or both, we excluded all 90 references as they clearly did not meet all criteria required for considering studies for this review. Scanning the reference lists of relevant studies, conference proceedings and ongoing trials databases did not identify any additional eligible (ongoing) studies.
For the second update, running the searches in the electronic databases of CENTRAL, MEDLINE (PubMed) and Embase (Ovid) (15 March 2018) yielded a total of 88 references after duplicates were removed. Following initial screening of the titles, abstracts, or both, we excluded all 88 references as they clearly did not meet all criteria required for considering studies for this review. Scanning the reference lists of relevant studies, conference proceedings and ongoing trials databases did not identify any additional eligible (ongoing) studies.
For the third update, running the searches in the electronic databases of CENTRAL, MEDLINE (PubMed) and Embase (Ovid) (14 November 2019) yielded a total of 106 references after duplicates were removed. Following initial screening of the titles, abstracts, or both, we excluded 105 references as they clearly did not meet all criteria required for considering studies for this review. One reference (Biswar 2017) was assessed in full and was excluded for reasons described in the Characteristics of excluded studies table. Scanning the reference lists of relevant studies, conference proceedings and ongoing trials databases did not identify any additional eligible (ongoing) studies.
In summary, the total number of included studies was one. No ongoing studies were identified. See Figure 1 for a flow diagram of the selection of studies for this systematic review.
Included studies
Characteristics of the included study are summarised below. For more detailed information see the Characteristics of included studies table.
We identified one randomised controlled trial (RCT) evaluating a continuous cisplatin infusion versus a one‐hour bolus cisplatin infusion (Coze 1997). For the continuous infusion, cisplatin was administered on days one to five of the cycle, but it is unclear if the infusion duration was thus five days. The total number of randomised children was 91; 43 were randomised to the continuous infusion group and 48 to the bolus infusion group. Please note that this randomisation was part of a larger study; the total number of eligible participants was unclear. All participants had a newly diagnosed neuroblastoma stage four and were aged over one year at diagnosis. For detailed information on treatment see the Characteristics of included studies table. Treatment other than induction therapy was not included in the manuscript. Regarding other ototoxic drugs, participants received anthracyclines (i.e. doxorubicin) and vincristine; it was not stated if participants received gentamycin or furosemide. It was also unclear if participants had prior hearing dysfunction; at least some of the participants had pre‐treatment renal impairment. Participants did not receive prior platinum treatment, prior radiotherapy to the head and neck region or prior cranial surgery. Genetic variants of platinum ototoxicity were not reported. The length of follow‐up was not mentioned. Only results from shortly after induction therapy were provided. The influence of the funder and the declarations of interest of the authors were not reported.
Risk of bias in included studies
See the 'Risk of bias' section of the Characteristics of included studies table and Figure 2 for the exact scores and the support for the judgements made.
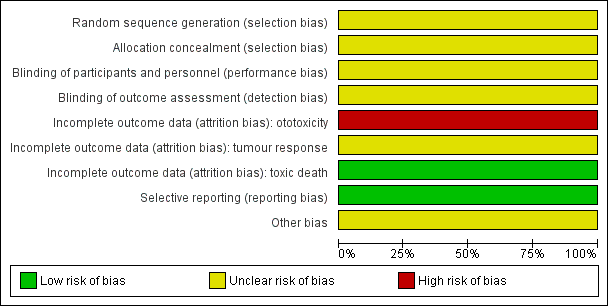
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
For evaluating selection bias we assessed the random sequence generation and the allocation concealment. Both of these items, and thus the risk of selection bias, were unclear.
Blinding
For evaluating performance bias we assessed the blinding of participants and personnel. The study did not provide information on blinding of participants and personnel, but since children in both treatment groups received their platinum therapy with different infusion durations this was most likely not the case. However, we judged the risk of performance bias as unclear.
For evaluating detection bias we assessed the blinding of outcome assessors for all separate outcomes. For all reported outcomes, that is ototoxicity, tumour response and adverse effects (toxic death), no information on blinding of outcome assessors was provided and the risk of detection bias was thus unclear.
