مداخلات شناختی‐رفتاری در مدیریت اختلال نقص توجه و بیشفعالی (ADHD) در بزرگسالان
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
| Participants | Country: Iceland Setting: ambulatory Age: adults; specific ages not given Sample size: 54 Sex: 34 women, 20 men Inclusion criteria: clinical diagnosis of ADHD; and stable on prescribed ADHD medication for at least a month Exclusion criteria: severe mental illness; active drug abuse; verbal intelligence quotient (IQ) estimated from clinical records to be below 85; and no valid ADHD diagnosis or not prescribed/taking ADHD medication | |
| Interventions | Intervention: CBT (15 sessions total, twice weekly, each lasting 90 minutes) + pharmacotherapy (n = 27) Control: treatment as usual (n = 27) Methylphenidate dosages ranged between 18‐180 mg, with a mean dosage of 60.5 mg at baseline. By the end of treatment, the dosage range was 36‐162 mg, with a mean dosage of 62.5 mg. | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | We contacted authors to get the information about random sequence generation and allocation concealment that we included in this table (Young 2014 [pers comm]). Study start date: not specified Study end date: not specified Funding source: Support for the study was received from research grants awarded by RANNIS the Icelandic Centre for Research (Nr. 080443022), the Landspitali Science Fund, and Janssen‐Cilag, Iceland. Declarations of interest: Brynjar Emilsson, Jon F Sigurdsson, Gisli Baldursson, Emil Einarsson and Halldora Olafsdottir declare that they have no competing interests. Susan Young has been a consultant for Janssen‐Cilag, Eli‐Lilly and Shire. She has given educational talks at meetings sponsored by Janssen‐Cilag, Shire, Novatis, Eli‐Lilly and Flynn‐Pharma and has received research grants from Janssen‐Cilag, Eli‐Lilly and Shire. Susan Young is a consultant for the Cognitive Centre of Canada and is co‐author of 'R&R2 for ADHD Youths and Adults'. Gisli Baldursson has been a consultant for Eli‐Lilly and given educational talks at meetings sponsored by Janssen‐Cilag and Shire. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The independent evaluators were psychiatrists who were blind to the treatment condition." |
| Incomplete outcome data (attrition bias) | High risk | Comment: the proportion of dropouts was 37% in both groups. Quote: "Missing values were not imputed because the ANCOVA calculates outcome whilst adjusting for all baseline data. Between group effect sizes for the outcome assessments were measured using Cohen's d using unadjusted means for the dependent variables and SD pooled for unequal group sizes. Fisher's exact test was used to compare proportions of medication changes. Since this study follows an ITT protocol, statistical analysis of the outcome variables were completed for all participants regardless of medication changes." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | High risk | Quote: "The participants in both conditions were not asked to refrain from engaging in other interventions during the study period." |
| Conflict of interest | Low risk | Comment: no evidence of conflicts of interest |
| Methods | Randomised controlled trial | |
| Participants | Country: USA Setting: ambulatory Age: adults (18 to 24 years old) Sample size: 33 Sex: 14 women, 19 men Inclusion criteria: currently enrolled undergraduate students; meeting criteria for ADHD in adulthood, including symptom onset by age 12 and functional impairment in multiple domains Exclusion criteria: current substance abuse/dependence; or active suicidal ideation, major depressive episode, and history of psychotic disorder, bipolar disorder, or pervasive developmental disorder. | |
| Interventions | Intervention: dialectical behaviour therapy (DBT) (8 weekly 90 min group sessions focused on skills acquisition and strengthening, and 7 weekly 10‐15 min individual coaching phone calls focused on skills generalisation) (n = 17; 12 participants with pharmacotherapy and 5 without pharmacotherapy) Control: skills handouts control condition (34 pages of skills handouts, drawn from a manual for treatment of adults with ADHD and designed to reflect publicly available self‐help materials for ADHD) (n = 16; 13 participants with pharmacotherapy and 3 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. | |
| Outcomes | Primary outcomes
| |
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: University of Washington – Robert C Bolles Doctoral Research Fellowship Declarations of interest: the author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it is not possible to blinding personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: participants were assessed by an interviewer who was blind to participant condition. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The intent‐to‐treat sample included 17 and 16 participants in the DBT group skills training and self‐guided SH, respectively. One participant dropped out of DBT after four sessions and did not complete the post‐treatment or follow‐up assessments; all other participants completed treatment and the three study assessments. Missing data from this participant were imputed conservatively using the last observation carried forward (LOCF) method ... Two participants receiving DBT and one receiving SH had substantial ADHD medication changes during the study (> 25% change in dose or change in medication type). One participant in each treatment condition met four (rather than five) ADHD inattentive symptom criteria. All analyses were conducted with and without medication changes, and with and without participants who did not meet full DSM‐V criteria. The pattern of results did not differ; thus, results from the full intent‐to‐treat sample are reported." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Quote: "This study cannot rule out therapist effects or non‐specific factors of group psychotherapy, although the latter concern is mitigated by the fact that response rates with SH approximate those of supportive group psychotherapy or similar control conditions in previous trials for adults with ADHD." |
| Conflict of interest | Low risk | Quote: "The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article." |
| Methods | Randomised controlled trial | |
| Participants | Country: China Setting: ambulatory Age: undergraduate students between the ages of 19 and 24 Sample size: 54 Sex: 24 women, 30 men Inclusion criteria: meeting DSM‐5 criteria for ADHD in adulthood Exclusion criteria: major depressive episode, bipolar disorder, substance abuse/dependence within the last 6 months; actively suicidal ideation; history of psychotic disorder, and learning difficulties or other cognitive impairments | |
| Interventions | Intervention: mindfulness‐based cognitive therapy (8 weekly 2.5‐h sessions) (n = 28; 20 participants with pharmacotherapy and 8 without pharmacotherapy) Control: waiting list group (n = 26; 20 participants with pharmacotherapy and 6 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. | |
| Outcomes | Primary outcomes
| |
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: the author(s) received no financial support for the research, authorship or publication of this article. Declarations of interest: the author(s) declared no potential conflicts of interest with respect to the research, authorship or publication of this article. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: participants were assessed by an interviewer who was blind to participant condition. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The intent‐to‐treat sample consisted of 30 and 26 participants from the MBCT treatment group and [waiting list] control group, respectively. Two participants dropped out of [mindfulness‐based cognitive therapy] after six sessions and did not complete the post‐treatment or follow‐up assessments; all other participants completed treatment and the three study assessments." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Quote: "The majority of the sample was Chinese students who were recruited through general psychology courses." |
| Conflict of interest | Low risk | Comment: the author(s) declared no potential conflicts of interest with respect to the research, authorship or publication of this article. |
| Methods | Randomised controlled trial | |
| Participants | Country: the Netherlands Setting: ambulatory Age: adults, 18‐65 years old Sample size: 103 Sex: 56 women, 47 men Inclusion criteria: meeting criteria for ADHD in adulthood with all subtypes Exclusion criteria: substance abuse/ dependence within the last 6 months; comorbid psychotic disorders; borderline‐ and/or antisocial personality disorders; learning difficulties; chronic suicidal ideation; and automutilation. | |
| Interventions | Intervention: mindfulness‐based cognitive therapy (12 sessions) (n = 55; 33 participants with pharmacotherapy and 22 without pharmacotherapy) Control: waiting list group (n = 48; 26 participants with pharmacotherapy and 22 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: the author(s) received no financial support for the research, authorship, and/or publication of this article. Declarations of interest: the author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Cornelis C. Kan has also been a member of the advisory board and consultancy team of Eli Lilly BV and was a speaker at the Adult‐ADHD Academy of Eli Lilly. The other authors declared that they have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: in this study, randomisation was done by shuffling cards on which an identifier number for each participant was written. |
| Allocation concealment (selection bias) | Low risk | Comment: an independent researcher randomly assigned the participant to the MBCT or control group. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The clinical interviews were conducted single blindly by a psychiatrist." |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: the proportion of dropouts was 25% for the mindfulness‐based cognitive therapy and 12% for the waiting list group. Quote: "To provide a more conservative estimate of the treatment effect, the authors performed ITT analyses with imputation of missing data according to LOCF. In addition, the smaller sample size at the end of the study might have led to Type II errors, that is, not establishing differences that were present, due to insufficient power." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Quote: "The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Cornelis C Kan has also been a member of the advisory board and consultancy team of Eli Lilly BV and was a speaker at the Adult‐ADHD Academy of Eli Lilly. The other authors declared that they have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article." |
| Methods | Randomised controlled trial | |
| Participants | Country: Sweden Setting: hospital (outpatients) Age: adults, 18 years old or older Sample size: 51 Sex: 32 women, 19 men Inclusion criteria: ADHD as the main neurodevelopmental diagnosis; if on any psychoactive drug treatment (for ADHD or other diagnoses), the treatment should have been stable for at least three months Exclusion criteria: ongoing substance abuse (during the last 3 months); mental retardation (Intelligence Quotient 70); organic brain injury; autism spectrum disorder; suicidal ideation; with clinically unstable psychosocial circumstances or psychiatric disorders that were of such a severity that participation was impossible, such as being homeless, or having severe depression, psychosis, or bipolar syndrome not under stable pharmacological treatment (judged by a clinical psychologist and a psychiatrist). | |
