Liječenje žena koje nakon porođaja imaju anemiju zbog nedostatka željeza
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centre, double‐blind, randomised controlled trial involving iron deficient anaemic mothers and a non‐anaemic control group. Per protocol analysis. Follow‐up was 9 months postpartum. | |
| Participants | 500 puerperal women were screened, and 95 were included. South African population with low socioeconomic status. 21 women were non‐anaemic (control group). 64 anaemic women were randomised to 2 groups: Inclusion criteria: Hb 90 ‐ 115 g/L, and at least 2 of the following: MCV < 80 fL, TSAT < 15%, serum ferritin < 12 µg/L. Age between 18 and 30 years, primary caregivers, breastfeeding for the duration of the study, no chronic diseases, and healthy by physical health screen. Gestation age > 38 weeks, birthweight > 2500 g, no hospitalisation during the neonatal period, and Apgar scores consistent with normal intrauterine growth and development. Exclusion criteria: Hb < 90 g/L. | |
| Interventions | Intervention: oral ferrous sulphate 125 mg, oral vitamin C 25 mg and 10 μg folic acid daily for 6 months, starting at inclusion 6‐8 weeks postpartum. Total non‐elemental iron dose ≈ 22,500 mg. Comparator: oral vitamin C 25 mg and 10 μg folic acid daily for 6 months. | |
| Outcomes | Aim was to determine the association between mothers with postpartum iron deficiency anaemia and behavioural changes and present the data on the effect of maternal iron deficiency anaemia on maternal emotions and cognition. Specific preplanned outcome measures were not described. Reported outcomes: Scores on EPDS, STAI, Perceived Stress, Raven’s test, and Digit Symbol. | |
| Notes | Source of funding: The ILSI Foundation. We only analysed the anaemic women, as per our inclusion criteria. The authors did not respond to the request on additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method described. |
| Allocation concealment (selection bias) | Low risk | One person who was aware of the code did the allocation. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study was described as double‐blind. However, it was not clearly described who was blinded. Also, it was unclear whether there was an actual placebo pill, or perhaps if all of the treatment components were dosed in 1 tablet. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Same as for performance bias. If the patients were able to guess their treatment group, this could have influenced the subjective scales used as outcome measures for psychological well being. |
| Incomplete outcome data (attrition bias) | High risk | High dropout rate, twice as many in placebo group. Reasons for dropout not described. |
| Selective reporting (reporting bias) | High risk | Intended aim investigated and reported. However, adverse events and maternal mortality were not reported. |
| Other bias | Low risk | None known. |
| Methods | Single‐centre open‐label randomised controlled trial. Per protocol analysis. Follow‐up period was 40 days. | |
| Participants | 44 puerperal anaemic women from the United Kingdom (socioeconomic conditions not described), were randomised to 2 groups of 22. 1 woman from the comparator group dropped out due to secondary PPH. Inclusion criteria: age > 18 years, Hb < 90 g/L. | |
| Interventions | Intervention: IV ferrous sucrose 200 mg (Venofer®) on day 2 and 4 postpartum. Total dose IV iron was 400 mg. Comparator: oral ferrous sulphate 200 mg twice daily for 42 days. Total dose non‐elemental iron was 16,800 mg. | |
| Outcomes | Preplanned outcomes were laboratory values. Compliance to treatment and adverse events during treatment were reported. | |
| Notes | Source of funding was not stated. Authors did not respond to the request on additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes were prepared and marked with a sequential numerical code by an independent person. After obtaining consent, the next consecutive envelope was opened by the recruiter, who was blinded to the envelope preparation. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 patient dropped out. However, no report on number of screened and excluded patients prior to randomisation. |
| Selective reporting (reporting bias) | High risk | Intended outcome measures reported. However, maternal mortality was not reported. |
| Other bias | Low risk | None known. |
| Methods | Single‐centre, open‐label, randomised, controlled trial conducted in Switzerland. ITT analysis. Follow‐up was 6 weeks. | |
| Participants | 90 anaemic puerperal women were randomised into 3 groups of 30. Socioeconomic conditions were not described. Inclusion criteria: Hb < 100 g/L 48 to 72 hours after delivery, normal cardiac and renal function, oral iron substitution during pregnancy. Exclusion criteria: anaemia during pregnancy, peripartum infection, peripartum blood transfusion, haematological disease, previous myelosuppressive medication, history of thromboembolism, haemosiderosis, iron intolerance, or rheumatoid polyarthritis. | |
| Interventions | Intervention referred to rhEPO (intravenously in group 3, subcutaneously in group 2). Group 1 (no EPO): IV ferric carboxymaltose (Ferrum Hausmann®) 100 mg single dose + oral combined tablet containing iron sulphate 160 mg elemental iron and 0.7 mg folic acid daily for 42 days. Total elemental iron dose was 6820 mg (non‐elemental iron dose unknown). Group 2: SC rhEPO (Eprex®) 300 U/kg as a single dose + IV ferric carboxymaltose (Ferrum Hausmann®) 100 mg single dose + oral combined tablet containing iron sulphate 160 mg elemental iron and 0.7 mg folic acid daily for 42 days. Total elemental iron dose was 6820 mg. Total rhEPO dose depended on weight, approximately 20,000 for a person weighing 70 kg. Group 3: IV rhEPO (Eprex®) 300 U/kg as a single dose + IV ferric carboxymaltose (Ferrum Hausmann®) 100 mg single dose + oral combined tablet containing iron sulphate 160 mg elemental iron and 0.7 mg folic acid daily for 42 days. Total elemental iron dose was 6820 mg. Total rhEPO dose depended on weight, approximately 20,000 for a person weighing 70 kg. Treatment was started from 48 to 72 hours after delivery. | |
| Outcomes | No preplanned outcome measures stated. Adverse events during treatment were reported. | |
| Notes | Source of funding was not stated. Adverse events of iron infusion were reported for the 3 groups combined. Authors did not provide additional information on request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method described. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label ‐ no EPO placebo. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not stated whether any women dropped out. The number of screened and excluded patients prior to randomisation was not reported. |
| Selective reporting (reporting bias) | High risk | No preplanned outcome measures stated. Maternal mortality was not reported. |
| Other bias | Low risk | None known. |
| Methods | Single‐centre, randomised, controlled trial, conducted in Switzerland. ITT analysis. Follow‐up was 14 days. | |
| Participants | 60 anaemic puerperal women (socioeconomic conditions not described) randomised into 3 groups of 20. No women dropped out. Inclusion criteria: postpartum Hb < 100 g/L 24 to 72 hours postpartum. Exclusion criteria: anaemia during pregnancy peripartum blood transfusion, anaemia from causes other than blood loss, history of thromboembolism, signs of infection, history of seizures, alcohol and/or drug abuse, renal or hepatic dysfunction, previous myelosuppressive medication, haemosiderosis, history of iron intolerance, and rheumatoid polyarthritis. | |
| Interventions | Intervention referred to rhEPO (group 1). Group 3: oral iron sulphate (Gynotardiferon ®, Robapharm) containing 80 mg elemental iron and folic acid 0.35 mg daily for 14 days. Total elemental iron dose was 1120 mg. | |
| Outcomes | No preplanned outcome measures. Incidence and severity of serious or unusual adverse events were recorded. Information on maternal mortality was extrapolated from the numbers of blood tests. | |
| Notes | Financial support from the University of Zurich. Authors did not provide additional information on request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method described. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Low risk for groups 1 and 2 (EPO and placebo), where the patients appear to have been blinded to what drug they received, but high in oral group. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Oral group was not blinded. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts. However, the number of screened and excluded patients prior to randomisation was not reported. |
| Selective reporting (reporting bias) | Unclear risk | No preplanned outcome measures mentioned. Data on adverse events were not group specific. |
| Other bias | Low risk | None known. |
| Methods | Multicentre, open‐label, randomised, controlled trial. Trial was conducted from June 2004 to August 2005 in 20 centres in Poland, Romania and Russia. Randomisation ratio was 2:1, stratified by country and severity of anaemia. Efficacy analyses was both ITT and per protocol. Follow‐up was 12 weeks. | |
| Participants | 824 puerperal anaemic women were screened, 349 were randomised: 118 women to the comparator group, where 117 represented the ITT group and 89 represented the per protocol group. Socioeconomic conditions were not described. Inclusion criteria: Hb < 105 g/L. | |
| Interventions | Intervention referred to IV ferric carboxymaltose. Intervention: IV infusion of ferric carboxymaltose (Ferinject®) at a maximum dose of 1000 mg iron over 15 min (15 mg iron/kg body weight if body weight < 66 kg) on day 1, with subsequent doses at 1‐week intervals until each patient's calculated total iron requirement was reached (up to 3 weekly infusions). Patients' total iron requirement was calculated using the modified formula of Ganzoni. Comparator: oral ferrous sulphate (Plastufer®) 100 mg twice daily for 12 weeks. Total non‐elemental iron dose was 16,800 mg. Treatment was initiated within 7 days postpartum. | |
| Outcomes | Preplanned outcome measures were laboratory values, and safety of the mother and child. Infections and compliance to treatment were reported. | |
| Notes | This study was supported by an unrestricted scientific grant from Vifor International Inc., Switzerland. Authors did not provide additional information on request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised 2:1 ratio, method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | High risk | Unknown reason for consent withdrawal and high dropout rate may have led to selection in the population. |
| Selective reporting (reporting bias) | High risk | Intended outcomes reported. However, maternal mortality was not reported. |
| Other bias | Low risk | None known. |
| Methods | Single‐centre, open‐label, randomised, controlled trial, conducted in Australia from 2009 to 2010. Per protocol analyses. Follow‐up was 6 weeks. | |
| Participants | Both pregnant and puerperal anaemic women. Originally 5950 women were screened. Of the postpartum population 90 women were randomised into 2 groups: 37 to the intervention group, where 31 completed the trial; 53 to the comparator group, where 43 completed the trial. This population came from a very low socioeconomic background (unemployment, teenage pregnancies, (qualitative) nutritional deficiencies, migrants from the Asian Pacific region, Africa and the subcontinent). Inclusion: Hb < 110 g/L and ferritin < 12 μg/L either antepartum or within 72 hours postpartum, following caesarian section and vaginal delivery with at blood loss > 500 mL. | |
| Interventions | Intervention referred to IV iron sucrose (Venofer®). Intervention group (n = 31): IV iron sucrose 200 mg given twice with a minimum of 24 hours apart + oral folic acid 600 μg daily for 42 days. Total iron dose was 400 mg. Treatment was initiated between days 1 and 3 postpartum. | |
| Outcomes | The aim was to determine if treatment could decrease the incidence of severe anaemia, and to measure if an increase in haematological indices was associated with a reduction in the rate of blood transfusions and associated complications, as well as improvement in maternal and neonatal outcomes. Severe adverse events were reported. | |
| Notes | The source of funding was not stated. The authors declare no conflict of interest. The author of this trial provided additional information on request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was done through a telephone service. |
| Allocation concealment (selection bias) | Low risk | Allocation was conducted by a third party who was blinded to patient data. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | High risk | The proportion of dropouts is similar in the 2 groups. However, the numbers are high: 16.2% and 18.9%. The reason for dropout is lost to follow‐up and decline of further participation. |
| Selective reporting (reporting bias) | High risk | Preplanned aim was investigated. However, mild to moderate adverse events and maternal mortality were not reported. |
| Other bias | Low risk | None known. |
| Methods | Single‐centre, comparative, open‐label, randomised trial, conducted in Spain between March 1 and May 31, 2008. ITT analysis. Follow‐up period was 6 weeks. | |
| Participants | 180 puerperal women were screened. 13 women were randomised into 2 groups: Socioeconomic conditions were not described. Inclusion criteria: age > 18 years, Hb 70 to 100 g/L, and ferritin > 15 µg/L at 24 hours postpartum. Exclusion criteria: iron intolerance, anaemia not caused by iron deficiency, peripartum blood transfusion, severe asthma and atopy, thromboembolism, alcohol or drug abuse, hepatitis B, C or HIV, infection, renal or hepatic dysfunction, no consent. | |
| Interventions | Intervention referred to IV iron sucrose (Venofer ®). Intervention group A: IV Iron sucrose (Venofer ®) 200 mg on day 2 and 4 after delivery. Total iron dose was 400 mg. Comparator group B: oral ferrous sulphate (Tardyferon®) containing 200 mg twice daily before meals for 42 days. Total dose of non‐elemental iron was 16,800 mg. | |
| Outcomes | No preplanned outcomes. The aim of the study was to compare efficacy and safety between IV and oral iron treatment. Maternal mortality, infections, compliance to treatment and adverse events during treatment were registered. | |
| Notes | Source of funding not stated. Authors declare no conflict of interest. Trial authors provided unpublished information and corrections to the published text on request. They reported an error in table 2 on page 193, where the values for group A and B are reversed. Table 3 is correct. The article is published in Spanish. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | System based on random distribution of envelopes in 1:1 ratio. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 out of 7 in the comparator group dropped out. Although, the authors state that dropout was unrelated to treatment, it is not specified what the exact reasons were. It is therefore not possible to assess if the dropouts were in fact not related to the trial. |
| Selective reporting (reporting bias) | Unclear risk | No preplanned outcome measures stated. |
| Other bias | Low risk | None known. |
| Methods | Single‐centre, open‐label, randomised (block randomisation), controlled trial conducted in India. Per protocol analyses. Follow‐up was 14 days. | |
| Participants | 46 women with postpartum anaemia were randomised into 2 groups: 23 to the intervention group where 21 completed per protocol; 23 to the comparator group where 20 completed per protocol. Socioeconomic conditions were not described. Inclusion criteria: age > 18 years, Hb < 80 g/L within 48 hours postpartum. Exclusion criteria: placenta previa, placental abruption, preeclampsia, clotting disorders, and peripartum blood transfusion. | |
| Interventions | Intervention referred to IV iron sucrose. Intervention: IV iron sucrose 300–600 mg divided into 3 doses every alternate day for 3 days. Total iron dose was individually calculated. Comparator: oral ferrous fumarate 300 mg daily for 14 days. Each dose contained 99 mg elemental iron. Total elemental iron dose was 1386 mg. Total non‐elemental iron dose was 4200 mg. Treatment was initiated between 24 and 48 hours after delivery. | |
| Outcomes | No preplanned outcomes stated. The objective was to compare effectiveness of IV iron sucrose vs oral ferrous fumarate in postpartum anaemia. Adverse events were reported. | |
| Notes | Source of funding was not stated. Trial authors did not respond to our request for further details. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Low dropout rate. However, reason for dropout not known and number of screened and excluded patients prior to randomisation was not reported. |
| Selective reporting (reporting bias) | High risk | No preplanned outcome measures stated. Maternal mortality was not reported. |
| Other bias | Low risk | None known. |
| Methods | Single‐centre, open‐label, randomised trial conducted in Switzerland. ITT analysis. Follow‐up was 15 days. | |
| Participants | 40 severely anaemic puerperal women were randomised 1:1 to 2 groups. There were no dropouts. Socioeconomic conditions were not described. Inclusion criteria: prepartal Hb > 100 g/L, followed by severe postpartum anaemia, defined by a Hb < 85 g/L 24 to 48 hours after delivery. Exclusion criteria: haematological, chronic inflammatory or malignant disease, cardiac or renal dysfunction, haemosiderosis, history of iron intolerance, peripartum blood transfusion. | |
| Interventions | Intervention referred to EPO. Group 1 (Comparator): IV iron sucrose (Venofer®) 200 mg for 4 days. Total iron dose was 800 mg. Group 2 (Intervention): IV rhEPO (Eprex®) 10,000 U for 4 days + IV iron sucrose (Venofer®) for 4 days. Treatment started at the day of delivery. | |
| Outcomes | Preplanned outcome measures: secondary: laboratory values and identification of subgroups which benefit from additional rhEPO treatment. Maternal mortality was extrapolated as lack of dropouts. Infections, compliance to treatment, breastfeeding and adverse events during treatment were also reported. | |
| Notes | Source of funding was not stated. Trial authors provided unpublished information on request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes containing numbers randomly allocated to 1 of the 2 groups. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | There were no dropouts. However, the number of screened and excluded patients prior to randomisation was not reported. |
| Selective reporting (reporting bias) | Low risk | Preplanned outcomes were reported. |
| Other bias | Low risk | None known. |
| Methods | Single‐centre, single‐blinded, randomised, controlled trial conducted in Germany. For each 3 women with similar parity, pre‐treatment Hb (± 0.3 g%) and age (± 3 years) 1 was allocated to each treatment group. Analysis of laboratory values was done per protocol, analyses on harms was ITT. Follow‐up was 30 days. | |
| Participants | 101 puerperal women were randomised to 3 groups: 34 to intervention group S, 32 completed trial; | |
| Interventions | Intervention referred to oral iron. Group S: oral tablet Eryfer® containing 152 mg iron sulphate (elemental 50 mg), 222 mg ascorbic acid and 84 mg sodium bicarbonate twice daily for 30 days. Total non‐elemental iron dose was 9120 mg, total elemental iron dose was 3000 mg. Group K: oral tablet containing 324 mg ferrous sulphate (elemental iron 102 mg), 25 mg magnesium oxide and yeast extract containing vitamin B once daily for 30. Total non‐elemental iron dose was 9720 mg, total elemental iron dose was 3060 mg. Group L: oral tablet empty preparation containing 1 g of milk sugar twice daily for 30 days. | |
| Outcomes | No preplanned outcomes stated. Adverse events during treatment were reported. | |
| Notes | Source of funding was not stated. Hb values for inclusion in this study were not defined. In table 1 it is shown, that the mean Hb in all groups was < 120 g/L prior to treatment. The table also shows a value of ± s for each Hb measurement. Assuming that "s" is the standard deviation, pre‐treatment Hb plus 2 standard deviations exceeds the value of 120 g/L, which is criteria for this review. However, the population is small and not necessarily normally distributed. Thus, in theory the results can be skewed to the left and not contain any values above 120 g/L. Therefore we chose to include the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratification based on parity, pre‐treatment Hb (± 0.3 g%) and age (± 3 years). However, the method of this sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | No method described |
| Blinding of participants and personnel (performance bias) | High risk | The dose regiment for group K is different than that of group S and L. Thus, the blinding of the patients was inadequate. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | The blinding of the patients was inadequate. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Few dropouts. Reasons for dropout were reported. However, the number of screened and excluded patients prior to randomisation was not reported. |
| Selective reporting (reporting bias) | High risk | No preplanned outcomes measures stated. However, maternal mortality was not reported. |
| Other bias | Unclear risk | The Hb value as a criteria for inclusion was not stated, thus the study may include non‐anaemic women. |
| Methods | Single‐centre, double‐blind, randomised controlled trial conducted in 1992 in Germany. Per protocol analysis. Follow‐up was 4 weeks. | |
| Participants | 36 puerperal anaemic women were randomised to 2 groups: Socioeconomic conditions were not described. Inclusion criteria: Hb < 90 g/L on 2nd day postpartum. Anaemia caused by childbirth or pregnancy, healthy baby with gestational age of minimum 38 weeks. | |