Incomplete outcome data
For evaluating attrition bias we assessed incomplete outcome data for all separate outcomes. The risk of attrition bias was high for ototoxicity, unclear for tumour response and low for adverse effects (toxic death).
Selective reporting
For evaluating reporting bias we assessed selective reporting. There was no study protocol mentioned in the manuscript (and we did not separately search for it), but all expected outcomes were reported taking into consideration the fact that only short‐term outcomes following induction therapy were reported. We judged the risk of reporting bias to be low.
Other potential sources of bias
For evaluating other potential sources of bias we assessed the following items: block randomisation in unblinded trials, baseline imbalance between treatment groups related to outcome (prior ototoxic treatment, age, sex, prior hearing loss), difference in ototoxic drugs other than platinum analogues between treatment groups (furosemide, gentamycin, anthracyclines, vincristine), difference in cumulative platinum dose between treatment groups, difference in length of follow‐up between treatment groups, difference in impaired renal function at time of platinum treatment between treatment groups, and if an insensitive instrument was used to evaluate ototoxicity. All these items, and thus the risk of other bias, were unclear. For a more detailed description of all different items see the risk of bias section of the Characteristics of included studies table.
Effects of interventions
Coze 1997 did not allow data extraction for all outcome measures (see the Characteristics of included studies table for a more detailed description of the extractable outcome measures).
Hearing loss
Coze 1997 provided data on hearing loss (see Table 1 for the used definitions; based on the available information we were not able to distinguish between asymptomatic and symptomatic hearing loss).
| Grade | Description |
| A | None: bilateral hearing loss, less than 40 dB in all frequencies |
| B | Mild: bilateral hearing loss, greater than 40 dB at 8000 Hz |
| C | Moderate: bilateral hearing loss, greater than 40 dB at 6000 Hz |
| D | Marked: bilateral hearing loss, greater than 40 dB at 4000 Hz |
| E | Severe: bilateral hearing loss, greater than 40 dB at 2000 Hz |
* As reported in the methods section of the included study (Coze 1997). Coze and colleagues refer to Brock 1987 for these criteria. However, Brock and colleagues define grade E as "Severe, bilateral hearing loss greater than 40 dB at 8000 Hz" (so identical to grade B).
dB: decibel; Hz: Hertz
For 24 of the 91 children no data on hearing loss were available (12 in each treatment group). The 'available data' analysis of asymptomatic and symptomatic hearing loss (that is grade B and higher) showed no clear evidence of a difference between the treatment groups but results were imprecise (risk ratio (RR) 1.39, 95% confidence interval (CI) 0.47 to 4.13; P = 0.55; see Figure 3; low quality of evidence; see summary of findings Table for the main comparison). There were six cases among the 31 available children in the continuous infusion group and five cases among the 36 available children in the bolus infusion group. Intention‐to‐treat analyses (data not shown) also showed no clear evidence of a difference between the treatment groups: the RR for the worst case scenario (that is 18 cases among the 43 children in the continuous infusion group and 17 cases among the 48 children in the bolus infusion group) was 1.18 (95% CI 0.70 to 1.99; P = 0.53; low quality of evidence), while the RR for the best case scenario (that is 6 cases among the 43 children in the continuous infusion group and 5 cases among the 48 children in the bolus infusion group) was 1.34 (95% CI 0.44 to 4.08; P = 0.61; low quality of evidence).
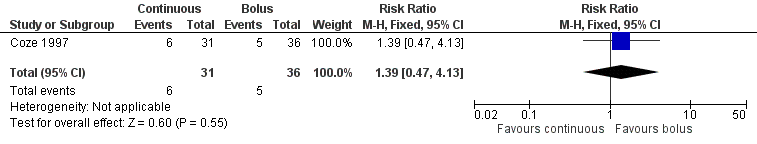
Forest plot of comparison: 1 Continuous platinum infusion versus bolus platinum infusion, outcome: 1.1 Hearing loss (asymptomatic and symptomatic disease).