| Interventions | Intervention: dialectical behaviour therapy (DBT) (14 structured sessions) (n = 26; 5 participants with pharmacotherapy and 11 without pharmacotherapy) Control: structured discussion group (14 sessions of loosely structured discussion group) (n = 25; 14 participants with pharmacotherapy and 11 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. | |
| Outcomes | Primary outcome
| |
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: the clinical part of the study was conducted as part of the clinical work at Neuropsychiatric Unit Karolinska, Psychiatry Northwest, Stockholm County Council. The scientific parts of the projects were supported by the foundations Psykiatrifonden and Bror Gadelius Minnesfond. The funding sources had no role in study design, in the collection, analysis or interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. Declarations of interest: all authors declare that they have no conflicts of interest related to this work. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: the study authors did not describe this aspect. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: the proportion of dropout was 19% for the DBT group and 20% for the structured discussion group. The study authors executed an ITT analysis. Quote: "Although the study plan described on treatment analysis, i.e. analysis of those that completed the treatment staying stable on medication (if they had any), we also wanted to a posteriori explore whether the results would change if those cases who did not fulfill these criteria were included in the analyses (Intention To Treat, ITT, analyses with LOCF, last observation carried forward)." In general well‐being, the effect was significant also in the ITT analyses (P < 0.05). |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Comment: all study authors declared that they have no conflicts of interest related to this work. |
| Methods | Randomised controlled trial | |
| Participants | Country: Sweden Setting: ambulatory Age: adults over the age of 18 Sample size: 57 Sex: 39 women, 18 men Inclusion criteria: confirmed or probable diagnosis of ADHD; current problems with organising daily activity and inattention defined, as 17 or more points on the ADHD Self‐Report Scale (ASRS; R. C. Kessler et al., 2005) subscale for Inattention (items 1–4 and 7–11); has access to a smart phone (android or Iphone) with Internet access; speaks, writes and reads Swedish; and cannot foresee any practical barriers to participation such as travels or medical operations. Exclusion criteria: high alcohol or drug use assessed by the AUDIT/DUDIT and assessment interview; somatic or psychiatric problems that are directly contraindicated or seriously hamper the implementation of the treatment (e.g. psychotic disorders). | |
| Interventions | Intervention: CBT‐inspired Internet‐based course with support (Living Smart) (n = 29). The course consisted of 7 text modules distributed over 6 weeks. The weekly modules taught the use of an online calendar (via computer and smartphone) and applications for reminders and to‐do lists. Furthermore, additional apps were introduced that previously had been shown beneficial for adults with ADHD. Control: waiting list group (n = 28) The authors did not explicitly state how many patients received pharmacological treatment. | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: October 2012 Study end date: March 2013 Funding source: Karolinska Institutet Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: blind evaluators also assessed improvement in organisation and inattention at post‐treatment. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: the proportion of dropout was 10% for the CBT‐inspired Internet‐based course with support (Living Smart) and 4% for the WL group. Quote: "The analyses were done according to the principles of intent‐to treat. All participants, including those who ended the course prematurely, were asked to fill out the post‐measurement after the 6‐week period of the online course. For all statistical analyses, observed data were used in the primary analyses. To evaluate the effect of missing data, additional sensitivity analyses were performed using last‐observation‐carried forward where the last ASRS‐score of the weekly measures was used to replace missing data at post‐treatment." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | High risk | Comment: the lack of confirmed ADHD‐diagnoses for some of the participants and the fact that 12% did not receive an ADHD diagnose after their previous neuropsychiatric assessment and therefore were classified as sub‐clinical ADHD |
| Conflict of interest | Low risk | Comment: no evidence of conflicts of interest |
| Methods | Randomised controlled trial | |
| Participants | Country: Sweden Setting: hospital (outpatients) Age: adults (specific ages not given) Sex: 29 women, 16 men Exclusion criteria: diagnosis of borderline or antisocial personality disorder and bipolar disorder; ongoing substance abuse; suicidal ideation; dyslexia; mental retardation; ongoing psychotherapy | |
| Interventions | Intervention:
Both the iCBT‐G and iCBT‐S groups followed the iCBT programme In Focus, developed by the Swedish company Livanda – Internet Clinic, Ltd., in collaboration with the NPC. Control: waiting list group (n = 18; 9 participants with specific pharmacotherapy for ADHD and 9 without specific pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | We could not contact the authors due to incorrect email address. Study start date: not specified Study end date: not specified Funding source: the author(s) disclosed receipt of financial support for the research, authorship, and/or publication of this article: this study was financed by the 'Sjukskrivningsmiljarden', an economic fund established by the Swedish government to encourage Swedish county councils to give higher healthcare priority to sick leave and to develop processes and methods to reduce its frequency. In addition, Kent W Nilsson, as the principal investigator, received research grants from Forskningsrådet för samhällsvetenskap och arbetsliv (FAS), Systembolagets råd för alkoholforskning (SRA), the Swedish Brain Foundation, the Uppsala and Örebro Regional Research Council, Fredrik and Ingrid Thurings Foundation, the County Council of Västmanland, the König‐Söderströmska Foundation, the Swedish Psychiatric Foundation, and Svenska Spel Research Foundation. None of these organisations had a role in the study design, data collection, data analysis, data interpretation, or writing of the report. Declarations of interest: the author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Richard Pettersson is a partner and shareholder in the company Livanda – Internet Clinic, Ltd, that constructed and owns the rights to the Internet‐based treatment programme In Focus. Richard Pettersson was also involved in the design and construction of the programme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A series of 54 patients were randomised in blocks to one of the study conditions over a period of four semesters (spring 2009 to autumn 2010). The results were kept in sealed envelopes, each coupled to the number in the consecutive series of patients referred to the study and who met the inclusion criteria. Unfortunately, the study had to be adjusted before the planned sample of 54 patients had been recruited because of the referral of fewer patients than expected, as well as limited financial resources and access to personnel. A total of 45 patients had been randomised to the study at the time of adjustment." |
| Allocation concealment (selection bias) | Low risk | Comment: the randomisation protocol was created by an independent statistician. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Independent evaluators, blinded to group assignment, administered the self‐report measures and conducted the semi‐structured interview." |
| Incomplete outcome data (attrition bias) | High risk | Quote: "The treatment dropout rate, defined as patients who did not complete all nine treatment modules, was 50% (seven patients) in the iCBT‐G group and 46% (six patients) in the iCBT‐S group. This gave a total dropout rate of 50% in the iCBT‐G group and 77% in the iCBT‐S group." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | High risk | Comment: the principal author was a partner and shareholder in the company Livanda – Internet Clinic, Ltd, that constructed and owns the rights to the Internet‐based treatment programme, In Focus. The principal author was also involved in the design and construction of the programme. |
| Methods | Randomised controlled trial | |
| Participants | Country: USA Setting: ambulatory Age: adults (18‐65 years old) Sample size: 31 Sex: 17 women, 14 men Inclusion criteria: have had a principal diagnosis of Attention‐Deficit Hyperactivity Disorder with external validation of childhood onset and clinical severity of at least a moderate level (Clinical Global Impression; CGI of 4 or above); have been able to give informed consent and comply with study procedures; and have been stabilised on medications for ADHD or related symptoms. Stabilisation on medications was defined as no more than 10% change in medication dose over a 2‐month period with clinical evidence of improvement compared to the patients' unmedicated status. Exclusion criteria: moderate to severe major depression; clinically significant panic disorder; organic mental disorders, psychotic spectrum disorders, bipolar disorders, active substance abuse or dependence (past three months), pervasive developmental disorder; active suicidal ideation; history of cognitive‐behavioral therapy (CBT); estimated or documented verbal intelligence quotient (IQ) of less than 90 | |
| Interventions | Intervention: CBT (12‐15 weekly sessions) + continued psychopharmacology (n = 16) Control: continued psychopharmacology alone (n = 15) Dosage, timing of dosage and administration of pharmacotherapy were not specified. | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | We contacted authors to get the information about random sequence generation and allocation concealment that we included in this table (Safren 2014 [pers comm]) Study start date: September 2001 Study end date: August 2003 Funding source: this study was supported by grant NIMH 60940 (Steven A Safren, PhD). Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: the study authors created a randomisation table in blocks of 2, stratified by severity (CGI scale) and sex. After the person was assessed and the team agreed that they met the inclusion/criteria, they were randomised based on the table. |
| Allocation concealment (selection bias) | Low risk | Comment: the interventionist was blinded to randomisation until the team had met and it was deemed that the person met criteria. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The baseline and outcome assessments consisted of a clinician‐administered interview by an evaluator who was blind to treatment condition, and a battery of self‐report measures". |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Comment: no evidence of conflicts of interest. |