| Interventions | Intervention referred to EPO. Intervention: IV rhEPO 20,000 IU single dose + IV iron 400 mg (Ferrum Hausmann®) single dose + oral iron 200 mg (Ferrum Hausman®) + folic acid 1 mg daily for 28 days (starting on second day). Total non‐elemental iron dose was 6000 mg. | |
| Outcomes | No preplanned outcome measures stated. Objective was to show if it is enough to use combined oral and IV iron therapy for a quick correction of anaemia, or if it is necessary to supplement with EPO. A brief comment on life quality (unsupported by data), and adverse events were stated. Maternal mortality was extrapolated as lack of lost to follow‐up. | |
| Notes | Authors did not respond to the request on additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method described. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Placebo‐controlled, double‐blinded, however not stated who exactly was blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is not stated how the personnel were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 dropout. However, number of screened and excluded patients prior to randomisation not reported. |
| Selective reporting (reporting bias) | High risk | No statement of preplanned outcomes. The authors comment briefly on quality of life but method is not adequately described and the evaluation was not supported by data. |
| Other bias | Low risk | None known. |
| Methods | Single‐centre randomised controlled trial, conducted in Greece. ITT analysis. Follow‐up was 40 days. | |
| Participants | 40 puerperal anaemic women were randomised into 2 groups of 20 on the first day following delivery. There were no dropouts. Socioeconomic conditions were not described. | |
| Interventions | Intervention referred to EPO. Intervention: SC injection rhEPO 200 IU/kg/day for 15 days, oral iron 200 mg/day for 40 days and folic acid 5 mg/day for 40 days. Total rhEPO dose varied according to weight (3000 IU/kg for a person weighing 70 kg). Total non‐elemental iron dose was 8000 mg. Comparator: oral iron 200 mg/day and folic acid 5 mg/day for 40 days. Total non‐elemental iron dose was 8000 mg. | |
| Outcomes | Preplanned outcomes were subjective symptoms, the ability to lactate and psychological well being. Length of hospital stay was reported. Maternal mortality was extrapolated as lack of dropouts. | |
| Notes | Source of funding was not stated. Improvement in psychological well being was a preplanned measure, however no results were reported. Many of the results were reported as medians. Authors did not respond to the request on additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method described. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial, no method of blinding described. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. However, low risk for maternal mortality, irrespective of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | There were no dropouts, small study. However, the number of screened patients prior to randomisation was not reported. |
| Selective reporting (reporting bias) | High risk | Psychological well being was planned to be investigated (mentioned in methods), but results are not reported, except an undocumented statement in discussion. |
| Other bias | Low risk | None known. |
| Methods | Multicentre, double‐blind, randomised, controlled trial conducted in 2 German centres from 1991 to 1992. Analysis appears to be per protocol. Follow‐up was 5 days. | |
| Participants | 90 puerperal women were selected, 71 were randomised: 35 to the intervention group and 36 to the placebo group. Socioeconomic conditions were not described. Inclusion criteria: Hb < 100 g/L. Exclusion criteria were not stated. | |
| Interventions | Intervention referred to EPO. Intervention: IV rhEPO (Eprex) 10,000 U twice with a 24‐hour interval during the first 5 days postpartum. Total EPO dose 20,000 U. Comparator: IV placebo twice with a 24 hour interval during the first 5 days postpartum. | |
| Outcomes | Preplanned outcome measures were not specified. Objectives were to test the 2 hypotheses: Psychologic status was measured using 2 questionnaires; the “Blues Questionnaire” during the first 5 consecutive days postpartum and the “SCL‐90‐R”, used on the 5th day postpartum. | |
| Notes | Source of funding not stated. The data on psychological well being were not eligible for analysis due to missing standard deviations. Authors did not respond to the request on additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method described. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Trial was described as double‐blinded and placebo‐controlled, however it was not stated who exactly was blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is not stated who of the personnel was blinded. Low risk for the subjective questionnaire since the patients were blinded. |
| Incomplete outcome data (attrition bias) | High risk | There was a high dropout rate of more than 20% caused by consent withdrawal or transferal of the child to an intensive care unit. These were excluded prior to randomisation. Unknown if the dropouts would have been equally distributed in intervention and placebo. Number of screened patients prior to randomisation was not reported. |
| Selective reporting (reporting bias) | High risk | Intended objectives were investigated and reported. However, adverse events and maternal mortality were not reported. |
| Other bias | Low risk | None known. |
| Methods | Multicentre, open‐label, randomised, controlled trial, conducted in 2009 in 2 centres in Pakistan. Per protocol analysis. Follow‐up was 40 days. | |
| Participants | 86 women were recruited to the trial, 80 were randomised into 2 groups of 40. 76 of the women had a caesarean section. Socioeconomic conditions were not described. Inclusion criteria: Hb < 90 g/L, ferritin < 15 μg/dL at 24 to 48 hours postpartum. | |
| Interventions | Intervention referred to IV iron sucrose. Intervention: IV iron sucrose infusion 200 mg on day 2 and 4. Total iron dose was 400 mg. Comparator: the women were advised to take oral ferrous sulphate 200 mg twice daily together with meals for 42 days. Total non‐elemental iron dose was 16,000 mg. | |
| Outcomes | No preplanned outcome measures stated. The aim was to compare the efficacy of IV ferrous sucrose vs oral ferrous sulphate on postpartum iron deficiency anaemia. Adverse events during treatment were reported. | |
| Notes | Source of funding not stated. Several errors were detected: adverse events were reported as twice as many in the text (page 3) compared to Table 3. Unknown which data are correct. We chose to use the lowest reported. The authors did not respond to our request for further details. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Low dropout rate. We assume that initial randomisation was 1:1, which would make the 6 dropouts equally distributed among the groups. Reasons for dropout were non‐compliance and complications. Number of screened and excluded patients prior to randomisation was not reported. |
| Selective reporting (reporting bias) | High risk | No preplanned outcome measures stated. Maternal mortality was not reported. |
| Other bias | High risk | Several errors and inconsistencies were detected. |
| Methods | Single‐centre, double‐blind, randomised controlled trial conducted from November 2005 to January 2008 in Barcelona, Spain. Per protocol analysis. Follow‐up was 6 weeks. | |
| Participants | 103 puerperal women were screened, 72 of these were randomised into 2 groups of 36. 31 women in the intervention group, and 29 women in the comparator group completed the trial. Inclusion criteria: age > 18 years; postpartum haemorrhage or severe anaemia symptoms and Hb 60 to 80 g/L within the 48 hours after delivery. Written informed consent. Exclusion criteria: antenatal chronic anaemia, infection, asthma, eczema, topical allergy, oral iron intolerance, women with blood transfusion criteria (Hb < 60 g/L or intolerable symptoms of anaemia), anaemia due to other causes than blood loss or iron deficiency, cirrhosis, hepatitis, elevation of liver enzymes, overload or alteration in iron metabolism, hypersensitivity to IV iron, or unwillingness to participate. | |
| Interventions | Intervention referred to IV iron sucrose. Intervention: IV iron sucrose (Venofer) 200 mg daily for 2 days. Then 2 tablets of ferrous sulphate 525 mg (containing 105 mg of elemental iron per tablet) daily for 30 days. Total elemental iron dose was 6700 mg, total dose of non‐elemental oral iron was 31,500 mg. Comparator: IV NaCl 0,9% equal volume for 2 days. Then 2 tablets of ferrous sulphate 525 mg (containing 105 mg of elemental iron per tablet) daily for 30 days. Total elemental iron dose was 6300 mg, total dose of non‐elemental oral iron was 31,500 mg. | |
| Outcomes | Preplanned outcome measures: primary: between‐group‐difference in the mean Hb and HCT at 6 weeks postpartum; secondary: ferritin, iron‐binding capacity, reticulocyte count, serum iron, and MCV. Longitudinal progression of Hb and HCT levels within groups. Clinical anaemic signs (pulse and blood pressure), and symptoms (headache, fatigue, tinnitus, dyspnoea, palpitations, tingling, dizziness, nausea, and difficulty in concentration). Levels of depression and anxiety. | |
| Notes | The trial was partially financed by J Uriach & Co. Information for this description is collected from trial registry and the main report. Authors did not respond to the request on additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation system. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | An opaque perfusion system was used in both groups to avoid the identification of the treatment received and maintain the double‐blind nature of the study. Thus, low risk for outcomes evaluated by the patients, such as adverse events. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is not stated whether persons who handled patient data were blinded. We are only sure that the patients and the clinicians were blinded. |
| Incomplete outcome data (attrition bias) | High risk | Lost to follow‐up not equally distributed among groups (14% vs 8%). |
| Selective reporting (reporting bias) | High risk | Intended outcomes (trial register and full report) are reported. However, adverse events are pooled for each group, which makes it impossible to know which adverse events occurred. Mortality is not mentioned and 8 patients were lost to follow‐up. |
| Other bias | Low risk | None known. |
| Methods | Multicentre, open‐label, randomised, controlled trial, conducted from May 2004 to February 2011 in 37 centres in the Netherlands. ITT analyses. Follow‐up was 6 weeks. | |
| Participants | 1011 puerperal women were screened, 521 were randomised into 2 groups: 259 to intervention group, 1 did not meet inclusion criteria, 258 represented the ITT population, 251 completed the trial per protocol; 262 to comparator group, 1 did not meet inclusion criteria, 261 represented the ITT population, 228 completed the trial per protocol. The socioeconomic status of the population was considered above average based on education level: non/low (3%), lower/senior secondary vocational education (51% to 56%), higher professional education and university (41% to 46%). Participants were mainly of western ethnic origin (76% to 78%). Inclusion criteria: Hb 48 to 79 g/L 12 to 24 hours postpartum, post partum haemorrhage (> 1000 mL and/or a decrease in Hb > 19 g/L), good knowledge of the Dutch language. Exclusion criteria: dyspnoea, syncope, tachycardia, angina pectoris and/or transient ischaemic attacks, RBC transfusion administered within 12 hours of delivery, severe pre‐eclampsia, severe infection, congenital haemolytic disease, compromised immunological status, malignancy, severe co‐morbidity, and death or critical condition of the neonate. | |
| Interventions | Intervention referred to RBC transfusion. Intervention: At least 1 unit of RBCs with the aim to a Hb of at least 89 g/L. | |
| Outcomes | Primary outcome: physical fatigue at day 3, measured with the Multidimensional Fatigue Inventory. Secondary outcomes: remaining health related quality of life scores, general and mental fatigue scores (from protocol), number of RBC units transfused, transfusion reactions, length of hospital stay, and physical complications during follow‐up. Data on breastfeeding and compliance were also reported. | |
| Notes | Source of funding: grants from the Landsteiner Foundation for Blood Transfusion Research (file number 0904) and Stichting Vrienden van de Bloedtransfusie (file number 1201005). Previous funding by Sanquin Blood Supply Foundation, the Netherlands, and the Department of Obstetrics, Erasmus Medical Centre, Rotterdam, the Netherlands. The authors responded to our request for further detail. This trial was registered in ClinicalTrials.gov (NCT00335023) and at the Dutch Trial Register (NTR335). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based application for block randomisation with a variable block size of 2 to 8 women. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed through the study web site (www.studies‐obsgyn.nl/womb); thus allocation was concealed. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | High risk | Differences in baseline characteristics of questionnaire responders vs non‐responders (western ethnicity in 81% vs 54%, mean age 31 vs 28 years, median blood loss 1500 vs 1150 mL). Big difference in compliance to allocated treatment: 8 vs 34. The design of this trial carries a high risk for selecting the study population. |
| Selective reporting (reporting bias) | High risk | Intended outcomes reported. Maternal mortality was not reported. |
| Other bias | Low risk | None known. |
| Methods | Multicentre, open‐label, randomised controlled trial, conducted from May 9, 2006 to December 27, 2006 in 28 centres in USA. Participants were stratified based on Hb, ferritin levels, and mode of delivery. ITT analyses. Follow‐up was 6 weeks. | |
| Participants | 291 anaemic puerperal women were randomised to 2 groups: 148 to comparator group,where, 144 competed the study, modified ITT population was 147 (65.3% were Caucasian, 13.6% Hispanic, 18.4% African American, 2% Asian, 0.7% other). Socioeconomic conditions were not described, study population were of mixed ethnic origin. Inclusion criteria: healthy women, Hb < 100 g/L 10 days or less postpartum on 2 or more laboratory tests conducted at least 12 hours apart. Exclusion criteria: estimated blood loss > 1 litre 24 hours prior to randomisation, history of anaemia other than iron deficiency anaemia or peripartum bleeding, current treatment with myelosuppressive therapy or asthma therapy, recent blood transfusions, or EPO treatment within 3 months prior to screening. | |
| Interventions | Intervention referred IV ferric carboxymaltose. Intervention: IV ferric carboxymaltose (brand unknown) given weekly until individual calculated cumulative dose was reached or a maximum of 2500 mg was administered. The maximum single weekly dose was 15 mg/kg, not to exceed 1000 mg per dose. Comparator: oral ferrous sulphate 325 mg (65 mg elemental iron) 3 times daily for 6 weeks. Total dose of elemental iron was 8190 mg. Total dose of non‐elemental iron was 40,950 mg. | |
| Outcomes | Preplanned outcomes were laboratory values and adverse events. Adverse events for participants who were randomised to ferric carboxymaltose and withdrew from the study early were reported for 28 days after the last treatment. | |
| Notes | Trial funded by research grants from American Regent, Inc. Authors provided additional information on request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised computer randomisation system. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Low dropout rate, detailed flowchart which accounts for all dropouts. However, reason for voluntary dropouts was not stated and the number of screened and excluded patients prior to randomisation was not reported. |
| Selective reporting (reporting bias) | High risk | Intended outcome measures reported. However, maternal mortality was not reported. |
| Other bias | Low risk | None known. |
| Methods | Single‐centre, double‐blind, randomised, controlled trial, conducted from August 1998 to July 1999 in China. Per protocol analysis. Follow‐up was 6 weeks. | |
| Participants | 170 puerperal anaemic women were screened, 150 were included and randomised into 2 groups: 75 to the comparator group, 59 completed the trial per protocol. Socioeconomic conditions were not described, ethnic origin was 76% to 81% Chinese, 19% to 24% Filippino. Inclusion criteria: Hb 80 to 99 g/L 2 days postpartum. Exclusion criteria: MCV < 80 fL, significant anaemia symptoms (tachycardia, severe dizziness, and shortness of breath), estimated blood loss > 500 mL. | |
| Interventions | Intervention referred to oral ferrous sulphate. Intervention: oral ferrous sulphate 200 mg (65 mg elemental iron) 3 times daily for 42 days. Total dose elemental iron was 8200 mg. Total dose non‐elemental iron was 25,200 mg. Comparator: placebo tablets containing lactose and drug binder 3 times daily for 42 days. | |
| Outcomes | No preplanned outcome measures stated. Aim was to determine effects of mild postpartum anaemia and iron supplementation in women. Laboratory values, subjective evaluation of general well being score on 4‐point scale, anaemia symptoms, ability to lactate and adverse events during treatment were reported. | |
| Notes | Source of funding was not stated. Placebo tablets contained lactose. Majority of Asian people are lactose intolerant. This may have influenced GI adverse events. In this trial anaemia symptoms in the anaemic group were compared to that of the non‐anaemic group, which did not describe the effect of treatment of the anaemic women. Authors did not respond to the request on additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table. |
| Allocation concealment (selection bias) | Low risk | Pharmacy responsible for randomisation, identical tablets. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Both patient and clinicians were blinded to the given treatment. However, intervention group's stool turned black. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assuming all parties involved in the trial were unaware of treatment, risk of bias is low for all outcomes. However, women in the intervention group may have been able to guess their allocation, as it was reported that their stool turned black due to iron supplementation. |
| Incomplete outcome data (attrition bias) | High risk | High dropout rate. Reason for dropout after randomisation was not described. |
| Selective reporting (reporting bias) | High risk | No preplanned outcome measures stated. Aim was to determine effects of mild postpartum anaemia and iron supplementation in women. Maternal mortality was not reported. |
| Other bias | Low risk | None known. |
| Methods | Multicentre, open‐label, randomised, controlled trial conducted from February 8, 2005 to November 11, 2005 at 43 sites, including 40 in USA and 3 in Mexico. Per protocol analysis. Follow‐up was 6 weeks. | |
| Participants | 660 women were screened, 361 were randomised to 2 groups: 182 to intervention group 168 completed the trial per protocol. Inclusion criteria: Hb ≤ 100 g/L, use of acceptable contraception, enrolment within 10 days after delivery. Exclusion criteria: previous non‐adherence to oral iron therapy, history of anaemia from other causes than iron deficiency or blood loss secondary to pregnancy or delivery, estimated blood loss > 100 mL 24 hours before randomisation, active severe infection, TSAT > 50 %, serum ferritin > 500 ng/mL, serum creatinine > 2.0 mg/dL, serum transaminases > 1.5 times upper limit, untreated B12 or folate deficiency; erythropoiesis‐stimulating treatment within 3 months before screening, history of myelosuppressive therapy, asthma under treatment, hepatitis, HIV, or hematologic disorder other than iron deficiency. | |
| Interventions | Intervention referred to IV ferric carboxymaltose. Comparator: oral ferrous sulphate 325 mg (65 mg elemental iron) 3 times daily for 42 days. Total elemental iron dose was approximately 8190 mg. Total dose of non‐elemental iron was 40,950 mg. | |
| Outcomes | Preplanned outcome measures were the proportion of patients with improved quality of life. Maternal mortality, fatigue, psychological well being, infections, compliance and adverse events during treatment were reported. | |
| Notes | Supported by American Regent, Inc, the human drug division of Luitpold Pharmaceuticals, Shirley, New York. The authors provided unpublished information and corrections to the published text on request. They reported 3 errors in figure 1: ‐ ITT population in the IV iron group was corrected to 168; ‐ ITT population in the oral iron group was 169; ‐ In oral iron group 2 women had a Hb not less than 110 g/L at baseline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generation, blocked randomisation, interactive voice response system. |
| Allocation concealment (selection bias) | Low risk | Computerised system. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | High risk | Different frequency of dropouts prior to dosing in the 2 groups. Discrepancy between groups in reasons for dropout. |
| Selective reporting (reporting bias) | Low risk | Preplanned outcome measures reported. |
| Other bias | High risk | Several errors detected in publication. |
| Methods | Multicentre, randomised trial, conducted from January 2010 to July 2010 in 2 Indian centres. ITT analysis. Follow‐up was 30 days. | |
| Participants | 150 puerperal anaemic women were randomised to 2 groups of 75. No dropouts were reported. Socioeconomic conditions were not described in detail. 93.3% of the women came from rural areas. Inclusion criteria: Hb < 80 g/L 24 hours after delivery. Exclusion criteria: anaemia from other cause than nutritional deficiency during pregnancy, immuno‐compromised patients, terminal illness, severe cardiac, hepatic, renal, cerebrovascular, malignant, or chronic uncontrolled systemic disease, other serious medical illness, allergy/reaction to iron complex and unwillingness to participate. | |