Tinnitus
No information on tinnitus was provided.
Overall survival
No information on overall survival was provided.
Event‐free survival
No information on event‐free survival was provided.
Tumour response
The number of children with a complete or partial remission (according to International Neuroblastoma Response Criteria) was not provided, but it was stated that tumour response was equivalent in both treatment arms (low quality of evidence: see summary of findings Table for the main comparison). However, it was not mentioned in how many children this outcome was assessed.
Adverse effects (grade three or higher) other than hearing loss and tinnitus
In the methods section of Coze 1997, it was stated that toxicities were graded according to the World Health Organization (WHO) criteria (WHO 1979). However, the information reported in the results section was not completely in accordance with the WHO criteria, thus making grading impossible. We did not receive clarification from the authors and as a result we were only able to include results on toxic death (death is always higher than grade three; for the other reported adverse effects grading was not possible).
The analysis of toxic death (no definition provided) showed no clear evidence of a difference between the treatment groups, but results were imprecise (RR 1.12, 95% CI 0.07 to 17.31; P = 0.94; see Figure 4; low quality of evidence; see summary of findings Table for the main comparison). There was one toxic death among the 43 children in the continuous infusion group and one toxic death among the 48 children in the bolus infusion group.

Forest plot of comparison: 1 Continuous platinum infusion versus bolus platinum infusion, outcome: 1.2 Adverse effects: toxic death.
Quality of life
No information on quality of life was provided.
Discussion
Summary of main results
Platinum‐based therapy is used to treat a variety of paediatric malignancies. Unfortunately, one of the most important adverse effects is the occurrence of hearing loss or ototoxicity (McHaney 1983). Although it is not life‐threatening, loss of hearing can have important implications, for example difficulties with school performance and psychosocial functioning (Gregg 2004; Skinner 2004; Dean 2008). Thus, prevention of platinum‐induced hearing loss is very important. This is the third update of the first systematic review evaluating all evidence on the use of different platinum infusion durations for the prevention of platinum‐induced hearing loss in children with cancer (Van As 2014b; Van As 2016c; Van As 2018).
To adequately ascertain the efficacy of different platinum infusion durations, the best study design — provided that the design and execution are correct — is a randomised controlled trial (an RCT) in which the only difference between the intervention and control group is the platinum infusion duration. Controlled clinical trials (CCTs) can also provide reliable information, keeping in mind their limitations, but other study designs (including historical control groups) were not eligible for this review due to the high risk of bias associated with such designs.
We identified one RCT evaluating the use of a continuous cisplatin infusion versus a one‐hour bolus cisplatin infusion in children with newly diagnosed neuroblastoma; in the updates no new eligible studies were identified. For the continuous infusion, cisplatin was administered on days one to five of the cycle, but it is unclear if the infusion duration was a total of five days. The total number of randomised children was 91; 43 were randomised to the continuous infusion group and 48 to the bolus infusion group. This randomisation was part of a larger study; the total number of eligible participants was unclear.
No clear evidence of a difference in hearing loss (asymptomatic and symptomatic disease combined) between the different infusion durations was identified. An important question regarding any possible otoprotective measure during platinum treatment is whether it could decrease the ototoxicity of platinum agents without reducing the anti‐tumour efficacy (that is tumour response and survival) and without negative effects on other toxicities or quality of life. The number of children with a complete or partial remission was not provided and it was unclear in how many children this outcome was assessed. However, it was stated that tumour response was equivalent in both treatment arms. With regard to adverse effects of grade three or higher, other than ototoxicity, we were only able to assess toxic deaths. Again, no clear evidence of a difference between the treatment groups was identified. No data were available for the other outcomes of interest (i.e. tinnitus, overall survival, event‐free survival and quality of life) (see summary of findings Table for the main comparison).
For other (combinations of) infusion durations and other platinum analogues, no eligible studies were identified.