| Methods | Randomised controlled trial | |
| Participants | Country: USA Setting: ambulatory Age: adults (18‐65 years old) Sample size: 86 Sex: 38 women, 48 men Inclusion criteria: principal diagnosis of ADHD (with childhood onset); Clinical Global Impression scale score for severity of 3 (mildly ill) or greater; able to provide informed consent and comply with study procedures; and stabilised on psychotropic medications Exclusion criteria: moderate to severe major depression, clinically significant (i.e., Clinical Global Impression scale score for severity 4) panic disorder, organic mental disorders, psychotic spectrum disorders, bipolar disorders, active substance abuse or dependence, mental retardation, or pervasive developmental disorder; active suicidal ideation; history of CBT; antisocial personality disorder or a learning disability that would interfere with treatment | |
| Interventions | Intervention: CBT (12 weekly sessions) for Medication‐Treated Adults (n = 43) Control: Relaxation with Educational Support for Medication‐Treated Adults (n = 43) Dosage, timing of dosage and administration of pharmacotherapy were not specified. | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | We contacted authors to get the information about random sequence generation and allocation concealment that we included in this table (Safren 2014 [pers comm]) Study start date: September 2004 Study end date: July 2010 Funding source: this study was funded by National Institutes of Health grant 5R01MH69812. Declarations of interest: Drs Safren, Sprich, and Otto reported receiving royalty payments from Oxford University Press. Dr Surman reported receiving research support from Abbott, Alza, Cephalon, Eli Lilly, El Minda the Hildaand Preston Davis Foundation, McNeil, Merck, New River, National Institutes of Health, Organon, Pfizer, Shire, and Takeda; being a speaker for Janssen‐Ortho, McNeil, Novartis, Shire, and MGH Academy/Reed Medical Education (which receives funding from multiple pharmaceutical companies); and being a consultant or advisor for McNeil, Shire, and Takeda. Dr Knouse reported receiving consulting income from Eli Lilly. Dr Otto reported receiving consulting income from Jazz Pharmaceuticals, Organon (Schering‐Plough), Pfizer, and Sanofi‐Aventis; research support from Organon (Schering‐Plough); and royalty payments for use of the SIGH‐A from Lilly. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: the method used was coin flip. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: blinding was maintained by having a single independent assessor who would not participate in meetings when cases were discussed. The blinded assessments were conducted by a doctoral‐level clinician with specific training from the Massachusetts General Hospital ADHD programme. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: the proportion of dropout was 5% for CBT and 14% for relaxation with educational support. Quote: "Following intent‐to‐treat principles, data were analysed for all participants regardless of whether they changed their medications postrandomisation, despite the consent and inclusion criteria that specified that only those with a stable regimen of medications with no plans to change should enroll and agree not to do this during the acute treatment period of approximately 15 weeks." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Comment: no evidence of conflicts of interest |
| Methods | Randomised controlled trial | |
| Participants | Country: the Netherlands Setting: ambulatory Age: adults (18 to 65 years old) Sample size: 44 Sex: 23 women, 21 men Inclusion criteria: primary diagnosis of ADHD, DSM‐IV‐TR confirmed by 3 psychiatrists Exclusion criteria: substance abuse/dependence within the last 6 months; co‐morbid psychotic‐, borderline‐, antisocial‐, and behavioural disorders; and learning difficulties | |
| Interventions | Intervention: mindfulness‐based cognitive therapy (12 weekly sessions) (n = 24; 15 participants with pharmacotherapy and 9 without pharmacotherapy). Control: waiting list group (n = 20; 16 participants with pharmacotherapy and 4 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. | |
| Outcomes | Primary outcome
| |
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: this research was supported by BrainGain SmartMix Programme of the Netherlands Ministry of Economic Affairs and Netherlands Ministry of Education, Culture and Science. Declarations of interest: all authors declare that they have no conflicts of interest related to this work. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: a random number table was used – whether the number was even or odd would dictate allocation to MBCT or WL. |
| Allocation concealment (selection bias) | Low risk | Comment: this procedure was carried out by a member of staff unrelated to the data collection, and was witnessed by the data manager of the project. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | High risk | Comment: the outcomes were potentially prone to risk of bias without blinding of the assessor. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: of the remaining 44 participants, complete clinical data sets were not available for 2 (1 MBCT, 1 WL); in 1 case the baseline, the other the post‐treatment, questionnaires were not completed at the time of testing due to practical/time constraints. |
| Selective reporting (reporting bias) | Low risk | Comment: the study authors reported all proposed outcomes. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Comment: no evidence of conflicts of interest |
| Methods | Randomised controlled trial | |
| Participants | Country: USA Setting: ambulatory Age: adults (18‐65 years old) Sex: 58 women, 30 men Exclusion criteria: active substance abuse or dependence; suicidal ideation; overtly hostile or aggressive behaviour likely to alienate group members; 'asocial' characteristics (e.g. pervasive developmental disorder); cognitive disability (estimated intelligence quotient (IQ) < 80); psychosis; borderline personality disorder; Alzheimer's disease or other dementia; overt neurological disorder; and childhood history of abuse or trauma or other severe psychiatric condition that confounded ascertainment of childhood ADHD symptoms. | |
| Interventions | Intervention: meta‐cognitive therapy (12‐week manualised meta‐cognitive therapy group intervention; 2‐h sessions) (n = 45; 19 participants with pharmacotherapy and 26 without pharmacotherapy) Control: supportive therapy (12 weeks; 2‐h sessions) (n = 43; 20 participants with pharmacotherapy and 23 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | We contacted authors to get the information about random sequence generation and allocation concealment that we included in this table (Solanto 2014 [pers comm]) Study start date: May 2005 Study end date: October 2008 Funding source: NIMH grant 1R34MH071721 to Dr Solanto Declarations of interest: Dr Solanto has served on the medical advisory board of Shire Pharmaceuticals and has served as a consultant and speaker for Ortho‐McNeil‐Janssen Pharmaceuticals. Dr Abikoff has received research funding from NIMH, the Hughes, Lemberg, and Heckscher Foundations, Ortho‐McNeil, Shire, and Eli Lilly, has served as a consultant to Shire, Eli Lilly, Cephalon, and Novartis, and has a financial interest in the Children's Organizational Skills Scale, published by Multi‐Health Systems. Dr Alvir is an employee of Pfizer. Drs Marks, Wasserstein, Mitchell, and Kofman report no financial relationships with commercial interests. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: a random number sequence was electronically generated. |
| Allocation concealment (selection bias) | Unclear risk | Comment:participants were stratified by whether or not they were currently receiving medication treatment for ADHD, and otherwise randomly assigned to either the CBT or the support group. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: although individuals were, of course, not told to which group they were assigned, most were able to ultimately discern this because of the very different nature of the intervention (i.e. the participants were savvy enough to know that formal CBT is much more structured than a supportive intervention). Also, it is not possible to maintain a blinding of personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: response was assessed via a structured interview completed by an independent (blind) evaluator, and by questionnaires completed by the patient and a significant other, immediately pre‐ and post‐treatment. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The proportion of drop‐out was 11% for Meta‐cognitive therapy and 12% for Supportive Therapy. All data were analysed both with and without non‐completers and medication changers." "The pattern of treatment contrasts indicated that the larger the score at baseline (that is, the more severe the symptoms), the greater the differential improvement observed with meta‐cognitive therapy; this occurred whether the data were analyzed with or without those who did not complete the program and those who made proscribed medication changes (interaction coefficients, 0.66 and 0.72, respectively)." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Quote: "Dr. Solanto is currently on the Medical Advisory Board for Shire Pharmaceuticals. She has previously served as a consultant and speaker for Ortho‐McNeil‐Janssen Pharmaceuticals, Inc. During the past five years, Dr. Abikoff has received research funding from the National Institute of Mental Health, the Hughes, Lemberg and Heckscher Foundations, Ortho‐McNeil, Shire, and Eli Lilly; has consulted to Shire, Eli Lilly, Cephalon, and Novartis; and has a financial interest in the Childrens Organizational Skills Scale, published by Multi‐Health Systems. Drs. Marks, Wasserstein, Mitchell, Alvir, and Kofman have no competing interests." |
| Methods | Randomised controlled trial | |
| Participants | Country: Australia Setting: ambulatory Age: adults (21 years old or older) Sample size: 43 Sex: 14 women, 29 men Inclusion criteria: ADHD symptoms from childhood (i.e. a score of 36 or over on the Wender Utah Rating Scale), current endorsement of the DSM‐IIIR criteria for ADHD by the applicant; lifelong history consistent with ADHD; evidence that the symptoms were causing impairment in day‐to‐day functioning; and willingness to participate in a study and sign a consent form Exclusion criteria: no ADHD like symptoms; under 21 years of age; current drug or alcohol problem; history of psychosis; reported involvement in criminal activities; and mental retardation | |
| Interventions | Intervention: cognitive remediation programme (intensive format with 8, 2‐h weekly sessions) (n = 22; 13 participants with pharmacotherapy and 9 without pharmacotherapy) Control: waiting list group (n = 21; 11 participants with pharmacotherapy and 10 without pharmacotherapy) Dosage, timing of dosage and administration of pharmacotherapy were not specified. | |
| Outcomes |
| |