| Interventions | Intervention referred to IV iron sucrose. Comparator: oral ferrous sulphate 200 mg twice daily for 1 month. Total non‐elemental was approximately 12,000 mg. | |
| Outcomes | Preplanned outcomes were laboratory values, quality of life, patient satisfaction, impact on cost and hospital stay, blood transfusion frequency, impact on stress, depression and cognitive function, impact on breastfeeding compared to oral iron therapy and recommendation of iron sucrose to postpartum anaemic patients. Compliance and adverse events during treatment were also reported. | |
| Notes | Source of funding was not reported. Two errors were detected in figure 2: first Hb value of oral iron does not correspond to text on page 68 (Haemoglobin Response); last Hb value for oral iron does not correspond to the graph, decimal error. Total IV iron dose was listed both as a fixed dose of 600 mg, and as a weight‐dependant dose calculated by the Ganzoni formula. On page 68 the authors state: "In oral group this mean rise of Hb was noted from 9.65 ± 0.88 gm/dl to 11.02 ± 1.02 gm/dl (p < 0.0001) in 30 days (Fig. 2)." These values cannot be found in figure 2. Adverse events in oral group stated but their rate not given. Authors did not respond to the request on additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study claimed to be randomised in abstract, but not elsewhere, and no method was described. |
| Allocation concealment (selection bias) | Unclear risk | No method described. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear description of participants, no mention of dropouts. Number of screened and excluded patients prior to randomisation was not reported. |
| Selective reporting (reporting bias) | High risk | No report on the following preplanned outcomes: patient satisfaction, quality of life, cost of treatment, length of hospital stay, use of blood transfusion, impact on stress, depression and cognitive function, lactation. Maternal mortality was not reported. |
| Other bias | High risk | Study offers limited amount of information and is difficult to evaluate. Several errors detected. |
| Methods | Multicentre, open‐label, randomised, controlled trial, conducted from November 1999 to May 2001 in 2 Swedish centres. Per protocol analysis. Follow‐up was 14 days. | |
| Participants | 60 puerperal anaemic women were randomised to 3 equal groups of 20: intervention: group 1 (20,000 U rhEPO), 15 completed the trial; intervention: group 2 (10,000 U rhEPO), 19 completed the trial; comparator: group 3, 16 completed trial. Socioeconomic conditions were not described. Inclusion criteria: age > 18 years, Hb < 80 g/L within 72 hours after delivery. All women had complicated deliveries: emergency caesarean, vacuum extractions, uterine explorations, lacerations or uterine atony. Exclusion criteria: malignant, infectious, epileptic, hypertensive, haematological, or cardiac disease, rheumatoid arthritis, diseases treated with cytostatic drugs. | |
| Interventions | Intervention referred to EPO. Intervention group 2: SC rhEPO (NeoRecormon®) 10,000 U on day 0 and 3 + IV iron sucrose (Venofer) 250 mg on day 0 and 200 mg on day 3. Total rhEPO dose was 20,000 U. Total iron IV dose was 450 mg. Comparator group: IV iron sucrose (Venofer) 250 mg on day 0 and 200 mg on day 3. Total IV iron dose was 450 mg. All women were advised to take supplementary iron, 100 mg daily, after 1 week. Doses were not registered, thus total iron dose is not known. | |
| Outcomes | The primary objective was to evaluate laboratory values. Infections and adverse events during treatment were reported. | |
| Notes | Source of funding: Roche AB, Stockholm, Sweden and the Swedish Research Council, Karolinska Institutet. Trial authors provided additional data on request. We included the discontinued patients in the analysis of adverse events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequentially numbered envelopes. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes unknown to recruiter. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | High risk | The patients who dropped out had significantly lower Hb than the rest. Dropout rate was high. Authors provided reasons for dropout according to treatment group. Infections (endometritis) were the most frequent reason for dropout, potentially selecting the population. The number of screened and excluded patients prior to randomisation was not reported. |
| Selective reporting (reporting bias) | High risk | Outcomes for intended objectives were reported. In the original published paper reason for dropout and serious complications was not given by group. However this information was provided by trial author on request. Maternal mortality was not reported. |
| Other bias | Low risk | None known. |
| Methods | Multicentre, open‐label, randomised, controlled trial, conducted from June 2004 to September 2006 in 5 Norwegian centres. Randomisasion according to the minimization method, controlling for age and Hb at inclusion, parity and iron treatment during the third trimester. ITT analysis. Follow‐up was 12 weeks. | |
| Participants | 128 puerperal women were randomised to 2 groups: Totally, 117 women completed the first 4 weeks and 93 completed 12 weeks per protocol. Socioeconomic conditions were not described. Inclusion criteria: age 18 to 45, Hb 65 to 85 g/L. Exclusion criteria: inability to read and understand the Norwegian language, prior commencement of postpartum iron supplementation, clinically significant disease, serum creatinine > 130 µmol/L or contraindications for Venofer® or Duroferon®. | |
| Interventions | Interventions referred to IV ferrous sucrose. Intervention: IV iron sucrose (Venofer®) 200 mg daily over 3 consecutive days. Total iron dose given IV was 600 mg. After 4 weeks the women were given ferrous sulphate tablets containing 100 mg elemental iron twice daily from week 4 to week 12 postpartum. Total dose of elemental iron after 12 weeks was 11,800 mg. Comparator: oral ferrous sulphate (Duroferon®) containing 100 mg elemental iron twice daily from inclusion until 12 weeks. Total dose of elemental iron was 5600 mg after 4 weeks and 16,800 mg after 12 weeks. Treatment was initiated within 48 hours after delivery. | |
| Outcomes | Preplanned primary outcomes were laboratory values. Secondary outcomes were quality of life measured by SF‐36 and the Fatigue Score after 4, 8 and 12 weeks of treatment. Compliance to treatment and adverse events during treatment were reported. | |
| Notes | The trial was sponsored by Renapharma AB, the Swedish representative of the manufacturer of iron sucrose (Venofer®). Trial authors provided unpublished information on request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Internet‐based central randomisation. |
| Allocation concealment (selection bias) | Low risk | Internet‐based. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. High risk for subjective outcomes such as adverse events. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. High risk for most outcomes. |
| Incomplete outcome data (attrition bias) | High risk | There was a big difference in number of dropouts in the 2 groups at 4 weeks (2 vs 9) and thereby high risk of selection of population regarding all outcomes. Dropout rate was very high 27% by the end of the trial. Number of screened and excluded patients prior to randomisation was not reported. |
| Selective reporting (reporting bias) | High risk | Intended outcomes reported. Reason for choosing 4 out of 8 scales from the SF‐36 was not explained. Maternal mortality was not reported. |
| Other bias | Low risk | None known. |
dl: decilitre
EPDS: Edinburgh Postnatal Depression Scale
EPO: erythropoietin
fL: femtolitres
g: grams
g/L: grams per litre
GI: gastrointestinal
Hb: haemoglobin
HCT: haematocrit
HIV: human immunodeficiency virus
ITT: intention‐to‐treat
IU: international units
IV: intravenous
kg: kilograms
MCV: mean corpuscular volume
mg: milligrams
mL: millilitres
n: number
NaCl: Sodium chloride
PPH: postpartum haemorrhage
RBC: red blood cell
rhEPO: recombinant human erythropoietin
SC: subcutaneous
SCL‐90‐R: Symptom Checklist‐90‐Revised
SF‐36: Short Form 36
STAI: State‐Trait Anxiety Inventory
TSAT: transferrin saturation
U: units
vs: versus
µg/L: micrograms per litre
Characteristics of excluded studies [author‐defined order]
| Study | Reason for exclusion |
| An observational cohort study, not randomised controlled trial. | |
| Population consists of both women with anaemia during pregnancy and postpartum anaemia, with no subgroup analyses. | |
| Not a randomised trial. This was clear from authors response on requested method description: "The patients were selected and placed to each group only according to their consent since from 135 women only 109 agreed to be treated with IV iron and the rest who refused intravenous treatment were treated with oral supplements". | |
| It was not stated in the text that the women were randomised. Therefore, we do not consider this trial a randomised controlled trial. | |
| It was not stated in the text that the women were randomised. Therefore, we do not consider this trial a randomised controlled trial. | |
| Not randomised controlled trial. This was clear from authors reply on requested method description. Authors used same allocation method as in the trial by Daniilidis 2011. | |
| Study design did not define the postpartum period and the period for enrolment, most probably including women who were enrolled more than 6 weeks postpartum, which conflicts with the review author’s inclusion criteria. | |
| The study population consists of both pregnant and postpartum patients and subgroup analyses were not reported. | |
| Quasi‐randomised trial. The women were assigned treatment based on their name in alphabetical order. | |
| It was not stated in the text of the translation that the women were randomised. Therefore, we do not consider this trial a randomised controlled trial. Trial ID: 00264270 and 00413969. | |
| Population reported in the study included non‐anaemic women. Trial ID 00328429 and 00324138. | |
| This study has a non‐anaemic control group which is therefore not relevant according to our predefined criteria. The remaining 2 groups received the exact same treatment and are therefore not comparable by intervention. The study investigates differences in screening strategies. It does not compare the effect of different treatment regiments as we predefined for this review. | |
| The intervention focuses on crude liver extract given intramuscularly, an intervention not accepted for treatment of postpartum iron deficiency anaemia in current time. | |
| The study assessed the usefulness of iron therapy in prevention, not treatment, of postpartum anaemia. | |
| This report summarises three trials: Breymann 1996 (included), Huch 1992 and Zimmermann 1994 (excluded). | |
| This trial does not have a control arm and is therefor not a randomised controlled trial. | |
| This study compares oral iron with folate supplementation with oral iron alone. In our review we did not consider folate as an independent treatment of iron deficiency anaemia. In this study, folate is the only difference between the two treatments and thus the study does not evaluate the effect of iron treatment. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Multicentre, open‐label, randomised controlled trial, conducted in Norway. Randomisation was performed by use of opaque envelopes. Laboratory analyses were provided by a recognised Swedish biochemical laboratory. |
| Participants | 200 patients with postpartum anaemia, included within 48 postpartum. Hb 65 to 85 g/L. |
| Interventions | Intervention: IV iron carboxymaltose (Ferinject) in a dose calculated by the Ganzoni formula. Comparator: oral iron sulphate (Duroferon) 100 mg twice daily. |
| Outcomes | Preplanned outcomes were laboratory values, fatigue (Fatigue Scale), quality of life (SF‐36), post partum depression, (Edinburgh Post Partum Depression Scale). |
| Notes | The trial was initiated, partially conducted and then terminated by sponsor (Renapharma Vifor) because of slow progress (citation B. Backe). Trial registered in ClinicalTrials.gov (Trial ID: NCT00929409). Renapharma was contacted March 7th and March 27th 2013 for a report of the trial and reasons for discontinuation. However, no‐one responded to our request. |
g/L: grams per litre
Hb: haemoglobin
IV: intravenous
mg: milligrams
SF‐36: Short Form 36
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Public Title of Study: Comparison of beneficial effects of IV iron with oral iron in treating anemia following childbirth. Scientific Title of Study: IV iron‐sucrose complex versus oral iron in the treatment of postpartum anemia. |
| Methods | Computer‐generated randomisation. Sequentially‐numbered, sealed, opaque envelopes. Open‐label trial. |
| Participants | Women with anaemia in the postnatal period. Sample size: 100 |
| Interventions | Intervention: IV iron‐sucrose injection 200 mg every alternate days until the total calculated dose is given (method not described). Comparator: oral ferrous sulphate tablet 200 mg (60 mg elemental iron) 3 times a day for 6 weeks. |
| Outcomes | Primary outcomes: Hb, HCT, red cell indices, serum ferritin. Time points: 0, 7, 14, 40 days. |
| Starting date | Date of first enrolment 02/10/2012. |
| Contact information | Dr. Picklu Chaudhuri, associate professor, N.R.S Medical College, Kolkata Phone: 9432277443 Fax: 22658179 Email: [email protected] |
| User defined 1 | CTRI Number: CTRI/2013/05/003624 [Registered on: 09/05/2013] Trial registered retrospectively. |
| Notes |
| Trial name or title | Intravenous iron isomaltoside 1000 administered by high single‐dose infusions or standard medical care for the treatment of fatigue in women after postpartum haemorrhage: study protocol for a randomised controlled trial. Name in trial register: A randomized comparative, open‐label study of IV iron isomaltoside 1000 (Monofer®) administered by high single dose in‐fusions or RBC transfusion in women with severe postpartum iron deficiency anaemia ‐ P‐Monofer‐PP‐02 |
| Methods | Single‐centre, open‐label, randomised comparative study. |
| Participants | Estimated enrolment: 200 Inclusion criteria: PPH > 1000 mL, Hb 55 to 80 g/L, and signed informed consent. Exclusion criteria: age < 18 years, multiple births, peripartum RBC transfusion, iron overload or disturbances in utilisation of iron (e.g. haemochromatosis and haemosiderosis), hypersensitivity to parenteral iron or any excipients in the investigational drug products, history of active asthma within the last 5 years or a history of multiple allergies, decompensated liver cirrhosis and active hepatitis, HELLP syndrome, active acute infection assessed by clinical judgement, active rheumatoid arthritis, history of anaemia caused by e.g. thalassaemia, hypersplenism or haemolytic anaemia (known haematologic disorder other than iron deficiency), not able to read, speak and understand the Danish language, participation in any other clinical study where the study drug has not passed 5 half‐lives prior to the baseline, any other medical condition that, in the opinion of the investigator, may cause the patient to be unsuitable for completion of the study or place the patient at potential risk from being in the study. |
| Interventions | Intervention arm: IV iron isomaltoside 1000 (Monofer®) given as a single dose of 1200 mg. Comparator arm: standard medical care. Standard medical Care is most often to recommend women with PPH to continue oral iron supplementation as recommended during pregnancy or to advise the participant to take 100 mg oral iron 1‐2 times a day. |
| Outcomes | Primary outcome measures: physical fatigue. The primary objective of this study is to compare efficacy of IV high single dose infusion of iron isomaltoside 1000 to standard medical care in women with PPH evaluated as physical fatigue. Other outcome measures: change in anaemia symptoms, change in GI symptoms. Time frame for all outcome measures: from exposure to day 3, week 1, 3, 8 and 12 post‐exposure. |
| Starting date | Study start date: June 2013. Estimated study completion date: Febuary 2015. |
| Contact information | Correspondence: [email protected] Department of Obstetrics, Juliane Marie Centre, Copenhagen University Pharmacosmos A/S Clinical R & D |
| User defined 1 | Trial ID: NCT01895218; 2012‐005782‐12 |
| Notes |
| Trial name or title | Use of Iron Isomaltoside 1000 (Monofer) in Postpartum Anemia. |
| Methods | Open‐label, randomised controlled trial. |
| Participants | Estimated enrolment: 300 women. Inclusion criteria: Hb < 100 g/L 24 to 48 hours after delivery. Exclusion criteria: history of PPH, or significant blood loss in last 24 hours, history of allergy to iron preparation, Hb < 70 g/L, sign and symptoms of cardiac failure, blood transfusion in last 3 months, chronic liver diseases, increased creatinine. |
| Interventions | Intervention: IV infusion of isomaltoside 1000 (Monofer), calculated according to Ganzoni formula. |
| Outcomes | Primary: to see the rise in Hb concentration of 2 g/dL or more, measured at day 14 and at 3 months. Secondary: time required for rise in haemoglobin concentration. Both groups will be compared in terms of time interval, to see the rise in Hb concentration. |
| Starting date | May 2012. |
| Contact information | Nazli Hossain, Dow University of Health Sciences. |
| User defined 1 | Trial ID: NCT01628770. |
| Notes |
| Trial name or title | Public title: A clinical trial to compare oral iron ferrous sulfate with newer intravenous iron (ferric carboxymaltose) injection in patients of iron deficiency anemia in post delivery period Scientific title: Comparison of ferric carboxymaltose injection with oral iron in treatment of postpartum iron deficiency anemia ‐ a randomized controlled clinical trial |
| Methods | Open‐label, randomised controlled trial. Computerised sequence generation for randomisation. |
| Participants | Target sample size: 140 women. Age: 20 to 40 years. Inclusion criteria: Women between 20 and 40 years of age, within 10 days of normal delivery with a Hb between 7 and 10 g% and iron deficiency measured by PCV < 36%, MCV < 80 fl, MCH < 27 pg and MCHC < 33 g/dL) with negative NESTROF test. Exclusion criteria: Weight < 35 kg, puerperal pyrexia, known drug allergy or intolerance to iron therapy, history of chronic medical illness (tuberculosis, asthma, liver diseases, kidney diseases, diabetes mellitus, hypertension, HIV infection), other anaemia treatment (blood transfusion, erythropoietin) within the last three months. |
| Interventions | Intervention: Injection ferric carboxymaltose, calculated by dose not exceeding 1000 mg per infusion. Comparator: Ferrous sulphate tablet containing 60 mg elemental iron thrice a day for 6 weeks. |
| Outcomes | Primary outcomes: Percentage of patients achieving Hb rise 3 g/dL from baseline at 3 and 6 weeks. Secondary outcomes: Percentage of patients achieving Hb 12 g/dL at 3 and 6 weeks. |
| Starting date | 1 November 2012 |
| Contact information | Dr Amita Suneja Department of obstetrics and gynaecology, 110095 East, DELHI, India Telephone: 9868399728 Email: [email protected] Affiliation: UCMS & GTB Hospital Dr Shikha Dilshad Garden, 110095, Thiruvananthapuram, DELHI, India Telephone: 9868399728 Email: [email protected] Affiliation: UCMS & GTB Hospital |
| User defined 1 | Trial ID: CTRI/2014/10/005099 |
| Notes |
fL: femtolitres
GI: gastrointestinal
g/dL: grams per decilitre
g/L: grams per litre
Hb: haemoglobin
HCT: haematocrit (= PCV)
HELLP: haemolysis elevated liver enzymes and low platelets
IV: intravenous
kg: kilograms
MCH: mean corpuscular haemoglobin
MCHC: mean corpuscular haemoglobin concentration
MCV: mean corpuscular volume
mg: milligrams
NESTROF: Naked Eye Single Tube Red Cell Osmotic Fragility Test
PPH: postpartum haemorrhage
PCV: packed cell volume (= HCT)
pg: picograms
RBC: red blood cell
µg/L: micrograms per litre
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal mortality Show forest plot | 2 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.12, 71.96] |
| Analysis 1.1  Comparison 1 Intravenous iron versus oral iron, Outcome 1 Maternal mortality. | ||||
| 2 Fatigue ‐ 14 days Show forest plot | 1 | 322 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐8.04, 1.44] |
| Analysis 1.2  Comparison 1 Intravenous iron versus oral iron, Outcome 2 Fatigue ‐ 14 days. | ||||
| 3 Fatigue ‐ 42 days Show forest plot | 1 | 329 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐6.77, 2.57] |
| Analysis 1.3  Comparison 1 Intravenous iron versus oral iron, Outcome 3 Fatigue ‐ 42 days. | ||||
| 4 SF‐36: Physical F(x) ‐ 14 days Show forest plot | 1 | 320 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐3.84, 5.64] |
| Analysis 1.4  Comparison 1 Intravenous iron versus oral iron, Outcome 4 SF‐36: Physical F(x) ‐ 14 days. | ||||
| 5 SF‐36: Physical role ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 3.50 [‐2.03, 9.03] |
| Analysis 1.5 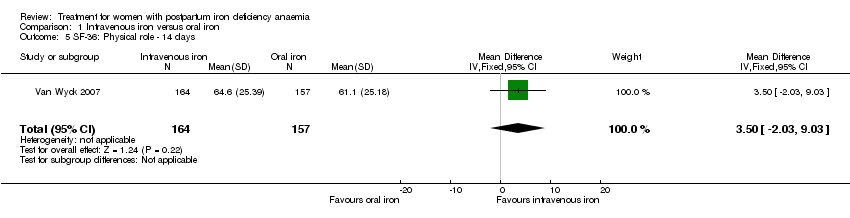 Comparison 1 Intravenous iron versus oral iron, Outcome 5 SF‐36: Physical role ‐ 14 days. | ||||
| 6 SF‐36: Bodily pain ‐ day 14 Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐6.00, 4.60] |
| Analysis 1.6 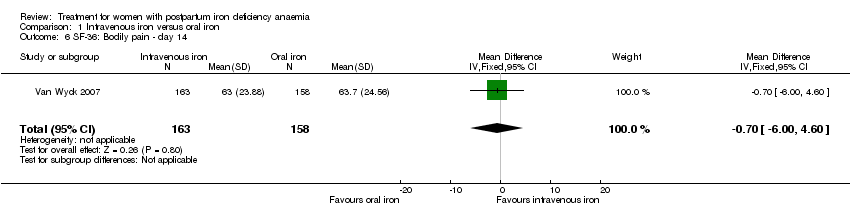 Comparison 1 Intravenous iron versus oral iron, Outcome 6 SF‐36: Bodily pain ‐ day 14. | ||||
| 7 SF‐36: General health ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐3.09, 4.49] |
| Analysis 1.7  Comparison 1 Intravenous iron versus oral iron, Outcome 7 SF‐36: General health ‐ 14 days. | ||||
| 8 SF‐36: Vitality ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐3.64, 5.44] |