Overall completeness and applicability of evidence
'No evidence of effect', as identified in this review, is not the same as 'evidence of no effect'. The reason that no clear evidence of a difference between treatment groups was identified could be the fact that the number of children included in this study was too small to detect a difference (that is low power). Also, hearing loss not only develops during platinum‐based therapy but also years after completion of the therapy (Bertolini 2004; Knight 2005), so the length of follow‐up could have been too short to detect a difference between the treatment groups since only results from shortly after induction therapy were provided.
It was stated that participants received a continuous cisplatin infusion and that cisplatin was administered on days one to five of the cycle, but it is unclear if the infusion duration was thus five days. No further information on the exact infusion duration was provided, making it impossible to use the results of this study in clinical practice. Also, the study did not provide a description of the exact test that was used to evaluate hearing loss so we cannot comment on its appropriateness (for example, if age‐specific tests were used or if children were checked for otitis media, common in this age group (Brock 1991)). Furthermore, the study was executed between 1987 and 1992. Since then, supportive care and anti‐cancer treatments have improved substantially and the applicability of its results to current clinical practice is unclear. Finally, data were not available for all outcomes of interest. As a result we cannot draw conclusions regarding those outcomes, but they are of course important for clinical practice.
Quality of the evidence
The quality of evidence was low for all evaluated outcomes; we downgraded one level each for both study limitations and imprecision. However, at this time this is the best available evidence based on RCTs and CCTs evaluating different platinum infusion durations in children with cancer.
It should be noted that although children in both treatment groups should have received the same platinum dosage schedule, the included study did not report the exact cumulative platinum dose received. If children in the bolus infusion group received a higher cumulative platinum dose than those treated with a continuous infusion, this could have led to an overestimation of the otoprotective effect of the continuous infusion (and vice versa). This uncertainty should also be kept in mind when interpreting the results of the other outcomes (response rate and adverse effects). The same is true for prior hearing loss, impaired renal function at the time of platinum treatment and the use of other ototoxic drugs like aminoglycosides (anthracyclines, gentamycin), vincristine and furosemide (Gallagher 1979; Skinner 2004; Jenney 2005; Meyer 2009). It was not clear if there were important imbalances between the treatment groups regarding these factors.
Potential biases in the review process
This systematic review used a very broad search strategy for identifying eligible studies. Thus, although it is unlikely that eligible studies were missed, it is never possible to completely rule out reporting bias. The search strategy included search terms for ototoxicity and as a result it is possible that for outcomes other than hearing loss and tinnitus more studies are available than the one identified in this review.
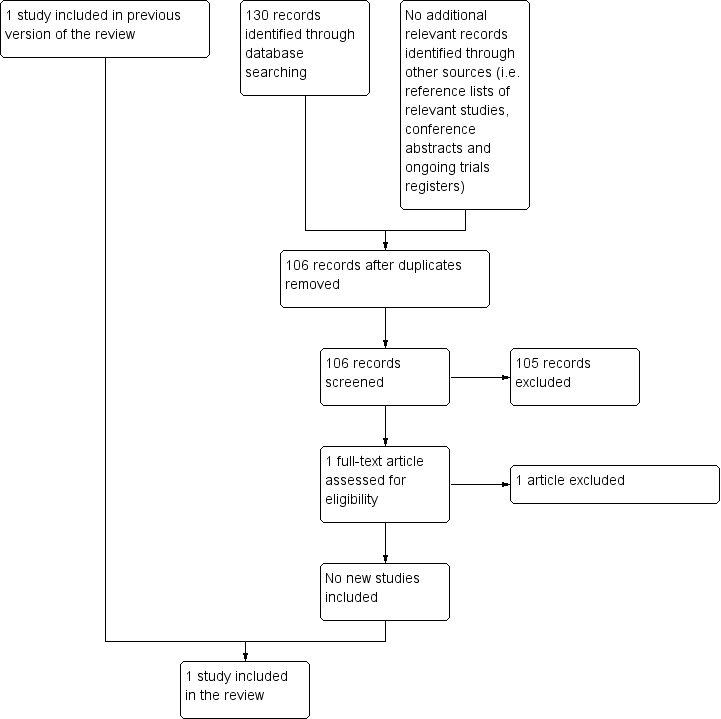
Flow diagram of selection of studies

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Continuous platinum infusion versus bolus platinum infusion, outcome: 1.1 Hearing loss (asymptomatic and symptomatic disease).