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: this research was supported by a grant from the Department of Psychology at Sydney University. Declarations of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Low risk | Comment: the study authors used opaque envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: no evidence of other bias |
| Conflict of interest | Low risk | Comment: no evidence of conflicts of interest |
| Methods | Randomised controlled trial | |
| Participants | Country: Spain Setting: hospital (outpatients) Age: adults (older than 18 years) Sex: 17 women, 15 men Exclusion criteria: history of substance abuse in the past 6 months or current comorbidity of other axis I or II disorders of DSM‐IV; history of psychiatric comorbidity with non‐stabilised symptoms at the moment of the study were also included. | |
| Interventions | Intervention: psychoeducation in medication‐treated adults (12 weeks; 2‐h sessions) (n = 17) Control: CBT in medication‐treated adults (12 weeks; 2‐h sessions) (n = 15) Dosage, timing of dosage and administration of pharmacotherapy were not specified. | |
| Outcomes | Primary outcome
Secondary outcomes
| |
| Notes | We contacted authors to get more information, but they had not responded at the time of writing. Study start date: not specified Study end date: not specified Funding source: this study was supported by a non‐restricted grant from Departament de Salut, Government of Catalonia, and from ADANA Foundation Declarations of interest: the authors declare no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: the participants were randomised using the Statistical Package for the Social Sciences (SPSS) software and were assigned either to psychoeducation or to CBT. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the allocation concealment process was not described. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: self‐report measures and the CGI‐S clinician version were completed at pretreatment (time 1). Outcome measures were repeated at the end of the treatment (time 2). The pretreatment and post‐treatment evaluations were conducted by a psychologist blinded to this study. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: the proportion of dropouts was 7% for the the CBT group and 6% for the psychoeducation group. Quote: "Data were analyzed (using SPSS version 20) according to intent‐to‐treat principles using a last observation carried forward procedure." |
| Selective reporting (reporting bias) | Low risk | Comment: the study authors report all proposed outcomes. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Comment: the authors declare no conflict of interest. |
| Methods | Randomised controlled trial | |
| Participants | Country: Finland Setting: ambulatory Age: adults (18–49 years old) Sample size: 29 Sex: 15 women, 14 men Inclusion criteria: ADHD diagnosis made by a physician; no diagnosis of psychosis, severe depression or paranoia; deficits of attention, executive functions or working memory identified in an earlier neuropsychological evaluation; no current alcohol dependency or drug use; not receiving a disability pension; no participation in our previous group rehabilitation study; currently not undergoing any other psychological rehabilitation; no medication or medication that has been stable for at least three months Exclusion criteria: no neuropsychological examination; diagnosis of psychosis, severe depression or paranoia; older age, retired, or current psychological rehabilitation. | |
| Interventions | Intervention:
Control: control group (not specified) (n = 10; 7 participants with specific pharmacotherapy for ADHD and 3 without specific pharmacotherapy). Dosage, timing of dosage and administration of pharmacotherapy were not specified. | |
| Outcomes |
| |
| Notes | We contacted authors to get the information about random sequence generation and allocation concealment that we included in this table (Virta 2014 [pers comm]). Study start date: not specified Study end date: not specified Funding source: this study was supported by RAY, Finland's Slot Machine Association. Maarit Virta received funding for preparation of this manuscript from the Rinnekoti Research Foundation. Declarations of interest: the first author report no conflicts of interest in this work. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: the randomisation was done before the study started by raffling/draw lots. The authors had a randomised list of the rehabilitation methods beforehand. They had 4 groups: 1. CBT; 2. computerised training; 3. hypnotherapy; and 4. control. So the list looked like: 2, 1, 1, 3, 4, 1, 4, etc. Then every enrolled participant was assigned to the next group. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) | High risk | Comment: it is not possible to blind personnel in a psychosocial intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: the independent evaluator was a clinical psychologist who was blind to the actual study group of participants. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified. |
| Other bias | Low risk | Comment: there was no evidence of other bias. |
| Conflict of interest | Low risk | Comment: the study authors report no conflicts of interest in this work. |
ADHD: attention deficit hyperactivity disorder; ANCOVA: analysis of covariance; ASRS: Adult ADHD Self‐Report Scale; CBT: cognitive‐behavioural therapy; CGI‐S: Clinical Global Impressions ‐ Severity; DBT: dialectical behaviour therapy; DSM: Diagnostic and Statistical Manual of Mental Disorders; ITT: intention‐to‐treat; LOCF: last observation carried forward; MBCT: mindfulness‐based cognitive therapy; SD: standard deviation; SH: skills handout; WL: waiting list.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The comparison between CBT alone versus CBT combined with medication was not a type of comparison prespecified in our protocol (Lopez 2013). | |
| The goal of the current study was to assess the preliminary efficacy of mindfulness, without introducing other treatment modalities such as CBT. | |
| This study compared group psychotherapy versus individual psychotherapy, affecting the comparability of the intervention of interest for our review. | |
| The goal of the current study was to assess the efficacy of virtual reality and mindfulness, without introducing other treatment modalities such as CBT. | |
| The comparison between CBT plus dextroamphetamine versus CBT plus placebo was not a type of comparison prespecified in our protocol (Lopez 2013) | |
| The comparison between medication plus TAU versus medication plus CBT was not a type of comparison prespecified in our protocol (Lopez 2013), due to the fact that TAU was defined by the study authors as receiving usual treatment, which included both pharmacological and non‐pharmacological treatments | |
| The comparison between medication plus TAU versus medication plus CBT was not a type of comparison prespecified in our protocol (Lopez 2013), due to the fact that TAU was defined by the study authors as receiving usual treatment, which included both pharmacological and non‐pharmacological treatments |
CBT: cognitive‐behavioural therapy; TAU: treatment as usual.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Protocol for a proof of concept randomized controlled trial of cognitive‐behavioural therapy for adult ADHD as a supplement to treatment as usual, compared with treatment as usual alone |
| Methods | Two‐arm randomised controlled trial |
| Participants | Sample size: 60 participants Inclusion criteria
Exclusion criteria
|
| Interventions | TAU plus 16 sessions of individual CBT vs TAU alone |
| Outcomes | Primary outcomes, rated by participant (self‐report)
Secondary outcomes, rated by participant (self‐report)
Nominated informant ratings
Independent evaluator ratings
Therapist ratings
Other
|
| Starting date | ISRCTN register number: ISRCTN03732556, assigned 4 November 2010 Start date: 21 April 2010 End date: 30 April 2014 Recruitment status: no longer recruiting |
| Contact information | Principal investigator: Dr Antonia J Dittner Email: [email protected] Address: King's College London, King's Health Partners, Behavioural and Developmental Psychiatry Clinical Academic Group, Maudsley Adult ADHD Service, South London and Maudsley NHS Foundation Trust, London, UK |
| Notes | Funding source: South London and Maudsley NHS Foundation Trust (UK) Declarations of interest: not reported |
| Trial name or title | A randomized controlled study of cognitive behavioral therapy for adults with attention deficit disorder |
| Methods | Randomised controlled trial, parallel assignment |
| Participants | 108 participants Inclusion criteria
Exclusion criteria
|
| Interventions | CBT vs CBT plus booster sessions |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | Current Controlled Trials: NCT02062411, date of registration: 12 February 2014 Start date: October 2013 End date: March 2016 Recruitment status: completed |
| Contact information | Dr Fang Huang. Correspondence: [email protected]. Peking University Sixth Hospital/Institute of Mental Health, National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), No. 51, Hua Yuan Bei Lu, Haidian District, Beijing 100191, China. Key Laboratory of Mental Health, Ministry of Health, Peking University, No. 51, Hua Yuan Bei Lu, Haidian District, Beijing 100191, China. |
| Notes | Funding source: Peking University Sixth Hospital Declarations of interest: not reported |
| Trial name or title | Efficacy of cognitive behavioral therapy in treatment of adults with attention deficit hyperactivity disorder |
| Methods | Allocation: randomised Intervention Model: parallel Assignment |
| Participants | This study is ongoing, but not recruiting participants. Inclusion criteria
Exclusion criteria
|
| Interventions | Stimulant medication only versus CBT only vs combined CBT and stimulant medication group |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | Start date: April 2006 End date: October 2017 Recruitment status: active, not recruiting |
| Contact information | Dr Lily Hechtman. McGill University Health Center |
| Notes | Funding source: McGill University Health Center Declarations of interest: not reported |
| Trial name or title | Mindfulness based cognitive therapy versus treatment as usual in adults with attention deficit hyperactivity disorder (ADHD) |
| Methods | Multicentre, parallel‐group, randomised controlled trial |
| Participants | 120 adults with ADHD Inclusion criteria
Exclusion criteria
|
| Interventions | Mindfulness‐based cognitive therapy (MBCT) plus TAU or TAU alone |
| Outcomes | Primary outcome measure will be severity of ADHD symptoms rated by a blinded clinician. Secondary outcome measures will be self‐reported ADHD symptoms, executive functioning, mindfulness skills, self‐compassion, positive mental health and general functioning. In addition, a cost‐effectiveness analysis will be conducted. |
| Starting date | Start date: September 013 End date: December 2017 Recruitment status: active, not recruiting |
| Contact information | Dr Lotte Janssen. Correspondence: [email protected]. Department of Psychiatry, Radboud University Medical Center, Nijmegen, the Netherlands |
| Notes | Funding source: ZonMw grant number: 837001501 Declarations of interest: not reported |