| Analysis 1.8 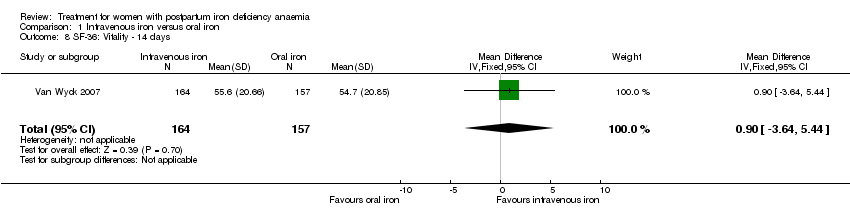 Comparison 1 Intravenous iron versus oral iron, Outcome 8 SF‐36: Vitality ‐ 14 days. | ||||
| 9 SF‐36: Emotional role ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐4.06, 6.26] |
| Analysis 1.9  Comparison 1 Intravenous iron versus oral iron, Outcome 9 SF‐36: Emotional role ‐ 14 days. | ||||
| 10 SF‐36: Social function ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐4.08, 6.08] |
| Analysis 1.10  Comparison 1 Intravenous iron versus oral iron, Outcome 10 SF‐36: Social function ‐ 14 days. | ||||
| 11 SF‐36: Mental health ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐4.84, 2.44] |
| Analysis 1.11  Comparison 1 Intravenous iron versus oral iron, Outcome 11 SF‐36: Mental health ‐ 14 days. | ||||
| 12 Depression Show forest plot | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.00] |
| Analysis 1.12 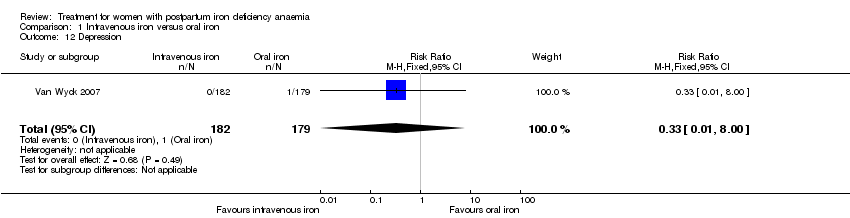 Comparison 1 Intravenous iron versus oral iron, Outcome 12 Depression. | ||||
| 13 Infections Show forest plot | 3 | 718 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.58, 5.03] |
| Analysis 1.13 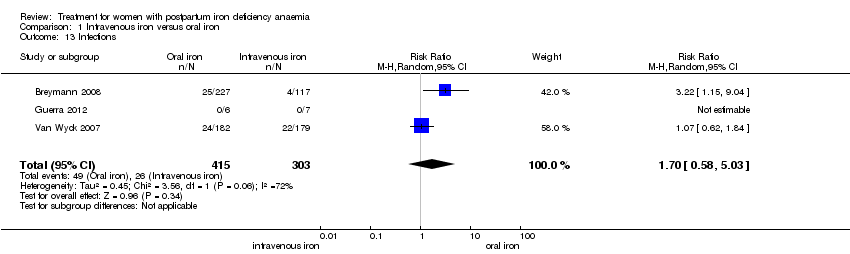 Comparison 1 Intravenous iron versus oral iron, Outcome 13 Infections. | ||||
| 14 Compliance to treatment Show forest plot | 5 | 890 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.01, 1.35] |
| Analysis 1.14  Comparison 1 Intravenous iron versus oral iron, Outcome 14 Compliance to treatment. | ||||
| 15 All gastrointestinal symptoms Show forest plot | 8 | 1307 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.20, 0.47] |
| Analysis 1.15 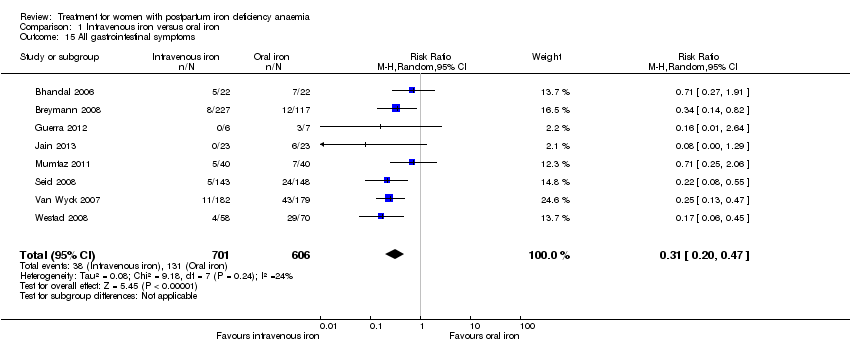 Comparison 1 Intravenous iron versus oral iron, Outcome 15 All gastrointestinal symptoms. | ||||
| 16 Constipation Show forest plot | 6 | 1217 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.11, 0.39] |
| Analysis 1.16 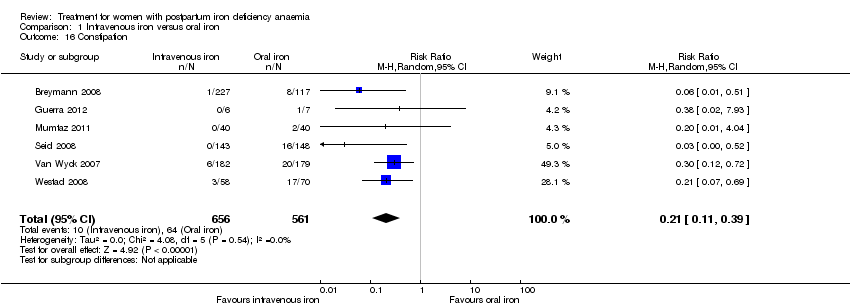 Comparison 1 Intravenous iron versus oral iron, Outcome 16 Constipation. | ||||
| 17 Nausea Show forest plot | 4 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.11, 0.81] |
| Analysis 1.17  Comparison 1 Intravenous iron versus oral iron, Outcome 17 Nausea. | ||||
| 18 Gastrointestinal pain Show forest plot | 4 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.04, 0.83] |
| Analysis 1.18  Comparison 1 Intravenous iron versus oral iron, Outcome 18 Gastrointestinal pain. | ||||
| 19 Diarrhoea Show forest plot | 3 | 569 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.02, 0.59] |
| Analysis 1.19  Comparison 1 Intravenous iron versus oral iron, Outcome 19 Diarrhoea. | ||||
| 20 Vomiting Show forest plot | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 9.66] |
| Analysis 1.20  Comparison 1 Intravenous iron versus oral iron, Outcome 20 Vomiting. | ||||
| 21 Dyspepsia Show forest plot | 2 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.04, 3.20] |
| Analysis 1.21  Comparison 1 Intravenous iron versus oral iron, Outcome 21 Dyspepsia. | ||||
| 22 Dysgeusia Show forest plot | 4 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 7.20 [1.63, 31.76] |
| Analysis 1.22  Comparison 1 Intravenous iron versus oral iron, Outcome 22 Dysgeusia. | ||||
| 23 Headache Show forest plot | 4 | 1124 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.87, 4.29] |
| Analysis 1.23  Comparison 1 Intravenous iron versus oral iron, Outcome 23 Headache. | ||||
| 24 Hepatic involvement Show forest plot | 3 | 996 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.12, 1.71] |
| Analysis 1.24  Comparison 1 Intravenous iron versus oral iron, Outcome 24 Hepatic involvement. | ||||
| 25 Injection site discomfort Show forest plot | 4 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 4.72 [1.03, 21.54] |
| Analysis 1.25  Comparison 1 Intravenous iron versus oral iron, Outcome 25 Injection site discomfort. | ||||
| 26 Skin rash Show forest plot | 2 | 489 | Risk Ratio (M‐H, Random, 95% CI) | 2.34 [0.79, 6.97] |
| Analysis 1.26  Comparison 1 Intravenous iron versus oral iron, Outcome 26 Skin rash. | ||||
| 27 Urticaria Show forest plot | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [0.47, 36.59] |
| Analysis 1.27  Comparison 1 Intravenous iron versus oral iron, Outcome 27 Urticaria. | ||||
| 28 Flush Show forest plot | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [1.18, 68.81] |
| Analysis 1.28  Comparison 1 Intravenous iron versus oral iron, Outcome 28 Flush. | ||||
| 29 Muscle cramp Show forest plot | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 6.05 [0.74, 49.68] |
| Analysis 1.29  Comparison 1 Intravenous iron versus oral iron, Outcome 29 Muscle cramp. | ||||
| 30 Pain (not specified) Show forest plot | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.42 [0.44, 159.82] |
| Analysis 1.30 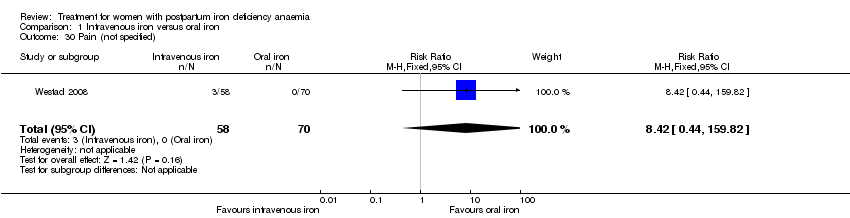 Comparison 1 Intravenous iron versus oral iron, Outcome 30 Pain (not specified). | ||||
| 31 Seriouse adverse events (not specified) Show forest plot | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.26, 4.06] |
| Analysis 1.31  Comparison 1 Intravenous iron versus oral iron, Outcome 31 Seriouse adverse events (not specified). | ||||
| 32 Anaphylaxis or evidence of hypersensitivity Show forest plot | 8 | 1454 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.31, 24.92] |
| Analysis 1.32 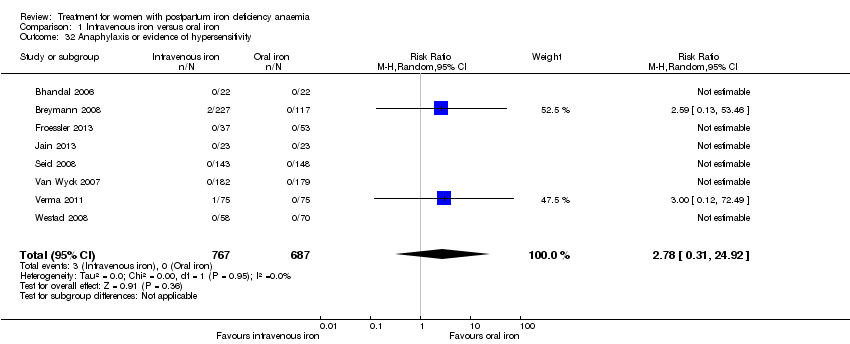 Comparison 1 Intravenous iron versus oral iron, Outcome 32 Anaphylaxis or evidence of hypersensitivity. | ||||
| 33 Arythmia Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.26 [0.18, 101.86] |
| Analysis 1.33  Comparison 1 Intravenous iron versus oral iron, Outcome 33 Arythmia. | ||||
| 34 Red blood cell transfusion Show forest plot | 4 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.19, 1.23] |
| Analysis 1.34  Comparison 1 Intravenous iron versus oral iron, Outcome 34 Red blood cell transfusion. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 General fatigue ‐ 3 days Show forest plot | 1 | 388 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.53, ‐0.07] |
| Analysis 2.1 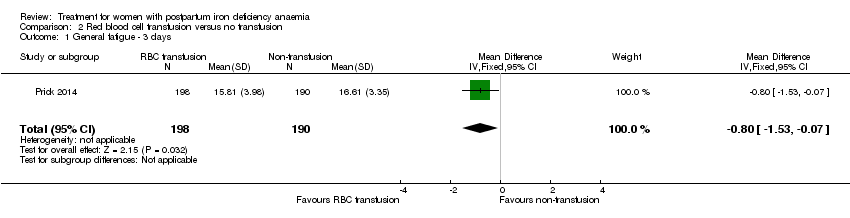 Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 1 General fatigue ‐ 3 days. | ||||
| 2 General fatigue ‐ 6 weeks Show forest plot | 1 | 318 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.22, 0.72] |
| Analysis 2.2 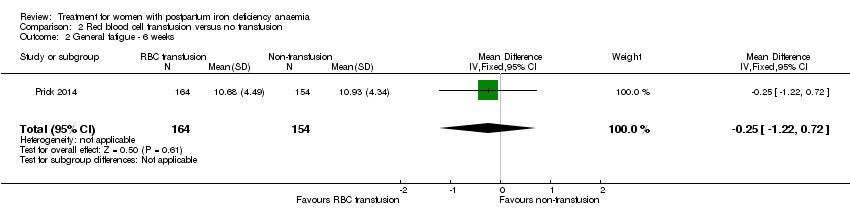 Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 2 General fatigue ‐ 6 weeks. | ||||
| 3 SF‐36: Physical functioning ‐ 1 week Show forest plot | 1 | 368 | Mean Difference (IV, Fixed, 95% CI) | 5.67 [0.84, 10.50] |
| Analysis 2.3  Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 3 SF‐36: Physical functioning ‐ 1 week. | ||||
| 4 SF‐36: Social function ‐ 1 week Show forest plot | 1 | 369 | Mean Difference (IV, Fixed, 95% CI) | 5.34 [0.11, 10.57] |
| Analysis 2.4 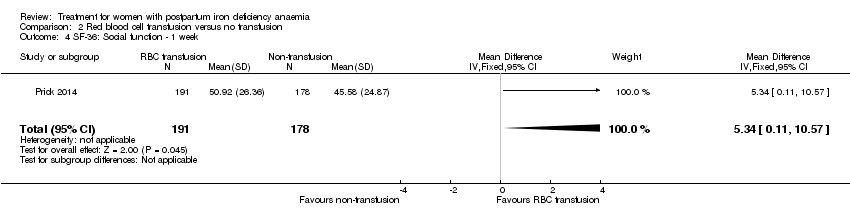 Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 4 SF‐36: Social function ‐ 1 week. | ||||
| 5 SF‐36: Physical role ‐ 1 week Show forest plot | 1 | 366 | Mean Difference (IV, Fixed, 95% CI) | 4.56 [‐1.41, 10.53] |
| Analysis 2.5  Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 5 SF‐36: Physical role ‐ 1 week. | ||||
| 6 SF‐36: Bodily pain ‐ 1 week Show forest plot | 1 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.90, 1.90] |
| Analysis 2.6 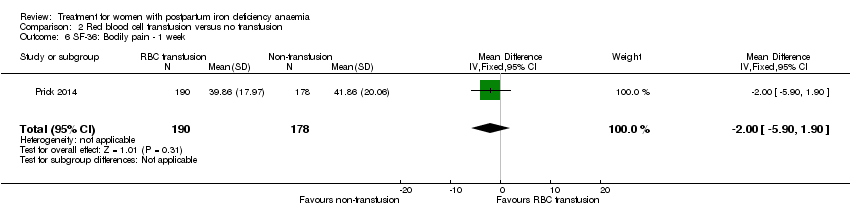 Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 6 SF‐36: Bodily pain ‐ 1 week. | ||||
| 7 SF‐36: General health ‐ 1 week Show forest plot | 1 | 369 | Mean Difference (IV, Fixed, 95% CI) | 2.18 [‐1.47, 5.83] |
| Analysis 2.7 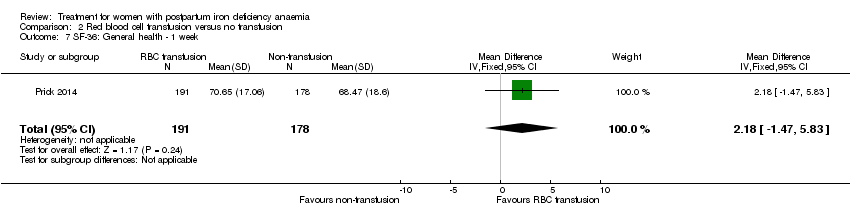 Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 7 SF‐36: General health ‐ 1 week. | ||||
| 8 SF‐36: Vitality ‐ 1 week Show forest plot | 1 | 369 | Mean Difference (IV, Fixed, 95% CI) | 1.88 [‐2.01, 5.77] |
| Analysis 2.8 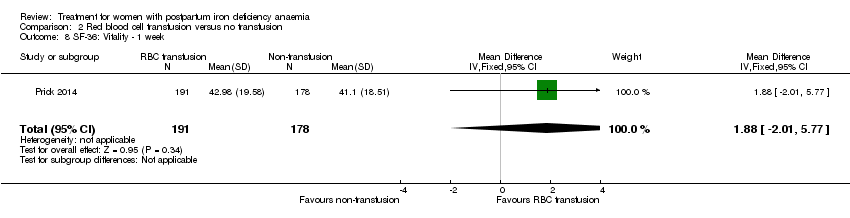 Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 8 SF‐36: Vitality ‐ 1 week. | ||||
| 9 SF‐36: Emotional role ‐ 1 week Show forest plot | 1 | 368 | Mean Difference (IV, Fixed, 95% CI) | 4.37 [‐4.51, 13.25] |
| Analysis 2.9  Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 9 SF‐36: Emotional role ‐ 1 week. | ||||
| 10 SF‐36: Mental health ‐ 1 week Show forest plot | 1 | 369 | Mean Difference (IV, Fixed, 95% CI) | 1.21 [‐2.29, 4.71] |
| Analysis 2.10  Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 10 SF‐36: Mental health ‐ 1 week. | ||||
| 11 Infections Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.53, 1.61] |
| Analysis 2.11 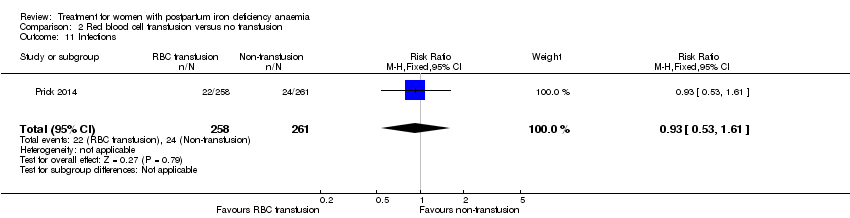 Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 11 Infections. | ||||
| 12 Compliance to treatment Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.06, 1.17] |
| Analysis 2.12  Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 12 Compliance to treatment. | ||||
| 13 Breastfeeding at six weeks Show forest plot | 1 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.07] |
| Analysis 2.13  Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 13 Breastfeeding at six weeks. | ||||
| 14 Erythrocyte alloantibody formation Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 74.15] |
| Analysis 2.14  Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 14 Erythrocyte alloantibody formation. | ||||
| 15 Rash Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 74.15] |
| Analysis 2.15  Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 15 Rash. | ||||
| 16 Fever Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.06 [0.24, 104.84] |
| Analysis 2.16  Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 16 Fever. | ||||
| 17 Thromboembolic events Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.13] |
| Analysis 2.17  Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 17 Thromboembolic events. | ||||
| 18 Parenteral iron intolerance Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.24] |
| Analysis 2.18  Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 18 Parenteral iron intolerance. | ||||
| 19 Transfusion reactions Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.08 [0.37, 136.41] |
| Analysis 2.19  Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 19 Transfusion reactions. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Digit Symbol Substitution test ‐ 10 weeks Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.76, 2.76] |
| Analysis 3.1 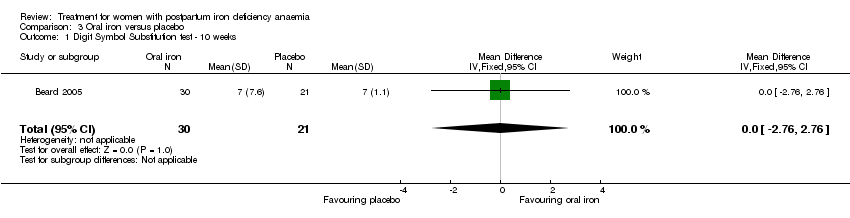 Comparison 3 Oral iron versus placebo, Outcome 1 Digit Symbol Substitution test ‐ 10 weeks. | ||||
| 2 EPDS ‐ 10 weeks Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.86, 1.06] |
| Analysis 3.2  Comparison 3 Oral iron versus placebo, Outcome 2 EPDS ‐ 10 weeks. | ||||
| 3 STAI ‐ 10 weeks Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐3.18, 2.38] |
| Analysis 3.3  Comparison 3 Oral iron versus placebo, Outcome 3 STAI ‐ 10 weeks. | ||||
| 4 Percieved Stress ‐ 10 weeks Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 4.1 [1.70, 6.50] |
| Analysis 3.4  Comparison 3 Oral iron versus placebo, Outcome 4 Percieved Stress ‐ 10 weeks. | ||||
| 5 Breastfeeding at two days postpartum Show forest plot | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.58, 1.17] |
| Analysis 3.5  Comparison 3 Oral iron versus placebo, Outcome 5 Breastfeeding at two days postpartum. | ||||
| 6 Back pain Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.42, 1.03] |
| Analysis 3.6 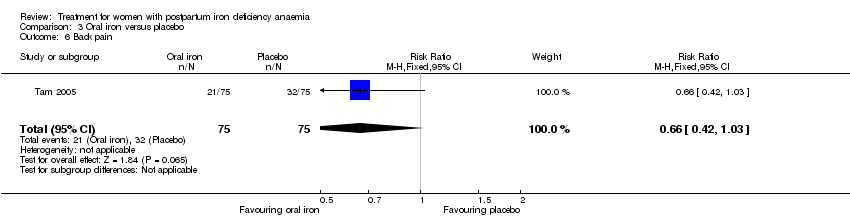 Comparison 3 Oral iron versus placebo, Outcome 6 Back pain. | ||||
| 7 All gastrointestinal symptoms Show forest plot | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.36, 2.79] |
| Analysis 3.7 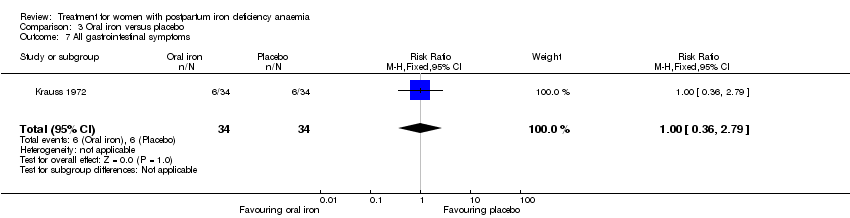 Comparison 3 Oral iron versus placebo, Outcome 7 All gastrointestinal symptoms. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All gastrointestinal symptoms Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.23, 6.16] |
| Analysis 4.1  Comparison 4 Oral iron, magnesium oxide and yeast extract versus placebo, Outcome 1 All gastrointestinal symptoms. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All gastrointestinal symptoms Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.83, 2.45] |
| Analysis 5.1 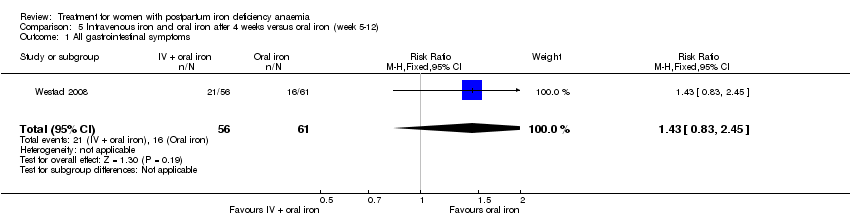 Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 1 All gastrointestinal symptoms. | ||||
| 2 Abdominal pain Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [0.55, 13.48] |
| Analysis 5.2  Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 2 Abdominal pain. | ||||
| 3 Constipation Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.55, 2.60] |
| Analysis 5.3  Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 3 Constipation. | ||||
| 4 Diarrhoea Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.35, 30.51] |
| Analysis 5.4 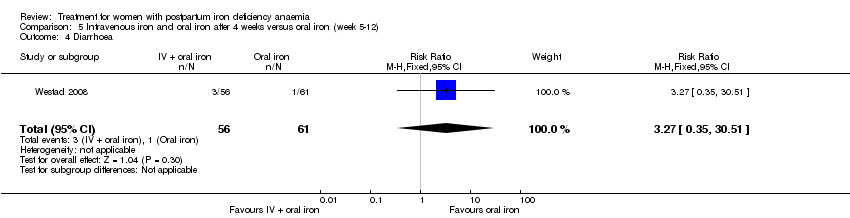 Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 4 Diarrhoea. | ||||
| 5 Nausea Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.14, 78.49] |
| Analysis 5.5  Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 5 Nausea. | ||||
| 6 Dysgeusia Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.14, 78.49] |
| Analysis 5.6  Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 6 Dysgeusia. | ||||
| 7 Flatulence Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.72] |
| Analysis 5.7  Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 7 Flatulence. | ||||
| 8 Melaena Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.72] |
| Analysis 5.8  Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 8 Melaena. | ||||
| 9 Headache Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.72] |
| Analysis 5.9  Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 9 Headache. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistent anaemia symptoms on a VAS scale: 1 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.46] |
| Analysis 6.1  Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 1 Persistent anaemia symptoms on a VAS scale: 1 week. | ||||
| 2 Persistent anaemia symptoms on a VAS scale: 2 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.33] |
| Analysis 6.2 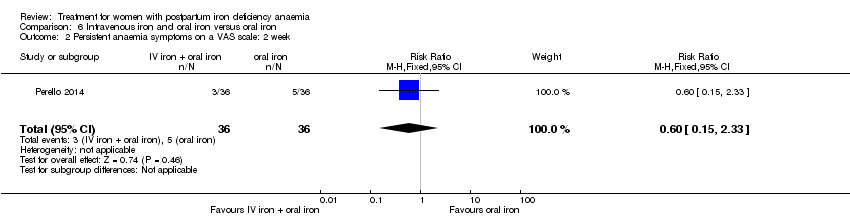 Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 2 Persistent anaemia symptoms on a VAS scale: 2 week. | ||||
| 3 Persistent anaemia symptoms on a VAS scale: 6 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.50] |
| Analysis 6.3 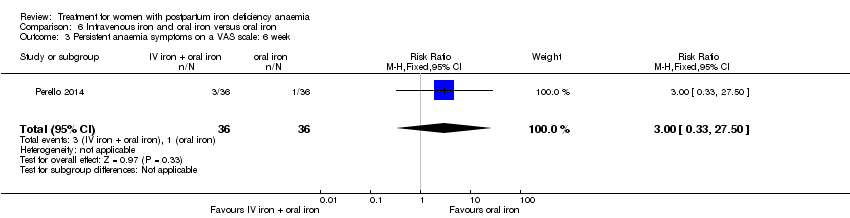 Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 3 Persistent anaemia symptoms on a VAS scale: 6 week. | ||||
| 4 EPDS ‐ 1 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.65, 13.88] |
| Analysis 6.4  Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 4 EPDS ‐ 1 week. | ||||
| 5 Length of hospital stay Show forest plot | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.02, 0.42] |
| Analysis 6.5  Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 5 Length of hospital stay. | ||||
| 6 Adverse events (pooled) ‐ 1 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.21, 2.16] |
| Analysis 6.6 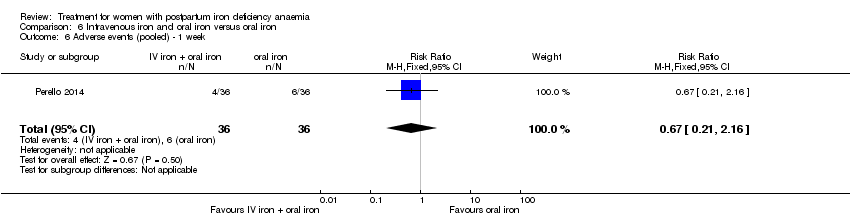 Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 6 Adverse events (pooled) ‐ 1 week. | ||||
| 7 Adverse events (pooled) ‐ 2 weeks Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.28] |
| Analysis 6.7 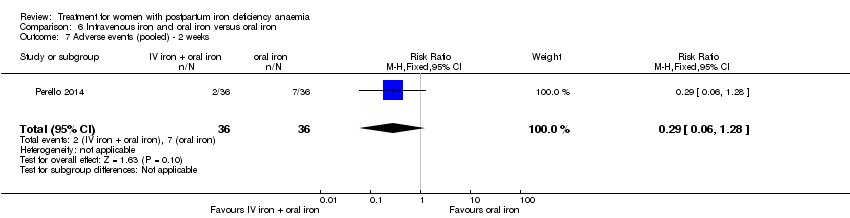 Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 7 Adverse events (pooled) ‐ 2 weeks. | ||||
| 8 Adverse events (pooled) ‐ 6 weeks Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 1.93] |
| Analysis 6.8 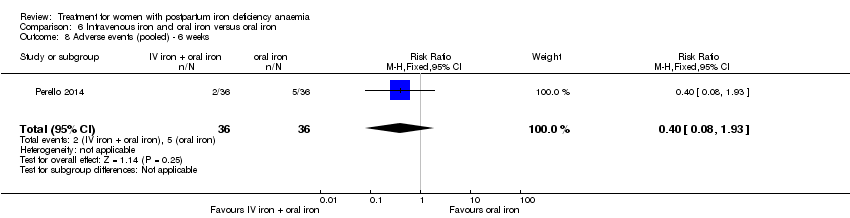 Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 8 Adverse events (pooled) ‐ 6 weeks. | ||||
| 9 Red blood cell transfusion Show forest plot | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.72] |
| Analysis 6.9  Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 9 Red blood cell transfusion. | ||||
| 10 Anaphylaxis or evidence of hypersensitivity Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 6.10  Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 10 Anaphylaxis or evidence of hypersensitivity. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postpartum depression Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| Analysis 7.1  Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 1 Postpartum depression. | ||||
| 2 Infections Show forest plot | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.72, 5.59] |
| Analysis 7.2  Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 2 Infections. | ||||
| 3 Compliance to treatment Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.91, 1.10] |
| Analysis 7.3  Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 3 Compliance to treatment. | ||||
| 4 Breasfeeding Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.91, 1.10] |
| Analysis 7.4  Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 4 Breasfeeding. | ||||
| 5 Dysgeusia Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.27, 1.88] |
| Analysis 7.5  Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 5 Dysgeusia. | ||||
| 6 Flush Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.20, 20.33] |
| Analysis 7.6  Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 6 Flush. | ||||
| 7 Diarrhoea Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| Analysis 7.7  Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 7 Diarrhoea. | ||||
| 8 Headache Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.60] |
| Analysis 7.8  Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 8 Headache. | ||||
| 9 Itching (including elevated liver enzymes) Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| Analysis 7.9  Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 9 Itching (including elevated liver enzymes). | ||||
| 10 Dizziness Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| Analysis 7.10  Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 10 Dizziness. | ||||
| 11 Thrombophlebitis Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 98.00] |
| Analysis 7.11  Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 11 Thrombophlebitis. | ||||
| 12 Red blood cell transfusion Show forest plot | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.52] |
| Analysis 7.12  Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 12 Red blood cell transfusion. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postpartum depression Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| Analysis 8.1  Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 1 Postpartum depression. | ||||
| 2 Infections Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.19, 2.93] |
| Analysis 8.2  Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 2 Infections. | ||||
| 3 Headache Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.57] |
| Analysis 8.3  Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 3 Headache. | ||||
| 4 Low blood pressure Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| Analysis 8.4  Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 4 Low blood pressure. | ||||
| 5 Diarrhoea Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| Analysis 8.5  Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 5 Diarrhoea. | ||||
| 6 Dizziness Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| Analysis 8.6  Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 6 Dizziness. | ||||
| 7 Itching (including elevated liver enzymes) Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| Analysis 8.7  Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 7 Itching (including elevated liver enzymes). | ||||
| 8 Red blood cell transfusion Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 8.8  Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 8 Red blood cell transfusion. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leg paraesthesia Show forest plot | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.08, 6.65] |
| Analysis 9.1  Comparison 9 Intravenous EPO, intravenous iron and oral iron versus intravenous iron and oral iron, Outcome 1 Leg paraesthesia. | ||||
| 2 Red blood cell transfusion Show forest plot | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 9.2  Comparison 9 Intravenous EPO, intravenous iron and oral iron versus intravenous iron and oral iron, Outcome 2 Red blood cell transfusion. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breastfeeding Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.9 [1.21, 2.98] |
| Analysis 10.1 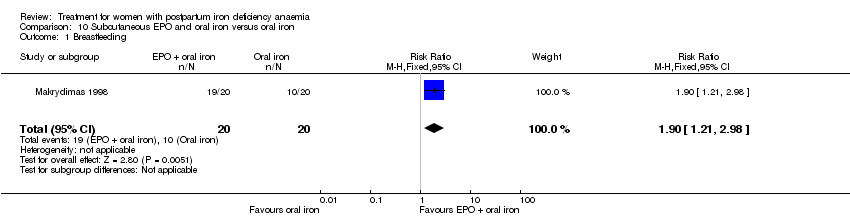 Comparison 10 Subcutaneous EPO and oral iron versus oral iron, Outcome 1 Breastfeeding. | ||||
| 2 Red blood cell transfusions Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| Analysis 10.2 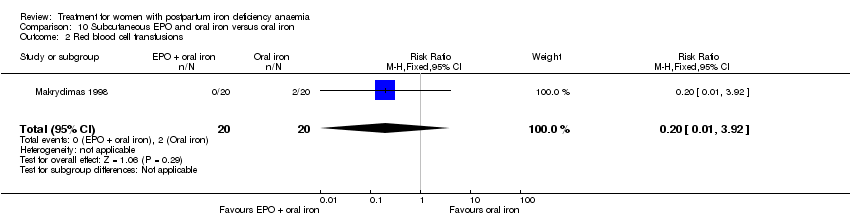 Comparison 10 Subcutaneous EPO and oral iron versus oral iron, Outcome 2 Red blood cell transfusions. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Heterogeneity ‐ Infections ‐ comparison 1 Show forest plot | 2 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.62, 1.84] |
| Analysis 14.1  Comparison 14 Sensitivity analysis, Outcome 1 Heterogeneity ‐ Infections ‐ comparison 1. | ||||
| 2 Heterogeneity, fixed effect ‐ Infections ‐ comparison 1 Show forest plot | 3 | 718 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.93, 2.38] |
| Analysis 14.2  Comparison 14 Sensitivity analysis, Outcome 2 Heterogeneity, fixed effect ‐ Infections ‐ comparison 1. | ||||
| 3 Heterogeneity ‐ Hepatic involvement ‐ comparison 1 Show forest plot | 2 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.06, 0.75] |
| Analysis 14.3 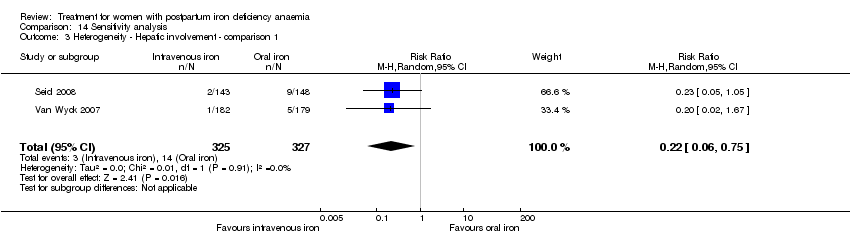 Comparison 14 Sensitivity analysis, Outcome 3 Heterogeneity ‐ Hepatic involvement ‐ comparison 1. | ||||
| 4 Heterogeneity, fixed effect ‐ Hepatic involvement ‐ comparison 1 Show forest plot | 3 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.21, 1.07] |
| Analysis 14.4  Comparison 14 Sensitivity analysis, Outcome 4 Heterogeneity, fixed effect ‐ Hepatic involvement ‐ comparison 1. | ||||