Forest plot of comparison: 1 Continuous platinum infusion versus bolus platinum infusion, outcome: 1.2 Adverse effects: toxic death.

Comparison 1 Continuous platinum infusion versus bolus platinum infusion, Outcome 1 Hearing loss (asymptomatic and symptomatic disease).

Comparison 1 Continuous platinum infusion versus bolus platinum infusion, Outcome 2 Adverse effects: toxic death.
| Continuous platinum infusion compared to bolus platinum infusion for children with cancer treated with platinum‐based therapy | ||||||
| Patient or population: children with cancer treated with platinum‐based therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Bolus platinum infusion | Continuous platinum infusion | |||||
| Hearing loss (asymptomatic and symptomatic disease according to Brock criteria) | 139 per 10001 | 193 per 1000 | RR 1.39 | 67 | ⊕⊕⊝⊝ | Length of follow‐up was not mentioned, but the median duration of induction was 102 days in the continuous infusion arm and 107 days in the bolus infusion arm (no significant difference) and only results from shortly after induction therapy were provided. For 24 of the 91 children included in the study no data on hearing loss were available (12 in each treatment group). The RR presented here results from the 'available data' analysis. Intention‐to‐treat analyses (i.e. worst‐ and best‐case scenarios) also showed no significant difference between the treatment groups. The GRADE assessment for the worst‐ and best‐case scenarios was identical to that of the 'available data' analysis. |
| Tinnitus ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on tinnitus was provided. |
| Overall survival ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on overall survival was provided. |
| Event‐free survival ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on event‐free survival was provided. |
| Tumour response (complete or partial remission according to INRC) | See comment | See comment | Not estimable (see comments) | Unclear | ⊕⊕⊝⊝ | The number of children with a complete or partial remission was not provided, but it was stated that tumour response was equivalent in both treatment arms. Length of follow‐up was not mentioned, but the median duration of induction was 102 days in the continuous infusion arm and 107 days in the bolus infusion arm (no significant difference) and only results from shortly after induction therapy were provided. |
| Adverse effects: toxic death (no definition provided) | 21 per 10001 | 23 per 1000 | RR 1.12 | 91 | ⊕⊕⊝⊝ | Length of follow‐up was not mentioned, but the median duration of induction was 102 days in the continuous infusion arm and 107 days in the bolus infusion arm (no significant difference) and only results from shortly after induction therapy were provided. |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No information on quality of life was provided. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The assumed risk is based on the prevalence in the control group of the included study. 4 We downgraded 1 level based on the fact that the presence of selection bias, performance bias, detection bias, attrition bias and other bias is unclear. 5 We downgraded 1 level based on the fact that the presence of selection bias, performance bias, detection bias and other bias is unclear. | ||||||
| Grade | Description |
| A | None: bilateral hearing loss, less than 40 dB in all frequencies |
| B | Mild: bilateral hearing loss, greater than 40 dB at 8000 Hz |
| C | Moderate: bilateral hearing loss, greater than 40 dB at 6000 Hz |
| D | Marked: bilateral hearing loss, greater than 40 dB at 4000 Hz |
| E | Severe: bilateral hearing loss, greater than 40 dB at 2000 Hz |
| * As reported in the methods section of the included study (Coze 1997). Coze and colleagues refer to Brock 1987 for these criteria. However, Brock and colleagues define grade E as "Severe, bilateral hearing loss greater than 40 dB at 8000 Hz" (so identical to grade B). dB: decibel; Hz: Hertz | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hearing loss (asymptomatic and symptomatic disease) Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.47, 4.13] |
| 2 Adverse effects: toxic death Show forest plot | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.07, 17.31] |