| Trial name or title | Behavioral activation to reduce problem alcohol use in college students with ADHD |
| Methods | Allocation: randomised Intervention model: parallel assignment |
| Participants | Estimated enrollment: 80 participants Inclusion criteria
Exclusion criteria
|
| Interventions | SUCCEEDS programme (Psychoeducation, Brief Motivational Interviewing and Behavioral Activation) vs Living a Healthy College Lifestyle (Psychoeducation, Brief Motivational Interviewing and Supportive Counseling) |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | Start date: September 2015 End date: September 2018 Recruitment status: recruiting |
| Contact information | Dr Andrea Chronis‐Tuscano ([email protected]), University of Maryland |
| Notes | Funding source: University of Maryland Declarations of interest: not reported |
ADHD: attention deficit hyperactivity disorder; AUDIT: Alcohol Use Disorders Identification Test; CBT: cognitive‐behavioural therapy; DSM: Diagnostic and Statistical Manual of Mental Disorders;IQ: intelligence quotient; MBCT: mindfulness‐based cognitive therapy; SD: standard deviation; TAU: treatment as usual.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ADHD symptoms (observer) Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 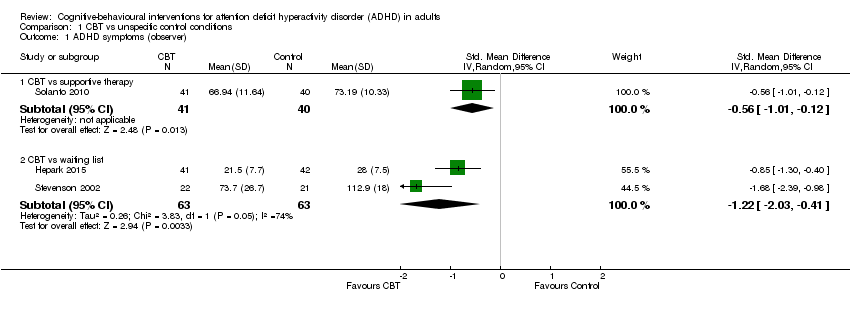 Comparison 1 CBT vs unspecific control conditions, Outcome 1 ADHD symptoms (observer). | ||||
| 1.1 CBT vs supportive therapy | 1 | 81 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.01, ‐0.12] |
| 1.2 CBT vs waiting list | 2 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.03, ‐0.41] |
| 2 ADHD symptoms (self‐reported) Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 CBT vs unspecific control conditions, Outcome 2 ADHD symptoms (self‐reported). | ||||
| 2.1 CBT vs supportive therapy | 2 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.52, 0.19] |
| 2.2 CBT vs waiting list | 5 | 251 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.18, ‐0.50] |
| 3 Inattention (clinician) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 CBT vs unspecific control conditions, Outcome 3 Inattention (clinician). | ||||
| 3.1 CBT vs supportive therapy | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐2.47 [‐4.43, ‐0.51] |
| 3.2 CBT vs waiting list | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐4.1 [‐4.00, ‐2.20] |
| 4 Inattention: CBT vs waiting list (self‐reported) Show forest plot | 4 | 244 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.37, ‐0.82] |
| Analysis 1.4  Comparison 1 CBT vs unspecific control conditions, Outcome 4 Inattention: CBT vs waiting list (self‐reported). | ||||
| 5 Hyperactivity‐impulsivity: CBT vs waiting list (clinician) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 CBT vs unspecific control conditions, Outcome 5 Hyperactivity‐impulsivity: CBT vs waiting list (clinician). | ||||
| 6 Hyperactivity‐impulsivity: CBT vs waiting list (self‐reported) Show forest plot | 4 | 244 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.98, ‐0.22] |
| Analysis 1.6 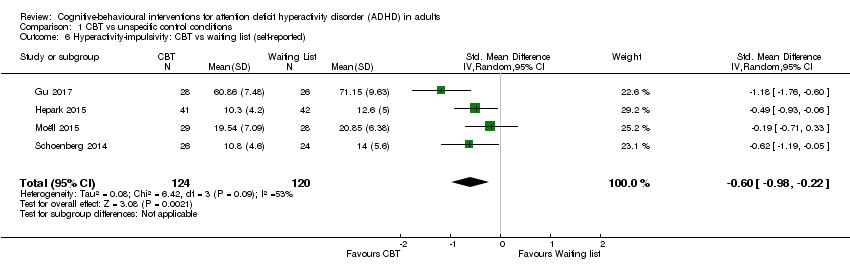 Comparison 1 CBT vs unspecific control conditions, Outcome 6 Hyperactivity‐impulsivity: CBT vs waiting list (self‐reported). | ||||
| 7 Depression (self‐reported) Show forest plot | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 CBT vs unspecific control conditions, Outcome 7 Depression (self‐reported). | ||||
| 7.1 CBT vs supportive therapy | 1 | 81 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.36, 0.51] |
| 7.2 CBT vs waiting list | 5 | 258 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.60, ‐0.11] |
| 8 Anxiety: CBT vs supportive therapy (clinician) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8 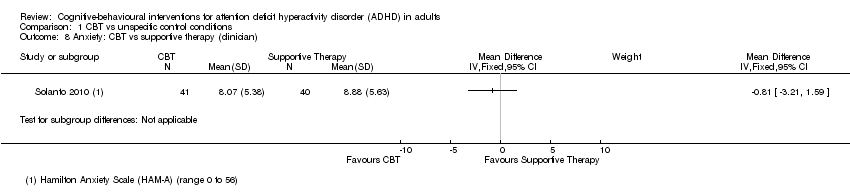 Comparison 1 CBT vs unspecific control conditions, Outcome 8 Anxiety: CBT vs supportive therapy (clinician). | ||||
| 9 Anxiety: CBT vs waiting list (self‐reported) Show forest plot | 4 | 239 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.71, ‐0.19] |
| Analysis 1.9 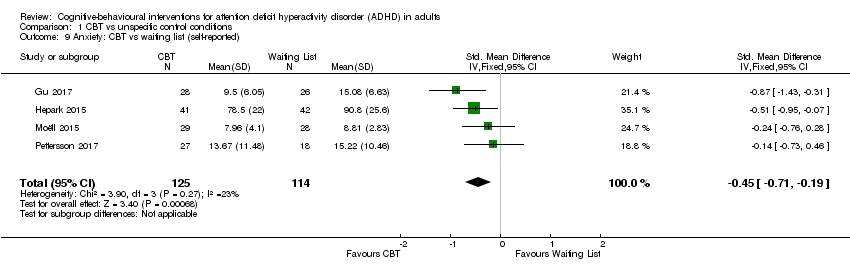 Comparison 1 CBT vs unspecific control conditions, Outcome 9 Anxiety: CBT vs waiting list (self‐reported). | ||||
| 10 State anger: CBT vs waiting list (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10 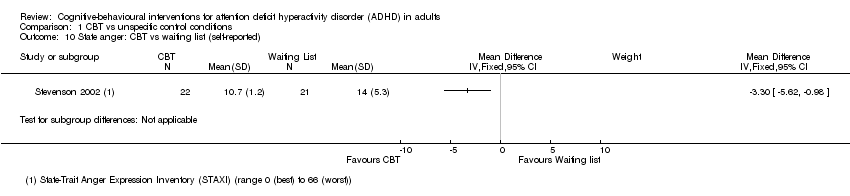 Comparison 1 CBT vs unspecific control conditions, Outcome 10 State anger: CBT vs waiting list (self‐reported). | ||||
| 11 Trait anger: CBT vs waiting list (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11 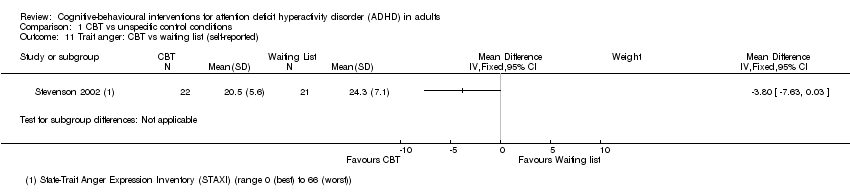 Comparison 1 CBT vs unspecific control conditions, Outcome 11 Trait anger: CBT vs waiting list (self‐reported). | ||||
| 12 Self‐esteem (self‐reported) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 CBT vs unspecific control conditions, Outcome 12 Self‐esteem (self‐reported). | ||||
| 12.1 CBT vs Supportive Therapy | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.85, 1.85] |
| 12.2 CBT vs Waiting list | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 12.40 [4.55, 20.25] |
| 13 Quality of life: CBT vs waiting list (self‐reported) Show forest plot | 2 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.29, 0.71] |
| Analysis 1.13 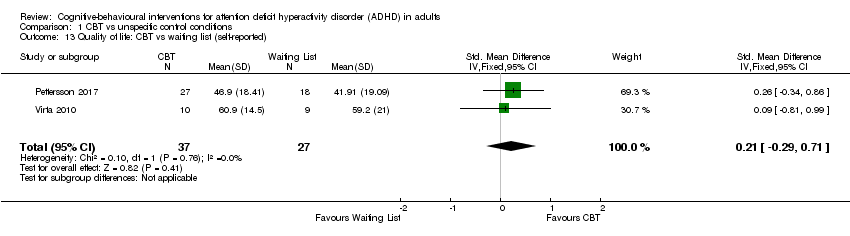 Comparison 1 CBT vs unspecific control conditions, Outcome 13 Quality of life: CBT vs waiting list (self‐reported). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ADHD symptoms (clinician) Show forest plot | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.31, ‐0.30] |
| Analysis 2.1  Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 1 ADHD symptoms (clinician). | ||||
| 2 ADHD symptoms (self‐reported) Show forest plot | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐7.42 [‐11.63, ‐3.22] |
| Analysis 2.2  Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 2 ADHD symptoms (self‐reported). | ||||
| 3 Inattention (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 3 Inattention (self‐reported). | ||||
| 4 Hyperactivity‐impulsivity (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 4 Hyperactivity‐impulsivity (self‐reported). | ||||
| 5 Clinical Global Impression (clinician) Show forest plot | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.21, ‐0.30] |
| Analysis 2.5  Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 5 Clinical Global Impression (clinician). | ||||
| 6 Depression (clinician) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6 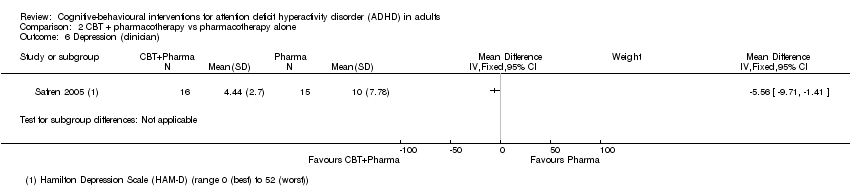 Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 6 Depression (clinician). | ||||
| 7 Depression (self‐reported) Show forest plot | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐6.09 [‐9.55, ‐2.63] |
| Analysis 2.7  Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 7 Depression (self‐reported). | ||||
| 8 Anxiety (clinician) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 8 Anxiety (clinician). | ||||
| 9 Anxiety (self‐reported) Show forest plot | 2 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐1.08, ‐0.08] |
| Analysis 2.9  Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 9 Anxiety (self‐reported). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ADHD symptoms (clinician) Show forest plot | 2 | 97 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.98, ‐0.17] |
| Analysis 3.1 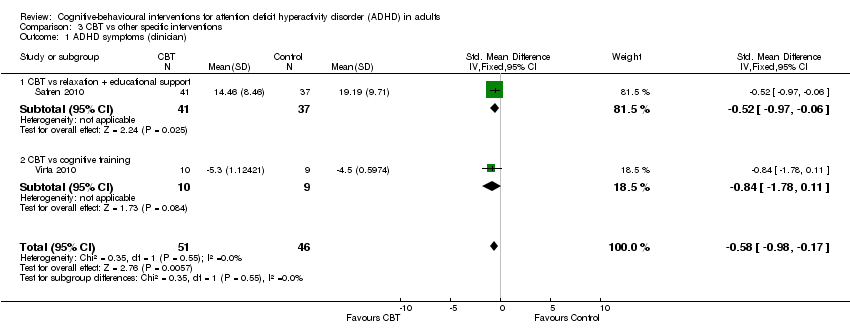 Comparison 3 CBT vs other specific interventions, Outcome 1 ADHD symptoms (clinician). | ||||
| 1.1 CBT vs relaxation + educational support | 1 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.97, ‐0.06] |
| 1.2 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.78, 0.11] |
| 2 ADHD symptoms (self‐reported) Show forest plot | 4 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.88, ‐0.01] |
| Analysis 3.2 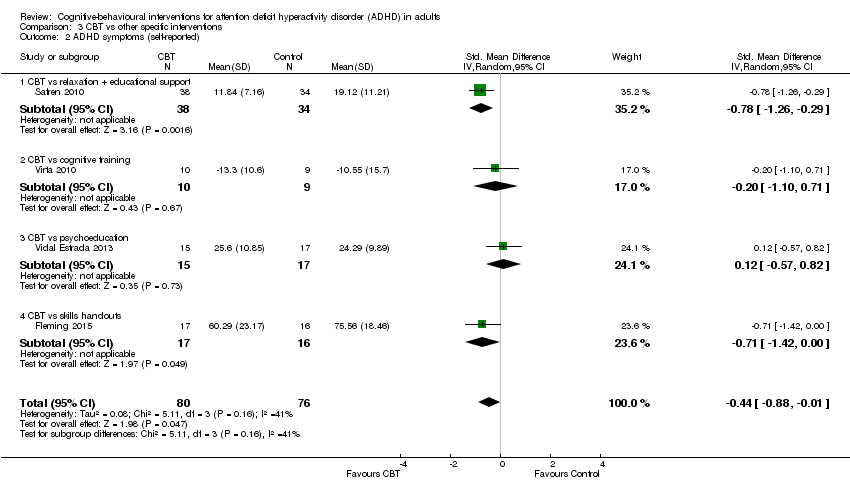 Comparison 3 CBT vs other specific interventions, Outcome 2 ADHD symptoms (self‐reported). | ||||
| 2.1 CBT vs relaxation + educational support | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.26, ‐0.29] |
| 2.2 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.10, 0.71] |
| 2.3 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.57, 0.82] |
| 2.4 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.42, ‐0.00] |
| 3 Inattention (self‐reported) Show forest plot | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.61, 0.37] |
| Analysis 3.3  Comparison 3 CBT vs other specific interventions, Outcome 3 Inattention (self‐reported). | ||||
| 3.1 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.54, 0.85] |
| 3.2 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.08, 0.30] |
| 4 Hyperactivity: CBT vs psychoeducation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4 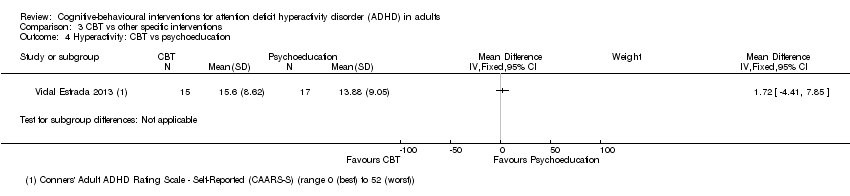 Comparison 3 CBT vs other specific interventions, Outcome 4 Hyperactivity: CBT vs psychoeducation. | ||||
| 5 Impulsivity: CBT vs psychoeducation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5 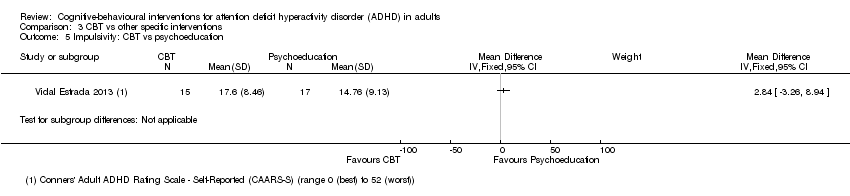 Comparison 3 CBT vs other specific interventions, Outcome 5 Impulsivity: CBT vs psychoeducation. | ||||
| 6 Clinical Global Impression (clinician) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 CBT vs other specific interventions, Outcome 6 Clinical Global Impression (clinician). | ||||
| 6.1 CBT vs relaxation + educational support | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.09, 0.03] |
| 6.2 CBT vs psychoeducation | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.19, 0.55] |
| 7 Clinical Global Impression: CBT vs psychoeducation (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.7  Comparison 3 CBT vs other specific interventions, Outcome 7 Clinical Global Impression: CBT vs psychoeducation (self‐reported). | ||||
| 8 Depression (self‐reported) Show forest plot | 3 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.70, 0.16] |
| Analysis 3.8  Comparison 3 CBT vs other specific interventions, Outcome 8 Depression (self‐reported). | ||||
| 8.1 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.32, 0.51] |
| 8.2 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.80, 0.59] |
| 8.3 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐1.04, 0.34] |
| 9 Anxiety (self‐reported) Show forest plot | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.95, 0.04] |
| Analysis 3.9 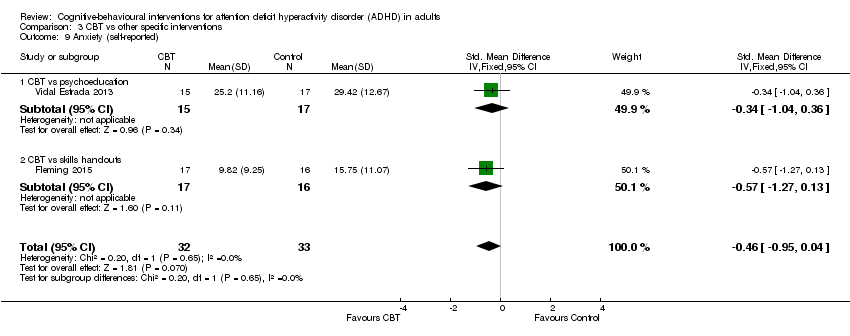 Comparison 3 CBT vs other specific interventions, Outcome 9 Anxiety (self‐reported). | ||||
| 9.1 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.04, 0.36] |
| 9.2 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐1.27, 0.13] |
| 10 Quality of life (self‐reported) Show forest plot | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.10  Comparison 3 CBT vs other specific interventions, Outcome 10 Quality of life (self‐reported). | ||||
| 10.1 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐1.19, 0.62] |
| 10.2 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.37, 1.03] |
| 10.3 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.17 [0.42, 1.92] |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
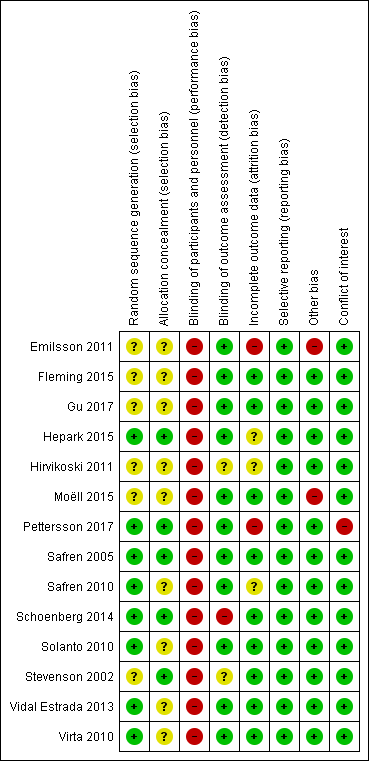
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
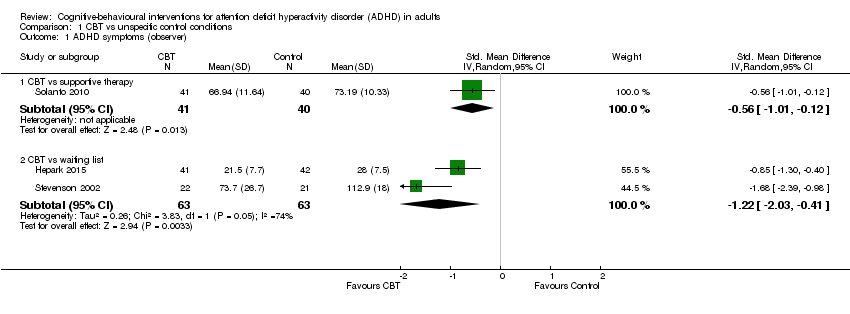
Comparison 1 CBT vs unspecific control conditions, Outcome 1 ADHD symptoms (observer).