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
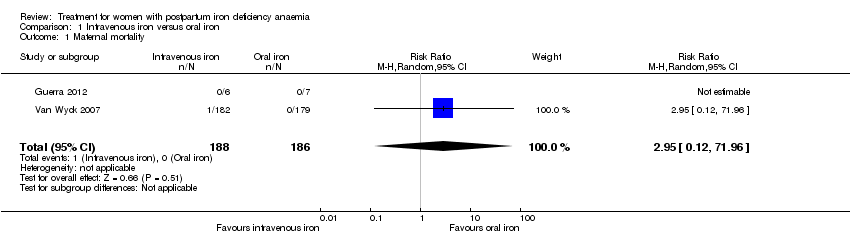
Comparison 1 Intravenous iron versus oral iron, Outcome 1 Maternal mortality.

Comparison 1 Intravenous iron versus oral iron, Outcome 2 Fatigue ‐ 14 days.

Comparison 1 Intravenous iron versus oral iron, Outcome 3 Fatigue ‐ 42 days.
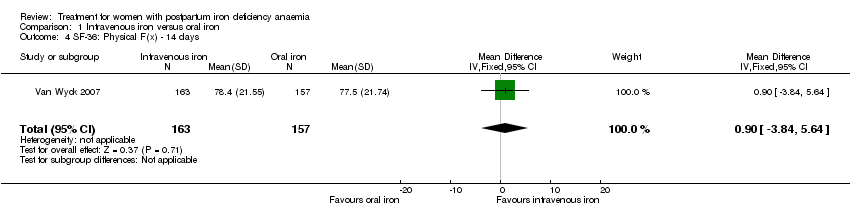
Comparison 1 Intravenous iron versus oral iron, Outcome 4 SF‐36: Physical F(x) ‐ 14 days.
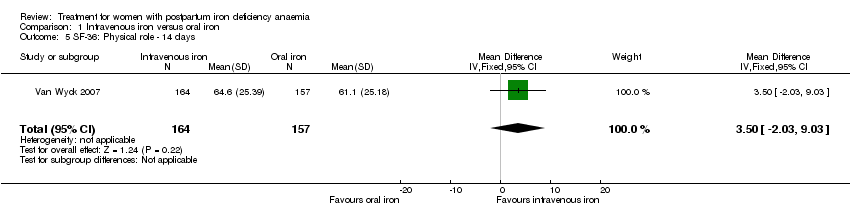
Comparison 1 Intravenous iron versus oral iron, Outcome 5 SF‐36: Physical role ‐ 14 days.
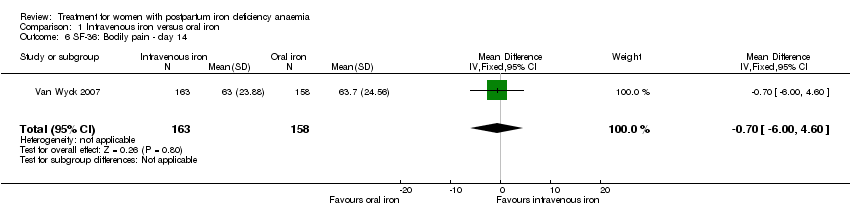
Comparison 1 Intravenous iron versus oral iron, Outcome 6 SF‐36: Bodily pain ‐ day 14.