Comparison 1 CBT vs unspecific control conditions, Outcome 2 ADHD symptoms (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 3 Inattention (clinician).

Comparison 1 CBT vs unspecific control conditions, Outcome 4 Inattention: CBT vs waiting list (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 5 Hyperactivity‐impulsivity: CBT vs waiting list (clinician).

Comparison 1 CBT vs unspecific control conditions, Outcome 6 Hyperactivity‐impulsivity: CBT vs waiting list (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 7 Depression (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 8 Anxiety: CBT vs supportive therapy (clinician).

Comparison 1 CBT vs unspecific control conditions, Outcome 9 Anxiety: CBT vs waiting list (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 10 State anger: CBT vs waiting list (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 11 Trait anger: CBT vs waiting list (self‐reported).

Comparison 1 CBT vs unspecific control conditions, Outcome 12 Self‐esteem (self‐reported).
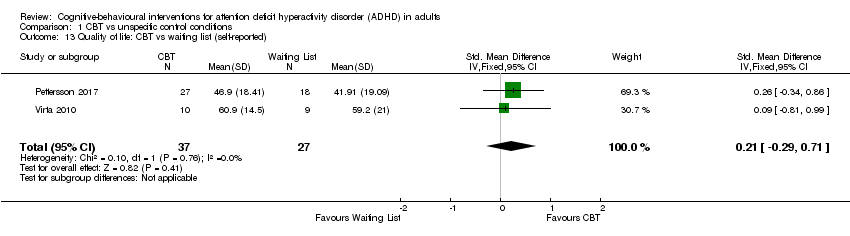
Comparison 1 CBT vs unspecific control conditions, Outcome 13 Quality of life: CBT vs waiting list (self‐reported).
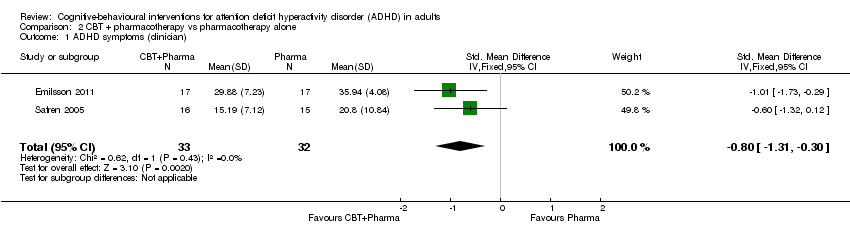
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 1 ADHD symptoms (clinician).
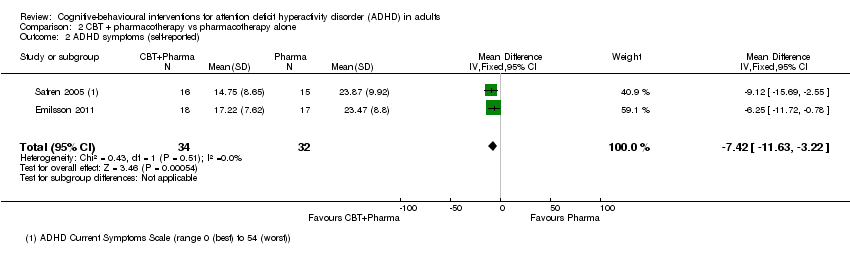
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 2 ADHD symptoms (self‐reported).

Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 3 Inattention (self‐reported).

Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 4 Hyperactivity‐impulsivity (self‐reported).
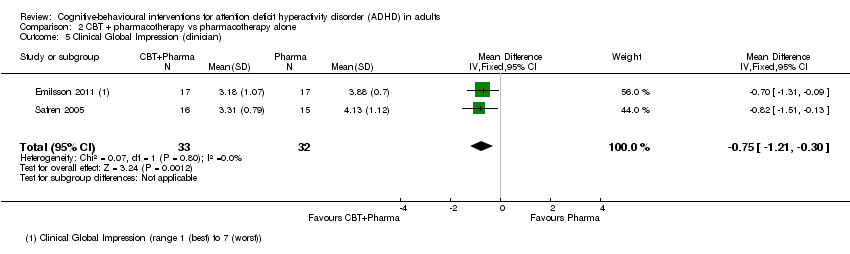
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 5 Clinical Global Impression (clinician).

Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 6 Depression (clinician).
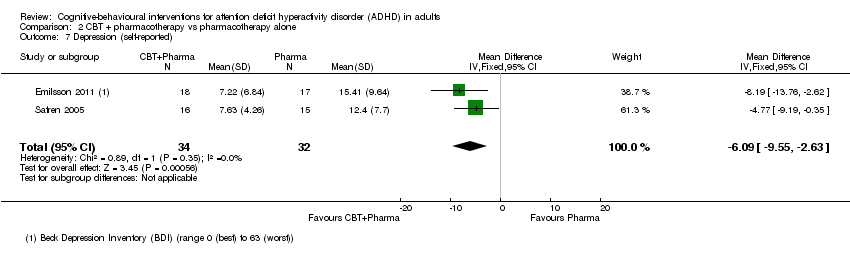
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 7 Depression (self‐reported).
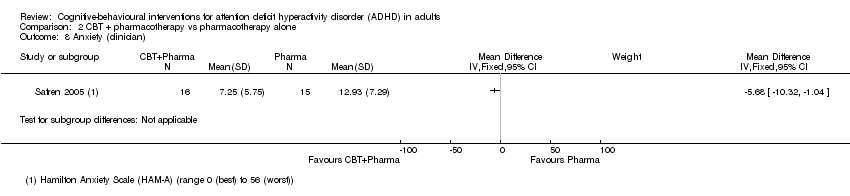
Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 8 Anxiety (clinician).