Comparison 1 Intravenous iron versus oral iron, Outcome 7 SF‐36: General health ‐ 14 days.

Comparison 1 Intravenous iron versus oral iron, Outcome 8 SF‐36: Vitality ‐ 14 days.
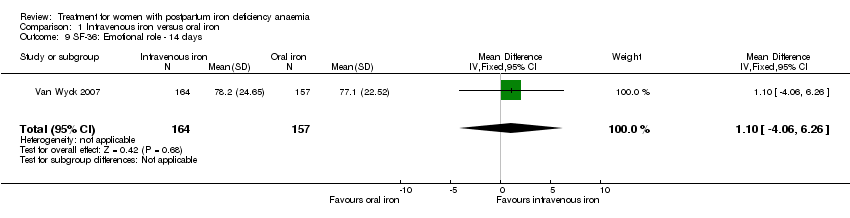
Comparison 1 Intravenous iron versus oral iron, Outcome 9 SF‐36: Emotional role ‐ 14 days.

Comparison 1 Intravenous iron versus oral iron, Outcome 10 SF‐36: Social function ‐ 14 days.
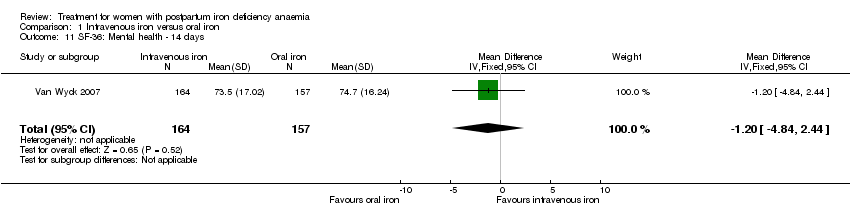
Comparison 1 Intravenous iron versus oral iron, Outcome 11 SF‐36: Mental health ‐ 14 days.

Comparison 1 Intravenous iron versus oral iron, Outcome 12 Depression.

Comparison 1 Intravenous iron versus oral iron, Outcome 13 Infections.

Comparison 1 Intravenous iron versus oral iron, Outcome 14 Compliance to treatment.

Comparison 1 Intravenous iron versus oral iron, Outcome 15 All gastrointestinal symptoms.
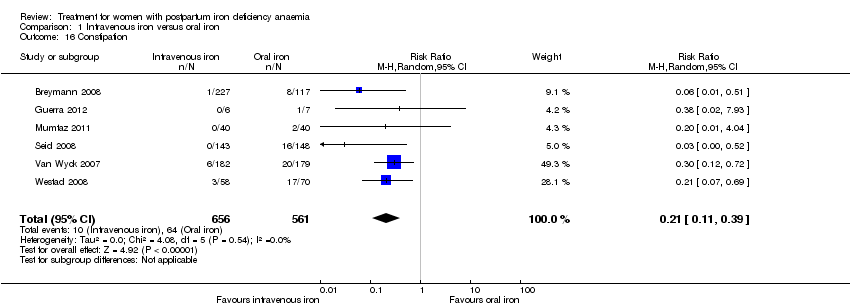
Comparison 1 Intravenous iron versus oral iron, Outcome 16 Constipation.
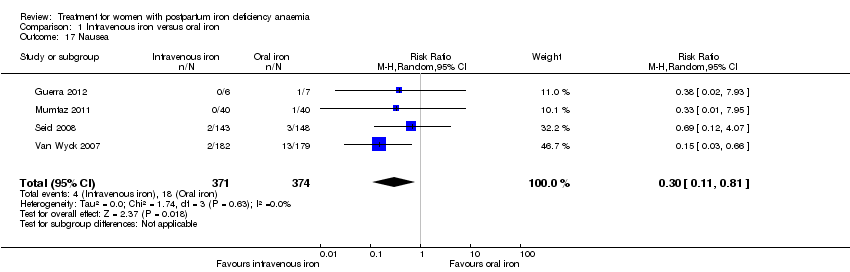
Comparison 1 Intravenous iron versus oral iron, Outcome 17 Nausea.

Comparison 1 Intravenous iron versus oral iron, Outcome 18 Gastrointestinal pain.

Comparison 1 Intravenous iron versus oral iron, Outcome 19 Diarrhoea.

Comparison 1 Intravenous iron versus oral iron, Outcome 20 Vomiting.

Comparison 1 Intravenous iron versus oral iron, Outcome 21 Dyspepsia.

Comparison 1 Intravenous iron versus oral iron, Outcome 22 Dysgeusia.
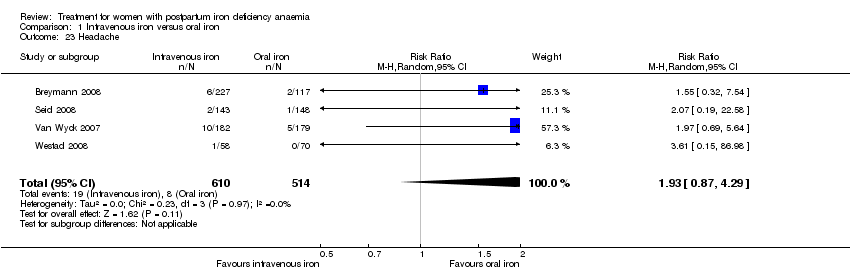
Comparison 1 Intravenous iron versus oral iron, Outcome 23 Headache.

Comparison 1 Intravenous iron versus oral iron, Outcome 24 Hepatic involvement.
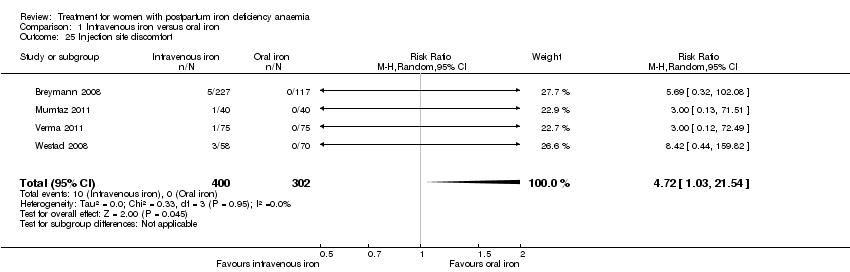
Comparison 1 Intravenous iron versus oral iron, Outcome 25 Injection site discomfort.
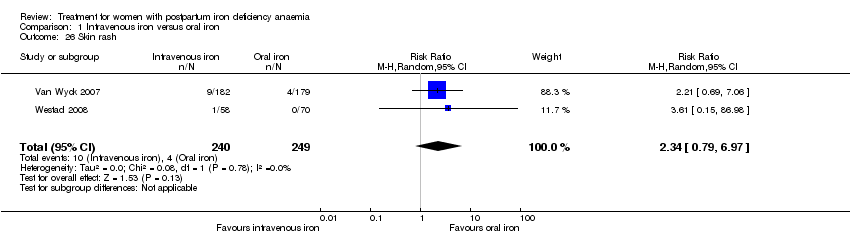
Comparison 1 Intravenous iron versus oral iron, Outcome 26 Skin rash.