Comparison 2 CBT + pharmacotherapy vs pharmacotherapy alone, Outcome 9 Anxiety (self‐reported).

Comparison 3 CBT vs other specific interventions, Outcome 1 ADHD symptoms (clinician).

Comparison 3 CBT vs other specific interventions, Outcome 2 ADHD symptoms (self‐reported).

Comparison 3 CBT vs other specific interventions, Outcome 3 Inattention (self‐reported).
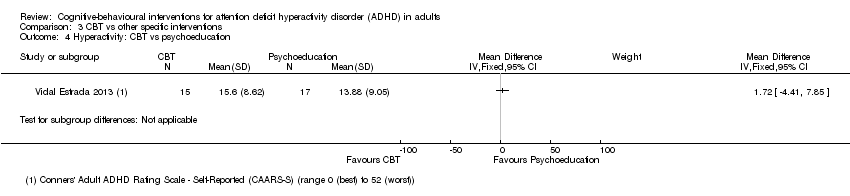
Comparison 3 CBT vs other specific interventions, Outcome 4 Hyperactivity: CBT vs psychoeducation.

Comparison 3 CBT vs other specific interventions, Outcome 5 Impulsivity: CBT vs psychoeducation.

Comparison 3 CBT vs other specific interventions, Outcome 6 Clinical Global Impression (clinician).
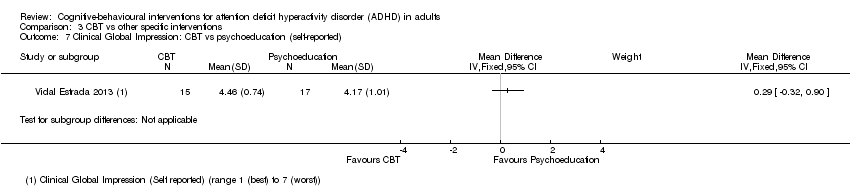
Comparison 3 CBT vs other specific interventions, Outcome 7 Clinical Global Impression: CBT vs psychoeducation (self‐reported).

Comparison 3 CBT vs other specific interventions, Outcome 8 Depression (self‐reported).

Comparison 3 CBT vs other specific interventions, Outcome 9 Anxiety (self‐reported).