Comparison 1 Intravenous iron versus oral iron, Outcome 27 Urticaria.

Comparison 1 Intravenous iron versus oral iron, Outcome 28 Flush.
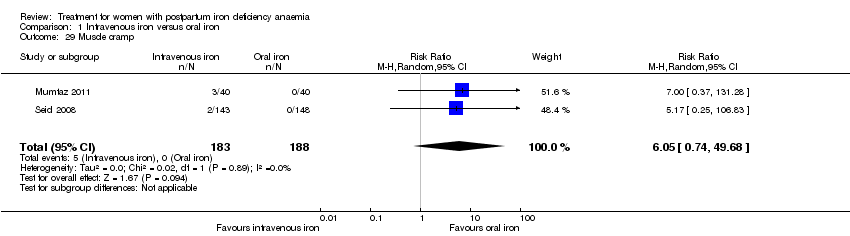
Comparison 1 Intravenous iron versus oral iron, Outcome 29 Muscle cramp.

Comparison 1 Intravenous iron versus oral iron, Outcome 30 Pain (not specified).

Comparison 1 Intravenous iron versus oral iron, Outcome 31 Seriouse adverse events (not specified).

Comparison 1 Intravenous iron versus oral iron, Outcome 32 Anaphylaxis or evidence of hypersensitivity.
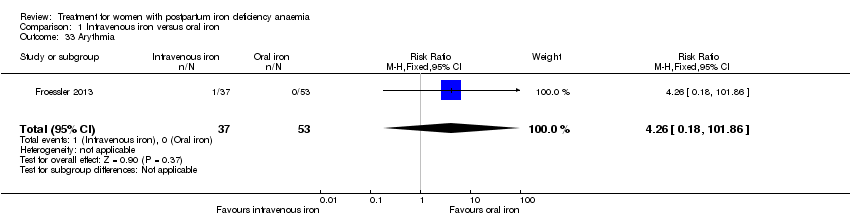
Comparison 1 Intravenous iron versus oral iron, Outcome 33 Arythmia.

Comparison 1 Intravenous iron versus oral iron, Outcome 34 Red blood cell transfusion.
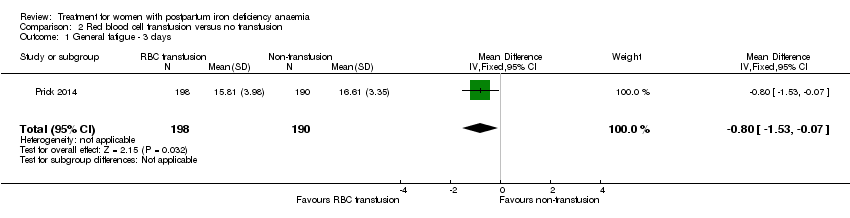
Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 1 General fatigue ‐ 3 days.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 2 General fatigue ‐ 6 weeks.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 3 SF‐36: Physical functioning ‐ 1 week.
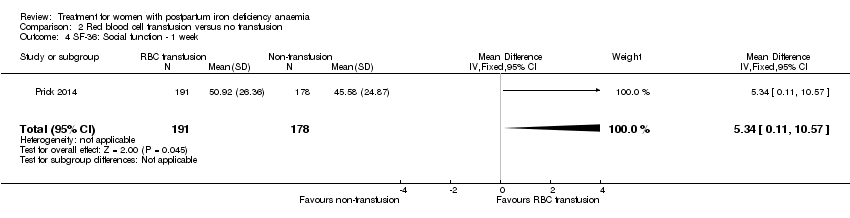
Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 4 SF‐36: Social function ‐ 1 week.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 5 SF‐36: Physical role ‐ 1 week.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 6 SF‐36: Bodily pain ‐ 1 week.
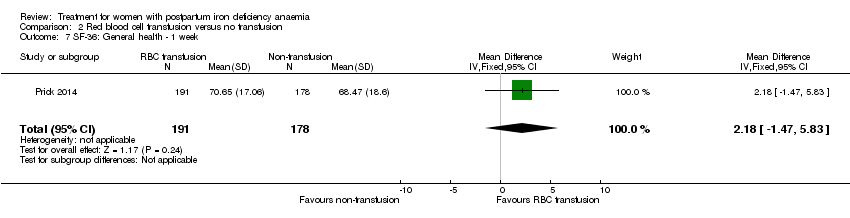
Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 7 SF‐36: General health ‐ 1 week.
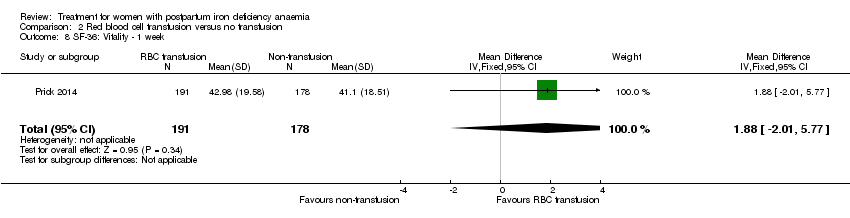
Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 8 SF‐36: Vitality ‐ 1 week.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 9 SF‐36: Emotional role ‐ 1 week.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 10 SF‐36: Mental health ‐ 1 week.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 11 Infections.
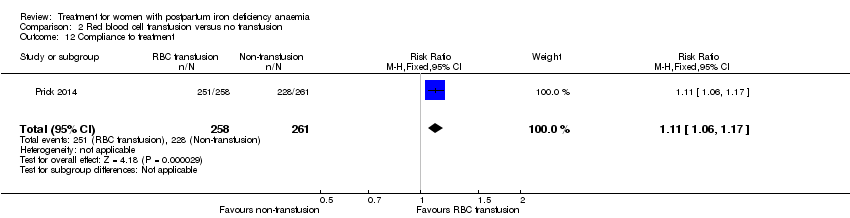
Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 12 Compliance to treatment.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 13 Breastfeeding at six weeks.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 14 Erythrocyte alloantibody formation.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 15 Rash.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 16 Fever.
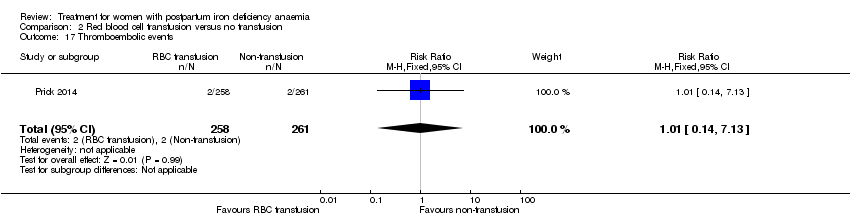
Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 17 Thromboembolic events.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 18 Parenteral iron intolerance.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 19 Transfusion reactions.

Comparison 3 Oral iron versus placebo, Outcome 1 Digit Symbol Substitution test ‐ 10 weeks.

Comparison 3 Oral iron versus placebo, Outcome 2 EPDS ‐ 10 weeks.

Comparison 3 Oral iron versus placebo, Outcome 3 STAI ‐ 10 weeks.

Comparison 3 Oral iron versus placebo, Outcome 4 Percieved Stress ‐ 10 weeks.
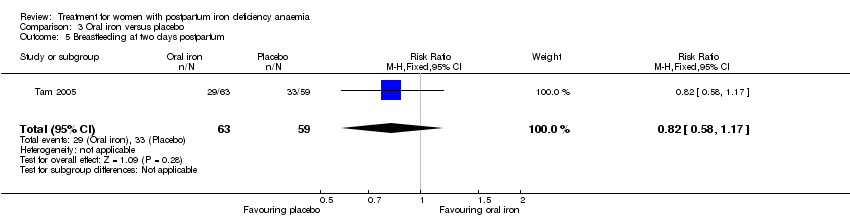
Comparison 3 Oral iron versus placebo, Outcome 5 Breastfeeding at two days postpartum.

Comparison 3 Oral iron versus placebo, Outcome 6 Back pain.

Comparison 3 Oral iron versus placebo, Outcome 7 All gastrointestinal symptoms.
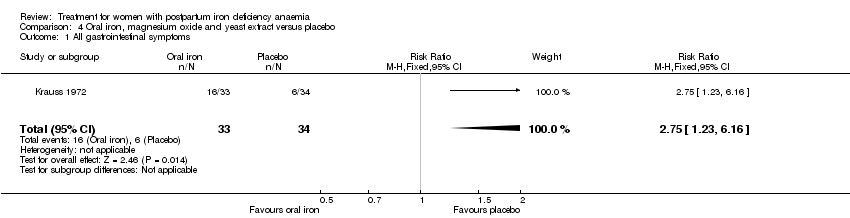
Comparison 4 Oral iron, magnesium oxide and yeast extract versus placebo, Outcome 1 All gastrointestinal symptoms.

Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 1 All gastrointestinal symptoms.
Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 2 Abdominal pain.

Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 3 Constipation.

Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 4 Diarrhoea.
Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 5 Nausea.

Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 6 Dysgeusia.

Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 7 Flatulence.

Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 8 Melaena.
Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 9 Headache.

Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 1 Persistent anaemia symptoms on a VAS scale: 1 week.

Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 2 Persistent anaemia symptoms on a VAS scale: 2 week.
Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 3 Persistent anaemia symptoms on a VAS scale: 6 week.
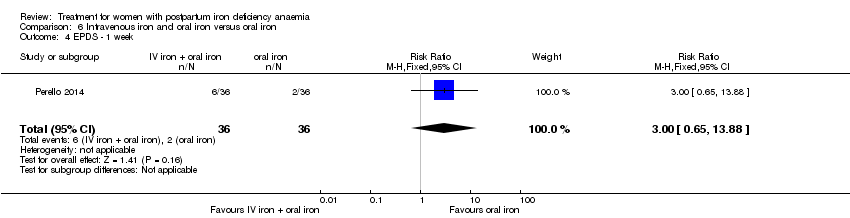
Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 4 EPDS ‐ 1 week.

Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 5 Length of hospital stay.
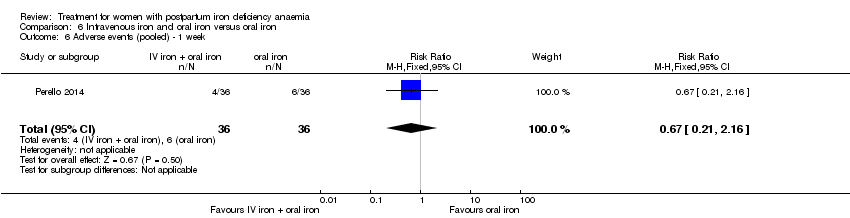
Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 6 Adverse events (pooled) ‐ 1 week.
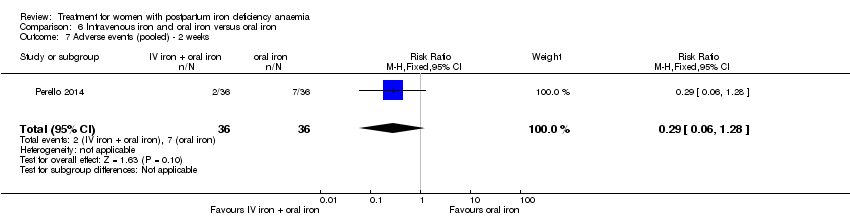
Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 7 Adverse events (pooled) ‐ 2 weeks.
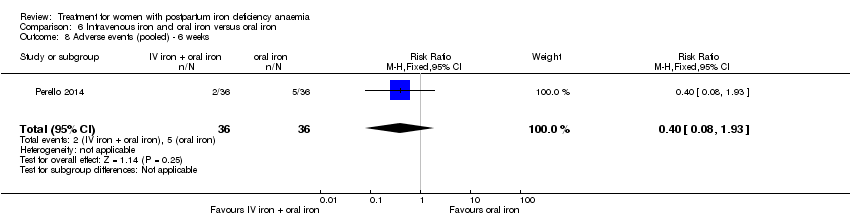
Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 8 Adverse events (pooled) ‐ 6 weeks.

Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 9 Red blood cell transfusion.

Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 10 Anaphylaxis or evidence of hypersensitivity.

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 1 Postpartum depression.
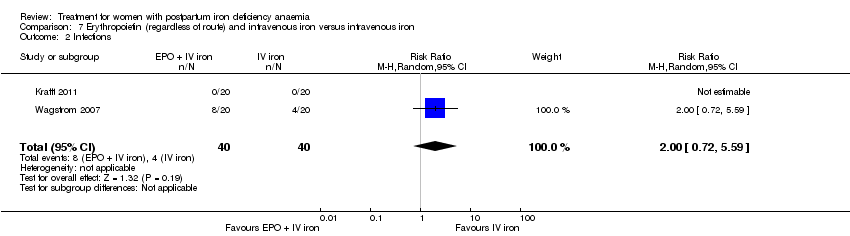
Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 2 Infections.
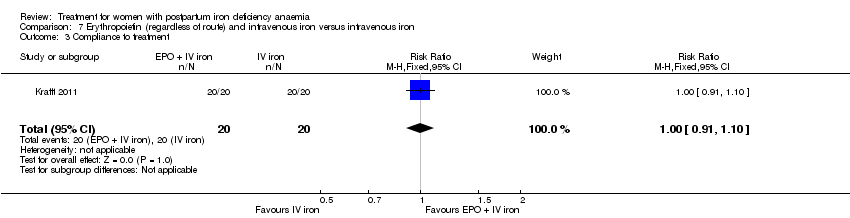
Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 3 Compliance to treatment.

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 4 Breasfeeding.

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 5 Dysgeusia.
Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 6 Flush.
Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 7 Diarrhoea.

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 8 Headache.

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 9 Itching (including elevated liver enzymes).

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 10 Dizziness.
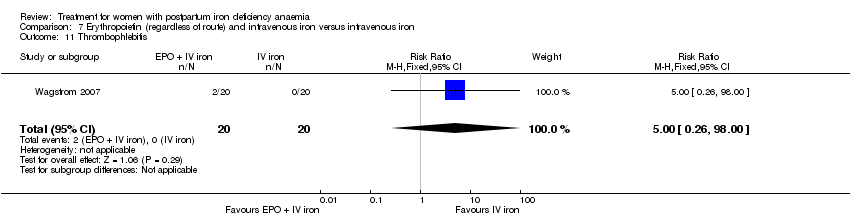
Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 11 Thrombophlebitis.

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 12 Red blood cell transfusion.
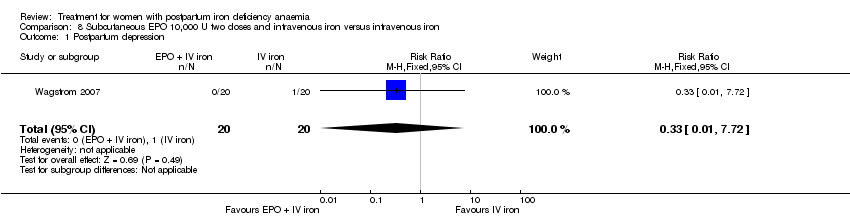
Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 1 Postpartum depression.
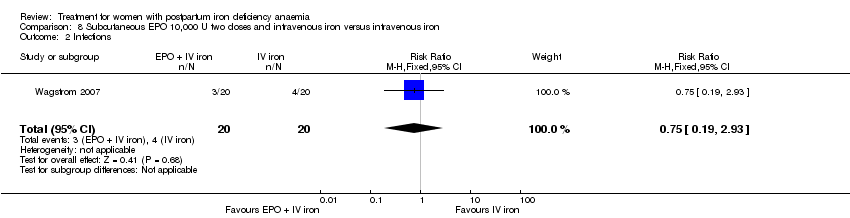
Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 2 Infections.
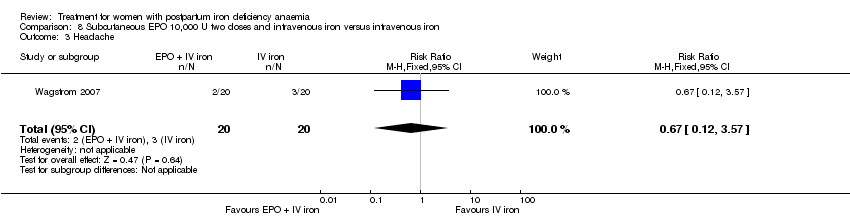
Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 3 Headache.

Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 4 Low blood pressure.

Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 5 Diarrhoea.

Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 6 Dizziness.
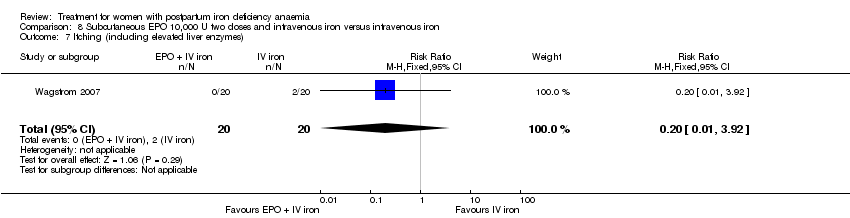
Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 7 Itching (including elevated liver enzymes).

Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 8 Red blood cell transfusion.