Comparison 3 CBT vs other specific interventions, Outcome 10 Quality of life (self‐reported).
| Cognitive‐behavioural interventions versus unspecific control for ADHD in adults | ||||||
| Patient or population: adults with ADHD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control conditions | Risk with CBT | |||||
| CBT versus supportive therapy | ||||||
| ADHD symptoms: observer‐rated Assessed by: various scales Follow‐up: 12 weeks | — | The mean ADHD observer‐rated symptoms score in the intervention groups was 0.56 standardised deviations lower (1.01 lower to 0.12 lower) | — | 81 | ⊕⊕⊝⊝ | Moderate effect sizeb |
| ADHD symptoms: self‐reported Assessed by: various scales Follow‐up: 12 to 14 weeks | — | The mean ADHD self‐rated symptoms score in the intervention groups was 0.16 standardised deviations lower (0.52 lower to 0.19 higher) | — | 122 | ⊕⊕⊝⊝ | Small effect sizeb |
| CBT versus waiting list control | ||||||
| ADHD symptoms: observer‐rated Assessed by: various scales Follow‐up: 8 to 12 weeks | ‐ | The mean ADHD self‐rated symptoms score in the intervention groups was 1.22 standardised deviations lower (2.03 lower to 0.41 lower) | — | 126 | ⊕⊝⊝⊝ | Large effect sizeb |
| ADHD symptoms: self‐reported Assessed by: various scales Follow‐up: 8 to 12 weeks | — | The mean ADHD self‐rated symptoms score in the intervention groups was 0.84 standardised deviations lower (1.18 lower to 0.50 lower) | — | 251 | ⊕⊕⊕⊝ | Large effect sizeb |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a We downgraded the quality of evidence due to imprecision (considering the width of the CI), methodological limitations (due to high risk of bias in blinding of participants and personnel), and because the evidence is based on a single study. | ||||||
| Cognitive‐behavioural therapy plus pharmacotherapy versus pharmacotherapy alone for ADHD in adults | ||||||
| Patient or population: adults with ADHD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control conditions | Risk with CBT plus pharmacotherapy | |||||
| ADHD symptoms: clinician rated Assessed by: various scales Follow‐up: 8 to 15 weeks | — | The mean ADHD clinician‐rated symptoms score in the intervention groups was 0.80 standardised deviations lower (1.31 lower to 0.30 lower) | — | 65 | ⊕⊝⊝⊝ | Large effect sizec |
| ADHD symptoms: self‐reported Assessed by: ADHD Current Symptoms Scale (range 0 (best) to 54 (worst)) Follow‐up: 8 to 15 weeks | The mean ADHD self‐rated symptoms score in the control groups ranged from 14.75 to 17.22. | The mean ADHD self‐rated symptoms score in the intervention groups was 7.42 lower (11.63 lower to 3.22 lower) | — | 66 | ⊕⊕⊝⊝ | Large effect size |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded the quality of evidence due to methodological limitations (high risk of bias in blinding of participants and personnel, and the fact that Emilsson 2011 had a high risk of bias in three domains in one of the two included studies). | ||||||
| Cognitive‐behavioural therapy versus other interventions for ADHD in adults | ||||||
| Patient or population: adults with ADHD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control conditions | Risk with CBT | |||||
| ADHD symptoms: clinician‐rated Assessed by: various scales Follow‐up: 10 to 12 weeks | — | The mean ADHD clinician‐rated symptoms score in the intervention groups was 0.58 standardised deviations lower (0.98 lower to 0.17 lower) | — | 97 | ⊕⊕⊝⊝ | Moderate effect sizeb |
| ADHD symptoms: self‐reported Assessed by: various scales Follow‐up: 8 to 12 weeks | — | The mean ADHD self‐reported symptoms score in the intervention groups was 0.44 standardised deviations lower (0.88 lower to 0.01 lower) | — | 156 | ⊕⊕⊝⊝ | Small effect sizeb |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a We downgraded the quality of evidence because of imprecision (considering the width of the CI) and methodological limitations (due to the high risk of bias in blinding of participants and personnel and three other domains with unclear risk of bias). | ||||||
| Criteria | Description |
| Random sequence generation (selection bias ‐ biased allocation to interventions ‐ due to inadequate generation of a randomised sequence) |
|
| Allocation concealment (selection bias ‐ biased allocation to interventions ‐ due to inadequate concealment of allocations prior to assignment) |
|
| Blinding of participants and personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the study) |
|
| Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessors) |
|
| Incomplete outcome data (attrition bias due to the amount, nature or handling of incomplete outcome data) |
|
| Selective reporting (reporting bias due to selective outcome reporting) |
|
| Other bias (other sources of bias not captured by the other domains) |
|
| Method | Approach |
| Measures of treatment effect | Dichotomous data We did not find dichotomous data. Should these data become available in future updates of this review, we will calculate the risk ratio (RR) with 95% confidence intervals (CI), as most readers find it easier to understand the RR and 95% CI than the odds ratio and risk difference. |
| Unit of analysis issues | Cluster‐RCTs We did not find cluster‐RCTs. This design is uncommon in this field. Should such studies become available in the future, and if the investigators report cluster‐randomised trial data as though the randomisation was performed on individuals rather than on clusters, we will request individual participant data to calculate an estimate of the intra‐cluster correlation coefficient (ICC). If the individual participant data are not available, we will obtain external estimates of the ICC from similar studies or available resources (Campbell 2000). Once established, we will use the ICC to re‐analyse the trial data to obtain approximate, correct analyses as described in section 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will combine the effect estimates and their corrected standard errors from cluster‐RCTs with those from parallel‐group designs using the generic inverse variance method (Higgins 2011). If the available information is insufficient to control for clustering in this manner, we will enter the data into Review Manager 5 (RevMan 2014), using individuals as the unit of analysis. We planned to perform sensitivity analyses to assess the potential bias that may occur as a result of the inadequately controlled clustered trials. Additionally, if the ICCs are obtained from external sources, we will perform sensitivity analyses to assess the potential biasing effects of inadequately controlled cluster‐randomised trials (Donner 2001). |
| Cross‐over trials We did not find cross‐over trials. Should we find these types of studies in future updates of this review, and as the duration of any effect of CBT is unknown, we will use only first‐period data from any cross‐over trials that fit the inclusion criteria to avoid a possible carry‐over effect. | |
| Dealing with missing data | If the studies do not report the standard deviation (SD), we will calculate it from the P values, t values, CIs or standard errors (as described in section 7.7.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011). If this information is not reported or is unattainable, we will impute the SD from the study with the highest SD for that outcome, and assess the effects of this assumption on the analysis by conducting a sensitivity analysis. If the outcome data are reported as a median, a range or as a mean without a variance, we will report the data in additional tables. |
| Sensitivity analysis | We will conduct sensitivity analyses to:
|
| CBT: cognitive‐behavioural intervention; CI: confidence interval; ICC: intracluster correlation coefficient; RCT: randomised controlled trial; SD: standard deviation. See also Lopez 2013. | |
| Outcomes | Studies and instruments |
| ADHD symptom severity |
|
| Inattention |
|
| Hyperactivity |
|
| Impulsivity |
|
| Hyperactivity‐impulsivity |
|
| Clinical Global Impression |
|
| ADHD: attention deficit hyperactivity disorder; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders ‐ Third Edition ‐ Revised; NIMH: National Institutes of Mental Health. | |
| Outcomes | Studies and instruments |
| Depression |
|
| Anxiety |
|
| State anger |
|
| Trait anger |
|
| Self‐esteem |
|
| Quality of life |
|
| Employment status | No study included this outcome. |
| ADHD: attention deficit hyperactivity disorder. | |
| Section of Review | Protocol | Full review |
| "The International Classification of Diseases (ICD‐10) offers a similar definition for hyperkinetic disorders (WHO 1993). Along with these three main symptomatic clusters, people with ADHD also present with deficits in executive functions, behaviour and emotion regulation, and motivation (Brown 2000; Wender 2001; Davidson 2008; Torrente 2011). There is a high prevalence of comorbid disorders, estimated at 50% to 75% (Kessler 2006), including anxiety, depression and substance abuse (Biederman 1993; Murphy 1996). Epidemiological studies estimate that the prevalence of ADHD is approximately 5% in childhood (Polanczyk 2007) and approximately 2.5% in adulthood (Simon 2009)." | "The International Classification of Diseases (ICD‐10) offers a similar definition for hyperkinetic disorders (WHO 1993), but the required number of symptoms and the age of onset are different. Along with these three main symptomatic clusters, people with ADHD also present with deficits in executive functions, behaviour and emotion regulation, and motivation (Brown 2000; Davidson 2008; Torrente 2011; Wender 2001). There is a high prevalence of comorbid disorders, estimated at 50% to 75% (Kessler 2006), including anxiety, depression and substance abuse (Biederman 1993; Murphy 1996). Epidemiological studies estimate that the prevalence of ADHD is approximately 5% in childhood and around 2.5% in adulthood (Polanczyk 2014; Simon 2009)." | |
| "Between 20% and 50% of people with ADHD do not respond to drug treatment (Wilens 2002)." | "Between 20% and 50% of people with ADHD do not respond to drug treatment (Wilens 2002). Also, pharmacological treatment is frequently associated with relevant side effects in both children and adults (AJCD 2001; Castells 2013; Cunill 2013; Graham 2011; King 2006; Lim 2006; Morton 2000; Perrin 2008; Prescrire 2007). Due to these concerns, it is important to have non‐pharmacological interventions for treating adults with ADHD." | |
| "To date, no systematic review has examined the effects of CBT in adults with ADHD. The growing number of randomised controlled trials assessing the efficacy of CBT for this population (Knouse 2008) suggest that this review is timely." | "To our knowledge, three systematic reviews have compared the effects of CBT in adults with ADHD (Jensen 2016; Knouse 2017; Young 2016). However, there are important methodological differences between them, also with respect to our review. Both Jensen 2016 and Young 2016 employed more restrictive criteria for defining CBT treatments that excluded relevant CBT variants such as mindfulness‐based cognitive therapy and dialectical behavioural therapy. Knouse 2017 did not report grades of quality of evidence of the included studies." | |
| We considered the following comparisons: Monotherapy
Combined therapy
| We considered the following comparisons:
| |
| We considered psychometrically validated self‐report measures or those completed by an independent rater or relative. The measures were considered short‐ (up to six months), medium‐ (six months to 12 months) and long‐term (more than 12 months)." | "We considered psychometrically validated self‐report measures or those completed by an independent rater or relative. "We presented clinical and self‐reported outcomes separately, as do most studies about this topic, because assessing ADHD is more accurate when symptom information comes from more than one source (Barkley 1998a). "We considered the measures as short term (up to 6 months), medium term (6 months to 12 months) and long term (more than 12 months). "We included studies that assessed at least one primary outcome or at least one secondary outcome." | |
| "We will include studies that have assessed at least one primary or secondary outcome." | "We included studies that assessed at least one primary outcome or at least one secondary outcome." | |
| The safety outcome 'All‐cause treatment discontinuation' was considered as secondary outcome | We considered the safety outcome 'All‐cause treatment discontinuation' to be a primary outcome. | |
| We planned to search Ovid MEDLINE | We used MEDLINE PubMed because of the availability of this interface. | |
| "Additionally, we searched dissertations and abstracts from the following.
| "Additionally, we searched dissertations and abstracts from the following.
| |
| "We independently evaluated the risk of bias using EROS. This process followed the six criteria described in Table 8.5.d, "Criteria for judging risk of bias in the 'Risk of bias' assessment tool" of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (See Table 1)." | "We evaluated the risk of bias in each included trial using the seven criteria described in Table 8.5.d ('Criteria for judging risk of bias in the "Risk of bias' assessment tool") of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (PL and FT) independently assessed each included study as being at low, high or unclear (uncertain) risk of bias for each domain, using EROS software (Ciapponi 2011; Glujovsky 2011; Glujovsky 2010); see Table 1. If there were discrepancies between their assessments, and the two review authors were unable to reach a consensus, a third review author (AC) joined the decision‐making process. All three review authors discussed the issue and made a final decision." | |
| The bands that we reported for I2 were:
| The bands that we reported for I2 were:
| |
| "When we considered studies to be sufficiently homogenous in terms of participants, interventions and outcomes, we synthesised the results in a meta‐analysis using RevMan (RevMan 2014)." | "We synthesised the results in a meta‐analysis using Review Manager 5 (RevMan 5) when we considered studies to be sufficiently homogenous in terms of population (regarding sex, age and diagnosis), interventions (comparable modalities of CBT) and comparisons (as a monotherapy or a part of a combined treatment) to avoid clinical heterogeneity, and in terms of outcome measurement methods to avoid methodological heterogeneity (RevMan 2014). Two authors assessed homogeneity independently and solved discrepancies by consensus." | |
| We did not include a subsection about summary of findings | We included a 'Summary of findings table' subsection: "We prepared a 'Summary of findings' table for our three main comparisons (see Types of interventions) according to GRADE methodology (Atkins 2004; Guyatt 2011), using GRADEpro GDT software (GRADEpro 2015). We included our primary outcome, the core symptoms of ADHD (self‐, clinician‐ or observer‐reported), in the tables. "Two review authors (AC and PL) independently assessed the quality of the evidence as high, moderate, low or very low, downgrading the rating according to the presence of the following criteria: study limitations, in which we considered the studies' 'Risk of bias' level; imprecision; inconsistency of results; indirectness of evidence; and likely publication bias. "To assess the magnitude of effect for continuous outcomes, we used the criteria suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect." | |
| We considered the type of CBT as a possible subgroup analysis | We did not include the type of CBT as a possible subgroup analysis after we redefined the comparisons. | |
| For this review, we had planned to undertake sensitivity analyses to determine the effect of restricting the analysis to: "(a) only studies with low risk of selection bias (associated with sequence generation or allocation concealment), (b) only studies with low risk of performance bias (associated with issues of blinding), and (c) only studies with low risk of attrition bias (associated with completeness of data). In addition, we will assess the sensitivity of findings to any imputed data within a study." | "[W]e undertook sensitivity analyses to determine the effect of removing from the analysis: studies with high risk of selection bias (associated with sequence generation or allocation concealment); studies with high risk of performance bias (associated with issues of blinding); and studies with high risk of attrition bias (associated with completeness of data). In addition, we assessed the sensitivity of findings to any imputed data within a study." | |
| The transformation of the continuous results to relative percentage changes had not been foreseen. | We included the transformation of continuous results to relative percentage changes in Appendix 2. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ADHD symptoms (observer) Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 CBT vs supportive therapy | 1 | 81 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.01, ‐0.12] |
| 1.2 CBT vs waiting list | 2 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.03, ‐0.41] |
| 2 ADHD symptoms (self‐reported) Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 CBT vs supportive therapy | 2 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.52, 0.19] |
| 2.2 CBT vs waiting list | 5 | 251 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.18, ‐0.50] |
| 3 Inattention (clinician) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 CBT vs supportive therapy | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐2.47 [‐4.43, ‐0.51] |
| 3.2 CBT vs waiting list | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐4.1 [‐4.00, ‐2.20] |
| 4 Inattention: CBT vs waiting list (self‐reported) Show forest plot | 4 | 244 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.37, ‐0.82] |
| 5 Hyperactivity‐impulsivity: CBT vs waiting list (clinician) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Hyperactivity‐impulsivity: CBT vs waiting list (self‐reported) Show forest plot | 4 | 244 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.98, ‐0.22] |
| 7 Depression (self‐reported) Show forest plot | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 CBT vs supportive therapy | 1 | 81 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.36, 0.51] |
| 7.2 CBT vs waiting list | 5 | 258 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.60, ‐0.11] |
| 8 Anxiety: CBT vs supportive therapy (clinician) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Anxiety: CBT vs waiting list (self‐reported) Show forest plot | 4 | 239 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.71, ‐0.19] |
| 10 State anger: CBT vs waiting list (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11 Trait anger: CBT vs waiting list (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12 Self‐esteem (self‐reported) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 CBT vs Supportive Therapy | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.85, 1.85] |
| 12.2 CBT vs Waiting list | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 12.40 [4.55, 20.25] |
| 13 Quality of life: CBT vs waiting list (self‐reported) Show forest plot | 2 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.29, 0.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ADHD symptoms (clinician) Show forest plot | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.31, ‐0.30] |
| 2 ADHD symptoms (self‐reported) Show forest plot | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐7.42 [‐11.63, ‐3.22] |
| 3 Inattention (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Hyperactivity‐impulsivity (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Clinical Global Impression (clinician) Show forest plot | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.21, ‐0.30] |
| 6 Depression (clinician) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Depression (self‐reported) Show forest plot | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐6.09 [‐9.55, ‐2.63] |
| 8 Anxiety (clinician) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Anxiety (self‐reported) Show forest plot | 2 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐1.08, ‐0.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ADHD symptoms (clinician) Show forest plot | 2 | 97 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.98, ‐0.17] |
| 1.1 CBT vs relaxation + educational support | 1 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.97, ‐0.06] |
| 1.2 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.78, 0.11] |
| 2 ADHD symptoms (self‐reported) Show forest plot | 4 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.88, ‐0.01] |
| 2.1 CBT vs relaxation + educational support | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.26, ‐0.29] |
| 2.2 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.10, 0.71] |
| 2.3 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.57, 0.82] |
| 2.4 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.42, ‐0.00] |
| 3 Inattention (self‐reported) Show forest plot | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.61, 0.37] |
| 3.1 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.54, 0.85] |
| 3.2 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.08, 0.30] |
| 4 Hyperactivity: CBT vs psychoeducation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Impulsivity: CBT vs psychoeducation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Clinical Global Impression (clinician) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 CBT vs relaxation + educational support | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.09, 0.03] |
| 6.2 CBT vs psychoeducation | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.19, 0.55] |
| 7 Clinical Global Impression: CBT vs psychoeducation (self‐reported) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Depression (self‐reported) Show forest plot | 3 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.70, 0.16] |
| 8.1 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.32, 0.51] |
| 8.2 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.80, 0.59] |
| 8.3 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐1.04, 0.34] |
| 9 Anxiety (self‐reported) Show forest plot | 2 | 65 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.95, 0.04] |
| 9.1 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.04, 0.36] |
| 9.2 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐1.27, 0.13] |
| 10 Quality of life (self‐reported) Show forest plot | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 CBT vs cognitive training | 1 | 19 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐1.19, 0.62] |
| 10.2 CBT vs psychoeducation | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.37, 1.03] |
| 10.3 CBT vs skills handouts | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.17 [0.42, 1.92] |