Comparison 9 Intravenous EPO, intravenous iron and oral iron versus intravenous iron and oral iron, Outcome 1 Leg paraesthesia.
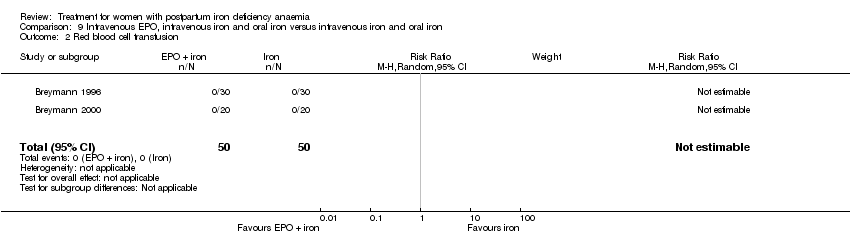
Comparison 9 Intravenous EPO, intravenous iron and oral iron versus intravenous iron and oral iron, Outcome 2 Red blood cell transfusion.

Comparison 10 Subcutaneous EPO and oral iron versus oral iron, Outcome 1 Breastfeeding.
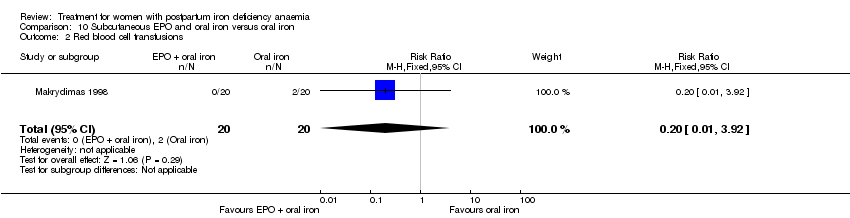
Comparison 10 Subcutaneous EPO and oral iron versus oral iron, Outcome 2 Red blood cell transfusions.
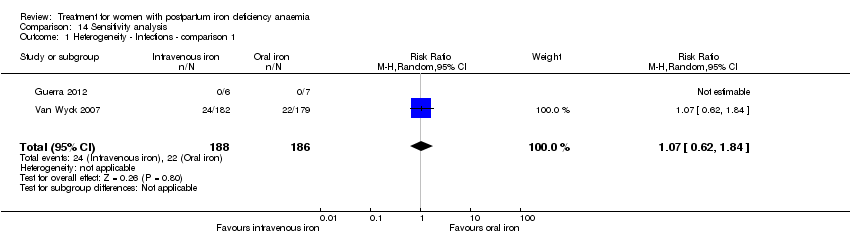
Comparison 14 Sensitivity analysis, Outcome 1 Heterogeneity ‐ Infections ‐ comparison 1.

Comparison 14 Sensitivity analysis, Outcome 2 Heterogeneity, fixed effect ‐ Infections ‐ comparison 1.
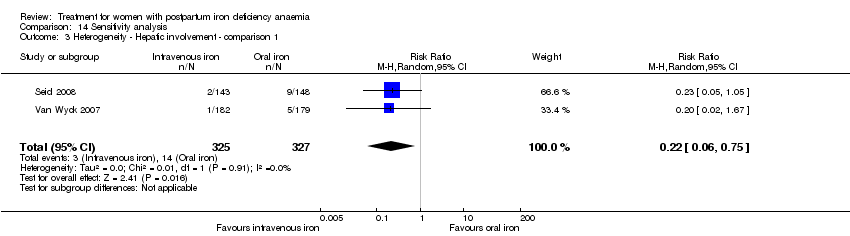
Comparison 14 Sensitivity analysis, Outcome 3 Heterogeneity ‐ Hepatic involvement ‐ comparison 1.

Comparison 14 Sensitivity analysis, Outcome 4 Heterogeneity, fixed effect ‐ Hepatic involvement ‐ comparison 1.
| Intravenous iron compared with oral iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron | Intravenous iron | |||||
| Maternal mortality | Study population | RR 2.95 | 374 | ⊕⊕⊝⊝ | 1 maternal death was reported across the included studies. | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Fatigue at 14, 28, and 42 days | See comment | See comment | Not estimable | 361 | ⊕⊝⊝⊝ | No statistically significant difference was found at days 14 and 42 days. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Infections | Study population | RR 1.7 | 718 | ⊕⊝⊝⊝ | ||
| 86 per 1000 | 146 per 1000 | |||||
| Moderate | ||||||
| 34 per 1000 | 58 per 1000 | |||||
| Constipation | Study population | RR 0.21 | 1217 | ⊕⊝⊝⊝ | ||
| 114 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 112 per 1000 | 24 per 1000 | |||||
| All gastrointestinal symptoms | Study population | RR 0.31 | 1307 | ⊕⊝⊝⊝ | ||
| 216 per 1000 | 67 per 1000 | |||||
| Moderate | ||||||
| 261 per 1000 | 81 per 1000 | |||||
| Anaphylaxis or evidence of hypersensitivity | Study population | RR 2.78 | 1454 | ⊕⊕⊝⊝ | 3 cases of allergic reactions all occurred in the group treated with intravenous iron. | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The outcome is unlikely to be influenced by risk of bias and so we did not downgrade the evidence for this outcome: open‐label design combined with a objective outcome measure. | ||||||
| Red blood cell transfusion compared with non‐transfusion for postpartum iron deficiency anaemia | ||||||
| Patient or population: patients with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐transfusion | RBC transfusion | |||||
| Maternal mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Fatigue | See comment | See comment | 519 | ⊕⊕⊝⊝ | General fatigue at 3 days was 0.8 lower (1.53 to 0.07) in the transfused group. No statistically significant difference was seen at six weeks. | |
| Persistent anaemia symptoms | Study population | Not estimable | 519 | ⊕⊝⊝⊝ | The outcome was not systematically registered/reported. | |
| See comment | See comment | |||||
| Moderate | ||||||
| Infections | Study population | RR 0.93 | 519 | ⊕⊕⊕⊝ | ||
| 92 per 1000 | 86 per 1000 | |||||
| Moderate | ||||||
| 92 per 1000 | 86 per 1000 | |||||
| Erythrocyte alloantibody formation | Study population | RR 3.03 | 519 | ⊕⊝⊝⊝ | There was no systematical screening for this outcome in the study population. | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Thromboembolic events | Study population | RR 1.01 | 519 | ⊕⊕⊝⊝ | ||
| 8 per 1000 | 8 per 1000 | |||||
| Moderate | ||||||
| 8 per 1000 | 8 per 1000 | |||||
| Transfusion reactions | Study population | RR 7.08 | 519 | ⊕⊝⊝⊝ | 3 cases of transfusion reactions occurred in the transfusion group. | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to risk of bias: open‐label design combined with a subjective outcome measure. | ||||||
| Oral iron compared with placebo for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oral iron | |||||
| Maternal mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms | Study population | Not estimable | (1) | See comment | Symptoms of anaemia were not reported for the anaemic groups separately. | |
| See comment | See comment | |||||
| Moderate | ||||||
| All gastrointestinal symptoms | Study population | RR 1 | 68 | ⊕⊝⊝⊝ | ||
| 176 per 1000 | 176 per 1000 | |||||
| Moderate | ||||||
| 177 per 1000 | 177 per 1000 | |||||
| Constipation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to risk of bias: open‐label design combined with a subjective outcome measure. | ||||||
| Intravenous iron with oral iron compared with oral iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron | Intravenous iron with oral iron | |||||
| Maternal mortality | See comment | See comment | Not estimable | ‐ | See comment | In 1 study no maternal deaths were reported. The other study did not report on maternal mortality. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ 1 week | Study population | RR 1.75 | 72 | ⊕⊝⊝⊝ | ||
| 111 per 1000 | 194 per 1000 | |||||
| Moderate | ||||||
| 111 per 1000 | 194 per 1000 | |||||
| Persistent anaemia symptoms ‐ 2 weeks | Study population | RR 0.6 | 72 | ⊕⊝⊝⊝ | ||
| 139 per 1000 | 83 per 1000 | |||||
| Moderate | ||||||
| 139 per 1000 | 83 per 1000 | |||||
| Persistent anaemia symptoms ‐ 6 weeks | Study population | RR 3 | 72 | ⊕⊝⊝⊝ | ||
| 28 per 1000 | 83 per 1000 | |||||
| Moderate | ||||||
| 28 per 1000 | 84 per 1000 | |||||
| Infections ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Anaphylaxis or evidence of hypersensitivity | Study population | Not estimable | 0 | ⊕⊕⊝⊝ | 1 study reported 0 events, other study pooled adverse events, not reporting allergic reactions separately. Thus the effect was not estimable. | |
| See comment | See comment | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to risk of bias: the included study had high risk of attrition and reporting bias. | ||||||
| Erythropoietin (regardless of rout) with intravenous iron compared with intravenous iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous iron | EPO (regardless of rout) with IV iron | |||||
| Maternal mortality | See comment | See comment | Not estimable | ‐ | See comment | In 1 study no maternal deaths were reported. The other study did not report on maternal mortality. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Thromboembolic events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Subcutaneous EPO 10,000 U of doses with intravenous iron compared with intravenous iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: patients with women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous iron | Erythropoietin 10,000 U 2 doses with intravenous iron | |||||
| Maternal mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Thromboembolic events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Subcutaneous EPO with oral iron compared with oral iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron | Subcutaneous EPO with oral iron | |||||
| Maternal mortality | See comment | See comment | Not estimable | 40 | See comment | No maternal deaths were reported. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Thromboembolic events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Subcutaneous EPO with IV iron and oral iron compared with intravenous iron with oral iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous iron + oral iron | Subcutaneous EPO + IV iron + oral iron | |||||
| Maternal mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Thromboembolic events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal mortality Show forest plot | 2 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.12, 71.96] |
| 2 Fatigue ‐ 14 days Show forest plot | 1 | 322 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐8.04, 1.44] |
| 3 Fatigue ‐ 42 days Show forest plot | 1 | 329 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐6.77, 2.57] |
| 4 SF‐36: Physical F(x) ‐ 14 days Show forest plot | 1 | 320 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐3.84, 5.64] |
| 5 SF‐36: Physical role ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 3.50 [‐2.03, 9.03] |
| 6 SF‐36: Bodily pain ‐ day 14 Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐6.00, 4.60] |
| 7 SF‐36: General health ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐3.09, 4.49] |
| 8 SF‐36: Vitality ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐3.64, 5.44] |
| 9 SF‐36: Emotional role ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐4.06, 6.26] |
| 10 SF‐36: Social function ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐4.08, 6.08] |
| 11 SF‐36: Mental health ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐4.84, 2.44] |
| 12 Depression Show forest plot | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.00] |
| 13 Infections Show forest plot | 3 | 718 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.58, 5.03] |
| 14 Compliance to treatment Show forest plot | 5 | 890 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.01, 1.35] |
| 15 All gastrointestinal symptoms Show forest plot | 8 | 1307 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.20, 0.47] |
| 16 Constipation Show forest plot | 6 | 1217 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.11, 0.39] |
| 17 Nausea Show forest plot | 4 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.11, 0.81] |
| 18 Gastrointestinal pain Show forest plot | 4 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.04, 0.83] |
| 19 Diarrhoea Show forest plot | 3 | 569 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.02, 0.59] |
| 20 Vomiting Show forest plot | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 9.66] |
| 21 Dyspepsia Show forest plot | 2 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.04, 3.20] |
| 22 Dysgeusia Show forest plot | 4 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 7.20 [1.63, 31.76] |
| 23 Headache Show forest plot | 4 | 1124 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.87, 4.29] |
| 24 Hepatic involvement Show forest plot | 3 | 996 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.12, 1.71] |
| 25 Injection site discomfort Show forest plot | 4 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 4.72 [1.03, 21.54] |
| 26 Skin rash Show forest plot | 2 | 489 | Risk Ratio (M‐H, Random, 95% CI) | 2.34 [0.79, 6.97] |
| 27 Urticaria Show forest plot | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [0.47, 36.59] |
| 28 Flush Show forest plot | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [1.18, 68.81] |
| 29 Muscle cramp Show forest plot | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 6.05 [0.74, 49.68] |
| 30 Pain (not specified) Show forest plot | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.42 [0.44, 159.82] |
| 31 Seriouse adverse events (not specified) Show forest plot | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.26, 4.06] |
| 32 Anaphylaxis or evidence of hypersensitivity Show forest plot | 8 | 1454 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.31, 24.92] |
| 33 Arythmia Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.26 [0.18, 101.86] |
| 34 Red blood cell transfusion Show forest plot | 4 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.19, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 General fatigue ‐ 3 days Show forest plot | 1 | 388 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.53, ‐0.07] |
| 2 General fatigue ‐ 6 weeks Show forest plot | 1 | 318 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.22, 0.72] |
| 3 SF‐36: Physical functioning ‐ 1 week Show forest plot | 1 | 368 | Mean Difference (IV, Fixed, 95% CI) | 5.67 [0.84, 10.50] |
| 4 SF‐36: Social function ‐ 1 week Show forest plot | 1 | 369 | Mean Difference (IV, Fixed, 95% CI) | 5.34 [0.11, 10.57] |
| 5 SF‐36: Physical role ‐ 1 week Show forest plot | 1 | 366 | Mean Difference (IV, Fixed, 95% CI) | 4.56 [‐1.41, 10.53] |
| 6 SF‐36: Bodily pain ‐ 1 week Show forest plot | 1 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.90, 1.90] |
| 7 SF‐36: General health ‐ 1 week Show forest plot | 1 | 369 | Mean Difference (IV, Fixed, 95% CI) | 2.18 [‐1.47, 5.83] |
| 8 SF‐36: Vitality ‐ 1 week Show forest plot | 1 | 369 | Mean Difference (IV, Fixed, 95% CI) | 1.88 [‐2.01, 5.77] |
| 9 SF‐36: Emotional role ‐ 1 week Show forest plot | 1 | 368 | Mean Difference (IV, Fixed, 95% CI) | 4.37 [‐4.51, 13.25] |
| 10 SF‐36: Mental health ‐ 1 week Show forest plot | 1 | 369 | Mean Difference (IV, Fixed, 95% CI) | 1.21 [‐2.29, 4.71] |
| 11 Infections Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.53, 1.61] |
| 12 Compliance to treatment Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.06, 1.17] |
| 13 Breastfeeding at six weeks Show forest plot | 1 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.07] |
| 14 Erythrocyte alloantibody formation Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 74.15] |
| 15 Rash Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 74.15] |
| 16 Fever Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.06 [0.24, 104.84] |
| 17 Thromboembolic events Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.13] |
| 18 Parenteral iron intolerance Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.24] |
| 19 Transfusion reactions Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.08 [0.37, 136.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Digit Symbol Substitution test ‐ 10 weeks Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.76, 2.76] |
| 2 EPDS ‐ 10 weeks Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.86, 1.06] |
| 3 STAI ‐ 10 weeks Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐3.18, 2.38] |
| 4 Percieved Stress ‐ 10 weeks Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 4.1 [1.70, 6.50] |
| 5 Breastfeeding at two days postpartum Show forest plot | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.58, 1.17] |
| 6 Back pain Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.42, 1.03] |
| 7 All gastrointestinal symptoms Show forest plot | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.36, 2.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All gastrointestinal symptoms Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.23, 6.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All gastrointestinal symptoms Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.83, 2.45] |
| 2 Abdominal pain Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [0.55, 13.48] |
| 3 Constipation Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.55, 2.60] |
| 4 Diarrhoea Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.35, 30.51] |
| 5 Nausea Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.14, 78.49] |
| 6 Dysgeusia Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.14, 78.49] |
| 7 Flatulence Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.72] |
| 8 Melaena Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.72] |
| 9 Headache Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistent anaemia symptoms on a VAS scale: 1 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.46] |
| 2 Persistent anaemia symptoms on a VAS scale: 2 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.33] |
| 3 Persistent anaemia symptoms on a VAS scale: 6 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.50] |
| 4 EPDS ‐ 1 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.65, 13.88] |
| 5 Length of hospital stay Show forest plot | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.02, 0.42] |
| 6 Adverse events (pooled) ‐ 1 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.21, 2.16] |
| 7 Adverse events (pooled) ‐ 2 weeks Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.28] |
| 8 Adverse events (pooled) ‐ 6 weeks Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 1.93] |
| 9 Red blood cell transfusion Show forest plot | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.72] |
| 10 Anaphylaxis or evidence of hypersensitivity Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postpartum depression Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 2 Infections Show forest plot | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.72, 5.59] |
| 3 Compliance to treatment Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.91, 1.10] |
| 4 Breasfeeding Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.91, 1.10] |
| 5 Dysgeusia Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.27, 1.88] |
| 6 Flush Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.20, 20.33] |
| 7 Diarrhoea Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 8 Headache Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.60] |
| 9 Itching (including elevated liver enzymes) Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| 10 Dizziness Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 11 Thrombophlebitis Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 98.00] |
| 12 Red blood cell transfusion Show forest plot | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postpartum depression Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 2 Infections Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.19, 2.93] |
| 3 Headache Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.57] |
| 4 Low blood pressure Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| 5 Diarrhoea Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 6 Dizziness Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 7 Itching (including elevated liver enzymes) Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| 8 Red blood cell transfusion Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leg paraesthesia Show forest plot | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.08, 6.65] |
| 2 Red blood cell transfusion Show forest plot | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breastfeeding Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.9 [1.21, 2.98] |
| 2 Red blood cell transfusions Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Heterogeneity ‐ Infections ‐ comparison 1 Show forest plot | 2 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.62, 1.84] |
| 2 Heterogeneity, fixed effect ‐ Infections ‐ comparison 1 Show forest plot | 3 | 718 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.93, 2.38] |
| 3 Heterogeneity ‐ Hepatic involvement ‐ comparison 1 Show forest plot | 2 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.06, 0.75] |
| 4 Heterogeneity, fixed effect ‐ Hepatic involvement ‐ comparison 1 Show forest plot | 3 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.21, 1.07] |

